Abstract
Objectives: To examine the prognostic value of interim 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) findings after 2–4 cycles of rituximab, plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with diffuse large B-cell lymphoma (DLBCL) receiving standardized treatment.
Results: After a median 3.36 years (range 0.33 to 9.14 years), 24 of the 80 patients had documented relapse. In Interim-PET findings, 2-year PFS was significantly shorter for PET-positive as compared with PET-negative patients (50.0% vs. 86.4%; p = 0.0012). In End-PET findings, 2-year PFS was significantly shorter for PET-positive as compared with PET-negative patients (25.0% vs. 84.7%; p < 0.0001). The positive predictive value (PPV) and negative predictive value (NPV) of Interim-PET for predicting relapse or disease progression were 57.1% and 75.8%, respectively, while those for End-PET were 75.0% and 75.0%, respectively.
Methods: Eighty DLBCL patients treated with first-line 6–8 R-CHOP courses regardless of interim imaging findings were enrolled. Each underwent FDG-PET/CT scanning at staging, and again during (Interim-PET) and at the end of (End-PET) therapy. PET positivity or negativity at Interim-PET and End-PET as related to progression-free survival (PFS) was examined using Kaplan–Meier analysis.
Conclusion: Mid-treatment FDG-PET/CT findings may be useful for determining disease status in patients with DLBCL undergoing induction R-CHOP chemotherapy, though are not recommended for treatment decisions as part of routine clinical practice.
Keywords: non-Hodgkin lymphoma, PET-CT, progression-free survival
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin lymphoma worldwide, has been reported to be curable in about 60–70% of patients treated with standard rituximab, plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy [1]. On the other hand, current salvage therapy strategies seem to be inadequate for nonresponding patients, with only 30% to 35% of resistant or relapsed patients in this rituximab era able to achieve prolonged progression-free survival (PFS) with high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). Thus, a possible good alternative approach might be first-line risk-tailored therapy in patients with poor prognosis.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is a widely used imaging modality, and known to be reliable for staging and response assessment of aggressive malignant lymphomas, including DLBCL. For assessment of response, FDG-PET adds valuable information to that obtained with CT, because, unlike that modality, FDG-PET findings can often differentiate a viable tumor from posttreatment fibrosis or necrosis. Currently, FDG-PET/CT is the standard recommended method for response assessment at the end of first-line treatment, with FDG positivity at that point considered predictive of survival in patients with malignant lymphoma [2, 3]. Although previous studies have found that application of FDG-PET during therapy (Interim-PET) allows for successful PET-guided management of patients with Hodgkin lymphoma [4, 5], the use of Interim-PET for early response assessment and management of patients with DLBCL remains controversial, with varying results reported [6–20]. In the present study, we examined the predictive value of Interim-PET regarding PFS of DLBCL patients using visual dichotomous analysis.
RESULTS
This study included 80 DLBCL patients (45 males, 35 females; mean age 64.3 years, range 22–84 years), of whom 38 were stage I or II and 42 were stage III or IV. After a median 3.36 years (0.33 to 9.14 years), 24 were documented as relapse cases. Of the 24 patients with recurrence or progression, the follow-up period was a median 1.74 years (0.33 to 5.74 years), while that was a median 3.73 years (1.84 to 9.14 years) for the 56 without recurrence or progression.
