Abstract
Hypertension treatment has been implicated in falls, syncope, and orthostatic hypotension (OH), common events among older adults. Whether choice of antihypertensive agent influences the risk of falls, syncope, and OH in older adults is unknown. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) was a randomized clinical trial that compared the effects of hypertension first-step therapy on fatal coronary heart disease or nonfatal myocardial infarction (1994–2002). In a subpopulation of ALLHAT participants, age 65 years and older, we determined the relative risk of falls, syncope, OH, or a composite based on CMS and VA claims, using Cox regression. We also determined the adjusted association of self-reported atenolol use (ascertained at the 1-month visit for indications other than hypertension) on outcomes in Cox models adjusted for age, sex, and race. Among 23,964 participants (mean age 69.8±6.8 years, 45% women, 31% non-Hispanic black) followed for a mean of 4.9 years, we identified 267 fall, 755 syncope, 249 OH, and 1,157 composite claims. There were no significant differences in the cumulative incidences of events across randomized drug assignments. However, amlodipine increased risk of falls during the first year of follow-up compared to chlorthalidone (HR[95%CI]: 2.24 [1.06–4.74];P=0.03) or lisinopril (HR[95%CI]: 2.61 [1.03–6.72];P=0.04). Atenolol use (N=928) was not associated with any of the 3 individual or composite claims. In older adults, choice of antihypertensive agent had no effect on risk of fall, syncope, or OH long-term. However, amlodipine increased risk of falls within 1-year of initiation. These short-term findings require confirmation.
Keywords: falls, syncope, orthostatic hypotension, hypertension treatment, trial, amlodipine, lisinopril, chlorthalidone
Blood pressure (BP) is an important modifiable risk factor for cardiovascular disease, stroke, and mortality.1 Recently, the SPRINT trial demonstrated that intensive (systolic BP [SBP] < 120 mm Hg) versus moderate (SBP of 135–139 mm Hg) BP treatment reduced the risk of cardiovascular disease in older adults.2 More intensive therapy was also associated with a higher risk of syncope, but had no effect on falls or orthostatic hypotension (OH).2 Given the high prevalence of hypertension and hypertension treatment among older adults, a greater understanding of agents associated with a lower risk of falls or syncope might benefit older adults being treated for hypertension. However, SPRINT did not incorporate a randomized comparison of antihypertensive drug choice as part of its study design.
The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) compared the effect of first-step therapy with different classes of antihypertensive agents (amlodipine, lisinopril, doxazosin, chlorthalidone) for prevention of coronary heart disease or nonfatal myocardial infarction.3 While the doxazosin arm was ended prematurely due to increased harm, ALLHAT ultimately reported that chlorthalidone was superior in preventing 1 or more major forms of CVD. Recently in secondary analyses, ALLHAT investigators utilized linked CMS and VA claims data with participants assigned amlodipine, lisinopril, or chlorthalidone, to demonstrate that chlorthalidone also reduced risk of hip and pelvic fractures compared to other agents4 and that atenolol did not contribute to higher risk of fracture.4 However, the mechanism of these relationships are unclear. Paradoxically, diuretics and beta blockers are known contributors toward OH,5,6 a risk factor for syncope, falls, and fracture,7 although the effect of thiazide diuretics on calcium reabsorption may explain the benefit of chlorthalidone on fractures.8
Our objectives in this study were to determine whether amlodipine, lisinopril, or chlorthalidone were associated with falls, syncope, or OH among ALLHAT participants age 65 and older with valid Medicare or Social Security identifiers during or in extended follow-up after the trial period. We also examined whether atenolol use at 1-month was associated with fall, syncope, or OH during the trial or in extended follow-up after the trial period. In addition, follow-up was lagged by 1-year to account for potential latency or follow-up was curtailed at 1-year to examine short-term effects. We hypothesized that chlorthalidone and atenolol would be associated with a greater risk of falls, syncope, and OH compared with amlodipine or lisinopril.
Methods
Study Overview
ALLHAT was a randomized, double-blind, controlled trial, comparing the effect of first-step antihypertensive therapies on major cardiovascular disease outcomes. Details are published elsewhere.3,9 In brief, ALLHAT recruited adults age 55 years and older with hypertension and at least one other risk factor for CHD at 623 centers throughout the United States and Canada (1994–1998). Participants were randomly assigned to chlorthalidone (N=15,255), amlodipine (N=9,048), doxazosin (N=9,061), or lisinopril (N=9,054) and followed through March 2002 (see Figure 1). After the trial, participants underwent an extended follow-up phase through 2006, using national databases (passive surveillance). The doxazosin arm was ended early by the trial’s safety and monitoring board due to a higher risk of congestive heart failure compared to chlorthalidone. Of the original trial participants assigned to chlorthalidone, amlodipine, or lisinopril, 23,964 were successfully linked with Centers for Medicare & Medicaid Services (CMS) and Veterans Affairs (VA) hospitalization claims. The initial trial was approved by the institutional review board of the University of Texas Health Science Center at Houston. While data for the ALLHAT trial is available via the NHLBI Biolincc Repository (Bethesda, Maryland), the CMS and VA claims data are restricted.
