Abstract
In this review, we summarize studies investigating the types and distribution of voltage- and calciumgated ion channels in the different classes of retinal neurons: rods, cones, horizontal cells, bipolar cells, amacrine cells, interplexiform cells, and ganglion cells. We discuss differences among cell subtypes within these major cell classes, as well as differences among species, and consider how different ion channels shape the responses of different neurons. For example, even though second-order bipolar and horizontal cells do not typically generate fast sodium-dependent action potentials, many of these cells nevertheless possess fast sodium currents that can enhance their kinetic response capabilities. Ca2+ channel activity can also shape response kinetics as well as regulating synaptic release. The L-type Ca2+ channel subtype, CaV1.4, expressed in photoreceptor cells exhibits specific properties matching the particular needs of these cells such as limited inactivation which allows sustained channel activity and maintained synaptic release in darkness. The particular properties of K+ and Cl− channels in different retinal neurons shape resting membrane potentials, response kinetics and spiking behavior. A remaining challenge is to characterize the specific distributions of ion channels in the more than 100 individual cell types that have been identified in the retina and to describe how these particular ion channels sculpt neuronal responses to assist in the processing of visual information by the retina.
Keywords: retina, ion channels, horizontal cell, amacrine cell, retinal bipolar cell, retinal ganglion cell, photoreceptor cell
1. Introduction
Investigators have explored the complement of ion channels in retinal neurons using an array of electrophysiological, immunohistochemical and molecular approaches. Early electrophysiological studies focused largely on non-mammalian vertebrates but later investigations provided greater insight into the properties of mammalian retinas. In recent years, the number of identified cell types in retina has increased considerably. For example, initial studies distinguished ON and OFF types of bipolar cells but we now recognize more than a dozen subtypes of bipolar cells. There is an even larger number of amacrine and ganglion cell types. Accompanying this expansion of recognized cell types has been a tremendous expansion in our understanding of the molecular diversity of ion channels. In that context, we thought it useful to summarize the current state of knowledge regarding the types of ion channels present in different types of retinal neurons. We focus on voltage- and Ca2+-dependent ion channels that transform photocurrents and synaptic currents into voltage responses. We do not focus on other ligand-gated ion channels such as the cyclic nucleotide-gated channels in photoreceptor outer segments or ion channels that couple directly to neurotransmitter receptors. Nor do we focus on aquaporins, gap junction hemichannels, TRP channels, or transporters. We use nomenclature recommended by the International Union of Pharmacology (IUPHAR) as summarized in “The Concise Guide to Pharmacology 2017/18”(Alexander et al., 2017a; Alexander et al., 2017b; Alexander et al., 2017c), supplemented by some of the more commonly used terms. Before turning to the different cell types, we begin with a summary of the subtypes and structural features of the ion channels that are the focus of this review.
1.1. K+ channels
1.1.1 Inwardly rectifying K+ channels are formed from a tetrameric complex of 4 individual subunit proteins that each possess 2 transmembrane domains linked by a short pore-forming reentrant loop (P-loop) (Hibino et al., 2010; Tao et al., 2009). These channels lack a genuine voltage sensor but nevertheless exhibit an inwardly rectifying voltage-dependence that arises from blockade of outward currents by divalent cations at the intracellular surface of the channel pore. Some inwardly rectifying K+ channels (KIR1.1-7.1) are constitutively active, some are activated by Gβγ subunits of G-proteins (GIRK), and others are activated by a fall in intracellular ATP (KATP).
1.1.2 Two-pore K+ channels are formed from dimers with each subunit containing 4 transmembrane alpha helices (M1-4) along with two P-loops linking M1 to M2 and M3 to M4 (Brohawn et al., 2012; Miller and Long, 2012). The presence of two P-loops in each subunit endows this group with its name. Like KIR channels, two-pore channels (K2P1.1-12.1) lack a genuine voltage sensor. Constitutive activity of two pore channels contributes to the leak K+ current in many cells and is important for setting the resting membrane potential (Feliciangeli et al., 2015; Renigunta et al., 2015).
1.1.3 Voltage-gated K+ channels (Armstrong, 2003; Kim and Nimigean, 2016; Kuang et al., 2015) are constructed from heteromeric or homomeric combinations of 4 individual subunits. Each subunit possesses 6 trans-membrane domains (S1-S6) with a P-loop located between S5 and S6. These channels are activated by depolarizing potentials. The voltage sensor in these and other similar voltage-dependent channels is the S4 trans-membrane domain that contains a number of positively charged amino acid residues (typically arginine). Membrane depolarization causes these residues to move towards the extracellular side of the membrane and the resulting conformational change in the protein opens the channel pore. It was originally proposed that voltage-sensing involves an outward helical screw motion of the S4 segment (Cha et al., 1999; Glauner et al., 1999), but subsequent structural analysis suggested that the S4 domain undergoes a paddle-like outward movement in response to depolarization (Jiang et al., 2003). Functional subtypes of voltage-gated K+ channels include delayed rectifier currents (IKDR) in which outward currents inactivate slowly and A-type currents (IKA) that inactivate rapidly. Rapid inactivation occurs through a “ball-and-chain” mechanism in which the amino terminus swings towards the channel pore to block conductance, involving either the K+ channel subunit itself or a segment of an accessory β subunit (Hille, 2001; Kurata and Fedida, 2006). Slow inactivation of IKDR involves conformational changes that restrict pore conductance. There are a few dozen subtypes of voltage-gated K+ channels (Kv1.1 to 12.3). Kv1-4 channels can form both homomeric and heteromeric channels with members of the same subclass (e.g., Kv1.1 with Kv1.2). Homomeric and heteromeric combinations of different Kv7 subunits form a special type of delayed rectifier current known as M-type currents. M currents were named for the ability of muscarinic agonists to inhibit these channels. Other agents that activate Gq/11 signaling pathways can also inhibit these channels (Brown and Passmore, 2009; Greene and Hoshi, 2017). Kv5, 6, 8 and 9 subunits have a similar structure as other K+ channels, but do not form functional homomeric channels. However, they can form functional channels in heteromeric combination with Kv2 subunits (Bocksteins, 2016).
Kv10-12 subunits encode ether-a-gogo (eag, Kv10), ether-a-gogo-related (erg, KV11) and ether-a-gogo-like (elk, Kv12) channels (Bauer and Schwarz, 2018). Ether-a-go-go channels received their name because under ether anesthesia, Drosophila with mutations in this channel shake their legs like go-go dancers (Vandenberg et al., 2012). These channels have a much shorter domain linking S4 and S5 domains compared to Kv1-2 channels that suggests a different gating mechanism (Whicher and MacKinnon, 2016). Kv10-12 channels have a C-terminal domain that is homologous to the cyclic nucleotide binding domain of CNG and HCN channels but lacks certain key residues so that it does not bind cyclic nucleotides.
In addition to the many pore-forming Kv channel subunits, a number of accessory K+ channel subunits have also been identified (Pongs and Schwarz, 2010). The many possible combinations of subunits and accessory proteins allows for an extremely large number of functionally and molecularly distinct K+ channels tuned to meet the particular needs of different cells.
1.1.4 Calcium-activated K+ channels (Adelman et al., 2012; Christophersen and Wulff, 2015; Kaczmarek et al., 2017; Kshatri et al., 2018; Latorre et al., 2017) are functionally classified as small, intermediate and large conductance channels. Like voltage-gated K+ channels, Ca2+-activated K+ channels with small (KCa2.1-2.3; SK) and intermediate (KCa3.1; IK) single channel conductance are formed from four subunits, each containing 6 trans-membrane domains with one P-loop. Ca2+ activates these channels in a voltage-independent way by binding to calmodulin (CaM) associated with a CaM binding domain on the C-terminus. Ca2+-activated K+ channels (KCa1.1) with a large single channel conductance (~250 pS in symmetrical K+) are referred to as big K+ (BK) or Maxi K+ channels. In addition to the 6 transmembrane domains possessed by most other voltage-dependent channels, BK channels have an additional S0 trans-membrane domain, placing the N-terminus on the extracellular rather than the intracellular surface as is typical of channels with six transmembrane domains. In BK channels, binding of Ca2+ to domains on the intracellular surface can directly activate the channels (Yuan et al., 2011; Yuan et al., 2010). The accompanying allosteric changes to the protein also lower the threshold for voltage-dependent activation by shifting voltage-dependence to more negative potentials. There is only a single gene for BK channels, but as with other channels, there are multiple splice variants. Accessory beta and gamma subunits can further modify the activity of BK channels.
1.1.5 Sodium-activated K+ channels (KNa1.1-1.2) (Kaczmarek, 2013; Kaczmarek et al., 2017) are formed from 6 transmembrane domains and a P-loop, but the S4 segment appears less free to move and does not possess the sequence of positively charged amino acid characteristic of voltage-dependent K+ channels (Hite et al., 2015). Elevation of intracellular Na+ and Cl− can both activate these channels. KNa channels are expressed in many neurons but, to our knowledge, their presence in retinal neurons has not been investigated.
1.2. Voltage-gated Na+ channels
Voltage-gated Na+ (NaV) channels are the key class of ion channels used to generate action potentials and are responsible for Na+ entry during the rising phase of the action potential (Ahern et al., 2016; Catterall, 2017). Unlike K+ channels that are formed from combinations of 2-4 individual subunits, the Na+ channel pore is formed from a single large α1 subunit protein. The α1 subunit consists of 4 similar sequences (I-IV), each possessing six transmembrane alpha helices (S1-6) with a short P-loop between S5 and S6, similar to individual voltage-dependent K+ channel subunits. As with most other voltage-dependent channels, the S4 domains function as the voltage sensor. Na+ channels underlying regenerative spiking are characterized by rapid and pronounced inactivation. Na+ channel inactivation involves a “hinged lid” mechanism in which the cytoplasmic loop between domains III and IV folds into the channel mouth to prevent conductance. There are currently 9 known isoforms of mammalian NaV channel alpha subunits (NaV1.1-1.9). NaV1.1, NaV1.2, and NaV1.6 are highly expressed in neurons from the central nervous system including retinal ganglion cells. In addition to the α subunit, functional channels typically associate with β subunits that can modify voltage-sensitivity and gating of the channel.
1.3. Voltage-gated Ca2+ channels
Voltage-gated Ca2+ channels share a common structure with a large pore-forming α1 subunit that assembles with an intracellular β subunit and extracellular α2δ subunit (Catterall, 2011; Dolphin, 2016). Skeletal muscle channels (CaV1.1) also have accessory γ subunits but these do not appear to associate with Ca2+ channels in neurons. Similar to voltage-gated Na+ channels, the pore-forming α subunit is a single large protein composed of four domains each with six transmembrane alpha helices, a voltage sensor on the transmembrane segment S4 and a P-loop between S5 and S6. Ca2+ channels are functionally classified into two major classes: low- and high-voltage activated (LVA and HVA). LVA currents (CaV3.1-3.3) activate at more negative potentials than HVA currents. Because of their tiny single channel conductance and rapid inactivation resulting in transient currents, LVA currents are also referred to as T-type currents. HVA L-type currents (CaV1.1-1.4) were originally defined by their large single channel conductance and long-lasting activation due to slow inactivation. Pharmacologically, L-type Ca2+ currents (ICa) are selectively sensitive to dihydropyridine agonists (e.g., BayK8644) and antagonists (e.g., nifedipine). N-type currents (CaV2.2) are HVA channels that show intermediate properties between T and L-type channels. N-type currents were found to be neither too long-lasting nor too transient and N-type single channel conductance was neither too large nor too tiny. N-type currents are also predominantly expressed in neurons. Selective block of another current by funnel web spider toxin revealed additional HVA Ca2+ channels in cerebellar Purkinje cells (P-type). Keeping to this largely alphabetical arrangement, the next subtype identified by use of selective blockers was then named Q. P and Q type channels (CaV2.1) both derive from a single gene, CACNA1A. Finally, the residual current that remains after blocking the other HVA types with a cocktail of toxins was named R (CaV2.3).
1.4. HCN and CNG channels
HCN and CNG channels are cation channels that share considerable homology with other voltage-gated channels. The channels consist of 4 subunits that each possess 6 transmembrane domains (S1-S6) with a pore-forming P-loop between S5 and S6. The S4 segment contains a number of positively charged amino acids, but despite this similarity to other voltage-dependent channels, CNG channels show little or no voltage-dependence (James and Zagotta, 2018) and HCN channels (HCN1-4) are activated by membrane hyperpolarization rather than depolarization (Craven and Zagotta, 2006; Wahl-Schott and Biel, 2009). CNG and HCN channels have an intracellular cyclic nucleotide binding domain. CNG channels are opened by cyclic nucleotide binding and the voltage-dependence of HCN channels is strongly modulated by cyclic nucleotides (James and Zagotta, 2018).
HCN subunits form cation channels that are weakly selective for K+ over Na+ (PNa/PK = 0.2-0.3) and show little Ca2+ permeability. Unlike other voltage-gated ion channels, depolarization of HCN channels causes the S4 segment to move inward rather than outward towards the extracellular surface (Lee and MacKinnon, 2017). HCN channels are therefore activated by hyperpolarization and are typically active only at quite negative membrane potentials. Binding of cAMP can shift HCN voltage-dependence to more positive potentials and thereby promote HCN activity at membrane potentials that are more often attained under physiological conditions. HCN channel activity promotes oscillatory behavior in many neurons where it is sometimes referred to as an anomalous rectifier current (Ia). It also contributes to pacemaker currents in the heart where it is termed the “funny” current (If). In this review, we refer to the current carried by HCN channels as “Ih” for hyperpolarization-activated current.
Our focus is on voltage- and Ca2+-gated ion channels and so we touch only briefly on CNG channels. There are six mammalian subunits: CNGA1-3 form functional homotetrameric channels but CNGA4, CNGB1 and CNGB3 can only form functional channels in combination with CNGA1-3 subunits. CNG channels are non-selective for monovalent cations and also conduct Ca2+, allowing it to serve as a second messenger in regulating phototransduction and olfactory transduction. We refer the interested reader to other reviews (Biel, 2009; Craven and Zagotta, 2006; James and Zagotta, 2018; Kaupp and Seifert, 2002).
1.5. Ca2+-activated Cl− channels
Anoctamin 1 and 2 (Ano1 and 2, also known as TMEM16A and B) are Ca2+-activated Cl− channels (Falzone et al., 2018; Kunzelmann, 2015; Whitlock and Hartzell, 2017). Ano1 and 2 are members of a larger family of anoctamin proteins (1-10) that also includes lipid scramblases and some cation channels. Ano1 and 2 anion channels are synergistically activated by voltage and Ca2+. The name “anoctamin” was given to TMEM proteins because it was originally thought that they possessed 8 transmembrane domains although it now appears that they have 10 transmembrane domains. Bestrophin proteins (Best1-4) can also form anion channels in expression system but there remains some question about whether these are truly Ca2+-activated Cl− channels (Hartzell et al., 2008). Best1 is strongly expressed in retinal pigment epithelium cells and mutations in this protein can cause Best vitelliform macular dystrophy (Johnson et al., 2017).
2. Rod and cone photoreceptor cells
There are two main classes of photoreceptor cells in the retina: rods and cones. Cones can be further classified into subtypes based on their spectral sensitivity. While the mechanisms of phototransduction are broadly similar in rods and cones, specific protein isoforms and structural differences promote greater sensitivity in rods and faster kinetics in cones. As we discuss below, rod and cone photoreceptors share many, but not all, of the same ion channels.
The outer segments of rods contain very few or no channels besides CNG channels involved in phototransduction (Baylor et al., 1984; Baylor and Nunn, 1986). Whole cell patch clamp recordings from dissociated rods and cones of amphibian retina revealed the presence of five types of ion channels in the inner segment and synaptic terminal (Attwell and Wilson, 1980; Bader et al., 1982; Barnes and Hille, 1989; MacLeish and Nurse, 2007): 1) inwardly rectifying cation currents activated by membrane hyperpolarization below −50 mV (Ih), 2) voltage-dependent K+ currents activated by depolarization above −60 mV (IKx), 3) sustained voltage-dependent ICa activated by depolarization above −50 mV, 4) Ca2+-activated K+ currents, and 5) Ca2+-activated Cl− currents. Rods and cones from mammalian retina share many of the same currents although Ca2+-activated K+ currents have not been observed in mammals (Cia et al., 2005; Demontis et al., 1999; Demontis et al., 2002; Han et al., 2000).
2.1. Voltage-gated Na+ channels
Recordings from many species, including non-human primates, have failed to reveal evidence for voltage-dependent Na+ currents in rods or cones. However, in a series of studies on small pieces of human retina excised during surgery for severe retinal detachment, Kawai and colleagues observed prominent, tetrodotoxin-sensitive action potentials in human rods and cones (Kawai et al., 2005; Kawai et al., 2001). The presence of NaV1.2 channels was confirmed in these cells by single cell PCR (Kawai et al., 2005). Anode break activation of these channels by a hyperpolarizing voltage step generated spikes and so the authors suggested that these channels might speed depolarization at the end of a light flash. There is also some immunohistochemical evidence for Na+ channels in rodent retina with labeling of cones by antibodies to NaV1.9 and labeling of photoreceptor terminals by antibodies to NaV1.1 (Mojumder et al., 2007; O'Brien et al., 2008). As discussed in a perspective by Copenhagen, the consistent observation that Na+ channels are absent from all other preparations, including non-human primates (Gayet-Primo et al., 2018; Yagi and Macleish, 1994), suggests that these channels are either uniquely present in human retina or, more likely, up-regulated in photoreceptors cultured after severe retinal detachment (Copenhagen, 2001).
2.2. Ca2+ channels
At the output end of the cell, release of glutamate-filled vesicles from the synaptic terminals of rods and cones is controlled by the influx of Ca2+ through L-type Ca2+ channels (Schmitz and Witkovsky, 1997; Thoreson et al., 1997; Wilkinson and Barnes, 1996). L-type Ca2+ channels are the only type of Ca2+ channels found in rods and cones (Bader et al., 1982; Barnes and Hille, 1989; Corey et al., 1984; Lasater and Witkovsky, 1991; Taylor and Morgans, 1998; Wilkinson and Barnes, 1996; Yagi and Macleish, 1994). We highlight some key aspects of Ca2+ channels and their properties at photoreceptor synapses. For additional details, we refer the reader to recent reviews that focus in depth on the properties of Ca2+ channels at photoreceptor synapses (Pangrsic et al., 2018; Waldner et al., 2018).
Sites of Ca2+ influx and labeling by antibodies to L-type Ca2+ channels are both localized close to individual synaptic ribbons of rods and cones (Cadetti et al., 2006; Choi et al., 2008; Firth et al., 2001; Lee et al., 2015; Lv et al., 2012; Morgans, 2001; Morgans et al., 2001; Nachman-Clewner et al., 1999; Taylor and Morgans, 1998). Immuno-electron micrographs show that Ca2+ channels sit just beneath ribbons (tom Dieck et al., 2005). Beneath each ribbon, Ca2+ channels are clustered in tiny sub-domains (Lv et al., 2012). Ca2+ channels show limited membrane mobility, behaving as if tethered in place by a weak spring (Mercer et al., 2011a). The vast majority of channels appear to be located near ribbons since salamander rods lacking synaptic terminals exhibit reductions in ICa of 95% (Xu and Slaughter, 2005).
L-type Ca2+ channels in rods and cones are formed principally from the pore-forming α1 subunit, CaV1.4, in combination with accessory β2 and α2δ4 subunits. CaV1.4 channels are expressed almost exclusively in retina although they also appear to be present in skeletal muscle (An et al., 2015) and T-lymphocytes (Kotturi and Jefferies, 2005). In the retina of many species (mouse, rat, chicken, human), labeling with antibodies to CaV1.4 is concentrated at synaptic ribbons of rods and cones (Firth et al., 2001; Lee et al., 2015; Liu et al., 2013b; Morgans, 2001; Morgans et al., 2001; Taylor and Morgans, 1998).
One of the initial findings suggesting a role for CaV1.4 at rod synapses was that mutations in this protein can lead to diminished synaptic output from rods and congenital stationary night blindness (Bech-Hansen et al., 1998; Strom et al., 1998; see review by Zeitz et al., 2015). Over 100 different nonsense, missense or frame-shift mutations in CaV1.4 have since been identified. These mutations can lead to loss of function, altered function, or gain of function. The impact of a specific mutation on channel function influences the nature and extent of night blindness (Zeitz et al., 2015). Mice in which CaV1.4 is completely eliminated exhibit total loss of both rod and cone responses suggesting that this channel subtype is responsible for mediating release from both types of photoreceptors, at least in this species (Mansergh et al., 2005).
Like L-type channels in other tissues, photoreceptor Ca2+ channels are sensitive to dihydropyridines. However, photoreceptor Ca2+ channels in vivo and heterologously expressed CaV1.4 channels show a weaker sensitivity to dihydropyridine antagonists and the benzothiazepine, diltiazem, than cardiac CaV1.2 channels (Baumann et al., 2004; Hart et al., 2003; Koschak et al., 2003; Wilkinson and Barnes, 1996). Together with poor penetration across the blood-retinal barrier, this explains why dihydropyridines and other Ca2+ channel blockers used for cardiovascular treatment do not cause vision changes (Uchida et al., 1997).
2.2.1. Voltage-dependence.
The L-type Ca2+ channels in rods and cones begin to activate above −60 mV and reach a peak around −20 mV. Voltage dependence of ICa measured in rods and cones from a number of species yields a midpoint activation voltage near −38 mV, very close to the dark resting membrane potential of photoreceptors (Babai and Thoreson, 2009; Grassmeyer and Thoreson, 2017; Schneeweis and Schnapf, 1999; Taylor and Morgans, 1998; Wu, 1985).
More than 20 splice isoforms of CaV1.4 have been identified and splice variants can differ in their voltage-dependence (Lee et al., 2015; Tan et al., 2012). While most variants activate at voltages that are more positive than those that activate the native channel, truncation of exon 47 allows channels to activate at more hyperpolarized potentials (Haeseleer et al., 2016; Tan et al., 2012). The Ca2+-binding protein, CaBP4, complexes with CaV1.4 and can shift activation to more negative potentials, although not in channels lacking exon 47 (Haeseleer et al., 2004; Haeseleer et al., 2016; Park et al., 2014; Shaltiel et al., 2012; Yang et al., 2014). For most channel isoforms, the presence of CaBP4 is thus essential for rods and cones to activate at potentials necessary to span the normal physiological voltage range in dark and light. Loss of CaBP4 can cause congenital stationary night blindness or cone-rod degeneration (Aldahmesh et al., 2010; Haeseleer et al., 2004; Khan et al., 2013; Littink et al., 2009; Maeda et al., 2005; Zeitz et al., 2006).
2.2.2. Inactivation.
L-type ICa in rods and cones show little or no voltage-dependent inactivation when activated by lengthy depolarizing voltage steps (Bader et al., 1982; Barnes and Hille, 1989; Corey et al., 1984; Rabl and Thoreson, 2002; Taylor and Morgans, 1998). This property allows them to remain active in darkness when photoreceptors are continuously depolarized. CaV1.4 channels originally characterized in heterologous expression systems showed very slow voltage-dependent inactivation along with little or no Ca2+-dependent inactivation (Baumann et al., 2004; Koschak et al., 2003; McRory et al., 2004). Apo-CaM binds to the IQ domain and the conformational change that occurs when Ca2+ ions bind to CaM leads to Ca2+-dependent inactivation. The absence of Ca2+-dependent inactivation in most CaV1.4 channels is due to the presence of an autoinhibitory domain in the C terminus that competes with the binding of apo-CaM to an IQ domain on the C-terminus. Because of these competitive interactions between apo-CaM and the autoinhibitory domain, higher endogenous levels of CaM promotes stronger Ca2+-dependent inactivation by promoting more binding of apo-CaM to the IQ domain. Phosphorylation of the autoinhibitory domain of CaV1.4 by protein kinase (PKA) also promotes apo-CaM binding to the IQ domain, thus further promoting Ca2+-dependent inactivation (Sang et al., 2016). Some splice isoforms of CaV1.4 have truncated C-termini that lack this autoinhibitory domain, thereby allowing Ca2+-dependent inactivation (Haeseleer et al., 2016; Lee et al., 2015; Tan et al., 2012). Thus, differences in the level of endogenous CaM, PKA activity, and the expression of splice isoforms can all potentially influence the degree of Ca2+-dependent inactivation.
2.2.3. Accessory subunits.
β2 subunits are the predominant accessory β subunits at rod and cone synapses. In electroretinogram (ERG) recordings, eliminating β2 subunits in a mouse knockout model almost completely eliminated rod- and cone-driven b-waves (that reflect On bipolar cell responses), with a-waves (that reflect photoreceptor responses) unchanged, showing a loss of synaptic transmission from photoreceptors (Ball et al., 2002). ERGs appear normal in mice lacking β1, 3 or 4 subunits. Antibodies to β2 label the OPL whereas antibodies to other β subunits do not (Ball et al., 2002; Lee et al., 2015). Direct interactions between β2 and CaV1.4 were confirmed with proximity ligation assays. A variant of β2 with an alternate exon 7, β2X13, appears to be the predominant subtype in human retina. This variant imparts greater voltage-dependent inactivation to the channel (Lee et al., 2015).
Mutations in α2δ4 also cause greatly attenuated b-waves and cone-rod dystrophy (Kerov et al., 2018; Wycisk et al., 2006a; Wycisk et al., 2006b). Deletion of α2δ4 in knockout mice eliminated rod-driven b-waves and reduced cone-driven b-waves, with little or no change in a-waves or rod and cone photocurrents (Kerov et al., 2018; Wang et al., 2017). Antibodies to α2δ4 label synaptic ribbons of rods and cones, forming a macromolecular complex with CaV1.4 and β2 (De Sevilla Muller et al., 2013; Lee et al., 2015; Mercer et al., 2011a). This suggests that α2δ4 is the predominant subunit at rod synapses although other isoforms may contribute in cones. α2δ4 subunits link to the extracellular membrane surface via glycosyl-phosphatidyl inositol interactions (Davies et al., 2010). In the photoreceptor synaptic cleft, α2δ4 interacts with ELFN1 and this interaction is important for proper formation of rod synapses (Kerov et al., 2018; Wang et al., 2017). Eliminating either α2δ4 or ELFN1 disrupts the formation of rod synapses (Cao et al., 2015; Kerov et al., 2018; Wang et al., 2017). Cone synapses do not possess ELFN1 and are less strongly affected by deletion of α2δ4 (Kerov et al., 2018; Wang et al., 2017). α2δ and β2 subunits assist in trafficking Ca2+ channel α1 subunits to the membrane (Dolphin, 2016) and so eliminating either subunit can reduce expression of functional CaV1.4 channels (Kerov et al., 2018; Wang et al., 2017). Diminished expression of CaV1.4 channels (Kerov et al., 2018; Liu et al., 2013b) may explain the diminished cone responses and impaired cone synapse formation seen after eliminating α2δ4 or β2 subunits (Katiyar et al., 2015; Kerov et al., 2018; Wang et al., 2017; Zabouri and Haverkamp, 2013) and may also contribute to impaired formation of rod synapses.
2.2.4. Single channel properties.
Single channel recordings of Ca2+ channels from salamander rod terminals and mean-variance analysis of ICa in salamander cones have both yielded single channel properties similar to other L-type channels including a single channel conductance in 82 mM Ba2+ of 22 pS and maximal open probability of 0.2-0.36 (Thoreson et al., 2000)(Bartoletti et al., 2011). By contrast, recordings of CaV1.4 channels expressed in tsA-201 cells yielded a single channel conductance of only 4 pS with 100 mM Ba2+ as the charge carrier and a peak open probability of <0.015 (Doering et al., 2005). Another expression study found a slightly larger single channel conductance of 10 pS but also a very low open probability (Burtscher et al., 2014). Is the unusually low open probability unique to CaV1.4 in mammalian preparations or does it only emerge in expression systems that lack protein partners such as CaBP4? Are the same properties present in different splice variants of CaV1.4? Using channels with a hundredfold lower open probability means that a hundredfold more channels would be needed to achieve the same current, which in turn implies a need for thousands of Ca2+ channels beneath each ribbon (Bartoletti et al., 2011). This appears inconsistent with freeze fracture electron micrographs showing ~400 particles thought to be Ca2+ channels beneath each macaque cone ribbon (each of which is 700-1000 nm long) (Raviola and Gilula, 1975).
2.2.5. Other Ca2+ channel subtypes.
In situ hybridization and immunohistochemical studies have suggested the presence of CaV1.3 in inner segments and synaptic terminals of rods and cones in a number of different species (Cristofanilli et al., 2007; Henderson et al., 2001; Kamphuis and Hendriksen, 1998; Kersten et al., 2010; Ko et al., 2007; Morgans, 1999; Morgans et al., 2005; Xiao et al., 2007; Zou et al., 2012). It has been suggested that CaV1.3 may interact with whirlin in a periciliary membrane complex to promote Usher disease (Kersten et al., 2010) but this interaction was not confirmed by a subsequent study (Zou et al., 2012). Zou et al. also showed that much of the labeling with various CaV1.3 Ca2+ channel antibodies was non-specific since it was not altered by elimination of CaV1.3 (Zou et al., 2012). However, elimination of CaV1.3 from mouse retina did cause some changes in ribbon structure (Busquet et al., 2010; Shi et al., 2017) and one study showed a reduction in ERG a- and b-waves (Shi et al., 2017). Another study on mice lacking CaV1.3 showed a small but statistically insignificant reduction in the b-wave and no significant changes in visual behavior assessed with a Morris water maze (Busquet et al., 2010). These data suggest that CaV1.3 channels may be present in photoreceptors but the role they play remains unclear. There is also immunohistochemical and in situ hybridization evidence for weak expression of CaV1.2 channels in photoreceptors (Kamphuis and Hendriksen, 1998; Ko et al., 2007; Nachman-Clewner et al., 1999; Xiao et al., 2007).
2.2.6. Ca2+ channel modulation.
Photoreceptor ICa can be modulated by many different signaling agents and pathways. Rods and cones can often be modulated differently by the same substance, suggesting differences in the regulation and channel composition at rod and cone synapses. For example, if we consider only salamander photoreceptors, activation of dopamine D4 receptors acts through pertussis toxin-sensitive G proteins to inhibit adenylate cyclase which in turn inhibits L-type ICa in large single cones, but these same pathways enhance ICa in rods and short wavelength-sensitive Scones (Stella and Thoreson, 2000). Likewise, inhibition of adenylate cyclase activity by CB1 cannabinoid receptors also inhibits ICa in large single cones but enhances ICa in rods (Straiker and Sullivan, 2003). Nitric oxide acts through a different pathway not involving guanylate cyclase but also inhibits ICa in cones and enhances ICa in rods (Kourennyi et al., 2004; Kurenny et al., 1994). By contrast with these agents, somatostatin 2A receptors acts through pertussis toxin-sensitive G proteins similar to dopamine, but has the opposite effect, enhancing cone ICa and inhibiting rod ICa (Akopian et al., 2000). Stimulation of adenylate cyclase by activation of adenosine A2a receptors inhibits rod ICa. This is consistent with effects of PKA on rod ICa observed with dopamine or cannabinoids, but activation of A2A receptors also inhibits cone ICa, rather than stimulating cone ICa as occurs by direct stimulation of PKA (Stella et al., 2002; Stella et al., 2007). Finally, activation of Group III metabotropic glutamate receptors inhibits ICa in cones but not rods (Hosoi et al., 2005; Van Hook et al., 2017). Thus, even agents that act through some of the same signaling pathways (e.g., pertussis toxin-sensitive G proteins or adenylate cyclase) can have different effects on rod and cone ICa. In addition to divergent intracellular signaling pathways, one possible source for such differences could be the presence of splice variants of CaV1.4 that differ in the C-terminal autoinhibitory domain sensitive to phosphorylation by PKA (see section 2.2.2). Splice variants of CaV1.4 that lack this C-terminal autoinhibitory domain would be expected to be insensitive to PKA modulation (Sang et al., 2016). Non-GPCR signaling pathways can also regulate photoreceptor ICa. For example, insulin inhibits ICa in salamander rods by mechanisms that involve tyrosine kinase activity (Stella et al., 2001). Polyunsaturated fats and retinoids, including 11-cis-retinal, also inhibit ICa in salamander rods (Vellani et al., 2000). Levels of dopamine, adenosine, and glutamate vary with light and dark and so it is hypothesized that these modulatory effects on ICa help to adjust gain at photoreceptor synapses with changing illumination (Hosoi et al., 2005; Stella et al., 2007; Thoreson et al., 2002) but details of how these different signaling pathways interact with one another remain unknown.
Evidence from chicken cones suggests that modulation of ICa is under circadian regulation. For example, somatostatin and nitric oxide both inhibit cone ICa in subjective night but not subjective day (Jian et al., 2009; Ko et al., 2013). Expression of Ca2+ channels in chicken cones is also under circadian regulation by pathways involving Ras-ERK, PI3-Kinase-Akt, and microRNA 26a (Ko et al., 2007).
ICa can be regulated by a number of negative feedback mechanisms that operate locally at the synapse. Protons are a powerful regulator of synaptic release from photoreceptors, altering both voltage-dependence and amplitude of ICa. Extracellular protons inhibit the amplitude of ICa and shift voltage-dependence of activation in a positive direction with the net effect of reducing Ca2+ channel activity in the normal physiological voltage range for photoreceptors. Protons released during synaptic vesicle fusion in rods and cones can feed back to inhibit presynaptic ICa and synaptic release (DeVries, 2001). Synaptic cleft proton levels are also regulated by changes in horizontal cell membrane potential (Hirasawa and Kaneko, 2003; Wang et al., 2014). The ability of horizontal cells to alter cleft proton levels is central to the mechanism of surround antagonism in which depolarization of horizontal cells leads to cleft acidification which in turn inhibits rod and cone ICa (Thoreson and Mangel, 2012). In addition to containing protons, glutamatergic vesicles in rods and cones also contain Zn2+ ions that can inhibit ICa (Chappell et al., 2008; Wu et al., 1993).
The binding of Cl− ions to the intracellular surface of L-type Ca2+ channels in photoreceptors promotes channel open probability and so reductions in intracellular Cl− can inhibit ICa (Babai et al., 2010; Thoreson et al., 1997). In rods, ECl is positive to the resting membrane potential and so activation of Ca2+-activated Cl− channels in rod terminals promotes Cl− efflux that can act as a feedback mechanism to inhibit ICa(Thoreson et al., 2003; Thoreson et al., 1997; Thoreson et al., 2002). In addition to effects of reducing cell input resistance during activation of ICl(Ca), a reduction in intracellular [Cl−] of only 10 mM can reduce ICa by 20% (Thoreson et al., 2003; Thoreson et al., 1997). In cones, ECl is close to the dark resting membrane potential (Thoreson and Bryson, 2004) so this sort of feedback inhibition will only occur when cones are hyperpolarized (e.g., by light). Local negative feedback mechanisms involving Cl− ions, zinc, and protons may help to limit regenerative activation of ICa and the generation of Ca2+ spikes in rods and cones.
