Abstract
Objective:
The present longitudinal study examined the role of neural cognitive control in the relation between negative and positive life events and depressive symptoms in adolescents.
Method:
The sample comprised 138 adolescents (52% male, Mage = 13.49 at baseline) and their parents. At Time 1, adolescents participated in a functional neuroimaging session in which neural cognitive control was measured as hemodynamic activity during an inhibitory control task, and parents reported on adolescents’ positive and negative life events within the past year. Adolescents and parents reported on adolescent depressive symptoms at Time 1, Time 2 (one year later), and Time 3 (two years later).
Results:
Conditional latent growth curve model was used to test the main and interaction effects of neural cognitive control and positive/negative life events on the growth factors of depressive symptoms. Higher neural cognitive control moderated the relation between negative life events and the intercept of depressive symptoms. Adolescents with higher neural cognitive control did not experience higher depressive symptoms when confronted with more negative life events, whereas their counterparts with lower neural cognitive control did. The interaction effect between neural cognitive control and positive life events on depressive symptoms was not significant.
Conclusions:
Results suggest that neural cognitive control acts as a protective factor such that adolescents with higher neural cognitive control are protected against depressionogenic effects of negative life events, whereas adolescents with lower cognitive control are at greater risk for depressive symptoms in response to negative life events.
Keywords: Depression, cognitive control, functional neuroimaging, life events, adolescence
Adolescence is a period in which youths undergo significant changes, including changes in the brain (Casey, Getz, & Galvan, 2008), adjusting to pubertal changes (Blakemore, Burnett, & Dahl, 2010), and transformations in their interpersonal relationships (Collins & Laursen, 2004). These changes can be perceived as demanding and make some adolescents more vulnerable to developing depressive symptoms during this particular developmental period. Indeed, research indicates increases in incidence and severity of depression during adolescence (Merikangas et al., 2010; Van Oort, Greaves-Lord, Verhulst, Ormel, & Huizink, 2009). Depressive symptoms during adolescence have been associated with impairments in several domains in adulthood, including continued mental health problems (Copeland, Shanahan, Costello, & Angold, 2009), suicidal behaviors (Fergusson, Horwood, Ridder, & Beautrais, 2005), and unemployment and welfare dependence (Fergusson, Boden, & Horwood, 2007), underscoring the necessity to better understand and prevent depression during adolescence.
Previous research has indicated that negative life events are a salient risk factor for depression (Hammen, 2005). The association between negative life events (and the associated perceived stress) and depression has been consistently replicated cross-sectionally and longitudinally, using community and clinical samples, for depressive disorders and depressive symptoms, and for minor and major stressors (Asselmann, Wittchen, Lieb, Höfler, & Beesdo-Baum, 2015; Braet, Van Vlierberghe, Vandevivere, Theuwis, & Bosmans, 2012; Carter & Garber, 2011; Low et al., 2012; Pettit, Lewinsohn, Seeley, Roberts, & Yaroslavsky, 2010). Because adolescence is a period with increases in stressful life events (Ge, Lorenz, Conger, Elder, & Simons, 1994) and because depression often originates in adolescence (Merikangas et al., 2010), examining the link between stressful life events and depression during this period is particularly relevant. Despite the strong link between negative life events and depressive symptoms, not all adolescents facing negative life events experience increases in depressive symptoms. Identification of the factors that may buffer the detrimental effects of negative life events on depression is critical given the long-term consequences and economic burden for society related to depression.
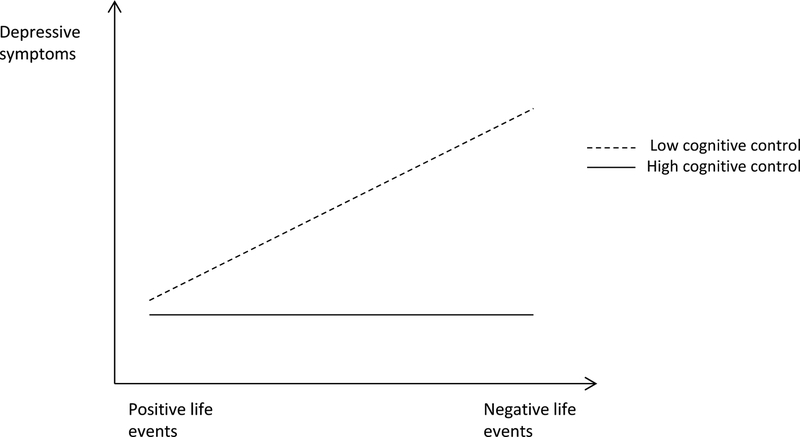
Previous research indicates that high cognitive control can protect against depression (Gotlib & Joormann, 2010). In their review on cognitive processes in emotion regulation related to depression, Joormann and D’Avanzato (2010) argued that cognitive control is important for responding in a flexible way and adapting behaviors and thoughts to changing contexts. That is, high cognitive control could be viewed as a protective factor, where adolescents with high cognitive control are protected against the effect of negative life events, whereas adolescents with low cognitive control experience more depressive symptoms in response to more negative life events. An illustration of cognitive control as a protective factor is depicted in Figure 1a. The figure is based on the protective-stabilizing factor from Luthar, Ciccetti and Becker (2000). In this figure, adolescents with high cognitive control do not experience more depressive symptoms in response to negative life events (i.e., are protected against the effect of negative life events), whereas adolescents with low cognitive control experience increasing depressive symptoms in response to negative life events. Consistent with this proposition, several studies indicate that high cognitive control predicts more effective emotion regulation and less stress reactivity (Compton et al., 2008, 2011; Hendricks & Buchanan, 2016; McRae, Jacobs, Ray, John, & Gross, 2012). In contrast, deficits in cognitive control may make it difficult to override attention and interpretation biases, diminish the ability for reappraisal, and make rumination more likely (Gotlib & Joormann, 2010; Joormann & D’Avanzato, 2010). Consistent with this notion, poor cognitive control could also be viewed as a vulnerability factor, in which adolescents with poor cognitive control are in general more susceptible to the effect of negative life events compared to individuals with high cognitive control. An illustration of low cognitive control as a vulnerability factor can be seen in Figure 1b. The figure is based on the vulnerable-reactive factor as described in Luthar et al. (2000). In this figure, both adolescents with high and low neural cognitive control experience increasing depressive symptoms in response to negative life events, but adolescents with low cognitive control experience more depressive symptoms in response to negative life events (i.e., are more vulnerable to the effect of negative life events) than adolescents with high cognitive control. Indeed, research has shown that depressed individuals experience deficits in cognitive control tasks (Snyder, 2013) and exhibit abnormalities in associated brain regions, including the prefrontal cortex (Disner, Beevers, Haigh, & Beck, 2011).
Figure 1a.
Cognitive control as a protective factor. In this Figure, adolescents with low cognitive control experience increasing depressive symptoms in response to negative life events, whereas adolescents with high cognitive control do not experience increasing depressive symptoms in response to negative life events, i.e., are protected against the effect of negative life events. The figure is based on Figure 1b in the article of Luthar et al., (2000).
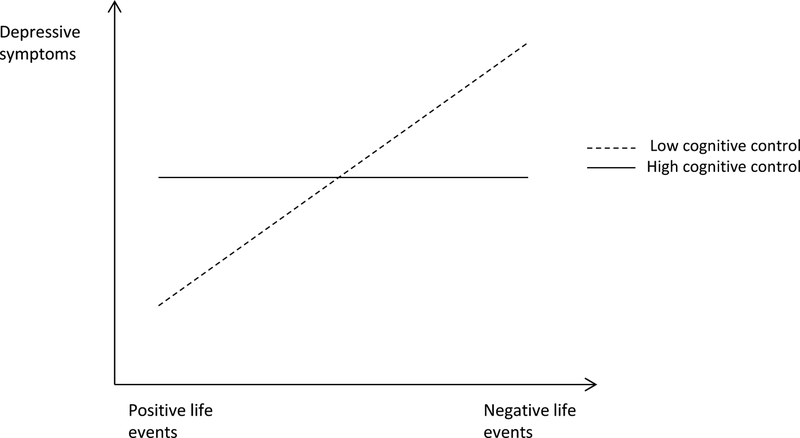
Figure 1b.
Cognitive control as a vulnerability factor. In this figure, both adolescents with high and low cognitive control experience more depressive symptoms in response to negative life events, however adolescents with low cognitive control experience stronger depressive symptoms compared to adolescents with high cognitive control, i.e., are more vulnerable to the effect of negative life events. The figure is based on Figure 1f in the article of Luthar et al., (2000).
