Abstract
Chronic kidney disease (CKD) is a strong risk factor for cardiovascular disease (CVD), but clinical kidney measures (eGFR and albuminuria) do not fully reflect the multiple aspects of kidney tubules influencing cardiovascular health. Applied methods are needed to integrate numerous tubule biomarkers into useful prognostic scores. In SPRINT participants with CKD at baseline (eGFRcr&cys <60mL/min/1.73m2), we measured eight biomarkers from urine (alpha-1, beta-2, umod, KIM-1, MCP-1, YKL-40, NGAL, IL-18) and two biomarkers from serum (iPTH, iFGF-23). We used an unsupervised method, exploratory factor analysis, to create summary scores of tubule health dimensions. Adjusted Cox models evaluated each tubule score with CVD events, heart failure (HF), and all-cause mortality. We examined CVD discrimination using Harrell’s C-statistic. Factor analysis of ten biomarkers from 2376 SPRINT-CKD participants identified four unique dimensions of tubular health: tubule injury/repair (NGAL, IL-18, YKL-40), tubule injury/fibrosis (KIM-1, MCP-1), tubule reabsorption (alpha-1, beta-2), and tubular reserve/mineral metabolism (umod, iPTH, iFGF-23). After adjustment for CVD risk factors, eGFR, and ACR, two of four tubule scores were associated with CVD (HR per SD, reabsorption: 1.21 (1.06–1.38), reserve: 1.24 (1.08–1.38)), one with HF (reserve: 1.41 (1.13–1.74)), and none with mortality. Compared to a base model (C-statistic = 0.674), adding eGFR and ACR improved the C-statistic (C=0.704, p=0.001); further adding tubule scores additionally improved the C-statistic (C=0.719, p=0.009). In the setting of CKD, dimensions of tubule health quantified using factor analysis improved CVD discrimination beyond contemporary kidney measures.
Clinical Trial Registration
Keywords: kidney, chronic kidney disease, biomarker, cardiovascular disease, epidemiology
INTRODUCTION
Chronic kidney disease (CKD) is a well-established risk factor for cardiovascular disease (CVD)1 and yet the mechanisms connecting the most common measure of kidney function, estimated glomerular filtration rate (eGFR), to atherosclerosis are unclear.2 The eGFR is often used clinically as a global marker of kidney health, despite its much narrower purpose of reflecting glomerular filtration.3 However, eGFR does not adequately capture the health of the kidney tubules, the site of numerous important homeostatic processes.4 These metabolically active processes, such as acid/base balance and toxin secretion, have been hypothesized to prevent endothelial dysfunction and atherosclerosis. By measuring injury and dysfunction in the kidney tubules, we may be able to understand better how kidney impairment leads to CVD and to improve CVD risk prediction, beyond eGFR and albuminuria which have shown mixed results for CVD risk prediction.5
Novel biomarkers have enabled non-invasive measurement of kidney tubule injury and dysfunction. These biomarkers represent a wide range of tubular processes, including tubular injury (IL-18, KIM-1, NGAL),6–8 repair (YKL-40),9 fibrosis (MCP-1),10 proximal tubule reabsorptive capacity (alpha-1 microglobulin, beta-2 microglobulin),11 defense from infections (uromodulin),12 and response to systemic hormones (intact FGF-23, intact PTH). Several of these biomarkers have been shown to improve detection of in-hospital acute kidney injury compared to traditional measures,13 therefore, there is also interest in using these urine proteins for CKD prognostication in ambulatory patients. Prior studies have seen mixed associations of individual biomarkers with cardiovascular and mortality risk, with numerous biomarkers having associations with risk, but not improving discrimination when evaluated as single markers, based on the C-statistic or other measure.14–22
It seems unlikely that any individual marker would fully capture all aspects of kidney tubule health. While these biomarkers may hold important information about CVD, approaches are needed to distill the complexities of multiple biomarkers into clinically informative and actionable results, and to capture the full spectrum of tubule health in an integrated approach. To optimize the biomarkers’ predictive capacity and reduce redundancy from correlated biomarkers, a crucial next step is to integrate the information from a panel of biomarkers into summary scores of kidney tubule health that can be incorporated and interpreted within clinical practice and might more accurately reflect the global relationship of kidney tubule health with CVD.
We had four objectives. First, to create scores for different dimensions of kidney tubule health, using factor analysis to summarize the 10 biomarkers. Second, to examine the correlation of traditional kidney measures (eGFR and albuminuria) with these summary scores of kidney tubule health. Third, to evaluate the association of the scores of tubular health with risk of cardiovascular disease events, heart failure, and all-cause mortality. Finally, to determine if the addition of tubular health scores improved CVD risk discrimination in comparison with eGFR and ACR.
METHODS
Study Population
The Systolic Blood Pressure Intervention Trial (SPRINT) recruited 9,361 participants at high risk of CVD to test whether a lower systolic blood pressure (BP) target (<120 mm Hg) would improve cardiovascular outcomes compared to usual BP goal (systolic BP target <140 mm Hg). Main results and trial details are published elsewhere.23, 24 Briefly, participants were eligible if they were aged ≥50 years and had systolic BP 130–180mmHg and at least one of the following: prevalent subclinical or clinical CVD, 10-year Framingham risk score ≥15%, CKD (eGFR by MDRD formula 20- <60 ml/min/1.73m2), or age ≥75 years. Notable exclusion criteria were diabetes, prior stroke, and proteinuria >1g/day. Participants were randomized between November 2010 and March 2013; on August 20, 2015, the trial was terminated on the recommendation of the data safety monitoring board.