2.3. K+ channels
2.3.1. Voltage-dependent K+ channels
Beech and Barnes (Beech and Barnes, 1989) described the properties of a voltage-dependent K+ current in cones that they named IKx. IKx activates quickly with depolarization and de-activates slowly upon hyperpolarization. This current is active between −70 and −30 mV with a midpoint activation value of −45 to −55 mV (Beech and Barnes, 1989; Gayet-Primo et al., 2018; Kurennyi and Barnes, 1997). A more transient K+ current that activates at more positive potentials than IKx has also been identified in primate rods and cones, (Gayet-Primo et al., 2018; Yagi and Macleish, 1994) as well as lizard cones (Maricq and Korenbrot, 1990b).
IKx shares a number of similarities with M-type K+ currents (Kv7) and there is evidence for M-type Kv7 channels in cone inner segments from immunohistochemistry and in situ hybridization (Zhang et al., 2011). However, IKx shows a different pharmacological profile from M-type currents, being more sensitive to Ba2+, insensitive to acetylcholine and LHRH, and insensitive to a Kv7 blocker XE991 (Beech and Barnes, 1989; Gayet-Primo et al., 2018). In situ hybridization suggests the presence of ether-a-gogo-related (EAG; Kv11) channels in the inner segments of bovine rods (Frings et al., 1998). However, the pharmacological properties do not support a substantial contribution from this subtype in primate rods (Gayet-Primo et al., 2018).
Using a combination of immunohistochemistry, electrophysiology and pharmacology, Gayet-Primo et al. (Gayet-Primo et al., 2018) established the presence of Kv8.2 and Kv2 channels localized to the inner segments of primate rods and cones. Studies also indicate the presence of Kv2.1 and 8.2 in photoreceptor inner segments from human and mouse retina (Klumpp et al., 1995b; Pinto and Klumpp, 1998; Wu et al., 2006). Kv8.2 subunits do not form functional channels by themselves but can form functional heteromers with other subunits. The presence of Kv8.2 subunits in heteromeric channels shifts Kv2 current activation to more negative potentials, yielding electrophysiological properties similar to those of native IKx currents (Czirjak et al., 2007). Mutations to the Kv8.2 gene cause a cone dystrophy with supernormal rod ERGs (Ben Salah et al., 2008; Vincent et al., 2013; Wissinger et al., 2008; Wissinger et al., 2011; Zobor et al., 2012). Some of the disease-causing mutations result in complete elimination of Kv8.2 whereas others impair its interaction with Kv2 subunits. When co-expressed with Kv2.1 in Xenopus oocytes, both types of mutations in Kv8.2 eliminate currents with properties similar to IKx (Czirjak et al., 2007). Cones express both Kv2.1 and Kv2.2, while rods predominantly express Kv2.1 (Gayet-Primo et al., 2018). Kv2.2 was also absent from mouse photoreceptors (Klumpp et al., 1995b). Using a combination of molecular, electrophysiological and pharmacological approaches, Gayet-Primo et al. concluded that the high voltage-activated K+ currents in primate rods and cones arise from homomeric Kv2 channels (Kv2.1 in rods and a combination of Kv2.1 and Kv2.2 in cones) whereas lower threshold IKx are likely to arise from heteromeric Kv2/Kv8.2 channels (Gayet-Primo et al., 2018).
2.3.2. Ca2+-activated K+ channels
In rods and cones of salamander retina, strong depolarizing steps that activate ICa (see section 2.2) also activate noisy outward currents carried by large conductance Ca2+-activated K+ currents (BK) currents (Bader et al., 1982; Barnes and Hille, 1989; MacLeish and Nurse, 2007; Moriondo et al., 2001; Pelucchi et al., 2008; Xu and Slaughter, 2005). Antibodies to BK (KCa1.1) and IK (KCa3.1) channels also label salamander rods, but not antibodies to SK channels (Pelucchi et al., 2008). The presence of IK and BK channels is also supported by pharmacology. Ca2+-dependent K+ currents in rods can be inhibited by a BK channel blocker, iberiotoxin; partially inhibited by the mycotoxin clotrimazole which inhibits IK channels (Pelucchi et al., 2008); but not inhibited by apamin which blocks SK channels (Pelucchi et al., 2008; Xu and Slaughter, 2005). IK channels are gated exclusively by Ca2+ (Sforna et al., 2018) whereas BK channels can be opened by both depolarizing voltage and Ca2+ (Latorre et al., 2017). These differences in gating may account for the finding that IK channels appear to contribute more strongly at positive voltages than BK channels (Pelucchi et al., 2008).
Blocking Ca2+-activated K+ channels enhances excitability and promotes regenerative spiking in photoreceptors (Fain et al., 1977; Moriondo et al., 2001), suggesting that one role for these channels may be to prevent regenerative activation of Ca2+ channels and thus maintain the membrane voltage in darkness near −40 mV. On the other hand, it has also been proposed that efflux of K+ during activation of these channels can enhance ICa in rods which would promote excitability (Xu and Slaughter, 2005).
While there is clear evidence for these channels in salamander retina, there is no evidence for Ca2+-activated K+ currents in cones from lizard or primate retina (Cia et al., 2005; Maricq and Korenbrot, 1990b; Yagi and Macleish, 1994).
2.4. HCN channels
Both rods and cones exhibit prominent inwardly rectifying currents activated by hyperpolarization (Ih). Ih was first identified from its blockade by low millimolar concentrations of cesium (Fain et al., 1978). Although blocked by cesium, Ih is relatively insensitive to tetraethylammonium (TEA) (Bader and Bertrand, 1984; Bader et al., 1982; Demontis et al., 1999; Demontis et al., 2002; Hestrin, 1987; Maricq and Korenbrot, 1990a). Ih are similar to inwardly rectifying currents in a variety of other cells, including so-called “funny” currents in cardiac myocytes. Accordingly, Ih can be selectively inhibited by various bradycardic agents including ZD7288, ivabradine and zatebradine (Demontis et al., 2009; Satoh and Yamada, 2000, 2002). Ih shows slow kinetics and a hyperpolarized voltage-dependence, activating below ca. −50 mV with an activation midpoint around −70 to −80 mV (Barrow and Wu, 2009; Demontis et al., 1999; Demontis et al., 2002; Malcolm et al., 2003; Maricq and Korenbrot, 1990a). Ih channels show a permeability ratio PNa/PK of 0.2-0.3 (Demontis et al., 1999; Demontis et al., 2002; Hestrin, 1987; Mao et al., 2003; Wollmuth and Hille, 1992), with a reversal potential under physiological conditions of −30-35 mV (Bader and Bertrand, 1984; Bader et al., 1982; Barnes and Hille, 1989; Demontis et al., 1999; Demontis et al., 2002; Maricq and Korenbrot, 1990a). The properties of Ih in cones are similar to those of rods (Barnes and Hille, 1989; Barrow and Wu, 2009; Maricq and Korenbrot, 1990a; Wollmuth and Hille, 1992; Yagi and Macleish, 1994). Properties of Ih are also similar in human rods and primate cones (Kawai et al., 2002; Yagi and Macleish, 1994). HCN1-type Ih channels are concentrated in the inner segment (Barrow and Wu, 2009; Della Santina et al., 2012; Demontis et al., 2002; MacLeish and Nurse, 2007). These channels have a small single channel conductance of <1 pS with an average of ~2,000 channels per rod or cone (Barrow and Wu, 2009). The low single channel conductance helps to reduce membrane noise.
In response to a bright light flash, rods show a transient hyperpolarization followed by a rapid depolarizing recovery of the membrane potential. This depolarizing rollback is due to the activation of Ih triggered during the initial light-evoked hyperpolarization of the rod. By eliminating this rollback, blocking Ih makes hyperpolarizing rod light responses more sustained and increases their peak amplitude. Cones do not normally show a prominent transient “nose” in response to light but blocking Ih increases the overall amplitude of their hyperpolarizing light responses (Barrow and Wu, 2009; Fain et al., 1978; Satoh and Yamada, 2000, 2002). In addition to changes in response waveform, the slow activation kinetics of Ih produces high-pass filtering of the hyperpolarizing photoreceptor light response (Attwell, 1986; Barrow and Wu, 2009; Demontis et al., 1999; Mao et al., 2003). Combined with low-pass filtering by the passive membrane properties and photocurrent, this yields a net band-pass filtering of photoreceptor light responses. By lowering cell input resistance to speed the membrane time constant, activation of Ih by membrane hyperpolarization improves the high frequency responses of cones (Howlett et al., 2017). This contributes to a form of light adaptation whereby high contrast changes that produce voltage excursions large enough to activate Ih can speed up cone responses. Ih also improves the ability of rods to adapt to light; rod photocurrents show a more significant reduction in sensitivity with increasing light levels than photovoltage (Pahlberg et al., 2017; Sothilingam et al., 2016). Eliminating Ih abolished these differences in the adaptation of photovoltage and photocurrent responses.
While loss of HCN1 does not directly cause retinal degeneration, it can worsen retinal degeneration caused by other mutations such as loss of CNG channel β subunits from rods or loss of CNG α subunits from cones (Schon et al., 2016). This worsening of degeneration does not appear to be due to an effect on resting membrane potential which did not differ in HCN1 KO rods but instead involves increased levels of calpain activity (Schon et al., 2016).
2.5. Ca2+-activated Cl− channels
Another prominent current in rods and cones is the Ca2+-activated Cl− current (Bader et al., 1982; Barnes and Hille, 1989). Immunohistochemical studies in salamander and mouse retina suggested the presence of Ano1 in both rod and cone terminals (Caputo et al., 2015; Jeon et al., 2013; Mercer et al., 2011b; Yang et al., 2008). Stohr et al. cloned Ano2 (aka TMEM16B) from mouse and human retina and showed that it formed Ca2+-activated anion channels (Stohr et al., 2009). They went on to show that Ano2 was selectively expressed at photoreceptor ribbon synapses. In rat retina, Ano2 is selectively expressed in rods but not cones; Ano1 expression was not seen in either cell type (Dauner et al., 2013).
Ca2+-activated Cl− currents were almost wholly eliminated in salamander rods lacking synaptic terminals (MacLeish and Nurse, 2007). Antibodies to Ano1 and Ano2 label the entire synaptic terminal and are not tightly confined to ribbons like antibodies to Ca2+ channels (Dauner et al., 2013; Mercer et al., 2011b; Stohr et al., 2009). Effects of Ca2+ buffers on Ca2+-activated Cl− currents in salamander rods and cones also suggest that these channels are distributed throughout the terminal. However, the ability of Ca2+-activated Cl− currents to persist in the presence of the fast Ca2+ buffer BAPTA suggests that some of these channels are located within 100 nm of Ca2+ channels (Mercer et al., 2011b). Consistent with tight co-localization between Ca2+-activated Cl− channels and Ca2+ channels, Ano1 channels can coimmunoprecipitate with CaV1.4 Ca2+ channels when expressed in tsa201 cells (Caputo et al., 2015).
The evidence for Ano1 in photoreceptors rests largely on immunohistochemistry while there is both immunohistochemical and molecular evidence for Ano2. Transcriptome analyses of rods and cones also suggest significant levels of Ano2 but not Ano1 (Busskamp et al., 2014; Hartl et al., 2017; Mo et al., 2016). On the other hand, Ano1 channels are 10 times less sensitive to Ca2+ than Ano2 channels (Vocke et al., 2013) and so the ability of submicromolar Ca2+ to stimulate Ca2+-activated Cl− currents in salamander rods and cones is more consistent with Ano1 (Mercer et al., 2011b).
2.6. CNG channels
The only ion channels in the outer segments of intact rods and cones are CNG cation channels gated open by cGMP (Baylor et al., 1984). The channels in rods consist of CNGA1 and CNGB1 heteromers while cones have CNGA3 and CNGB3 heteromers. Cation influx through these channels support the dark current that is terminated by their closure during phototransduction. The reduced Ca2+ influx that accompanies channel closure plays a key role in adjusting the gain of phototransduction during light adaptation. Mutations in CNGA1 and CNGB1 cause autosomal recessive retinitis pigmentosa while mutations in CNGA3 and CNGB3 cause achromatopsia. A detailed consideration of phototransduction and outer segment CNG channels is beyond the scope of this review and is reviewed in detail elsewhere (Arshavsky and Burns, 2012; Biel, 2009; Burns and Baylor, 2001; Fu and Yau, 2007; Kaupp and Seifert, 2002; Michalakis et al., 2018).
CNG channels are also present in the synaptic terminals of cones. Ca2+ influx through these channels can trigger fusion of glutamate-filled vesicles (Rieke and Schwartz, 1994; Savchenko et al., 1997). It has been suggested that the opening of CNG channels may extend the cone operating range, allowing release of glutamate at more negative potentials where the activity of Ca2+ channels begins to diminish. However, because of the increased driving force for cations, CNG currents typically increase with hyperpolarization, rather than diminishing like ICa. CNG channels in cone terminals can be regulated by constitutive levels of cGMP but can also be opened by increases in cGMP triggered by nitric oxide released from neighboring neurons and glia (Savchenko et al., 1997). Thus, these channels may help to regulate glutamate release in response to changes in nitric oxide levels.
2.7. Summary
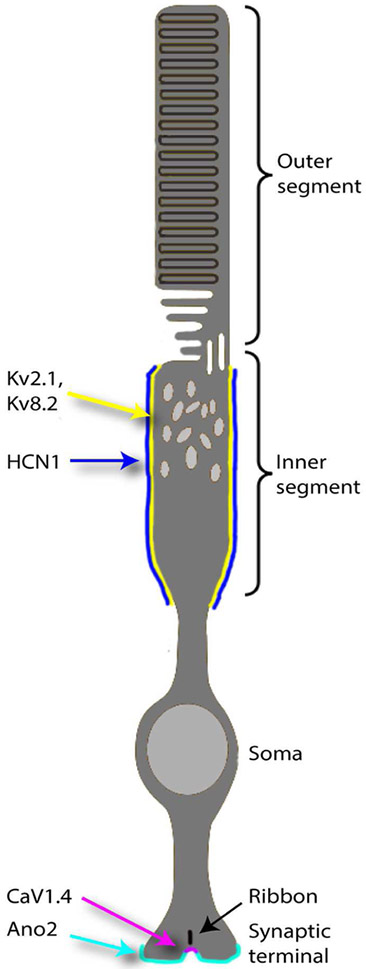
The distribution of the principal ion channels in mammalian rods is summarized in Fig. 1 showing that homomeric KV2.1 and heteromeric KV2.1/KV8.2 channels are distributed throughout the inner segment, along with HCN1 channels. CaV1.4 channels in a complex with β2a and α2δ4 subunits are clustered beneath the synaptic ribbon. Ano2 Ca2+-activated Cl− channels are distributed more diffusely throughout the synaptic terminal membrane. Ca2+-activated Cl− channels in cones appear to be a different subtype from Ano2. In primate cones, inner segments also possess Kv2.2.
Figure 1.
Diagram of the ion channel distribution in a mammalian rod. HCN1, homomeric KV2.1 and heteromeric KV2.1/KV8.2 channels are distributed throughout the inner segment. CaV1.4 channels in a complex with β2a and α2δ4 subunits are clustered beneath synaptic ribbons. Ano2 channels are distributed more diffusely in the synaptic terminal membrane. See text for details.
Measurements of the membrane potential of rods and cones show it to be near −40 mV in darkness. These potentials are close to the activation midpoint value for IKx whereas Ih is minimally active at this potential (Attwell, 1986; Barnes, 1994). Thus, when light closes CNG channels in the outer segments, the dominant conductance will be IKx, and K+ efflux through these channels will drive the membrane potential in a hyperpolarizing direction. Strong hyperpolarization will activate Ih, driving the membrane potential back in a depolarizing direction. Activation of Ih thus limits the amplitude of hyperpolarizing rod and cone light responses and makes rod responses more transient. There is immunohistochemical evidence for KCNK2 two pore channels in mouse cones suggesting that along with IKx, K+ leak channels might also contribute to the negative driving force (Hughes et al., 2017).
The dark resting membrane potential of −40 mV in darkness is close to the activation midpoint value for ICa. Positioning the membrane potential close to the activation midpoint maximizes the changes in ICa caused by light-evoked voltage changes in membrane potential, which in turn maximizes the sensitivity of Ca2+-dependent glutamate release. However, this also places the cone in an unstable region of negative slope conductance. The likelihood for regenerative activation of Ca2+ channels is limited by the activation of strong countervailing conductances, especially IKX, and mechanisms that reduce Ca2+ channel activity during maintained darkness. The activation of Ca2+-activated Cl− channels leads to a conductance increase that tends to drive the membrane potential towards ECl. In cones, ECl is near the dark resting membrane potential; ECl is somewhat more depolarized in rods (Thoreson and Bryson, 2004; Thoreson et al., 2003). At potentials below ECl, the efflux of Cl− through Ca2+-activated Cl− channels will also directly inhibit ICa. In those photoreceptors that possess them, activation of BK channels can also provide a hyperpolarizing driving force to limit excitability. With maintained depolarization as occurs in maintained darkness, ICa will slowly inactivate as a result of Ca2+ and/or voltage-dependent inactivation. In addition, as found at calyceal synapses (Borst and Sakmann, 1999; Stanley, 2000), the constant influx of Ca2+ ions into tonically open Ca2+ channels at rod ribbons depletes extracellular Ca2+ ions from the synaptic cleft to further inhibit ICa (Rabl and Thoreson, 2002). The maintained activity of Ca2+ channels in darkness stimulates continuous release of glutamate-filled synaptic vesicles. The release of protons and Zn2+ ions from synaptic vesicles can further inhibit ICa. Acidification of the synaptic cleft accompanying negative feedback from depolarized horizontal cells will also inhibit rod and cone ICa.
While these various mechanisms work to limit the likelihood of regenerative Ca2+ action potentials, depolarizing stimulation can trigger regenerative activation of Ca2+ channels under certain conditions. Illumination of the receptive field surround acting through horizontal cell feedback can produce a leftward (negative) shift in ICa activation and increase in ICa peak amplitude. This causes a net increase in rod and cone ICa at physiological potentials that can in turn generate depolarizing Ca2+ spikes in rods and cones (Burkhardt et al., 1988; Burkhardt et al., 1991; Lasansky, 1986; Maricq and Korenbrot, 1988; Piccolino and Gerschenfeld, 1978, 1980; Thoreson and Burkhardt, 1991). The likelihood of such spikes can be dramatically increased by enhancing ICa with application of Sr2+ or Ba2+ (Piccolino and Gerschenfeld, 1980). When ECl is more positive than the resting membrane potential, the activation of Ca2+-activated Cl− currents helps to maintain the membrane in a depolarized state, promoting regenerative potentials that can last for seconds (Thoreson and Burkhardt, 1991). Thus, elevating intracellular Cl− enhances the likelihood of these events (Barnes and Deschenes, 1992; Maricq and Korenbrot, 1988; Thoreson and Burkhardt, 1990). These long-lasting regenerative potentials are terminated when intracellular Ca2+ levels fall (Krizaj, 2012) and the activation of ICI(Ca) diminishes. These prolonged Ca2+ action potentials arise from bistability in the membrane voltage (Barnes and Deschenes, 1992; Kamiyama et al., 1996) that can be successfully simulated by computational models incorporating biophysical parameters of rod currents (Kamiyama et al., 1996). These long-lasting regenerative events are probably not normally experienced by healthy photoreceptors that have robust Ca2+ handling mechanisms. However, occurrence of such events in unhealthy photoreceptors might impair signaling in disease states.
3. Horizontal cells
Most vertebrate species have four types of horizontal cells while most mammals have only two types (Gallego, 1986; Peichl et al., 1998). Rodent retinas have only a single type of horizontal cell (Peichl and Gonzalez-Soriano, 1994). Five major types of ion currents are present in horizontal cells of most species: fast TTX-sensitive Na+ current, Ca2+ current (ICa), transient outwardly rectifying K+ current (IKA), delayed rectifier outward K+ current (IKDR), and inwardly rectifying K+ current (IKIR) (Golard et al., 1992; Lasater, 1986; Lohrke and Hofmann, 1994; Malchow et al., 1990; Picaud et al., 1998; Shingai and Christensen, 1983, 1986; Tachibana, 1983a; Ueda et al., 1992). There is no evidence for significant voltage-dependent Cl− currents in horizontal cells (Byzov and Trifonov Yu, 1981; Waloga and Pak, 1978). While the composition of ion channels is generally similar among horizontal cells, it can vary among species and among different types of horizontal cells. For example, rod-dominated H4 cells in white perch retina lack A-type K+ currents that are present in H1-H3 cells (Lasater, 1986) but in white bass retina, IKA is present in H4 cells but not H1 cells (Sullivan and Lasater, 1990a). In rod-dominated skate retina, external horizontal cells lying closer to rods have a greater density of IKIR and lower density of sustained outward currents than internal horizontal cells (Malchow et al., 1990). In cat retina, axonless A-type cells showed fast sodium currents whereas axon-bearing B-type cells did not (Ueda et al., 1992).
In every species, there appears to be at least one type of horizontal cell that has an axon that extends laterally through the OPL and then expands into a functionally distinct, large, axon-terminal compartment. The other horizontal cell subtype(s) are axonless. In fish retina, the axon terminal compartment does not appear to contact any photoreceptors but nevertheless shows light-evoked voltage responses similar in amplitude and spectral characteristics to responses recorded in the cell body (Stell, 1975; Weiler and Zettler, 1979). It has therefore been concluded that light responses generated in the soma pass almost without decrement to the axon terminal. Transmission along the axon does not appear to be boosted by activation of voltage-dependent Na+ channels (Djamgoz and Stell, 1984; Weiler and Zettler, 1979). Recordings from enzymatically isolated axon terminals in fish retina show a similar complement of channels as somas, but a higher specific membrane resistance (Yagi and Kaneko, 1988). Similarly, the input resistance of axon terminals is much higher than somas of horizontal cells isolated from turtle retina (Golard et al., 1992). Thus, small currents that reach the high resistance axon terminal compartment can generate large voltage responses (Golard et al., 1992; Yagi and Kaneko, 1988).
In rodents and other mammals, the soma compartment contacts only cones whereas the axon terminal compartment contacts only rods. The only type of horizontal cell in rodent retina (B-type) is an axon-bearing horizontal cell. In mice that lack gap junctions between rods and cones, recordings from axon terminals that contact only rods nevertheless show the presence of cone inputs in their responses (Trumpler et al., 2008). By contrast, rod responses were not observed in somas of these same connexin 36 knockout mice. Trumpler et al. therefore concluded that cone signals can pass from soma to terminal but rod signals cannot go the other direction, from terminal to soma (Trumpler et al., 2008). On the other hand, Szikra et al. observed small depolarizing responses in cones evoked by light flashes that should only activate rods and concluded that rod signals can travel from terminal to soma (Szikra et al., 2014). However, the cone recordings were similar in size (< 2 mV) and waveform to intraretinal ERGs raising the possibility of contamination by extracellular field potentials. As discussed above, the ability of signals to flow between the two compartments depends on their relative input resistances. Lowering the somatic resistance by reducing glutamatergic input or uncoupling of gap junctions would be one mechanism for improving transmission of voltage signals from axon terminal to soma. Differences in the expression of ion currents between soma and axon terminals might also contribute. While the types of ion channels in the two compartments do not appear to differ in most species, this may not be the case for mouse horizontal cells (Feigenspan et al., 2009).
3.1. Voltage-gated Na+ channels
Fast, TTX-sensitive Na+ currents that activate above −50 mV have been observed in isolated horizontal cells from a variety of species (Golard et al., 1992; Lasater, 1986; Lohrke and Hofmann, 1994; Malchow et al., 1990; Shingai and Christensen, 1983; Ueda et al., 1992). Antibodies to 1.1, 1.2 and 1.6 sodium channels show labeling throughout horizontal cells in rodent and rabbit retina (Mojumder et al., 2007). Na+ currents in horizontal cells are not as large as those found in ganglion cells and action potentials are not normally observed in horizontal cells in situ. However, Na+ channels can facilitate regenerative action potentials in horizontal cells that are isolated from the retina or uncoupled from their neighbors (Blanco et al., 1996; Shingai and Christensen, 1986; Tachibana, 1981). While it seems plausible that rapid activation of Na+ channels might assist in speeding membrane depolarization at light offset, blocking these channels with TTX had no obvious effect on light responses (Akopian et al., 1997; Djamgoz and Stell, 1984; Perlman et al., 1993).
3.2. Ca2+ channels
Horizontal cells in all species studied exhibit a small, sustained inward ICa that begins to activate around −40 to −30 mV (Golard et al., 1992; Liu et al., 2013a; Lohrke and Hofmann, 1994; Malchow et al., 1990; Picaud et al., 1998; Schubert et al., 2006; Shingai and Christensen, 1983; Sullivan and Lasater, 1992; Tachibana, 1983a; Ueda et al., 1992). This sustained current is sensitive to dihydropyridine agonists and antagonists (Chapot et al., 2017; Golard et al., 1992; Liu et al., 2013a; Lohrke and Hofmann, 1994; Pfeiffer-Linn and Lasater, 1996b; Picaud et al., 1998; Ueda et al., 1992) indicating that it involves L-type channels. The single channel conductance is similar to other L-type channels (Pfeiffer-Linn and Lasater, 1996b). In mouse and fish retina, sustained ICa can also be weakly inhibited by ω-agatoxin IVA (Liu et al., 2013a; Pfeiffer-Linn and Lasater, 1996b; Schubert et al., 2006), suggesting the additional presence of CaV2.1 (P/Q-type) channels (Bourinet and Zamponi, 2017). In mouse horizontal cells, ω-conotoxin also inhibited ICa consistent with the presence of N-type channels (Liu et al., 2013a; Schubert et al., 2006). Immunohistochemical studies from mouse retina also show the presence of L, N and P/Q-type channels in the dendritic tips of horizontal cells. It has been proposed that Ca2+ channels in horizontal cell dendrites may mediate Ca2+-dependent release of GABA (Liu et al., 2013a).
There is evidence for transient ICa in horizontal cells from fish, Xenopus, and rabbit (Akopian et al., 1997; Lohrke and Hofmann, 1994; Pfeiffer-Linn and Lasater, 1996b; Shingai and Christensen, 1983; Sullivan and Lasater, 1992) but not turtle, cat or mouse (Golard et al., 1992; Liu et al., 2013a; Schubert et al., 2006; Ueda et al., 1992). This transient ICa is insensitive to dihydropyridines, activates at more negative potentials than sustained inward currents, and can be inhibited by Ni2+ (Akopian et al., 1997; Pfeiffer-Linn and Lasater, 1996b; Sullivan and Lasater, 1992), consistent with T-type ICa. Although these currents are generally small in horizontal cells, voltage-dependent activation of Ca2+ channels as horizontal cells depolarize during light offset may help speed repolarization of the membrane (Akopian et al., 1997).
3.3. K+ channels
3.3.1. Inwardly rectifying K+ channels
One of the most prominent currents in horizontal cells is IKIR. IKIR is also referred to as the anomalous rectifier current. Unlike the inward rectifying cation current Ih in photoreceptors, the inward rectifier in horizontal cells is selective for K+ ions (Golard et al., 1992; Shingai and Christensen, 1986; Yagi and Kaneko, 1988). Unlike Ih, IKIR is also not blocked by ZD7288 (Feigenspan et al., 2009). Like IKIR in other preparations, horizontal cell currents are relatively insensitive to TEA or 4-AP but blocked by low concentrations of extracellular Cs+ or Ba2+ (Shingai and Christensen, 1986; Tachibana, 1983a; Ueda et al., 1992). The single channel conductance of 20 pS in 125 mM external K+ (Shingai and Quandt, 1986) is similar to that of IKIR in other preparations (Newman, 1993; Park et al., 2008; Sakmann and Trube, 1984).
Small outward currents through inward rectifier K+ channels at potentials above EK contribute to maintenance of the resting membrane potential in many neurons (Hibino et al., 2010). IKIR is active throughout the normal physiological voltage range of horizontal cells (−30 to −90 mV), contributing to the resting membrane potential of these cells in darkness and to the driving force for hyperpolarizing excursions during light (Dong and Werblin, 1995; Feigenspan et al., 2009). However, IKIR is not the only current responsible for this hyperpolarizing driving force since even after blocking IKIR, the light-evoked hyperpolarization of horizontal cells approaches EK. Small leak K+ currents in horizontal cells (Lasater, 1986; Tachibana, 1983a) may contribute the additional driving force. Transcriptome data from horizontal cells show significant levels of KCNK1 two pore channel mRNA (Hartl et al., 2017). Immunohistochemical studies also show evidence for KCNK1 and KCNK3 channels in horizontal cells early in development and KCNK2 channels in adult mouse retina (Hughes et al., 2017).
IKIR may play other roles in horizontal cells besides setting the resting membrane potential. In adult rabbit retina, Kir2.1 channels are localized to a macromolecular complex with glutamate receptors and scaffold proteins at the dendritic tips of B-type horizontal cells in the OPL (Vila et al., 2017). The authors suggested that currents flowing through these channels could generate ephaptic changes in the extracellular voltage within the invaginating cone synapse that might contribute to negative feedback modulation of cone ICa by horizontal cells (Vila et al., 2017).
IKIR in horizontal cells are larger than similar currents in many other neurons. Kir4.1 channels in glial Müller cells have been shown to be important for buffering extracellular K+ changes by siphoning K+ from regions of high extracellular K+ (e.g., synaptic plexiform layers) to regions of lower K+ (e.g., adjacent to the vitreous and vasculature) (Kofuji and Newman, 2004). In newborn mice, Kir4.1 channels are expressed in horizontal cells prior to their expression in Müller cells, leading Bosco et al. to propose that before Müller cells are fully developed, horizontal cells may play a similar role in buffering and siphoning of K+ from the OPL (Bosco et al., 2005). In support of this, they noted close contacts between horizontal cells and outer retinal blood vessels. Kir4.1 expression disappears from horizontal cells in adult mice (Bosco et al., 2005), but these cells nevertheless continue to express prominent IKIR. In rabbit retina, Kir 2.1 channels are expressed at the tips of horizontal cell dendrites within the synaptic invaginations of cone pedicles (Vila et al., 2017). We suggest that such channels would be well positioned to assist in buffering extracellular K+ changes that can occur near the terminals of rods and cones in the OPL (Dick and Miller, 1985; Dick et al., 1985; Karwoski et al., 1985). Elevation of extracellular K+ also substantially increases the conductance of IKIR and shifts its reversal potential to more positive values (Dong and Werblin, 1995), promoting the influx of K+ at more positive potentials. Thus, localized changes in extracellular K+ within invaginating rod and cone synapses might be buffered by the flux of K+ in and out of horizontal cells via IKIR.
3.3.2. Outwardly rectifying K+ channels
Two types of outwardly rectifying K+ currents are observed in most horizontal cells: rapidly inactivating A-type currents and sustained delayed rectifier currents. A-type K+ currents activate around −40 mV whereas sustained K+ currents activate at −30 to −10 mV (Shingai and Christensen, 1986; Sullivan and Lasater, 1990a, b; Tachibana, 1983a; Ueda et al., 1992). Sustained currents are therefore less likely to contribute to responses in the normal physiological voltage range (−30 to −90 mV). Similar to other preparations, sustained outward currents are more sensitive to extracellular TEA and intracellular Cs+ whereas A-type currents are more readily blocked by 4-AP (Lasater, 1986; Lohrke and Hofmann, 1994; Malchow et al., 1990; Shingai and Christensen, 1986; Sullivan and Lasater, 1990a; Tachibana, 1983a; Ueda et al., 1992). The molecular identities of these channels have not been characterized.
3.3.3. Ca2+-activated K+ channels
While Ca2+- activated K+ channels have not been found in horizontal cells from fish, turtle, cat, and human retina (Golard et al., 1992; Picaud et al., 1998; Sullivan and Lasater, 1990a; Tachibana, 1983a; Ueda et al., 1992), a careful study of B-type horizontal cells established the presence of BK channels in mouse retina (Sun et al., 2017). Single channel recordings also showed evidence for large conductance Ca2+-activated K+ channels in B-type horizontal cells from rabbit retina (Lohrke and Hofmann, 1994). From the rapid inactivation kinetics of BK channels in mouse horizontal cells, Sun et al. suggested that the channel complex may incorporate β2 subunits (Sun et al., 2017).
3.4. Bistable membrane behavior in horizontal cells
Despite the presence of voltage-dependent Na+ and Ca2+ currents, horizontal cells do not typically generate Na+- or Ca2+- dependent action potentials in vivo. This is because of the low input resistance of horizontal cells that arises from strong gap junction coupling between horizontal cells and from the tonic activation of ionotropic glutamate receptors by glutamate released from photoreceptors (Aoyama et al., 2005; Miyachi and Murakami, 1989; Winslow and Ma, 1990). However, Na+ and Ca2+ - dependent action potentials are readily observed in solitary horizontal cells after enzymatic isolation (Blanco et al., 1996; Johnston and Lam, 1981; Shingai and Christensen, 1983; Tachibana, 1981, 1983b) and can be evoked in horizontal cells in vivo after inhibiting countervailing K+ currents (Murakami and Takahashi, 1987). The membrane potential of isolated horizontal cells typically shows two stable values: one at a negative value approaching EK and the other at a more positive value matching the plateau potential for action potentials. As in cardiac muscle cells, slow inactivation of ICa ultimately allows the continued activity of countervailing K+ currents to drive an abrupt transition from the more positive potential back to the more negative stable membrane potential value, terminating the action potential. In mammalian horizontal cells, erg1 K+ channels appear to contribute to this balancing act between Ca2+ and K+ since blocking erg1 channels with haloperidol enhances depolarizing responses generated at light offset by horizontal cells in vivo and promotes Ca2+ spikes in isolated cells (Feigenspan et al., 2009). Similar to its role in ventricular myocytes (Hibino et al., 2010), diminished activity of KIR channels at more depolarized potentials also promotes Ca2+ action potentials in isolated horizontal cells. In intact fish retina, blocking A-type K+ currents with 4AP enhanced depolarizing spikes at light offset in horizontal cells (Perlman et al., 1993). Computational models incorporating biophysical properties can reproduce horizontal cell responses, including the long-lasting action potentials seen in isolated horizontal cells (Aoyama et al., 2005; Usui et al., 1996; Winslow and Ma, 1990). While these long-lasting depolarizing action potentials do not appear to occur often in healthy tissue in vivo, the presence of two stable membrane potential values may help to speed both depolarizing responses at light offset and hyperpolarizing deflections at light onset.
