Abstract
Obesity-related hypertension is a major public health concern. We recently demonstrated that plasma levels of the soluble form of the prorenin receptor (sPRR) were elevated in obesity-associated hypertension. Therefore, in the present study, we investigated the contribution of sPRR to blood pressure elevation in the context of obesity. High-fat fed C57BL/6 male mice were infused with vehicle or sPRR (30 µg/kg/day) via subcutaneously implanted osmotic minipump for 4 weeks. Blood pressure parameters were recorded using radiotelemetry devices. Male mice infused with sPRR exhibited higher systolic blood pressure and mean arterial pressure and lower spontaneous baroreflex sensitivity than mice infused with vehicle. To define mechanisms involved in systolic blood pressure elevation, mice were injected with an angiotensin-II type 1 receptor antagonist (losartan), a muscarinic receptor antagonist (atropine), a β-adrenergic antagonist (propranolol) and a ganglionic blocker (chlorisondamine). Losartan did not blunt sPRR-induced elevation in systolic blood pressure. Chlorisondamine treatment exacerbated the decrease in mean arterial pressure in male mice infused with sPRR. These results demonstrated that sPRR induced autonomic nervous dysfunction. Interestingly, plasma leptin levels were increased in high-fat fed C57BL/6 male mice infused with sPRR. Overall, our results indicated that sPRR increased systolic blood pressure through an impairment of the baroreflex sensitivity and an increase in the sympathetic tone potentially mediated by leptin in high-fat fed C57BL/6 male mice.
Keywords: soluble prorenin receptor, blood pressure, obesity
Graphical Abstract
Summary
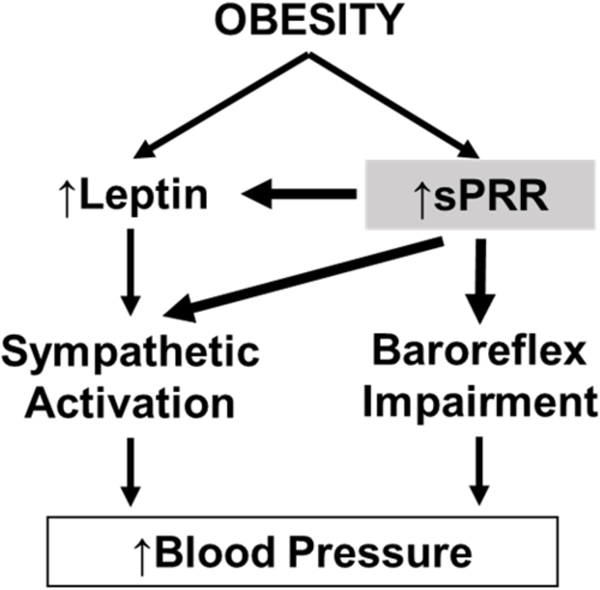
The soluble form of the prorenin receptor elevates blood pressure during the development of obesity, demonstrating an important role for sPRR in the control of blood pressure in obese male mice. In obese male mice, sPRR may elevate blood pressure primarily through a dysregulation of the autonomic nervous system via an impairment of the baroreflex sensitivity and an increase in the sympathetic tone.
Introduction
Obesity is one of the major causes of the rise in prevalence of hypertension1. According to the National Health and Nutrition Examination Survey (2011–14), 36.5% of adults are obese and 29.1 % are hypertensive in the US 2,3.Treating hypertension associated with obesity has become the most challenging healthcare epidemic for the medical field. Despite treatments (Angiotensin-converting enzyme inhibitors, angiotensin-II type 1 receptor blockers, adrenergic receptor antagonists and calcium channel blockers), 20–30% of the patients have resistant hypertension 4,5. Therefore, to advance medical care, a clear need exists for better understanding the mechanisms responsible for the pathogenesis of hypertension associated with obesity.
The renin angiotensin system (RAS) plays a pivotal role in blood pressure control and fluid homeostasis. We and others recently found that (pro)renin receptor (PRR), a component of the RAS, is up-regulated during the development of obesity 6–9. The PRR, a 350 amino protein with a single transmembrane domain, is the receptor for (pro)renin, renin in its active form and prorenin in its inactive form 10,11. PRR can be cleaved to generate a soluble form of PRR (sPRR). The sPRR can be retained inside cells 12 and secreted into plasma 13, urine and into the extra-cellular space 14,15. An increasing body of evidence suggested that sPRR has a biological function. Previous studies have found that sPRR increased renin activity of prorenin in vitro 16. sPRR up-regulated renal aquaporin 2 gene through LRP6/FZD8 and participated in urine concentration 17. Moreover, we demonstrated that infusion of sPRR activated the RAS by increasing plasma renin levels and by up-regulating renal and hepatic angiotensinogen (AGT) genes in C57BL/6 female mice fed a standard diet 18. In human and rodents, sPRR has also been shown to be a potential biomarker of cardiovascular pathologies. For instance, plasma sPRR levels were elevated in patients with heart failure 19. In patients with essential hypertension, circulating sPRR levels correlated positively with urinary AGT excretion and negatively with glomerular filtration rate (GFR) 20. High circulating levels of plasma sPRR in early pregnancy predicted elevated systolic blood pressure (SBP) and high sPRR levels at delivery were associated with preeclampsia 21. Interestingly, we recently demonstrated that sPRR levels are elevated during the development of obesity-hypertension and in mouse models of lipodystrophy 9,18. However, whether sPRR is directly involved in blood pressure control during the development of obesity remained to be investigated.