Our study population included SPRINT participants with CKD, as defined by eGFR (by CKD-Epi creatinine-cystatin C equation) <60ml/min/1.73m2 (n=2514).25 We excluded participants with missing or invalid biomarker measurements (n=86, due to poor sample quality) and with missing covariates (n=52). Our final study population included 2376 participants.
Data and study materials will be publically available to other researchers at the National Heart, Lung, and Blood Institute’s data repository (https://biolincc.nhlbi.nih.gov/home/) in November 2020.
Biomarker Measurement
Urine and venous blood specimens were collected at the baseline visit and stored at −80°C at a central laboratory. Urine biomarkers were measured in duplicate and averaged to improve precision at the Laboratory for Clinical Biochemistry Research at the University of Vermont. Alpha-1 microglobulin (alpha-1) was measured using a Siemens nephelometric assay (Tarrytown, NY) with inter-assay CV of 3.5–8.8% and detectable range of 5–480 mg/L. Beta-2 microglobulin (beta-2), uromodulin (umod), and NGAL were measured on a multiplex assay on a MESO Scale Diagnostics platform (Rockville, MD) with inter-assay CVs of 15–16%, 13–16%, and 11–19%, respectively. The ranges of detection were 1.2–5,020 ng/mL for beta-2, 0.6–2,510 ng/mL for uromodulin, and 6–251,000 ng/mL for NGAL. IL-18, KIM-1, MCP-1, and YKL-40 were also measured together using a multi-plex assay on a MESO Scale Diagnostics platform (Rockville, MD). Inter-assay CVs were 4.9–13.7%, 6.1–13.0%, 7.1–12.0%, and 6.5–11.1%, respectively. The analytic ranges were 2–10,000 ng/mL for IL-18, 4–200,000 pg/mL for KIM-1, 3–10,000pg/ml for MCP-1, and 10–500,000 ng/mL for YKL-40. Samples with biomarker values below the limit of detection were assigned a value equivalent to the lower limit of detection divided by the square root of two.
Intact serum PTH and intact serum FGF-23 were measured at the SPRINT Central Laboratory at the University of Minnesota, Minneapolis in 2015. An intact PTH immunoassay (e411 analyzer, Roche, Indianapolis, IN) was used with an analytic measurement range of 1.2–5,000 pg/mL and an inter-assay CV of 4.9% at 35.1 pg/mL and 2.5% at 210.4 pg/mL. A two-site ELISA (Kainos Laboratories, Tokyo, Japan) was used to measure intact FGF-23 with an analytic measurement range of 2.2–800 pg/mL and an inter-assay CV of 8.6% at 22.5 pg/mL and 3.2% at 85.1 pg/mL.
Outcome Ascertainment
Our primary outcome was the same as the SPRINT primary outcome: CVD events, defined as a composite of myocardial infarction, acute coronary syndrome, stroke, acute decompensated heart failure, and cardiovascular death. We also separately examined all-cause mortality and heart failure, defined as hospitalization or emergency department visit with signs or symptoms of heart failure and requiring infusion therapy treatment.23
Covariates
Demographic characteristics were age, race, and sex. Clinical characteristics measured prior to randomization included systolic and diastolic BP, number of hypertension medications, statin use, serum total cholesterol, serum HDL cholesterol, and history of clinical CVD or heart failure.23
Both serum and urine creatinine were measured by an enzymatic procedure with calibration traceable to an isotope dilution mass spectrometry (IDMS) procedure on Roche Chemistry Analyzers (Roche, Indianapolis, IN).26 Urine albumin was measured by a nephelometric method using the Siemens ProSpec nephelometer (Siemens, Tarrytown, NY). The inter-assay CV for urine creatinine was 1.5–4.3% and for urine albumin was 2.2–6.9%. Albuminuria was calculated as the albumin-to-creatinine ratio (ACR). Serum cystatin C was measured (Gentian, Moss, Norway) in all participants at baseline. eGFR was calculated using the CKD-Epi equation for creatinine and cystatin C.25
Statistical Analysis
Urine biomarkers were indexed to urine creatinine and log base-2 transformed. We examined all pairwise Spearman correlations of creatinine-index kidney tubule biomarkers. To determine the number of factors to extract, we initially performed principal components analysis and examined the number of eigenvalues >1 and the scree plot; we also conducted parallel analysis.27, 28 From these results, a model with 4 distinct factors was determined to be appropriate for the data. We then used factor analysis with principal components factors estimation and examined different rotations to determine the best fit to the data by Thurstone’s rules.29 To derive factor scores, we used the regression scoring method, which creates standardized scores for each factor (i.e., mean 0 and standard deviation 1).
We stratified participants by those who did vs. did not have CVD events during follow-up and examined baseline characteristics across groups. We calculated Pearson correlations among the factor scores and eGFR and albuminuria. Cox proportional hazards regression was used to evaluate associations with CVD events, heart failure events, and all-cause mortality. We first used restricted cubic splines for each factor score, adjusted for age, sex, race, and randomization arm, to evaluate whether the factor score had an approximately linear association with cardiovascular disease events. In our main analysis, we used the factor scores as continuous, linear predictors and nested adjusted models: Model 1 adjusted for age, sex, race, intervention arm, systolic blood pressure, diastolic blood pressure, number of hypertension agents at baseline, total cholesterol, HDL cholesterol, statin use (yes/no), smoking status (current/former/never), and history of CVD/HF. Model 2 additionally adjusted for eGFR and ACR; Model 3 additionally included all factor scores simultaneously. Both the hazard ratios for the factors and for eGFR and ACR were of interest across the sequence of models, to compare relative strengths of associations with outcomes, and to explore the degree of attenuation of different markers of kidney function in nested models before and after mutual adjustment.
