Abstract
Objectives:
To determine the contribution of specific uropathogenic Escherichia coli (UPEC) lineages, drug resistance genes, and plasmid incompatibility/replicon (Inc) groups to the prevalence of β-lactam-resistant urinary tract infections (UTIs) in a university community.
Methods:
Urine samples were consecutively collected and cultured over a two-year period from patients presenting to a university health center with symptoms of UTI. Isolated UPEC were subtyped by MLST and fimH typing, and tested by PCR and sequencing for ß-lactamase genes and plasmid Inc groups.
Results:
Among 273 UPEC isolates, 85 (31%) were ampicillin-resistant (AMP-R) and 188 (69%) were susceptible to all ß-lactam drugs (AMP-S). Six lineages accounted for two-thirds of the isolates: ST95 (21%), ST69 (11%), ST420 (11%), ST73 (10%), ST127 (8%), and ST404 (3%). ST69 and ST404 were associated with AMP-R (P=0.003, P=0.0005), while ST420 and ST127 were associated with AMPS (P<0.0001, P=0.027). ST95 contained four fimH types; the ST95/f-6 sublineage was more frequently identified among the AMP-R population (P=0.009), while the ST95/f-47 sublineage was more frequently identified among the AMP-S population (P=0.007). blaTEM was the most common ß-lactamase gene, identified in 81 (95%) AMP-R isolates. IncFIB, IncFIA, and IncB/O type plasmids were the most common types identified, and were associated with ß-lactam resistance (P<0.001 for all).
Conclusions:
These observations indicate that the prevalence of β-lactam-resistant UTIs in this community was largely determined by a limited set of circulating UPEC STs and sublineages, carrying TEM ß-lactamase genes that were likely encoded on one of three Inc type plasmids.
Keywords: ß-lactamase, E. coli, fimH, MLST, plasmid incompatibility group, urinary tract infection
1. Introduction
Urinary tract infections (UTIs) are among the most common types of infection in community and healthcare settings. Uropathogenic Escherichia coli (UPEC) is the main cause of UTI in both environments, typically accounting for 75% of community-acquired UTIs (CA-UTIs) and 50–65% of healthcare-associated UTIs [1, 2]. Empirical antimicrobial therapy is frequently administered to treat UTIs and prevent the spread of infection to other sites, such as the bloodstream; however, selection of an appropriate antimicrobial agent is a growing challenge due to the increasing prevalence of antimicrobial resistance in E. coli and other urinary tract pathogens [3, 4]. In particular, ß-lactam-resistant E. coli, especially those producing extended-spectrum ß-lactamases (ESBLs) or carbapenemases, have become a global concern [3–6]. Understanding factors that affect the prevalence and dissemination of antimicrobial resistance may provide insights into strategies to slow the spread of resistance and inform better empirical treatment decisions.
UPEC are a subset of extraintestinal pathogenic E. coli (ExPEC), which cause disease outside the intestinal tract [7]. Classic pandemic ExPEC clones, identified through multilocus sequence typing (MLST), include ST393, ST69, ST73, ST95, and ST131 [7]. The predominant clones identified in a population, and the proportional composition, can vary and depend on the study population [8]. Few studies have fully examined the distribution of ExPEC STs across both antimicrobial-susceptible and antimicrobial-resistant UPEC, and even fewer studies have examined the distribution of sublineages [8–14]. As a result, it is not well understood whether the clonal composition of the antimicrobial-resistant UPEC population in a community is distinct from the susceptible population, and whether resistant UPEC emerge from prevalent sublineages in the drug-susceptible population. To address these questions, we compared the distribution of genotypes among ß-lactam-resistant and susceptible UPEC isolated from a university community. In addition, isolates were tested for ß-lactamase genes and plasmid incompatibility/replicon (Inc/rep) groups to identify the most prevalent resistance genes and plasmid types associated with resistance.
2. Materials and methods
2.1. Strain collection and susceptibility testing
The CA-UTI E. coli were isolated from urine specimens consecutively collected from patients presenting to the University Health Services at the University of California, Berkeley with symptoms of UTI from April 2003 to June 2004 and October 2004 to January 2005 [15, 16]. E. coli isolated from urine samples demonstrating a viable bacterial count of ≥104 CFU/ml were used in this study. Isolates were tested for susceptibility to antimicrobial agents by broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, which included the following classes and agents: ß-lactams (ampicillin [AMP], piperacillin, ampicillin/sulbactam, piperacillin/tazobactam, amoxicillin/clavulanic acid, ticarcillin/clavulanic acid, cephalothin, cefazolin, cefoxitin, cefotetan, cefuroxime, ceftriaxone, ceftizoxime, cefotaxime, ceftazidime, aztreonam, cefepime, imipenem, meropenem), folate pathway inhibitors (trimethoprim/sulfamethoxazole [SXT]), aminoglycosides (amikacin, gentamicin, tobramycin), fluoroquinolones (ciprofloxacin, gatifloxacin, and levofloxacin), nitrofurantoin, tetracycline (TET), and chloramphenicol (CHL) [15, 16]. Multidrug resistance (MDR) was defined as resistance to at least one agent in three or more classes of tested drugs. For the ß-lactams, isolates resistant to at least ampicillin were designated “AMP-R,” and isolates susceptible to all tested ß-lactams were designated “AMP-S.”
2.2. DNA extraction
Bacteria were subcultured from frozen glycerol stocks and grown overnight in LB broth in a 37°C shaker. The next day, 1ml of culture was pelleted in a microfuge tube by spinning in a microcentrifuge at maximum speed for one min. Pellets were resuspended in 300μl of sterile water and boiled for 10 min. Samples were pelleted, and the supernatant was collected and stored at −20°C.