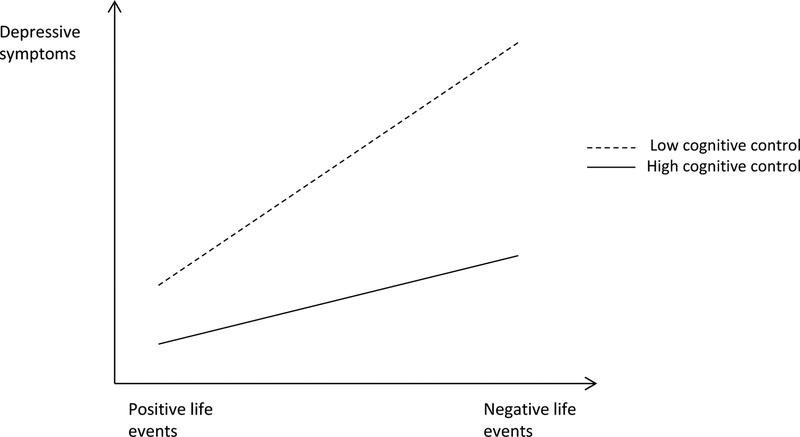
Figure 1c.
Cognitive control as a plasticity factor. In this figure, adolescents with low cognitive control experience more depressive symptoms in response to negative life events, but less depressive symptoms in response to positive life events compared to adolescents with high cognitive control. Figure is based on Figure 1a in the article of Belsky et al., (2007).
Two recent studies support the idea that high cognitive control can buffer the impact of negative life events on depression in adolescents. The first study included adolescent offspring of parents with a mood disorder, who completed an affective Go/No Go and a Verbal Fluency task (Davidovich et al., 2016). The results showed that a current parental depressive episode, a severe stressful life event for adolescents, increased adolescents’ depressive symptoms. However, adolescents with higher inhibitory control and mental flexibility showed significantly fewer depressive symptoms in the presence of parental depression, compared to their counterparts with lower inhibitory control and mental flexibility. The second study used two datasets of adolescents (one using a community sample, and the other using a sample with heightened familial risk for depression) to examine the associations among stressful life events, cognitive abilities, and depressive symptoms (Riglin et al., 2016). In both samples, more stressful life events predicted more depressive symptoms. However, this effect was moderated by cognitive control such that higher cognitive ability buffered the effect of stressful life events on depressive symptoms, albeit for girls only.
Taken together, these two studies implicate that high cognitive control may be a protective factor against the effects of negative life events on depression in adolescents. However, there are gaps in our understanding of the relation between life events, cognitive control, and depression in adolescence. First, the two previous studies by Davidovich and colleagues (2016) and Riglin and colleagues (2016) used a cross-sectional design. Extending this model to longitudinal data could help illuminate whether cognitive control acts as a protective factor in the development of depressive symptoms. Second, most available studies have focused on behavioral indices of cognitive control. Gottlib and Joorman (2010) argue that more research needs to be conducted on the neural underpinnings of cognitive control and its relation with depression. Extending evidence to the neural level is especially important when considering that the adolescent brain undergoes significant changes in prefrontal cortices underlying cognitive control. Specifically, research indicates that prefrontal cortex regions undergo maturation, including increased myelination and experience-dependent synaptogenesis and pruning (Paus, 2005) as well as strengthening of connections within prefrontal circuitry to adapt to changing environmental demands (Liston et al., 2006) throughout adolescence and into early adulthood. To our knowledge, it is not known whether cognitive control at a neural level could also work as a protective factor in the relation between negative life events and depression in adolescents.
Third, previous studies have predominantly focused on the effect of negative life events on depressive symptoms. However, there is evidence that positive life events can decrease depressive symptoms (Disabato, Kashdan, Short, & Jarden, 2017; Haeffel & Vargas, 2011; Spinhoven et al., 2011). In thinking of the moderating role of cognitive control for the effects of both stressful and supportive environmental conditions on adolescent depression, it will be particularly informative for prevention efforts to know (i) whether the detrimental effects of negative life events on the development of depressive symptoms would be reduced for adolescents with higher cognitive control (i.e., protective factor; Luthar et al., 2000), compared to adolescents with lower cognitive control (i.e., vulnerability factor; Sameroff, 1983), and (ii) whether the beneficial effects of positive life events against the development of depressive symptoms would be amplified for adolescents with lower cognitive control relative to their counterparts (i.e., plasticity factor; Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007). In other words, if low cognitive control is a plasticity factor, then adolescents with low cognitive control experience more depressive symptoms in response to negative life events, but fewer depressive symptoms in response to positive life events compared to adolescents with high cognitive control. An illustration of the three models can be found in Figure 1 (protective factor in Figure 1a, vulnerability factor in Figure 1b, and plasticity factor in Figure 1c).
Specifically, the differential susceptibility model (Belsky, 1997; Boyce et al., 1995) proposes individual differences in sensitivity to environmental influence, for better and for worse, such that those who are disproportionately vulnerable to adversity are also disproportionately likely to benefit from supportive environments. Although the differential susceptibility model does not theorize what may distinguish those individuals who are more susceptible from those who are less, evidence suggests that children at risk due to low self-regulation, difficult temperament, and negative affect show greater susceptibility to environmental influence (Ellis, Boyce, Belsky, Bakersman-Kranenburg, & Van IJzendoorn, 2011; Kim-Spoon, Haskett, Longo, & Nice, 2012). At the level of neural functioning, differential sensitivity to environmental exposures may be determined by systematic differences in the functioning of brain regions implicated in the filtering of incoming sensory information, including the prefrontal cortex (Ellis et al., 2011). Given the developmental literature indicating that adolescents’ prefrontal cortex functioning shows greater sensitivity to positive and negative environmental conditions compared to adults (Fuhrmann, Knoll, & Blakemore, 2015), then those with more immature and less efficient neural functioning related to cognitive control might arguably show heightened sensitivity to environmental contexts. However, to our knowledge, the moderating roles of neural cognitive control as a protective, vulnerability, or plasticity factor in the relation between positive and negative life events and depressive symptoms in adolescence have not yet been investigated.
In the present study, we used functional magnetic resonance imaging (fMRI) to investigate whether neural cognitive control (operationalized as hemodynamic activity during an inhibitory control task) moderated the link between positive and negative life events and adolescent depressive symptoms using a three-year longitudinal design. We hypothesized that negative life events predicted more, and positive life events fewer, depressive symptoms. Concerning the moderating effects of neural cognitive control, we did not form a specific hypothesis. We expected that if higher neural cognitive control were a protective factor, then the effects of negative life events on depressive symptoms would be attenuated for adolescents with higher neural cognitive control (Luthar et al., 2000). It could be that negative life events predict more depressive symptoms only for adolescents with lower neural cognitive control, in line with the dual-risk model (Sameroff, 1983). Alternatively, if data were to support the differential susceptibility model (Belsky, 1997), it was expected that adolescents with lower neural cognitive control would suffer from negative life events, but also benefit from positive life events. Specifically, adolescents with higher versus lower neural cognitive control were expected to react differentially to negative and positive life events such that those with lower neural cognitive control would be more susceptible to the effects of life events, whether the effects were detrimental or beneficial. Given the indication from prior research that adolescent girls react more strongly to negative life events than boys (Hankin, Mermelstein, & Roesch, 2007) and that the protective effect of cognitive control might only be evident for girls (Riglin et al., 2016), we additionally tested whether the moderated associations between negative life events and depressive symptoms varied by sex.
Methods
Participants and Procedure
Participants were 167 adolescents and their primary caregivers. Of the primary caregivers, 83% were the mother, 12% were the father, and 5% were others (e.g., grandparent, foster parent). The median family income ranged from $35,000 to 49,999 per year at Time 1. The sample represents an understudied sample of adolescents from economically disadvantaged rural communities in the Appalachian area of the United States. This sample faces unique challenges, such as relatively low income, geographical isolation, and limited prosocial recreational opportunities (Moreland, Raup-Krieger, Hecht, & Miller-Day, 2013). The adolescents showed a mean verbal IQ, as measured with the Kaufman Brief Intelligence Test (Kaufman & Kaufman, 2004), of 106.40 (SD = 14.08) which is within range of a normal IQ.
Of the 167 adolescents, 29 adolescents were not included in the final analyses due to missing or unusable imaging data (e.g., due to excessive motion), leaving a final sample of 138 adolescents (52% male, Mage = 13.49 at baseline, SD = 0.50, age range 13–14 years). Of the adolescents, 84.8% identified as white, 8.7% as African-American, and 6.5% as other. The excluded adolescents did not differ in demographic (age, income, sex) or study (positive and negative life events, depressive symptoms) variables at baseline (all ps > .15). They were however more likely to identify themselves as non-white (p = .001).