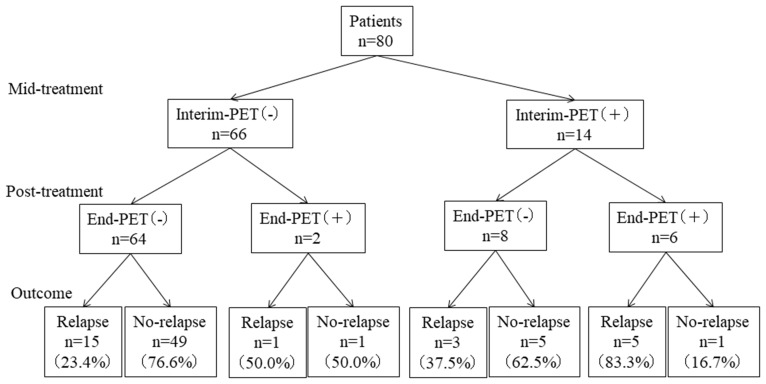
An Interim-PET score of 1 was noted in 41 patients, a score of 2 was noted in 11, a score of 3 was noted in 14, a score of 4 was noted in 10, and a score of 5 was noted in 4. As for End-PET, a score of 1 was noted in 49 patients, a score of 2 in 17, a score of 3 in 6, a score of 4 in 6, and a score of 5 in 2. Therefore, 66 patients (82.5%) were negative and 14 (17.5%) were positive at the time of Interim-PET, and 72 (90.0%) were negative and 8 (10%) were positive at the time of End-PET. Eight of 14 (57.1%) patients positive in Interim-PET findings were negative in End-PET results, whereas only 2 of 66 (3.0%) Interim-PET negative cases were positive at End-PET (Figure 1).
Figure 1. Patient-outcomes according to Interim-PET and End-PET.
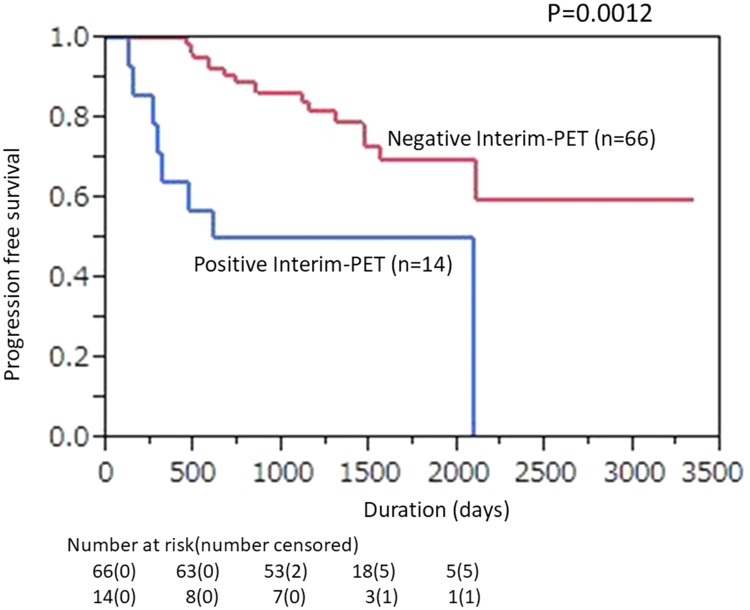
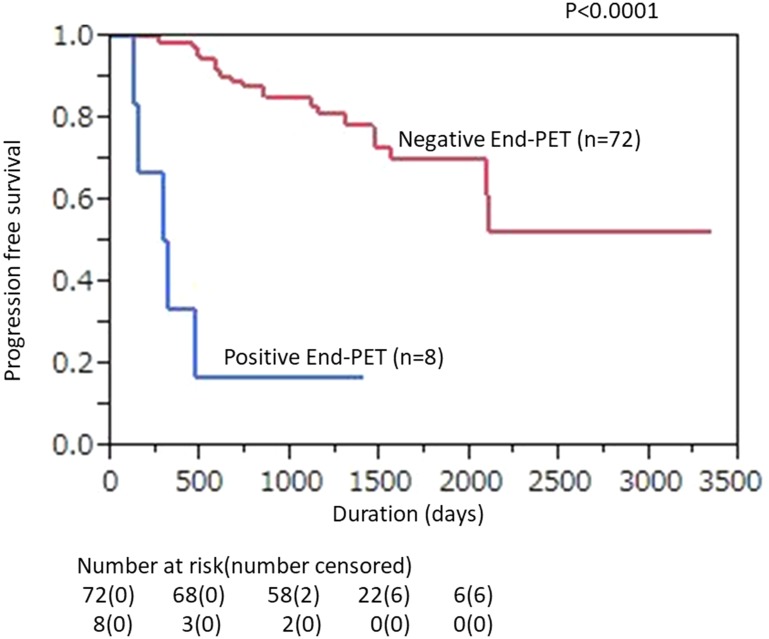
The ratio of 2-year PFS shown by Interim-PET/CT findings was significantly lower for PET-positive as compared with -negative patients (50.0% [95% CI, 23.8% to 76.2%] vs. 86.4% [95% CI, 78.1% to 94.6%]; p = 0.0012, Figure 2). In End-PET/CT findings, 2-year PFS was also significantly lower for PET-positive as compared with -negative patients [25.0% (95% CI, 0% to 55.0%) vs. 84.7% (95% CI, 76.4% to 93.0%); p < 0.0001, Figure 3].