To determine if kidney tubule factor scores improve CVD discrimination, we constructed a base model with CVD risk factors (Model 1 as described above), and added 1) all factor scores 2) eGFR and ACR, 3) all factor scores, eGFR, and ACR. We calculated Harrell’s C-statistic with 95% confidence intervals and tested the differences between each model using Stata’s roccomp. We calculated the C-statistics overall and in individuals without a history of CVD.
We also conducted several sensitivity analyses. First, to determine whether the association of the factor scores with CVD events was different by trial arm, we included an interaction term between the factor score and randomization. Second, we added an interaction term between the factor score and history of CVD/HF; we hypothesized that the associations would be stronger in individuals without a history of CVD/HF, similar to other risk factors associations that are often weaker in people with prior CVD events.30 We used a likelihood ratio test to evaluate the significance of these interaction terms. Statistical analyses were conducted using Stata Version 15.1 (StataCorp LLC, College Station, TX); p-values < 0.05 were considered statistically significant for all analyses. There was no adjustment for multiple comparisons for interaction terms.
RESULTS
There were 2376 SPRINT-CKD participants, including 306 who developed CVD during a median of 3.8 years of follow-up. On average, CVD risk factors were more prevalent in the group that subsequently experienced CVD during follow-up (Table 1). Among the persons who experienced CVD events, the median and 75th percentile values of urine and serum biomarkers were generally higher than in participants who did not develop CVD. Correlations between serum biomarkers and creatinine-indexed urine biomarkers were absent to moderate in strength, ranging from 0 to |0.57| with the highest correlations among the two markers of proximal tubule reabsorptive capacity (alpha-1 and beta-2) (Supplementary Table S1).
Table 1.
Baseline characteristics of SPRINT-CKD participants, by CVD during follow-up, n=2376
| Characteristics | No CVD during follow-up n=2070 | CVD during follow-up n=306 |
|---|---|---|
| Age | 72.7 ± 9.0 | 76.0 ± 9.1 |
| Female | 861 (41.6) | 98 (32.0) |
| Race | ||
| Non-Hispanic White | 1352 (65.3) | 218 (71.2) |
| Non-Hispanic Black | 538 (26.0) | 67 (21.9) |
| Other | 180 (8.7) | 21 (6.9) |
| Intensive BP arm | 1074 (51.9) | 142 (46.4) |
| Systolic blood pressure | 139.4 ± 16.2 | 140.5 ± 17.7 |
| Diastolic blood pressure | 74.6 ± 12.2 | 71.8 ± 12.9 |
| Number of hypertensive medications at baseline | 2 [1, 3] | 2 [2, 3] |
| History of CVD or HF | 471 (22.8) | 125 (40.9) |
| Total cholesterol | 184.3 ± 40.2 | 179.3 ± 44.9 |
| HDL cholesterol | 52.6 ± 14.4 | 50.2 ± 13.9 |
| Statin use | 1067 (51.6) | 173 (56.5) |
| Smoking | ||
| Current smoker | 178 (8.6) | 33 (10.8) |
| Former smoker | 940 (45.4) | 148 (48.4) |
| eGFRcr&cysC | 48.8 [39.4, 54.9] | 44.4 [34.1, 53.0] |
| Albumin-to-Creatinine Ratio | 13.1 [6.5, 40.7] | 28.5 [10.4, 95.9] |
| Alpha-1 microglobulin mg/L | 13 [7, 24] | 19 [10, 33] |
| Beta-2 microglobulin ng/mL | 94 [33, 299] | 153 [42, 479] |
| Uromodulin ng/mL | 7 [4, 10] | 6 [4, 9] |
| IL-18 ng/mL | 30 [16, 56] | 34 [17, 59] |
| KIM-1 pg/mL | 840 [384, 1556] | 881 [395, 1699] |
| MCP-1 pg/mL | 178 [90, 324] | 184 [86, 367] |
| YKL-40 ng/mL | 532 [212, 1200] | 602 [219, 1594] |
| NGAL ng/mL | 27 [15, 56] | 29 [16, 70] |
| iPTH pg/mL | 47 [35, 65] | 52 [35, 75] |
| iFGF-23 pg/mL | 66 [52, 87] | 69 [55, 95] |
Data displayed are mean ± SD, n (%), or median [25th percentile, 75th percentile].
Umod, uromodulin. IL-18, interleukin 18. KIM-1, kidney injury molecule-1. MCP-1, monocyte chemoattractant protein-1. YKL-40, chitinase-3-like protein-1. NGAL, neutrophil gelatinase-associated lipocalin. iPTH, intact parathyroid hormone. iFGF-23, intact fibroblast growth factor-23.
The factor loading matrix from principal components estimation with promax rotation is displayed in Table 2, in which each coefficient can roughly be interpreted as a correlation, with values close to −1 or 1 indicating a strong correlation with the factor and values close to 0 indicating no correlation of the individual biomarker with the factor itself. The solution showed good model fit, with no biomarker loading higher than 0.32 on more than one factor.29 Uniqueness, meaning the proportion of the biomarker’s variance not explained by the factors, was generally low (0.2–0.5). We created names for the factors according to the hypothesized physiology of the most highly loading biomarkers’: we labeled Factor 1, comprised by IL-18, NGAL, and YKL-40, as “tubule injury/repair”; Factor 2, comprised by KIM-1, MCP-1, as “tubule injury and fibrosis”; Factor 3, comprised by alpha-1 and beta-2, as “tubule reabsorption”; and Factor 4, comprised by umod, iFGF-23, and iPTH, as “tubular reserve/mineral metabolism.”