Data were collected across the course of two years. At Time 1, adolescents participated in fMRI sessions, in which they completed an inhibitory control task in the scanner, and parents completed a questionnaire about positive and negative life events of the adolescent. Moreover, at baseline (Time 1), one year later (Time 2), and two years later (Time 3), adolescents and their parents rated the adolescent’s depressive symptoms. Attrition was relatively low in our sample, with 122 adolescents (88%) still participating at Time 3. Participants were recruited at 13 – 14 years of age by diverse advertisement methods including flyers, recruitment letters, and e-mail distributions. Research assistants described the nature of the study to interested individuals over the telephone and invited them to participate. The exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for more than 10 minutes, orthodontia impairing image acquisition, and contraindications to magnetic resonance imaging. Data collection took place at the university’s offices where adolescents and their primary caregivers were interviewed by trained research assistants. During the sessions, adolescents and caregivers filled out questionnaires and adolescents participated in the fMRI session. All adolescent participants provided written assent and their parents provided written consent for a protocol approved by the university’s institutional review board. Both parents and adolescents received monetary compensation for their time.
Measures
Positive and negative life events.
At baseline, parents filled out the 38-item Parent version of the Child and Adolescent Survey of Experiences (CASE; Allen, Rapee, & Sandberg, 2012). Parents were required to indicate whether one of the listed life events had occurred to their child in the previous 12 months (e.g., “Someone in our family was really sick or injured”). If a specific event occurred, parents were asked to rate the impact of the events (ranging from 1 = “really good” to 6 = “really bad”). If an event was rated as a little good/quite good/really good, then the event was categorized as a positive event. If an event was rated as a little bad/quite bad/really bad, then the event was categorized as a negative event. Examples of potentially negative events included “My child was teased or bullied”, “My child was really sick or injured”, and “My child saw something bad happen (e.g., car accident, someone being robbed)”. Examples of potentially positive events included “My child made a new special friend”, “My child was chosen to be class monitor, prefect or school captain”, and “My child went on a special vacation”. All events could either be rated as positive or negative events. That means that not all events were rated as positive or negative in the same way. For instance, there are some ambiguous events that could be either perceived as positive or negative, such as “We moved house”, “My partner or I started a new job”, or “My partner or I had a baby / found out we are going to have a baby”. The number of positive and negative life events was calculated as the sum of the life events rated as having a positive or negative impact, respectively. The CASE demonstrates good test-retest reliability, good agreement between mothers and children concerning the number of life events reported (Allen et al., 2012), and good validity, showing significant associations with anxiety and depression (e.g., Allen & Rapee, 2009).
Depressive symptoms.
At Time 1, Time 2, and Time 3, parents filled out the Child Behavior Checklist (CBCL; Achenbach, 1991) and adolescents filled out the Youth Self Report (YSR; Achenbach, 1991). Depressive symptoms were calculated from the 13-item affective problems subscale (e.g., “There is very little I enjoy”), which correspond closely to DSM-IV diagnostic criteria for major depression and dysthymia (Achenbach, Dumenci, & Rescorla, 2003). The items are rated on a 3-point scale with response options ranging from 0 = “not true” to 2 = “very true or often true.” Previous research has provided strong evidence for the reliability and convergent and discriminant validity of the affective problems subscale (Ferdinand, 2008). In the present sample, Cronbach’s alpha ranged from .79-.80 for parent report and .76-.81 for adolescent report across the three time-points. Adolescent and parent reports (r = .29-.43, p < .001) were averaged into a composite score with higher scores indicating higher depressive symptoms.
Imaging acquisition and analysis.
Adolescents participated in an fMRI session at baseline. Functional neuroimaging data were acquired on a 3T Siemens Tim Trio MRI scanner with a standard 12-channel head matrix coil. Echo-planar images (EPIs) were collected using the following parameters: slice thickness = 4mm, 34 axial slices, field of view (FoV) = 220 × 220mm, repetition time (TR) = 2 s, echo time (TE)= 30 ms, flip angle= 90 degrees, voxel size = 3.4375 × 3.4375 × 4 mm (during analysis the images were resliced so that voxels were 3 × 3 × 3mm), 64 × 64 grid, and slices were hyperangulated at 30 degrees from anterior-posterior commissure. The structural scan was acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence with the following parameters: TR = 1200 ms, TE = 3.02 ms, FoV = 245 × 245mm, and 192 slices with the spatial resolution of 1 × 1 × 1mm. FMRI data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). For each scan, functional imaging data were corrected for head motion using a six-parameter rigid body transformation and realigned. Functional volumes were normalized using parameters from a segmented anatomical image coregistered to the average EPI and smoothed using a 6mm full-width-half-maximum Gaussian filter.
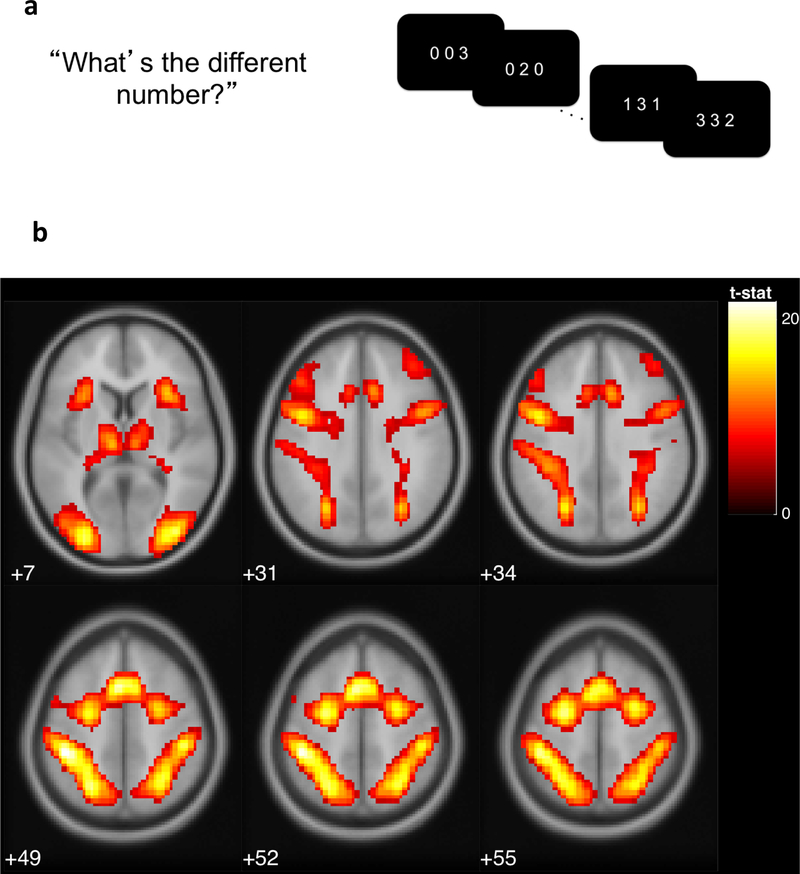
While in the scanner, adolescents completed a multi-source interference task (MSIT; Bush, Shin, Holmes, Rosen, & Vogt, 2003), as illustrated in Figure 2a, during which blood oxygenation level dependent (BOLD) responses were assessed. In the task, adolescents were presented with three digits, two of which were identical. Adolescents were required to indicate the identity, but not the position of the oddball digit. In neutral trials, the identity of the target digit was congruent with its presented location (e.g., “2” was in the second position in the sequence “020”). In interference trials, the identity of the target digit was incongruent with its presented location (e.g., “2” was in the third position in the sequence “112”). In total, there were 96 interference and 96 neutral trials. Mean reaction time for neutral and interference trials were 1.11 and 0.64 seconds, respectively, and mean accuracy for neutral and interference trials were 98.32% and 88.35%, respectively.
Figure 2.
a) In the multi-source interference task (MSIT), adolescents were asked to identify the digit that differed from two other concurrently presented digits, ignoring its position in the sequence. b) Adolescents exhibited greater activation for interference relative to neutral conditions in the regions of left posterior-medial frontal cortex, right and left inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus, displayed at p(FWE) < .001 (see Appendix A). Figure adapted from Kim-Spoon, Maciejewski, Lee, Deater-Deckard, & King-Casas (2017).
We found a significant interference effect, indicating that accuracy was lower, t(137) = −14.33, p < .001, and reaction time was higher (i.e., slower), t(137) = 69.40, p < .001, in interference compared to neutral trials. We calculated difference scores (i.e., interference minus neutral condition) for accuracy and reaction time for correct responses (reverse coded) and used these two standardized variables as indicators of a latent behavioral cognitive control variable with higher scores indicating higher behavioral cognitive control during the MSIT. The measurement model was a fully saturated model with the factor loadings constrained to be equal between the two indicators for identification purposes (standardized factor loadings = .69, ps < .001).