Figure 2. Kaplan–Meier plot showing progression-free survival (PFS) according to mid-therapy FDG-PET/CT findings in patients with diffuse large B-cell lymphoma (DLBCL) treated with 6–8 courses of rituximab, plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
Correlations of Interim-PET results (positivity vs. negativity) with PFS are shown (p = 0.0012).
Figure 3. Kaplan–Meier plot showing PFS according to posttherapy FDG-PET/CT findings in DLBCL patients treated with 6-8 R-CHOP courses.
Correlations of End-PET results (positivity vs. negativity) with PFS are shown (p < 0.0001).
The rates for PPV, NPV, sensitivity, and specificity for Interim-PET to predict relapse or progression were 57.1% (95% CI, 31.2% to 82.9%), 75.8% (95% CI, 65.4% to 86.1%), 33.3% (95% CI, 14.5% to 52.2%), and 89.3% (95% CI, 81.2% to 97.4%), respectively, while those for End-PET were 75.0% (95% CI, 30.0% to 100%), 75.0% (95% CI, 65.0% to 85.0%), 25.0% (95% CI, 7.7% to 42.3%), and 96.4% (95% CI, 91.6% to 100%), respectively.
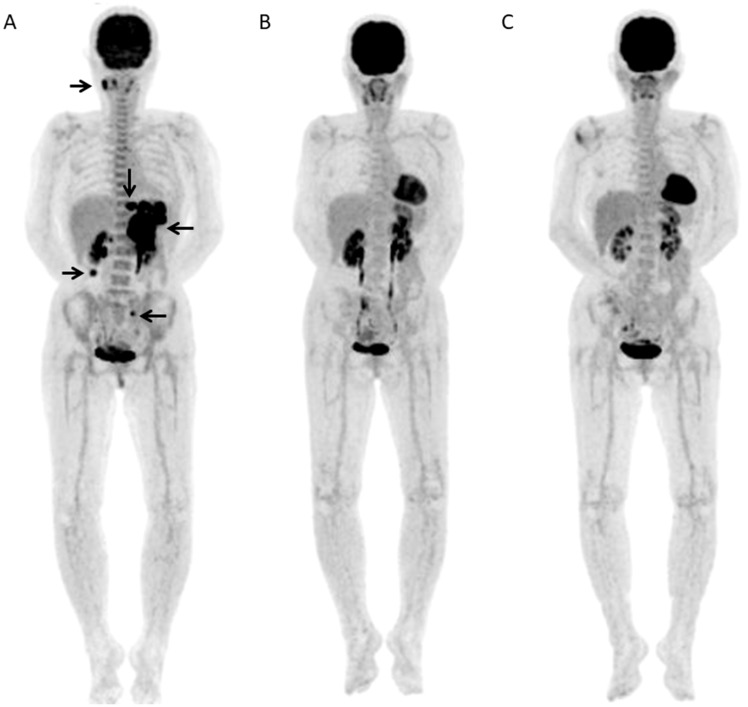
Two representative cases were shown (Figures 4, 5).
Figure 4. A 67-year-old female with DLBCL received 6 R-CHOP courses and no relapse was seen after 3.82 years.
(A) Baseline FDG-PET maximum intensity projection (MIP) showed several areas of abnormal FDG uptake in right neck, abdomen, and left pelvis (arrows). (B) FDG-PET scan MIP after 2 courses of R-CHOP (Interim-PET) showed complete resolution of abnormal metabolic activity. (C) FDG-PET scan MIP after chemotherapy (End-PET) showed complete resolution of abnormal metabolic activity.