Therefore, the objectives of our study were to determine whether sPRR infusion affects the blood pressure regulation during the development of obesity in male mice and to investigate whether elevated blood pressure was mediated by an AngII-dependent mechanism in male mice.
Methods and Animals
The data, analytic methods, and study materials that support the findings of this study are available from the corresponding author upon reasonable request.
Experimental protocol.
All animal protocols described below were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky (IACUC protocol number 2013–1109).
Six weeks old C57BL/6 male mice (The Jackson Laboratory, DIO C57BL/6J, Jax # 380050) were fed a high-fat diet (HF, 60% fat; catalog #D12492, Research Diets Inc, New Brunswick, NJ). Mice were provided ad libitum access to food and drinking water. Body weight was recorded once a week. After 15 weeks of HF feeding, a total of 12 out of 14 male mice were implanted with a catheter connected to a telemetry transmitter (PA-C10 model, DSI, St Paul, MN) in the left carotid artery for blood pressure measurement. Surgery was performed under isoflurane anesthesia and followed by carprofen injection for 3 days (10mg/kg daily, i.p). After radiotelemetry device implantation, mice were housed individually. Blood pressure (BP) was recorded prior vehicle or sPRR infusion to design 2 groups of mice with similar BP. On week 17 of HF feeding, all mice (n=14) were implanted with an osmotic pump (Alzet Mini osmotic pump, Model 2004, Durect Corporation, Cupertino, CA) under isoflurane anesthesia and infused for 4 weeks with vehicle (saline) (Veh, n=7) or sPRR (mouse recombinant sPRR-HisTag, 30 μg/kg/day) (sPRR, n=7), as previously described 18. On day 27 of infusion, mice were placed in individual metabolic cages for 24h with free access to food and water (Tecniplast Solo Mouse Metabolic Cages, Tecniplast USA, Exton, PA) for urine collection.
Quantification of plasma and urine parameters.
Plasma AGT levels were determined using mouse total AGT assay kit (IBL Co, Minneapolis, MN). Plasma renin activity was evaluated using Renin Assay Kit (Sigma, St Louis, MO) according to the manufacturer’s instructions. Results were expressed in renin equivalent calculated from the change in relative fluorescence units for each sample compared with that of recombinant renin provided in the kit. Plasma leptin levels were measured using Mouse/Rat Leptin ELISA (Alpco, Salem, NH). Plasma total prorenin/renin levels were assessed using Mouse Prorenin and Renin Total Antigen ELISA Kit (Molecular Innovation, Novi, MI). Urinary vasopressin levels were determined with Arg8-Vasopressin ELISA kit (Enzo Life Sciences, Farmingdale, NY).Urinary sodium excretion was quantified using a dual channel flame photometer (Model 2655–10, Cole-Parmer Instrument Company, Vernon Hills, IL).
LC-MS/MS based quantification of equilibrium plasma angiotensin levels.
Angiotensins levels were measured by Attoquant Diagnostics as previously described 18. Equilibrium angiotensin concentrations were analyzed by mass spectrometry following 30 min of equilibration of conditioned heparin plasma at 37°C and subsequent stabilization of equilibrium peptide levels. Stabilized samples were spiked with 200 pg of stable isotope-labeled internal standard for each individual angiotensin metabolite (AngI, AngII, Ang1–7, Ang1–5, Ang2–8, Ang3–8, Ang2–10, Ang2–7, Ang1–9, and Ang3–7). Following C18-based solid-phase-extraction, samples were subjected to LC-MS/MS analysis using a reversed-phase analytical column (Acquity UPLC® C18, Waters) operating in line with a XEVO TQ-S triple quadrupole mass spectrometer (Waters) in MRM mode.
Immunostaining.
Retroperitoneal fat pads were fixed in paraformaldehyde and embedded in paraffin blocks. Sections were stained with hematoxylin and eosin and examined with a Nikon Eclipse 80i light microscope. Cells size and number were determined at 10x magnification using NIS Elements BR.3.10 software.
RNA extraction and quantitative RT-PCR.
RNA was extracted from kidney using the SV Total RNA Isolation System (Promega, Madison, WI) and quantified with a NanoDrop 2000 spectrophotometer (Wilmington, DE). PerfeCTa SYBR Green FastMix (Quanta BioSciences, Gaithersburg, MD) was used to perform real-time quantitative PCR after cDNA synthesis using qscript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD).
Western blotting.