Following preprocessing, we estimated a General Linear Model (GLM) for each participant using Statistical Parametric Mapping (SPM8; Welcome Department of Cognitive Neurology, London). Interference and neutral trials were each modeled using a boxcar function convolved with a canonical hemodynamic response function. Six motion realignment parameters were included as nuisance covariates. A low-pass filter was applied with a cutoff of 128 seconds to model out low-frequency noise. The contrast of interest was interference minus neutral. These contrasts of interest were entered into a second-level one-sample t-test to obtain whole-brain maps of voxels with significant differences in interference and neutral mean activation.
For each participant, individual-level regions-of-interest (ROI) values were extracted at coordinates corresponding to peak activations the second-level analysis of interference minus neutral contrasts. Specifically, the first eigenvariate values of the contrast images were extracted using spherical masks of 6 mm surrounding MNI coordinates, thresholded at p < .001, family-wise error corrected. Among these extracted ROI values (see Appendix A for all activated areas), (1) regions known to be engaged by cognitive control related to interference- and error-processing (Fitzgerald et al., 2010; Koechlin, Ody, & Kouneliher, 2003; Roberts & Hall, 2008) and (2) regions significantly correlated with behavioral performance (i.e., absolute magnitude of correlation > .2 with the behavioral cognitive control factor score) were chosen as manifest indicators of the neural cognitive control factor. These ROIs included left posterior-medial frontal cortex, right and left inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus (see Figure 2b).
Using these ROIs, we conducted confirmatory factor analyses, in which the selected indicators loaded on an overall neural cognitive control factor. Based on modification indices, we included residual correlations between left and right inferior parietal lobules, right inferior frontal gyrus and left middle frontal gyrus, right superior frontal gyrus and left middle frontal gyrus, and right inferior frontal gyrus and left posterior-medial frontal cortex. The final model showed a good fit (χ2 = 23.48, df = 16, p = .10, CFI = .99, RMSEA = .06). Standardized factor loadings ranged from .58 to .86 (all ps < .001). This neural cognitive control factor score correlated significantly with the behavioral cognitive control score (r = −.43, p < .001), indicating that higher interference-related BOLD responses (i.e., higher activation) were associated with lower behavioral cognitive control. Previous papers illustrate how creating latent factor scores based on confirmatory factor analysis (CFA) is well suited for integrating multiple ROIs based on whole-brain analysis (e.g., Kim-Spoon et al., 2016; Nees et al., 2012). As has been shown in many functional neuroimaging studies, a single region can be involved in a broad range of tasks. Thus, it is unlikely that there is always one core region that is crucial for a particular function (Kanai & Rees, 2011). Therefore, we believe that the multivariate approach to analyzing multiple ROIs related to a particular function during a behavioral task is a promising way to address correlations between ROIs for the same function, as it can potentially reveal common underlying components and any neural substrates. Verbal IQ was significantly correlated with the latent behavioral cognitive control score (r = .28, p < .001), but not with the latent neural cognitive control score (r = −.10, p =.23).
Analytic Strategy
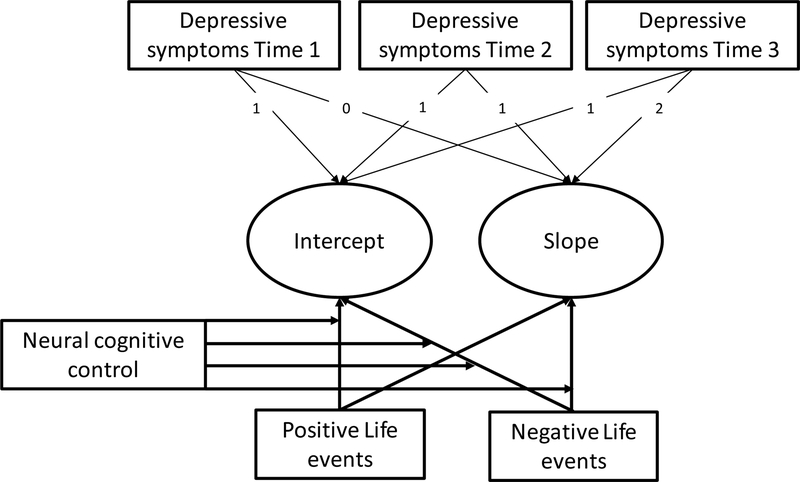
To test our research question concerning the joint effects of positive and negative life events and neural cognitive control on adolescent depressive symptoms and whether these effects differed by sex, we conducted latent growth curve moderation analyses in Mplus 7.4 (Muthén & Muthén, 1998-2012). The analyses followed recommendations for moderated models by Hayes (2013) using Mplus scripts based on the codes developed by Stride, Gardner, Catley, and Thomas (2015). We started by fitting an unconditional latent growth curve model (LGM; i.e., without covariates). In LGM, developmental trajectories are captured by two latent factors; the intercept (or the starting level) and a slope (or the change over time). Residual variances of the manifest depressive scores Time 1 to Time 3 were estimated to be equal over time. Using this LGM, we conducted conditional LGM for moderation analyses (see Figure 3 for an explanation of the model). In Step 1 (i.e., main effects model), we regressed the intercept and slope of depressive symptoms on positive and negative life events and the neural cognitive control score. In Step 2 (i.e., interaction effects model), we calculated two-way interaction terms between positive life events and neural cognitive control scores as well as between negative life events and neural cognitive control scores. Again the intercept and the slopes were regressed on these two two-way interaction scores. We followed up significant interaction effects using simple slope analyses, contrasting adolescents with higher interference-related BOLD responses during MSIT (+1 SD; i.e., lower neural cognitive control) and adolescents with lower interference-related BOLD responses during MSIT (−1 SD; i.e., higher neural cognitive control). In order to test whether sex moderated the associations, we added three two-way interaction terms between sex and positive life events, sex and negative life events, and sex neural cognitive control, as well as two three-way interaction terms between sex, positive life events, and neural cognitive control and between sex, negative life events and neural cognitive control. All continuous predictors were mean-centered to avoid multicollinearity and for better interpretation of significant moderation effects. The data resembled a Complete Missing at Random Pattern (Little’s Missing Completely At Random (MCAR) test: χ2(17) = 23.04, p = .15). Therefore, we used maximum likelihood estimation with robust standard errors (MLR) to control for missing data and non-normality. Model fit was evaluated by examining the Comparative Fit Index (CFI) and the root mean square error of approximation (RMSEA). Hu and Bentler (1999) recommend cutoff values of a value > .95 for CFI and < .05 for RMSEA. We performed simple slope analyses for significant interaction effects (contrasting 1 SD below the mean and 1 SD above the mean).
Figure 3.
Graphical representation of model testing for the influence of positive/negative life events on the development of depressive symptoms, moderated by neural/behavioral cognitive control
Results
Prior to the analyses, values that deviated more than 3 SD from the mean were Winsorized to the next value that was not an outlier (n = 11). Multivariate general linear modeling revealed that of the demographic variables (i.e., race, sex, income, age), only sex was a significant predictor of depressive symptoms at all time-points (all ps < .03); race, income, and age were not significant predictors for depressive symptoms at any time-point (all ps > .22). Thus, we only controlled for sex in all of our models. We additionally tested the strengths of the relations between the demographic factors and the predictor variables (i.e., life events and cognitive control). The only significant associations were between income and positive life events (r = .19, p = .02), indicating that higher income was associated with more positive life events; and between sex and behavioral cognitive control [t(152) = 2.54, p = .01], indicating that boys showed higher behavioral cognitive control than girls. Descriptive statistics and zero-order correlations among study variables are presented in Table 1.
Table 1.
Descriptive Statistics and Zero-order Correlations Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | M | SD | Min | Max | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Depressive symptoms Time 1 | 3.16 | 2.76 | 0.00 | 11.00 | ||||||
| 2. Depressive symptoms Time 2 | .74*** | 3.25 | 2.55 | 0.00 | 9.50 | |||||
| 3. Depressive symptoms Time 3 | .67*** | .72*** | 3.64 | 2.83 | 0.00 | 12.00 | ||||
| 4. Number of positive life events Time 1 | .06 | .12 | .04 | 5.82 | 2.28 | 0.00 | 12.00 | |||
| 5. Number of negative life events Time 1 | .21* | .14 | .22* | −.01 | 3.19 | 2.40 | 0.00 | 10.00 | ||
| 6. Neural cognitive control Time 1 | .16 | .17 | .20* | .01 | −.09 | 0.80 | 0.39 | −0.24 | 1.81 |
Note.
p < .001,
p < .05
We first started with fitting an unconditional LGM for the depressive symptoms across the three time-points. Results of this LGM are presented in Table 2. This LGM showed an excellent fit. The analyses revealed that there was significant variation around the intercept (p < .001). In addition, there was a positive, yet only marginally significant linear slope (p = .07) and marginally significant variation around the slope (p = .06).
Table 2.