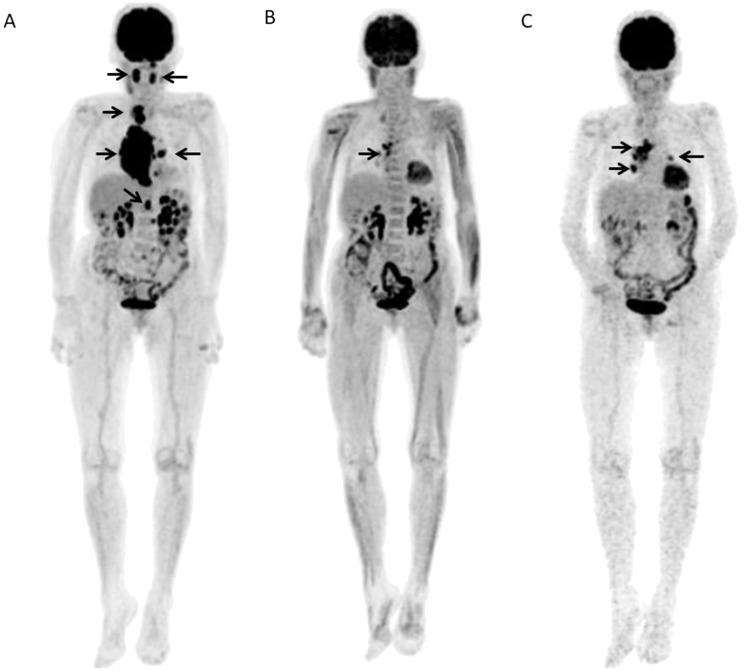
Figure 5. A 80-year-old female with DLBCL received 6 R-CHOP courses and then showed further progression at 0.41 years after the end of chemotherapy.
(A) Baseline FDG-PET MIP showed several areas of abnormal FDG uptake in bilateral neck, mediastinum/hilum, and abdomen (arrows). (B) FDG-PET scan MIP after 3 courses of R-CHOP (Interim-PET) showed residual uptake in the mediastinum (arrows). (C) FDG-PET scan MIP after the chemotherapy (End-PET) showed progression of residual uptake in mediastinum and reappearance of abnormal uptake in left hilum (arrows).
DISCUSSION
In the present series, mid-treatment FDG-PET/CT (Interim-PET after 2-4 cycles of R-CHOP therapy) results examined using visual dichotomous analysis proved to be useful for predicting PFS in patients with DLBCL receiving R-CHOP chemotherapy, though were slightly inferior to post-treatment FDG-PET/CT (End-PET) results.
Interim-PET has been reported to provide relatively good NPV and low to modest PPV findings [22]. A recent systematic review and meta-analysis demonstrated that the pooled summary false-positive proportions in patients with non-Hodgkin lymphoma were 83.0% (95% CI: 72.0%–90.2%) for Interim-PET (fixed effects, I2 = 27.7%) and 31.5% (95% CI: 3.9%–83.9%) for End-PET (random effects, I2 = 68.3%), and the authors concluded that patients with non-Hodgkin lymphoma often have a very high number of false-positive FDG-avid lesions in Interim-PET scanning findings [21]. Furthermore, Moscowitz et al. [10] analyzed Interim-PET results after 4 cycles of dose-dense R-CHOP in 97 patients with DLBCL and found that 33 of 38 found positive at the time of Interim-PET showed negativity for residual lymphoma in biopsy results.
There are some potential explanations for the high rate of false positivity. FDG does not have such a high specificity as a marker, because it is also taken up in infections and inflammatory processes, while it is also possible that use of immunotherapy may increase lesion inflammation. In addition, antibody-mediated cellular cytotoxicity and complement activation are important mechanisms related to the activity of rituximab [7, 14, 22]. Therefore, a biopsy should be considered for patients with positive PET findings if those indicate a change in treatment. On the other hand, Interim-PET is known to be associated with false-negative findings. Kwon et al. [17] investigated the prognostic value of negative Interim-PET results in 92 patients with DLBCL and found that 39.1% of cases shown negative later experienced relapse during the follow-up course (median 30.8 months).