2.3. Multilocus sequence typing (MLST)
Primer sequences and protocols for PCR amplification of seven housekeeping genes for MLST—adk, fumC, gyrB, icd, mdh, purA, and recA— were obtained from the EnteroBase website (https://enterobase.warwick.ac.uk/species/ecoli/allele_st_search) [17]. Amplicons were cleaned and sequenced by Sanger sequencing at the University of California, Berkeley DNA Sequencing Facility (Berkeley, CA). Geneious® v.9.1.3 (Biomatters Ltd., New Zealand) was used to visually inspect, edit, then align forward and reverse sequences to obtain a consensus sequence. Trimmed consensus sequences were submitted to the EnteroBase MLST database curator for sequence type (ST) and sequence complex assignment.
2.4. fimH typing
SNP analysis of fimH can be used to further differentiate isolates within lineages identified by MLST. PCR for fimH SNP analysis was performed according to Tartof et al. [18]. Amplicons were cleaned, sequenced, then analyzed with Geneious®. The known fimH alleles, corresponding to bp positions 360 to 781, were obtained from GenBank then aligned with trimmed fimH consensus sequences to type the alleles [18–20]. A FASTA file of the known fimH alleles can be found in the Supplementary section.
2.5. Identification of ß-lactamase genes
PCR for ß-lactamase genes—blaTEM, blaSHV, blaOXA, blaCTX-M, blaKPC, and blaCMY—was performed according to Dallenne et al. [21]. Amplicons were cleaned, sequenced, then analyzed with Geneious®. DNA sequence variants for blaTEM, blaSHV, blaOXA, and blaCTX-M genes were obtained from the database of K. Bush, T. Palzkill, and G. Jacoby (http://www.lahey.org/Studies/) and GenBank, then aligned with our ß-lactamase consensus sequences to identify the ß-lactamase variants present in our collection.
2.6. Plasmid incompatibility/replicon (Inc/rep) typing
PCR for plasmid Inc/rep tying for Inc groups A/C, B/O, F, FIA, FIB, FIC, FIIA, HI2, I1-Iγ, L/M, N, P, and Y was performed according to Carattoli et al. [22]. Amplicons were cleaned, sequenced, then analyzed with Geneious®. Our Inc/rep consensus sequences were compared to sequences deposited in the NCBI database using the BLAST program.
2.7. Statistical analysis
Categorical variables were compared with a chi-square or Fisher’s exact test (two-tailed). A P value of <0.05 was considered statistically significant.
3. Results
3.1. Antimicrobial susceptibility profile of E. coli causing CA-UTIs
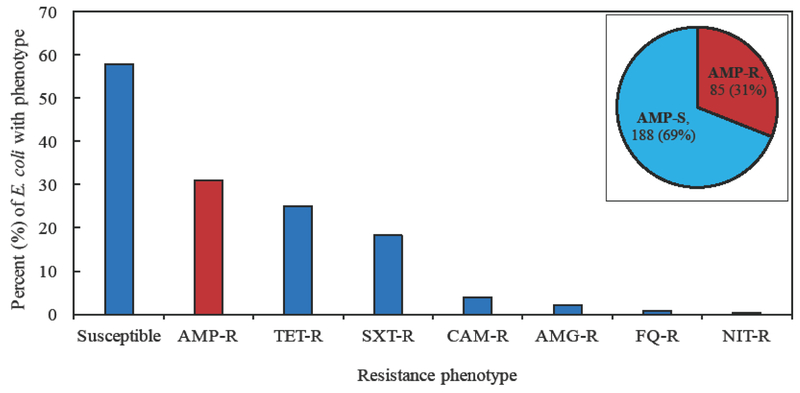
Of the 273 consecutively collected CA-UTI E. coli isolates, 85 (31%) were AMP-R (resistant at least to ampicillin for the ß-lactams tested) and 188 (69%) were AMP-S (susceptible to all ß-lactams tested) (Figure 1). In addition, 68 (25%) isolates were resistant to TET, 48 (18%) were resistant to SXT, 11 (4%) were resistant to CHL, six (2%) were resistant to an aminoglycoside, two (0.7%) were resistant to a fluoroquinolone, and one (0.4%) was resistant to nitrofurantoin. Of 188 AMP-S isolates, 157 (84%) were susceptible to all antimicrobial agents tested; the other 31 (16%) were mainly resistant to SXT (16%), TET (52%), or both (29%). Of 85 AMP-R isolates, 54 (64%) were resistant to another class of antimicrobial agent, which typically included SXT (20%), TET (37%), or both (43%).
Figure 1.
Distribution of antimicrobial resistance among CA-UTI E. coli isolates. Susceptible, isolates were susceptible to all agents tested; AMP-R, resistant to at least ampicillin for the ß-lactams tested; TET-R, tetracycline-resistant; SXT-R, trimethoprim/sulfamethoxazole-resistant; CAM-R, chloramphenicol-resistant; AMG-R, resistant to at least one tested aminoglycoside; FQ-R, resistant to at least one tested fluoroquinolone; NIT-R, resistant to nitrofurantoin. The figure inset displays the number and percent of isolates that are AMP-S (susceptible to all ß-lactams tested) and AMP-R.
While resistance to different classes of antimicrobial agents typically occurred together, ß-lactam resistance was the most common phenotype identified among isolates resistant to a single class of drugs; 31 (36%) of 85 ß-lactam-resistant, 15 (22%) of 68 TET-resistant, five (10%) of 48 SXT-resistant, and one (9%) of 11 CHL-resistant isolates were resistant only to the indicated drug class. Thirty (11%) isolates in this collection were MDR; ß-lactam/SXT/TET resistance was the most common MDR phenotype (76% of MDR isolates). One AMP-R isolate was resistant to third-generation cephalosporins (cefotaxime and ceftriaxone)—this isolate was also MDR.