Results of Unconditional Growth Curve Model For Developmental of Depressive Symptoms from Time 1 to Time 3.
| χ2 | df | CFI | RMSEA | |||
| Model Fit | 1.76 | 3 | 1.00 | 0.00 | ||
| Mean | Variance | |||||
| Estimate | SE | p | Estimate | SE | p | |
| Parameters Intercept | 3.13 | 0.23 | <0.001 | 5.59 | 1.05 | <0.001 |
| Parameters Slope | 0.19 | 0.10 | 0.07 | 0.41 | 0.22 | 0.06 |
The results of the moderation analyses can be found in Table 3. Analyses from Step 1 showed main effects of both negative life events and neural cognitive control on the intercept of depressive symptoms. Specifically, more negative life events and lower levels of neural cognitive control were associated with higher depressive symptoms at Time 1. No effects of positive life events and on the slope of depressive symptoms were found.
Table 3.
Parameter Estimates for Effects of Negative Life Events on Adolescent Depressive Symptoms, Moderated by Neural Cognitive Control
| Model Fit | ||||
| χ2 | df | CFI | RMSEA | |
| Main effects model | 4.85 | 7 | 1.00 | 0.00 |
| Interaction effects model | 10.73 | 9 | 0.99 | 0.04 |
| Parameter estimates | ||||
| Effect on intercept | Effect on slope | |||
| B [95% CI] | b* [95% CI] | B [95% CI] | b* [95% CI] | |
| Step 1: Main effects | ||||
| Positive life events | 0.11 [−0.06; 0.29] | .11 [−.05; .27] | −0.03 [−0.11; 0.06] | −.09 [−.39; .21] |
| Negative life events | 0.22* [0.05; 0.38] | .22* [.05; .38] | −0.01 [−0.09;0.07] | −.03 [−.34; .27] |
| Neural cognitive control | 1.14* [0.15; 2.13] | .19* [.03; .34] | 0.07 [−0.41; 0.56] | .05 [−.25; .34] |
| Step 2: Interaction effects | ||||
| Positive life events*Neural cognitive control | −0.06 [−0.33; 0.21] | −.03 [−.15; .10] | −0.11 [−0.25; 0.03] | −.18 [−.43; .06] |
| Negative life events*Neural cognitive control | 0.55* [0.07; 1.02] | .17* [.02; .32] | −0.02 [−0.23; 0.20] | −.02 [−.27; .23] |
Note. Model is controlled for adolescent sex. Neural cognitive control was operationalized as interference-related BOLD responses during MSIT with higher values indicating lower neural cognitive control.
p < .05.
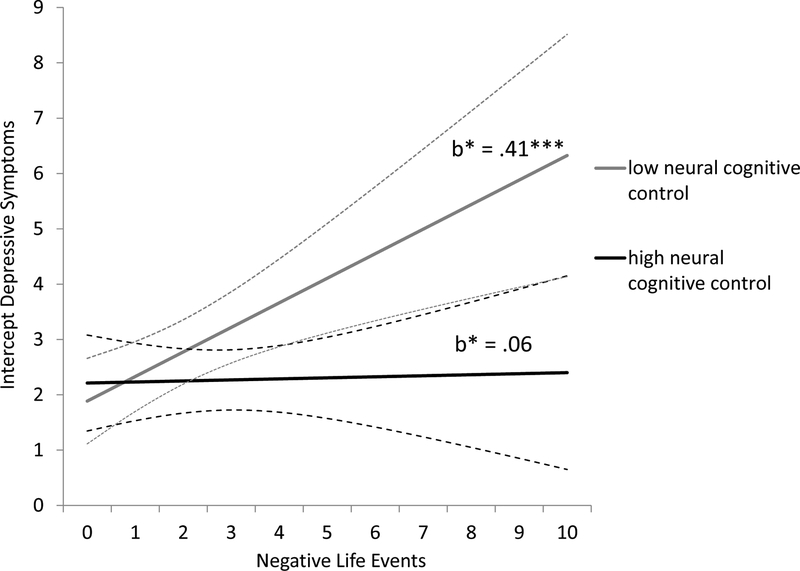
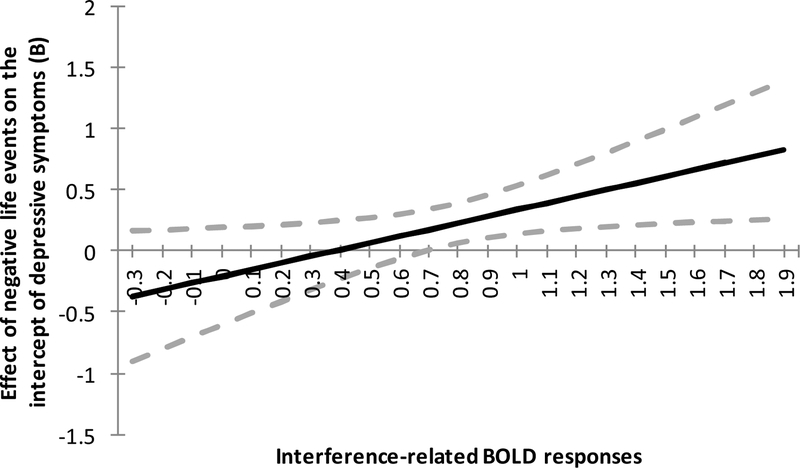
In Step 2, analyses revealed a significant interaction effect between negative life events and neural cognitive control on the intercept of depressive symptoms. This significant interaction effect was probed by contrasting adolescents with higher interference-related BOLD responses during MSIT (+1 SD; i.e., lower neural cognitive control) versus adolescents with lower interference-related BOLD responses during MSIT (−1 SD; i.e., higher neural cognitive control). As shown in Figure 4, more negative life events were related to more depressive symptoms at Time 1 (i.e., the starting level) only for adolescents with lower neural cognitive control (B = 0.44, 95% CI [0.18; 0.71], b* = 0.41), but not for adolescents with higher neural cognitive control (B = 0.02, 95% CI [−0.22; 0.25], b* = .06). We additionally present the results using the Johnson-Neyman technique (Johnson & Fay, 1950) to plot regions of significance and identify regions of significance. Figure 5 shows the conditional effect of negative life events on the intercept of depressive symptoms for different levels of the neural cognitive control variable for the minimum and maximum value of the scale. Regions of significance calculations suggested that the regions of significance extended from 0.69 and above, indicating that any simple slope at or above this value is statistically significant, which included 53% of the current sample.
Figure 4.
Simple slope analyses for the effect of negative life events at Time 1 on the intercept of depressive symptoms for adolescents with high cognitive control versus low cognitive control. 95% confidence intervals are indicated by dashed lines. Standardized estimates are given. Low interference-related BOLD responses (i.e., indicating higher cognitive control) is defined as 1 SD below the mean, high interference-related BOLD responses (i.e., indicating lower cognitive control) as 1 SD above the mean. *** p < .001.
Figure 5.
Johnson-Neyman plot showing the moderating effects of interference-related BOLD responses (i.e., neural cognitive control) on the association between negative life events at Time 1 and the intercept of depressive symptoms. 95% confidence intervals are indicated by dashed lines. Scale goes from minimum to maximum of the interference-related BOLD responses (−0.24 to 1.81). Higher values of interference-related BOLD responses mean lower neural cognitive control.
The interaction effect between neural cognitive control and positive life events on the intercept of depressive symptoms was not significant. Moreover, we found no significant interaction effects between neural cognitive control and negative or positive life events on the slope of depressive symptoms. This finding suggests that adolescents with more negative life events coupled with lower neural cognitive control already start out with more depressive symptoms than their adolescent counterparts, but that all adolescents exhibited similar developmental trajectories of depressive symptoms, regardless of their neural cognitive control.
In order to examine whether the interaction effect on depressive symptoms persisted over time, we reran the LGM and rescaled the intercept at Time 3 (i.e., intercept was scaled as −2, −1, 0). In this model, we also found a significant interaction effect between negative life events and neural cognitive control on the intercept (B = 0.51, 95% CI [0.02; 1.01], b* = .16). Simple slope analyses confirmed that more negative life events predicted more depressive symptoms at Time 3 for adolescents with lower neural cognitive control (B = 0.42, 95% CI [0.13; 0.70], b* = .38), but not if they showed higher neural cognitive control (B = 0.01, 95% CI [−0.20; 0.23], b* = .06).
We tested whether any of the main or interaction effects involving neural cognitive control were moderated by sex. We found no significant interaction effects by sex, indicating that these effects were comparable between boys and girls (Step 3, all ps > .07). As supplemental analyses, we reran the models using the behavioral cognitive control score (i.e., factor scores based on accuracy and reaction time difference scores as described in the Methods section) instead of neural cognitive control (i.e., factor scores based on BOLD responses). In this model, neither the main effects of behavioral cognitive control nor the interaction effects between behavioral cognitive control and positive or negative life events were significant (all ps > .39; see Appendix B for estimates), indicating that the protective effect of cognitive control against negative life events was evident only at the neural, but not at the behavioral level.