Findings in the present study support the recommendation that Interim-PET should be performed only in the context of a clinical trial and is not reliable for treatment decisions in routine clinical practice. Ongoing clinical trials that alter therapy on the basis of Interim-PET may be difficult to interpret, as it is not clear that a positive FDG-PET scan result accurately defines a group of patients with poor risk for whom a change in therapy is warranted. For example, Kasamon et al. [8] used a risk-adapted approach to treat 59 patients with aggressive lymphoma, of whom 56 had DLBCL treated with R-CHOP. Using criteria similar to the IHP criteria, patients with a positive FDG-PET scan result after cycle 2 had their treatment changed to salvage chemotherapy, followed by autologous stem cell transplantation. More than half of their DLBCL patients had a positive interim FDG-PET scan result. For those who underwent ASCT, 74% demonstrated 2-year event-free survival (EFS), as compared to 88% of those who had a negative interim FDG-PET scan result and completed R-CHOP. Dührsen et al. [19] assessed whether Interim-PET guided therapy could improve outcome in 862 patients with aggressive non-Hodgkin lymphoma, including 609 with DLBCL. Those who showed R-CHOP positive results in Interim-PET after 2 cycles were assigned to receive either 6 additional cycles of R-CHOP or an intensive Burkitt protocol, whereas patients with negative FDG-PET results were assigned to either 4 additional cycles of R-CHOP, or 4 additional cycles of R-CHOP as well as 2 additional cycles of rituximab. After 2 cycles of R-CHOP, only 108 (12.5%) were classified as FDG-PET positive, whereas 754 (87.5%) were classified as FDG-PET negative. There was no significant difference regarding the EFS of patients with positive Interim-PET results and treated with 6 additional cycles of R-CHOP (2-year EFS, 42.0%) as compared with those treated with 6 additional cycles of the intensive Burkitt protocol (2-year EFS, 31.6%), and also no significant difference in EFS between Interim-PET negative patients treated with 4 additional cycles of R-CHOP (2-year EFS, 76.4%) and those treated with 4 additional cycles of R-CHOP plus 2 cycles of rituximab (2-year EFS, 73.5%). They concluded that treatment adapted based on interim FDG-PET findings did not improve outcome, but also speculated that Interim-PET could be a powerful tool to distinguish chemotherapy-sensitive from chemotherapy-resistant lymphoma.
Our study has several limitations. First, the design was retrospective and relatively few patients from a single institution were enrolled, which may have limited our ability to make generalized conclusions and introduced statistical errors. A larger prospective multicenter study is necessary to further explore and validate the potential of Interim-PET in patients with DLBCL. Second, the Interim-PET examinations were performed at different time points. Although the optimal time point remains to be established, a prospective study that utilizes the same time point of Interim-PET in the analyzed cases is needed. Third, we used the recommended standard method for evaluation of FDG-PET results, i.e., the Deauville criteria (D 5PS) [21]. On the other hand, earlier studies used custom visual criteria, such as criteria presented by the international Harmonization Project [2], the D 5PS, and maximum standardized uptake value reduction [9, 12, 15, 16, 20]. The use of different response criteria among studies makes comparisons of results challenging. We consider that consistent standardized criteria for FDG-PET/CT interpretation in this setting are needed in the future. Forth, the regimen and course of the treatment were not identical in 80 patients.
MATERIALS AND METHODS
Patients
This retrospective study was approved by our institutional review board, which waived the requirement for informed consent. Eighty newly diagnosed DLBCL patients referred to 3 different hematology departments between March 2009 and December 2015 were included. Demographic and tumor-related details are shown in Table 1. Each underwent FDG-PET/CT scanning at staging, then again during (Interim-PET) and at the end of (End-PET) therapy at Hyogo College of Medicine Hospital. Among all of the cases, Interim-PET was performed after 2 courses of chemotherapy for 28, after 3 courses for 34, and after 4 courses for 18 patients.