3.2. Distribution of MLSTs and identification of ß-lactamase genes in E. coli
All isolates were genotyped by MLST, and 57 STs were identified (Table 1). Only six STs accounted for 175 (64%) of 273 isolates: ST95 (21%), ST69 (11%), ST420 (11%), ST73 (10%), ST127 (8%), and ST404 (3%). The pandemic lineages ST95, ST69, and ST73 were common in both AMP-R and AMP-S isolates, but they accounted for 51 (60%) isolates in the former and 63 (34%) isolates in the latter group (P<0.0001). ST69 and ST404 were more frequently identified among ß-lactam-resistant isolates, accounting for 17 (20%) and 8 (9%) AMP-R isolates, compared to 14 (7%) and 1 (0.5%) AMP-S isolate (P=0.003, P=0.0005, respectively). In contrast, ST420 and ST127 were more frequently identified among ß-lactam-susceptible isolates, accounting for 31 (17%) and 19 (10%) AMP-S isolates, but none and 2 (2%) AMP-R isolates (P<0.0001, P=0.027, respectively). In addition to ß-lactam resistance, ST69 was also more frequently identified as resistant to other antimicrobial agents compared to other STs; of 31 ST69 isolates, 19 (61%) were SXT-R (P<0.0001), 14 (45%) were TET-R (P=0.0056), five (16%) were CHL-R (P=0.0039), and 11 (35%) were MDR (P<0.0001).
Table 1.
Distribution of MLSTs among AMP-S and AMP-R E. coli, and identification of ß-lactamase genes in ß-lactam-resistant isolates.
| No. (%) of isolates that are: | |||||
|---|---|---|---|---|---|
| MLST (complex) | No. (%) of isolates | AMP-Sb | AMP-R | P valuec | bla genes in AMP-R isolates (no. of isolates)d |
| ST95 (ST95 cplx) | 56 (20.5) | 34(18.1) | 22 (25.9) | 0.140 | blaTEM (22) |
| ST69 (ST69 cplx) | 31 (11) | 14 (7.4) | 17 (20.0) | 0.003 | blaTEM (17), blaOXA (1) |
| ST420 | 31 (11) | 31 (16.5) | 0 | <0.0001 | |
| ST73 (ST73 cplx) | 27 (10) | 15 (8.0) | 12 (14.1) | 0.116 | blaTEM (11), blaSHV (1) |
| ST127 | 21 (8) | 19 (10.1) | 2 (2.4) | 0.027 | blaTEM (2) |
| ST404 (ST14 cplx) | 9 (3) | 1 (0.5) | 8 (9.4) | 0.0005 | blaTEM (8) |
| ST10 (ST10 cplx) | 8 (3) | 5 (2.7) | 3 (3.5) | 0.707 | blaTEM (3) |
| ST12 (ST12 cplx) | 8 (3) | 5 (2.7) | 3 (3.5) | 0.707 | blaTEM (1), blaSHV (2) |
| ST62 | 6 (2) | 5 (2.7) | 1 (1.2) | 0.669 | blaTEM (1) |
| ST141 | 5 (2) | 5 (2.7) | 0 | 0 329 | |
| ST131 (ST131 cplx) | 4 (1.5) | 2 (1.1) | 2 (2.4) | 0 591 | blaTEM (2) |
| ST144 | 4 (1.5) | 3 (1.6) | 1 (1.2) | 1.0 | blaTEM (1) |
| ST405 (ST405 cplx) | 4 (1.5) | 1 (0.5) | 3 (3 5) | 0 001 | blaTEM (3) |
| ST14 (ST14 cplx) | 3 (1) | 2 (1.1) | 1 (12) | 1 0 | blaTEM (1) |
| ST372 | 3 (1) | 3 (1.6) | 0 | 0 555 | |
| ST555 (ST538 cplx) | 3 (1) | 2 (1.1) | 1 (12) | 1.0 | blaTEM (1) |
| ST58 (ST155 cplx) | 2 (0.8) | 1 (0.5) | 1 (12) | 0 527 | blaTEM (1) |
| ST88 (ST23 cplx) | 2 (0.8) | 1 (0.5) | 1 (12) | 0 527 | blaTEM (1) |
| ST106 (ST69 cplx) | 2 (0.8) | 2 (1.1) | 0 | 1.0 | |
| ST297 | 2 (0.8) | 2 (1.1) | 0 | 1.0 | |
| ST393 (ST31 cplx) | 2 (0.8) | 1 (0.5) | 1 (1.2) | 0.527 | blaTEM (1) |
| ST410 (ST23 cplx) | 2 (0.8) | 2 (1.1) | 0 | 1.0 | |
| ST491 | 2 (0.8) | 2 (1.1) | 0 | 1.0 | |
| ST706 | 2 (0.8) | 2 (1.1) | 0 | 1.0 | |
| ST998 | 2 (08) | 1 (0.5) | 1 (1.2) | 0.527 | blaTEM (1), blaCTX-M (1) |
| ST38 (ST38 cplx) | 1 (04) | 0 | 1 (1.2) | 0.311 | blaOXA (1) |
| ST394 (ST394 cplx) | 1 (04) | 0 | 1 (1.2) | 0.311 | blaOXA (1) |
| ST1604 | 1 (04) | 0 | 1 (1.2) | 0.311 | blaTEM (1) |
| ST2328 | 1 (04) | 0 | 1 (1.2) | 0.311 | blaTEM (1) |
| ST2617 | 1 (04) | 0 | 1 (1.2) | 0.311 | blaTEM (1) |
| Other STsa | 27 (10) | 27 (14.4) | 0 | ||
| Total (% of total) | 273 (100) | 188 (69) | 85 (31) | blaTEM (81; 95%), blaSHV (3; 4%), blaOXA (2; 2%), blaCTX-M (1; 1%) | |
AMP-S, susceptible to ampicillin/all ß-lactams tested; AMP-R, resistant to at least ampicillin.
Includes ST59, ST80, ST93, ST101, ST126, ST136, ST155, ST348, ST357, ST550, ST641, ST847, ST906, ST963, ST1147, ST1155, ST1633, ST1859, ST1946, ST2178, ST2227, ST2562, ST2567, ST4044, ST6092, and two unknown STs.