Additionally, we re-ran the final model with only adolescent-reported depressive symptoms to check for specificity of informants. The moderating effect between negative life events and neural cognitive control was in the same direction, however, not significant in these analyses (B = .52, SE = .33, p = .12, b* = .13). We also tested for specificity of our findings to depressive symptoms by rerunning the final moderation model with two highly comorbid psychopathology problems, namely attention deficit hyperactivity disorder (ADHD) and anxiety, both assessed by the YSR and CBCL. In both models, we did not find moderating effects of neural cognitive control on the relation between negative life events and the intercept of ADHD (B = −0.11, SE = 0.32, p = .73) and anxiety symptoms (B = −0.12, SE = 0.19, p = .52), suggesting that the protective effect of neural cognitive control is specific to depressive symptoms.
Discussion
The aim of the present longitudinal study was to test whether neural cognitive control, as measured by interference-related BOLD responses during an inhibitory control task, moderated the effects of positive and negative life events on the level and developmental change of depressive symptoms in adolescents. Our results of significant interactions suggested that adolescents with higher neural cognitive control did not experience elevated depressive symptoms when being confronted with negative life events, whereas their counterparts with lower neural cognitive control did. However, we did not find indications that neural cognitive control moderated the relation between positive life events and depressive symptoms. Our results further suggested that the associations applied to both adolescent boys and girls. The findings emphasize that higher neural cognitive control may make adolescents less susceptible to depressive symptoms that result from the detrimental effects of negative life events.
Our findings of the protective effects of neural cognitive control supports the classic view of resilience that regards traits such as intelligence and cognitive skills as protective processes residing within the child (Garmezy, 1991). Specifically, the pattern of the negative life events by neural cognitive control interaction follows the protective-stabilizing model (Luthar et al., 2000), showing that the presence of high neural cognitive control confers stability in good functioning despite increasing risk (i.e., high levels of stress related to negative life events). Thus, the current findings lend support to the theoretical perspectives emphasizing the top-down modulation of prefrontal cortex functioning in adolescent brain development (Casey, Galván, & Somerville, 2016; Kim-Spoon et al., 2017). Emotion regulation models of depression may elucidate why high cognitive control demonstrated protective effects against depression. According to Gross’s (2002) process model of emotion regulation and depression, better cognitive control may facilitate reappraisal of a situation which in turn modifies one’s emotional response. Thus, adolescents who have higher cognitive control may be better able to reinterpret stressful or negative life events to reduce resulting negative emotions. In this way, individuals with strong cognitive control may be able to avoid maladaptive emotion regulation strategies, such as rumination, which maintain negative affect and perpetuate depressive symptomatology (Joormann & D’Avanzato, 2010).
The results of the present study significantly contribute to our understanding of the modulating role of cognitive control above and beyond the findings from previous behavioral studies demonstrating that high cognitive control/abilities can protect adolescents against the negative effects of stressful events on depression (Davidovich et al., 2016; Riglin et al., 2016). First, our longitudinal data spanning three years suggest that neural cognitive control acted as a moderator not only for the relation between negative life events and depressive symptoms cross-sectionally at baseline, but that it also buffered the relation between negative life events at baseline and depressive symptoms two years later. This result clarifies prior findings based on cross-sectional data by evidencing a long-lasting protective effect of neural cognitive control against the detrimental effect of negative life events. That is, these results suggest that even events happening in early adolescence have a lingering effect on depressive symptoms in middle adolescence, highlighting the important role of negative life events on depressive symptoms that can be moderated by neural cognitive control. However, we did not find that neural cognitive control moderated the effect of negative life events on the slope of depressive symptoms. This finding indicates that while adolescents with lower neural cognitive control in general showed more depressive symptoms in the face of negative life events compared to adolescents with higher cognitive control, they did not differ with respect to the rate of change of their depressive symptoms.
Second, our study focused on the neural underpinnings of cognitive control, whereas both Davidoch and colleagues (2016) and Riglin and colleagues (2016) used behavioral indices of cognitive control. We found moderating effects of neural activation during a cognitive control task, demonstrating how neurobiological processes related to cognitive control can modulate environmental effects on emotional problems. In contrast to the two previous studies, we did not evidence a moderating effect of behavioral indicators during inhibitory control. However, there are some differences between our study and the other two studies that might explain this discrepancy. Our study involved a community sample of adolescents, whereas prior studies by Riglin and colleagues (2016) and Davidoch and colleagues (2016) included at-risk adolescents whose parents had mood disorders. Thus, further research is warranted to clarify whether the modulating roles of neural versus behavioral cognitive control may vary across samples of differing levels of risk for depression. In addition, we used an inhibitory control paradigm that does not involve affect or motivation, whereas Davidovich and colleagues (2016) used a task that assesses cognitive control over affective stimuli (one of their two tasks). Specifically, the first task was a verbal fluency task to assess mental flexibility (i.e., thinking of as many words as possible with beginning letters F, A, and S). The second task was an affective go/no go task to measure inhibitory control and set-shifting (i.e., pressing a button when a presented word with positive or negative valence was matched the target valence, while withholding their response when the word did not match the target valence). Riglin and colleagues (2016) measured cognitive ability, rather than cognitive control per se, using a standardized assessment of verbal reasoning (i.e., sentence completion, verbal classification, and verbal analogies), nonverbal reasoning (figure matrices, paper folding, and figure classification), and quantitative reasoning (i.e., number analogies, number puzzles, and number series). Finally, one explanation for the discrepancy between the behavioral and neural results in our study may be that the modulating role may be specific to the neural computations occurring during inhibitory control, rather than manifested behavior. This may be in part due to the fact that behavior tested using a laboratory task may be limited in representing real-life behaviors, whereas task-related neural responses more reliably capture individual differences in neurobiological vulnerability (Richards, Plate, & Ernst, 2013).
Third, unlike prior research, we examined the effect of positive life events on depressive symptoms, given the evidence suggesting that positive events can decrease depressive symptoms (e.g., Spinhoven et al., 2011). Here, we were interested in testing whether the data are consistent with the differential susceptibility model (Belsky, 1997; Boyce et al., 1995) showing that lower neural cognitive control may act as a plasticity factor, in which the most susceptible individuals are disproportionately negatively affected by negative life events, but also benefit disproportionately from positive life events. The non-significant interaction between neural cognitive control and positive life events indicated the lack of evidence to support the differential susceptibility model. Instead, our data are most consistent with the protective-stabilizing model (Luthar et al., 2000), suggesting that higher neural cognitive control attenuated the association between negative life events and depressive symptoms.
Limitations of the present study suggest fruitful directions for future research. First, life events were measured only by a single assessment at Time 1 and their interactive effects with neural cognitive control on the development of depressive symptoms may reflect the effects of concurrent life events, rather than prospective effects of earlier life events. Further, it is also plausible that early life stress, which is putatively correlated with Time 1 life events, might be the driving force. Future studies will benefit by involving repeated-measures data of life events to achieve developmentally nuanced effects of life events. Likewise, it is important for future research to examine the interaction between cognitive control and life events over time, particularly for testing how the moderating effects of cognitive control may vary within individuals over time. Second, depressive symptoms were based on adolescent and parent self-reports, rather than on diagnostic interviews. Although there is the advantage of using multiple informants to reduce method variance due to possible single informant bias, it might be that parents did not adequately rate all depressive symptoms of adolescents, because some symptoms regard internal states and might not be visible to parents. Nevertheless, we believe that using parent reports of depressive symptoms adds incremental validity. Third, to measure neural cognitive control, we used the MSIT which combines multiple dimensions of cognitive interference (i.e., Stroop, Eriksen, and Simon) with decision-making and other factors known to activate the cingulo-frontal-parietal cognitive/attention network, which is related to target detection, error detection, response selection, and stimulus/response competition (Bush et al., 2003). Yet, the question of whether the current findings regarding neural cognitive control may be generalized to tasks focusing on other important aspects of cognitive control, such as working memory, awaits further replications using diverse tasks to assess neural cognitive control. Finally, life events were only measured by parent report. Using parent reports of adolescents’ life events could have resulted in missing important life events that were experienced by the adolescent, but which the parent did not know about. In addition, parental perception of life events may not accurately reflect events perceived by the adolescent as positive or negative. We recommend that future research will consider diverse approaches to comprehensively capture the effects of life events, such as using interview-based methods of identifying life events and comparing subjectively versus objectively defined positive and negative events.