3.5. Modulation of ion channels in horizontal cells
As in other neurons, ion currents in horizontal cells are subject to modulation by many factors. In fish retina, dopamine acting through D1 receptors of horizontal cells can stimulate both PKA and PKC to enhance L-type currents but depress T-type currents (Pfeiffer-Linn and Lasater, 1993; Pfeiffer-Linn and Lasater, 1996a, 1998). On the other hand, in mouse retina, activation of D1 receptors acting through Gβγ subunits inhibits L-type ICa (Liu et al., 2016). Activation of G proteins can also regulate A-type K+ currents by shifting their voltage-dependence of inactivation towards more positive potentials (Akopian and Witkovsky, 1994).
Glutamate can modulate horizontal cell ion currents by acting through G proteins and by modulation of intracellular pH. Activation of Group I and III mGluRs enhances L-type ICa in catfish horizontal cells (Linn and Gafka, 1999). Group III mGluRs can also suppress inward rectifier currents, likely by acting through PKG (Dixon and Copenhagen, 1997; Kaneko and Tachibana, 1985a). Glutamate application to horizontal cells also lowers intracellular pH to inhibit L-type ICa (Dixon et al., 1993; Takahashi et al., 1993). Extracellular acidification can also inhibit L-type currents in horizontal cells (Jonz and Barnes, 2007). Extracellular acidification inhibits inward rectifier currents in horizontal cells (Jonz and Barnes, 2007) and intracellular alkalinization enhances those currents (Takahashi et al., 1993).
4. Bipolar cells
In mouse retina, at least 14 different types of bipolar cells have been identified based on functional, morphological and genetic criteria (Seung and Sümbül, 2014; Vlasits et al., 2018). All bipolar cells can be divided into two categories of roughly equal size based on the polarity of their light response, a fundamental classification criterion first described half a century ago (Dowling and Werblin, 1969; Kaneko, 1970; Werblin and Dowling, 1969). On cells depolarize to light and their axons terminate in the more proximal half of the inner plexiform layer (IPL) closer to the vitreous (sublamina B). Conversely, Off type bipolar cells hyperpolarize to light and their axons terminate in the distal sublamina A. The reason for this dichotomy is the type of glutamate receptor each class expresses: On bipolar cells express the L-AP4 sensitive metabotropic mGluR6 receptor that closes a cation conductance leading to membrane hyperpolarization (Masu et al., 1995; Nawy and Copenhagen, 1987; Shiells et al., 1981; Slaughter and Miller, 1981) while Off bipolar cells express ionotropic AMPA or kainate receptors whose activation generates cationic current (DeVries, 2000; Saito and Kaneko, 1983; Slaughter and Miller, 1983). Voltage-gated channels play no role in this aspect of signaling. Bipolar cells are numbered from 1 to 9 according to the depth of their axon terminals, proceeding from the distal edge of sublamina A to the proximal border of sublamina B. An additional rod bipolar cell (RBC) projects to the most proximal position of any bipolar cell. Types 5 and 3 are further divided into subtypes, and an X-type has also recently been added. In retrospect, it might have been prudent to name bipolar cells using only odd or even numbers, leaving room for addition of newly discovered subtypes.
Why are 14 types necessary? Part of the explanation lies in the specificity of photoreceptor input. For example, the RBC receives input primarily from rods while other bipolar cells principally receive input from cones and are referred to as cone bipolar cells (CBCs). The RBC is functionally an On cell (Dacheux and Raviola, 1986; Dolan and Schiller, 1989; Karschin and Wassle, 1990). Another On type is specialized to receive input only from short wavelength-sensitive cones (Dacey and Lee, 1994; Li and DeVries, 2006; Mariani, 1984). In addition, bipolar cells may be optimally tuned to respond preferentially at specific temporal frequencies. There is wide consensus across species and studies that RGCs partly inherit their response characteristics from presynaptic bipolar cells. This is true for both On and Off subtypes. Thus each type of bipolar cell is thought to form a functional channel that carries kinetically distinct information to downstream RGCs. In support of this idea, ganglion cells that respond transiently to illumination and those with a sustained response collect input from distinct populations of bipolar cells within highly organized layers of the IPL. Both imaging and patch clamp studies have confirmed that synaptic Interactions between bipolar and transient RGCs are confined to the midline of the IPL, while the outer borders contain synapses formed between cells that have sustained responses to illumination (Baden et al., 2013; Borghuis et al., 2014; Borghuis et al., 2013; Ichinose et al., 2014). Perhaps more remarkably, synaptic input from bipolar cells carrying kinetically distinct information is segregated into separate dendritic compartments on the same postsynaptic starburst amacrine cell (Greene et al., 2016; Kim et al., 2014).
Evidence presented below suggests that selective expression of voltage-gated channels contributes to diversity of bipolar cell responses to light. An alternative possibility is that this diversity originates from differences in the response kinetics of the glutamate receptors expressed by bipolar cell subtypes at photoreceptor - bipolar cell synapses. Currently, support for this idea is not compelling. For example, it was postulated that sustained Off bipolar cells express slowly desensitizing kainate receptors while more transiently responding cells express rapidly desensitizing AMPAR receptors in squirrel retina (DeVries, 2000). However, this does not seem to be the case in mouse retina; as kainate receptors are thought to be expressed in transient bipolar cells (Borghuis et al., 2014; Ichinose and Hellmer, 2016; Puthussery et al., 2014). Differential expression of AMPAR auxiliary subunits such as TARPs, NETOs or cornichons would be expected to add complexity to both AMPA and kainate currents (Jackson and Nicoll, 2011; Tomita, 2010) and could potentially contribute to response diversity, but this next level analysis of Off bipolar cell glutamate receptors has not yet been undertaken.
As mentioned above, synaptic input to On bipolar cells is mediated by a single type of glutamate receptor, the metabotropic receptor mGluR6. During the presentation of light, unbinding of glutamate from mGluR6 triggers opening of the downstream synaptic channel, Trpm1 (Koike et al., 2010; Morgans et al., 2009; Shen et al., 2009). There is a general consensus that the length of time that Trpm1 channels remain open in response to light or pharmacological block of mGluR6 can vary from cell to cell, thus generating relatively sustained or transient responses (Awatramani and Slaughter, 2000; Kaur and Nawy 2012; Zhao et al., 2017). However, analysis of the duration of synaptic currents suggests that they generate a single broad distribution, rather than discrete groups corresponding to transient vs. sustained On bipolar cells (Kaur and Nawy, 2012), and the role of the transduction cascade in generating a transient-sustained dichotomy amongst On bipolar cell types is still in doubt. As discussed below, intrinsic voltage gated channels appear to play a critical role in the generation of sustained and transien bipolar cell subtypes.
4.1. Voltage-gated Na+ channels
4.1.1. Voltage-gated Na+ channels and transient vs. sustained responses to light
A classic view of bipolar cells is that regenerative voltage- gated channels are absent and that information passes from dendrites through the soma to the axon terminal in a passive way. A number of papers have challenged this assertion, showing functional evidence for Na+ channel expression in bipolar cells of many species (Cui and Pan, 2008; Hellmer et al., 2016; Ichinose and Lukasiewicz, 2007; Ma et al. 2005; Margolis et al., 2014; Puthussery et al., 2013; Saszik and DeVries, 2012; Trenholm and Awatramani, 2015; Zenisek et al., 2001). Although a complete consensus has not been reached regarding their function, the specific types of bipolar cells that express them, their cellular location, or their regulation, several themes have emerged. One is the observation that they are expressed in CBCs, but not RBCs. These observations thus far hold across species varying from teleosts to primates. Anothe theme is that Na+ channels are expressed in transient type bipolar cells, perhaps responsible for generating the transient responses observed in current clamp or imaging studies, but at the very least enhancing this response. Strict verification of this idea requires that several criteria be met. First, all types of bipolar cells must be identified, and this now appears to be the case, although such a claim has been made before. Second, the temporal properties of the light response of each type must be known. Third, evidence for Na+ channel expression of each type must be known, and the effect of either genetic or pharmacologic Na+ channel block on the temporal properties of the light response must be determined. The recent discovery of new types of bipolar cells, due in large part to efforts to define the mouse retinal connectome (Helmstaedter et al., 2013), has been invaluable in this regard.
Imaging of bipolar cell terminals In mouse using the Ca2+ dye OG1 revealed that cells near the IPL midline have more transient responses to light than cells at the IPL margins (Baden et al., 2013). These authors concluded that axon terminal clusters corresponding to cone bipolar cell (CBC) types 3a and 3b (Off cells) and CBC types 5 and 6 (On cells) exhibited the most transient responses to changes in illumination. This approach is valuable for sampling multiple bipolar cells to the same stimuli. However, without the use of genetic tools or markers to identify specific bipolar cell types, conclusive identification of individual bipolar cell types with bulk dye loading is difficult. Qualitatively similar results were obtained by expressing the glutamate “sniffer” iGluSnFR in cells postsynaptic to bipolar cells to monitor glutamate release (Borghuis et al., 2013). To date, supporting data from patch clamp studies has produced mixed results. For example, CBC type 2 might be expected to provide sustained input to sustained α Off RGCs based on their co-stratification (Della Santina et al., 2016), but their responses appear transient (Della Santina et al., 2016; Ichinose and Hellmer, 2016), perhaps important for providing input to On-Off direction selective RGCs (Duan et al., 2014). On CBC types 5a and 5b have been classified as transient, consistent with predictions based on their layers of axon termination (Hellmer et al., 2016), although it is unclear which 2 of the 3 currently accepted subtypes (Greene et al., 2016) were recorded from. CBC types X and 7 have been classified as transient (Ichinose et al., 2014). Based on its positioning near type 5 CBCs, this is expected for type X. However, the light response of type 7 might be expected to be sustained based on position in the IPL, and from imaging of Ca2+ signals from cells whose axons terminate in the proximal layers of sublamina b (Baden et al., 2013). A caveat to the designation of transient vs. sustained bipolar cell light responses is that the shape of the light response depends critically on a number of factors including adaptation state, stimulus intensity, the presence or absence of inhibition, and current vs. voltage clamp. For example, the response of type 2 CBCs is often sustained when measured in current clamp, but the excitatory component, isolated by measuring in voltage clamp near the reversal potential for inhibitory conductances, is transient (Della Santina et al., 2016).
Examination of bipolar cell types that exhibit regenerative Na+ currents in mouse retina is roughly consistent with the idea that Na+ channels are preferentially expressed in transient bipolar cells. Currents were absent or rarely seen in type 2 and type 7 bipolar cells, both of which are predicted to produced sustained responses based on stratification layer (Hellmer et al., 2016). Conversely, type XBC and type 5-2 CBCs, which most likely is identical to the type 5f CBC of a previous study (Ichinose et al., 2014), expressed robust Na+ currents, as did type 3A. The same study showed evidence for labeling of axons in these same bipolar cell populations by a Na+ channel antibody (Hellmer et al., 2016). A TTX-sensitive current was also detected in one subtype of the rat homolog of CBC type 5 and type 3 (Cui and Pan, 2008). Both types of cells responded to depolarization with regenerative spike-like activity, but effects of light were not examined, nor was the contribution of Na+ channels to transient signaling. Thus, although Na+ currents have been clearly documented in mouse and rat retina, their role in shaping the output of bipolar cells needs further investigation: they appear to be expressed preferentially in bipolar cells that contact transiently responding RGCs, but it is unclear if selective block of Na+ channels that are expressed in bipolar cells would significantly alter RGC response properties.
In ground squirrel retina, only one type of bipolar cell, the On type cb5, was found to have Na+ currents (Saszik and DeVries, 2012). The authors went on to divide cb5 bipolar cells into two groups: one group, termed cb5b, had large Na+ currents (>400 pA) and was immunoreactive for both calbindin and PKC, while another group, cb5a, had smaller Na+ currents and was labeled only by calbindin antibodies. Using perforated patch recording to maintain the native resting potential of cb5b cells, the authors showed that light rarely initiated spiking, as Na+ channels were largely inactivated at the dark membrane potential. Importantly, cb5b cells were capable of generating spikes during presentation of flickering stimuli, as rebound hyperpolarization during the dark phase of the stimulus was sufficient to remove channel inactivation. Thus, Na+ channels may amplify responses to rapidly changing visual input, but stay silent during low temporal frequency stimuli. As expected, cb5b bipolar cells co-stratified with transient On RGC dendrites.
In primate retina, large Na+ currents (≈ 400 pA at −60 mV) were observed in an Off (DB3a) and On (DB4) bipolar cell, both of which are part of the rapidly responding magnocellular pathway of primates. In current clamp, depolarizing pulses from resting potential were able to evoke fast TTX-sensitive spikes (Puthussery et al., 2013). These authors went on to show localization of Na+ channels to the initial segment of the axon using antisera to Nav1.1. Finally, following TTX application, they showed a marked reduction in excitatory input to parasol RGCs that likely collect input from these bipolar cells. Although the reduction was relatively modest, this may be due to the nature of the light stimulus paradigm, which did not contain temporal frequencies that are likely to be optimal for spike generation in presynaptic bipolar cells, and so the contribution to ganglion cell signaling may have been underestimated.
Expression of Na+ channels would seem to be a strategy for amplifying or speeding responses in bipolar cells that mediate photopic, but not scotopic vision. This is evident in mouse retina, where Na+ currents are not observed in rod bipolar cells (Ma et al., 2005; Tian et al., 2010), and in teleost retina, where Mb1 cells that received mixed rod/cone input also lack Na+ currents (Zenisek et al., 2001). In primate retina there is anatomical evidence that a mixture of rod and cone input is conveyed to parasol RGCs by giant bipolar (GB) On type cells and cb3b Off bipolar cells (Tsukamoto and Omi, 2014, 2016). It will be interesting to determine whether primate bipolar cells that receive rod input are capable of producing Na+ spikes.
4.1.2. Na+ channel modulation
There is substantial evidence that Nav channel function in cone bipolar cells is subject to modulation, particularly by dopamine receptors. Not surprisingly, results vary depending upon variables such as species, adaptation state, bipolar cell type, and time of day. Dopamine released in the light-adapted state inhibits Nav function in amphibian retina. Thus, TTX reduces light responses of On cone bipolar cells in the dark-adapted but not light-adapted retina (Ichinose and Lukasiewicz, 2007). The underlying mechanism is primarily a shift in the voltage-dependence of Na+ channel inactivation, such that Na+ channels are mostly inactivated at resting potentials in the light adapted state. These authors went on to show that the suppressive effects of light adaptation on Na+ current were mimicked by application of dopamine receptor agonists in the dark adapted state, and that D1 antagonists prevented inhibition of Na+ current in the light-adapted state. Presumably this form of dopamine-dependent plasticity allows for amplification of bipolar cell pathways by Na+ channels during times when light is scarce, and reduces saturation when light is plentiful. Roles for dopamine receptors may differ in mammalian retina, as the b-wave, an indicator of On bipolar cell activity, is reduced in D1 receptor knockouts in the light, but not dark-adapted retina (Jackson et al., 2012). In addition, the b wave is reduced in the light-adapted state following intravitreal injection of TTX or in mice lacking functional Nav1.6 channels (Mojumder et al., 2008; Smith and Cote, 2012; Smith et al., 2015a). This apparent conflict may be explained by the observation that the suppressive effect of dopamine on the b wave in the light adapted retina is not due to a direct effect of dopamine on Na+ channels of bipolar cells, but rather through an amacrine cell disinhibition circuit (Smith et al., 2015b). In this scenario, inhibition of amacrine cell Na+ channels decreases tonic inhibitory drive onto a downstream amacrine cell. This downstream amacrine cell now more strongly inhibits rod bipolar cells via GABAC mediated feedback (Smith et al., 2015b). Modulation of Na+ currents may be a critical factor in extending the dynamic range of bipolar cells as lighting conditions change, and our current knowledge is insufficient to draw firm conclusions regarding the role of dopamine or other potential modulators.
4.2. Ca2+ channels
High voltage-activated L-type Ca2+ channels mediate transmitter release from bipolar cell ribbon synapses (Heidelberger and Matthews, 1992; Tachibana and Okada, 1991; Tachibana et al., 1993), as they do for photoreceptors. The molecular composition of L-type channels in photoreceptors is more clearly established (see section 2.2), but the identity or even the number of isoforms expressed in bipolar cells remains unclear. In goldfish, CaV1.3 was identified using RT-PCR as the major constituent of Ca2+ channels (Logiudice et al., 2006), but the field of candidates appears to be more crowded in mammalian bipolar terminals, as there is also evidence for expression of CaV1.4 (Baumann et al., 2004; Berntson et al., 2003) and CaV1.2 (Satoh et al., 1998). T-type channels are also expressed in rat bipolar cells (de la Villa et al., 1998; Kaneko et al., 1989; Pan, 2000; Protti and Llano, 1998), but their role in transmission is controversial: experiments showing that T-type, but not L-type ICa are present when recording from the cell body of bipolar cells with severed axons suggest that T type channels are not present on the axon terminal (Hartveit, 1999), a conclusion supported by immunolabeling and local application of L- and T-type antagonists to axon terminals (Satoh et al., 1998). This has led to the idea that T-type channels play a role in signal propagation rather than the gating of transmitter release. Conversely, work from the Pan lab suggests that transmitter release persists following pharmacological blockade of L-type channels at voltages that activate primarily T-type channels (Pan et al., 2001). A potential explanation for this paradox is the differential expression of Ca2+ channel isoform by cone- and rod-driven bipolar cells, as T-type channels are preferentially expressed in CBCs, particularly type 3 (Cui et al., 2012; Hu et al., 2009). Understanding why T-type channels are selectively expressed on a single type of CBC would provide insight into channel-specific processing of visual information.
Regenerative currents originating from activation of Ca2+ channels have also been detected in bipolar cells of goldfish (Burrone and Lagnado, 1997; Cui and Pan, 2008; Palmer, 2006; Protti et al., 2000; Zenisek and Matthews, 1998) and zebrafish (Baden et al., 2011; Dreosti et al., 2011). In some cases, depolarizing current pulses resulted in maintained and stereotyped electrical resonance, due to the interaction of L-type and Ca2+-sensing BK channels (Burrone and Lagnado, 1997). Non-invasive monitoring of Ca2+ using the GCaMP reporter demonstrated that the occurrence of spikes did not alter the frequency, but enhanced the amplitude of Ca2+ transients locked to the frequency of the light stimulus (Baden et al., 2011; Dreosti et al., 2011). Thus, L-type channels appear to perform two functions in fish bipolar cells, gating transmitter release and generating spikes that allow for periods of maintained Ca2+ influx. A single spike is thought to be sufficient to empty the readily releasable pool of vesicles (Mennerick and Matthews, 1996; Palmer, 2006). Although such an event would prevent further signaling until the pool is replenished, such signals are produced relatively rarely (Baden et al., 2011). Thus, a single presynaptic terminal has the potential to signal both tonic and phasic information.
Although Ca2+ channels underlie regenerative currents in goldfish Mb1 cells, they appear to play a more limited role in generating spikes in bipolar cells of mammalian retina than Na+ channels. Instead, their role seems to be more focused on gating transmitter release. In mouse rod bipolar cells, the opening of a single L-type channel per active zone is sufficient to support univesicular release from bipolar cell terminals (Jarsky et al., 2010) but the opening of multiple channels per active zone is required in goldfish Mb1 bipolar cells (Coggins and Zenisek, 2009). This difference may arise because spikes generated by the interplay of Na+ and K+ channels allow for higher spike frequency than L-type Ca2+ channels. Importantly, expression of Ca2+ and Na+ channels allows for separate populations of channels dedicated to the tasks of synaptic transmission and spiking, allowing for selective placement of spike-generating channels in specific bipolar cell populations.
4.3. K+ channels
4.3.1. Voltage-gated K+ channels
An outwardly rectifying K+ channel was first described by Kaneko and Tachibana in isolated On bipolar cells of the goldfish (Kaneko and Tachibana, 1985a) and subsequently found in perch (Kaneko and Tachibana, 1985b; Lasater, 1988), zebrafish (Connaughton and Maguire, 1998), mouse and rat (Kaneko et al., 1989; Karschin and Wassle, 1990; Klumpp et al., 1995a; Klumpp et al., 1995b). This current can be regulated by a variety of second messenger pathways, potentiated by dopamine via D1 receptors and inhibited by endocannabinoids via CB1 receptors (Fan and Yazulla, 2005). There may be differences in expression patterns of outward rectifiers in cone- and rod-driven bipolar cells in rat as currents recorded from CBCs were of larger magnitude and activated at more negative voltages than their counterparts in RBCs (Hu and Pan, 2002; Ma et al., 2005). The reason for these differences is unclear. They could potentially play a role in repolarization following Na+ channel activation since, as discussed above (section 4.1.1), Na+ channels are preferentially expressed in CBCs. Indeed, CBCs with robust Na+ currents had matching outward rectifying currents (Ma et al., 2005), although correlation with specific CBC subtypes was not attempted. Immunohistochemical evidence suggests that shaker (KV1.2) and shab (KV1.3) channels are expressed in bipolar cells of goldfish retina (Yazulla and Studholme, 1998) and in mouse RBCs (Klumpp et al., 1995a; Klumpp et al., 1995b). The role of these channels is unknown. Mammalian RBCs have a dark potential of about −45 to −50 mV (Berntson and Taylor, 2000; Euler and Masland, 2000; Oesch and Diamond, 2011), and peak depolarizing light responses are generally less than 20 mV in amplitude (Berntson and Taylor, 2000; Euler and Masland, 2000; Trexler et al., 2005), a voltage excursion that is not sufficient to substantially activate the delayed rectifier expressed in RBCs (Kaneko et al., 1989; Karschin and Wassle, 1990). Furthermore, comparison of current and voltage-clamped light responses obtained from the same cell confirm that voltage-gated channels do not significantly shape the light response in RBCs (Berntson and Taylor, 2000). In salamander retina, activation of outwardly rectifying K+ currents restrains the membrane potential from depolarizing above −30 mV (Thoreson and Burkhardt, 2003).
A-type currents have been described in bipolar cells from cold blooded vertebrates (Connaughton and Maguire, 1998; Lasater, 1988; Tessier-Lavigne et al., 1988) but not mouse (Klumpp et al., 1995a) or rat (Karschin and Wassle, 1990). The cell type-specific distribution of these and other K+ currents among different types of bipolar cells in cold-blooded vertebrates has not been carefully investigated.
4.3.2. BK channels
As discussed in section 1.1.4, BK channels are large conductance K+ channels activated by both voltage and micromolar concentrations of Ca2+. Opening in response to local increases in Ca2+, they repolarize membrane potential. They often co-localize with Ca2+ channels, together regulating Ca2+ levels on a nanoscale (Lee and Cui, 2010). Depending upon their relative distance and numbers, these two channels can act in concert to generate oscillating responses at specific frequencies (Roberts et al., 1990). In bipolar cells, such an intimate relationship between BK and L-type Ca2+ channels also exists and has been studied extensively (Burrone and Lagnado, 1997; Llobet et al., 2003; Palmer, 2006; Protti et al., 2000; Sakaba et al., 1997; Zenisek and Matthews, 1998). In particular, work from the Lagnado laboratory using whole cell and cell-attached patch clamp recording has demonstrated that co-localized BK and Ca2+ channels contribute to electronic resonance that serves to amplify bipolar cell responses (Burrone and Lagnado, 1997; Llobet et al., 2003).
It should be noted that all of the studies cited above were carried out in On type bipolar cells of the goldfish retina. The situation in mammalian retina may be quite different. To date BK channels have not been detected immunohistochemically on terminals of bipolar cells in mouse or rat retina, but rather on A17 amacrine cell processes in close apposition to rod bipolar cell terminals (Grimes et al., 2009; Tanimoto et al., 2012). Furthermore, an ERG study taking advantage of a BK channel knockout mouse failed to demonstrate any functional deficit other than a change in the duration of the b-wave at low light intensities that was attributed to increased inhibitory feedback from BK-expressing A17 amacrine cells (Tanimoto et al., 2012), although horizontal cells, which also express BK channels, might be expected to play a role as well (Sun et al., 2017). It remains to be determined whether BK channels have thus far escaped detection in higher mammals, or are confined to horizontal, amacrine and ganglion cells. If so, this serves as a reminder that findings in lower vertebrates may not necessarily translate to mammalian retina. It is tempting to speculate that regenerative activity in mammalian bipolar cells relies on Na+-dependent action potentials, rather than Ca2+ channels, reducing the need for a Ca2+-sensing K+ channel.
4.4. HCN channels
In rat retina, type 3 Off cells stain for HCN4, while type 5 cells express HCN1, HCN2 and HCN4 (Fyk-Kolodziej and Pourcho, 2007; Müller et al., 2003). Interestingly, only the CB5b subtype of type 5 CBC was shown to express HCN channels, while the CB5a subtype did not (Fyk-Kolodziej and Pourcho, 2007). Anatomically, the two subtypes can be distinguished by the pattern of axon stratification, as CB5b exhibit diffuse axon terminals, whereas CB5a have a narrower stratification. Functionally, CB5a cells have low pass filtering characteristics, while CB5b have band pass characteristics (Ichinose et al., 2014), consistent with the predicted roles of HCN channels. Unlike Nav expression, there is functional and immunohistochemical evidence for expression of HCN channels in the rod pathway. HCN2 channels are found in rod bipolar cells (Cangiano et al., 2007; Müller et al., 2003), although differing reports on the location of the channels point to localization in either dendrites (Cangiano et al., 2007) or axon terminals(Müller et al., 2003). In mouse, they appear restricted to bipolar cell dendrites, colocalizing with mGluR6. In primate retina, an HCN current with kinetic properties similar to HCN1 has been described for DB3a, DB3b and DB4 cells (Puthussery et al., 2013), suggesting that HCN currents are highly conserved amongst bipolar cells that comprise transient signaling in the retina. In primate bipolar cells, HCN channels are localized to axon terminals.
4.5. Summary
Bipolar cells are high resistance, electrically compact cells, ensuring that small currents evoked by fluctuations in photoreceptor transmitter release can be reliably transmitted to the inner retina. The distribution of ion channels among different subtypes of bipolar cells is summarized in Table 1. In addition to channels that maintain resting potential and passive flow of information, strategically placed Na+, Ca2+ and K+ channels in specific subsets of bipolar cells generate regenerative responses that allow for the encoding of information with greater fidelity at high temporal frequencies. In mammalian retina evidence collected to date suggests that these channels are preferentially expressed in bipolar cells that terminate in the middle layers of the IPL, providing input to transiently responding RGCs. In addition to intrinsic, voltage-gated channels, retinal circuitry undoubtedly contributes to generating sustained or transiently responding bipolar cells. In particular, the precise timing of negative feedback from amacrine cells is of critical importance (Eggers and Lukasiewicz, 2011; Eggers et al., 2007; Moore-Dotson et al., 2015). A thorough understanding of the role of voltage-gated channels will require a complete wiring diagram of bipolar cell to ganglion cell connections, and bipolar cell specific knockouts of voltage-gated channels, allowing for the assessment of the impact of each channel on RGC kinetics and sensitivity.
Table 1.
Ion channels in bipolar cells. Summary of voltage-gated ion channel expression in specific classes of bipolar cells in mammalian and non-mammalian retina. Evidence for expression is based on immunohistochemical and electrophysiological findings (see text for details). Na+ channels are preferentially expressed in On and Off transient, cone-driven bipolar cells. The same appears true for HCN channels, although the differences in expression are not as dramatic. Kvx refers to unidentified K+ channel isoforms, as no studies to date have molecularly characterized K+ channels in cone bipolar cells.
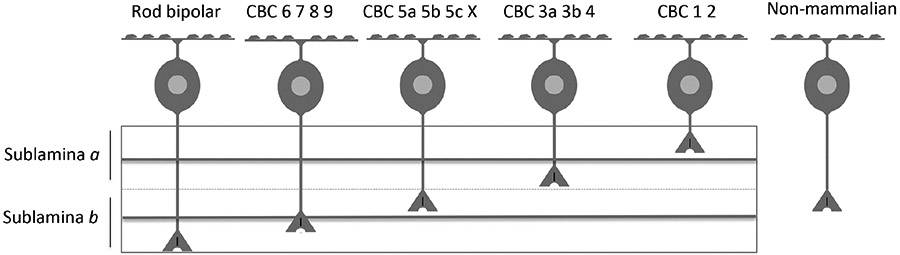 | ||||||
|---|---|---|---|---|---|---|
| Transient/ sustained |
sustained | sustained | transient | transient | sustained | |
| Na+ channels | – | – | Type X: ++ Type 5-1: − Type 5-2: + |
Type 3a: ++ | – | Mb1: − CBC: + |
| Ca2+ channels | L-Type T-Type |
L-Type | L-Type | L-Type T-Type |
L-Type | Mb1: L-Type, |
| K+ channels | Kv1.2 Kv1.3 |
Kvx | Kvx | Kvx | Kvx | BK, Kv1.2, Kv1.3 A-type |
| HCN channels | HCN2 | HCN2 | HCN1,3,4 Type X: ++ Type 5-1: + Type 5-2: ++ |
HCN4 | – | ? |
5. Amacrine cells
Amacrine cells (ACs) are the most diverse population of neurons in the retina and are responsible for shaping the visual signal as it is passed from bipolar cells to RGCs. They do this by making feedback inhibitory synapses (GABAergic or glycinergic) onto bipolar cell axon terminals and by providing feed-forward inhibition onto RGCs (Diamond, 2017; Eggers and Lukasiewicz, 2011; Masland, 2012b). ACs also provide inhibition to other ACs, creating complex feedback and feed-forward inhibitory networks.
AC cell bodies occupy the inner nuclear layer (INL) and the RGC layer and are often referred to as “displaced” ACs when found in the RGC layer. Their dendrites and, in some cases, axons occupy strata of the IPL along with the bipolar cell axon terminals and ganglion cell dendrites. The selective layering of AC processes in the IPL gives clues to the identities of the bipolar cells and retinal ganglion cells with which they communicate. Bi- or multi-stratified ACs (with dendrites spanning multiple sublayers of the IPL) provide “crossover inhibition”, the relay of signals between On and Off channels of the retina (Diamond, 2017) and are conventionally thought to be glycinergic (Menger et al., 1998). Wide-field ACs, on the other hand, are conventionally thought to be GABAergic (Pourcho and Goebel, 1983; Vaney, 1990). As discussed in the following sections, wide-field ACs are able to fire action potentials, which allow them to carry signals along the length of their dendrites and axons, while narrow-field ACs are thought to signal via passive mechanisms (Bloomfield, 1992). However, as we describe for AII ACs below, this “rule” is not hard and fast.
ACs also release other neurotransmitters and neuromodulators. Dopaminergic ACs (DACs), for instance, are the sole source of dopamine, a signal for light adaptation in many retinas (Witkovsky, 2004), although they also release GABA (Hirasawa et al., 2012; Hirasawa et al., 2009). Starburst ACs (SACs) are cholinergic and are identified in immunolabeling experiments by their expression of choline acetyltransferase (Taylor and Smith, 2012). Like DACs, they also release GABA, which is key for providing directional selectivity of direction-selective ganglion cells (DSGCs). An additional strange AC class was recently identified based on expression of a vesicular glutamate transporter, vGlut3. The vGlut3 AC has numerous identified post-synaptic targets, providing glutamatergic input to some and glycinergic input to others (Grimes et al., 2011; Lee et al., 2016).
Current estimates from mouse and rat studies indicate that there are >45 distinct AC classes (Diamond, 2017; Helmstaedter et al., 2013; MacNeil et al., 1999; MacNeil and Masland, 1998; Masland, 2012a; Masland, 2012b), yet only a handful have been studied in detail. Generally, several ion channel subunits as well as voltage-gated currents such as ICa, INa, Ih, IKA, and IKDR have been described in various ACs from several species (Barnes and Werblin, 1986; Bloomfield and Völgyi, 2007; Cameron et al., 2017; Eliasof et al., 1987; Horio et al., 2018; Huba et al., 1992; Koizumi et al., 2004; Lasater and Witkovsky, 1990; Maguire, 1999; Mitra and Slaughter, 2002; Solessio et al., 2002; Taylor, 1996; Yang et al., 1991). A particularly nice body of literature from groups studying amphibian retinas has shown the presence of multiple voltage-gated currents in ACs and explored their roles in shaping spiking and synaptic output. In salamander retina, 95% of ACs exhibit INa (Heflin and Cook, 2007). Some ACs are capable of repetitive spiking whereas others can generate only a single spike in response to depolarizing voltage steps and often fail to spike altogether during light-evoked depolarization. ACs that generate repetitive spiking have larger INa and those that generate a single spike have smaller INa (Heflin and Cook, 2007). The tendency to fire a single spike can also be promoted by the presence of a slowly activating IK that fails to provide sufficient hyperpolarizing relief of Na+ channel inactivation (Barnes and Werblin, 1986; Eliasof et al., 1987). Although a number of exceptions to this generalization were observed, wide-field ACs are more likely to generate bursts of spikes whereas small-field ACs are more likely to generate only a single spike (Heflin and Cook, 2007). Cells that are only capable of firing single spikes are not always transient ACs but can also exhibit sustained post-synaptic potentials. Similarly, not all ACs that exhibit repetitive spiking are “sustained” ACs (Heflin and Cook, 2007).
Release of GABA and glycine from salamander ACs involves both Na+-dependent spiking and graded potentials (Bieda and Copenhagen, 1999; Cook and Werblin, 1994). Release evoked by widefield illumination is more sensitive to inhibition of Na+ channels than release evoked by illumination restricted to the receptive field center (Bieda and Copenhagen, 1999). The generation of dendritic spikes in large field ACs can boost excitatory post-synaptic potentials and thereby coordinate release from multiple sites (Cook and Werblin, 1994; Miller and Dacheux, 1976; Miller et al., 2006; Werblin, 1977). Both N- and L-type Ca2+ channels localized primarily to dendrites (Maguire, 1999) contribute to the Ca2+ influx that triggers glycine release from ACs (Bieda and Copenhagen, 2004). Although widefield ACs of teleost retina also possess both N and L-type Ca2+ channels, only L-type channels appear to mediate release from these cells (Vigh and Lasater, 2004). This was also true for GABAergic ACs from chick retina (Gleason et al., 1994).