Table 1. Patient and tumor characteristics.
| Character | N | % |
|---|---|---|
| Sex | ||
| Male | 45 | 56.3 |
| Female | 35 | 43.7 |
| Age | ||
| Mean | 64.3 ± 13.6 | |
| Range | 22–84 | |
| Lactate dehydrogenase | ||
| Normal | 35 | 43.8 |
| Abnormal | 45 | 56.3 |
| Initial clinical stage | ||
| I | 15 | 18.8 |
| II | 23 | 28.7 |
| III | 12 | 15 |
| IV | 30 | 37.5 |
| IPI risk group | ||
| Low | 28 | 35.0 |
| Low-intermediate | 22 | 27.5 |
| High-intermediate | 16 | 20.0 |
| High | 14 | 17.5 |
| Treatment | ||
| R-CHOP | 61 | 76.3 |
| R-THP-COP | 19 | 23.7 |
| Total courses of chemotherapy | ||
| Six courses | 56 | 70.0 |
| Eight courses | 24 | 30.0 |
| Timing of Interim-PET after chemotherapy | ||
| After two courses | 28 | 35.0 |
| After three courses | 34 | 42.5 |
| After four courses | 18 | 22.5 |
Abbreviaitons: IPI: International prognostic index; R-CHOP: rituximab, plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-THP-COP: rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisone.
All treatments were performed according to departmental protocol. Patients were given standard R-CHOP (n = 61) or R-THP-COP (rituximab, pirarubicin, cyclophosphamide, vincristine, prednisone) (n = 19) for 6 (n = 56) or 8 (n = 24) courses. Therapy for each was performed as planned and never modified based on Interim-PET results. Involved field radiotherapy was performed to areas of bulky disease regardless of PET results (n = 8).
PET scanning
PET scans (Gemini GXL16 or Gemini TF64; Philips Medical Systems, Eindhoven, The Netherlands) were performed for all patients at the time of diagnosis, during (Interim-PET) after 2, 3, or 4 cycles of chemotherapy, and at the end (End-PET) of treatment at Hyogo College of Medicine Hospital. Patients were instructed to fast for 5 hours before the scan, and blood glucose was measured immediately before injection of FDG at 4.0 MBq/kg body weight for the GXL16 or 3.0 MBq/kg for the TF64. None of the present cohort had a blood glucose level greater than 150 mg/dL. Static emission images were obtained approximately 60 minutes after injection. For attenuation correction and anatomic localization, helical CT scans from the top of the head to the bottom of the feet were obtained using the following parameters: tube voltage, 120 kV; effective tube current auto-mA (up to 120 mAs for GXL16, up to 100 mAs for TF64); gantry rotation speed, 0.5 seconds; detector configuration, 16 × 1.5 mm for GXL16, or 64 × 0.625 mm for TF64); slice thickness, 2 mm; and transverse FOV, 600 mm. Immediately after completion of CT scanning, PET images of the region from the head to mid-thigh were acquired for 90 seconds per bed position and from the mid-thigh to toes for 30 seconds per bed position employing the variable sampling method. Thereafter, images at 12–14 bed positions, each for 90 seconds, and 6–7 bed positions, each for 30 seconds, were acquired in three-dimensional mode, thus requiring between 22 to 26 minutes of emission scanning per patient. The patients were allowed to breathe normally during PET acquisitions. Attenuation-corrected PET images were reconstructed using a line-of-response row-action maximum likelihood algorithm (n/a subsets, 2 iterations) for the GXL16 or an ordered-subset expectation maximization iterative reconstruction algorithm (33 subsets, 3 iterations) for the TF64.
PET findings interpretation
Baseline, Interim-PET, and End-PET scan images were retrospectively reviewed by 2 experienced nuclear medicine physicians (10 and 2 years of experience with oncologic FDG-PET/CT scanning, respectively) who had no knowledge of the other imaging results, or clinical and histopathologic results other than those obtained before, during, and after chemotherapy for DLBCL, with consensus obtained by discussion.
All Interim-PET and End-PET results were interpreted as positive or negative by visual dichotomous response criteria using a 5-point score system defined as the Deauville criteria (D 5PS) [22], as follows: 1, no uptake; 2, uptake equal or less than mediastinum; 3, uptake more than mediastinum but less than liver; 4, uptake moderately increased as compared with liver at any site; 5, uptake markedly increased as compared with liver at any site and/or new site of disease. A score of 1 through 3 was regarded as indicating negative and that of 4 or 5 as indicating positive findings.