Table 2.
Factor loading matrix from exploratory factor analysis of kidney tubule biomarkers
| Biomarkers | Factor 1: Tubule Injury/Repair | Factor 2: Tubule Injury/Fibrosis | Factor 3: Tubule Reabsorption | Factor 4: Tubular Reserve/Mineral Metabolism | Uniqueness |
|---|---|---|---|---|---|
| Alpha-1 | −0.085 | 0.072 | 0.873 | 0.117 | 0.235 |
| Beta-2 | 0.083 | −0.011 | 0.858 | −0.126 | 0.223 |
| Umod | 0.089 | −0.071 | 0.258 | −0.683 | 0.456 |
| IL-18 | 0.596 | 0.253 | −0.061 | 0.114 | 0.485 |
| KIM-1 | −0.020 | 0.894 | 0.026 | 0.036 | 0.200 |
| MCP-1 | −0.035 | 0.912 | 0.035 | −0.073 | 0.200 |
| YKL-40 | 0.838 | −0.001 | −0.016 | −0.025 | 0.304 |
| NGAL | 0.877 | −0.110 | 0.033 | −0.016 | 0.259 |
| iFGF-23 | −0.043 | −0.059 | 0.157 | 0.587 | 0.628 |
| iPTH | 0.105 | −0.067 | 0.121 | 0.707 | 0.470 |
Bold indicates factor loading >0.5. Uniqueness is defined as the proportion of each biomarker’s variance not explained by the factors.
The Pearson correlations among tubule factor scores were low, ranging from −0.02 between the injury/fibrosis score and the reabsorption score, to 0.28 for the injury/repair score with the injury/fibrosis score (Supplementary Table S2). There was minimal correlation of eGFR with the two injury scores (−0.03 for injury/repair and −0.06 for injury/fibrosis), weak inverse correlation of eGFR with the tubule reabsorption score (−0.32), and moderate inverse correlation with the tubular reserve/mineral metabolism score (−0.51). ACR had weak to moderate correlations with all tubular factor scores, ranging from 0.19 to 0.42.
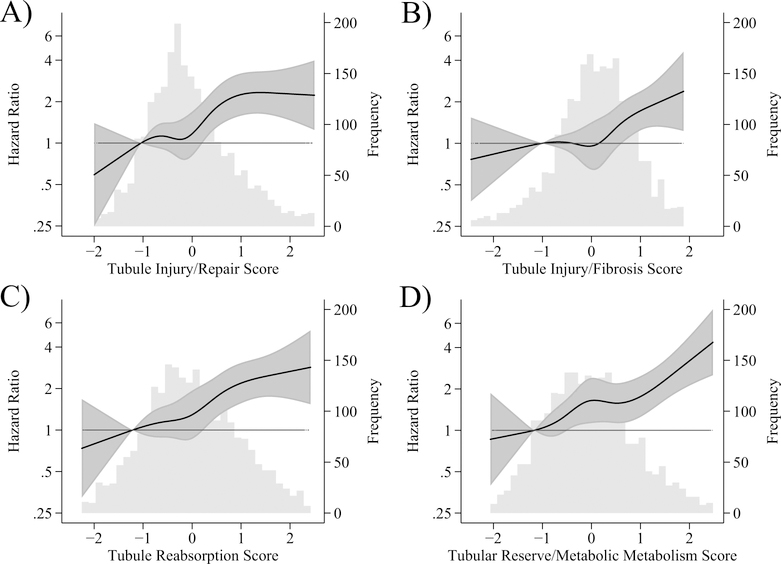
There was a strong association between the factor scores and risk of CVD events after adjustment for demographics (age, sex, race) and intervention arm (Figure 1) and associations appeared fairly linear in spline analyses.
Figure 1. Restricted cubic splines of hazard ratios and 95% confidence intervals for the association of factor scores and cardiovascular disease events in SPRINT-CKD, overlaid on histogram of factor score, n=2376.
Panel A: Tubule Injury/Repair Score. Panel B: Tubule Injury/Fibrosis Score. Panel C: Tubule Reabsorption Score. Panel D: Tubular Reserve/Mineral Metabolism Score. Models adjusted for age, sex, race, and randomization arm.
The associations of each factor with CVD events and HF, across the sequence of multivariable adjusted models is shown in Table 3. In Model 1, adjusted for demographics, intervention arm, and CVD risk factors, each factor was individually associated with risk of CVD and HF, as were eGFR and ACR. After adjustment of the factor scores for eGFR and ACR, the associations with CVD and HF were attenuated: the injury/fibrosis score was no longer significantly associated with CVD or HF, and the tubule reabsorption score was not significantly associated with HF. When all six dimensions of kidney health were included simultaneously in the model (4 tubule factor scores + eGFR + ACR), the tubule reabsorption score, the tubular reserve/mineral metabolism score, and ACR were independently associated with CVD, whereas eGFR was completely attenuated (HR 0.98, 95%CI: 0.85 to 1.13). In the same model for HF, only the tubular reserve/mineral metabolism score and ACR were significant, and eGFR was similarly null (1.03, 0.82 to 1.29).
Table 3.