Protein from frozen brain and kidney were extracted in ice-cold Tris buffer with a Geno/Grinder® 2010 (SPEX SamplePrep, Metuchen, NJ) and were submitted to SDS-Page on precast polyacrylamide gel (Mini-PROTEAN® TGX™, 4–20%, Bio-Rad Laboratories, Hercules, CA). After transfer to polyvinylidene difluoride membrane and blocking in 5% non-fat dried milk in Tris-buffered saline with 0.1 % Tween 20 (TBST), membranes were incubated with anti-PRR antibody (Sigma, St Louis, MO), anti-AQP2 antibody (Cell Signaling Technology, Inc., Danvers, MA) or anti-GAPDH (Cell Signaling Technology, Inc., Danvers, MA) in TBST 5% non-fat dried milk. Following incubation with HRP-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), proteins were imaged using a Syngene PXi imager (Syngene, Frederick, MD). The levels of proteins were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH levels.
Assessment of cardiovascular parameters using radiotelemetry.
Systolic blood pressure (SBP), mean arterial pressure (MAP), diastolic blood pressure (DBP) and heart rate (HR) were examined for 5 consecutive days after 2 weeks of infusion with vehicle or sPRR. The spontaneous baroreflex sensitivity (SBRS) was analyzed with HemoLab Software Ver. 20.7, using the sequence method described by Bertinieri et al22 after 2 weeks of infusion with vehicle or sPRR. After 3 weeks of infusion, the contribution of the RAS to BP control was evaluated using losartan (20 mg/kg body weight, i.p). The autonomic function was assessed using atropine sulfate (5 mg/kg body weight, i.p), propranolol (5 mg/kg body weight, i.p) and chlorisondamine diiodide (1 mg/kg body weight, i.p).
Statistical Analysis.
Data are represented as means ± the standard error of the mean (SEM). Statistical differences between groups were assessed by one-way ANOVA or two-way ANOVA followed by Holm-Sidak post-hoc analysis for multiple comparisons. The normal distribution of the data was tested using Shapiro-Wilk test. When required, data were transformed to ensure normal distribution. Statistical outliers were identified using Grubbs test (GraphPad QuickCalcs). Based on Grubbs test outcomes, one male mouse infused with vehicle was excluded from blood pressure measurement. Values of P<0.05 were considered statistically significant.
Results
sPRR infusion tends to increase fat mass in high-fat fed male mice
To determine whether sPRR influenced body weight and adipose deposition during the development of obesity, male mice were fed a high fat diet for 17 weeks and infused with sPRR or with vehicle. After 4 weeks of infusion, there was no significant difference in body weight of HF-fed C57BL/6 male mice infused with sPRR compared with HF-fed male mice infused with vehicle (Figure 1A). White fat pad weights (retro-peritoneal and subcutaneous fat) tended to increase in HF-fed C57BL/6 male mice infused with sPRR compared with HF-fed male mice infused with vehicle (Figure 1B), likely due to an increased number of enlarged adipocytes (Supplemental figure S1, please see http://hyper.ahajournals.org). sPRR infusion did not change liver, kidney and heart weights but slightly affected the weights of the pancreas and the spleen (Supplemental Table S1).
Figure 1.
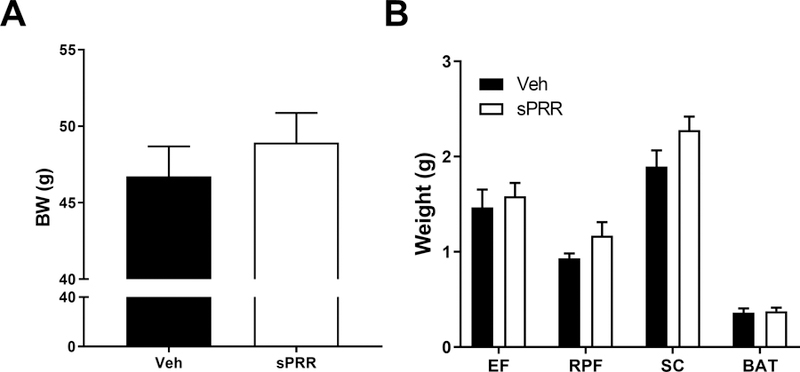
The infusion of sPRR tended to increase adipose depot weight in obese male mice. (A) Body weight of HF-fed C57BL/6 male mice after 4 weeks of infusion with vehicle (Veh, n=7) or sPRR (sPRR, n=7). (B) Weight of epididymal fat (EF), retroperitoneal fat (RPF), subcutaneous fat (SC) and brown adipose tissue (BAT) of mice after 4 weeks of infusion. Values are mean±SEM. One–way ANOVA with post hoc Holm-Sidak multiple comparison was performed to detect differences between groups.
sPRR infusion induced an increase in blood pressure during the development of obesity
After 2 weeks of infusion, SBP and MAP were significantly increased during light and dark cycles in HF-fed male mice infused with sPRR compared to male mice infused with vehicle (Figure 2A and Supplemental Table S2), suggesting that sPRR infusion potentiated the elevation of blood pressure during the development of obesity. We next examined the participation of the autonomic nervous system to SBP elevation by assessing the baroreflex sensitivity. HF-fed mice infused with sPRR displayed impaired baroreflex function compared to vehicle suggesting that sPRR might affect baroreflex sensitivity during the development of obesity in male mice (Figure 2B).