157 (84%) of 188 AMP-S isolates were susceptible to other tested agents.
The P value compares the AMP-S column and AMP-R column. Bolded values are statistically significant.
Sequences identified include blaOXA-1, blaSHV-1, and blaCTX-9/51, and of the 36 blaTEM sequences typed, 8% were blaTEM-1A, 69% were blaTEM-1B, and 22% were blaTEM-1C.
All AMP-R isolates were tested for ß-lactamase genes (Table 1). Eighty-one (95%) of 85 AMP-R isolates tested positive for blaTEM. Thirty-six (44%) blaTEM sequences were analyzed and found to be blaTEM-1; three (8%) were blaTEM-1A, 25 (69%) were blaTEM-1B (also aligned with blaTEM-104, 122, 163, 198, 206, or 214), and eight (22%) were blaTEM-1C. In addition, three (4%) AMP-R isolates carried blaSHV-1, two (2%) AMP-R isolates carried blaOXA-1, and one ST38 AMP-R isolate carried blaCTX-M-9/51; this was the only ESBL gene identified.
3.3. Distribution of fimH genotypes among different MLSTs, and identification of prevalent sublineages of AMP-S and AMP-R E. coli
Of the 273 isolates, 269 (99%) tested positive for fimH, and 35 fimH SNP types were identified (Table 2). Only six fimH types accounted for 180 (65%) isolates: f-1 (20%), f-6 (15%), f-29 (11%), f-2 (8%), f-47 (6%), and f-3 (5%). The f-1 and f-6 SNP types were more frequently identified among ß-lactam-resistant isolates, accounting for 30 (35%) and 20 (24%) AMP-R isolates, compared to 25 (13%) and 21 (11%) AMP-S isolates (P<0.0001, P=0.008, respectively). In contrast, f-29 and f-2 were more frequently identified among ß-lactam-susceptible isolates, accounting for 30 (16%) and 20 (11%) AMP-S isolates, but none and 2 (2%) AMP-R isolates (P<0.0001, P=0.017, respectively). The six main fimH types— f-1, f-6, f-29, f-2, f-47, and f-3—were well represented among the six major STs (Table 2). One or two fimH types typically predominated an ST group; the main exception was in ST73, where the most prevalent fimH type (f-8) represented only 10 (37%) of 27 isolates in the group. Alleles f-47 and f-29 were found only among ST95 and ST420 isolates, respectively.
Table 2.
Distribution of fimH alleles among AMP-S and AMP-R isolates in different MLST groups.
| Isolates that have fimH type (no.): | ||
|---|---|---|
| MLST (no. of isolates) | AMP-S | AMP-R |
| ST95 (56) | f-1 (2), f-6 (17), f-47 (15) | f-6 (19), f-9 (1), f-47 (2) |
| ST69 (31) | f-1 (12), f-43 (2) | f-1 (17) |
| ST420 (31) | f-29 (30), f-29+3bp del (1) | 0 |
| ST73 (27) | f-3 (7), f-8 (4), f-9 (4) | f-3 (2), f-8 (6), f-9 (2), f-60 (2) |
| ST127 (21) | f-2 (18), U (1) | f-2 (2) |
| ST404 (9) | f-1 (1) | f-1 (8) |
| ST10 (8) | f-1 (1), f-4 (1), f-7 (1), f-15 (1), f-28 (1) | f-4 (2), f-15 (1) |
| ST12 (8) | f-1 (4), f-6 (1) | f-1 (1), f-12 (1), f-51 (1) |
| ST62 (6) | f-5 (5) | f-5 (1) |
| ST141 (5) | f-12 (5) | 0 |
| ST131 (4) | f-6 (1), f-17 (1) | f-6 (1), f-17 (1) |
| ST144 (4) | f-1 (2), f-4 (1) | f-4 (1) |
| ST405 (4) | f-6 (1) | f-1 (2), f-13 (1) |
| ST14 (3) | f-1 (2) | f-1 (1) |
| ST372 (3) | f-3 (3) | 0 |
| ST555 (3) | U (2) | U (1) |
| ST58 (2) | Neg (1) | f-15 (1) |
| ST88 (2) | f-1 (1) | f-1 (1) |
| ST106 (2) | f-43 (2) | 0 |
| ST297 (2) | f-10 (2) | 0 |
| ST393 (2) | f-4 (1) | f-4 (1) |
| ST410 (2) | f-15 (2) | 0 |
| ST491 (2) | f-12 (2) | 0 |
| ST706 (2) | f-14 (2) | 0 |
| ST998 (2) | f-3 (1) | f-3 (1) |
| ST38 (1) | 0 | f-12 (1) |
| ST394 (1) | 0 | Neg (1) |
| ST1604 (1) | 0 | f-4 (1) |
| ST2328 (1) | 0 | f-15 (1) |
| ST2617 (1) | 0 | f-24 (1) |
| Other STs (27) | Neg, f-2, f-5, f-7, f-9, f-26 (2’s); f-3, f-4, f-6, f-11, f-12, f-16, f-45, f-57 (1’s); U (7) | 0 |
| Prevalent fimH types (total; % of group)a | f-1 (25; 13%), f-6 (21; 11%), f-29 (30; 16%), f-2 (20; 11%), f-47 (15; 8%), f-3 (12; 6%), | f-1 (30; 35%), f-6 (20; 24%), f-29 (0; 0%), f-2 (2; 2%), f-47 (2; 2%), f-3 (3; 4%), |
U, unique fimH type; Neg, tested negative for fimH PCR.