In ACs that have been enzymatically isolated from salamander retina, the close association between L-type Ca2+ channels and Ca2+-activated K+ (BK) channels promote the appearance of spontaneous outward currents (Mitra and Slaughter, 2002). Similar to muscle cells and a number of other neurons, Ca2+ influx that accompanies opening of a Ca2+ channel can be boosted by Ca2+-induced Ca2+ release from intracellular stores leading to activation of nearby BK channels that generate an outward current. Spontaneous outward currents involving similar mechanisms have also been observed in teleost ACs, although there is a role for both BK and SK channels in these cells (Solessio et al., 2002; Vigh et al., 2003). These currents contribute to oscillatory behavior and shape bandpass filtering characteristics of the AC membrane (Vigh et al., 2003). Amacrine cells in salamander retina also exhibit IKA and IKDR (Eliasof et al., 1987; Mitra and Slaughter, 2002).
Rodent retinas are the current preferred model system for studying retinal function. Because of the diverse structure and function of retinal ACs in rodent retinas, it is challenging to describe function of specific ion channels in ACs in general terms. Therefore, rather than organizing this section by ion channel type, we have organized it by AC type, describing the role of various ion channels in the function of several well-characterized AC classes: AII’s, A17’s, SACs, DACs, and wide-field CRH ACs. The principal ion channels in these different subtypes, as well as amacrine cells from salamander retina, are summarized in Table 2.
Table 2.
Summary of ion channels in interplexiform cells and different types of amacrine cells.
| Salamander ACs |
AII | A17 | Starburst | Dopaminergic | Widefield CRH |
Interplexiform cells (IPCs) |
|
|---|---|---|---|---|---|---|---|
| Na+ channels | INa (most ACs) | INa(NaV1.1) | Weak INa | TTX-insensitive NaV1.8 | TTX-sensitive and insensitive | INa (CRH2, 3) No INa (CRH1) |
INa |
| Ca2+ channels | N- & L-type | L-type (CaV1.3) | L-type | N, P/Q | L- (CaV1.2), N- (CaV2.2), P/Q (CaV2.1), & R- (CaV2.3) type | ? | L-type |
| K+ channels | IKDR IKA |
IKDR IKA |
IKDR IKA BK |
IKDR (mostly Kv3.1-3.2) | IKDR (Kv1.1, 1.3, 2.1) IKA (KV4.3) BK, SK |
? | IKDR IKA (some IPCs) BK (mouse) |
| HCN channels | Ih | ? | Ih (mouse) |
5.1. AII amacrine cells
AII ACs are a key hub for information flow in the inner retina and largely function to transmit signals arising from rods and RBCs to CBCs under scotopic conditions as part of the “primary rod pathway” (Bloomfield and Vülgyi, 2009; Demb and Singer, 2012). These retinal interneurons have been studied extensively in retinas of mammals such as rat, mouse, and rabbit. All’s receive excitatory glutamatergic synaptic input from RBCs in their distal dendrites near the border of the IPL and GCL. Alls then relay that On depolarization to On cone bipolar cells via gap junction electrical synapses in their distal dendrites. Off signals are relayed to Off CBCs via inhibitory glycinergic synapses at All lobular dendrites. All’s are also important in photopic (cone-driven) signaling conditions, where they contribute to crossover inhibition and provide a direct inhibitory drive to some classes of Off RGCs (Manookin et al., 2008).
Glycinergic synaptic transmission from All’s is mediated by Ca2+ influx exclusively through CaV1.3 L-type channels (Balakrishnan et al., 2015; Bieda and Copenhagen, 2004; Habermann et al., 2003). L-type channels are key for synaptic transmission at excitatory ribbon synapses of photoreceptors, bipolar cells, and hair cells, where sustained calcium entry supports tonic neurotransmitter release (Joiner and Lee, 2015; Matthews and Fuchs, 2010). Despite their lack of a ribbon, AII synaptic output is also quite sustained, which appears to result in part from the use of L-type channels (Balakrishnan et al., 2015). ICa are present in early postnatal AII’s, but dramatically increase in amplitude by P9 in mice, which is around the same time at which glycinergic inputs can be detected in Off cone BCs (Balakrishnan et al., 2015; Schubert et al., 2008). This time course is also associated with a refinement of Ca2+ channel localization; Ca2+ imaging studies indicate that Ca2+ influx is more diffuse in early postnatal AII’s, but is refined by adulthood and localized exclusively to the lobular dendrites (Balakrishnan et al., 2015; Habermann et al., 2003).
Despite being narrow-field ACs, AII’s are known to possess voltage-gated Na+ channels and fire small and relatively slow action potentials that are blocked by TTX (Bloomfield and Xin, 2000; Boos et al., 1993; Cembrowski et al., 2012; Mørkve et al., 2002; Tamalu and Watanabe, 2007; Tian et al., 2010; Veruki and Hartveit, 2002). These are likely mediated largely by NaV1.1 channels, which have been identified in AII’s by in situ hybridization in rats (Kaneko and Watanabe, 2007). The sluggish action potential kinetics in somatic recordings suggest that the action potentials are generated in a distal compartment of the AII (Cembrowski et al., 2012; Tamalu and Watanabe, 2007). Consistent with this, labeling with anti-NaV1.1 and anti-pan NaV antibodies has been shown to localize to a short AII process that branches off of dendrites near the AII somata in mice (Cembrowski et al., 2012; Wu et al., 2011). This region also appears enriched for ankyrin-G and neurofascin, which localize Na+ channels to the axon initial segment in many neurons (Cembrowski et al., 2012; Wu et al., 2011). Extracellular stimulation near these processes triggers a spike and excision or localized application of TTX will block AII spiking behavior and Na+ currents (Cembrowski et al., 2012; Tamalu and Watanabe, 2007). AII’s also possess K+ currents that support spiking behavior including a delayed-rectifier and A-type current (Boos et al., 1993; Tian et al., 2010). Much of the K+ current in AII’s is sensitive to TEA, while a smaller portion is blocked by 4-AP (Boos et al., 1993; Tian et al., 2010).
The consequences of AII sodium currents and spiking are still largely unclear. AII spike frequency encodes the strength of excitatory input (Tamalu and Watanabe, 2007) and AII Na+ currents also appear to play a major role in the oscillatory activity detected in RGCs and other inner retinal neurons (Margolis et al., 2008; Stasheff, 2008) following photoreceptor degeneration (Trenholm et al., 2012).
Work by Tian and colleagues (Tian et al., 2010) examined the influence of AII Na+ channels on the inputs from rod bipolar cells and how that process affects propagation of scotopic signals from AII’s to cone BCs and RGCs in mouse retinas. In paired recordings from AII’s and RBCs, they found that TTX blockade of Na+ channels had the dual effect of attenuating and slowing post-synaptic potentials. Thus, Na+ channels accelerate and amplify synaptic responses in AIIs, which, along with the kinetics of RBC exocytosis (Singer and Diamond, 2003), might contribute to the phenomenon that AII light responses are faster than those of presynaptic RBCs (Nelson, 1982). Additionally, this effect depended on the AII membrane potential; the EPSP was not enhanced at either depolarized potentials (>−45mV), where most Na+ channels would be unavailable due to inactivation, or at hyperpolarized potentials (<−80 mV), below the Na channel activation threshold (Tian et al., 2010). Recordings of light responses from RGCs showed that inhibition of Na+ channels slowed inhibitory and excitatory synaptic inputs to RGCs without affecting amplitude. Thus, while potentially serving to threshold synaptic inputs to AII’s, Na+ currents appear only to accelerate AII output to downstream RGCs (Tian et al., 2010). The implications of this for RGC output are still unclear. Because the AII resting membrane potential appears to vary with background lighting (Dunn, 2006; Tian et al., 2010), this might slightly shift the strength of AII-mediated synaptic acceleration as a result of changing Na+ channel availability.
5.2. A17 amacrine cells
The A17 AC (which likely corresponds to S2 ACs in rabbit) receives input from, and sends its output to, the same RBC, regulating synaptic transmission from RBC to AII ACs (Menger and Wassle, 2000; Nelson and Kolb, 1985; Raviola and Dacheux, 1987; Vaney, 1986; Volgyi et al., 2002; Zhang et al., 2002). A17 ACs extend individual thin processes to the innermost layer of the IPL, contacting only RBCs and no other cell type. Each of these contacts has been likened to an electrically-isolated, individual microcircuit (Grimes et al., 2010). Elegant support for this idea comes from 2-photon imaging of Ca2+ transients in synaptic boutons during electrical stimulation. These experiments revealed that stimulation of individual boutons generated ICa that could be detected in somatic patch clamp recordings, but did not activate neighboring synaptic boutons. Such electrical isolation would imply the absence of action potential generation in A17 cells. Indeed, although Na+ channels are expressed in A17 ACs, they do not appear capable of supporting robust regenerative currents (Bloomfield, 1996; Grimes et al., 2010; Menger and Wassle, 2000; Nelson and Kolb, 1985). Both A-type and outward rectifying K+ currents have been reported in A17 ACs as well (Grimes et al., 2010). What might be the function of Na+ and K+ channels if not to augment signal propagation? Modeling of signal spread through A17 processes shows that the presence of these voltage-gated channels, expressed at low density, actually reduce spread of signals produced by opening of postsynaptic AMPA channels, compared to a purely passive membrane. Channel activation increases membrane conductance and reduces the length constant of the cell, effectively reducing signal conduction. This curious arrangement serves as a reminder that the presence of voltage-gated channels does not always result in the enhancement of signal conduction.
A17 ACs express L-type Ca2+ channels (Grimes et al., 2009; Menger and Wassle, 2000), but it was initially thought that Ca2+ influx through Ca2+-permeable AMPA receptors (CP-AMPARs), rather Ca2+ channels, triggers release of GABA onto RBC terminals (Chavez et al., 2006). These authors also showed that Ca2+-induced Ca2+ release from stores provided a second source of Ca2+ for triggering GABA release. NMDA receptors, which are highly Ca2+ permeable, situated close to GABA release sites, and activated by glutamate released from RBC terminals, may also contribute to GABA release in this feedback circuit (Zhou et al., 2016). However, an additional layer of complexity was revealed by experiments demonstrating a role for Ca2+ channels at some, but not all A17 release sites (Grimes et al., 2009; Grimes et al., 2015). Specifically, they found that terminals using CP-AMPARs to couple Ca2+ to GABA release were presynaptic to rapidly activating GABAA receptors, while a second terminal, contacting the same RBC, used Ca2+ channels to gate GABA release. This second terminal was presynaptic to slower activating GABAC receptors.
What mechanisms are used by A17 ACs to terminate transmitter release? Expression of BK channels at synaptic varicosities has been reported (Grimes et al., 2009). As discussed previously, BK channels are both voltage and Ca2+-sensitive, and their opening repolarizes the membrane and contributes to rapid closure of L-type Ca2+ channels. Close apposition of BK and L-type channels appears to be an important mechanism for presynaptic regulation of synapses (Roberts et al., 1990; Skinner et al., 2003; Xu and Slaughter, 2005). Conversely, closure of AMPA receptors is not voltage-dependent, but is dictated by the lifetime of glutamate in the synaptic cleft (Diamond and Jahr, 1995). Thus, association of CP-AMPARs with BK channels affords no obvious advantage to regulation of GABA release from terminals that express CP-AMPARs. In support of this, both immunogold labeling of BK channels and electrophysiological evidence suggest that BK channels colocalize with L-type channels, but are too far from CP-AMPARs to be activated by Ca2+ influx through these receptors (Grimes et al., 2015). The authors go on to speculate that in the dark, release rates of RBCs are sufficient to depolarize A17 boutons and activate BK channels, thus limiting GABA release from L-type Ca2+ channel-expressing boutons and allowing for high throughput from RBCs to AII’s. As ambient light increases, this concomitantly raises release rates of presynaptic RBCs, leading to further depolarization of A17 dendrites. This triggers a voltage-dependent switch within the BK channel to an inactive state, mediated by the auxiliary β2 subunit (Hicks and Marrion, 1998; Wallner et al., 1999; Xia et al., 2003). Inactivation of BK channels would allow for maintained L-type channel activation, allowing for strong feedback inhibition onto RBC terminals. In this way, BK channels expressed in A17 ACs are proposed to play a role in extending the operating range of the RBC-AII AC synapse by reducing RBC transmitter release at higher light intensities (Grimes et al., 2009; Grimes et al., 2015). A powerful tool to further explore this hypothesis would be cre-mediated deletion of the β2 subunit gene kcnmb2, provided that a cre line specific for A17 ACs becomes available.
5.3. Starburst amacrine cells (SACs)
SACs provide GABAergic inhibition to DSGCs. Glutamatergic excitation from bipolar cells to DSGCs lacks direction selectivity. Rather it is the direction-selective inhibitory output of SACs onto DSGCs that generates preferred and null directions. Mechanisms responsible for generation of direction selectivity of SACs have been studied extensively and there are a number of recent reviews summarizing this progress (Franke and Baden, 2017; Mauss et al., 2017; Wei, 2018). A key feature of information processing in the SAC is the preference of motion away from (centrifugal) rather than toward (centripetal) the soma (Euler et al., 2002; Hausselt et al., 2007; Lee and Zhou, 2006). As an object moves through the receptive field of a SAC, it stimulates bipolar cells that subsequently release glutamate along the entire length of the radially extending dendrites of the SAC in a sequential manner. Objects moving centrifugally generate a wave of depolarization that ultimately results in Ca2+ dependent release of GABA from distal processes, while the depolarizing wave generated from centripetal movement, presumably activating the same presynaptic bipolar cells as an object moving in the centrifugal direction, is much more modest. The reason for this differential effect of direction is still unclear, but a recent model suggesting that it is due to differences in the response kinetics of bipolar cell types providing input is particularly intriguing (Greene et al., 2016; Kim et al., 2014). However, this so-called “space time wiring” hypothesis is controversial (Fransen and Borghuis, 2017; Morrie and Feller, 2018; Stincic et al., 2016) and probably cannot account for the directional effect entirely.
Although no single mechanism has been shown to be necessary or sufficient to generate direction selectivity of SAC processes, voltage-gated channels appear to enhance this selectivity (Hausselt et al., 2007; Jensen, 1995a; Oesch and Taylor, 2010; Tukker et al., 2004). Studies of the contribution of Na+ channels to the physiological properties of SACs have provided conflicting results, with some reporting robust spiking (Bloomfield, 1992; Cohen, 2001; Jensen, 1995b) and others suggesting that they lack Na+ currents completely (Kaneda et al., 2007; Ozaita et al., 2004; Taylor and Wassle, 1995; Zhou and Fain, 1996). The resolution may be provided by more recent studies showing that SACs express TTX-resistant NaV1.8 channels (O'Brien et al., 2008; Oesch and Taylor, 2010), and thus studies using TTX to block inward or regenerative currents would have attributed them to another type of channel. Using a blocker of TTX-insensitive Na+ channels, Oesch and Taylor (2010) demonstrated a significant reduction in centrifugal, compared to centripetal light stimulation. However, even in the absence of TTX-resistant Na+ channels the difference in the response to centrifugal and centripetal stimulation was still quite robust, suggesting only a modest effect on direction selectivity. There is evidence for expression of N, P and Q (but not L) type ICa (Cohen, 2001; Kaneda et al., 2007; Lee et al., 2010). Interestingly, SACs release both acetylcholine and GABA, and it has been suggested that release of each transmitter is gated by a different subset of Ca2+ channels: agatoxin, a P/Q channel antagonist blocked release of predominantly GABA, while the N type channel blocker ω-conotoxin almost completely prevented release of acetylcholine, but not GABA (Lee et al., 2010).
Models of SAC direction selectivity require significant compartmentalization of processing (Miller and Bloomfield, 1983; Poznanski, 1996; Tukker et al., 2004; Velte and Miller, 1997). Specifically, the soma must have a low input resistance to prevent rapid spread of signals across to neighboring proximal dendrites. It has been proposed that delayed rectifier KV3 channels fulfill this role (Ozaita et al., 2004). Immunolabeling of KV3.1 and KV3.2 channels showed a high to low gradient of expression from soma to distal dendrites, consistent with a role for somatic shunting of signals. Furthermore, outward rectification was nearly absent in SACs from KV3.1- KV3.2 double knockout mice, suggesting that these are the predominant isoforms expressed by this cell (Ozaita et al., 2004). It remains to be determined if direction selectivity is reduced in DSGCs in these knockout mice as a result of the loss of somatic shunting. Alternatively, or perhaps in addition, mGluR2 mediated regulation of Ca2+ channels may also participate in dynamically regulating somatic resistance (Koren et al., 2017). These authors showed that inhibition of mGluR2 increased spread of Ca2+ signals across the soma to dendrites on the opposite side of the SAC. Recordings from DSGCs revealed an increase in inhibition in response to movement in the preferred direction when mGluR2 receptors were blocked, thus reducing the selectivity for movement in the preferred direction. Inhibition of mGluR2 receptors also decreased the amplitude of N, P and Q Ca2+ channels, but not KV3 channels (Koren et al., 2017). Thus, voltage-gated channels in SACs may play a greater role in preventing spread of signals across compartments than they do in generating the signals themselves.
5.4. Dopaminergic Amacrine Cells (DACs)
DAC somas reside on the very inner border of the INL (Dacey, 1990). These neurons possess a fairly sparse localized dendritic arbor with a diameter of ~500 microns and a much wider-reaching arbor of thin axons (Dacey, 1988; Dacey, 1990; Keeley and Reese, 2009; Witkovsky et al., 2005). Both the dendrites and axons stratify in the very outer sublamina of the IPL adjacent to the INL, although a few processes occasionally reach into other layers of the retina. DACs are excited by light (On cells) due to excitatory en passant or ectopic synaptic inputs from On bipolar cells and M1-type melanopsin-expressing ganglion cells, as revealed in studies of both mouse and rabbit retinas (Dumitrescu et al., 2009; Hoshi et al., 2009; Prigge et al., 2016; Zhang et al., 2008; Zhang et al., 2007). This triggers action potential firing and release of dopamine (Puopolo et al., 2001), which functions as a neuromodulatory signal for light adaptation in the retina (Witkovsky, 2004). Dopamine appears to act in a paracrine fashion (Puopolo et al., 2001; Witkovsky, 2004), diffusing to DA receptors expressed by all classes of neurons in all layers of the retina without requiring direct synaptic contacts. Additionally, DACs are also GABAergic and co-release GABA with dopamine (Hirasawa et al., 2012, 2009). DACs appear to play a major role in regulating the function of AII ACs, as DAC processes encircle and synapse onto the thick proximal dendrites of AII ACs (Contini and Raviola, 2003; Voigt and Wässle, 1987).
Several classes of voltage-gated current have been identified in recordings from DACs in multiple species. In keeping with their axons and relatively wide dendritic fields, DACs in mice fire action potentials (Gustincich et al., 1997) and can exhibit several distinct patterns of spiking, including silent, sustained, irregular, and bursting (Newkirk et al., 2013; Zhang et al., 2007). Bursting behavior appears to be a key trigger for dopamine release by DACs (Puopolo et al., 2001). In voltage-clamp recordings, both a transient, TTX-sensitive and persistent, TTX-insensitive Na+ current have been characterized (Feigenspan et al., 1998; Steffen et al., 2003; Xiao et al., 2004). While TTX blocks regenerative spiking activity and transient Na+ currents in DACs (Feigenspan et al., 1998), modeling indicates that the persistent INa is required for sustained spiking (Steffen et al., 2003).
DACs also have delayed rectifier and A-type K+ currents (IKDR and IKA, respectively). A Kv4.3 channel (which generates an IKA) has been found localized to the somatodendritic compartment of DACs, while Kv3.1 is not present in DACs (Tian et al., 2003). Other K+ channel subunits including IKDR channels Kv1.1, 1.3, and 2.1 are present in DACs, as is the IKA channel Kv4.3 (Tian et al., 2003). Inhibiting IKDR with a low concentration of TEA slightly broadened action potentials and blocked the action potential after-hyperpolarization (AHP) in mice (Feigenspan et al., 1998). A high concentration of TEA, on the other hand (40 mM), depolarized DACs and dramatically slowed the falling phase of the action potential (Feigenspan et al., 1998). 4-AP application, which is often used to inhibit IKA, depolarized the DAC and increased spike rate (Feigenspan et al., 1998). This is consistent with modeling indicating that IKA plays a major role in regulating DAC firing rate (Xiao et al., 2004).
HVA ICa are detectable in voltage-clamp recordings of DACs and several CaV isoforms including the L-type channel CaV1.2, P/Q channel CaV2.1, and R-type channel CaV2.3 have been localized to DAC somata and processed by immunofluorescence (Xu et al., 2002). The N-type channel CaV2.2 is found only in DAC processes (Xu et al., 2002). It is likely that some combination of these channels is responsible for dopamine and/or GABA release by DACs, which is known to be Ca2+-dependent (Hirasawa et al., 2012, 2009). Ca2+ influx through Ca2+ channels is also likely responsible for gating Ca2+-activated K+ channels (Feigenspan et al., 1998; Xiao et al., 2004). SK2 channels have been identified in DACs (Klöcker et al., 2001). However, application of the SK channel blocker apamin had minimal effect on DAC action potential waveforms or spiking frequency while the BK channel blocker charybdotoxin slightly accelerated spiking and blocked the action potential after-hyperpolarization (Feigenspan et al., 1998). Co2+ application, which blocks Ca2+ influx through voltage-gated Ca2+ channels, caused a small reduction in action potential amplitude without affecting frequency (Feigenspan et al., 1998).
Finally, DACs in current clamp display a modest depolarizing voltage “sag” in response to hyperpolarization and a small Cs+-sensitive inwardly-rectifying hyperpolarization-activated current, consistent with the presence of the hyperpolarization activated cation current Ih. Cs+ blockade of Ih, however, had minimal effect on DAC spiking behavior (Feigenspan et al., 1998), although modeling of DAC membrane currents indicated that Ih can function to subtly increase DAC firing rate (Xiao et al., 2004).
Thus, DACs respond to excitatory synaptic input by firing action potentials and releasing dopamine and GABA. The various classes of voltage-gated and Ca2+-activated ion channels in DACs support this behavior, although only a handful have been shown to substantially alter DAC spike frequency when blocked. It remains to be determined what combinations of ion channel properties and synaptic inputs lead to the different spiking behaviors of DACs (Newkirk et al., 2013; Zhang et al., 2007) and whether or how these are dynamically regulated by patterns of synaptic input to favor dopamine and/or GABA release by DACs.
5.5. Wide-field CRH amacrine cells
The use of genetic lines has enabled identification of specific types of ACs not easily identified otherwise. An example is the CRH family of ACs, identified by screening of a corticotropin releasing hormone (CRH)-cre mouse line (Jacoby et al., 2015; Zhu et al., 2014). In the initial screen, two types of CRH ACs were described: a medium field cell with dendrites that extend approximately 200-300 μm in diameter and terminate deep in the IPL, and an axon-bearing bistratified wide field cell with dendrites that extend >1 mm (Zhu et al., 2014). The CRH-1 cell was later shown to provide feedforward inhibition to the “suppressed by contrast” RGC, a bistratified RGC that is inhibited by both positive and negative contrast (Jacoby et al., 2015). A subsequent study demonstrated a third type of axon-bearing wide field CRH AC (Park et al., 2018). This study went on to show that two of three classes of CRH cells, type 1 (medium field) and type 3 (axon-bearing wide field) provide inhibitory input to On alpha RGCs. Thus CRH-1 ACs provide input to both alpha ON and suppressed by contrast RGCs.
Comparison of CRH-1 and CRH-3 ACs illustrates a basic principle of Na+ channel expression in ACs. CRH-1, a medium field cell, lacks Na+ channels and communicates to postsynaptic RGCs using passive electronic spread (Jacoby et al., 2015; Park et al., 2018). Conversely, CRH-3 and CRH-2 are axonbearing wide field cells that contact postsynaptic RGCs over a distance of 1 mm or more, express Na+ channels and fire at high frequency in response to light (Park et al., 2018). They appear similar to a group of wide field axon-bearing ACs, including polyaxonal ACs, that are capable of generating Na+ spikes (Cook and McReynolds, 1998; Flores-Herr et al., 2001; Freed et al., 1996; Greschner et al., 2014; Murphy-Baum and Taylor, 2015; Stafford and Dacey, 2009; Taylor, 1999; Volgyi et al., 2001). In primate A1 ACs, which are morphologically similar to the mouse CRH-2 (Zhu et al., 2014), action potentials recorded at the soma are initiated within the dendritic arbor (Freed et al., 1996; Stafford and Dacey, 2009). This is presumably in addition to axonal Na+ channels required for propagation of signals to distant locations within the retina. Although the site of Na+ channel expression on CRH-2/3 cells has not been determined, they appear necessary for propagation of signals to downstream α RGCs. Park et al (2018) drove IPSCs in ON α RGCs by expressing channelrhodopsin in CRH-1 and CRH-3 ACs. Blocking Na+ channels with TTX reduced the inhibitory post-synaptic current by approximately 50%. Since both types of ACs drive ON α RGCs, but only CRH-3 ACs spike, this result implies that input from CRH-3 cells was severely reduced or perhaps eliminated following Na+ channel blockade. Ca2+ and K+ channels have not yet been characterized in CRH ACs.
Convergence of narrow and wide-field ACs onto a single RGC implies that postsynaptic inhibition is shaped by both global and local patterns of illumination. In addition, CRH-1 and CRH-3 ACs appear to differentially temporally filter input, as CRH-1 but not CRH-3 cells low pass filters flickering stimuli (Park et al., 2018). Differences in filtering between the two cell types are most apparent in current, rather than voltage clamp, highlighting the role of voltage-gated channels in temporal filtering. However, an analysis of other voltage-gated channels that may contribute to this temporal filtering has yet to be carried out.
6. Interplexiform cells
Interplexiform cells were first identified in teleost retina by Ehinger et al. (1969). Gallego (1971) found the same class of cells in cat retina and named them interplexiform cells because they have processes that terminate in both the inner and outer plexiform layers. Like amacrine cells, their cell bodies reside in the proximal INL and they have dendrites in the IPL, so some investigators have classified interplexiform cells as a subtype of amacrine cells (Witkovsky, 1980). However, unlike other amacrine cells, they extend processes into the OPL to terminate adjacent to bipolar, horizontal and cone photoreceptor cells (Dowling and Ehinger, 1975; Boycott et al., 1975; Kolb and West, 1977; Linberg and Fisher, 1986; Jiang and Shen, 2010). There are at least three neurochemically distinct subtypes in mouse retina: one that contains dopamine, one with GABA, and one with glycine (Dedek et al; Witkovsky et al 2008; Haverkamp and Wassle, 2000). At least three anatomically distinct types of interplexiform cells have also been identified in salamander retina (Maguire et al. 1990). There were no obvious differences in the types of ion channels in these three types (Maguire et al. 1990).
There are relatively few interplexiform cells in the retina and their ion currents have received little study. The few studies on these cells show currents similar to those of amacrine cells. In both salamander and mouse, interplexiform cells possess fast voltage-dependent INa, HVA L-type ICa, and IKDR that can be blocked by extracellular TEA and intracellular Cs+ (Feigenspan et al., 1998; Gustincich et al., 1997; Maguire et al., 1990). T-type ICa appear to be absent from interplexiform cells. Some interplexiform cells in mouse and salamander exhibit modest A-type K+ currents that can be inhibited by 4-AP (Feigenspan et al., 1998; Maguire et al., 1990). In mouse retina, charybdotoxin-sensitive BK channels and weak Ih have also been found (Feigenspan et al., 1998). The ion channels that have been identified in interplexiform cells are summarized in the last column of Table 2.
7. Retinal Ganglion cells (RGCs)
RGCs are the output neurons of the retina and responsible for relaying information to visual areas of the brain. The combination of distance along which RGCs must carry visual information – several millimeters in mice to several centimeters in humans – and the speed necessary to support visually-guided behaviors requires that RGCs relay vision information as trains of regenerative action potentials. They accomplish this task by integrating excitatory synaptic inputs from bipolar cells and inhibitory inputs from ACs into a train of action potentials that propagate along RGC axons.
To date, anatomical and functional studies indicate that there are >30 classes of RGCs in mice (Baden et al., 2016; Bae et al., 2018) while a single-cell transcriptomics approach has clustered RGCs into 40 distinct subtypes (Rheaume et al., 2018). As with bipolar cells, the most fundamental classification scheme for RGCs segregates them by the sign of their response – whether they are excited by light onset (On cells) or light offset (Off cells) or respond to both light and dark (On-Off cells). Other classifying response properties include whether RGC responses are sustained or transient, whether RGCs are sensitive to directional motion, or whether they have chromatic preference. RGCs of the same class also share anatomical features including dendritic co-stratification in the IPL, similar soma sizes, similar dendritic branching patterns, and regular spacing across the retinal surface.
7.1. Voltage-gated Na+ channels
Like most neurons that fire regenerative action potentials, RGCs do so using a combination of voltage-gated Na+ and K+ channels in a manner that follows Hodgkin and Huxley’s experiments on the squid giant axon. RGCs in rodents have a resting potential of approx. −60 to −75 mV (O'Brien et al., 2002; Qu and Myhr, 2008; Wong et al., 2012), which appears to vary systematically by RGC type (Hu et al., 2013; O'Brien et al., 2002; Qu and Myhr, 2008; Wong et al., 2012). Upon depolarization, voltage-gated Na+ channels open, allowing Na+ ion entry that rapidly pulls the membrane potential toward the Na+ equilibrium potential (approx. +50 mV) before inactivating. This is balanced by the slower gating of voltage-gated K+ channels that act to pull Vm toward EK (approx. −90 mV) to repolarize the membrane.
7.1.1. Functional compartmentalization of Na+ channels in RGCs.
Several distinct compartments of RGCs each express different complements of ion channels and play unique roles in synaptic integration, spike generation, and spike propagation. Unmyelinated RGC axons fasciculate and course across the retina surface toward the optic nerve head, where they converge and form the optic nerve. An elegant study of amphibian RGCs demonstrated the existence of a short region of axonal thinning (~40-140 microns in length) located just after the axon initial segment (Carras et al., 1992). Detailed studies of RGC axons in rat revealed that NaV1.1 channels occupy a microdomain corresponding to the first ~10 microns of the axon immediately adjacent to the soma, while a region slightly more distal from the soma (10-40 microns) is occupied by NaV1.6 channels (Boiko et al., 2003; Van Wart et al., 2005; Van Wart et al., 2007; Wollner and Catterall, 1986; Wollner et al., 1988). This might correspond to the thin region seen in amphibian RGCs (Carras et al., 1992; Fohlmeister and Miller, 1997b) and might therefore be the site of action potential generation in RGCs. Indeed, NaV1.6 channels are localized to the axon initial segment of many neurons and appear to be ideally suited for action potential initiation. These channels typically have a more hyperpolarized activation curve and recover from inactivation at fairly hyperpolarized potentials (Qiao et al., 2014; Rush et al., 2005). Additionally, NaV1.6 channel currents potentiate during repetitive depolarization (Zhou and Goldin, 2004), which can support higher-frequency firing. In healthy RGCs, the properties of NaV1.6 and its localization to the axon initial segment would likely enhance excitability to promote efficient spike initiation. During early stages of glaucoma, an increase in NaV1.6 expression appears to contribute to increased RGC excitability in mice (Risner et al., 2018). The opposite seems to be the case in a rodent model of multiple sclerosis in which NaV1.6 expression and node localization is reduced and replaced by NaV1.2 (Craner et al., 2003; Craner et al., 2004).
NaV1.2 channels are present in unmyelinated RGC axons and likely underlie propagation of the action potential to the optic nerve head (Boiko et al., 2001; Boiko et al., 2003). Once the axon leaves the eye and becomes myelinated, Na+ channels are localized principally at nodes of Ranvier. These are largely NaV1.6 channels, although Boiko and colleagues have shown that some nodes have NaV1.2 (Boiko et al., 2001). It is unclear whether those nodes contain a combination of NaV1.2/NaV1.6 or whether NaV1.2 is present instead of NaV1.6. In a NaV1.6 null mouse, NaV1.2 and NaV1.1 localize to nodes in the optic nerve (Van Wart and Matthews, 2006b; Vega et al., 2008), suggesting that the presence of NaV1.6 might play a role in excluding them from that location. A tetrodotoxin-resistant Na+ channel (NaV1.8) has also been shown to be present at nodes of very large RGC axons in the optic nerve and somata of very large RGCs in mouse retina (O'Brien et al., 2008). NaV1.8 currents are relatively slow and sustained, showing little inactivation (Renganathan et al., 2000; Sangameswaran et al., 1996). This appears to aid in sustained high-frequency spiking, as blockade using a specific NaV1.8 inhibitor A803467 attenuates light-driven spiking of sustained On αRGCs (Smith et al., 2017).
RGC dendrites and cell bodies also participate in spike generation that appears to play important roles in visual processing. In a “textbook” model of neuronal structure and function, excitatory neurotransmitter release onto dendrites alters the gating of ion channels, leading to excitatory post-synaptic potentials (EPSPs) that passively propagate to the soma. If sufficient numbers of EPSPs of sufficiently large amplitude occur in a sufficiently narrow time window, they depolarize the membrane at the axon initial segment past the action potential threshold and initiate a spike. It is now clear, however, that dendrites of many neurons, including amacrine cells as discussed earlier, possess numerous active conductances that allow them to amplify EPSPs, participate in antidromic spiking, and generate their own regenerative action potentials (Holthoff et al., 2006; Johnston et al., 1996). The same is true for RGCs as well. For instance, Na+ channel α and β subunits are expressed in the IPL of rats (Van Wart et al., 2005; Wollner et al., 1988). While these might in part be the result of Na+ channel expression in spiking ACs (Kaneko and Watanabe, 2007; Wu et al., 2011), faint NaV1.1 expression has been observed in proximal dendrites and somata of rat RGCs (Van Wart et al., 2007) and slowly-inactivating, TTX-resistant NaV1.8 channels are present in somata and dendrites of mouse RGCs (O'Brien et al., 2008).
A particularly elegant study by Velte and Masland used simultaneous paired whole-cell recordings of RGC somata and dendrites to show that dendrites are capable of generating action potentials (Velte and Masland, 1999). Using current steps to depolarize the soma evoked a train of spikes measured at both somatic and dendritic electrodes. Stimulation of dendrites likewise evoked a train of spikes recorded with the dendritic electrode. These could be measured as spikelets with the somatic electrode and, in some cases, dendritic spikes initiated full-amplitude somatic spikes. Additionally, while introducing the Na+-channel blocker and lidocaine derivative QX-314 through the somatic patch pipette blocked somatic spikes, the spikes recorded with the dendritic electrode persisted. This indicates that RGC dendrites are able to generate action potentials.