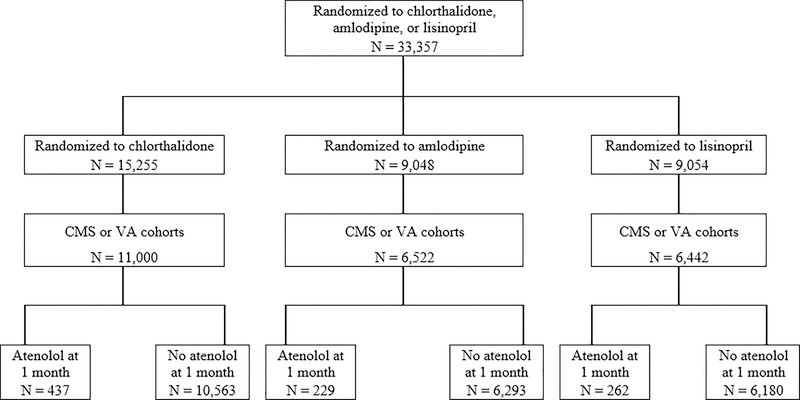
Figure 1.
CONSORT diagram for participants randomized to chlorthalidone, amlodipine, or lisinopril from baseline onward for the falls, syncope, and orthostatic hypotension, who are in the CMS or VA cohort during the trial follow-up period.
Study Participants
Adults were required to have a SBP ≥ 140 mm Hg or a DBP ≥ 90 mm Hg and at least one other risk factor for coronary heart disease. These risk factors included previous myocardial infarction, previous stroke, left ventricular hypertrophy, type 2 diabetes, current cigarette smoking, or low high density lipoprotein cholesterol. Excluded were participants with a myocardial infarction, stroke, or angina within 6 months of the study, symptomatic heart failure or ejection fracture < 35%, an elevated serum creatinine (>2 mg/dL), a SBP > 180 mm Hg, or a diastolic BP (DBP) > 110 mm Hg. All participants provided written informed consent prior to participation.
Study Interventions
Unless there was a safety concern, participants were continued on their antihypertensive regimen until the day they began their randomized study drug. Participants were assigned to 1 of 3 first-line study medications (amlodipine, 2.5–10 mg; chlorthalidone, 12.5–25 mg; or lisinopril, 10–40 mg) that were identical in appearance to maintain the study’s masking scheme. During the first year of the study, participants were seen as needed to titrate first-line medications to achieve the BP goal of SBP <140 mm Hg and DBP <90 mm Hg. If the maximum dose first-line agent was reached without achieving this goal, participants were started on open label second-line (reserpine, clonidine, atenolol) or third-line (hydralazine) agents. Participants on atenolol for hypertension were weaned prior to randomization as this was a second-line agent. If participants were on atenolol prior to study initiation for reasons other than hypertension, they were not required to discontinue it. As a result, since atenolol use was not ascertained at baseline, participants taking atenolol at 1-month were assumed to be taking it at baseline.
Fall, Syncope, or Orthostatic Hypotension
First occurrence of a fall, syncope, and OH event after randomization was based on claims from the CMS and VA hospitalization data (February 1, 1994 through December 31, 2006).4 This linkage was limited to participants with valid Medicare or Social Security identifiers. Participants from Canada or below the age of 65 years at baseline were excluded as they would not have continuous surveillance. VA data was not available for the post-trial follow-up period (2002–2006). As a result, these analyses were limited to US citizens with Medicare Part A insurance at randomization. Falls were based on external cause of injury codes: E880.X-E888.X. Syncope was based on International Classification of Diseases, Ninth Revision (ICD9), code 780.2. OH was based on code 458.0. Events were examined individually and as a composite outcome. Time to first event was based on the minimum follow-up period between baseline and either an event or administrative censoring (death or end of surveillance). In sensitivity analyses, we examined both a lagged start (by 1 year) and a short-term follow-up period (end at 1 year) after baseline.
Other Covariates
Trained study personnel followed standardized data collection protocols to document population characteristics of interest. Age, sex, race (white, black, other), ethnicity (Hispanic, non-Hispanic) were self-reported. SBP and DBP were measured at baseline via auscultation after 5 minutes of seated rest. Body mass index (BMI) was derived from height and weight measurements and obesity defined as a BMI ≥ 30 kg/m2. History of type 2 diabetes, and smoking status (current, former, never) were self-reported. History of cardiovascular disease (yes, no) was based on self-report or medical records (if available).
Statistical Analysis
Baseline characteristics were reported as means and proportions across drug assignments and 1-month atenolol use. We determined the mean SBP, DBP, and number of BP medications during the 1-year visit to assess for post-randomization differences between treatment arms.