Bolded values indicate that the P value is statistically significant (P<0.05) for the comparison of the indicated fimH type between the AMP-S and AMP-R group.
fimH typing allowed for subtyping of isolates within an ST (Table 3). ST95 contained four subtypes: f-1, f-6, f-9, and f-47. The ST95/f-47 subtype was more frequently identified among ß-lactam-susceptible isolates; 44% of AMP-S ST95 were f-47, but only 9% of AMP-R ST95 were f-47 (P=0.007). The ST95/f-6 subtype was more frequently identified among ß-lactam-resistant isolates; 86% of AMP-R ST95 were f-6, while 50% of AMP-S ST95 were f-6 (P=0.0093). ST73 also contained four subtypes: f-3, f-8, f-9, and f-60. While 50% of AMP-R ST73 isolates were subtype ST73/f-8, and nearly 50% of AMP-S ST73 isolates were subtype ST73/f-3, this difference in subtype distribution was not statistically significant.
Table 3.
Subtype distribution among prevalent STs that demonstrate more than one fimH type.
| No. (%) of ST that are: | ||||
|---|---|---|---|---|
| Subtype | No. (%) of ST | AMP-S | AMP-R | P valuea |
| ST95/f-1 | 2 (4) | 2 (5.9) | 0 | 0.514 |
| ST95/f-6 | 36 (64) | 17 (50.0) | 19 (86.4) | 0.009 |
| ST95/f-9 | 1 (2) | 0 | 1 (4.5) | 0.393 |
| ST95/f-47 | 17 (30) | 15 (44.1) | 2 (9.1) | 0.007 |
| ST69/f-1 | 29 (94) | 12 (85.7) | 17 (100) | 0.196 |
| ST69/f-43 | 2 (6) | 2 (14.3) | 0 | 0.196 |
| ST73/f-3 | 9 (33) | 7 (46.7) | 2 (16.7) | 0.217 |
| ST73/f-8 | 10 (37) | 4 (26.7) | 6 (50.0) | 0.257 |
| ST73/f-9 | 6 (22) | 4 (26.7) | 2 (16.7) | 0.662 |
| ST73/f-60 | 2 (7) | 0 | 2 (16.7) | 0.188 |
The P value compares the AMP-S group with the corresponding AMP-R group for each subtype. Bolded values are statistically significant.
3.4. Identification of plasmid Inc groups in AMP-S and AMP-R E. coli
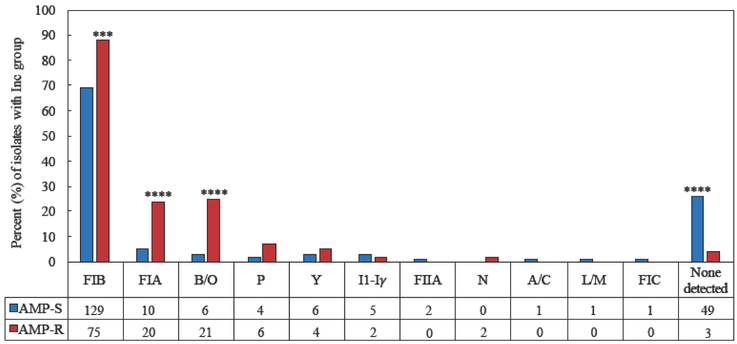
E. coli were tested for plasmid Inc groups commonly found in Enterobacteriaceae [22]. Of 273 isolates, 52 (19%) tested negative by the Inc/rep typing PCR (Figure 2). The IncFIB replicon was the most common replicon identified, detected in 204 (75%) isolates. IncFIA and IncB/O replicons were detected in 30 (11%) and 27 (10%) isolates, respectively. The prevalent Inc groups were more frequently identified in AMP-R isolates than in AMP-S isolates (Figure 2); among the 85 AMP-R isolates, 75 (88%) harbored IncFIB, 20 (24%) harbored IncFIA, and 21 (24%) harbored IncB/O replicons, while among the 188 AMP-S isolates 129 (69%) harbored IncFIB, 10 (5%) harbored IncFIA, and 6 (3%) harbored IncB/O replicons (P=0.0006, P<0.0001, and P<0.0001, respectively). In addition to ß-lactam resistance, the IncFIA replicon was more frequently identified among isolates resistant to other classes of antimicrobial agents. Fifteen (31%) of 48 SXT-R isolates harbored IncFIA compared to 15 (6%) of 225 SXT-S isolates (P<0.0001); 22 (32%) of 68 TET-R isolates harbored IncFIA compared to 8 (4%) of 205 TET-S isolates (P<0.0001); 5 (45%) of 11 CHL-R isolates harbored IncFIA compared to 25 (10%) of 262 CHL-S isolates (P=0.0033); and 17 (57%) of 30 MDR isolates harbored IncFIA compared to 13 (5%) of 243 non-MDR isolates (P<0.0001). All 10 isolates with IncP replicons were resistant to TET.
Figure 2.
Distribution of plasmid Inc groups among AMP-S and AMP-R E. coli. The bar graph represents the percent of isolates within each group that contain the Inc type, while the values under the graph are the number of isolates that contain the Inc type. The P value compares the AMP-S group with the corresponding AMP-R group for each Inc type; ***P<0.001, and ****P<0.0001.