Statistical analysis
PFS was chosen as the study endpoint, and was defined as the time from diagnosis to either disease progression or relapse, or to death from any cause. Data were censored if the patient was alive and free of progression/relapse at the final follow-up examination. Survival curves were calculated using the method of Kaplan and Meier according to the Interim-PET or End-PET result (positive vs. negative). Log-rank tests were performed, with p-values obtained to estimate survival probability after 2 years.
For predicting relapse or disease progression, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with the 95% confidence interval (95% CI) were analyzed for both Interim-PET/CT and End-PET/CT scanning results.
Each test was 2-sided and the level of significance was set at 0.05. All analyses were performed using SAS software.
Ethical approval
All included patients gave written and informed consent for the diagnostic procedure. Due to the retrospective character of the patient data evaluation ethical commitment was waived by the institutional ethics board.
CONCLUSIONS
In conclusion, mid-treatment FDG-PET/CT may prove useful for providing predictive information for patients with DLBCL undergoing R-CHOP chemotherapy. Nevertheless, a larger prospective multicenter study is necessary to further explore and validate the potential of Interim-PET in patients with DLBCL. Furthermore, mid-treatment FDG-PET/CT results should not be used at this time in routine clinical practice to make treatment decisions.
Abbreviations
- FDG
18F-fluorodeoxyglucose
- PET/CT
positron emission tomography/computed tomography
- R-CHOP
rituximab, plus cyclophosphamide, doxorubicin, vincristine, and prednisone
- DLBCL
diffuse large B-cell lymphoma
- PFS
progression-free survival
- PPV
positive predictive value
- NPV
negative predictive value
- ASCT
autologous stem cell transplantation
- R-THP-COP
rituximab, pirarubicin, cyclophosphamide, vincristine, prednisone
- 95% CI
95% confidence interval
- EFS
event-free survival
Footnotes
Author contributions
All authors have contributed to (1) conception and design, or acquiring data, or analyzing and interpreting data; (2) Drafting the manuscript, or critically contributing to or revising the manuscript, or enhancing its intellectual content; and (3) approval of the final manuscript.
Specific contributions
Conception and design: Kazuhiro Kitajima, Masaya Okada, Koichiro Yamakado; Recruiting of DLBCL patients: Masaya Okada, Kyoko Yoshihara, Tazuko Tokugawa, Akihiro Sawada, Satoshi Yoshihara, Hiroya Tamaki, Yoshihiro Fujimori, Syuji Ueda, Hiroyuki Kawamoto; Analyzing and Interpreting PET data: Kazuhiro Kitajima, Junichi Taniguchi; Statistical Analysis: Kazuhiro Kitajima, Masaya Okada; Drafting the manuscript: Kazuhiro Kitajima, Masaya Okada; Critically contributing to or revising the manuscript: Masaya Okada, Kyoko Yoshihara, Tazuko Tokugawa, Akihiro Sawada, Satoshi Yoshihara, Hiroya Tamaki, Yoshihiro Fujimori, Syuji Ueda, Hiroyuki Kawamoto, Junichi Taniguchi, Koichiro Yamakado.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
This work was supported by JSPS KAKENHI grant numbers 19K08187.