We examined the effects of drug assignment on falls, syncope, OH, and a composite outcome. The 5-yr cumulative incidence was determined via Cox regression adjusted for age, sex, and race (black versus non-black). Unadjusted Kaplan-Meier curves were used to visualize cumulative incidence during 4 follow-up timeframes: (1) in-trial, (2) in-trial plus the extended follow-up period, (3) in-trial lagged by 1-year (to account for a potential latency period), and (4) short-term in-trial with follow-up ending at 1-year (to examine short-term effects). For the lagged analysis, we did not exclude participants with events occurring within the first year, but instead began event surveillance 1 year after baseline. The effects of randomized drug assignment on the relative risk of the 4 outcomes were determined via unadjusted Cox proportional hazards models. Models were performed overall and in strata of age (≥75 vs <75 years), sex (women, men), race (black, non-black), obesity (BMI >30 vs ≤ 30 kg/m2), history of type 2 diabetes (yes, no), and history of cardiovascular disease (yes, no). Differences across strata were evaluated via interaction terms.
We also determined the unadjusted and adjusted cumulative incidence of falls, syncope, OH, and the composite outcome each year up to 5 years by atenolol status. In addition, we determined the association of atenolol use with each of the 4 outcomes using Cox proportional hazard models, overall and in strata of drug assignment, unadjusted and adjusted for age, sex, and race.
Models were not adjusted for multiple comparisons. However, as sensitivity analyses we compared amlodipine versus either chlorthalidone or lisinopril as a common reference. We also examined a previously published outcome (hip and pelvic fractures, International Classification of Diseases, Ninth Revision, codes 820.x and 808.x)4 as a potential downstream effect of a fall.
Results
Baseline and 1-year characteristics
The study population (N = 23,964) was 45% women and 31% non-Hispanic black with a mean age of 69.8 (SD, 6.8) years at baseline (Table 1). There were no major differences between randomization assignments at baseline. Only 928 (3.9%) reported atenolol use at the 1-month visit. Atenolol use was more common among men and non-Hispanic white participants and participants with prior cardiovascular disease. Mean SBP, DBP, and number of medications were similar at 1-year across randomized assignments (Supplement Table S1).
Table 1.
Baseline characteristics by randomization assignment and by atenolol status, Mean (SD) or %
| Chlorthalidone | Amlodipine | Lisinopril | No Atenolol | Atenolol | |
|---|---|---|---|---|---|
| Characteristic | N=11,000* | N=6,522* | N=6,442* | N=23,036 | N=928 |
| Age, years | 69.8 (6.8) | 69.8 (6.8) | 69.9 (6.8) | 69.9 (6.8) | 69.1 (6.3) |
| Age ≥ 75 years, % | 23.2 | 23.8 | 24.2 | 23.8 | 19.9 |
| Women, % | 45.1 | 45.5 | 44.2 | 45.2 | 39.9 |
| Race and Ethnicity, % | |||||
| White, non-Hispanic | 49.5 | 50.3 | 49.5 | 48.8 | 71.2 |
| Black, non-Hispanic | 31.1 | 31.3 | 31.0 | 31.8 | 15.0 |
| White Hispanic | 12.0 | 11.5 | 12.3 | 12.1 | 8.0 |
| Black Hispanic | 3.1 | 3.0 | 3.2 | 3.1 | 1.7 |
| Other | 4.4 | 4.0 | 4.1 | 4.2 | 4.1 |
| Systolic blood pressure, mm Hg | 146.6 (15.7) | 146.5 (15.6) | 146.8 (15.6) | 146.5 (15.6) | 149.2 (16.2) |
| Diastolic blood pressure, mm Hg | 83.1 (10.1) | 82.9 (10.1) | 83.0 (10.0) | 83.0 (10.1) | 83.4 (10.3) |
| Body mass index, kg/m2 | 29.3 (6.0) | 29.3 (5.9) | 29.3 (5.9) | 29.3 (6.0) | 29.0 (5.6) |
| Obesity, % | 38.9 | 39.0 | 38.8 | 39.0 | 37.7 |
| Type 2 diabetes status, % | 42.6 | 43.0 | 42.2 | 42.9 | 35.5 |
| Prior cardiovascular disease, % | 56.1 | 54.6 | 55.7 | 54.8 | 75.5 |
| Smoking Status, % | |||||
| Never | 38.4 | 38.6 | 38.5 | 38.6 | 35.6 |
| Former | 43.1 | 42.5 | 43.2 | 42.7 | 49.5 |
| Current | 18.5 | 18.9 | 18.2 | 18.7 | 15.0 |