Figure 2.
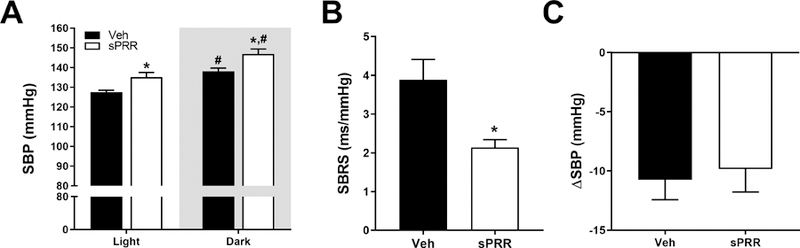
The infusion of sPRR increased systolic blood pressure and impaired baroreflex sensitivity in obese male mice. (A) Systolic blood pressure (SBP) of HF-fed C57BL/6 male mice during light and dark cycles infused with vehicle (Veh, n=5) or sPRR (sPRR, n=6). Differences were revealed using Two–way ANOVA followed by post hoc Holm-Sidak multiple comparison. *P<0.05 compared with Veh, #P<0.05 compared with light SBP. (B) Spontaneous baroreflex sensitivity (SBRS) of HF-fed C57BL/6 male mice infused with vehicle or sPRR. (C) SBP response (ΔSBP) represents the difference in SBP before and after 2 days of losartan injection (20 mg/kg body weight, i.p) in mice infused with vehicle or sPRR. Values are mean±SEM. Differences between groups were revealed using One–way ANOVA followed by post hoc Holm-Sidak multiple comparison. *P<0.05 compared with Veh.
Blood pressure response to losartan was not affected by sPRR infusion in HF-fed mice
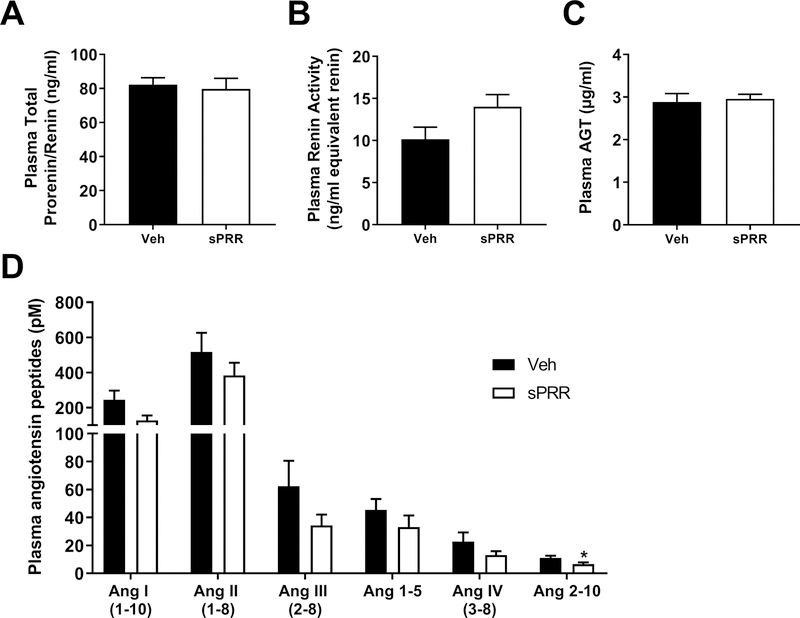
We next investigated the mechanism by which sPRR elevated SBP. To examine whether this elevation was mediated by an Angiotensin II (AngII)-dependent mechanism, male mice were treated with an angiotensin-II type 1 receptor (AT1R) antagonist (Losartan). The decrease in SBP induced by losartan was similar in HF-fed male mice infused with sPRR compared to male mice infused with vehicle suggesting that sPRR-induced increase in SBP is not primarily mediated by AT1R (Figure 2C). Plasma total prorenin/renin and AGT levels were not influenced by sPRR infusion in HF-fed male mice (Figure 3A and Figure 3C), while plasma renin activity tended to increase in HF-fed male mice infused with sPRR compared to male mice infused with vehicle (Figure 3B). The quantification of plasma angiotensin peptides in HF-fed male mice infused with sPRR did not reveal significant changes in angiotensin I, angiotensin II, angiotensin III, angiotensin 1–5 and angiotensin IV levels compared to mice infused with vehicle (Figure 3D). Interestingly, plasma Ang2–10 level was significantly decreased in HF-fed male mice infused with sPRR compared to mice infused with vehicle (Figure 3D). The infusion of sPRR in HF-fed male mice did not change urinary vasopressin (Supplemental figure S2A), urine volume (Supplemental figure S2B), nor urine sodium excretion (Supplemental figure S2C). sPRR infusion had no effect on PRR or aquaporin 2 mRNA abundance or protein levels in kidney (Supplemental figures S2D, S2E and S2F). In addition, sPRR infusion did not change PRR and sPRR protein contents in the brain (Supplemental figure S3).