Modeling studies based on empirically-recorded current properties indicate that voltage-gated Na+ and K+ channels present in dendrites are essential for regulating RGC spike rate (Fohlmeister et al., 1990; Fohlmeister and Miller, 1997a, b). Interestingly, these dendritic channels function to slow repetitive spiking by providing a shunt to discharge the membrane capacitance that would otherwise keep the membrane potential above spike threshold. This is similar to the shunting effect of Na+ and K+ channels in the dendrites of A17 ACs described in section 5.2.
For DSGCs, which preferentially respond to motion in one direction, active dendritic conductances amplify responses to motion in the preferred-direction by triggering dendritic action potentials that propagate and initiate somatic spiking (Oesch et al., 2005; Trenholm et al., 2011). Using simultaneous patch-clamp recordings of DSGC somata and dendrites, Sivyer and Williams showed that dendrites in the region of the dendritic field that is first activated by motion in the preferred direction fire spikes that precede spikes generated in the soma (Sivyer and Williams, 2013). This is consistent with work in amacrine cells and other RGC types showing that dendritic Na+ channels amplify synaptic potentials and that blockade of voltage-gated Na+ channels reduces the signal-to-noise ratio of RGC responses to stimuli of varying contrast (Dhingra, 2005). Dendritic spikes also allow for temporally-precise correlated spiking in a subpopulation of gap junction-coupled DSGCs, a process that likely favors strong temporal summation of their synaptic output to the dorsal lateral geniculate nucleus (Trenholm et al., 2013).
7.1.2. Na+ channels during development
RGC spiking behavior, as with all CNS neurons, matures over the course of development, with RGCs becoming more excitable with maturity. Rat RGCs, for instance, appear to reach full maturity by ~25-27 days postnatal (P25-P27), firing trains of repetitive spikes in response to sustained current injection. Earlier postnatal stages (P7-P9) are characterized predominantly by single-spike behavior with increasing numbers of RGCs being able to fire a rapidly adapting series of spikes by P13. A similar progression, albeit with slightly different timing relative to birth, is seen for other species (Chalupa et al., 1993; Guenther et al., 1999; Qu et al., 2009; Robinson and Wang, 1998; Schmid and Guenther, 1996; Skaliora et al., 1995; Skaliora et al., 1993; Wang et al., 1997; Wollner et al., 1988).
This development of RGC excitability corresponds with the developmental progression of membrane currents and might be explained as well by shifts in both the density of channels in the membrane and the specific channel isoforms expressed by RGCs during development. Voltage-gated Na+ current amplitudes and current density (current amplitude normalized to membrane capacitance) increase dramatically from embryonic RGCs and plateau around eye opening in both rodents and cats (Chalupa et al., 1993; Robinson and Wang, 1998; Rothe et al., 1999; Schmid and Guenther, 1996, 1998; Skaliora et al., 1993). This is accompanied by a hyperpolarizing shift in the INa activation curves progressing through development meaning that the population of Na+ channels requires a weaker depolarization in order to open (Robinson and Wang, 1998; Rothe et al., 1999; Skaliora et al., 1993). Similarly, INa steady-state inactivation curves show a depolarizing (rightward) shift, meaning that relatively more channels are available in the range of RGC resting potentials (Robinson and Wang, 1998; Skaliora et al., 1993).
These functional shifts generally correspond with changes in the expression and localization of Na+ channel isoforms (Miguel-Hidalgo et al., 1995). mRNA levels for both NaV1.2 and NaV1.6 increase during development along a similar time course (Van Wart and Matthews, 2006a). At both nodes and the axon initial segment, NaV1.2 is expressed earlier in development and later replaced by NaV1.6 (Boiko et al., 2001; Boiko et al., 2003; Van Wart and Matthews, 2006b; Vega et al., 2008). The increase in NaV1.6 is likely important in the development of repetitive spiking behavior; RGC spiking in NaV1.6 knockout is similar to wild-type at P12 (largely rapidly adapting spike behavior), but whereas WT RGCs develop repetitive spiking behavior by P14, NaV1.6 knockout RGCs do not. Interestingly, myelination appears to have an instructive and/or regulatory effect on channel localization; in the shiverer mouse which is deficient in myelin, NaV1.6 does not replace NaV1.2 at nodes. Additionally, in mice heterozygous for a NaV1.6 knockout (Scn8a+/−), the gene dose-dependent reduction in NaV1.6 protein leads to an increase in NaV1.2 at nodes in the optic nerve (Vega et al., 2008). The alterations in optic nerve Nav1.6 and NaV1.2 expression and localization in a rodent multiple sclerosis model appear to represent a recapitulation of some of these developmental stages (Craner et al., 2003; Craner et al., 2004).
RGCs have D1-type dopamine receptors (Chen and Yang, 2007; Hayashida and Ishida, 2004; Hayashida et al., 2009; Ogata et al., 2012; Van Hook et al., 2012; Vaquero et al., 2001) and dopamine is known to alter RGC spiking behavior in multiple species including rodents and fish (Hayashida et al., 2009; Ogata et al., 2012; Van Hook et al., 2012; Vaquero et al., 2001). Regulation of Na+ channel inactivation parameters appears to underlie some of this effect (Hayashida and Ishida, 2004; Hayashida et al., 2009), although HCN channels and K+ channels have also been implicated (see below) (Chen and Yang, 2007; Prigge et al., 2016).
7.2. Ca2+ channels
As discussed below, several classes of voltage-gated Ca2+ channels can be distinguished by current kinetics, voltage-dependence, and pharmacology in RGCs.
7.2.1. Low-voltage activated (LVA) Ca2+ currents
LVA ICa activates at fairly hyperpolarized potentials (positive to −70 mV) and in whole-cell recordings in which ICa is isolated by blocking Na+ and K+ currents, the remaining macroscopic membrane current I-V relationship will show a discernable hump at more hyperpolarized voltages that corresponds to the LVA component. Voltage-clamp studies from isolated postnatal rat RGCs (P10) have identified these LVA (or T-type) currents in approximately 1/3 of recorded RCGs (Guenther et al., 1994; Karschin and Lipton, 1989; Sargoy et al., 2014; Schmid and Guenther, 1996). In intact retinas or retinal slices, LVA currents are present in 100% of early embryonic RGCs and the percentage of LVA current-possessing RGCs declines to 13% by eye opening (~P12) and to 0% by adulthood (Schmid and Guenther, 1996). Recordings of RGCs cultured from P13-17 or adult rats show that the proportion of RGCs with LVA current fell from ~33% at P13-17 to ~15% at adulthood. In intact retinas, LVA current amplitudes decreased from embryonic through postnatal stages (Rothe et al., 1999). Interestingly, isolated cat RGCs appeared to lack any discernable T-type current, having an I-V plot without the LVA hump and a pharmacological signature consistent with a predominant L-type current (Kaneda and Kaneko, 1991a). It is unclear how the presence in a subpopulation of RGCs in adult mice is reconciled with the apparent loss of LVA currents detected in adult rat RGCs.
In recordings from isolated salamander RGCs, Henderson and Miller found that LVA current was detectable in RGC somata. However, in cells that had dendritic processes remaining, the LVA current was larger and estimates of membrane surface area from whole-cell capacitance suggested that LVA current density was ~5-fold greater in dendritic compartment than in the soma. LVA currents appear to be constrained to Off RGCs in mouse (Margolis and Detwiler, 2007; Margolis et al., 2010) and additional evidence suggests that it is Off-transient RGCs, but not Off-sustained RGCs, that have LVA currents (Murphy and Rieke, 2011; Van Wyk et al., 2009).
The resting membrane potential for RGCs is typically between −65 and −80 mV (Coleman and Miller, 1989; Lee et al., 2003; O'Brien et al., 2002), a point at which the LVA current is largely inactivated in physiological conditions. However, at this point on the inactivation curve, even a small hyperpolarization, such as that mediated by GABA or glycine-gated chloride channels or K+ channels gated by GABAB receptor activation, will be sufficient to relieve the LVA channel inactivation so that depolarization into LVA channels’ activation range is able to trigger a low-threshold Ca2+ spike (LTS). Studies in amphibian retina suggest that this process is a major contributor to the rebound spiking of RGCs (along with contributions from HCN channels, below) (Mitra and Miller, 2007a, b), where Na+ spikes ride atop the LTS following cessation of a hyperpolarizing stimulus. Indeed, in RGCs recorded in adult mouse flat-mount retinas (4-8 week postnatal), Ca2+ imaging combined with patch-clamp recordings showed that Ca2+ influx was associated with rebound spiking (depolarization and spiking at the termination of a hyperpolarization) only in Off RGCs, but not On RGCs (Margolis and Detwiler, 2007; Margolis et al., 2010). This was highly sensitive to Ni2+, which is a strong blocker of LVA Ca2+ channels, but less effective at blocking HVA channels. Such rebound depolarization and spiking may serve as a thresholding mechanism to enhance post-synaptic responses and promote temporally precise detection of changing light intensity (Mitra and Miller, 2007b) in a manner analogous to the phasic synaptic vesicle release at photoreceptor synapses (Jackman et al., 2009).
7.2.2. High voltage-activated (HVA) Ca2+ currents
HVA ICa have also been recorded from RGCs in multiple species and preparations and identified as L, P/Q, N, or R-type (Guenther et al., 1994; Henderson and Miller, 2003, 2007; Kaneda and Kaneko, 1991a; Karschin and Lipton, 1989; Lipton and Tauck, 1987; Schmid and Guenther, 1996). L-type currents have been identified in RGCs based on their sensitivity to dihydropyridines and large single channel conductance (Guenther et al., 1994; Kaneda and Kaneko, 1991a; Karschin and Lipton, 1989; Schmid and Guenther, 1996). Other HVA currents in RGCs are sensitive to ω-conotoxin-GVIA, which is a strong blocker of N-type CaV2.2 channels (Guenther et al., 1994; Karschin and Lipton, 1989; Schmid and Guenther, 1996). There is also a component of the HVA current in RGCs that is insensitive to dihydropyridines, ω-conotoxin, and ω-agatoxin-IVA (Guenther et al., 1994; Karschin and Lipton, 1989; Schmid and Guenther, 1996), possibly suggesting the presence of R-type currents (CaV2.3)
Immunofluorescence staining for L-type (CaV1.2 and CaV1.3), P/Q-type (CaV2.1) and N-type (CaV2.2) channel α subunits has demonstrated their localization to distinct compartments of RGCs (Ahlijanian et al., 1990; Sargoy et al., 2014; Xu et al., 2002). L-type channels are found in RGC somata (identified by RBPMS staining, which is a selective marker for RGCs) (Rodriguez et al., 2014) and strongly expressed in unmyelinated RGC axons within the retina, while P/Q and N-type channels appear largely constrained to RGC somata (Sargoy et al., 2014). HVA channels α subunits typically complex with α2δ and β subunits that affect membrane localization and gating. While photoreceptor L-type channels (CaV1.4) associate with α2δ4 and β2 accessory subunits, α2δ3 and α2δ1 subunits have been localized to RGC somata in rodents (Farrell et al., 2014).
7.2.3. Ca2+ channels during development.
The density of ICa in RGCs increases throughout development, consistent with an increase in channel insertion into the plasma membrane. As discussed above, LVA channels appear to be downregulated throughout development suggesting that the increase in ICa density from birth to adulthood is the result of an increase in HVA channels. However, the relative proportion of ω-conotoxin and nifedipine-sensitive current is relatively stable once they appear around embryonic day 20 or 21 in rats. The ω-conotoxin-sensitive current is ~50% of the total ICa at embryonic day 21, remains stable until eye opening, and declines to ~35% of the total ICa by adulthood. L-type currents make up ~10% of the whole-cell ICa around E21, ~20% throughout early postnatal period, and eventually settle at ~25% by adulthood. The residual ICa is toxin-resistant (Schmid and Guenther, 1996). T-type currents first appear around the same time as gap junction-mediated stage I retinal waves (Kerschensteiner, 2016; Schmid and Guenther, 1996). Retinal waves are important for refinement of RGC projections to targets in the brain (Firth et al., 2005; Kerschensteiner, 2016). Stage II waves (P1-10 in mouse) are mediated by cholinergic synaptic transmission while stage III are glutamatergic (P10-14). The similar timing of retinal waves with the appearance and gradual reduction in LVA currents raises the possibility that they might play a role in supporting wave-associated bursting behavior in RGCs, although this has not been tested.
7.2.4. Ca2+ channel function.
Action potential firing in myelinated axons triggers Ca2+ influx along the length of the axon (not just at the nodes of Ranvier). This does not appear to be the result of L-type channels, as Ca2+ influx along the axon is insensitive to nifedipine (Zhang et al., 2006). ω-conotoxin dramatically reduces action potential-triggered Ca2+ influx in neonatal rat optic nerves suggesting that N-type channels might instead play a major role (Sun and Chiu, 1999). In contrast, in unmyelinated RGC axons, L-type channels appear to contribute to depolarization-evoked calcium influx (Sargoy et al., 2014). The role for axonal Ca2+ influx is unclear, although it is may regulate axonal excitability by gating Ca2+-activated K+ or Cl− channels (Lev-Ram and Grinvald, 1986), which play important roles in spike frequency adaptation in neurons (Ha and Cheong, 2017). Additionally, autophosphorylation of CaM kinase II, a downstream effector enzyme for intracellular Ca2+ signals, alters spike propagation in the optic nerve (Partida et al., 2018).
While Na+ and K+ channels are key for changing membrane voltage and LVA Ca2+ channels contribute to the low-threshold spike and rebound spiking, HVA Ca2+ channels principally function to allow influx of Ca2+ so that it can act as a second messenger to mediate non-electrogenic cellular behaviors such as contraction, secretion, enzyme activity, and gene expression. For example, Ca2+ influx through L-type Ca2+ channels is essential for triggering tonic glutamate release at photoreceptors, bipolar cells, and hair cell ribbon synapses, while P/Q-, N- and R-type channels allow Ca2+ influx for action-potential-triggered release at most other CNS synapses. RGCs generally do not make intraretinal synapses. However, one exception is M1-type intrinsically photosensitive RGCs (ipRGCs), which have axon collaterals that are likely mediators of ipRGC glutamatergic synaptic output to DACs (Prigge et al., 2016; Zhang et al., 2008). ipRGC synaptic drive depends slightly on N-type channels as indicated by a modest inhibition (~30%) by ω-conotoxin. ipRGC-DAC synapses were insensitive to other L-, T-, R-, or P/Q-type blockers (Prigge et al., 2016). It is unclear what additional Ca2+ channel contributes at this synapse.
There is little firmly known about the Ca2+ channels that mediate synaptic release from RGC terminals at visual nuclei in the brain. In a RGC primary culture system in which RGCs form synapses with neighboring cultured neurons, Taschenberger and Grantyn used paired recordings and Ca2+ channel blockers to show that glutamate release from RGCs depends largely on N-type channels (~70% block of the post-synaptic current by ω-conotoxin) (Taschenberger and Grantyn, 1995). ω-agatoxin had no effect on glutamate release and release was slightly enhanced by an L-type blocker (nifedipine).
Glutamatergic output synapses of M1-type ipRGCs in the suprachiasmatic nucleus appear to depend on a combination of N-, P/Q-, T-, and R-type Ca2+ channels, without a contribution from L-type channels (Moldavan et al., 2006). RGC synaptic outputs to other visual brain nuclei presumably operate by a similar complement of presynaptic Ca2+ channel, although this has not yet been tested.
Modulation of presynaptic Ca2+ influx is a powerful means of regulating synaptic strength and this appears to occur by multiple mechanisms at RGC output synapses in both the SCN and dLGN. In the SCN, GABAB receptor activation reduces the amplitude of excitatory post-synaptic currents by inhibiting presynaptic Ca2+ channels (Moldavan et al., 2006). GABAB receptors likewise inhibit synaptic transmission at retinogeniculate synapses in the dLGN as does activation of 5HT1 receptors (Chen and Regehr, 2003). Metabotropic glutamate receptors also alter retinogeniculate synaptic transmission, possibly by impinging on presynaptic Ca2+ influx (Govindaiah et al., 2012; Lam and Sherman, 2013).
Although HVA channels (especially non-L-type HVA channels) are important for RGC synaptic output, it is not clear what role HVA channels expressed in RGC somata and axons play in the retina. In M1 ipRGCs, L-type channels are responsible for most of the Ca2+ influx during melanopsin-mediated depolarization, a process which might contribute to adaptation of the melanopsin transduction cascade (Do and Yau, 2013). L-type channels are also important in coupling excitation to transcription via CaM/CaM kinase (Catterall, 2011; Simms and Zamponi, 2014). Such a process has not been explored in RGCs. Additionally, L-type channels play a role in Ca2+-dependent AMPA receptor trafficking associated with synaptic plasticity (Voglis and Tavernarakis, 2006). AMPA-type glutamate receptors in RGCs are subject to light-induced and intracellular Ca2+-dependent plasticity, although that process appears to rely on NMDA-type receptors (Jones et al., 2012). As described above, Ca2+ influx might be important in regulating spike generation and firing properties via activation of Ca2+-activated K+ channels in RGCs (Wang et al., 1998).
7.3. K+ channels
7.3.1. Voltage-gated K+ channels
Voltage-clamp recordings from isolated adult rat RGCs revealed several distinguishable type of voltage-gated K+ currents (Lipton and Tauck, 1987; Lukasiewicz and Werblin, 1988; Reiff and Guenther, 1999; Rothe et al., 1999). These include a TEA-sensitive, sustained delayed rectifier current (IKDR), and a 4-AP-sensitive, transient A-type K+ current (IKA) (Lipton and Tauck, 1987; Reiff and Guenther, 1999). Several studies in rodent and amphibian RGCs have identified an additional TEA- and 4-AP-insensitive K+ current with especially slow inactivation kinetics (Lukasiewicz and Werblin, 1988; Reiff and Guenther, 1999; Sucher and Lipton, 1992). Voltage-gated K+ channels are necessary for proper membrane repolarization during the action potential. This is most clearly the case for delayed-rectifier currents in the classical Hodgkin-Huxley action potential model. A-type currents, however, because of their voltage-dependent inactivation, play important roles in regulating inter-spike timing and spike initiation (Hille, 2001).
There are >15 different isoforms of K+ channels responsible for IKDR and IKA (Alexander et al., 2017c). Several different K+ channels have been localized to RGCs using immunofluorescence techniques. KV1.2, for instance, is present in the axon initial segment, in the same general region as NaV1.6, and absent from the slightly more proximal NaV1.1-enriched region (Van Wart et al., 2007). It is also expressed strongly in unmyelinated RGC axons near the optic nerve head, where it co-localizes with NaV1.2 (Boiko et al., 2001). KV1.2 is also present in regions of optic nerve adjacent to the nodes of Ranvier (Boiko et al., 2001; Rasband et al., 1999). KV1.1, KV1.2, and KV1.3 are also found in RGC somata (Koeberle and Schlichter, 2010; Koeberle et al., 2010). KV4.2 channels which carry IKA have been identified in a subpopulation of RGCs in adult and developing mouse retina (Qu et al., 2009).
Whereas a developmental increase in Na+ current density is fairly consistent across species and corresponds to documented developmental increases in RGC excitability, developmental shifts in K+ currents are less well-defined (Sernagor et al., 2001). In mouse, for instance, both IKDR and IKA were largely unchanged from late embryonic through postnatal stages (Rothe et al., 1999). In rat, however, both K+ current types appear to increase (Reiff and Guenther, 1999) while in cats, IKA decrease and IKDR increase in amplitude (Skaliora et al., 1995). In addition to the changes in current densities, these developmental changes also accompany shifts in current kinetics and voltage-dependence (Robinson and Wang, 1998; Rothe et al., 1999; Skaliora et al., 1995).
7.3.2. Other K+ channels
Beyond delayed rectifier and A-type K+ currents, several other K+ channels have been identified in RGCs. A qRT-PCR and immunofluorescence analysis has shown the presence of numerous two-pore K+ channels (K2P) in RGCs including TASK-1, TREK-1, TWIK-1, TWIK-2 and TWIK-3 (Hughes et al., 2017). As discussed in section 1.1.2, K2P channels are leak K+ channels and therefore play important roles in setting RGC membrane potential. There appears to be some differences in expression of these channel genes in different RGC populations, as identified by gene clustering analysis (Rheaume et al., 2018). Varying levels of each K2P channel might help set unique resting potentials for distinct populations of RGCs (Hu et al., 2013; O'Brien et al., 2002; Schmidt and Kofuji, 2009).
Ca2+-activated K+ currents are important for coupling changes in intracellular Ca2+ to changes in neuronal spike patterns. Since Ca2+ entry and intracellular sequestration is relatively slow, the buildup of intracellular Ca2+ during a spike train can prolong the gating of Ca2+-activated K+ channels. This phenomenon regulates the both the fast after-hyperpolarization that occurs after a spike and a slow after-hyperpolarization that follows a train of spikes. Ca2+- activated K+ currents have been recorded from RGCs in trout, ferret, rat, and salamander and pharmacology studies indicate that both BK and SK channels contribute to these currents (Henne and Jeserich, 2004; Henne et al., 2000; Lipton and Tauck, 1987; Lukasiewicz and Werblin, 1988; Wang et al., 1998). SK2 channels have been localized to RGCs (Klöcker et al., 2001). Inhibition of either channel type increases RGC excitability (Wang et al., 1998) and SK channels appear to mediate an adenosine-evoked RGC hyperpolarization (Clark et al., 2009).
7.4. HCN channels
Like those of other preparations, including photoreceptors, HCN channels in RGCs are gated by hyperpolarization and are permeable to cations with a ~2- to 4-fold preference for K+ over Na+ (Wahl-Schott and Biel, 2009). This ion selectivity gives the resulting Ih an equilibrium potential of ~−20 mV so that gating of HCN channels in their activation range has a depolarizing influence. As a result, HCN channels in neurons are thought to play a role in setting the resting membrane potential, underlie rebound depolarization, and regulate the temporal summation of synaptic inputs by altering membrane resistance at hyperpolarized potentials. In intracellular recordings, many RGCs show a pronounced depolarizing voltage “sag” in response to hyperpolarizing current injection (Hu et al., 2013; O'Brien et al., 2002; Van Hook et al., 2012). A similar sag is seen in intra-axonal recordings from the rat optic nerve (Eng et al., 1990). Voltage-clamp recordings in mammalian RGCs have identified and characterized Ih, showing that it is activated at quite hyperpolarized potentials (approx. −80 to −100 mV) (Lee and Ishida, 2007; Van Hook and Berson, 2010).
Contributions from Ih vary by RGC class. In recordings of cat RGCs, the depolarizing sag was more pronounced in some RGC classes than others and absent from alpha and possibly lambda RGCs (O'Brien et al., 2002). In melanopsin-expressing RGCs in mouse, all five classes of ipRGCs display a voltage sag, but it varies in amplitude (Hu et al., 2013). In voltage-clamp recordings, Ih has been identified in M1-type ipRGCs in rats identified by retrograde labeling from tracer injection into the suprachiasmatic nucleus (Van Hook and Berson, 2010). Ih has also been recorded from M2 and M4 (On sustained αRGC)-type ipRGCs from mouse (Jiang et al., 2018).
There are four known HCN channel isoforms (HCN1-4) that vary in their kinetics and voltage dependence (Ludwig et al., 1998; Moosmang et al., 2001; Santoro et al., 1998; Stieber et al., 2005). HCN1 and HCN4 have been identified by immunofluorescence in retrolabeled RGCs (to avoid accidentally counting ACs displaced in the RGC layer) (Stradleigh et al., 2011). Some RGCs are unlabeled by either HCN1 or HCN4 antibodies, while some express a mix of both channels in varying proportions. This labeling pattern is also consistent with physiology. The soma size and dendritic stratification (both measures used to differentiate RGC classes) of HCN1 and HCN4-immunopositive and immuno-negative RGCs varies, indicating that many different RGC populations express HCN channels.
In cases where a single neuron expresses multiple Ih channel subtypes, the whole-cell Ih takes on properties of both expressed isoforms. In co-immunoprecipitation assays with retinal plasma membrane preparations, HCN1 and HCN4 appear to interact physically with each other, suggesting that they can function as HCN1/HCN4 heteromers (Stradleigh et al., 2011). In M1-type ipRGCs, Ih activation voltage is especially hyperpolarized (threshold at ~−75 mV) and the current is quite slow (activation time constant of ~900 ms at −120 mV) (Van Hook and Berson, 2010), consistent with the properties of HCN4. Other studies of Ih in RGCs have indicated that Ih is comprised of two kinetic components, with a fast activation time constant of ~100 ms and a slower component with a time constant of ~800-900 ms (Lee and Ishida, 2007; Stradleigh et al., 2011).
It is likely that a more extensive analysis of RGCs by subtype using traditional anatomical parameters (i.e., soma size, dendritic stratification, Sholl analysis, dendritic field diameter) will reveal subtype-specific patterns in HCN channel expression and Ih properties. Indeed, single-cell transcriptomics work shows that different HCN channel isoforms are enriched in different putative RGC populations (identified by clustering based on gene expression profiles) (Rheaume et al., 2018).
Immunostaining evidence shows that HCN channels are largely localized to RGC somata and axons, with some possible staining in proximal dendrites (Stradleigh et al., 2011). HCN channels might be expressed in more distal dendrites and simply escape detection due to low protein level, so this result does not necessarily indicate that they are absent from RGC dendrites. HCN1 and HCN4 staining has also been detected in dendrites of RGC primary cell cultures (Abbas et al., 2013). Interestingly, HCN4 colocalizes and coimmunoprecipitates with Thy1, a glycophosphatidylinositol-anchored cell surface protein expressed in RGC somata (Partida et al., 2012). Thus, HCN channels appear to be most highly concentrated in RGC somata. Substantial staining of HCN channel isoforms in the IPL is likely to arise principally from labeling of bipolar cell axons and synaptic terminals (Ivanova and Müller, 2006; Müller et al., 2003).
These results suggest that in RGCs, HCN channels principally act to shape firing patterns and integration of synaptic inputs in the soma rather than shaping individual synaptic potentials in the dendrites. Contrary to this, however, a study using sequential uncaging of glutamate along the length of an RGC dendrite has shown that Ih contributes to directional summing of inputs by enhancing sequential responses to stimuli as they move away from the RGC soma (Abbas et al., 2013). Such a mechanism might be a cell-autonomous process allowing for the detection of looming motion by RGCs.
More in keeping with a role in regulating spiking behavior, Ih in amphibian RGCs has been shown to contribute to rebound depolarization and spiking after the cessation of a hyperpolarizing stimulus (Mitra and Miller, 2007a). Some evidence from rat ipRGCs hints at a similar role in mammalian RGCs (Van Hook and Berson, 2010). HCN channels also play a role in regulating RGC membrane potential and excitability and apparently contribute to the effects of dopamine on excitability (but see (Hayashida et al., 2009)). Blockade of HCN channels in M1 ipRGCs does little to affect resting membrane potential and does not alter spiking evoked by melanopsin phototransduction, making it unclear what role Ih plays in that class of RGCs (Van Hook and Berson, 2010). Recall that HCN channels can also modulated by cyclic nucleotides such as cAMP. Activation of D1-type dopamine receptors in RGCs leads to an increase in intracellular cAMP production, which can in turn modulate RGC excitability and resting membrane potential by altering the voltage-dependence of Ih activation (Chen and Yang, 2007) (but see (Hayashida et al., 2009)).
An especially novel role for HCN channels as an endpoint for melanopsin-based phototransduction has recently been demonstrated for M2 and M4-type ipRGCs (Jiang et al., 2018). While M1-type ipRGCs rely largely on a Gq/PLC cascade that gates TRPC6/7 channels (Perez-Leighton et al., 2011), Jiang and colleagues have shown that melanopsin-activated photocurrents that linger following TRPC6/7 knockout in M2 and M4 cells were almost entirely blocked by the HCN channel blocker ZD7288 and severely reduced in cells transfected to express a dominant-negative HCN channel (Jiang et al., 2018). This is difficult to reconcile, however, with another recent study showing that M4 transduction culminates in the closure of leak K+ channels, as evidenced by voltage-dependence (reversal at EK) and barium block (which will not substantially affect Ih) of melanopsin-mediated responses (Sonoda et al., 2018). A very small component of the M1 photocurrent also appears to result from HCN channel gating (Jiang et al., 2018).
7.5. Summary
In many respects, the ion channel complement in RGCs is typical of other spiking neurons. The distribution of ion channels in different compartments of RGCs is summarized in Table 3 and its associated diagram. RGCs possess voltage-gated Na+ channels (predominantly NaV1.6 in nodes of Ranvier, NaV1.2 in unmyelinated axon, and NaV1.1 and 1.8 in the soma and dendrites) as well as voltagegated K+ channels that give rise to delayed rectifier and A-type K+ currents. INa and IKDR are responsible for the rising and falling phase of the action potential while IKA is important in shaping spike timing. Dendritic action potentials play important computational roles including motion detection and amplification of post-synaptic potentials. N-, P/Q- and R-type voltage-gated Ca2+ channels are important for synaptic output, either at intraretinal synapses made by M1 ipRGC axon collaterals or at output synapses in visual nuclei of the brain, although this has not been firmly established at outputs other than those made by ipRGCs in the SCN. T-type ICa in dendrites and somata shape spiking behavior. The role of L-type and N-, P/Q- and R-type ICa in RGC somata are unclear, although they likely shape spiking behavior by gating Ca2+-activated K+ currents.
Table 3.
Ion channels in retinal ganglion cells (RGCs). The distribution of multiple types and isoforms of voltage-gated ion channels in distinct RGC compartments is summarized in the table and illustrated in the diagram. See text for details.
| Synaptic terminal |
Nodes of Ranvier |
Unmyelinated axon |
Axon initial segment |
Soma | Dendrites | |
|---|---|---|---|---|---|---|
| Na+ channels | NaV1.6, also 1.2, 1.8 | NaV1.2 | NaV1.6 (distal segment) NaV1.1(proximal segment) |
NaV1.1, 1.8 | NaV1.1, 1.8 | |
| Ca2+ channels | N, P/Q, R-type | N-type | L-type | L, N, P/Q-type | LVA (Off cells) | |
| K+ channels | Kv1.2 | KV1.2 (distal segment) | KV1.1., 1.2, 1.3, 4.2, 4.3, SK2 | Kv4.2, others? | ||
| HCN channels | HCN1, 4 | |||||
 | ||||||
RGC receptive field centers are comprised of the pooled excitatory inputs arising from the population of presynaptic bipolar cells and fine substructure can be revealed using spatiotemporal white noise or naturalistic scene stimuli (Brown et al., 2000; Freed and Sterling, 1988; Freeman et al., 2015; Wienbar and Schwartz, 2018). Postsynaptic processes such as spike generation in dendrites and influences of voltage-gated conductances on local membrane properties are likely to influence spatial and temporal summation of synaptic inputs at these individual sub-regions of RGC receptive fields (Ujfalussy et al., 2018). This would be a fruitful avenue for future research.
A major open question concerns how and whether differential expression of ion channels is responsible for shaping the unique response properties of different RGC classes. There are at least 30 functionally-distinct classes of RGC identified in mouse retina, which is currently the principal animal model used for probing RGC function. Evidence from a variety of species indicates that the spiking properties of RGCs are heterogeneous (Hu et al., 2013; Kaneda and Kaneko, 1991b; O'Brien et al., 2002; Schmidt and Kofuji, 2009; Tabata and Kano, 2002). For instance, M1-type ipRGCs in mice are unable to maintain a high firing frequency and instead rapidly enter depolarization block (where Na+ channel inactivation prevents further regenerative spiking). In contrast, other ipRGC types can sustain much higher firing frequencies (Hu et al., 2013; Schmidt and Kofuji, 2009). This implies that different RGC classes differ in their complement and/or densities of Na+ and K+ channels, which would be consistent with the documented diversity of expression patterns seen among RGCs for HCN, Ca2+, and other channel types. There can be considerable variability even within distinct RGC subpopulations. For instance, M1-type ipRGCs display a strikingly heterogeneous range of biophysical parameters and melanopsin-driven light responses, hinting at variable ion channel expression and/or regulation in these RGCs (Emanuel and Do, 2015; Emanuel et al., 2017; Milner and Do, 2017). Studies of gene expression patterns along with exploration of the unique channel properties in distinct RGC classes and, in some cases, within RGC classes are needed to clarify how the diversity of channel expression and properties contribute to the unique signaling roles of different RGC populations.
8. Conclusions
Recent years have seen a dramatic expansion in the recognized number of individual cell types in the retina. In this review, we have outlined our current understanding of the numerous subtypes of voltage- and Ca2+-gated ion channels present in many of these different retinal neurons. In addition to differences in ion channel distributions between species, it is clear that there are notable differences between major cell types and even differences among subtypes of the same cell. A major remaining challenge is to understand how this diversity of ion channel complement across different populations of retinal neurons contributes to visual processing performed as information is relayed through the retinal network and conveyed to the brain. What is the role of the particular ion channel subunit combinations and their localization in each of these cells in shaping their unique response properties? Can we identify specific ion channel finger-prints for each type of cell? How does ion channel expression change in response to changes in illumination, metabolism, or disease? Answers to these questions will come to light as the field develops a more complete wiring diagram and probes the function of the retinal network using a combination of computational, molecular, and physiological approaches. Electrophysiological recordings remain the dominant approach for studying the membrane currents and voltage responses of neurons. However, these techniques are increasingly supplemented by molecular techniques, imaging with activity-dependent dyes, pharmacological tools, genetic elimination of target proteins, and genetic introduction of mutant proteins, sensor proteins, DREADDs, or optogenetic tools. This diverse and powerful array of experimental tools provides an opportunity to unravel the mysteries of signal transmission in the retina. Along with a detailed understanding of the basis for single cell properties in different cell types, these experiments are sure to reveal new principles concerning the strategies used by the nervous system to shape activity in response to a dynamically changing visual environment.
Highlights:
There are many types of voltage- and calcium-gated ion channels.
There are almost 100 subtypes of retinal neurons that differ in their ion channels.
Ion channel type and distribution shape responses of retinal neurons.
Ion channel dysfunction can contribute to retinal disease.
Acknowledgments
This work was supported the National Institutes of Health (grant EY10542, WT) and the Bright Focus Foundation National Glaucoma Research Program (grant G2017027, MVH).