Hazard ratios and 95% confidence intervals for association of factor scores, eGFR, and ACR (per standard deviation) with risk of cardiovascular disease and heart failure, n=2376
| Kidney measures | Model 1: Demographics + Intervention arm + CVD Risk Factors | Model 2: Model 1 + eGFR + ACR | Model 3: Model 2 + all factors |
|---|---|---|---|
| Cardiovascular Disease (events=306) | |||
| Tubule Injury/Repair Score | 1.25 (1.11 to 1.40) | 1.14 (1.01 to 1.28) | 1.08 (0.95 to 1.23) |
| Tubule Injury/Fibrosis Score | 1.17 (1.03 to 1.33) | 1.07 (0.94 to 1.22) | 1.05 (0.92 to 1.21) |
| Tubule Reabsorption Score | 1.37 (1.23 to 1.54) | 1.18 (1.04 to 1.34) | 1.21 (1.06 to 1.38) |
| Tubular Reserve/Mineral Metabolism Score | 1.35 (1.21 to 1.51) | 1.22 (1.06 to 1.39) | 1.24 (1.08 to 1.42) |
| eGFR (per SD =10.4 ml/min/1.73m2decrease) | 1.29 (1.15 to 1.43) | - | 0.98 (0.85 to 1.13) |
| ACR | 1.44 (1.29 to 1.60) | - | 1.23 (1.08 to 1.40) |
| Heart Failure (events = 123) | |||
| Tubule Injury/Repair Score | 1.36 (1.15 to 1.60) | 1.22 (1.03 to 1.44) | 1.16 (0.96 to 1.40) |
| Tubule Injury/Fibrosis Score | 1.34 (1.07 to 1.67) | 1.18 (0.94 to 1.48) | 1.11 (0.88 to 1.41) |
| Tubule Reabsorption Score | 1.41 (1.18 to 1.68) | 1.11 (0.92 to 1.35) | 1.13 (0.93 to 1.39) |
| Tubular Reserve/Mineral Metabolism Score | 1.62 (1.37 to 1.92) | 1.41 (1.14 to 1.74) | 1.41 (1.13 to 1.74) |
| eGFR (per SD=10.4 ml/min/1.73m2 decrease) | 1.47 (1.24 to 1.75) | - | 1.03 (0.82 to 1.29) |
| ACR | 1.64 (1.39 to 1.94) | - | 1.32 (1.08 to 1.62) |
Demographics: age, sex, race. CVD risk factors: SBP, DBP, number of hypertension medications at baseline, smoking, total cholesterol, HDL cholesterol, statin use, history of CVD or HF. ACR modeled per SD of log2-transformed ACR. Bold indicates p<0.05.
Associations of the four factors with all-cause mortality are shown in Table 4. In Model 1, all four factors were individually associated with mortality, as were eGFR and urine ACR. After adjustment for eGFR and ACR, only the tubule injury/repair score remained significant. In a single model containing all 6 dimensions of kidney health, only eGFR (HR 1.33, 1.13 to 1.58) and ACR (1.29, 1.10 to 1.49) were associated with death. although the tubule injury/repair score was marginally not associated with death (HR 1.15, 0.998 to 1.32, p=0.053).
Table 4.
Hazard ratios and 95% confidence intervals for association of factor scores, eGFR, and ACR (per standard deviation) with risk of all-cause death, n=2376
| All-cause death (events=233) | Model 1: Demographics + Intervention Arm + CVD Risk Factors | Model 2: Model 1 + eGFR + ACR | Model 3: Model 2 + all factors |
|---|---|---|---|
| Tubule Injury/Repair Score | 1.28 (1.13 to 1.45) | 1.17 (1.03 to 1.33) | 1.15 (0.998 to 1.32) |
| Tubule Injury/Fibrosis Score | 1.26 (1.07 to 1.47) | 1.14 (0.97 to 1.33) | 1.09 (0.92 to 1.28) |
| Tubule Reabsorption Score | 1.30 (1.15 to 1.49) | 1.01 (0.88 to 1.17) | 1.00 (0.86 to 1.16) |
| Tubular Reserve/Mineral Metabolism Score | 1.43 (1.26 to 1.62) | 1.12 (0.95 to 1.30) | 1.09 (0.93 to 1.28) |
| eGFR (per SD=10.4 ml/min/1.73m2 decrease) | 1.56 (1.38 to 1.77) | - | 1.33 (1.13 to 1.58) |
| ACR | 1.55 (1.37 to 1.75) | - | 1.29 (1.10 to 1.49) |
Demographics: age, sex, race. randomization arm. CVD risk factors: SBP, DBP, number of hypertension medications at baseline, smoking, total cholesterol, HDL cholesterol, statin use, history of CVD or HF. ACR modeled per SD of log2-transformed ACR. Bold indicates p<0.05.
The inclusion of factor scores significantly improved discrimination of CVD risk based on the C-statistics (Table 5). Compared to a base model (C-statistic 0.674), there was significant improvement either by adding both eGFR and ACR (C-statistic 0.704, p=0.001) or by adding all the factor scores (C-statistic 0.716, p=0.0001 to the base model). Including all dimensions of kidney health (eGFR, ACR, and all factor scores) resulted in the highest C-statistic (0.719), which was significantly better than the model with eGFR and ACR (p=0.009), but not than the model with the factor scores (p=0.30). Among participants without a history of CVD (n=1885), the tubule factors had a somewhat larger improvement in the C-statistic above and beyond eGFR and ACR (0.026) compared with the same models in the overall cohort (0.015). (Table 5)
Table 5.