Figure 3.
sPRR infusion in obese male mice did not change systemic RAS. (A) Plasma total prorenin/renin in HF-fed C57BL/6 male mice infused for 4 weeks with vehicle (Veh, n=7) or sPRR (sPRR, n=7). (B) Plasma renin activity. (C) Plasma angiotensinogen (AGT). (D) Angiotensin peptides levels at equilibrium. Values are mean±SEM. Differences between groups were revealed using One–way ANOVA followed by post hoc Holm-Sidak multiple comparison. *P<0.05 compared with Veh.
The elevation of blood pressure is likely mediated by the autonomic nervous system
We next determined the contribution of the autonomic nervous system to SBP elevation by assessing changes in MAP (∆MAP) and in HR (∆HR) using a non-selective muscarinic receptor antagonist (atropine-sulfate), a β-adrenergic receptor antagonist (propranolol) and a nicotinic receptor antagonist (chlorisondamine) as previously published 23. The diminution in MAP, observed after the injection of chlorisondamine, was amplified by sPRR infusion indicating that sPRR might compromise the autonomic nervous function likely by increasing vascular sympathetic activity (Figure 4A). The bradycardic response after propranolol injection (Figure 4B) and the tachycardic response after atropine injection (Figure 4C) were similar in HF-fed male mice infused with sPRR compared to male mice infused with vehicle. Interestingly, sPRR infusion increased plasma leptin levels in obese male mice (Figure 5) suggesting that sPRR effect on sympathetic activity could be partly mediated by leptin.
Figure 4.
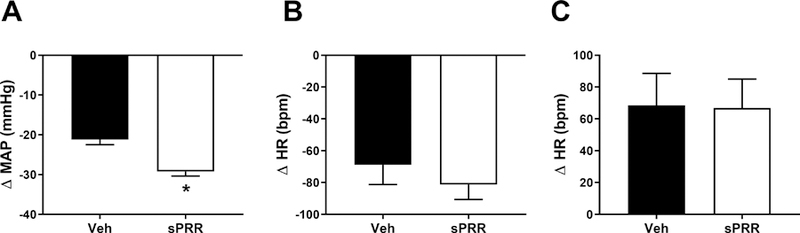
Infusion of sPRR impaired autonomous nervous system in obese male mice. (A) Mean arterial pressure response (ΔMAP) to chlorisondamine injection (1mg/kg body weight, i.p) represents the difference in MAP one hour before and one hour after drug injection in HF-fed C57BL/6 male mice infused with vehicle (Veh, n=5) or sPRR (sPRR, n=6). (B) Heart rate response (ΔHR) to propranolol injection (5mg/kg body weight, i.p) represents the difference in HR one hour before and one hour after drug injection. (C) HR response (ΔHR) to atropine injection (5mg/kg body weight, i.p) represents the difference in HR one hour before and one hour after drug injection. Values are mean±SEM. Differences between groups were revealed using One–way ANOVA followed by post hoc Holm-Sidak multiple comparison. *P<0.05 compared with Veh.
Figure 5.
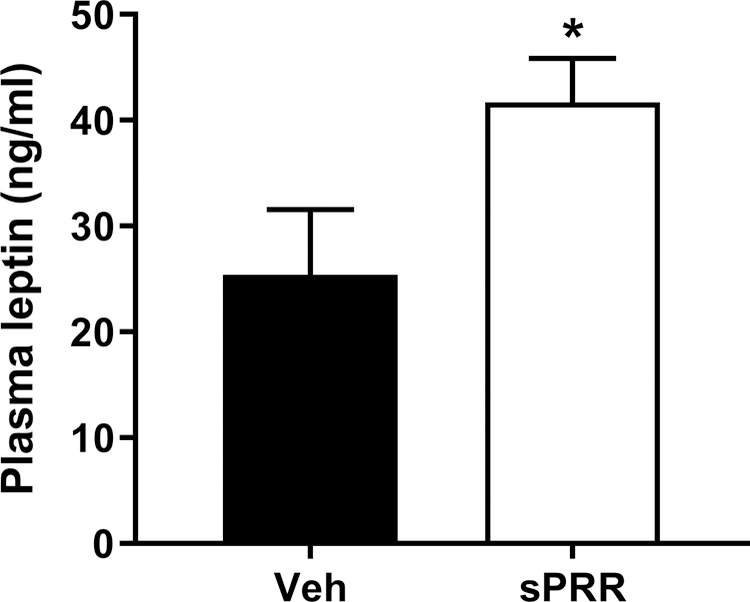
Infusion of sPRR increased plasma leptin levels in obese male mice. Plasma leptin levels in HF-fed C57BL/6 male mice infused for 4 weeks with vehicle (Veh, n=7) or sPRR (sPRR, n=7). Values are mean±SEM. Differences between groups were revealed using One–way ANOVA followed by post hoc Holm-Sidak multiple comparison. *P<0.05 compared with Veh.