Abbrevations:
- (IKA)
A-type K+ currents
- (AC)
Amacrine cell
- (ICa)
Ca2+ currents
- (CaM)
Calmodulin
- (CBC)
Cone bipolar cell
- (CRH)
Corticotropin releasing hormone
- (IKDR)
Delayed rectifier K+ currents
- (DSGC)
Direction-selective ganglion cells
- (DAC)
Dopaminergic amacrine cell
- (HVA)
High-voltage activated
- (Ih)
Hyperpolarization-activated current
- (IPL)
Inner plexiform layer
- (ipRGC)
Intrinsically photosensitive retinal ganglion cell
- (IKIR)
Inwardly rectifying K+ current
- (LVA)
Low-voltage activated
- (OPL)
Outer plexiform layer
- (PKA)
Protein kinase A
- (RGC)
Retinal ganglion cell
- (RBC)
Rod bipolar cell
- (SAC)
Starburst amacrine cell
- (TEA)
Tetraethylammonium
- (NaV)
Voltage-gated Na+
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas SY, Hamade KC, Yang EJ, Nawy S, Smith RG, and Pettit DL (2013). Directional Summation in Non-direction Selective Retinal Ganglion Cells. PLoS Computational Biology 9, e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman JP, Maylie J, and Sah P (2012). Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74, 245–269. [DOI] [PubMed] [Google Scholar]
- Ahern CA, Payandeh J, Bosmans F, and Chanda B (2016). The hitchhiker's guide to the voltage-gated sodium channel galaxy. J Gen Physiol 147, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlijanian MK, Westenbroek RE, and Catterall WA (1990). Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron 4, 819–832. [DOI] [PubMed] [Google Scholar]
- Akopian A, Johnson J, Gabriel R, Brecha N, and Witkovsky P (2000). Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina. J Neurosci 20, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Krizaj D, and Witkovsky P (1997). Both high- and low voltage-activated calcium currents contribute to the light-evoked responses of luminosity horizontal cells in the Xenopus retina. Brain Res 762, 121–130. [DOI] [PubMed] [Google Scholar]
- Akopian A, and Witkovsky P (1994). Modulation of transient outward potassium current by GTP, calcium, and glutamate in horizontal cells of the Xenopus retina. J Neurophysiol 71, 1661–1671. [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Al-Owain M, Alqahtani F, Hazzaa S, and Alkuraya FS (2010). A null mutation in CABP4 causes Leber's congenital amaurosis-like phenotype. Mol Vis 16, 207–212. [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, et al. (2017a). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Other ion channels. Br J Pharmacol 174 Suppl 1, S195–S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, et al. (2017b). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand-gated ion channels. Br J Pharmacol 174 Suppl 1, S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, et al. (2017c). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage-gated ion channels. Br J Pharmacol 174 Suppl 1, S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Zhang L, Jiao B, Lu F, Xia F, Yu Z, and Zhang Z (2015). Cacna1f gene decreased contractility of skeletal muscle in rat model with congenital stationary night blindness. Gene 562, 210–219. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kamiyama Y, and Usui S (2005). Simulation analysis of receptive-field size of retinal horizontal cells by ionic current model. Vis Neurosci 22, 65–78. [DOI] [PubMed] [Google Scholar]
- Armstrong CM (2003). Voltage-gated K channels. Sci STKE 2003, re10. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY, and Burns ME (2012). Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287, 1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D (1986). The Sharpey-Schafer lecture. Ion channels and signal processing in the outer retina. Q J Exp Physiol 71, 497–536. [PubMed] [Google Scholar]
- Attwell D, and Wilson M (1980). Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol 309, 287–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, and Slaughter MM (2000). Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci 20, 7087–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, Kanevsky N, Dascal N, Rozanski GJ, Singh DP, Fatma N, and Thoreson WB (2010). Anion-sensitive regions of L-type CaV1.2 calcium channels expressed in HEK293 cells. PLoS One 5, e8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, and Thoreson WB (2009). Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol 587, 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Berens P, Bethge M, and Euler T (2013). Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr Biol 23, 48–52. [DOI] [PubMed] [Google Scholar]
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, and Euler T (2016). The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Esposti F, Nikolaev A, and Lagnado L (2011). Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision. Curr Biol 21, 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CR, and Bertrand D (1984). Effect of changes in intra- and extracellular sodium on the inward (anomalous) rectification in salamander photoreceptors. J Physiol 347, 611–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, and Schwartz EA (1982). Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol 331, 253–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JA, Mu S, Kim JS, Turner NL, Tartavull I, Kemnitz N, Jordan CS, Norton AD, Silversmith WM, Prentki R, et al. (2018). Digital Museum of Retinal Ganglion Cells with Dense Anatomy and Physiology. Cell 173, 1293–1306.e1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Puthussery T, Kim M-H, Taylor WR, and von Gersdorff H (2015). Synaptic Vesicle Exocytosis at the Dendritic Lobules of an Inhibitory Interneuron in the Mammalian Retina. Neuron 87, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, and Gregg RG (2002). Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43, 1595–1603. [PubMed] [Google Scholar]
- Barnes S (1994). After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience 58, 447–459. [DOI] [PubMed] [Google Scholar]
- Barnes S, and Deschenes MC (1992). Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol 68, 745–755. [DOI] [PubMed] [Google Scholar]
- Barnes S, and Hille B (1989). Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol 94, 719–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, and Werblin F (1986). Gated currents generate single spike activity in amacrine cells of the tiger salamander retina. Proc Natl Acad Sci U S A 83, 1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow AJ, and Wu SM (2009). Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci 29, 5841–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti TM, Jackman SL, Babai N, Mercer AJ, Kramer RH, and Thoreson WB (2011). Release from the cone ribbon synapse under bright light conditions can be controlled by the opening of only a few Ca(2+) channels. J Neurophysiol 106, 2922–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CK, and Schwarz JR (2018). Ether-a-go-go K(+) channels: effective modulators of neuronal excitability. J Physiol 596, 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L, Gerstner A, Zong X, Biel M, and Wahl-Schott C (2004). Functional characterization of the L-type Ca2+ channel Cav1.4alpha1 from mouse retina. Invest Ophthalmol Vis Sci 45, 708–713. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Matthews G, and Nunn BJ (1984). Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol 354, 203–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, and Nunn BJ (1986). Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol 371, 115–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, and Boycott KM (1998). Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19, 264–267. [DOI] [PubMed] [Google Scholar]
- Beech DJ, and Barnes S (1989). Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron 3, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salah S, Kamei S, Senechal A, Lopez S, Bazalgette C, Bazalgette C, Eliaou CM, Zanlonghi X, and Hamel CP (2008). Novel KCNV2 mutations in cone dystrophy with supernormal rod electroretinogram. Am J Ophthalmol 145, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Berntson A, and Taylor WR (2000). Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol 524 Pt 3, 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Taylor WR, and Morgans CW (2003). Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. Journal of Neuroscience Research 71, 146–151. [DOI] [PubMed] [Google Scholar]
- Bieda MC, and Copenhagen DR (1999). Sodium action potentials are not required for light-evoked release of GABA or glycine from retinal amacrine cells. J Neurophysiol 81, 3092–3095. [DOI] [PubMed] [Google Scholar]
- Bieda MC, and Copenhagen DR (2004). N-type and L-type calcium channels mediate glycinergic synaptic inputs to retinal ganglion cells of tiger salamanders. Visual Neuroscience 21, 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M (2009). Cyclic nucleotide-regulated cation channels. The Journal of biological chemistry 284, 9017–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Vaquero CF, and de la Villa P (1996). Action potentials in axonless horizontal cells isolated from the rabbit retina. Neurosci Lett 203, 57–60. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA (1992). Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. Journal of neurophysiology 68, 711–725. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA (1996). Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. Journal of neurophysiology 75, 1878–1893. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, and Völgyi B (2007). Response properties of a unique subtype of wide-field amacrine cell in the rabbit retina. Visual neuroscience 24, 459–469. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, and Völgyi B (2009). The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Reviews Neuroscience 10, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, and Xin D (2000). Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. The Journal of Physiology 523 Pt 3, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocksteins E (2016). Kv5, Kv6, Kv8, and Kv9 subunits: No simple silent bystanders. J Gen Physiol 147, 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, and Matthews G (2001). Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, and Matthews G (2003). Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci 23, 2306–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos R, Schneider H, and Wassle H (1993). Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J Neurosci 13, 2874–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Looger LL, Tomita S, and Demb JB (2014). Kainate Receptors Mediate Signaling in Both Transient and Sustained OFF Bipolar Cell Pathways in Mouse Retina. Journal of Neuroscience 34, 6128–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Marvin JS, Looger LL, and Demb JB (2013). Two-Photon Imaging of Nonlinear Glutamate Release Dynamics at Bipolar Cell Synapses in the Mouse Retina. Journal of Neuroscience 33, 10972–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, and Sakmann B (1999). Depletion of calcium in the synaptic cleft of a calyx-type synapse in the rat brainstem. J Physiol 521 Pt 1, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Cusato K, Nicchia GP, Frigeri A, and Spray DC (2005). A developmental switch in the expression of aquaporin-4 and Kir4.1 from horizontal to Muller cells in mouse retina. Invest Ophthalmol Vis Sci 46, 3869–3875. [DOI] [PubMed] [Google Scholar]
- Bourinet E, and Zamponi GW (2017). Block of voltage-gated calcium channels by peptide toxins. Neuropharmacology 127, 109–115. [DOI] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, and MacKinnon R (2012). Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, and Passmore GM (2009). Neural KCNQ (Kv7) channels. Br J Pharmacol 156, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, He S, and Masland RH (2000). Receptive field microstructure and dendritic geometry of retinal ganglion cells. Neuron 27, 371–383. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Gottesman J, and Thoreson WB (1988). Prolonged depolarization in turtle cones evoked by current injection and stimulation of the receptive field surround. J Physiol 407, 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA, Zhang SQ, and Gottesman J (1991). Prolonged depolarization in rods in situ. Vis Neurosci 6, 607–614. [DOI] [PubMed] [Google Scholar]
- Burns ME, and Baylor DA (2001). Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci 24, 779–805. [DOI] [PubMed] [Google Scholar]
- Burrone J, and Lagnado L (1997). Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. J Physiol 505 (Pt 3), 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher V, Schicker K, Novikova E, Pohn B, Stockner T, Kugler C, Singh A, Zeitz C, Lancelot ME, Audo I, et al. (2014). Spectrum of Cav1.4 dysfunction in congenital stationary night blindness type 2. Biochim Biophys Acta 1838, 2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, and Singewald N (2010). CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol 13, 499–513. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Krol J, Nelidova D, Daum J, Szikra T, Tsuda B, Juttner J, Farrow K, Scherf BG, Alvarez CP, et al. (2014). miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 83, 586–600. [DOI] [PubMed] [Google Scholar]
- Byzov AL, and Trifonov Yu A (1981). Ionic mechanisms underlying the nonlinearity of horizontal cell membrane. Vision Res 21, 1573–1578. [DOI] [PubMed] [Google Scholar]
- Cadetti L, Bryson EJ, Ciccone CA, Rabl K, and Thoreson WB (2006). Calcium-induced calcium release in rod photoreceptor terminals boosts synaptic transmission during maintained depolarization. Eur J Neurosci 23, 2983–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Al Abed A, Buskila Y, Dokos S, Lovell NH, and Morley JW (2017). Differential effect of brief electrical stimulation on voltage-gated potassium channels. Journal of neurophysiology 117, 2014–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano L, Gargini C, Della Santina L, Demontis GC, and Cervetto L (2007). High-pass filtering of input signals by the Ih current in a non-spiking neuron, the retinal rod bipolar cell. PLoS One 2, e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sarria I, Fehlhaber KE, Kamasawa N, Orlandi C, James KN, Hazen JL, Gardner MR, Farzan M, Lee A, et al. (2015). Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron 87, 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Piano I, Demontis GC, Bacchi N, Casarosa S, Della Santina L, and Gargini C (2015). TMEM16A is associated with voltage-gated calcium channels in mouse retina and its function is disrupted upon mutation of the auxiliary alpha2delta4 subunit. Front Cell Neurosci 9, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carras PL, Coleman PA, and Miller RF (1992). Site of action potential initiation in amphibian retinal ganglion cells. Journal of neurophysiology 67, 292–304. [DOI] [PubMed] [Google Scholar]
- Catterall WA (2011). Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3, a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA (2017). Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem Res 42, 2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski Mark S., Logan Stephen M., Tian M, Jia L, Li W, Kath William L., Riecke H, and Singer Joshua H. (2012). The Mechanisms of Repetitive Spike Generation in an Axonless Retinal Interneuron. Cell Reports 1, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, Snyder GE, Selvin PR, and Bezanilla F (1999). Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature 402, 809–813. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Skaliora I, and Scobey RP (1993). Responses of isolated cat retinal ganglion cells to injected currents during development. Prog Brain Res 95, 25–31. [DOI] [PubMed] [Google Scholar]
- Chapot CA, Euler T, and Schubert T (2017). How do horizontal cells 'talk' to cone photoreceptors? Different levels of complexity at the cone-horizontal cell synapse. J Physiol 595, 5495–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell RL, Anastassov I, Lugo P, and Ripps H (2008). Zinc-mediated feedback at the synaptic terminals of vertebrate photoreceptors. Exp Eye Res 87, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, and Diamond JS (2006). Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443, 705–708. [DOI] [PubMed] [Google Scholar]
- Chen C, and Regehr WG (2003). Presynaptic modulation of the retinogeniculate synapse. J Neurosci 23, 3130–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, and Yang XL (2007). Hyperpolarization-activated cation current is involved in modulation of the excitability of rat retinal ganglion cells by dopamine. Neuroscience 150, 299–308. [DOI] [PubMed] [Google Scholar]
- Choi SY, Jackman S, Thoreson WB, and Kramer RH (2008). Light regulation of Ca2+ in the cone photoreceptor synaptic terminal. Vis Neurosci 25, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen P, and Wulff H (2015). Pharmacological gating modulation of small- and intermediate-conductance Ca(2+)-activated K(+) channels (KCa2.x and KCa3.1). Channels (Austin) 9, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, and Picaud S (2005). Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. J Neurophysiol 93, 1468–1475. [DOI] [PubMed] [Google Scholar]
- Clark BD, Kurth-Nelson ZL, and Newman EA (2009). Adenosine-Evoked Hyperpolarization of Retinal Ganglion Cells Is Mediated by G-Protein-Coupled Inwardly Rectifying K+ and Small Conductance Ca2+-Activated K+ Channel Activation. Journal of Neuroscience 29, 11237–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins M, and Zenisek D (2009). Evidence that exocytosis is driven by calcium entry through multiple calcium channels in goldfish retinal bipolar cells. J Neurophysiol 101, 2601–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED (2001). Voltage-gated calcium and sodium currents of starburst amacrine cells in the rabbit retina. Visual neuroscience 18, 799–809. [DOI] [PubMed] [Google Scholar]
- Coleman PA, and Miller RF (1989). Measurement of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. Journal of neurophysiology 61, 218–230. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, and Maguire G (1998). Differential expression of voltage-gated K+ and Ca2+ currents in bipolar cells in the zebrafish retinal slice. The European journal of neuroscience 10, 1350–1362. [DOI] [PubMed] [Google Scholar]
- Cook PB, and McReynolds JS (1998). Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1, 714–719. [DOI] [PubMed] [Google Scholar]
- Cook PB, and Werblin FS (1994). Spike initiation and propagation in wide field transient amacrine cells of the salamander retina. J Neurosci 14, 3852–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D (2001). Is the retina going digital? Neuron 30, 303–305. [DOI] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, and Schwartz EA (1984). The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol 354, 557–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Lo AC, Black JA, and Waxman SG (2003). Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain : a journal of neurology 126, 1552–1561. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, and Waxman SG (2004). Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proceedings of the National Academy of Sciences of the United States of America 101, 8168–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven KB, and Zagotta WN (2006). CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68, 375–401. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Mizuno F, and Akopian A (2007). Disruption of actin cytoskeleton causes internalization of Ca(v)1.3 (alpha 1D) L-type calcium channels in salamander retinal neurons. Mol Vis 13, 1496–1507. [PubMed] [Google Scholar]
- Cui J, Ivanova E, Qi L, and Pan ZH (2012). Expression of CaV3.2 T-type Ca(2)(+) channels in a subpopulation of retinal type-3 cone bipolar cells. Neuroscience 224, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, and Pan ZH (2008). Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis Neurosci 25, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G, Toth ZE, and Enyedi P (2007). Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J Neurophysiol 98, 1213–1222. [DOI] [PubMed] [Google Scholar]
- Dacey DM (1988). Dopamine-accumulating retinal neurons revealed by in vitro fluorescence display a unique morphology. Science (New York, NY) 240, 1196–1198. [DOI] [PubMed] [Google Scholar]
- Dacey DM (1990). The dopaminergic amacrine cell. The Journal of Comparative Neurology 301, 461–489. [DOI] [PubMed] [Google Scholar]
- Dacey DM, and Lee BB (1994). The 'blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 367, 731–735. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, and Raviola E (1986). The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6, 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauner K, Mobus C, Frings S, and Mohrlen F (2013). Targeted expression of anoctamin calcium-activated chloride channels in rod photoreceptor terminals of the rodent retina. Invest Ophthalmol Vis Sci 54, 3126–3136. [DOI] [PubMed] [Google Scholar]
- Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, and Dolphin AC (2010). The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A 107, 1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, and Kaneko A (1998). Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. The European journal of neuroscience 10, 317–323. [DOI] [PubMed] [Google Scholar]
- De Sevilla Muller LP, Liu J, Solomon A, Rodriguez A, and Brecha NC (2013). Expression of voltage-gated calcium channel alpha(2)delta(4) subunits in the mouse and rat retina. J Comp Neurol 521, 2486–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Piano I, Cangiano L, Caputo A, Ludwig A, Cervetto L, and Gargini C (2012). Processing of retinal signals in normal and HCN deficient mice. PLoS One 7, e29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Kuo Sidney P., Yoshimatsu T, Okawa H, Suzuki Sachihiro C., Hoon M, Tsuboyama K, Rieke F, and Wong Rachel O.L. (2016). Glutamatergic Monopolar Interneurons Provide a Novel Pathway of Excitation in the Mouse Retina. Current Biology 26, 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, and Singer JH (2012). Intrinsic properties and functional circuitry of the AII amacrine cell. Visual neuroscience 29, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis GC, Gargini C, Paoli TG, and Cervetto L (2009). Selective Hcn1 channels inhibition by ivabradine in mouse rod photoreceptors. Invest Ophthalmol Vis Sci 50, 1948–1955. [DOI] [PubMed] [Google Scholar]
- Demontis GC, Longoni B, Barcaro U, and Cervetto L (1999). Properties and functional roles of hyperpolarization-gated currents in guinea-pig retinal rods. J Physiol 515 (Pt 3), 813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis GC, Moroni A, Gravante B, Altomare C, Longoni B, Cervetto L, and DiFrancesco D (2002). Functional characterisation and subcellular localisation of HCN1 channels in rabbit retinal rod photoreceptors. J Physiol 542, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH (2000). Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28, 847–856. [DOI] [PubMed] [Google Scholar]
- DeVries SH (2001). Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Dhingra NK (2005). Voltage-Gated Sodium Channels Improve Contrast Sensitivity of a Retinal Ganglion Cell. Journal of Neuroscience 25, 8097–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS (2017). Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annual review of vision science 3, 1–24. [DOI] [PubMed] [Google Scholar]
- Diamond JS, and Jahr CE (1995). Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron 15, 1097–1107. [DOI] [PubMed] [Google Scholar]
- Dick E, and Miller RF (1985). Extracellular K+ activity changes related to electroretinogram components. I. Amphibian (I-type) retinas. J Gen Physiol 85, 885–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick E, Miller RF, and Bloomfield S (1985). Extracellular K+ activity changes related to electroretinogram components. II. Rabbit (E-type) retinas. J Gen Physiol 85, 911–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DB, and Copenhagen DR (1997). Metabotropic glutamate receptor-mediated suppression of an inward rectifier current is linked via a cGMP cascade. J Neurosci 17, 8945–8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, and Copenhagen DR (1993). L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron 11, 267–277. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, and Stell WK (1984). Tetrodotoxin does not block the axonal transmission of S-potentials in goldfish retina. Neurosci Lett 49, 233–238. [DOI] [PubMed] [Google Scholar]
- Do MTH, and Yau KW (2013). Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proceedings of the National Academy of Sciences 110, 7470–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering CJ, Hamid J, Simms B, McRory JE, and Zamponi GW (2005). Cav1.4 encodes a calcium channel with low open probability and unitary conductance. Biophys J 89, 3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RP, and Schiller PH (1989). Evidence for only depolarizing rod bipolar cells in the primate retina. Visual neuroscience 2, 421–424. [DOI] [PubMed] [Google Scholar]
- Dolphin AC (2016). Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol 594, 5369–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, and Werblin FS (1995). Inwardly rectifying potassium conductance can accelerate the hyperpolarizing response in retinal horizontal cells. J Neurophysiol 74, 2258–2265. [DOI] [PubMed] [Google Scholar]
- Dowling JE, and Werblin FS (1969). Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. Journal of neurophysiology 32, 315–338. [DOI] [PubMed] [Google Scholar]
- Dreosti E, Esposti F, Baden T, and Lagnado L (2011). In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat Neurosci 14, 951–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, and Sanes JR (2014). Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158, 793–807. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, and Berson DM (2009). Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of Comparative Neurology 517, 226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA (2006). Controlling the Gain of Rod-Mediated Signals in the Mammalian Retina. Journal of Neuroscience 26, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, and Lukasiewicz PD (2011). Multiple pathways of inhibition shape bipolar cell responses in the retina. Visual neuroscience 28, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, and Lukasiewicz PD (2007). Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582, 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, Barnes S, and Werblin F (1987). The interaction of ionic currents mediating single spike activity in retinal amacrine cells of the tiger salamander. J Neurosci 7, 3512–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel AJ, and Do MT (2015). Melanopsin tristability for sustained and broadband phototransduction. Neuron 85, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel AJ, Kapur K, and Do MTH (2017). Biophysical Variation within the M1 Type of Ganglion Cell Photoreceptor. Cell Rep 21, 1048–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, and Waxman SG (1990). Current-clamp analysis of a time-dependent rectification in rat optic nerve. The Journal of Physiology 421, 185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, and Denk W (2002). Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845. [DOI] [PubMed] [Google Scholar]
- Euler T, and Masland RH (2000). Light-evoked responses of bipolar cells in a mammalian retina. Journal of neurophysiology 83, 1817–1829. [DOI] [PubMed] [Google Scholar]
- Fain GL, Quandt FN, Bastian BL, and Gerschenfeld HM (1978). Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature 272, 466–469. [DOI] [PubMed] [Google Scholar]
- Fain GL, Quandt FN, and Gerschenfeld HM (1977). Calcium-dependent regenerative responses in rods. Nature 269, 707–710. [DOI] [PubMed] [Google Scholar]
- Falzone ME, Malvezzi M, Lee BC, and Accardi A (2018). Known structures and unknown mechanisms of TMEM16 scramblases and channels. J Gen Physiol 150, 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S-F, and Yazulla S (2005). Reciprocal inhibition of voltage-gated potassium currents (IK(V)) by activation of cannabinoid CB1 and dopamine D1 receptors in ON bipolar cells of goldfish retina. Visual neuroscience 22, 55–63. [DOI] [PubMed] [Google Scholar]
- Farrell SR, Sargoy A, Brecha NC, and Barnes S (2014). Modulation of voltage-gated Ca2+ channels in rat retinal ganglion cells by gabapentin. Visual neuroscience 31, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Gustincich S, Bean BP, and Raviola E (1998). Spontaneous activity of solitary dopaminergic cells of the retina. J Neurosci 18, 6776–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Trumpler J, Dirks P, and Weiler R (2009). Ether-a-gogo-related gene (erg1) potassium channels shape the dark response of horizontal cells in the mammalian retina. Pflugers Arch 458, 359–377. [DOI] [PubMed] [Google Scholar]
- Feliciangeli S, Chatelain FC, Bichet D, and Lesage F (2015). The family of K2P channels: salient structural and functional properties. J Physiol 593, 2587–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK, and Morgans CW (2001). Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clin Exp Ophthalmol 29, 183–187. [DOI] [PubMed] [Google Scholar]
- Firth SI, Wang C-T, and Feller MB (2005). Retinal waves: mechanisms and function in visual system development. Cell Calcium 37, 425–432. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, and Wassle H (2001). Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21, 4852–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister JF, Coleman PA, and Miller RF (1990). Modeling the repetitive firing of retinal ganglion cells. Brain Research 510, 343–345. [DOI] [PubMed] [Google Scholar]
- Fohlmeister JF, and Miller RF (1997a). Impulse Encoding Mechanisms of Ganglion Cells in the Tiger Salamander Retina. Journal of neurophysiology 78, 1935–1947. [DOI] [PubMed] [Google Scholar]
- Fohlmeister JF, and Miller RF (1997b). Mechanisms by Which Cell Geometry Controls Repetitive Impulse Firing in Retinal Ganglion Cells. Journal of neurophysiology 78, 1948–1964. [DOI] [PubMed] [Google Scholar]
- Franke K, and Baden T (2017). General features of inhibition in the inner retina. J Physiol 595, 5507–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen JW, and Borghuis BG (2017). Temporally Diverse Excitation Generates Direction-Selective Responses in ON- and OFF-Type Retinal Starburst Amacrine Cells. Cell Rep 18, 1356–1365. [DOI] [PubMed] [Google Scholar]
- Freed MA, Pflug R, Kolb H, and Nelson R (1996). ON-OFF amacrine cells in cat retina. Journal of Comparative Neurology 364, 556–566. [DOI] [PubMed] [Google Scholar]
- Freed MA, and Sterling P (1988). The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci 8, 2303–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Field GD, Li PH, Greschner M, Gunning DE, Mathieson K, Sher A, Litke AM, Paninski L, Simoncelli EP, et al. (2015). Mapping nonlinear receptive field structure in primate retina at single cone resolution. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Brull N, Dzeja C, Angele A, Hagen V, Kaupp UB, and Baumann A (1998). Characterization of ether-a-go-go channels present in photoreceptors reveals similarity to IKx, a K+ current in rod inner segments. J Gen Physiol 111, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, and Yau KW (2007). Phototransduction in mouse rods and cones. Pflugers Arch 454, 805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyk-Kolodziej B, and Pourcho RG (2007). Differential distribution of hyperpolarization-activated and cyclic nucleotide-gated channels in cone bipolar cells of the rat retina. J Comp Neurol 501, 891–903. [DOI] [PubMed] [Google Scholar]
- Gallego A (1986). Comparative studies on horizontal cells and a note on microglial cells. Progress in Retinal Research 5, 165–206. [Google Scholar]
- Gayet-Primo J, Yaeger DB, Khanjian RA, and Puthussery T (2018). Heteromeric KV2/KV8.2 Channels Mediate Delayed Rectifier Potassium Currents in Primate Photoreceptors. J Neurosci 38, 3414–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauner KS, Mannuzzu LM, Gandhi CS, and Isacoff EY (1999). Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature 402, 813–817. [DOI] [PubMed] [Google Scholar]
- Gleason E, Borges S, and Wilson M (1994). Control of transmitter release from retinal amacrine cells by Ca2+ influx and efflux. Neuron 13, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Golard A, Witkovsky P, and Tranchina D (1992). Membrane currents of horizontal cells isolated from turtle retina. J Neurophysiol 68, 351–361. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Wang T, Gillette MU, and Cox CL (2012). Activity-Dependent Regulation of Retinogeniculate Signaling by Metabotropic Glutamate Receptors. Journal of Neuroscience 32, 12820–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmeyer JJ, and Thoreson WB (2017). Synaptic Ribbon Active Zones in Cone Photoreceptors Operate Independently from One Another. Front Cell Neurosci 11, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DL, and Hoshi N (2017). Modulation of Kv7 channels and excitability in the brain. Cell Mol Life Sci 74, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene MJ, Kim JS, Seung HS, and EyeWirers (2016). Analogous Convergence of Sustained and Transient Inputs in Parallel On and Off Pathways for Retinal Motion Computation. Cell Rep 14, 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greschner M, Field GD, Li PH, Schiff ML, Gauthier JL, Ahn D, Sher A, Litke AM, and Chichilnisky EJ (2014). A polyaxonal amacrine cell population in the primate retina. J Neurosci 34, 3597–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE, and Diamond JS (2009). BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci 12, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Seal RP, Oesch N, Edwards RH, and Diamond JS (2011). Genetic targeting and physiological features of VGLUT3+ amacrine cells. Visual neuroscience 28, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, and Diamond JS (2010). Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron 65, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Tian H, Graydon CW, Hoon M, Rieke F, and Diamond JS (2015). Complex inhibitory microcircuitry regulates retinal signaling near visual threshold. Journal of neurophysiology 114, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther E, Rothe T, Taschenberger H, and Grantyn R (1994). Separation of calcium currents in retinal ganglion cells from postnatal rat. Brain Research 633, 223–235. [DOI] [PubMed] [Google Scholar]
- Guenther E, Schmid S, Reiff D, and Zrenner E (1999). Maturation of intrinsic membrane properties in rat retinal ganglion cells. Vision Res 39, 2477–2484. [DOI] [PubMed] [Google Scholar]
- Ha GE, and Cheong E (2017). Spike Frequency Adaptation in Neurons of the Central Nervous System. Experimental Neurobiology 26, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann CJ, O'Brien BJ, Wässle H, and Protti DA (2003). AII Amacrine Cells Express L-Type Calcium Channels at Their Output Synapses. The Journal of Neuroscience 23, 6904–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, and Palczewski K (2004). Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci 7, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Williams B, and Lee A (2016). Characterization of C-terminal Splice Variants of Cav1.4 Ca2+ Channels in Human Retina. J Biol Chem 291, 15663–15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Jacoby RA, and Wu SM (2000). Morphological and electrophysiological properties of dissociated primate retinal cells. Brain Res 875, 175–186. [DOI] [PubMed] [Google Scholar]
- Hart J, Wilkinson MF, Kelly ME, and Barnes S (2003). Inhibitory action of diltiazem on voltage-gated calcium channels in cone photoreceptors. Exp Eye Res 76, 597–604. [DOI] [PubMed] [Google Scholar]
- Hartl D, Krebs AR, Juttner J, Roska B, and Schubeler D (2017). Cis-regulatory landscapes of four cell types of the retina. Nucleic Acids Res 45, 11607–11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E (1999). Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. Journal of neurophysiology 81, 2923–2936. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Qu Z, Yu K, Xiao Q, and Chien LT (2008). Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev 88, 639–672. [DOI] [PubMed] [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, and Denk W (2007). A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol 5, e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, and Ishida AT (2004). Dopamine Receptor Activation Can Reduce Voltage-Gated Na + Current by Modulating Both Entry Into and Recovery From Inactivation. Journal of neurophysiology 92, 3134–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Rodriguez CV, Ogata G, Partida GJ, Oi H, Stradleigh TW, Lee SC, Colado AF, and Ishida AT (2009). Inhibition of Adult Rat Retinal Ganglion Cells by D1-Type Dopamine Receptor Activation. Journal of Neuroscience 29, 15001–15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin SJ, and Cook PB (2007). Narrow and wide field amacrine cells fire action potentials in response to depolarization and light stimulation. Vis Neurosci 24, 197–206. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, and Matthews G (1992). Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol 447, 235–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmer CB, Zhou Y, Fyk-Kolodziej B, Hu Z, and Ichinose T (2016). Morphological and physiological analysis of type-5 and other bipolar cells in the Mouse Retina. Neuroscience 315, 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, and Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168. [DOI] [PubMed] [Google Scholar]
- Henderson D, Doerr TA, Gottesman J, and Miller RF (2001). Calcium channel immunoreactivity in the salamander retina. Neuroreport 12, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Henderson D, and Miller RF (2003). Evidence for low-voltage-activated (LVA) calcium currents in the dendrites of tiger salamander retinal ganglion cells. Visual neuroscience 20, 141–152. [DOI] [PubMed] [Google Scholar]
- Henderson D, and Miller RF (2007). Low-voltage activated calcium currents in ganglion cells of the tiger salamander retina: Experiment and simulation. Visual neuroscience 24, 37–51. [DOI] [PubMed] [Google Scholar]
- Henne J, and Jeserich G (2004). Maturation of spiking activity in trout retinal ganglion cells coincides with upregulation of Kv3.1- and BK-related potassium channels. Journal of Neuroscience Research 75, 44–54. [DOI] [PubMed] [Google Scholar]
- Henne J, Pottering S, and Jeserich G (2000). Voltage-gated potassium channels in retinal ganglion cells of trout: A combined biophysical, pharmacological, and single-cell RT-PCR approach. Journal of Neuroscience Research 62, 629–637. [DOI] [PubMed] [Google Scholar]
- Hestrin S (1987). The properties and function of inward rectification in rod photoreceptors of the tiger salamander. J Physiol 390, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, and Kurachi Y (2010). Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90, 291–366. [DOI] [PubMed] [Google Scholar]
- Hicks GA, and Marrion NV (1998). Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol 508 (Pt 3), 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001). Ion channels of excitable membranes, 3rd edn (Sunderland, Mass: Sinauer; ). [Google Scholar]
- Hirasawa H, Betensky RA, and Raviola E (2012). Corelease of Dopamine and GABA by a Retinal Dopaminergic Neuron. Journal of Neuroscience 32, 13281–13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, and Kaneko A (2003). pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol 122, 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Puopolo M, and Raviola E (2009). Extrasynaptic Release of GABA by Retinal Dopaminergic Neurons. Journal of neurophysiology 102, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite RK, Yuan P, Li Z, Hsuing Y, Walz T, and MacKinnon R (2015). Cryo-electron microscopy structure of the Slo2.2 Na(+)-activated K(+) channel. Nature 527, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, and Konnerth A (2006). Dendritic spikes and activity-dependent synaptic plasticity. Cell and Tissue Research 326, 369–377. [DOI] [PubMed] [Google Scholar]