Reduced denominators (c/a/l) for smoking=11,000/6,521/6,441; BMI/obesity=10,964/6,494/6,422; diabetes=10,222/6,057/5,986
Effects of Randomized Drug Assignment
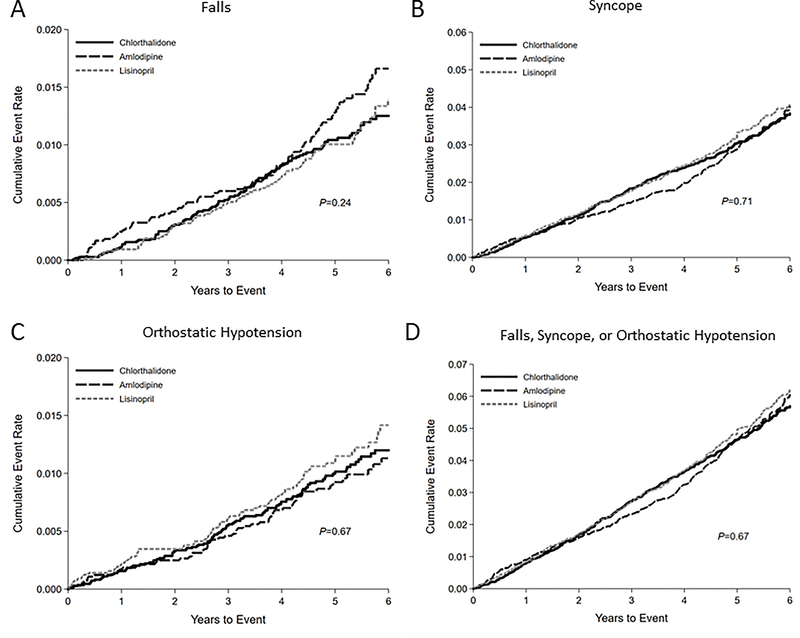
Over a mean of 4.9 years of follow-up, we identified 267 fall, 755 syncope, 249 OH, and 1,157 composite claims. The 5-year cumulative incidence of falls adjusted for age, sex, and race was higher with amlodipine at 1.16 per 100 person-years versus 0.80 and 0.79 per 100 person-years for chlorthalidone and lisinopril, respectively (Supplement Table S2). We observed no difference in cumulative incidence of falls, syncope, OH, or the composite outcome across drug assignments during the entire trial period (Figure 2, Supplement Table S3). Similarly, we observed no difference in cumulative incidence of any of the outcomes between drug assignments with extended follow-up (Supplement Figure S1, Supplement Table S4) or in follow-up lagged by 1 year (Supplement Figure S2, Supplement Table S5). However, the cumulative incidence of falls during the first year was significantly higher among those assigned amlodipine (Supplement Figure S3, Supplement Table S6).
Figure 2.
Kaplan-Meier cumulative incidence plots of (A) falls, (B) syncope, (C) orthostatic hypotension, or (D) a composite outcome (falls, syncope, or orthostatic hypotension) during the trial period according to chlorthalidone, amlodipine, or lisinopril assignment.
Similarly, drug assignment was not associated with falls, syncope, OH, or composite events during the in-trial follow-up period, extended follow-up period, or the in-trial period lagged by 1-year (Table 2). However, amlodipine was associated with a higher short-term risk of falls compared to both chlorthalidone (HR: 2.24; 95% CI: 1.06–4.74) and lisinopril (HR: 2.63; 95% CI: 1.03–6.72). This short-term association was not observed with syncope, OH, or the composite outcome. However, the short-term association persisted when amlodipine was compared to a common reference of both chlorthalidone and lisinopril (Supplement Table S7). Furthermore, in a sensitivity analysis, amlodipine was associated with non-significant, higher risks of hip and pelvic fracture short-term compared to chlorthalidone or lisinopril (Supplement Table S8).
Table 2.
Effect of anti-hypertensive assignment on falls, syncope, orthostatic hypotension, or a composite outcome, HR (95% CI)
| Falls (Events/Total) |
Syncope (Events/Total) |
OH (Events/Total) |
Composite (Events/Total) |
|||||
|---|---|---|---|---|---|---|---|---|
| Study Period | (267/23,964) | (755/23,964) | (249/23,964) | (1,157/23,964) | ||||
| In-trial follow-up | HR (95CI) | P | HR (95CI) | P | HR (95CI) | P | HR (95CI) | P |
| Amlodipine vs chlorthalidone | 1.26 (0.95–1.67) | 0.10 | 0.96 (0.81–1.15) | 0.67 | 1.00 (0.74–1.36) | 0.98 | 1.01 (0.88–1.16) | 0.89 |
| Lisinopril vs chlorthalidone | 1.04 (0.77–1.41) | 0.78 | 1.05 (0.88–1.24) | 0.61 | 1.14 (0.85–1.53) | 0.40 | 1.06 (0.93–1.22) | 0.39 |
| Amlodipine vs lisinopril | 1.21 (0.88–1.66) | 0.24 | 0.92 (0.76–1.12) | 0.41 | 0.88 (0.63–1.23) | 0.47 | 0.95 (0.81–1.11) | 0.52 |
| Extended follow-up | (351/19,157) | (1,197/19,157) | (340/19,157) | (1,696/19,157) | ||||
| Amlodipine vs chlorthalidone | 1.06 (0.83–1.36) | 0.65 | 0.95 (0.83–1.10) | 0.51 | 0.89 (0.69–1.15) | 0.38 | 0.97 (0.86–1.09) | 0.61 |
| Lisinopril vs chlorthalidone | 0.98 (0.76–1.27) | 0.89 | 0.98 (0.86–1.13) | 0.80 | 0.92 (0.71–1.19) | 0.53 | 0.99 (0.88–1.11) | 0.90 |
| Amlodipine vs lisinopril | 1.08 (0.81–1.43) | 0.60 | 0.97 (0.83–1.13) | 0.72 | 0.97 (0.72–1.30) | 0.84 | 0.98 (0.86–1.11) | 0.73 |
| In-trial follow-up lagged by 1-year | (233/23,964) | (627/23964) | (207/23,964) | (960/23,964) | ||||
| Amlodipine vs chlorthalidone | 1.15 (0.84–1.56) | 0.38 | 0.96 (0.79–1.16) | 0.64 | 0.99 (0.71–1.38) | 0.95 | 0.98 (0.84–1.15) | 0.83 |
| Lisinopril vs chlorthalidone | 1.07 (0.78–1.46) | 0.69 | 1.03 (0.86–1.25) | 0.73 | 1.09 (0.78–1.51) | 0.62 | 1.06 (0.91–1.23) | 0.46 |