C-statistics (95% confidence interval) for discrimination of CVD events, overall and in participants without a history of CVD.
| Models | Overall n=2376 | Participants without history of CVD n=1885 |
|---|---|---|
| Base model (Model 1) | 0.674 (0.641 to 0.706) | 0.667 (0.626 to 0.709) |
| Base + eGFR/ACR (Model 2a) | 0.704 (0.673 to 0.735)* | 0.705 (0.667 to 0.744)* |
| Base + factor scores (Model 2b) | 0.716 (0.686 to 0.745)* | 0.728 (0.692 to 0.765)*† |
| Base + eGFR/ACR + factor scores (Model 3) | 0.719 (0.690 to 0.749)*† | 0.731 (0.695 to 0.768)*† |
different than base model, p<0.05.
different than eGFR/ACR model, p<0.05
Base model is same as Model 1 in Tables 3 & 4; includes demographics (age, sex, race), intervention arm, and CVD risk factors (SBP, DBP, number of hypertension medications at baseline, smoking, total cholesterol, HDL cholesterol, statin use, history of CVD or HF).
In the sensitivity analysis stratified by intervention arm (Supplementary Table S3), one of twelve interactions was statistically significant: for the tubular reserve/mineral metabolism score and HF, the HR in the standard arm was 1.10 (95%CI: 0.83 to 1.45) and in the intervention arm was 1.63 (95%CI: 1.26 to 2.11) (p-for-interaction: 0.02). As alpha was not corrected for multiple comparisons, this interaction may be due to chance. In a second sensitivity analysis, stratification by prior history of CVD or HF (Supplementary Table S4) showed effect modification for the tubule reabsorption score with both CVD and HF. The association of the tubule reabsorption score was significantly stronger in those without a history of CVD/HF compared to those with a history of CVD/HF.
DISCUSSION
In this study among SPRINT participants with CKD at baseline, novel dimensions of kidney tubule health, measured by a panel of urine and serum biomarkers and quantified by factor analysis, were independently associated with risk of CVD, HF, and mortality. These new dimensions of kidney tubule health also improved discrimination of CVD risk beyond that provided by eGFR and ACR. The tubule factor scores were only weakly correlated with eGFR and ACR, indicating that they represent distinct dimensions of kidney health that are not captured by these kidney function measures available in contemporary clinical practice. In mutually adjusted models, while two of the factors remained strongly associated with CVD events, eGFR was completely attenuated, indicating that tubular health may, in large part, explain the association between eGFR and CVD events. These findings suggest that integrating multiple kidney biomarkers into scores representing physiologically distinct tubule processes can create new metrics of kidney health that are distinct from eGFR and ACR, and that may improve CKD diagnostics and prognostication.
To our knowledge, this is the first study to show gains in combining biomarkers using factor analysis in the setting of CKD.31 In our previous analyses examining biomarkers individually with risk of CVD and death in the same SPRINT CKD subset, we found that only urine alpha-1 microglobulin and uromodulin concentration were independently associated with CVD events, while alpha-1, YKL-40 and IL-18 were also associated with death.21, 22 The current study shows that by combining biomarkers using factor analysis, we created stronger, more statistically precise markers of tubule health, gaining power to detect associations that were not present for individual biomarkers. In combining multiple biomarkers into a few indices, factor analysis accounted for the correlation among biomarkers and reduced the influence of random error from both measurement error and intra-individual variability. Additionally, the factors were developed using an unsupervised statistical approach based solely on the inter-relationship of the biomarkers themselves, without any influence of their association with endpoints. What emerged were four factors that represented distinct physiologic processes based on the biomarkers that were grouped together. This reflects factor analysis’ ability to identify relevant underlying biological processes and provided new integrated scores that are useful both for research and clinical practice. For research, we envision the use of factor analysis to accommodate and sort an ever-expanding number of candidate biomarkers, to determine which biomarkers add substantial power to existing factor scores and to identify biomarkers that detect new dimensions of kidney health. In future clinical practice, we hope that biomarkers will be ordered in parsimonious panels depending on indication, such as a proximal tubule injury score for HIV drug toxicity32 and a secretion score for drug dosing.33
Ever since eGFR was found to predict CVD events, it has been posited that glomerular filtration may not be the primary kidney-related contributor to CVD risk.2 However, few studies have been able to pinpoint other causes that would explain the strong and consistent association between eGFR and CVD.22 In our study, eGFR was associated with CVD before, but not after, adjustment for kidney tubule factor scores. The attenuation of eGFR’s association with CVD may have several explanations. One possibility is that kidney tubule dysfunction and injury may directly contribute to atherosclerotic events more so than glomerular filtration. The numerous homeostatic functions performed by the kidney tubules likely have wide-ranging effects, including endothelial function. Another possibility is that kidney tubular health is not causally associated with CVD but rather is serving as a more sensitive marker of vascular damage or other aging processes that lead to increased risk of CVD. The tubules may reflect cumulative damage from environmental toxins and other sources that harm both the tubules and other vascular systems in parallel. As such, the health of the kidney tubules could be a marker of total vascular damage that is not captured by traditional cardiovascular risk factors such as high serum cholesterol and high blood pressure. Regardless of which of these hypotheses is proven true, the ability to non-invasively measure kidney tubule health, its ability to predict CVD, and its strong attenuating effects on eGFR suggest that these markers have important potential to provide new insights to the links between kidney disease and CVD beyond currently available clinical measures.
This study also provides insights on the relationships between eGFR, albuminuria, and kidney tubule health. Previous studies have hypothesized that albuminuria may be a marker of total kidney damage rather than glomerular or tubular damage exclusively.18 The findings reported here support this hypothesis, as we observed that albuminuria was somewhat correlated with tubule injury scores while eGFR was not correlated. Additionally, as albumin is reabsorbed after filtration,19 we reassuringly saw a modest correlation between ACR and the tubule reabsorption score.