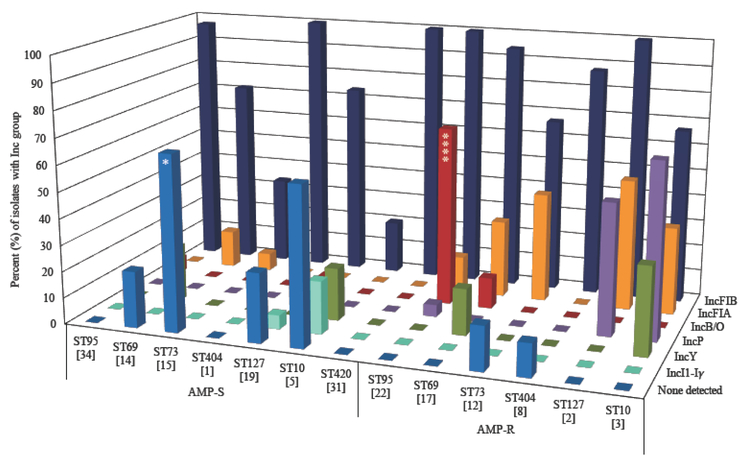
The distribution of plasmid Inc groups among the prevalent STs was different (Figure 3). While 10 (67%) of 15 AMP-S ST73 tested negative for the Inc types, only 2 (17%) of 12 AMP-R ST73 tested negative for the Inc types examined (P=0.0185). Over half of the 27 IncB/O containing isolates were ST95, with IncB/O observed in 15 (68%) of 22 AMP-R ST95 compared to 2 (6%) of 34 AMP-S ST95 isolates (P<0.0001). The IncFIB replicon was nearly ubiquitous in some STs. While all (100%) of 31 ST420 isolates and 55 (98%) of 56 ST95 isolates contained the IncFIB replicon, only 118 (63%) of 186 non-ST95/420 isolates contained this replicon (P<0.0001). Additionally, IncFIB was less frequently identified in SF73 compared to other STs; 13 (48%) of 27 ST73 isolates contained IncFIB compared to 191 (78%) of 246 non-ST73 isolates (P=0.0008). The IncFIA replicon was more frequently identified in ST69 and ST73 than other STs; while 7 (23%) of 31 ST69 and 6 (22%) of 27 ST73 isolates contained IncFIA, only 17 (8%) of 215 non-ST69/73 isolates contained this replicon (P=0.0017).
Figure 3.
Distribution of plasmid Inc groups among AMP-S and AMP-R E. coli from seven prevalent STs. Data for IncFIB, IncFIA, IncB/O, IncP, IncY, IncI1-Iγ, and no Inc detected are shown. The bar graph represents the percent of isolates within each group that contain the Inc type, while the values in parenthesis are the number of isolates per group. The P value compares the AMP-S group with the corresponding AMP-R group for each ST; *P<0.05, and ****P<0.0001.
4. Discussion
The prevalence of antimicrobial resistance in ExPEC is a global concern and major obstacle to empirical treatment of infections caused by these organisms. To better understand the factors that drive the prevalence of ß-lactam resistance in a community, we genetically characterized consecutively collected E. coli isolated from individuals with CA-UTI at a university health center, over two intervals between 2003 and 2005. By comparing ß-lactam-resistant (AMP-R) and ß-lactam-susceptible (AMP-S) UPEC, we found that the prevalence of ß-lactam resistance was multifactorial, and that a specific set of ST/fimH sublineages carrying specific plasmid Inc types and ß-lactamase genes contributed to a large proportion of ß-lactam-resistant CA-UTIs in our study community. Our findings are in accord with previous studies that used fimH SNP analysis to subtype E. coli MLST groups, which demonstrated that there are sublineages within specific STs that are associated with antimicrobial susceptibility or resistance [19, 23, 24].
Only four of the six most prevalent STs contributed appreciably to the AMP-R population (representing 69%): ST95, ST69, ST73, and ST404. Within ST95 and ST73, fimH typing enabled the identification of multiple sublineages, revealing differences in sublineage distribution among AMP-R and AMP-S isolates, indicating that the prevalence of ß-lactam resistance in the study community is dependent, in part, on the prevalence of several sublineages that are AMP-R. Sublineage ST95/f-6 represented 22% of the AMP-R population, and sublineage ST73/f-8 represented 7% of the AMP-R population. Interestingly, fimH typing did not offer further discriminatory power among other prevalent STs—including ST69, ST404, ST420, and ST127—indicating either that isolates that cause UTI from these lineages are more homogeneous than ST95 and ST73, or that another subtyping method would be more appropriate.
Interestingly, ST420 was tied for second-most prevalent ST in this study community, but none were resistant to the tested antimicrobial agents. In other studies that have identified ST420 among human clinical isolates, it is often reported as a low-prevalence clone that is susceptible to all antimicrobial agents; however, several antimicrobial-resistant ST420 E. coli have been isolated from animal sources [10, 12, 13, 19, 25, 26]. ST95 was previously identified as a lineage associated with a low prevalence of antimicrobial resistance [19]. Stephens et al. used whole-genome sequence analysis to examine ST95 isolates for factors associated with a lack of acquired antimicrobial resistance genes [27]. Carriage of a 114-kb IncFIB/IncFII plasmid called pUTI89 was significantly associated with a pan-susceptible ST95 sublineage in their study. Interestingly, the ST420 isolates in this study all contained IncFIB type plasmids. Further genomic analysis of ST420 could lead to identification of additional factors that limit the spread of antimicrobial resistance genes.
While the isolates examined in this study were collected between 2003–2005, comparison of these UPEC to those obtained from the same university health service in 2016–2017 demonstrates several important points. Four of the six most prevalent STs found in this study have remained prevalent clones in this community over time: ST95, ST127, ST73, and ST69, the latter two of which have remained prevalent clones in the AMP-R population [28]. Three significant changes include: a) the emergence of ST131 as a more prevalent ST in this community (P=0.02); the lack of ST404 (P=0.005), which was a prevalent AMP-R clone in 2003–2005; and the near disappearance of ST420 (P<0.0001), which nearly dominated the AMP-S population in 2003–2005. While some prevalent clones demonstrate stability over time, the emergence and disappearance of other prevalent clones in a population underscores the need for routine monitoring of the clonal composition of UPEC, since these changes can impact the prevalence of antimicrobial resistance in a community. For example, the frequency of fluoroquinolone resistance in the UPEC population increased from 0.7% (2003–2005) to 5.1% (2016–2017), and the occurrence of third-generation cephalosporin resistance increased from 0.4% to 4.3%—both largely due to the emergence of ST131 in this community. The reasons why some prevalent lineages remain stable over time, while others emerge or disappear, are unknown; the introduction of ingestible vehicles into the community, contaminated or not contaminated with particular lineages, is a possibility.