| Amlodipine vs lisinopril | 1.07 (0.76–1.51) | 0.68 | 0.92 (0.75–1.14) | 0.47 | 0.91 (0.63–1.32) | 0.62 | 0.93 (0.78–1.10) | 0.39 |
| Short-term follow-up (end at 1-year) | (34/23,964) | (128/23,964) | (42/23,964) | (197/23,964) | ||||
| Amlodipine vs chlorthalidone | 2.24 (1.06–4.74) | 0.03 | 1.00 (0.66–1.54) | 0.99 | 1.09 (0.51–2.32) | 0.83 | 1.15 (0.82–1.60) | 0.41 |
| Lisinopril vs chlorthalidone | 0.85 (0.32–2.27) | 0.75 | 1.11 (0.73–1.68) | 0.62 | 1.41 (0.69–2.85) | 0.34 | 1.09 (0.77–1.53) | 0.64 |
| Amlodipine vs Lisinopril | 2.63 (1.03–6.72) | 0.04 | 0.91 (0.57–1.44) | 0.68 | 0.77 (0.35–1.71) | 0.53 | 1.06 (0.73–1.53) | 0.76 |
Note: Consistent with the randomized design, these analyses were not adjusted.
We examined the effects of drug assignment in strata of age, sex, race, obesity, history of type 2 diabetes, and history of cardiovascular disease (Supplement Table S9). The only consistent interaction was related to a higher risk of OH with lisinopril among black participants.
Effects of Atenolol Use at the 1-Month Visit
Cumulative incidences of falls, syncope, OH, or the composite outcome were similar whether or not a participant reported atenolol use at 1-month (Supplement Tables S10–13). Similarly, atenolol use was not associated with outcomes overall or in strata of drug assignment (Table 3).
Table 3.
Association of baseline atenolol with fall, syncope, orthostatic hypotension, or a composite outcome during the trial period in strata of drug assignment or overall, HR (95% CI)
| Outcome | Unadjusted |
Age/Sex/Race Adjusted |
||||
|---|---|---|---|---|---|---|
| Did not use atenolol | Used atenolol | |||||
| Falls | Events/Total | Events/Total | HR (95% CI) | P | HR (95% CI) | P |
| Amlodipine | 84/6293 | 1/229 | 0.36 (0.05–2.56) | 0.31 | 0.37 (0.05–2.68) | 0.33 |
| Chlorthalidone | 111/10563 | 2/437 | 0.46 (0.11–1.84) | 0.27 | 0.45 (0.11–1.83) | 0.27 |
| Lisinopril | 66/6180 | 3/262 | 1.13 (0.35–3.59) | 0.84 | 1.06 (0.33–3.39) | 0.92 |
| Overall | 261/23036 | 6/928 | 0.60 (0.27–1.36) | 0.22 | 0.60 (0.27–1.35) | 0.22 |
| Syncope | ||||||
| Amlodipine | 194/6293 | 5/229 | 0.78 (0.32–1.89) | 0.58 | 0.89 (0.36–2.16) | 0.79 |
| Chlorthalidone | 334/10563 | 11/437 | 0.85 (0.47–1.55) | 0.59 | 0.92 (0.50–1.67) | 0.78 |
| Lisinopril | 198/6180 | 13/262 | 1.61 (0.92–2.82) | 0.10 | 1.65 (0.94–2.90) | 0.08 |
| Overall | 726/23036 | 29/928 | 1.06 (0.73–1.53) | 0.77 | 1.14 (0.79–1.66) | 0.48 |
| Orthostatic hypotension | ||||||
| Amlodipine | 62/6293 | 4/229 | 2.01 (0.73–5.52) | 0.18 | 1.99 (0.72–5.51) | 0.19 |
| Chlorthalidone | 103/10563 | 7/437 | 1.75 (0.81–3.77) | 0.15 | 1.81 (0.84–3.92) | 0.13 |
| Lisinopril | 73/6180 | 0/262 | - | - | ||
| Overall | 238/23036 | 11/928 | 1.22 (0.67–2.34) | 0.51 | 1.27 (0.69–2.33) | 0.44 |
| Composite | ||||||
| Amlodipine | 306/6293 | 8/229 | 0.78 (0.39–1.57) | 0.49 | 0.86 (0.42–1.73) | 0.67 |
| Chlorthalidone | 501/10563 | 19/437 | 0.97 (0.61–1.54) | 0.90 | 1.02 (0.65–1.62) | 0.92 |
| Lisinopril | 307/6180 | 16/262 | 1.29 (0.78–2.13) | 0.33 | 1.30 (0.78–2.15) | 0.31 |
| Overall | 1114/23036 | 43/928 | 1.02 (0.75–1.38) | 0.913 | 1.07 (0.79–1.46) | 0.65 |
Discussion
In this secondary analysis of older participants of the ALLHAT study, we found no differences in risk of fall, syncope, or OH between principal classes of antihypertensives during the trial period or extended follow-up. However, amlodipine increased fall claims in the short-term. Meanwhile, baseline atenolol use was not associated with higher risk of the individual or composite outcomes. Together these findings suggest that among older adults, hypertensive class is generally not an important determinant of long-term risk of falls, syncope, or OH; however, in the short-term amlodipine may increase risk of falls among older adults, which should be confirmed given the multiple comparisons performed in this study.