REFERENCES
- 1. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Brière J, Moskowitz CH, Schmitz N. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:4184–90. 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, et al. , and International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86. 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 3. Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011; 29:1844–54. 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 4. Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, d’Amore F, Enblad G, Franceschetto A, Fulham M, Luminari S, O’Doherty M, Patrick P, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016; 374:2419–29. 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mulé A, Picardi M, Romano A, Cantonetti M, La Nasa G, Trentin L, Bolis S, Rapezzi D, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 Trial. J Clin Oncol. 2018; 36:454–62. 10.1200/JCO.2017.75.2543. [DOI] [PubMed] [Google Scholar]
- 6. Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, Gaulard P, Garderet L, Lepage E, Reyes F, Meignan M. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005; 106:1376–81. 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 7. Han HS, Escalón MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009; 20:309–18. 10.1093/annonc/mdn629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasamon YL, Wahl RL, Ziessman HA, Blackford AL, Goodman SN, Fidyk CA, Rogers KM, Bolaños-Meade J, Borowitz MJ, Ambinder RF, Jones RJ, Swinnen LJ. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol Blood Marrow Transplant. 2009; 15:242–48. 10.1016/j.bbmt.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, Haioun C, Meignan M. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009; 50:527–33. 10.2967/jnumed.108.057703. [DOI] [PubMed] [Google Scholar]
- 10. Moskowitz CH, Schöder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, Straus D, Noy A, Palomba ML, O’Connor OA, Horwitz S, Weaver SA, Meikle JL, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010; 28:1896–903. 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, Véra P, Copie-Bergman C, Rahmouni A, Tilly H, Meignan M, Haioun C. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012; 30:184–90. 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 12. Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, Vera P, Bologna S, Brière J, Jais JP, Haioun C, Coiffier B, Morschhauser F, and Groupe d’étude des lymphomes de l’adulte (GELA) . SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011; 118:37–43. 10.1182/blood-2010-12-327767. [DOI] [PubMed] [Google Scholar]
- 13. Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011; 52:386–92. 10.2967/jnumed.110.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pregno P, Chiappella A, Bellò M, Botto B, Ferrero S, Franceschetti S, Giunta F, Ladetto M, Limerutti G, Menga M, Nicolosi M, Priolo G, Puccini B, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012; 119:2066–73. 10.1182/blood-2011-06-359943. [DOI] [PubMed] [Google Scholar]
- 15. Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, Tilly H, Siegel BA, Gallamini A, Casasnovas RO, Haioun C. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013; 40:1312–20. 10.1007/s00259-013-2435-6. [DOI] [PubMed] [Google Scholar]
- 16. Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, Mey U, Pless M, Bargetzi M, Krasniqi F, Gigli F, Hany T, Samarin A, et al. Final Results of a Prospective Evaluation of the Predictive Value of Interim Positron Emission Tomography in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015; 33:2523–29. 10.1200/JCO.2014.58.9846. [DOI] [PubMed] [Google Scholar]
- 17. Kwon SH, Kang DR, Kim J, Yoon JK, Lee SJ, Jeong SH, Lee HW, An YS. Prognostic value of negative interim 2-[¹8F]-fluoro-2-deoxy-d-glucose PET/CT in diffuse large B-cell lymphoma. Clin Radiol. 2016; 71:280–86. 10.1016/j.crad.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 18. Burggraaff CN, Cornelisse AC, Hoekstra OS, Lugtenburg PJ, De Keizer B, Arens AI, Celik F, Huijbregts JE, De Vet HC, Zijlstra JM, and HOVON Imaging Working Group . Interobserver Agreement of Interim and End-of-Treatment 18F-FDG PET/CT in Diffuse Large B-Cell Lymphoma: Impact on Clinical Practice and Trials. J Nucl Med. 2018; 59:1831–36. 10.2967/jnumed.118.210807. [DOI] [PubMed] [Google Scholar]
- 19. Dührsen U, Müller S, Hertenstein B, Thomssen H, Kotzerke J, Mesters R, Berdel WE, Franzius C, Kroschinsky F, Weckesser M, Kofahl-Krause D, Bengel FM, Dürig J, et al. , and PETAL Trial Investigators . Hüttmann A1 PETAL Trial Investigators. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): A multicenter, randomized phase III trial. J Clin Oncol. 2018; 36:2024–34. 10.1200/JCO.2017.76.8093. [DOI] [PubMed] [Google Scholar]
- 20. Yuan L, Kreissl MC, Su L, Wu Z, Hacker M, Liu J, Zhang X, Bo Y, Zhang H, Li X, Li S. Prognostic analysis of interim 18F-FDG PET/CT in patients with diffuse large B cell lymphoma after one cycle versus two cycles of chemotherapy. Eur J Nucl Med Mol Imaging. 2019; 46:478–88. 10.1007/s00259-018-4198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams HJ, Kwee TC. Proportion of false-positive lesions at interim and end-of-treatment FDG-PET in lymphoma as determined by histology: systematic review and meta-analysis. Eur J Radiol. 2016; 85:1963–70. 10.1016/j.ejrad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 22. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O’Doherty MJ, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014; 32:3048–58. 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]