Discussion
To our knowledge, no prior studies examined the role of sPRR in blood pressure regulation during diet-induced obesity in male mice. In this present study, we found that sPRR infusion elevated blood pressure in HF-fed male mice but that losartan treatment failed to restore SBP in male mice infused with sPRR. Interestingly, sPRR infusion impaired baroreflex sensitivity and autonomic nervous function potentially through leptin.
Our present study is in agreement with previous studies showing an association between sPRR levels and blood pressure. Indeed, we previously demonstrated that the deletion of adipocyte PRR led to an increase in plasma levels of sPRR, which were positively correlated with the increase in blood pressure in male mice 9. In addition, reduced PRR gene dosage in nephron progenitors cells resulted in an increase in urinary sPRR levels which were thought to contribute to developmental programming of hypertension in mice 24. In human, Narita et al., reported that plasma sPRR levels were higher in preeclamptic women than in normotensive pregnant women 25.
The role of the sympathetic nervous system in obesity-related hypertension is well recognized and has been extensively described in several studies 26–29. The underlying mechanisms involved endothelial dysfunction 30, increased leptin levels 31, decreased adiponectin and ghrelin levels 32,33 and baroreflex dysfunction 34. In the present study, the impairment of the baroreflex sensitivity observed in male mice infused with sPRR could partly explain the autonomic nervous system dysfunction. In addition, our data demonstrated that, chlorisondamine treatment induced a larger decrease in MAP in mice infused with sPRR than in mice infused with vehicle, suggesting that sPRR could increase vascular sympathetic tone. Since leptin has been reported to cause sympathetic activation leading to an increase in blood pressure 35–37, and since our data showed that sPRR infusion elevated plasma leptin, one could speculate that plasma leptin mediates sPRR effect on sympathetic tone.
Previous studies showed that the activation of PRR in the hypothalamic paraventricular nucleus induced sympathoexcitation in Sprague-Dawley rats 38. Additionally, PRR knockdown in the brain of human renin-AGT double transgenic hypertensive mice or intracerebroventricular infusion of PRO20 in DOCA salt treated mice improved baroreflex sensitivity and lowered cardiac and vasomotor sympathetic tone 39,40. However, in HF-fed male mice infused with sPRR, brain full length and sPRR protein contents were not increased indicating that the activation of the sympathetic activity is likely independent of cerebral PRR.
Losartan reduced SBP to the same extent in male mice infused with sPRR compared with male mice infused with vehicle. Moreover, plasma total prorenin/renin, AGT and Ang II levels at equilibrium were not increased in mice infused with sPRR compared with mice infused with vehicle. Together our data suggested that, in HF-fed male mice, sPRR-induced SBP increase is not likely mediated by an AT1R-dependent mechanism. Interestingly, the equilibrium peptide level analysis revealed that sPRR reduced Ang2–10 concentration, an angiotensin peptide known to induce opposite effect to AngII through AT1R and thus promote cardioprotection 41–43. Therefore, Ang2–10 reduction could also participate to SBP elevation.
A body of evidence suggested sex-specific mechanisms in the development of obesity-associated hypertension 44,45. Notably, it is recognized that obese males have a higher propensity to exhibit elevated sympathetic nerve activity than obese females 46,47. In the present study, we demonstrated that sPRR elevated sympathetic activity in HF-fed male mice. In addition, losartan did not blunt the elevation of blood pressure in C57BL/6 male mice infused with sPRR. Similar results from our group were observed in HF-fed adipose PRR KO male mice exhibiting elevated sPRR levels (unpublished data: ∆SBP after losartan injection, WT mice= −7.9 ± 1.5 mmHg; adipose PRR KO mice= −6.4 ± 3.1 mmHg). In contrast, we recently showed that the elevation of blood pressure in HF-fed adipose PRR KO female mice was mediated by an AngII/AT1R-dependent mechanism but not through the autonomic nervous system 18. Therefore, our data indicated that sex differences in sPRR-induced elevation in blood pressure could exist during the development of obesity. The underlying sex-specific mechanism needs further investigation.
The infusion of sPRR did not induce significant changes in body weight or organ weights. However, the presence of enlarged adipocytes, in line with a trend for an expanded adipose tissue suggested that sPRR plays a role in adipose tissue morphology and remodeling. In agreement with these results, we previously showed that adipose PRR KO significantly decreased adipose tissues weights 9,18. It would be interesting in future studies to determine whether higher dose of infused sPRR exacerbated those effects.
Perspectives
We demonstrated that, in obese male mice, sPRR participates in blood pressure regulation primarily through impairment of baroreflex sensitivity and elevation of the sympathetic tone likely mediated by leptin. Because of sexual dimorphism in obesity-hypertension, further investigation is needed to decipher sex-specific mechanism. Future studies should also investigate whether the inhibition of sPRR could prevent obesity-hypertension.
Supplementary Material
Novelty and Significance.
What is new?
Demonstration that sPRR is involved in obesity-hypertension in male mice
Demonstration that sPRR impairs the baroreflex sensitivity and increases the sympathetic tone
What is relevant?