A recent study analyzing UPEC from Switzerland found that ST131 was the most frequently occurring ST (equal to ST69) causing CA-UTI, while ST95 was not identified as a cause of CA-UTI in their community [29]. This is in contrast to our community, where ST95 has been identified as the predominant UPEC ST causing CA-UTIs at multiple points in time over the past 18 years, with ST131 more recently emerging in prevalence [28]. One reason for this difference in clonal composition may be due to differences in patient demographics; the median age of patients in this study representing a University community was 22 (range 18–44 years), while the median age of patients from Nüesch-Inderbinen et al. [29] representing a suburban community was 53 (range 16–87 years). Interestingly, ST95 was also identified as the predominant clone causing CA-UTI among children seen at a University hospital in South Korea [14]. Thus, while ST95 is a well-recognized pandemic lineage of ExPEC, it appears to represent a more prevalent cause of CA-UTI in certain patient populations and geographic regions, including the US, South Korea, and Australia [8, 14, 28, 30].
Non-ESBL blaTEM genes were a major driving factor for ß-lactam resistance in this population, across STs and sublineages; it was the sole ß-lactamase gene identified in 93% of AMP-R isolates. In fact, blaTEM genes have remained the most prevalent ß-lactam resistance determinant in this community, where blaTEM alone accounted for 65% of AMP-R isolates in 2016–2017 [28]. TEM is likely to persist as the main cause of early-generation ß-lactam resistance across communities; ß-lactamase gene analysis of other recent collections of ExPEC have demonstrated its continued importance [10, 30]. CTX-M genes were rare in the study collection—only one isolate produced CTX-M-9/51. By 2016–2017, CTX-M-producing UPEC became more frequent in this community (10% of AMP-R isolates), again mostly due to the emergence of ST131 containing CTX-M group 1 genes [28].
In E. coli, blaTEM, blaSHV, blaOXA, and blaCTX-M are horizontally acquired ß-lactamase genes. Plasmids contribute substantially to the acquisition and dissemination of these and other resistance genes in ExPEC. Based on the strong association between specific replicons and an AMP-R phenotype, we speculate that the acquired ß-lactamase genes are localized on IncFIB, IncFIA, or IncB/O replicon-containing plasmids in the majority of isolates. While these plasmids/replicons appear to be major factors in the dissemination of ß-lactam resistance in these isolates, as were IncF type plasmids in other studies [31], it is important to note that these replicons do not occur exclusively in antimicrobial-resistant isolates [27, 32]. In this study, we found these Inc replicons in AMP-S strains, although at a significantly lower frequency.
The association between IncFIA replicons and ß-lactam, SXT, TET and CHL resistance, along with the finding that these resistance phenotypes frequently occurred together, suggests that these resistance genes may be colocalized on IncFIA replicon-containing plasmids in some isolates. Although TET and CHL are not generally used to treat UTIs, these agents are commonly used to treat other infections and conditions. For instance, tetracyclines are the most common oral antibiotics used to treat acne vulgaris [33]. Thus, one significance of TET and CHL resistance in the CA-UTI isolates from this college community is the possibility that antibiotic selection from TET or CHL treatment of other conditions helps maintain resistance to agents used to treat UTIs, in the absence of the agents themselves (i.e. ß-lactams and SXT) [34]. While our Inc analysis was fairly comprehensive for common Inc groups found in Enterobacteriaceae, it was not exhaustive; other Inc replicons that we did not properly screen for may exist in the isolates. Additionally, our current Inc analysis may have underestimated the presence of different types of IncFII replicons.
In conclusion, we found that a limited set of circulating UPEC STs, fimH sublineages, TEM ß-lactamase genes, and Inc type plasmids likely harboring these genes, accounted for a substantial proportion of AMP-R UPEC infections in this study community. The distribution of STs and sublineages among ß-lactam resistant and susceptible UPEC was not the same, indicating that the selective pressure from ß-lactam drugs is not exerted equally on all UPEC. This observation may suggest that the AMP-R strains do not evolve from AMP-S strains that acquire antimicrobial resistance genes in this community; rather, these strains may become introduced into the community by vehicles contaminated with specific lineages of UPEC, that colonize the intestine and subsequently cause UTI. If these observations are found to be generalizable by similar studies elsewhere, such information could support targeting specific sublineages with vaccines or other anti-infective modalities.
Supplementary Material
Highlights.
ß-lactam resistance was the most common, found in 31% of the uropathogenic E. coli
The blaTEM ß-lactamase gene accounted for 93% of ß-lactam resistance
IncFIB, IncFIA, and IncB/O plasmid groups were associated with ß-lactam resistance
Lineage composition was different among ß-lactam-resistant and susceptible E. coli
Acknowledgements
We would like to thank Sherry P. Smith and Amee R. Manges for identification, phenotypic characterization, and archival of the E. coli isolates used in this study. Thank you to Clarissa A. Borges for technical support.
Funding: This work was supported by the National Institutes of Health [grant number AI108029]; and the R. B. Roberts Fund provided to the Riley Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Approval: Not required.
Competing Interests: None declared.
References
- 1.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–84. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection. 2007;35(3):150–3. doi: 10.1007/s15010-007-6180-2. [DOI] [PubMed] [Google Scholar]
- 3.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagnostic microbiology and infectious disease. 2016;85(4):459–65. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013;6(11):1335–46. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2013.
- 6.World Health Organization (WHO). Antimicrobial resistance: global report on surveillance. 2014.