- Horio K, Ohkuma M, and Miyachi E.-i. (2018). The Effect of Histamine on Inward and Outward Currents in Mouse Retinal Amacrine Cells. Cellular and Molecular Neurobiology 38, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, and Mills SL (2009). ON Inputs to the OFF Layer: Bipolar Cells That Break the Stratification Rules of the Retina. Journal of Neuroscience 29, 8875–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Arai I, and Tachibana M (2005). Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci 25, 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett MH, Smith RG, and Kamermans M (2017). A novel mechanism of cone photoreceptor adaptation. PLoS Biol 15, e2001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Bi A, and Pan ZH (2009). Differential expression of three T-type calcium channels in retinal bipolar cells in rats. Visual neuroscience 26, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Hill DD, and Wong KY (2013). Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. Journal of neurophysiology 109, 1876–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, and Pan ZH (2002). Differential expression of K+ currents in mammalian retinal bipolar cells. Visual neuroscience 19, 163–173. [DOI] [PubMed] [Google Scholar]
- Huba R, Schneider H, and Hofmann HD (1992). Voltage-gated currents of putative GABAergic amacrine cells in primary cultures and in retinal slice preparations. Brain Res 577, 10–18. [DOI] [PubMed] [Google Scholar]
- Hughes S, Foster RG, Peirson SN, and Hankins MW (2017). Expression and localisation of two-pore domain (K2P) background leak potassium ion channels in the mouse retina. Sci Rep 7, 46085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Fyk-Kolodziej B, and Cohn J (2014). Roles of ON cone bipolar cell subtypes in temporal coding in the mouse retina. J Neurosci 34, 8761–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, and Hellmer CB (2016). Differential signalling and glutamate receptor compositions in the OFF bipolar cell types in the mouse retina. J Physiol 594, 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, and Lukasiewicz PD (2007). Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci 27, 4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, and Müller F (2006). Retinal bipolar cell types differ in their inventory of ion channels. Visual neuroscience 23, 143–154. [DOI] [PubMed] [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, and Kramer RH (2009). Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci 12, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Alexander C., and Nicoll Roger A. (2011). The Expanding Social Network of Ionotropic Glutamate Receptors: TARPs and Other Transmembrane Auxiliary Subunits. Neuron 70, 178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, and McMahon DG (2012). Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Zhu Y, DeVries SH, and Schwartz GW (2015). An Amacrine Cell Circuit for Signaling Steady Illumination in the Retina. Cell Rep 13, 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ZM, and Zagotta WN (2018). Structural insights into the mechanisms of CNBD channel function. J Gen Physiol 150, 225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, and Singer JH (2010). Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci 30, 11885–11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RJ (1995a). Effects of Ca2+ channel blockers on directional selectivity of rabbit retinal ganglion cells. Journal of neurophysiology 74, 12–23. [DOI] [PubMed] [Google Scholar]
- Jensen RJ (1995b). Receptive-field properties of displaced starburst amacrine cells change following axotomy-induced degeneration of ganglion cells. Visual neuroscience 12, 177–184. [DOI] [PubMed] [Google Scholar]
- Jeon JH, Paik SS, Chun MH, Oh U, and Kim IB (2013). Presynaptic Localization and Possible Function of Calcium-Activated Chloride Channel Anoctamin 1 in the Mammalian Retina. PLoS One 8, e67989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian K, Barhoumi R, Ko ML, and Ko GY (2009). Inhibitory effect of somatostatin-14 on L-type voltage-gated calcium channels in cultured cone photoreceptors requires intracellular calcium. J Neurophysiol 102, 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ruta V, Chen J, Lee A, and MacKinnon R (2003). The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423, 42–48. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Yue WWS, Chen L, Sheng Y, and Yau K-W (2018). Cyclic-Nucleotide- and HCN-Channel-Mediated Phototransduction in Intrinsically Photosensitive Retinal Ganglion Cells. Cell 175, 652–664.e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA, Guziewicz KE, Lee CJ, Kalathur RC, Pulido JS, Marmorstein LY, and Marmorstein AD (2017). Bestrophin 1 and retinal disease. Prog Retin Eye Res 58, 45–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, and Lam DM (1981). Regenerative and passive membrane properties of isolated horizontal cells from a teleost retina. Nature 292, 451–454. [DOI] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, and Christie BR (1996). Active Properties of Neuronal Dendrites. Annual review of neuroscience 19, 165–186. [DOI] [PubMed] [Google Scholar]
- Jones Rebecca S., Carroll Reed C., and Nawy S (2012). Light-Induced Plasticity of Synaptic AMPA Receptor Composition in Retinal Ganglion Cells. Neuron 75, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonz MG, and Barnes S (2007). Proton modulation of ion channels in isolated horizontal cells of the goldfish retina. J Physiol 581, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK (2013). Slack, Slick and Sodium-Activated Potassium Channels. ISRN Neurosci 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, and Wulff H (2017). International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels. Pharmacol Rev 69, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama Y, Ogura T, and Usui S (1996). Ionic current model of the vertebrate rod photoreceptor. Vision Res 36, 4059–4068. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, and Hendriksen H (1998). Expression patterns of voltage-dependent calcium channel alpha 1 subunits (alpha 1A-alpha 1E) mRNA in rat retina. Brain Res Mol Brain Res 55, 209–220. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Ito K, Morishima Y, Shigematsu Y, and Shimoda Y (2007). Characterization of Voltage-Gated Ionic Channels in Cholinergic Amacrine Cells in the Mouse Retina. Journal of neurophysiology 97, 4225–4234. [DOI] [PubMed] [Google Scholar]
- Kaneda M, and Kaneko A (1991a). Voltage-gated calcium currents in isolated retinal ganglion cells of the cat. Jpn J Physiol 41, 35–48. [DOI] [PubMed] [Google Scholar]
- Kaneda M, and Kaneko A (1991b). Voltage-gated sodium currents in isolated retinal ganglion cells of the cat: relation between the inactivation kinetics and the cell type. Neuroscience research 11, 261–275. [DOI] [PubMed] [Google Scholar]
- Kaneko A (1970). Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol 207, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Pinto LH, and Tachibana M (1989). Transient calcium current of retinal bipolar cells of the mouse. Journal of Physiology 410, 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, and Tachibana M (1985a). Effects of L-glutamate on the anomalous rectifier potassium current in horizontal cells of Carassius auratus retina. J Physiol 358, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, and Tachibana M (1985b). A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol 358, 131–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, and Watanabe S-I (2007). Expression of Nav1.1 in rat retinal AII amacrine cells. Neuroscience Letters 424, 83–88. [DOI] [PubMed] [Google Scholar]
- Karschin A, and Lipton SA (1989). Calcium channels in solitary retinal ganglion cells from post-natal rat. The Journal of Physiology 418, 379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A, and Wassle H (1990). Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. Journal of neurophysiology 63, 860–876. [DOI] [PubMed] [Google Scholar]
- Karwoski CJ, Newman EA, Shimazaki H, and Proenza LM (1985). Light-evoked increases in extracellular K+ in the plexiform layers of amphibian retinas. J Gen Physiol 86, 189–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar R, Weissgerber P, Roth E, Dorr J, Sothilingam V, Garcia Garrido M, Beck SC, Seeliger MW, Beck A, Schmitz F, et al. (2015). Influence of the beta2-Subunit of L-Type Voltage-Gated Cav Channels on the Structural and Functional Development of Photoreceptor Ribbon Synapses. Invest Ophthalmol Vis Sci 56, 2312–2324. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, and Seifert R (2002). Cyclic nucleotide-gated ion channels. Physiol Rev 82, 769–824. [DOI] [PubMed] [Google Scholar]
- Kaur T, and Nawy S (2012). Characterization of Trpm1 desensitization in ON bipolar cells and its role in downstream signalling. The Journal of Physiology 590, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Ichinose H, Ohkuma M, Isobe R, and Miyachi E (2005). Suppression by an h current of spontaneous Na+ action potentials in human cone and rod photoreceptors. Invest Ophthalmol Vis Sci 46, 390–397. [DOI] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Suzuki H, and Miyachi E (2001). Na(+) action potentials in human photoreceptors. Neuron 30, 451–458. [DOI] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Suzuki H, and Miyachi E (2002). Modulation by hyperpolarization-activated cationic currents of voltage responses in human rods. Brain Res 943, 48–55. [DOI] [PubMed] [Google Scholar]
- Keeley PW, and Reese BE (2009). Morphology of dopaminergic amacrine cells in the mouse retina: Independence from homotypic interactions. The Journal of Comparative Neurology, 518, 1220–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerov V, Laird JG, Joiner ML, Knecht S, Soh D, Hagen J, Gardner SH, Gutierrez W, Yoshimatsu T, Bhattarai S, et al. (2018). alpha2delta-4 Is Required for the Molecular and Structural Organization of Rod and Cone Photoreceptor Synapses. J Neurosci 38, 6145–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D (2016). Glutamatergic Retinal Waves. Frontiers in Neural Circuits 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten FF, van Wijk E, van Reeuwijk J, van der Zwaag B, Marker T, Peters TA, Katsanis N, Wolfrum U, Keunen JE, Roepman R, et al. (2010). Association of whirlin with Cav1.3 (alpha1D) channels in photoreceptors, defining a novel member of the usher protein network. Invest Ophthalmol Vis Sci 51, 2338–2346. [DOI] [PubMed] [Google Scholar]
- Khan AO, Alrashed M, and Alkuraya FS (2013). Clinical characterisation of the CABP4-related retinal phenotype. Br J Ophthalmol 97, 262–265. [DOI] [PubMed] [Google Scholar]
- Kim DM, and Nimigean CM (2016). Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, Turaga SC, Purcaro M, Balkam M, Robinson A, Behabadi BF, et al. (2014). Space-time wiring specificity supports direction selectivity in the retina. Nature 509, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöcker N, Oliver D, Ruppersberg JP, Knaus H-G, and Fakler B (2001). Developmental Expression of the Small-Conductance Ca2+-Activated Potassium Channel SK2 in the Rat Retina. Molecular and Cellular Neuroscience 17, 514–520. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, Ito S, Sheng MH, Jan LY, and Pinto LH (1995a). The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current. J Neurosci 15, 5004–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, and Pinto LH (1995b). Identification and localization of K+ channels in the mouse retina. Vis Neurosci 12, 1177–1190. [DOI] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, and Ko GY (2007). The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem 103, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Shi L, Huang CC, Grushin K, Park SY, and Ko GY (2013). Circadian phase-dependent effect of nitric oxide on L-type voltage-gated calcium channels in avian cone photoreceptors. J Neurochem 127, 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle PD, and Schlichter LC (2010). Targeting K(V) channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels (Austin, Tex) 4, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle PD, Wang Y, and Schlichter LC (2010). Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death & Differentiation 17, 134–144. [DOI] [PubMed] [Google Scholar]
- Kofuji P, and Newman EA (2004). Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, et al. (2010). TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proceedings of the National Academy of Sciences 107, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Jakobs TC, and Masland RH (2004). Inward rectifying currents stabilize the membrane potential in dendrites of mouse amacrine cells: patch-clamp recordings and single-cell RT-PCR. Molecular vision 10, 328–340. [PubMed] [Google Scholar]
- Koren D, Grove JCR, and Wei W (2017). Cross-compartmental Modulation of Dendritic Signals for Retinal Direction Selectivity. Neuron 95, 914–927 e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M, and Striessnig J (2003). Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci 23, 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotturi MF, and Jefferies WA (2005). Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol 42, 1461–1474. [DOI] [PubMed] [Google Scholar]
- Kourennyi DE, Liu XD, Hart J, Mahmud F, Baldridge WH, and Barnes S (2004). Reciprocal modulation of calcium dynamics at rod and cone photoreceptor synapses by nitric oxide. J Neurophysiol 92, 477–483. [DOI] [PubMed] [Google Scholar]
- Krizaj D (2012). Calcium stores in vertebrate photoreceptors. Adv Exp Med Biol 740, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatri AS, Gonzalez-Hernandez A, and Giraldez T (2018). Physiological Roles and Therapeutic Potential of Ca(2+) Activated Potassium Channels in the Nervous System. Front Mol Neurosci 11, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Q, Purhonen P, and Hebert H (2015). Structure of potassium channels. Cell Mol Life Sci 72, 3677–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K (2015). TMEM16, LRRC8A, bestrophin: chloride channels controlled by Ca(2+) and cell volume. Trends Biochem Sci 40, 535–543. [DOI] [PubMed] [Google Scholar]
- Kurata HT, and Fedida D (2006). A structural interpretation of voltage-gated potassium channel inactivation. Progress in Biophysics and Molecular Biology 92, 185–208. [DOI] [PubMed] [Google Scholar]
- Kurenny DE, Moroz LL, Turner RW, Sharkey KA, and Barnes S (1994). Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron 13, 315–324. [DOI] [PubMed] [Google Scholar]
- Kurennyi DE, and Barnes S (1997). Regulation of M-like K+ current, IKx, by Ca(2+)-dependent phosphorylation in rod photoreceptors. Am J Physiol 272, C1844–1853. [DOI] [PubMed] [Google Scholar]
- Lam YW, and Sherman SM (2013). Activation of both Group I and Group II metabotropic glutamatergic receptors suppress retinogeniculate transmission. Neuroscience 242, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A (1986). Depolarizing responses of turtle rods. Neurosci Res Suppl 4, S59–67. [DOI] [PubMed] [Google Scholar]
- Lasater EM (1986). Ionic currents of cultured horizontal cells isolated from white perch retina. J Neurophysiol 55, 499–513. [DOI] [PubMed] [Google Scholar]
- Lasater EM (1988). Membrane currents of retinal bipolar cells in culture. Journal of neurophysiology 60, 1460–1480. [DOI] [PubMed] [Google Scholar]
- Lasater EM, and Witkovsky P (1990). Membrane currents of spiking cells isolated from turtle retina. J Comp Physiol A 167, 11–21. [DOI] [PubMed] [Google Scholar]
- Lasater EM, and Witkovsky P (1991). The calcium current of turtle cone photoreceptor axon terminals. Neurosci Res Suppl 15, S165–173. [PubMed] [Google Scholar]
- Latorre R, Castillo K, Carrasquel-Ursulaez W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C, and Alvarez O (2017). Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol Rev 97, 39–87. [DOI] [PubMed] [Google Scholar]
- Lee A, Wang S, Williams B, Hagen J, Scheetz TE, and Haeseleer F (2015). Characterization of Cav1.4 complexes (alpha11.4, beta2, and alpha2delta4) in HEK293T cells and in the retina. J Biol Chem 290, 1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, and MacKinnon R (2017). Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 168, 111–120 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, and Zhou ZJ (2010). Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang Y, Chen M, and Zhou ZJ (2016). Segregated Glycine-Glutamate Co-transmission from vGluT3 Amacrine Cells to Contrast-Suppressed and Contrast-Enhanced Retinal Circuits. Neuron 90, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, and Zhou ZJ (2006). The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51, 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Hayashida Y, and Ishida AT (2003). Availability of Low-Threshold Ca 2+ Current in Retinal Ganglion Cells. Journal of neurophysiology 90, 3888–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, and Ishida AT (2007). I h Without K ir in Adult Rat Retinal Ganglion Cells. Journal of neurophysiology 97, 3790–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee US, and Cui J (2010). BK channel activation: structural and functional insights. Trends in Neurosciences 33, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V, and Grinvald A (1986). Ca2+- and K+-dependent communication between central nervous system myelinated axons and oligodendrocytes revealed by voltage-sensitive dyes. Proceedings of the National Academy of Sciences of the United States of America 83, 6651–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, and DeVries SH (2006). Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat Neurosci 9, 669–675. [DOI] [PubMed] [Google Scholar]
- Linn CL, and Gafka AC (1999). Activation of metabotropic glutamate receptors modulates the voltage-gated sustained calcium current in a teleost horizontal cell. J Neurophysiol 81, 425–434. [DOI] [PubMed] [Google Scholar]
- Lipton SA, and Tauck DL (1987). Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. The Journal of Physiology 385, 361–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littink KW, van Genderen MM, Collin RW, Roosing S, de Brouwer AP, Riemslag FC, Venselaar H, Thiadens AA, Hoyng CB, Rohrschneider K, et al. (2009). A novel homozygous nonsense mutation in CABP4 causes congenital cone-rod synaptic disorder. Invest Ophthalmol Vis Sci 50, 2344–2350. [DOI] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC, and Barnes S (2016). Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, and Barnes S (2013a). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol 591, 3309–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA, and Lee A (2013b). Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin) 7, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet A, Cooke A, and Lagnado L (2003). Exocytosis at the ribbon synapse of retinal bipolar cells studied in patches of presynaptic membrane. J Neurosci 23, 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logiudice L, Henry D, and Matthews G (2006). Identification of calcium channel alpha1 subunit mRNA expressed in retinal bipolar neurons. Molecular vision 12, 184–189. [PMC free article] [PubMed] [Google Scholar]
- Lohrke S, and Hofmann HD (1994). Voltage-gated currents of rabbit A- and B-type horizontal cells in retinal monolayer cultures. Vis Neurosci 11, 369–378. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, and Biel M (1998). A family of hyperpolarization-activated mammalian cation channels. Nature 393, 587–591. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P, and Werblin F (1988). A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci 8, 4470–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Gould TJ, Bewersdorf J, and Zenisek D (2012). High-resolution optical imaging of zebrafish larval ribbon synapse protein RIBEYE, RIM2, and CaV 1.4 by stimulation emission depletion microscopy. Microsc Microanal 18, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YP, Cui J, and Pan ZH (2005). Heterogeneous expression of voltage-dependent Na+ and K+ channels in mammalian retinal bipolar cells. Vis Neurosci 22, 119–133. [DOI] [PubMed] [Google Scholar]
- MacLeish PR, and Nurse CA (2007). Ion channel compartments in photoreceptors: evidence from salamander rods with intact and ablated terminals. J Neurophysiol 98, 86–95. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, and Masland RH (1999). The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. The Journal of Comparative Neurology 413, 305–326. [PubMed] [Google Scholar]
- MacNeil MA, and Masland RH (1998). Extreme Diversity among Amacrine Cells: Implications for Function. Neuron 20, 971–982. [DOI] [PubMed] [Google Scholar]
- Maeda T, Lem J, Palczewski K, and Haeseleer F (2005). A critical role of CaBP4 in the cone synapse. Invest Ophthalmol Vis Sci 46, 4320–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G (1999). Spatial heterogeneity and function of voltage– and ligand–gated ion channels in retinal amacrine neurons. Proceedings of the Royal Society of London Series B: Biological Sciences 266, 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Qian HH, Ripps H, and Dowling JE (1990). Structural and functional properties of two types of horizontal cell in the skate retina. J Gen Physiol 95, 177–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm AT, Kourennyi DE, and Barnes S (2003). Protons and calcium alter gating of the hyperpolarization-activated cation current (I(h)) in rod photoreceptors. Biochim Biophys Acta 1609, 183–192. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, and Demb JB (2008). Disinhibition Combines with Excitation to Extend the Operating Range of the OFF Visual Pathway in Daylight. Journal of Neuroscience 28, 4136–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, and Bech-Hansen NT (2005). Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet 14, 3035–3046. [DOI] [PubMed] [Google Scholar]
- Mao BQ, MacLeish PR, and Victor JD (2003). Role of hyperpolarization-activated currents for the intrinsic dynamics of isolated retinal neurons. Biophys J 84, 2756–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, and Detwiler PB (2007). Different Mechanisms Generate Maintained Activity in ON and OFF Retinal Ganglion Cells. Journal of Neuroscience 27, 5994–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Euler T, and Detwiler PB (2010). Dendritic Calcium Signaling in ON and OFF Mouse Retinal Ganglion Cells. Journal of Neuroscience 30, 7127–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Singer JH, and Detwiler PB (2014). Network oscillations drive correlated spiking of ON and OFF ganglion cells in the rd1 mouse model of retinal degeneration. PLoS One 9, e86253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Newkirk G, Euler T, and Detwiler PB (2008). Functional Stability of Retinal Ganglion Cells after Degeneration-Induced Changes in Synaptic Input. Journal of Neuroscience 28, 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP (1984). Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature 308, 184–186. [DOI] [PubMed] [Google Scholar]
- Maricq AV, and Korenbrot JI (1988). Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron 1, 503–515. [DOI] [PubMed] [Google Scholar]
- Maricq AV, and Korenbrot JI (1990a). Inward rectification in the inner segment of single retinal cone photoreceptors. J Neurophysiol 64, 1917–1928. [DOI] [PubMed] [Google Scholar]
- Maricq AV, and Korenbrot JI (1990b). Potassium currents in the inner segment of single retinal cone photoreceptors. J Neurophysiol 64, 1929–1940. [DOI] [PubMed] [Google Scholar]
- Masland Richard H. (2012a). The Neuronal Organization of the Retina. Neuron 76, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH (2012b). The tasks of amacrine cells. Visual neuroscience 29, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. (1995). Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80, 757–765. [DOI] [PubMed] [Google Scholar]
- Mauss AS, Vlasits A, Borst A, and Feller M (2017). Visual Circuits for Direction Selectivity. Annual review of neuroscience 40, 211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRory JE, Hamid J, Doering CJ, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle AM, Feldcamp L, et al. (2004). The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci 24, 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, and Wässle H (1998). Glycinergic amacrine cells of the rat retina. The Journal of Comparative Neurology 401, 34–46. [DOI] [PubMed] [Google Scholar]
- Menger N, and Wassle H (2000). Morphological and physiological properties of the A17 amacrine cell of the rat retina. Visual neuroscience 17, 769–780. [DOI] [PubMed] [Google Scholar]
- Mennerick S, and Matthews G (1996). Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Mercer AJ, Chen M, and Thoreson WB (2011a). Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J Neurosci 31, 4397–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Rabl K, Riccardi GE, Brecha NC, Stella SL Jr., and Thoreson WB (2011b). Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. J Neurophysiol 105, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Becirovic E, and Biel M (2018). Retinal Cyclic Nucleotide-Gated Channels: From Pathophysiology to Therapy. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Angelides KJ, and Chalupa LM (1995). Distinct temporal patterns of expression of sodium channel-like immunoreactivity during the prenatal development of the monkey and cat retina. The European journal of neuroscience 7, 535–546. [DOI] [PubMed] [Google Scholar]
- Miller AN, and Long SB (2012). Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436. [DOI] [PubMed] [Google Scholar]
- Miller RF, and Bloomfield SA (1983). Electroanatomy of a unique amacrine cell in the rabbit retina. Proceedings of the National Academy of Sciences of the United States of America 80, 3069–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, and Dacheux R (1976). Dendritic and somatic spikes in mudpuppy amacrine cells: indentification and TTX sensitivity. Brain Res 104, 157–162. [DOI] [PubMed] [Google Scholar]
- Miller RF, Staff NP, and Velte TJ (2006). Form and function of ON-OFF amacrine cells in the amphibian retina. J Neurophysiol 95, 3171–3190. [DOI] [PubMed] [Google Scholar]
- Milner ES, and Do MTH (2017). A Population Representation of Absolute Light Intensity in the Mammalian Retina. Cell 171, 865–876.e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, and Miller RF (2007a). Mechanism underlying rebound excitation in retinal ganglion cells. Visual neuroscience 24, 709–731. [DOI] [PubMed] [Google Scholar]
- Mitra P, and Miller RF (2007b). Normal and rebound impulse firing in retinal ganglion cells. Visual neuroscience 24, 79–90. [DOI] [PubMed] [Google Scholar]
- Mitra P, and Slaughter MM (2002). Mechanism of generation of spontaneous miniature outward currents (SMOCs) in retinal amacrine cells. J Gen Physiol 119, 355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi E, and Murakami M (1989). Decoupling of horizontal cells in carp and turtle retinae by intracellular injection of cyclic AMP. J Physiol 419, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo A, Luo C, Davis FP, Mukamel EA, Henry GL, Nery JR, Urich MA, Picard S, Lister R, Eddy SR, et al. (2016). Epigenomic landscapes of retinal rods and cones. Elife 5, e11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumder DK, Frishman LJ, Otteson DC, and Sherry DM (2007). Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis 13, 2163–2182. [PubMed] [Google Scholar]
- Mojumder DK, Sherry DM, and Frishman LJ (2008). Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol 586, 2551–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Irwin RP, and Allen CN (2006). Presynaptic GABA B Receptors Regulate Retinohypothalamic Tract Synaptic Transmission by Inhibiting Voltage-Gated Ca 2+ Channels. Journal of neurophysiology 95, 3727–3741. [DOI] [PubMed] [Google Scholar]
- Moore-Dotson JM, Klein JS, Mazade RE, and Eggers ED (2015). Different types of retinal inhibition have distinct neurotransmitter release properties. Journal of neurophysiology 113, 2078–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, and Ludwig A (2001). Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. European journal of biochemistry 268, 1646–1652. [DOI] [PubMed] [Google Scholar]
- Morgans CW (1999). Calcium channel heterogeneity among cone photoreceptors in the tree shrew retina. Eur J Neurosci 11, 2989–2993. [DOI] [PubMed] [Google Scholar]
- Morgans CW (2001). Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 42, 2414–2418. [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, and Taylor WR (2005). Photoreceptor calcium channels: insight from night blindness. Vis Neurosci 22, 561–568. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Gaughwin P, and Maleszka R (2001). Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Mol Vis 7, 202–209. [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, and Brown RL (2009). TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A 106, 19174–19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriondo A, Pelucchi B, and Rispoli G (2001). Calcium-activated potassium current clamps the dark potential of vertebrate rods. Eur J Neurosci 14, 19–26. [DOI] [PubMed] [Google Scholar]
- Mørkve SH, Veruki ML, and Hartveit E (2002). Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. The Journal of Physiology 542, 147–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrie RD, and Feller MB (2018). A Dense Starburst Plexus Is Critical for Generating Direction Selectivity. Curr Biol 28, 1204–1212 e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, and Kaupp UB (2003). HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. European Journal of Neuroscience 17, 2084–2096. [DOI] [PubMed] [Google Scholar]
- Murakami M, and Takahashi K (1987). Calcium action potential and its use for measurement of reversal potentials of horizontal cell responses in carp retina. J Physiol 386, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Baum BL, and Taylor WR (2015). The Synaptic and Morphological Basis of Orientation Selectivity in a Polyaxonal Amacrine Cell of the Rabbit Retina. J Neurosci 35, 13336–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, and Rieke F (2011). Electrical synaptic input to ganglion cells underlies differences in the output and absolute sensitivity of parallel retinal circuits. J Neurosci 31, 12218–12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman-Clewner M, St Jules R, and Townes-Anderson E (1999). L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J Comp Neurol 415, 1–16. [PubMed] [Google Scholar]
- Nawy S, and Copenhagen DR (1987). Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature 325, 56–58. [DOI] [PubMed] [Google Scholar]
- Nelson R (1982). AII amacrine cells quicken time course of rod signals in the cat retina. Journal of neurophysiology 47, 928–947. [DOI] [PubMed] [Google Scholar]
- Nelson R, and Kolb H (1985). A17: a broad-field amacrine cell in the rod system of the cat retina. Journal of neurophysiology 54, 592–614. [DOI] [PubMed] [Google Scholar]
- Newkirk GS, Hoon M, Wong RO, and Detwiler PB (2013). Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. Journal of neurophysiology 110, 536–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA (1993). Inward-rectifying potassium channels in retinal glial (Muller) cells. J Neurosci 13, 3333–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien BJ, Caldwell JH, Ehring GR, Bumsted O'Brien KM, Luo S, and Levinson SR (2008). Tetrodotoxin-resistant voltage-gated sodium channels Nav1.8 and Nav1.9 are expressed in the retina. Journal of Comparative Neurology 508, 940–951. [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Isayama T, Richardson R, and Berson DM (2002). Intrinsic physiological properties of cat retinal ganglion cells. The Journal of Physiology 538, 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch N, Euler T, and Taylor WR (2005). Direction-Selective Dendritic Action Potentials in Rabbit Retina. Neuron 47, 739–750. [DOI] [PubMed] [Google Scholar]
- Oesch NW, and Diamond JS (2011). Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nature Neuroscience 14, 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch NW, and Taylor WR (2010). Tetrodotoxin-resistant sodium channels contribute to directional responses in starburst amacrine cells. PLoS One 5, e12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata G, Stradleigh TW, Partida GJ, and Ishida AT (2012). Dopamine and full-field illumination activate D1 and D2-D5-type receptors in adult rat retinal ganglion cells. Journal of Comparative Neurology 520, 4032–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Petit-Jacques J, Volgyi B, Ho CS, Joho RH, Bloomfield SA, and Rudy B (2004). A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J Neurosci 24, 7335–7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlberg J, Frederiksen R, Pollock GE, Miyagishima KJ, Sampath AP, and Cornwall MC (2017). Voltage-sensitive conductances increase the sensitivity of rod photoresponses following pigment bleaching. J Physiol 595, 3459–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ (2006). Modulation of Ca(2+)-activated K+ currents and Ca(2+)-dependent action potentials by exocytosis in goldfish bipolar cell terminals. J Physiol 572, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z-H (2000). Differential Expression of High- and Two Types of Low-Voltage–Activated Calcium Currents in Rod and Cone Bipolar Cells of the Rat Retina. Journal of neurophysiology 83, 513–527. [DOI] [PubMed] [Google Scholar]
- Pan ZH, Hu HJ, Perring P, and Andrade R (2001). T-type Ca(2+) channels mediate neurotransmitter release in retinal bipolar cells. Neuron 32, 89–98. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Singer JH, and Koschak A (2018). Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear. Physiol Rev 98, 2063–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li C, Haeseleer F, Palczewski K, and Ames JB (2014). Structural insights into activation of the retinal L-type Ca(2)(+) channel (Cav1.4) by Ca(2)(+)-binding protein 4 (CaBP4). J Biol Chem 289, 31262–31273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJH, Pottackal J, Ke JB, Jun NY, Rahmani P, Kim IJ, Singer JH, and Demb JB (2018). Convergence and Divergence of CRH Amacrine Cells in Mouse Retinal Circuitry. J Neurosci 38, 3753–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WS, Han J, and Earm YE (2008). Physiological role of inward rectifier K(+) channels in vascular smooth muscle cells. Pflugers Arch 457, 137–147. [DOI] [PubMed] [Google Scholar]
- Partida GJ, Fasoli A, Fogli Iseppe A, Ogata G, Johnson JS, Thambiaiyah V, Passaglia CL, and Ishida AT (2018). Autophosphorylated CaMKII Facilitates Spike Propagation in Rat Optic Nerve. The Journal of Neuroscience 38, 8087–8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida GJ, Stradleigh TW, Ogata G, Godzdanker I, and Ishida AT (2012). Thy1 Associates with the Cation Channel Subunit HCN4 in Adult Rat Retina. Investigative Opthalmology & Visual Science 53, 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, and Gonzalez-Soriano J (1994). Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci 11, 501–517. [DOI] [PubMed] [Google Scholar]
- Peichl L, Sandmann D, and Boycott BB (1998). Comparative anatomy and function of mammalian horizontal cells In Development and Organization of the Retina, Chalupa, and Finlay, eds. (New York: Plenum Press; ), pp. 147–172. [Google Scholar]
- Pelucchi B, Grimaldi A, and Moriondo A (2008). Vertebrate rod photoreceptors express both BK and IK calcium-activated potassium channels, but only BK channels are involved in receptor potential regulation. J Neurosci Res 86, 194–201. [DOI] [PubMed] [Google Scholar]
- Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, and Kofuji P (2011). Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol-sensitive TRPC subunits: TRPC and melanopsin phototransduction. European Journal of Neuroscience 33, 856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman I, Sullivan JM, and Normann RA (1993). Voltage- and time-dependent potassium conductances enhance the frequency response of horizontal cells in the turtle retina. Brain Res 619, 89–97. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C, and Lasater EM (1993). Dopamine modulates in a differential fashion T- and L-type calcium currents in bass retinal horizontal cells. J Gen Physiol 102, 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer-Linn CL, and Lasater EM (1996a). Dopamine modulates unitary conductance of single PL-type calcium channels in Roccus chrysops retinal horizontal cells. J Physiol 496 (Pt 3), 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer-Linn CL, and Lasater EM (1996b). Whole cell and single-channel properties of a unique voltage-activated sustained calcium current identified in teleost retinal horizontal cells. J Neurophysiol 75, 609–619. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn CL, and Lasater EM (1998). Multiple second-messenger system modulation of voltage-activated calcium currents in teleost retinal horizontal cells. J Neurophysiol 80, 377–388. [DOI] [PubMed] [Google Scholar]
- Picaud S, Hicks D, Forster V, Sahel J, and Dreyfus H (1998). Adult human retinal neurons in culture: Physiology of horizontal cells. Invest Ophthalmol Vis Sci 39, 2637–2648. [PubMed] [Google Scholar]
- Piccolino M, and Gerschenfeld HM (1978). Activation of a regenerative calcium conductance in turtle cones by peripheral stimulation. Proc R Soc Lond B Biol Sci 201, 309–315. [DOI] [PubMed] [Google Scholar]
- Piccolino M, and Gerschenfeld HM (1980). Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci 206, 439–463. [DOI] [PubMed] [Google Scholar]
- Pinto LH, and Klumpp DJ (1998). Localization of potassium channels in the retina. Prog Retin Eye Res 17, 207–230. [DOI] [PubMed] [Google Scholar]
- Pongs O, and Schwarz JR (2010). Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90, 755–796. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, and Goebel DJ (1983). Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: A combined Golgi and autoradiographic study. The Journal of Comparative Neurology 219, 25–35. [DOI] [PubMed] [Google Scholar]
- Poznanski RR (1996). Transient response in a tapering cable model with somatic shunt. Neuroreport 7, 1700–1704. [DOI] [PubMed] [Google Scholar]
- Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK, et al. (2016). M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina. J Neurosci 36, 7184–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, and von Gersdorff H (2000). Light Evokes Ca2+ Spikes in the Axon Terminal of a Retinal Bipolar Cell. Neuron 25, 215–227. [DOI] [PubMed] [Google Scholar]
- Protti DA, and Llano I (1998). Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J Neurosci 18, 3715–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, and Raviola E (2001). Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 30, 211–225. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, and Taylor WR (2014). Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J Neurosci 34, 7611–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Venkataramani S, Gayet-Primo J, Smith RG, and Taylor WR (2013). NaV1.1 channels in axon initial segments of bipolar cells augment input to magnocellular visual pathways in the primate retina. J Neurosci 33, 16045–16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Sun G, Clare JJ, Werkman TR, and Wadman WJ (2014). Properties of human brain sodium channel α-subunits expressed in HEK293 cells and their modulation by carbamazepine, phenytoin and lamotrigine: Properties of brain Na channel α-subunits. British Journal of Pharmacology 171, 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Mulo I, and Myhr KL (2009). The development of Kv4.2 expression in the retina. Neuroscience Letters 464, 209–213. [DOI] [PubMed] [Google Scholar]
- Qu J, and Myhr KL (2008). The development of intrinsic excitability in mouse retinal ganglion cells. Developmental Neurobiology 68, 1196–1212. [DOI] [PubMed] [Google Scholar]
- Rabl K, and Thoreson WB (2002). Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci 16, 2070–2077. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Peles E, Levinson SR, and Shrager P (1999). K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol 28, 319–331. [DOI] [PubMed] [Google Scholar]