This study demonstrates that sPRR is a contributor to the control of blood pressure in obese male mice
This study demonstrates that sPRR could represent a new therapeutic target for the treatment of hypertension related to obesity
Acknowledgments
We appreciated the work of Dr. Wen Su from Dr. Ming Gong’s laboratory for her expertise and skills with radiotelemetry device implantation. We thank Dr. Marko Poglitsch from Attoquant Diagnostics, Vienna, Austria for the quantification of angiotensin peptides.
Sources of Funding
This work was supported by National Institutes of Health Grants [R01-HL-142969]; the American Heart Association [13SDG17230008]; the National Institute of General Medical Sciences [P30 GM127211]; and the University of Kentucky, Center for Clinical and Translational Sciences [UL1TR001998].
Footnotes
Disclosures
None.
References
- 1.Cutler Jeffrey A., Sorlie Paul D., Wolz Michael, Thom Thomas, Fields Larry E., Roccella Edward J. Trends in Hypertension Prevalence, Awareness, Treatment, and Control Rates in United States Adults Between 1988–1994 and 1999–2004. Hypertension 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief 2015;(219):1–8. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 2012;(82):1–8. [PubMed] [Google Scholar]
- 4.Egan BM, Zhao Y, Li J, Brzezinski WA, Todoran TM, Brook RD, Calhoun DA. Prevalence of Optimal Treatment Regimens in Patients with Apparent Treatment Resistant Hypertension Based on Office BP in a Community-Based Practice Network. Hypertension 2013;62(4):691–697. doi: 10.1161/HYPERTENSIONAHA.113.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu-Isenberg KS. Initial Mono- And Combinaton Therapy And Hypertension Control The First Year. Hypertension 2012;59(6):1124–1131. doi: 10.1161/HYPERTENSIONAHA.112.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol - Regul Integr Comp Physiol 2007;292(1):R274–R282. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- 7.Achard V, Tassistro V, Boullu-Ciocca S, Grino M. Expression and nutritional regulation of the (pro)renin receptor in rat visceral adipose tissue. J Endocrinol Invest 2011;34(11):840–846. doi: 10.3275/7627. [DOI] [PubMed] [Google Scholar]
- 8.Tan P, Shamansurova Z, Bisotto S, Michel C, Gauthier M-S, Rabasa-Lhoret R, Nguyen TM-D, Schiller PW, Gutkowska J, Lavoie JL. Impact of the prorenin/renin receptor on the development of obesity and associated cardiometabolic risk factors. Obesity 2014;22(10):2201–2209. doi: 10.1002/oby.20844. [DOI] [PubMed] [Google Scholar]
- 9.Wu C-H, Mohammadmoradi S, Thompson J, Su W, Gong M, Nguyen G, Yiannikouris F. Adipocyte (Pro)Renin-Receptor Deficiency Induces Lipodystrophy, Liver Steatosis And Increases Blood Pressure In Male Mice. Hypertension 2016;68(1):213–219. doi: 10.1161/HYPERTENSIONAHA.115.06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabi AHMN, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 2006;18(3):483–488. [PubMed] [Google Scholar]
- 11.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer J-D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002;109(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki-Nakagawa C, Nishimura M, Noda M, Iwata H, Hattori M, Ebihara A, Suzuki F, Nakagawa T. Intracellular retention of the extracellular domain of the (pro)renin receptor in mammalian cells. Biosci Biotechnol Biochem 2014;78(7):1187–1190. doi: 10.1080/09168451.2014.915732. [DOI] [PubMed] [Google Scholar]
- 13.Cousin Christelle, Bracquart Diane, Contrepas Aurelie, Corvol Pierre, Muller Laurent, Nguyen Genevieve. Soluble Form of the (Pro)Renin Receptor Generated by Intracellular Cleavage by Furin Is Secreted in Plasma. Hypertension 2009;53(6):1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 14.Biswas KB, Nabi AN, Arai Y, Nakagawa T, Ebihara A, Ichihara A, Inagami T, Suzuki F. Qualitative and quantitative analyses of (pro)renin receptor in the medium of cultured human umbilical vein endothelial cells. Hypertens Res Off J Jpn Soc Hypertens 2011;34(6):735–739. doi: 10.1038/hr.2011.26. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. The soluble form of the (pro)renin receptor [s(P)RR] is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 2011;57(4):859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 2011;34(5):599–605. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou S-F, Gustafsson J-Å, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci U S A 2016;113(13):E1898–E1906. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatineau E, Cohn DM, Poglitsch M, Loria AS, Gong M, Yiannikouris F. Losartan prevents the elevation of blood pressure in adipose-PRR deficient female mice while elevated circulating sPRR activates the renin-angiotensin system. Am J Physiol-Heart Circ Physiol 2018;316(3):H506–H515. doi: 10.1152/ajpheart.00473.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 2013;168(4):4313–4314. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto S, Ando T, Niiyama M, Seki Y, Yoshida N, Watanabe D, Kawakami-Mori F, Kobori H, Nishiyama A, Ichihara A. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res Off J Jpn Soc Hypertens 2014;37(7):642–648. doi: 10.1038/hr.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe Noriyoshi, Bokuda Kanako, Fujiwara Takeo, Suzuki Tomo, Mito Asako, Morimoto Satoshi, Jwa Seung Chik, Egawa Makiko, Arai Yoshie, Suzuki Fumiaki, Sago Haruhiko, Ichihara Atsuhiro. Soluble (Pro)Renin Receptor and Blood Pressure During Pregnancy. Hypertension 2012;60(5):1250–1256. doi: 10.1161/HYPERTENSIONAHA.112.197418. [DOI] [PubMed] [Google Scholar]