The factor scores provided similar, if not slightly stronger, improvement in CVD discrimination than eGFR and ACR in this population. Adding factor scores to the eGFR/ACR model also improved CVD discrimination, while adding eGFR/ACR to the factor scores did not. We believe that, while these findings provide important proof of the concept that tubular dysfunction and injury may provide new insights into the link between kidney and vascular disease, the list of markers evaluated here may not be optimal. Further work is needed to refine the panel of biomarkers that would most improve CVD discrimination. However, whatever the ultimate panel is composed of, it is likely that factor analysis may assist in understanding unique physiological processes within the kidney that may contribute to CVD risk.
This study has important limitations. First, conducting this study in a population with CKD with a relatively restricted range of eGFR (20–59ml/min/1.73m2) may have limited our ability to detect an independent association between eGFR and outcomes. However, the association between eGFR and death provides more confidence in interpreting our null results for CVD and HF as being unlikely to result from a lack of power. Second, we did not have a validation cohort to ensure that our results are replicable in another population. Third, biomarkers were measured from frozen spot urine samples, and the influence of storage and diurnal variation in these biomarkers has not been extensively studied. Fourth, SPRINT excluded individuals with diabetes, polycystic kidney disease, and proteinuria >1g/day. Whether or not the results generalize to these populations requires future study. Finally, we selected 10 different biomarkers of kidney tubule health. The study benefitted from our ability to compare their inter-correlation and utilize them to develop factors. On the other hand, these are among a subset of novel markers of kidney tubule health, and additional markers may have further improved risk discrimination.
In conclusion, by combining kidney tubule health biomarkers using an unsupervised approach, we identified multiple factors that defined unique aspects of kidney tubule health. Several of these factors were strongly associated with CVD and death and improved discrimination of CVD above and beyond traditional CVD risk factors, eGFR, and ACR; replication is needed in other settings. When factors were employed, the strong association of eGFR with CVD was completely attenuated, while associations of several factors with CVD remained robust. Summary scores of kidney tubular health may help capture unique aspects of CKD not evident by eGFR, ACR, or individual biomarkers by themselves.
PERSPECTIVES
CKD is a strong risk factor for CVD, but current clinically used measures of kidney health (eGFR and ACR) focus on glomerular health. The kidney tubules perform a myriad of functions important for maintaining homeostasis and likely contain important prognostic information for CVD. We demonstrated that factor analysis can distill complex information from ten biomarkers of kidney tubule health into summary scores representing four distinct dimensions of tubule health. Several of these scores were significantly associated with cardiovascular events and mortality. Our study thereby showed both the importance of the kidney tubules in CVD risk discrimination as well as the utility of factor analysis for integrating numerous kidney tubule biomarkers into clinically useful scores for future research.
Supplementary Material
Novelty and Significance.
- What is New?
- We measured 10 biomarkers measured in blood and urine and applied statistical methods to condense them into four distinct aspects of kidney tubule health. This improved identification of people who developed cardiovascular disease, a common complication of chronic kidney disease
- What is Relevant?
- Hypertension is a strong risk factor for chronic kidney disease, and all our participants had hypertension
- By better characterizing the location and type of dysfunction in the kidney, we can improve treatments for chronic kidney disease
- Summary
- It is important to measure the health of the kidney tubules to improve management of chronic kidney disease, and the statistical method we used to condense numerous biomarkers into a small number of distinct scores is crucial to advance kidney research
ACKNOWLEDGMENTS
The Systolic Blood Pressure Intervention Trial (SPRINT) investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health (NIH) and the National Research Service Award through the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK; grants R01DK098234 to J.H.I. and M.G.S. and K24DK110427 to J.H.I.) and the American Heart Association (14EIA18560026 to J.H.I.). A.K.L was supported by the National Institute on Aging (NIA; grant 2T32AG000212).
SPRINT is funded with federal funds from the NIH, including the National Heart, Lung, and Blood Institute, the NIDDK, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke (contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C, and interagency agreement A-HL-13–002-001). It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. We also acknowledge the support from the following Clinical and Translation Science Awards funded by National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University: UL1RR025771; Stanford University: UL1TR000093; Tufts University: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas, Southwestern: 9U54TR000017–06; University of Utah: UL1TR000105–05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of CA, Davis: UL1TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337 Centers of Biomedical Research Excellence Award National Institute of General Medical Sciences; and Wake Forest University: UL1TR001420.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the US Government.
DISCLOSURES
Dr. Shlipak reports grant support from Cricket Health and TAI Diagnostics outside the submitted work. Dr. Ix reports grant support from Baxter International outside the submitted work. All other authors report no relevant financial, personal, or professional relationships.