- 7.Riley LW. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(5):380–90. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee R, Johnston B, Lohse C, Chattopadhyay S, Tchesnokova V, Sokurenko EV, et al. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrobial agents and chemotherapy. 2013;57(12):5912–7. doi: 10.1128/AAC.01065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alghoribi MF, Gibreel TM, Farnham G, Al Johani SM, Balkhy HH, Upton M. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. The Journal of antimicrobial chemotherapy. 2015;70(10):2757–62. doi: 10.1093/jac/dkv188. [DOI] [PubMed] [Google Scholar]
- 10.Croxall G, Hale J, Weston V, Manning G, Cheetham P, Achtman M, et al. Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. The Journal of antimicrobial chemotherapy. 2011;66(11):2501–8. doi: 10.1093/jac/dkr349. [DOI] [PubMed] [Google Scholar]
- 11.Dias RC, Marangoni DV, Smith SP, Alves EM, Pellegrino FL, Riley LW, et al. Clonal composition of Escherichia coli causing community-acquired urinary tract infections in the State of Rio de Janeiro, Brazil. Microbial drug resistance. 2009;15(4):303–8. doi: 10.1089/mdr.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. The Journal of antimicrobial chemotherapy. 2012;67(2):346–56. doi: 10.1093/jac/dkr451. [DOI] [PubMed] [Google Scholar]
- 13.Hertz FB, Nielsen JB, Schonning K, Littauer P, Knudsen JD, Lobner-Olesen A, et al. Population structure of drug-susceptible,-resistant and ESBL-producing Escherichia coli from community-acquired urinary tract. BMC Microbiol. 2016;16:63. doi: 10.1186/s12866-016-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun KW, Kim DS, Kim W, Lim IS. Molecular typing of uropathogenic Escherichia coli isolated from Korean children with urinary tract infection. Korean J Pediatr. 2015;58(1):20–7. doi: 10.3345/kjp.2015.58.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manges AR, Smith SP, Lau BJ, Nuval CJ, Eisenberg JN, Dietrich PS, et al. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathog Dis. 2007;4(4):419–31. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- 16.Smith SP, Manges AR, Riley LW. Temporal changes in the prevalence of community-acquired antimicrobial-resistant urinary tract infection affected by Escherichia coli clonal group composition. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(5):689–95. doi: 10.1086/527386. [DOI] [PubMed] [Google Scholar]
- 17.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Molecular microbiology. 2006;60(5):1136–51. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tartof SY, Solberg OD, Riley LW. Genotypic analyses of uropathogenic Escherichia coli based on fimH single nucleotide polymorphisms (SNPs). Journal of medical microbiology. 2007;56(Pt 10):1363–9. doi: 10.1099/jmm.0.47262-0. [DOI] [PubMed] [Google Scholar]
- 19.Adams-Sapper S, Diep BA, Perdreau-Remington F, Riley LW. Clonal composition and community clustering of drug-susceptible and -resistant Escherichia coli isolates from bloodstream infections. Antimicrobial agents and chemotherapy. 2013;57(1):490–7. doi: 10.1128/AAC.01025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias RC, Moreira BM, Riley LW. Use of fimH single-nucleotide polymorphisms for strain typing of clinical isolates of Escherichia coli for epidemiologic investigation. Journal of clinical microbiology. 2010;48(2):483–8. doi: 10.1128/JCM.01858-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. The Journal of antimicrobial chemotherapy. 2010;65(3):490–5. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 22.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–28. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. The Journal of infectious diseases. 2013;207(6):919–28. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchesnokova V, Avagyan H, Billig M, Chattopadhyay S, Aprikian P, Chan D, et al. A Novel 7-Single Nucleotide Polymorphism-Based Clonotyping Test Allows Rapid Prediction of Antimicrobial Susceptibility of Extraintestinal Escherichia coli Directly From Urine Specimens. Open Forum Infect Dis. 2016;3(1):ofw002. doi: 10.1093/ofid/ofw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo S, Wakeham D, Brouwers HJ, Cobbold RN, Abraham S, Mollinger JL, et al. Human-associated fluoroquinolone-resistant Escherichia coli clonal lineages, including ST354, isolated from canine feces and extraintestinal infections in Australia. Microbes Infect. 2015;17(4):266–74. doi: 10.1016/j.micinf.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Seiffert SN, Carattoli A, Schwendener S, Collaud A, Endimiani A, Perreten V. Plasmids Carrying blaCMY-2/4 in Escherichia coli from Poultry, Poultry Meat, and Humans Belong to a Novel IncK Subgroup Designated IncK2. Front Microbiol. 2017;8:407. doi: 10.3389/fmicb.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens CM, Adams-Sapper S, Sekhon M, Johnson JR, Riley LW. Genomic Analysis of Factors Associated with Low Prevalence of Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Sequence Type 95 Strains. mSphere. 2017;2(2). doi: 10.1128/mSphere.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaji R, Rubin J, Thys E, Friedman CR, Riley LW. Persistent Pandemic Lineages of Uropathogenic Escherichia coli in a College Community from 1999 to 2017. Journal of clinical microbiology. 2018;56(4). doi: 10.1128/JCM.01834-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuesch-Inderbinen MT, Baschera M, Zurfluh K, Hachler H, Nuesch H, Stephan R. Clonal Diversity, Virulence Potential and Antimicrobial Resistance of Escherichia coli Causing Community Acquired Urinary Tract Infection in Switzerland. Front Microbiol. 2017;8:2334. doi: 10.3389/fmicb.2017.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon DM, Geyik S, Clermont O, O’Brien CL, Huang S, Abayasekara C, et al. Fine-Scale Structure Analysis Shows Epidemic Patterns of Clonal Complex 95, a Cosmopolitan Escherichia coli Lineage Responsible for Extraintestinal Infection. mSphere. 2017;2(3). doi: 10.1128/mSphere.00168-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carattoli A. Plasmids the spread of resistance. Int J Med Microbiol. 2013;303(6–7):298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson S, Naseer U, Sundsfjord A, Kahlmeter G, Sundqvist M. Sequence types and plasmid carriage of uropathogenic Escherichia coli devoid of phenotypically detectable resistance. The Journal of antimicrobial chemotherapy. 2012;67(1):69–73. doi: 10.1093/jac/dkr421. [DOI] [PubMed] [Google Scholar]
- 33.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–73 e33. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Adams SJ, Cunliffe WJ, Cooke EM. Long-term antibiotic therapy for acne vulgaris: effects on the bowel flora of patients and their relatives. J Invest Dermatol. 1985;85(1):35–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.