- 22.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl Off J Int Soc Hypertens 1985;3(3):S79–81. [PubMed] [Google Scholar]
- 23.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res 2017;121(1):43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song R, Kidd L, Janssen A, Yosypiv IV. Conditional ablation of the prorenin receptor in nephron progenitor cells results in developmental programming of hypertension. Physiol Rep 2018;6(7). doi: 10.14814/phy2.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, Itoh H, Seki H. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 2016;37:72–78. doi: 10.1016/j.placenta.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 26.da Silva A, doCarmo J, Dubinion J, Hall JE. Role of Sympathetic Nervous System in Obesity Related Hypertension. Curr Hypertens Rep 2009;11(3):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116(6):976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seravalle G, Grassi G. Sympathetic Nervous System, Hypertension, Obesity and Metabolic Syndrome. High Blood Press Cardiovasc Prev 2016;23(3):175–179. doi: 10.1007/s40292-016-0137-4. [DOI] [PubMed] [Google Scholar]
- 29.Rahmouni K Obesity-Associated Hypertension: Recent Progress in Deciphering the Pathogenesis. Hypertension 2014;64(2):215–221. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamboa A, Figueroa R, Paranjape SY, Farley G, Diedrich A, Biaggioni I. Autonomic Blockade Reverses Endothelial Dysfunction in Obesity-Associated Hypertension. Hypertension 2016;68(4):1004–1010. doi: 10.1161/HYPERTENSIONAHA.116.07681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 2002;39(2 Pt 2):496–501. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension 2004;43(5):977–982. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- 33.Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med Maywood NJ 2007;232(3):390–397. [PubMed] [Google Scholar]
- 34.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension 2007;49(6):1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 35.Rahmouni K. Leptin-Induced Sympathetic Nerve Activation: Signaling Mechanisms and Cardiovascular Consequences in Obesity. Curr Hypertens Rev 2010;6(2):104–209. doi: 10.2174/157340210791170994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertens Dallas Tex 1979 2001;37(3):936–942. [DOI] [PubMed] [Google Scholar]
- 37.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertens Dallas Tex 1979 1998;31(1 Pt 2):409–414. [DOI] [PubMed] [Google Scholar]
- 38.Huber MJ, Basu R, Cecchettini C, Cuadra AE, Chen Q-H, Shan Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am J Physiol - Heart Circ Physiol 2015;309(5):H880–H887. doi: 10.1152/ajpheart.00095.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-Targeted (Pro)Renin Receptor Knockdown attenuates Angiotensin II-Dependent Hypertension. Hypertension 2012;59(6):1188–1194. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular Infusion of the (Pro)renin Receptor Antagonist PRO20 Attenuates Deoxycorticosterone Acetate-Salt–Induced Hypertension. Hypertension 2015;65(2):352–361. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh WM, Ling WC, Murugan DD, Lau YS, Achike FI, Vanhoutte PM, Mustafa MR. Des-aspartate angiotensin I (DAA-I) reduces endothelial dysfunction in the aorta of the spontaneously hypertensive rat through inhibition of angiotensin II-induced oxidative stress. Vascul Pharmacol 2015;71:151–158. doi: 10.1016/j.vph.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Min L, Sim MK, Xu XG. Effects of des-aspartate-angiotensin I on angiotensin II-induced incorporation of phenylalanine and thymidine in cultured rat cardiomyocytes and aortic smooth muscle cells. Regul Pept 2000;95(1–3):93–97. [DOI] [PubMed] [Google Scholar]
- 43.Sim M-K. Des-aspartate-angiotensin I, a novel angiotensin AT1 receptor drug. Eur J Pharmacol 2015;760:36–41. doi: 10.1016/j.ejphar.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Taylor LE, Sullivan JC. Sex differences in obesity-induced hypertension and vascular dysfunction: a protective role for estrogen in adipose tissue inflammation? Am J Physiol - Regul Integr Comp Physiol 2016;311(4):R714–R720. doi: 10.1152/ajpregu.00202.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulkner JL, de Chantemèle EJB. Sex Differences In Mechanisms Of Hypertension Associated With Obesity. Hypertension 2018;71(1):15–21. doi: 10.1161/HYPERTENSIONAHA.117.09980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks VL, Shi Z, Holwerda SW, Fadel PJ. Obesity-induced increases in sympathetic nerve activity: sex matters. Auton Neurosci Basic Clin 2015;187:18–26. doi: 10.1016/j.autneu.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briant LJB, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol 2016;101(2):219–229. doi: 10.1113/EP085368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.