With the advent of the new AHA/ACC High Blood Pressure Guidelines, the number of adults recommended for BP pharmacologic treatment has expanded to nearly 82 million US adults, including all healthy adults over 75 years of age with BP above 130/80 mm Hg.10 These recommendations were based in part on results from the SPRINT trial sub-study in adults over 75 years, which demonstrated a reduction in CVD events among those assigned to more intensive BP management. Despite the substantial survival benefit, intensive management did not increase risk of falls or OH, but did increase risk of syncope.11 These findings were supported by a secondary analysis of the ACCORD study, which showed no association between more intensive BP treatment and falls or OH.12,13 However, these studies did not examine the effects of class selection on falls, syncope, or OH.
There is evidence that different classes of medications might contribute to falls in older adults via OH. Diuretics are associated with hypovolemia and OH,14–16 which is a risk factor for both falls and syncope.7,17 Several observational studies have demonstrated an association between diuretics and falls.18–24 However, a number of studies have not demonstrated an association between diuretics and falls.25–29 Our study similarly did not show a greater risk of falls, syncope, or OH with chlorthalidone. This is consistent with a prior ALLHAT publication on chlorthalidone and lower fracture risk,4 which has been thought to be mediated by calcium handling, but may also be related to lower fall risk.
Our study unexpectedly demonstrated a short-term association between amlodipine use and falls at 1-year that became evident between 3 and 6 months after randomization. This difference did not remain significant over time and was unstable, disappearing between years 3–4.5 and then reappearing. This observation may help elucidate the inconsistency between studies showing that calcium channel blockers are associated with fall history30 and fracture after falls,31,32 but not chronic use.33 A temporary, short-term higher risk of falls after antihypertensive treatment initiation has been observed in the literature.34–36 However, these studies did not identify calcium channel blockers as higher risk agents. A few observational studies have also linked calcium channel blockers with falls.30,36 The mechanism by which amlodipine might contribute to falls is unknown; although, it is possible that lower extremity edema, a common complication in older adults,37 might contribute to reduced physical function. Notably there were no major differences in SBP, DBP, or medication use at 1-year of the study. Ultimately, these observations require replication, especially given the multiple comparisons performed in this study. It should also be noted that given the few number of falls within the first year, the absolute difference between randomized treatments was small.
Atenolol use was not associated with any of the outcomes examined in our study. This was unexpected as beta blockers are associated with a higher risk of OH,38–40 and in the AASK trial we observed that metoprolol increased risk of OH.6 Furthermore, several observational studies have demonstrated a relationship between beta blockers and falls18 or fracture;41 although, observational studies are inconsistent.19 Further research is needed to confirm a potential relationship between beta blockers and fall pathways.
This study has limitations. First, events were based on claims data. While claims have been shown to be specific, they are insensitive.17 As a result, many cases, for example non-injurious falls or asymptomatic OH, were likely missed. This could attenuate our findings. Second, some studies have indicated greater risk of falls in the first few weeks after antihypertensive initiation or intensification.34 While our findings support these concerns with amlodipine within 1-year of initiation, we were not adequately powered to examine the 1-month period after initiation. Third, the atenolol comparison was not randomized.
This study also has multiple strengths. First, ALLHAT is the largest, randomized trial of primary antihypertensive classes on long-term outcomes ever conducted. Thus, its design is ideal for comparing effects of drug class. Second, our study included a large and diverse population of older adults. As a result, our findings are directly applicable to a population at greatest risk of falls and often considered most likely to be harmed by antihypertensive treatment. Third, by using claims data, our findings reflect outcomes that impact the health system, which may be more applicable to the real world.
Our study has clinical implications. With the advent of the AHA/ACC hypertension management guidelines,42 an unprecedented number of older adults are recommended for hypertension treatment. There are ongoing concerns about the impact of these recommendations on falls in older adults.43 While more aggressive BP control has not been associated with greater fall risk in randomized trials,2,44 there is evidence that different classes do contribute to fall risk factors like OH.6 This has led to the speculation that some classes, i.e. those minimizing OH, might represent an optimal first-line therapy for this vulnerable population. Although our study did not show any differences in risk across drug assignments during the 5-year trial period, we did observe a short-term higher risk of falls with amlodipine. This should be monitored in older adults initiating antihypertensive therapy particularly among those with a prior history of falls or other fall risk factors.