Despite the limitations, there are also methodological strengths to note. First, we used longitudinal depressive symptom data, multiple informants (i.e., parent and adolescent reports), and multiple levels of data encompassing brain activation, behavioral performance, and questionnaires. Second, we used latent factor measurement models to create variables that best capture adolescent cognitive control at both neural and behavioral levels. In particular, our work illustrates using latent factor scores to integrate multiple ROIs based on whole brain analysis, guided by prior knowledge and empirical data. This multivariate approach offers a promising way not only to create conceptually sound neural constructs, but also to address correlations between ROIs for the same function and reduce multiple comparisons.
In closing, this is the first study demonstrating that neural cognitive control moderates the relation between negative life events and depressive symptoms among adolescents, both cross-sectionally, and two years later. Our findings provide critical evidence that supports the current theoretical views of adolescent brain development (e.g., Casey et al., 2016) by providing evidence suggesting that neural cognitive control systems interact with contextual factors (such as negative life events) to predict interindividual differences in adolescent psychopathology. Also, the current findings offer important implications for clinical prevention and intervention efforts. Our findings may be useful in identifying adolescents who are most vulnerable to developing depressive symptoms following negative life experiences. In addition, our findings illustrate cognitive control as a protective factor in the development of adolescent depressive symptoms, and point toward specific neural systems that can be targeted to elicit changes in symptoms. There is evidence that neurobehavioral therapies can be used to initiate change at a neural level. For example, Siegle, Ghinassi, and Thase (2007) introduced a neurobehavioral intervention involving cognitive control training for individuals diagnosed with depression and demonstrated decreased disruptions in neural activity in the dorsolateral prefrontal cortex while engaging in a cognitive task. Our findings suggest that these types of neurocognitive interventions may meaningfully benefit adolescents who are at increased risk for depression related to negative life events. Improving adolescents’ prefrontal control may enable them to attain cognitive resources that can mitigate the detrimental effects of negative experiences and impede trajectories of maladaptation.
Acknowledgement:
This work was supported by grants awarded to Jungmeen Kim-Spoon and Brooks King-Casas from the National Institute on Drug Abuse (DA036017). We are grateful to the adolescents and parents who participated in this study.
Appendix A:
Areas of significant activation for the contrast of Interference minus Neutral blocks of the Multi-Source Interference Task at Time 1
| Cluster | Peak | MNI Coordinates | Region | |||
|---|---|---|---|---|---|---|
| k | p(FWE) | t | x | y | z | |
| 759 | < .001 | 21.84 | −42 | −37 | 49 | L postcentral gyrus |
| 19.61 | −24 | −64 | 49 | L superior parietal lobule | ||
| 18.07 | −30 | −55 | 52 | L inferior parietal lobule | ||
| 265 | < .001 | 20.43 | −3 | 14 | 49 | L posterior-medial frontal |
| 506 | < .001 | 20.42 | −39 | −85 | −2 | L inferior occipital gyrus |
| 20.15 | −30 | −91 | −2 | L inferior occipital gyrus | ||
| 19.05 | −39 | −73 | −8 | L fusiform gyrus | ||
| 654 | < .001 | 19.21 | 42 | −64 | −8 | R fusiform gyrus |
| 19.17 | 42 | −82 | −2 | R inferior occipital gyrus | ||
| 18.46 | 33 | −91 | 1 | R inferior occipital gyrus | ||
| 15.78 | 39 | −67 | −23 | R cerebellum (crus 1) | ||
| 15.24 | 33 | −49 | −26 | R cerebellum (VI) | ||
| 245 | < .001 | 19.06 | −24 | −4 | 55 | L middle frontal gyrus |
| 431 | < .001 | 18.12 | 30 | −58 | 52 | R inferior parietal lobule |
| 18.11 | 45 | −31 | 49 | R postcentral gyrus | ||
| 16.52 | 30 | −64 | 40 | R superior occipital gyrus | ||
| 140 | < .001 | 17.38 | 27 | −4 | 55 | R superior frontal gyrus |
| 94 | < .001 | 16.89 | −45 | 2 | 34 | L inferior frontal gyrus (pars opercularis) |
| 46 | < .001 | 15.01 | −9 | −19 | 10 | L thalamus |
| 24 | < .001 | 14.50 | 33 | 20 | 7 | R insula lobe |
| 7 | < .001 | 13.47 | 6 | −73 | −20 | Cerebellar vermis (7) |
| 13 | < .001 | 13.32 | 48 | 8 | 31 | R inferior frontal gyrus (pars opercularis) |
| 12 | < .001 | 13.31 | −30 | 17 | 10 | L insula lobe |
| 5 | < .001 | 12.73 | 9 | −19 | 10 | R thalamus |
| 9 | < .001 | 12.66 | −27 | −55 | −23 | L cerebellum (VI) |
| 12.60 | −27 | −64 | −23 | L cerebellum (VI) | ||
Note. Voxel-wise thresholded at t = 12, equivalent to p = 2.00 × 10−23 uncorrected. k = the number of voxels in each significant cluster; FWE = family-wise error corrected; t = peak activation level in each cluster; x, y, z = MNI coordinates; L = left; R = right. Boldface indicates the regions included in the neural cognitive control factor scores. Reprinted from CITATION BLINDED
Appendix B:
Parameter Estimates for Effects of Negative Life Events on Adolescent Depressive Symptoms, Moderated by Behavioral Cognitive Control
| Model Fit | ||||
| χ2 | df | CFI | RMSEA | |
| 5.45 | 7 | 1.00 | 0.00 | |
| Interaction effects model | 6.46 | 9 | 1.00 | 0.00 |
| Parameter estimates | ||||
| Effect on intercept | Effect on slope | |||
| B [95% CI] | b* [95% CI] | B [95% CI] | b* [95% CI] | |
| Step 1: Main effects | ||||
| Positive life events | 0.12 [−0.06; 0.29] | .11 [−.05; .28] | −0.02 [−0.11; 0.06] | −.04 [−.39; .22] |
| Negative life events | 0.20* [0.03; 0.37] | .20* [.03; .37] | −0.01 [−0.09; 0.07] | −.08 [−.34; .26] |
| Behavioral cognitive control | 0.11 [−0.39; 0.60] | .03 [−.13; .19] | −0.05 [−0.30; 0.21] | −.05 [−.36; .25] |
| Step 2: Interaction effects | ||||
| Positive life events*Behavioral cognitive control | 0.11 [−0.13; 0.34] | .07 [−.09; .24] | 0.02 [−0.07; 0.11] | .06 [−.18; .29] |
| Negative life events*Behavioral cognitive control | 0.05 [−0.16; 0.27] | .04 [−.12; .20] | −0.03 [−0.11; 0.06] | −.07 [−.31; .17] |
Note.
p < .05.