REFERENCES
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- 2.Hostetter T Chronic Kidney Disease Predicts Cardiovascular Disease. N Engl J Med. 2004;351:1334–1346. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL and Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC and Stegall MD. The Association Between Age and Nephrosclerosis on Renal Biopsy Among Health Adults. Ann Intern Med. 2010;152:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Ballew SH and Coresh J. Cardiovascular risk prediction in people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han WK, Bailly V, Abichandani R, Thadhani R and Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. [DOI] [PubMed] [Google Scholar]
- 7.Parikh CR, Jani A, Melnikov VY, Faubel S and Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. American Journal of Kidney Diseases. 2004;43:405–414. [DOI] [PubMed] [Google Scholar]
- 8.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A and Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, Parikh CR and Cantley LG. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, Himmelfarb J and Zager RA. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol. 2011;22:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akerstrom B, Lodgdberg L, Berggard T, Osmakr P and Lindqvist A. alpha1-Microglobulin: a yellow-brown lipocalin. Biochimica et Biophysica Acta. 2000;1482:172–184. [DOI] [PubMed] [Google Scholar]
- 12.Garimella PS and Sarnak MJ. Uromodulin in kidney health and disease. Curr Opin Nephrol Hypertens. 2017;26:136–142. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR and Mansour SG. Perspective on Clinical Application of Biomarkers in AKI. J Am Soc Nephrol. 2017;28:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park M, Hsu CY, Go AS, Feldman HI, Xie D, Zhang X, Mifflin T, Waikar SS, Sabbisetti VS, Bonventre JV, Coresh J, Nelson RG, Kimmel PL, Kusek JW, Rahman M, Schelling JR, Vasan RS, Liu KD, Chronic Renal Insufficiency Cohort Study I and Consortium CKDB. Urine Kidney Injury Biomarkers and Risks of Cardiovascular Disease Events and All-Cause Death: The CRIC Study. Clin J Am Soc Nephrol. 2017;12:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park M, Katz R, Shlipak MG, Weiner D, Tracy R, Jotwani V, Hughes-Austin J, Gabbai F, Hsu CY, Pfeffer M, Bansal N, Bostom A, Gutierrez O, Sarnak M, Levey A and Ix JH. Urinary Markers of Fibrosis and Risk of Cardiovascular Events and Death in Kidney Transplant Recipients: The FAVORIT Trial. Am J Transplant. 2017;17:2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jotwani V, Katz R, Ix JH, Gutierrez OM, Bennett M, Parikh CR, Cummings SR, Sarnak MJ and Shlipak MG. Urinary Biomarkers of Kidney Tubular Damage and Risk of Cardiovascular Disease and Mortality in Elders. Am J Kidney Dis. 2018;72(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, Bennett M, Butch A, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C and Shlipak M. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med. 2014;15:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, Bennett MR, Fried L, Ix JH, Satterfield S, Simonsick EM, Parikh CR, Shlipak MG and Health ABCS. Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol. 2014;25:1545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driver TH, Katz R, Ix JH, Magnani JW, Peralta CA, Parikh CR, Fried L, Newman AB, Kritchevsky SB, Sarnak MJ, Shlipak MG and Health ABCS. Urinary kidney injury molecule 1 (KIM-1) and interleukin 18 (IL-18) as risk markers for heart failure in older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014;64:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Bottai M, Sundstrom J and Arnlov J. Urinary kidney injury molecule-1 and the risk of cardiovascular mortality in elderly men. Clin J Am Soc Nephrol. 2014;9:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jotwani VK, Lee AK, Estrella MM, Katz R, Garimella PS, Malhotra R, Rifkin DE, Ambrosius W, Freedman BI, Cheung AK, Raphael KL, Drawz P, Neyra JA, Oparil S, Punzi H, Shlipak MG, Ix JH. Urinary biomarkers of tubular damage are associated with mortality but not cardiovascular risk among Systolic Blood Pressure Intervention Trial participants with chronic kidney disease. Am J Nephrol. 2019;49(5):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garimella PS, Lee AK, Ambrosius WT, Bhatt U, Cheung AK, Chonchol M, Craven T, Hawfield AT, Jotwani, Killeen A, Punzi H, Sarnak MJ, Wall BM, Ix JH and Shlipak MG. Markers of Kidney Tubule Function and Risk of Cardiovascular Disease Events and Mortality in the SPRINT Trial. Eur H Journal. 2019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr., Whelton PK and Group SSR. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC Jr., Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS and Investigators C-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocco MV, Chapman A, Chertow GM, Cohen D, Chen J, Cutler JA, Diamond MJ, Freedman BI, Hawfield A, Judd E, Killeen AA, Kirchner K, Lewis CE, Pajewski NM, Wall BM, Yee J and Group SR. Chronic Kidney Disease Classification in Systolic Blood Pressure Intervention Trial: Comparison Using Modification of Diet in Renal Disease and CKD-Epidemiology Collaboration Definitions. Am J Nephrol. 2016;44:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello AB and Osborne JW. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most From your Analysis. Practical Assessment, Research and Evaluation. 2005;10. [Google Scholar]
- 28.Fabrigar LR, MacCallum RC, Wegener DT and Strahan EJ. Evaluating the Use of Exploratory Factor Analysis in Psychological Research. Psychological Methods. 1999;4:272–299. [Google Scholar]
- 29.Thurstone LL. Multiple Factor Analysis: A Development and expansion of vectors of the mind. Chicago, IL: University of Chicago; 1947. [Google Scholar]
- 30.Wattanakit K, Folsom AR, Chambless LE and Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149:606–12. [DOI] [PubMed] [Google Scholar]
- 31.Manhenke C, Orn S, von Haehling S, Wollert KC, Ueland T, Aukrust P, Voors AA, Squire I, Zannad F, Anker SD and Dickstein K. Clustering of 37 circulating biomarkers by exploratory factor analysis in patients following complicated acute myocardial infarction. Int J Cardiol. 2013;166:729–35. [DOI] [PubMed] [Google Scholar]
- 32.Jotwani V, Scherzer R, Glidden DV, Mehrotra M, Defechereux P, Liu A, Gandhi M, Bennett M, Coca SG, Parikh C, Grant RM and Shlipak M. Pre-exposure Prophylaxis With Tenofovir Disoproxil Fumarate/Emtricitabine and Kidney Tubular Dysfunction in HIV-Uninfected Individuals. J Acqui Immune Defic Syndr. 2018;78:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K and Kestenbaum B. Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin J Am Soc Nephrol. 2018; 13(8):1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.