- Raviola E, and Dacheux RF (1987). Excitatory dyad synapse in rabbit retina. Proceedings of the National Academy of Sciences of the United States of America 84, 7324–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, and Gilula NB (1975). Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol 65, 192–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff DF, and Guenther E (1999). Developmental changes in voltage-activated potassium currents of rat retinal ganglion cells. Neuroscience 92, 1103–1117. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Hormuzdiar WN, and Waxman SG (2000). α-SNS Produces the Slow TTX-Resistant Sodium Current in Large Cutaneous Afferent DRG Neurons. Journal of neurophysiology 84, 710–718. [DOI] [PubMed] [Google Scholar]
- Renigunta V, Schlichthorl G, and Daut J (2015). Much more than a leak: structure and function of K(2)p-channels. Pflugers Arch 467, 867–894. [DOI] [PubMed] [Google Scholar]
- Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, Sun L, Robson P, and Trakhtenberg EF (2018). Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, and Schwartz EA (1994). A cGMP-gated current can control exocytosis at cone synapses. Neuron 13, 863–873. [DOI] [PubMed] [Google Scholar]
- Risner ML, Pasini S, Cooper ML, Lambert WS, and Calkins DJ (2018). Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proceedings of the National Academy of Sciences 115, E2393–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Jacobs R, and Hudspeth A (1990). Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. The Journal of Neuroscience 10, 3664–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DW, and Wang G-Y (1998). Development of intrinsic membrane properties in mammalian retinal ganglion cells. Seminars in Cell & Developmental Biology 9, 301–310. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Muller LP, and Brecha NC (2014). The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol 522, 1411–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe T, Jüttner R, Bähring R, and Grantyn R (1999). Ion conductances related to development of repetitive firing in mouse retinal ganglion neurons in situ. J Neurobiol 38, 191–206. [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, and Waxman SG (2005). Electrophysiological properties of two axonal sodium channels, Na v 1.2 and Na v 1.6, expressed in mouse spinal sensory neurones: Sodium channels in sensory neurones. The Journal of Physiology 564, 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, and Kaneko A (1983). Ionic mechanisms underlying the responses of off-center bipolar cells in the carp retina. I. Studies on responses evoked by light. J Gen Physiol 81, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H, and Tachibana M (1997). Ca2+ -activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neuroscience research 27, 219–228. [DOI] [PubMed] [Google Scholar]
- Sakmann B, and Trube G (1984). Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol 347, 641–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Dick IE, and Yue DT (2016). Protein kinase A modulation of CaV1.4 calcium channels. Nat Commun 7, 12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, and Herman RC (1996). Structure and Function of a Novel Voltage-gated, Tetrodotoxin-resistant Sodium Channel Specific to Sensory Neurons. Journal of Biological Chemistry 271, 5953–5956. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, and Tibbs GR (1998). Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93, 717–729. [DOI] [PubMed] [Google Scholar]
- Sargoy A, Sun X, Barnes S, and Brecha NC (2014). Differential Calcium Signaling Mediated by Voltage-Gated Calcium Channels in Rat Retinal Ganglion Cells and Their Unmyelinated Axons. PLoS ONE 9, e84507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik S, and DeVries SH (2012). A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci 32, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Aoki K, Watanabe SI, and Kaneko A (1998). L-type calcium channels in the axon terminal of mouse bipolar cells. Neuroreport 9, 2161–2165. [DOI] [PubMed] [Google Scholar]
- Satoh TO, and Yamada M (2000). A bradycardiac agent ZD7288 blocks the hyperpolarization-activated current (I(h)) in retinal rod photoreceptors. Neuropharmacology 39, 1284–1291. [DOI] [PubMed] [Google Scholar]
- Satoh TO, and Yamada M (2002). Multiple inhibitory effects of zatebradine (UL-FS 49) on the electrophysiological properties of retinal rod photoreceptors. Pflugers Arch 443, 532–540. [DOI] [PubMed] [Google Scholar]
- Savchenko A, Barnes S, and Kramer RH (1997). Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature 390, 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, and Guenther E (1996). Developmental regulation of voltage-activated Na+ and Ca2+ currents in rat retinal ganglion cells. Neuroreport 7, 677–681. [DOI] [PubMed] [Google Scholar]
- Schmid S, and Guenther E (1998). Alterations in channel density and kinetic properties of the sodium current in retinal ganglion cells of the rat during in vivo differentiation. Neuroscience 85, 249–258. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, and Kofuji P (2009). Functional and Morphological Differences among Intrinsically Photosensitive Retinal Ganglion Cells. Journal of Neuroscience 29, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, and Witkovsky P (1997). Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience 78, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Schneeweis DM, and Schnapf JL (1999). The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci 19, 1203–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon C, Asteriti S, Koch S, Sothilingam V, Garcia Garrido M, Tanimoto N, Herms J, Seeliger MW, Cangiano L, Biel M, et al. (2016). Loss of HCN1 enhances disease progression in mouse models of CNG channel-linked retinitis pigmentosa and achromatopsia. Hum Mol Genet 25, 1165–1175. [DOI] [PubMed] [Google Scholar]
- Schubert T, Kerschensteiner D, Eggers ED, Misgeld T, Kerschensteiner M, Lichtman JW, Lukasiewicz PD, and Wong ROL (2008). Development of Presynaptic Inhibition Onto Retinal Bipolar Cell Axon Terminals Is Subclass-Specific. Journal of neurophysiology 100, 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Weiler R, and Feigenspan A (2006). Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol 96, 1278–1292. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, and Wong RO (2001). Development of retinal ganglion cell structure and function. Progress in Retinal and Eye Research 20, 139–174. [DOI] [PubMed] [Google Scholar]
- Seung HS, and Sümbül U (2014). Neuronal Cell Types and Connectivity: Lessons from the Retina. Neuron 83, 1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforna L, Megaro A, Pessia M, Franciolini F, and Catacuzzeno L (2018). Structure, Gating and Basic Functions of the Ca2+-activated K Channel of Intermediate Conductance. Curr Neuropharmacol 16, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel L, Paparizos C, Fenske S, Hassan S, Gruner C, Rotzer K, Biel M, and Wahl-Schott CA (2012). Complex regulation of voltage-dependent activation and inactivation properties of retinal voltage-gated Cav1.4 L-type Ca2+ channels by Ca2+-binding protein 4 (CaBP4). J Biol Chem 287, 36312–36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, and Nawy S (2009). A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci 29, 6088–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chang JY, Yu F, Ko ML, and Ko GY (2017). The Contribution of L-Type Cav1.3 Channels to Retinal Light Responses. Front Mol Neurosci 10, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G, and Naghshineh S (1981). Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature 294, 592–594. [DOI] [PubMed] [Google Scholar]
- Shingai R, and Christensen BN (1983). Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience 10, 893–897. [DOI] [PubMed] [Google Scholar]
- Shingai R, and Christensen BN (1986). Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol 56, 32–49. [DOI] [PubMed] [Google Scholar]
- Shingai R, and Quandt FN (1986). Single inward rectifier channels in horizontal cells. Brain Res 369, 65–74. [DOI] [PubMed] [Google Scholar]
- Simms Brett A., and Zamponi Gerald W. (2014). Neuronal Voltage-Gated Calcium Channels: Structure, Function, and Dysfunction. Neuron 82, 24–45. [DOI] [PubMed] [Google Scholar]
- Singer JH, and Diamond JS (2003). Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23, 10923–10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivyer B, and Williams SR (2013). Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nature Neuroscience 16, 1848–1856. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Robinson DW, Scobey RP, and Chalupa LM (1995). Properties of K+ conductances in cat retinal ganglion cells during the period of activity-mediated refinements in retinofugal pathways. The European journal of neuroscience 7, 1558–1568. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Scobey RP, and Chalupa LM (1993). Prenatal development of excitability in cat retinal ganglion cells: action potentials and sodium currents. J Neurosci 13, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, and Dulon D (2003). Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the Guinea pig cochlea. Journal of neurophysiology 90, 320–332. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, and Miller RF (1981). 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science (New York, NY) 211, 182–185. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, and Miller RF (1983). An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science (New York, NY) 219, 1230–1232. [DOI] [PubMed] [Google Scholar]
- Smith BJ, and Cote PD (2012). Reduced Retinal Function in the Absence of Na(v)1.6. PLoS One 7, e31476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BJ, Cote PD, and Tremblay F (2015a). D1 dopamine receptors modulate cone ON bipolar cell Nav channels to control daily rhythms in photopic vision. Chronobiol Int 32, 48–58. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Cote PD, and Tremblay F (2015b). Dopamine modulation of rod pathway signaling by suppression of GABAC feedback to rod-driven depolarizing bipolar cells. The European journal of neuroscience 42, 2258–2270. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Cote PD, and Tremblay F (2017). Contribution of Nav1.8 sodium channels to retinal function. Neuroscience 340, 279–290. [DOI] [PubMed] [Google Scholar]
- Solessio E, Vigh J, Cuenca N, Rapp K, and Lasater EM (2002). Membrane properties of an unusual intrinsically oscillating, wide-field teleost retinal amacrine cell. J Physiol 544, 831–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, Lee SK, Birnbaumer L, and Schmidt TM (2018). Melanopsin Phototransduction Is Repurposed by ipRGC Subtypes to Shape the Function of Distinct Visual Circuits. Neuron 99, 754–767.e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sothilingam V, Michalakis S, Garcia Garrido M, Biel M, Tanimoto N, and Seeliger MW (2016). HCN1 Channels Enhance Rod System Responsivity in the Retina under Conditions of Light Exposure. PLoS One 11, e0147728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DK, and Dacey DM (2009). Physiology of the A1 amacrine: A spiking, axon-bearing interneuron of the macaque monkey retina. Visual neuroscience 14, 507–522. [DOI] [PubMed] [Google Scholar]
- Stanley EF (2000). Presynaptic calcium channels and the depletion of synaptic cleft calcium ions. J Neurophysiol 83, 477–482. [DOI] [PubMed] [Google Scholar]
- Stasheff SF (2008). Emergence of Sustained Spontaneous Hyperactivity and Temporary Preservation of off Responses in Ganglion Cells of the Retinal Degeneration (rd1) Mouse. Journal of neurophysiology 99, 1408–1421. [DOI] [PubMed] [Google Scholar]
- Steffen MA, Seay CA, Amini B, Cai Y, Feigenspan A, Baxter DA, and Marshak DW (2003). Spontaneous Activity of Dopaminergic Retinal Neurons. Biophysical Journal 85, 2158–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell WK (1975). Horizontal cell axons and axon terminals in goldfish retina. J Comp Neurol 159, 503–520. [DOI] [PubMed] [Google Scholar]
- Stella SL Jr., Bryson EJ, and Thoreson WB (2002). A2 adenosine receptors inhibit calcium influx through L-type calcium channels in rod photoreceptors of the salamander retina. J Neurophysiol 87, 351–360. [DOI] [PubMed] [Google Scholar]
- Stella SL Jr., Hu WD, Vila A, and Brecha NC (2007). Adenosine inhibits voltage-dependent Ca2+ influx in cone photoreceptor terminals of the tiger salamander retina. J Neurosci Res 85, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL Jr., and Thoreson WB (2000). Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci 12, 3537–3548. [DOI] [PubMed] [Google Scholar]
- Stieber J, Stöckl G, Herrmann S, Hassfurth B, and Hofmann F (2005). Functional Expression of the Human HCN3 Channel. Journal of Biological Chemistry 280, 34635–34643. [DOI] [PubMed] [Google Scholar]
- Stincic T, Smith RG, and Taylor WR (2016). Time course of EPSCs in ON-type starburst amacrine cells is independent of dendritic location. J Physiol 594, 5685–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, and Schulz HL (2009). TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci 29, 6809–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradleigh TW, Ogata G, Partida GJ, Oi H, Greenberg KP, Krempely KS, and Ishida AT (2011). Colocalization of hyperpolarization-activated, cyclic nucleotide-gated channel subunits in rat retinal ganglion cells. The Journal of Comparative Neurology 519, 2546–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, and Sullivan JM (2003). Cannabinoid receptor activation differentially modulates ion channels in photoreceptors of the tiger salamander. J Neurophysiol 89, 2647–2654. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, et al. (1998). An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet 19, 260–263. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, and Lipton SA (1992). A slowly inactivating K+ current in retinal ganglion cells from postnatal rat. Visual neuroscience 8, 171–176. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, and Lasater EM (1990a). Sustained and transient potassium currents of cultured horizontal cells isolated from white bass retina. J Neurophysiol 64, 1758–1766. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, and Lasater EM (1990b). An unusually small potassium current that is well-suited to a retinal neuron which is chronically depolarized. Brain Res 528, 130–132. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, and Lasater EM (1992). Sustained and transient calcium currents in horizontal cells of the white bass retina. J Gen Physiol 99, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BB, and Chiu SY (1999). N-type calcium channels and their regulation by GABAB receptors in axons of neonatal rat optic nerve. J Neurosci 19, 5185–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Hirano AA, Brecha NC, and Barnes S (2017). Calcium-activated BKCa channels govern dynamic membrane depolarizations of horizontal cells in rodent retina. J Physiol 595, 4449–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Trenholm S, Drinnenberg A, Juttner J, Raics Z, Farrow K, Biel M, Awatramani G, Clark DA, Sahel JA, et al. (2014). Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nat Neurosci 17, 1728–1735. [DOI] [PubMed] [Google Scholar]
- Tabata T, and Kano M (2002). Heterogeneous Intrinsic Firing Properties of Vertebrate Retinal Ganglion Cells. Journal of neurophysiology 87, 30–41. [DOI] [PubMed] [Google Scholar]
- Tachibana M (1981). Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol 321, 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M (1983a). Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol 345, 329–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M (1983b). Solitary horizontal cells in culture--I. Their electrical properties. Vision Res 23, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Tachibana M, and Okada T (1991). Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J Neurosci 11, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, and Piccolino M (1993). Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci 13, 2898–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Dixon DB, and Copenhagen DR (1993). Modulation of a sustained calcium current by intracellular pH in horizontal cells of fish retina. J Gen Physiol 101, 695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamalu F, and Watanabe S-I (2007). Glutamatergic input is coded by spike frequency at the soma and proximal dendrite of AII amacrine cells in the mouse retina: Spike properties of mouse AII amacrine cells. European Journal of Neuroscience 25, 3243–3252. [DOI] [PubMed] [Google Scholar]
- Tan GM, Yu D, Wang J, and Soong TW (2012). Alternative splicing at C terminus of Ca(V)1.4 calcium channel modulates calcium-dependent inactivation, activation potential, and current density. J Biol Chem 287, 832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto N, Sothilingam V, Euler T, Ruth P, Seeliger MW, and Schubert T (2012). BK channels mediate pathway-specific modulation of visual signals in the in vivo mouse retina. J Neurosci 32, 4861–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Avalos JL, Chen J, and MacKinnon R (2009). Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science 326, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, and Grantyn R (1995). Several types of Ca2+ channels mediate glutamatergic synaptic responses to activation of single Thy-1-immunolabeled rat retinal ganglion neurons. J Neurosci 15, 2240–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR (1996). Response properties of long-range axon-bearing amacrine cells in the dark-adapted rabbit retina. Visual neuroscience 13, 599–604. [DOI] [PubMed] [Google Scholar]
- Taylor WR (1999). TTX attenuates surround inhibition in rabbit retinal ganglion cells. Visual neuroscience 16, 285–290. [DOI] [PubMed] [Google Scholar]
- Taylor WR, and Morgans C (1998). Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci 15, 541–552. [DOI] [PubMed] [Google Scholar]
- Taylor WR, and Smith RG (2012). The role of starburst amacrine cells in visual signal processing. Visual neuroscience 29, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, and Wassle H (1995). Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. The European journal of neuroscience 7, 2308–2321. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Attwell D, Mobbs P, and Wilson M (1988). Membrane currents in retinal bipolar cells of the axolotl. J Gen Physiol 91, 49–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, and Bryson EJ (2004). Chloride equilibrium potential in salamander cones. BMC Neurosci 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ, and Rabl K (2003). Reciprocal interactions between calcium and chloride in rod photoreceptors. J Neurophysiol 90, 1747–1753. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, and Burkhardt DA (1990). Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis Neurosci 5, 571–583. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, and Burkhardt DA (1991). Ionic influences on the prolonged depolarization of turtle cones in situ. J Neurophysiol 65, 96–110. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, and Burkhardt DA (2003). Contrast encoding in retinal bipolar cells: current vs. voltage. Vis Neurosci 20, 19–28. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, and Mangel SC (2012). Lateral interactions in the outer retina. Prog Retin Eye Res 31, 407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, and Miller RF (1997). Reducing extracellular Cl- suppresses dihydropyridine-sensitive Ca2+ currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol 77, 2175–2190. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, and Miller RF (2000). Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch-clamp recordings. Vis Neurosci 17, 197–206. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL Jr., Bryson EI, Clements J, and Witkovsky P (2002). D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci 19, 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Jarsky T, Murphy GJ, Rieke F, and Singer JH (2010). Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. J Neurosci 30, 4650–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Zhao JW, Yang XL, and Xie JX (2003). Voltage-gated K(+) channel subunits on cholinergic and dopaminergic amacrine cells. Neuroreport 14, 1763–1766. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, and Brandstatter JH (2005). Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol 168, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S (2010). Regulation of Ionotropic Glutamate Receptors by Their Auxiliary Subunits. Physiology 25, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, and Awatramani GB (2015). Origins of spontaneous activity in the degenerating retina. Front Cell Neurosci 9, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Borowska J, Zhang J, Hoggarth A, Johnson K, Barnes S, Lewis TJ, and Awatramani GB (2012). Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na + channels: Oscillatory networks in rd1 retina. The Journal of Physiology 590, 2501–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Johnson K, Li X, Smith Robert G., and Awatramani Gautam B. (2011). Parallel Mechanisms Encode Direction in the Retina. Neuron 71, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, and Awatramani GB (2013). Dynamic Tuning of Electrical and Chemical Synaptic Transmission in a Network of Motion Coding Retinal Neurons. Journal of Neuroscience 33, 14927–14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Li W, and Massey SC (2005). Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. Journal of neurophysiology 93, 1476–1485. [DOI] [PubMed] [Google Scholar]
- Trumpler J, Dedek K, Schubert T, de Sevilla Muller LP, Seeliger M, Humphries P, Biel M, and Weiler R (2008). Rod and cone contributions to horizontal cell light responses in the mouse retina. J Neurosci 28, 6818–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, and Omi N (2014). Some OFF bipolar cell types make contact with both rods and cones in macaque and mouse retinas. Front Neuroanat 8, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, and Omi N (2016). ON Bipolar Cells in Macaque Retina: Type-Specific Synaptic Connectivity with Special Reference to OFF Counterparts. Front Neuroanat 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Taylor WR, and Smith RG (2004). Direction selectivity in a model of the starburst amacrine cell. Visual neuroscience 21, 611–625. [DOI] [PubMed] [Google Scholar]
- Uchida S, Yamada S, Nagai K, Deguchi Y, and Kimura R (1997). Brain pharmacokinetics and in vivo receptor binding of 1,4-dihydropyridine calcium channel antagonists. Life Sci 61, 2083–2090. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A, and Kaneda M (1992). Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol 68, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Ujfalussy BB, Makara JK, Lengyel M, and Branco T (2018). Global and Multiplexed Dendritic Computations under In Vivo-like Conditions. Neuron 100, 579–592 e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S, Kamiyama Y, Ishii H, and Ikeno H (1996). Reconstruction of retinal horizontal cell responses by the ionic current model. Vision Res 36, 1711–1719. [DOI] [PubMed] [Google Scholar]
- Van Hook MJ, Babai N, Zurawski Z, Yim YY, Hamm HE, and Thoreson WB (2017). A Presynaptic Group III mGluR Recruits Gbetagamma/SNARE Interactions to Inhibit Synaptic Transmission by Cone Photoreceptors in the Vertebrate Retina. J Neurosci 37, 4618–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hook MJ, and Berson DM (2010). Hyperpolarization-Activated Current (Ih) in Ganglion-Cell Photoreceptors. PLoS ONE 5, e15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hook MJ, Wong KY, and Berson DM (2012). Dopaminergic modulation of ganglion-cell photoreceptors in rat: Dopamine and ipRGCs. European Journal of Neuroscience 35, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Boiko T, Trimmer JS, and Matthews G (2005). Novel clustering of sodium channel Nav1.1 with ankyrin-G and neurofascin at discrete sites in the inner plexiform layer of the retina. Molecular and Cellular Neuroscience 28, 661–673. [DOI] [PubMed] [Google Scholar]
- Van Wart A, and Matthews G (2006a). Expression of sodium channels Nav1.2 and Nav1.6 during postnatal development of the retina. Neuroscience Letters 403, 315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, and Matthews G (2006b). Impaired Firing and Cell-Specific Compensation in Neurons Lacking Nav1.6 Sodium Channels. Journal of Neuroscience 26, 7172–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, and Matthews G (2007). Polarized distribution of ion channels within microdomains of the axon initial segment. The Journal of Comparative Neurology 500, 339–352. [DOI] [PubMed] [Google Scholar]
- Van Wyk M, Wässle H, and Taylor WR (2009). Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Visual neuroscience 26, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, and Hill AP (2012). hERG K(+) channels: structure, function, and clinical significance. Physiol Rev 92, 1393–1478. [DOI] [PubMed] [Google Scholar]
- Vaney DI (1986). Morphological identification of serotonin-accumulating neurons in the living retina. Science (New York, NY) 233, 444–446. [DOI] [PubMed] [Google Scholar]
- Vaney DI (1990). Chapter 2 The mosaic of amacrine cells in the mammalian retina. Progress in Retinal Research 9, 49–100. [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, and Ishida AT (2001). A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci 21, 8624–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega AV, Henry DL, and Matthews G (2008). Reduced expression of Nav1.6 sodium channels and compensation by Nav1.2 channels in mice heterozygous for a null mutation in Scn8a. Neuroscience Letters 442, 69–73. [DOI] [PubMed] [Google Scholar]
- Vellani V, Reynolds AM, and McNaughton PA (2000). Modulation of the synaptic Ca2+ current in salamander photoreceptors by polyunsaturated fatty acids and retinoids. J Physiol 529 Pt 2, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velte TJ, and Masland RH (1999). Action Potentials in the Dendrites of Retinal Ganglion Cells. Journal of neurophysiology 81, 1412–1417. [DOI] [PubMed] [Google Scholar]
- Velte TJ, and Miller RF (1997). Spiking and nonspiking models of starburst amacrine cells in the rabbit retina. Vis Neurosci 14, 1073–1088. [DOI] [PubMed] [Google Scholar]
- Veruki ML, and Hartveit E (2002). AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron 33, 935–946. [DOI] [PubMed] [Google Scholar]
- Vigh J, and Lasater EM (2004). L-type calcium channels mediate transmitter release in isolated, wide-field retinal amacrine cells. Vis Neurosci 21, 129–134. [DOI] [PubMed] [Google Scholar]
- Vigh J, Solessio E, Morgans CW, and Lasater EM (2003). Ionic mechanisms mediating oscillatory membrane potentials in wide-field retinal amacrine cells. J Neurophysiol 90, 431–443. [DOI] [PubMed] [Google Scholar]
- Vila A, Whitaker CM, and O'Brien J (2017). Membrane-associated guanylate kinase scaffolds organize a horizontal cell synaptic complex restricted to invaginating contacts with photoreceptors. J Comp Neurol 525, 850–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Wright T, Garcia-Sanchez Y, Kisilak M, Campbell M, Westall C, and Heon E (2013). Phenotypic characteristics including in vivo cone photoreceptor mosaic in KCNV2-related "cone dystrophy with supernormal rod electroretinogram". Invest Ophthalmol Vis Sci 54, 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Euler T, and Franke K (2018). Function first: classifying cell types and circuits of the retina. Curr Opin Neurobiol 56, 8–15. [DOI] [PubMed] [Google Scholar]
- Vocke K, Dauner K, Hahn A, Ulbrich A, Broecker J, Keller S, Frings S, and Mohrlen F (2013). Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels. J Gen Physiol 142, 381–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, and Tavernarakis N (2006). The role of synaptic ion channels in synaptic plasticity. EMBO reports 7, 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Amarillo Y, and Bloomfield SA (2001). Morphology and physiology of the polyaxonal amacrine cells in the rabbit retina. J Comp Neurol 440, 109–125. [DOI] [PubMed] [Google Scholar]
- Volgyi B, Xin D, and Bloomfield SA (2002). Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol 539, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl-Schott C, and Biel M (2009). HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66, 470–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner DM, Bech-Hansen NT, and Stell WK (2018). Channeling Vision: CaV1.4-A Critical Link in Retinal Signal Transmission. Biomed Res Int 2018, 7272630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, and Toro L (1999). Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proceedings of the National Academy of Sciences of the United States of America 96, 4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waloga G, and Pak WL (1978). Ionic mechanism for the generation of horizontal cell potentials in isolated axolotl retina. J Gen Physiol 71, 69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-Y, Ratto GM, Bisti S, and Chalupa LM (1997). Functional Development of Intrinsic Properties in Ganglion Cells of the Mammalian Retina. Journal of neurophysiology 78, 2895–2903. [DOI] [PubMed] [Google Scholar]
- Wang G-Y, Robinson DW, and Chalupa LM (1998). Calcium-Activated Potassium Conductances in Retinal Ganglion Cells of the Ferret. Journal of neurophysiology 79, 151–158. [DOI] [PubMed] [Google Scholar]
- Wang TM, Holzhausen LC, and Kramer RH (2014). Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci 17, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fehlhaber KE, Sarria I, Cao Y, Ingram NT, Guerrero-Given D, Throesch B, Baldwin K, Kamasawa N, Ohtsuka T, et al. (2017). The Auxiliary Calcium Channel Subunit alpha2delta4 Is Required for Axonal Elaboration, Synaptic Transmission, and Wiring of Rod Photoreceptors. Neuron 93, 1359–1374 e1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W (2018). Neural Mechanisms of Motion Processing in the Mammalian Retina. Annual review of vision science 4, 165–192. [DOI] [PubMed] [Google Scholar]
- Weiler R, and Zettler F (1979). The axon-bearing horizontal cells in the teleost retina are functional as well as structural units. Vision Res 19, 1261–1268. [DOI] [PubMed] [Google Scholar]
- Werblin FS (1977). Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol 264, 767–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, and Dowling JE (1969). Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. Journal of neurophysiology 32, 339–355. [DOI] [PubMed] [Google Scholar]
- Whicher JR, and MacKinnon R (2016). Structure of the voltage-gated K(+) channel Eag1 reveals an alternative voltage sensing mechanism. Science 353, 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JM, and Hartzell HC (2017). Anoctamins/TMEM16 Proteins: Chloride Channels Flirting with Lipids and Extracellular Vesicles. Annu Rev Physiol 79, 119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbar S, and Schwartz GW (2018). The dynamic receptive fields of retinal ganglion cells. Prog Retin Eye Res 67, 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MF, and Barnes S (1996). The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol 107, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RL, and Ma S (1990). Bifurcation analysis of nonlinear retinal horizontal cell models. II. Network properties. J Neurophysiol 64, 248–261. [DOI] [PubMed] [Google Scholar]
- Wissinger B, Dangel S, Jagle H, Hansen L, Baumann B, Rudolph G, Wolf C, Bonin M, Koeppen K, Ladewig T, et al. (2008). Cone dystrophy with supernormal rod response is strictly associated with mutations in KCNV2. Invest Ophthalmol Vis Sci 49, 751–757. [DOI] [PubMed] [Google Scholar]
- Wissinger B, Schaich S, Baumann B, Bonin M, Jagle H, Friedburg C, Varsanyi B, Hoyng CB, Dollfus H, Heckenlively JR, et al. (2011). Large deletions of the KCNV2 gene are common in patients with cone dystrophy with supernormal rod response. Hum Mutat 32, 1398–1406. [DOI] [PubMed] [Google Scholar]
- Witkovsky P (2004). Dopamine and retinal function. Doc Ophthalmol 108, 17–40. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Arango-Gonzalez B, Haycock JW, and Kohler K (2005). Rat retinal dopaminergic neurons: Differential maturation of somatodendritic and axonal compartments. The Journal of Comparative Neurology 481, 352–362. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP, and Hille B (1992). Ionic selectivity of Ih channels of rod photoreceptors in tiger salamanders. J Gen Physiol 100, 749–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, and Catterall WA (1986). Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proceedings of the National Academy of Sciences of the United States of America 83, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Scheinman R, and Catterall WA (1988). Sodium channel expression and assembly during development of retinal ganglion cells. Neuron 1, 727–737. [DOI] [PubMed] [Google Scholar]
- Wong RCS, Cloherty SL, Ibbotson MR, and O'Brien BJ (2012). Intrinsic physiological properties of rat retinal ganglion cells with a comparative analysis. Journal of neurophysiology 108, 2008–2023. [DOI] [PubMed] [Google Scholar]
- Wu C, Ivanova E, Cui J, Lu Q, and Pan ZH (2011). Action Potential Generation at an Axon Initial Segment-Like Process in the Axonless Retinal AII Amacrine Cell. Journal of Neuroscience 31, 14654–14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Cowing JA, Michaelides M, Wilkie SE, Jeffery G, Jenkins SA, Mester V, Bird AC, Robson AG, Holder GE, et al. (2006). Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause "cone dystrophy with supernormal rod electroretinogram" in humans. Am J Hum Genet 79, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM (1985). Synaptic transmission from rods to bipolar cells in the tiger salamander retina. Proc Natl Acad Sci U S A 82, 3944–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Qiao X, Noebels JL, and Yang XL (1993). Localization and modulatory actions of zinc in vertebrate retina. Vision Res 33, 2611–2616. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nurnberg P, Ruether K, and Berger W (2006a). Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci 47, 3523–3530. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, et al. (2006b). Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet 79, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, and Lingle CJ (2003). Inactivation of BK channels by the NH2 terminus of the beta2 auxiliary subunit: an essential role of a terminal peptide segment of three hydrophobic residues. J Gen Physiol 121, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Chen X, and Steele EC Jr. (2007). Abundant L-type calcium channel Ca(v)1.3 (alpha1D) subunit mRNA is detected in rod photoreceptors of the mouse retina via in situ hybridization. Mol Vis 13, 764–771. [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Cai Y, Yen J, Steffen M, Baxter DA, Feigenspan A, and Marshak D (2004). Voltage-clamp analysis and computational model of dopaminergic neurons from mouse retina. Visual neuroscience 21, 835–849. [DOI] [PubMed] [Google Scholar]
- Xu H-P, Zhao J-W, and Yang X-L (2002). Expression of voltage-dependent calcium channel subunits in the rat retina. Neuroscience Letters 329, 297–300. [DOI] [PubMed] [Google Scholar]
- Xu JW, and Slaughter MM (2005). Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci 25, 7660–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, and Kaneko A (1988). The axon terminal of goldfish retinal horizontal cells: a low membrane conductance measured in solitary preparations and its implication to the signal conduction from the soma. J Neurophysiol 59, 482–494. [DOI] [PubMed] [Google Scholar]
- Yagi T, and Macleish PR (1994). Ionic conductances of monkey solitary cone inner segments. J Neurophysiol 71, 656–665. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Lukasiewicz P, Maguire G, Werblin FS, and Yazulla S (1991). Amacrine cells in the tiger salamander retina: Morphology, physiology, and neurotransmitter identification. The Journal of Comparative Neurology 312, 19–32. [DOI] [PubMed] [Google Scholar]
- Yang PS, Johny MB, and Yue DT (2014). Allostery in Ca(2)(+) channel modulation by calcium-binding proteins. Nat Chem Biol 10, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al. (2008). TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215. [DOI] [PubMed] [Google Scholar]
- Yazulla S, and Studholme KM (1998). Differential distribution of Shaker-like and Shab-like K+-channel subunits in goldfish retina and retinal bipolar cells. J Comp Neurol 396, 131–140. [DOI] [PubMed] [Google Scholar]
- Yuan C, Chen M, Covey DF, Johnston LJ, and Treistman SN (2011). Cholesterol tuning of BK ethanol response is enantioselective, and is a function of accompanying lipids. PLoS One 6, e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, and MacKinnon R (2010). Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science 329, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabouri N, and Haverkamp S (2013). Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One 8, e63853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, Matyas G, Borruat FX, Schorderet DF, Zrenner E, et al. (2006). Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet 79, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Robson AG, and Audo I (2015). Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res 45, 58–110. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Henry D, Studholme K, Yazulla S, and Matthews G (2001). Voltage-dependent sodium channels are expressed in nonspiking retinal bipolar neurons. J Neurosci 21, 4543–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, and Matthews G (1998). Calcium action potentials in retinal bipolar neurons. Visual neuroscience 15, 69–75. [DOI] [PubMed] [Google Scholar]
- Zhang C-L, Wilson JA, Williams J, and Chiu SY (2006). Action Potentials Induce Uniform Calcium Influx in Mammalian Myelinated Optic Nerves. Journal of neurophysiology 96, 695–709. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, and McMahon DG (2008). Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences 105, 14181–14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou TR, and McMahon DG (2007). Functional Heterogeneity of Retinal Dopaminergic Neurons Underlying Their Multiple Roles in Vision. Journal of Neuroscience 27, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Trexler EB, and Massey SC (2002). Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci 22, 10871–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang D, and Hughes BA (2011). KCNQ5/K(v)7.5 potassium channel expression and subcellular localization in primate retinal pigment epithelium and neural retina. Am J Physiol Cell Physiol 301, C1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Reifler AN, Schroeder MM, Jaeckel ER, Chervenak AP, and Wong KY (2017). Mechanisms creating transient and sustained photoresponses in mammalian retinal ganglion cells. The Journal of General Physiology 149, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, and Goldin AL (2004). Use-Dependent Potentiation of the Nav1.6 Sodium Channel. Biophysical Journal 87, 3862–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tencerova B, Hartveit E, and Veruki ML (2016). Functional NMDA receptors are expressed by both AII and A17 amacrine cells in the rod pathway of the mammalian retina. Journal of neurophysiology 115, 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, and Fain GL (1996). Starburst amacrine cells change from spiking to nonspiking neurons during retinal development. Proceedings of the National Academy of Sciences of the United States of America 93, 8057–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Hauswirth WW, and DeVries SH (2014). Genetically Targeted Binary Labeling of Retinal Neurons. The Journal of Neuroscience 34, 7845–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobor D, Kohl S, Wissinger B, Zrenner E, and Jagle H (2012). Rod and cone function in patients with KCNV2 retinopathy. PLoS One 7, e46762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lee A, and Yang J (2012). The expression of whirlin and Cav1.3alpha(1) is mutually independent in photoreceptors. Vision Res 75, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]