Model is controlled for adolescent sex. Behavioral cognitive control was a factor score with accuracy and reaction time (reverse coded) during MSIT as manifest indicators.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- Achenbach TM (1991). Integrative guide for the 1991 CBCL/4–18, YSR, and TRF profiles. University of Vermont: Department of Psychiatry. [Google Scholar]
- Achenbach TM, Dumenci L, & Rescorla LA (2003). DSM-oriented and empirically based approaches to constructing scales from the same item pools. Journal of Clinical Child and Adolescent Psychology, 32, 328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- Allen JL, & Rapee RM (2009). Are reported differences in life events for anxious children and controls due to comorbid disorders? Journal of Anxiety Disorders, 23, 511–518. doi: 10.1016/j.janxdis.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Allen JL, Rapee RM, & Sandberg S (2012). Assessment of maternally reported life events in children and adolescents: A comparison of interview and checklist methods. Journal of Psychopathology and Behavioral Assessment, 34, 204–215. doi: 10.1007/s10862-011-9270-5 [DOI] [Google Scholar]
- Asselmann E, Wittchen H-U, Lieb R, Höfler M, & Beesdo-Baum K (2015). Danger and loss events and the incidence of anxiety and depressive disorders: a prospective-longitudinal community study of adolescents and young adults. Psychological Medicine, 45, 153–163. doi: 10.1017/S0033291714001160 [DOI] [PubMed] [Google Scholar]
- Belsky J (1997). Variation in susceptibility to rearing influences: An evolutionary argument. Psychological Inquiry, 8, 182–186. doi: 10.1207/s15327965pli0803_3 [DOI] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, & Van IJzendoorn MH (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16, 300–304. doi: 10.1111/j.1467-8721.2007.00525.x [DOI] [Google Scholar]
- Blakemore S-J, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31, 926–933. doi: 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C, Van Vlierberghe L, Vandevivere E, Theuwis L, & Bosmans G (2012). Depression in early, middle and late adolescence: differential evidence for the cognitive diathesis-stress model. Clinical Psychology & Psychotherapy, 20, 369–83. doi: 10.1002/cpp.1789 [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B… Wara D (1995). Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine, 57, 411–422. doi: 10.1097/00006842-199509000-00001 [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, & Vogt BA (2003). The Multi-Source Interference Task: validation study with fMRI in individual subjects. Molecular Psychiatry, 8, 60–70. doi: 10.1038/sj.mp.4001217 [DOI] [PubMed] [Google Scholar]
- Carter JS, & Garber J (2011). Predictors of the first onset of a major depressive episode and changes in depressive symptoms across adolescence: stress and negative cognitions. Journal of Abnormal Psychology, 120, 779–796. doi: 10.1037/a0025441 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galván A, & Somerville LH (2016). Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience, 17, 128–30. doi: 10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28, 62–77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, & Laursen B (2004). Parent adolescent relationships and influences In Lerner RM & Steinberg L (Eds.), Handbook of adolescent psychology (2nd ed., pp. 331–361). New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, & Robinson MD (2011). Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion, 11, 379–90. doi: 10.1037/a0021776 [DOI] [PubMed] [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, & Carp J (2008). Error-monitoring ability predicts daily stress regulation. Psychological Science, 19, 702–8. doi: 10.1111/j.1467-9280.2008.02145.x [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Costello EJ, & Angold A (2009). Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Archives of General Psychiatry, 66, 764. doi: 10.1001/archgenpsychiatry.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich S, Collishaw S, Thapar AK, Harold G, Thapar A, & Rice F (2016). Do better executive functions buffer the effect of current parental depression on adolescent depressive symptoms? Journal of Affective Disorders, 199, 54–64. doi: 10.1016/j.jad.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disabato DJ, Kashdan TB, Short JL, & Jarden A (2017). What predicts positive life events that influence the course of depression? A longitudinal examination of gratitude and meaning in life. Cognitive Therapy and Research, 41, 444–458. doi: 10.1007/s10608-016-9785-x [DOI] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12, 467–477. doi: 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakersman-Kranenburg M, & Van IJzendoorn MH (2011). Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Development and Psychopathology, 23, 7–28. doi: 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Ferdinand RF (2008). Validity of the CBCL/YSR DSM-IV scales Anxiety Problems and Affective Problems. Journal of Anxiety Disorders, 22, 126–134. doi: 10.1016/J.JANXDIS.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, & Horwood LJ (2007). Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. The British Journal of Psychiatry, 191, 335–342. doi: 10.1192/bjp.bp.107.036079 [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, & Beautrais AL (2005). Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry, 62, 66. doi: 10.1001/archpsyc.62.1.66 [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Perkins SC, Angstadt M, Johnson T, Stern ER, Welsh RC, & Taylor SF (2010). The development of performance-monitoring function in the posterior medial frontal cortex. NeuroImage, 49, 3463–3473. doi: 10.1016/j.neuroimage.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore S-J (2015). Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences, 19, 558–566. doi: 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Garmezy N (1991). Resilience in children’s adaptation to negative life events and stressed environments. Pediatric Annals, 20, 459–60, 463–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1945543 [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, & Simons RL (1994). Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology, 30, 467–483. doi: 10.1037/0012-1649.30.4.467 [DOI] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. doi: 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. doi: 10.1017/S0048577201393198 [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, & Vargas I (2011). Resilience to depressive symptoms: The buffering effects of enhancing cognitive style and positive life events. Journal of Behavior Therapy and Experimental Psychiatry, 42, 13–18. doi: 10.1016/j.jbtep.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Hammen C (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, & Roesch L (2007). Sex differences in adolescent depression: stress exposure and reactivity models. Child Development, 78, 279–295. doi: 10.1111/j.1467-8624.2007.00997.x [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Hendricks MA, & Buchanan TW (2016). Individual differences in cognitive control processes and their relationship to emotion regulation. Cognition and Emotion, 30, 912–924. doi: 10.1080/02699931.2015.1032893 [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman technique, its theory and application.Psychometrika,15, 349–367. doi: 10.1007/BF02288864 [DOI] [PubMed] [Google Scholar]
- Joormann J, & D’Avanzato C (2010). Emotion regulation in depression: Examining the role of cognitive processes. Cognition & Emotion, 24, 913–939. doi: 10.1080/02699931003784939 [DOI] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman Brief Intelligence Test, Second Edition Bloomington, MN: Pearson, Inc. [Google Scholar]
- Kanai R, & Rees G (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12, 231–242. doi: 10.1038/nrn3000 [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes C, Lee J, Chiu P, & King-Casas B (2016). Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia, 91, 318–326. doi: 10.1016/j.neuropsychologia.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Haskett ME, Longo GS, & Nice R (2012). Longitudinal stufy of self-regulation, positive parenting, and adjustment problems among physically abused children. Child Abuse and Neglect, 36, 95–107. doi: 10.1016/j.chiabu.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Kahn RE, Lauharatanahirun N, Deater-Deckard K, Bickel WK, Chiu PH, & King-Casas B (2017). Executive functioning and substance use in adolescence: Neurobiological and behavioral perspectives. Neuropsychologia, 100, 79–92. doi: 10.1016/j.neuropsychologia.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Maciejewski D, Lee J, Deater-Deckard K, & King-Casas (2017). Longitudinal associations among family environment, neural cognitive control, and social competence among adolescents. Developmental Cognitive Neuroscience, 26, 69–76. doi: 10.1016/j.dcn.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, & Kouneiher F (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302, 1181–1185. doi: 10.1126/science.1088545 [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, & Casey BJ (2006). Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex, 16, 553–60. doi: 10.1093/cercor/bhj003 [DOI] [PubMed] [Google Scholar]
- Low NC, Dugas E, O’Loughlin E, Rodriguez D, Contreras G, Chaiton M, & O’Loughlin J (2012). Common stressful life events and difficulties are associated with mental health symptoms and substance use in young adolescents. BMC Psychiatry, 12, 116. doi: 10.1186/1471-244X-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, & Becker B (2000). The construct of resilience: A critical evaluation and guidelines for future work. Child Development, 71, 543–62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10953923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Jacobs SE, Ray RD, John OP, & Gross JJ (2012). Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality, 46, 2–7. doi: 10.1016/j.jrp.2011.10.003 [DOI] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49, 980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland JJ, Raup-Krieger JL, Hecht ML, & Miller-Day MM (2013). The conceptualization and communication of risk among rural Appalachian adolescents. Journal of Health Communication, 18, 668–685. doi: 10.1080/10810730.2012.743620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998). Mplus User’s Guide. Seventh Edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstädt-Klein S, Steiner S, Poustka L, … IMAGEN Consortium. (2012). Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology, 37, 986–995. doi: 10.1038/npp.2011.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2005). Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences, 9, 60–68. doi: 10.1016/j.tics.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Pettit JW, Lewinsohn PM, Seeley JR, Roberts RE, & Yaroslavsky I (2010). Developmental relations between depressive symptoms, minor hassles, and major events from adolescence through age 30 years. Journal of Abnormal Psychology, 119, 811–24. doi: 10.1037/a0020980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, & Ernst M (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews, 37, 976–991. doi: 10.1016/j.neubiorev.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglin L, Collishaw S, Shelton KH, McManus IC, Ng-Knight T, Sellers R, … Rice F (2016). Higher cognitive ability buffers stress-related depressive symptoms in adolescent girls. Development and Psychopathology, 28, 97–109. doi: 10.1017/S0954579415000310 [DOI] [PubMed] [Google Scholar]
- Roberts KL, & Hall DA (2008). Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience, 20, 1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ (1983). Developmental systems: Contexts and evolution In Mussen P (Ed.), Handbook of child psychology (pp. 237–294). New York, NY: Wiley. [Google Scholar]
- Siegle GJ, Ghinassi F, & Thase ME (2007). Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research, 31, 235–262. doi: 10.1007/s10608-006-9118-6 [DOI] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, Elzinga BM, Hovens JGFM, Roelofs K, van Oppen P, Zitman FG, & Penninx BWJH (2011). Positive and negative life events and personality traits in predicting course of depression and anxiety. Acta Psychiatrica Scandinavica, 124, 462–73. doi: 10.1111/j.1600-0447.2011.01753.x [DOI] [PubMed] [Google Scholar]
- Stride CB, Gardner S, Catley N, & Thomas F (2015). Mplus code for the mediation, moderation, and moderated mediation model templates from Andrew Hayes’ PROCESS analysis examples. Retrieved from http://offbeat.group.shef.ac.uk/FIO/mplusmedmod.htm
- Van Oort FVA, Greaves-Lord K, Verhulst FC, Ormel J, & Huizink AC (2009). The developmental course of anxiety symptoms during adolescence: the TRAILS study. Journal of Child Psychology and Psychiatry, 50, 1209–1217. doi: 10.1111/j.1469-7610.2009.02092.x [DOI] [PubMed] [Google Scholar]