Perspectives
In conclusion, our study of older adults demonstrated no difference in risk of falls, syncope, or OH between principal classes of antihypertensive agents long-term. However, there was evidence of a higher short-term risk of falls with amlodipine, which should be confirmed.
Supplementary Material
Novelty and Significance.
What is New?
Over a 5-year period, we observed similar risks of falls, syncope, or orthostatic hypotension among older adults randomly assigned amlodipine, chlorthalidone, or lisinopril
However, within the first year of treatment, there was a higher risk of falls from amlodipine
What is Relevant?
Our findings inform treatment selection in older adults, a population with prevalent hypertension and higher risk of falls, syncope, and orthostatic hypotension
Summary
Representatives of 3 principal classes of antihypertensive medications share similar risks for falls, syncope, or orthostatic hypotension in older adults
The short-term higher risk of falls from amlodipine requires further study
Acknowledgments
Funding
SPJ supported by a NIH/NHLBI K23HL135273 and R21HL144876.
Abbreviations used
- ALLHAT
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
- OH
orthostatic hypotension
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HR
heart rate or hazard ratio
- CVD
cardiovascular disease
- CHF
congestive heart failure
- OR
odds ratio
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- VA
Veterans Affairs
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
This trial is registered at clinicaltrials.gov, number: NCT00000542
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2.SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 4.Puttnam R, Davis BR, Pressel SL, Whelton PK, Cushman WC, Louis GT, Margolis KL, Oparil S, Williamson J, Ghosh A, Einhorn PT, Barzilay JI, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Collaborative Research Group. Association of 3 Different Antihypertensive Medications With Hip and Pelvic Fracture Risk in Older Adults: Secondary Analysis of a Randomized Clinical Trial. JAMA Intern Med. 2017;177:67–76. [DOI] [PubMed] [Google Scholar]
- 5.Heseltine D, Bramble MG. Loop diuretics cause less postural hypotension than thiazide diuretics in the frail elderly. Curr Med Res Opin. 1988;11:232–235. [DOI] [PubMed] [Google Scholar]
- 6.Juraschek SP, Appel LJ, Miller ER, Mukamal KJ, Lipsitz LA. Hypertension Treatment Effects on Orthostatic Hypotension and Its Relationship With Cardiovascular Disease. Hypertension. 2018;72:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG, Griswold ME, Heiss G, Selvin E. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med. 2017;177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasnich RD, Benfante RJ, Yano K, Heilbrun L, Vogel JM. Thiazide effect on the mineral content of bone. N Engl J Med. 1983;309:344–347. [DOI] [PubMed] [Google Scholar]
- 9.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342–360. [DOI] [PubMed] [Google Scholar]
- 10.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Whelton PK. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Pajewski NM, SPRINT Research Group. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, Pop-Busui R, Sperl-Hillen J, Cushman WC. Orthostatic Hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension. 2016;68:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juraschek SP, Lipsitz LA, Beach JL, Mukamal KJ. Association of Orthostatic Hypotension Timing With Clinical Events in Adults With Diabetes and Hypertension: Results From the ACCORD Trial. Am J Hypertens. 2019;32:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens. 2015;29:599–603. [DOI] [PubMed] [Google Scholar]
- 15.Zia A, Kamaruzzaman SB, Myint PK, Tan MP. The association of antihypertensives with postural blood pressure and falls among seniors residing in the community: a case-control study. Eur J Clin Invest. 2015;45:1069–1076. [DOI] [PubMed] [Google Scholar]
- 16.Guérin A, Bureau M-L, Ghazali N, Gervais R, Liuu E, Seité F, Bellarbre F, Ingrand P, Paccalin M. Factors associated with orthostatic hypotension in hospitalized elderly patients. Aging Clin Exp Res. 2016;28:513–517. [DOI] [PubMed] [Google Scholar]
- 17.Juraschek SP, Daya N, Appel LJ, Miller ER, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic Hypotension in Middle-Age and Risk of Falls. Am J Hypertens. 2017;30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribbin J, Hubbard R, Gladman J, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: self-controlled case series. Pharmacoepidemiol Drug Saf. 2011;20:879–884. [DOI] [PubMed] [Google Scholar]
- 19.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA. Meta-analysis of the Impact of 9 Medication Classes on Falls in Elderly Persons. Arch Intern Med. 2009;169:1952–1960. [DOI] [PubMed] [Google Scholar]
- 20.Williams T, Szekendi M, Thomas S. An analysis of patient falls and fall prevention programs across academic medical centers. J Nurs Care Qual. 2014;29:19–29. [DOI] [PubMed] [Google Scholar]
- 21.Testa G, Ceccofiglio A, Mussi C, Bellelli G, Nicosia F, Bo M, Riccio D, Curcio F, Martone AM, Noro G, Landi F, Ungar A, Abete P. Hypotensive Drugs and Syncope Due to Orthostatic Hypotension in Older Adults with Dementia (Syncope and Dementia Study). J Am Geriatr Soc. 2018;66:1532–1537. [DOI] [PubMed] [Google Scholar]
- 22.Beunza-Sola M, Hidalgo-Ovejero ÁM, Martí-Ayerdi J, Sánchez-Hernández JG, Menéndez-García M, García-Mata S. Study of fall risk-increasing drugs in elderly patients before and after a bone fracture. Postgrad Med J. 2018;94:76–80. [DOI] [PubMed] [Google Scholar]
- 23.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47:40–50. [DOI] [PubMed] [Google Scholar]
- 24.Gribbin J, Hubbard R, Gladman JRF, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: population-based case-control study. Age Ageing. 2010;39:592–597. [DOI] [PubMed] [Google Scholar]
- 25.Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing. 2015;44:90–96. [DOI] [PubMed] [Google Scholar]
- 26.Liu BA, Topper AK, Reeves RA, Gryfe C, Maki BE. Falls among older people: relationship to medication use and orthostatic hypotension. J Am Geriatr Soc. 1995;43:1141–1145. [DOI] [PubMed] [Google Scholar]
- 27.Tinetti ME, Han L, Lee DSH, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive Medications and Serious Fall Injuries in a Nationally Representative Sample of Older Adults. JAMA Intern Med. 2014;174:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis KL, Buchner DM, LaMonte MJ, Zhang Y, Di C, Rillamas‐Sun E, Hunt J, Ikramuddin F, Li W, Marshall S, Rosenberg D, Stefanick ML, Wallace R, LaCroix AZ. Hypertension Treatment and Control and Risk of Falls in Older Women. Journal of the American Geriatrics Society [Internet]. [cited 2019 Jan 30];0. Available from: https://onlinelibrary-wiley-com.ezp.welch.jhmi.edu/doi/abs/10.1111/jgs.15732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauley JA, Cummings SR, Seeley DG, Black D, Browner W, Kuller LH, Nevitt MC. Effects of thiazide diuretic therapy on bone mass, fractures, and falls. The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1993;118:666–673. [DOI] [PubMed] [Google Scholar]
- 30.Takaoka S, Yamaguchi T, Tanaka K, Morita M, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Fracture risk is increased by the complication of hypertension and treatment with calcium channel blockers in postmenopausal women with type 2 diabetes. J Bone Miner Metab. 2013;31:102–107. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho Ed E da SF, Silva SD da. [Medication as a risk factor for falls resulting in severe fractures in the elderly]. Cad Saude Publica. 2002;18:1359–1366. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Mogun H, Garneau K, Fischer MA. Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res. 2011;26:1561–1567. [DOI] [PubMed] [Google Scholar]
- 33.Lipsitz LA, Habtemariam D, Gagnon M, Iloputaife I, Sorond F, Tchalla AE, Dantoine TF, Travison TG. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension. 2015;66:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimbo D, Bowling CB, Levitan EB, Deng L, Sim JJ, Huang L, Reynolds K, Muntner P. Short-term Risk of Serious Fall Injuries in Older Adults Initiating and Intensifying Treatment with Antihypertensive Medication. Circ Cardiovasc Qual Outcomes. 2016;9:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuto H, Imakyure O, Matsumoto J, Egawa T, Jiang Y, Hirakawa M, Kataoka Y, Yanagawa T. Medication use as a risk factor for inpatient falls in an acute care hospital: a case-crossover study. Br J Clin Pharmacol. 2010;69:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of falls on initiation of antihypertensive drugs in the elderly. Osteoporos Int. 2013;24:2649–2657. [DOI] [PubMed] [Google Scholar]
- 37.Sapienza S, Sacco P, Floyd K, DiCesare J, Doan QD. Results of a pilot pharmacotherapy quality improvement program using fixed-dose, combination amlodipine/benazepril antihypertensive therapy in a long-term care setting. Clin Ther. 2003;25:1872–1887. [DOI] [PubMed] [Google Scholar]
- 38.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39:51–56. [DOI] [PubMed] [Google Scholar]
- 39.Svetkey LP, Brobyn R, Deedwania P, Graham R, Morganroth J, Klotman P. Double-blind comparison of doxazosin, nadolol and placebo in patients with mild-to-moderate hypertension. Curr Ther Res. 1988;43:969–978. [Google Scholar]
- 40.Canney M, O’Connell MDL, Murphy CM, O’Leary N, Little MA, O’Seaghdha CM, Kenny RA. Single Agent Antihypertensive Therapy and Orthostatic Blood Pressure Behaviour in Older Adults Using Beat-to-Beat Measurements: The Irish Longitudinal Study on Ageing. PLoS ONE. 2016;11:e0146156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;172:1739–1744. [DOI] [PubMed] [Google Scholar]
- 42.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 43.Smetana GW, Beach J, Lipsitz L, Libman H. What Should Be the Target Blood Pressure for This Older Patient With Hypertension?: Grand Rounds Discussion From Beth Israel Deaconess Medical Center. Ann Intern Med. 2018;169:175–182. [DOI] [PubMed] [Google Scholar]
- 44.Margolis KL, Palermo L, Vittinghoff E, Evans GW, Atkinson HH, Hamilton BP, Josse RG, O’Connor PJ, Simmons DL, Tiktin M, Schwartz AV. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.