Abstract
Cytokine-induced memory like (CIML) NK cells generated in response to pro-inflammatory cytokines in vitro and in vivo, can also be generated by vaccination, exhibiting heightened responses to cytokines stimulation months after their initial induction. Our previous study demonstrated that in vitro human NK cell responses to inactivated influenza virus were also indirectly augmented by very low doses of IL-15 which increased induction of myeloid cell-derived cytokine secretion. These findings led us to hypothesise that IL-15 stimulation could reveal a similar effect for active influenza vaccination and influence CIML NK cell effector functions. In this study, 51 healthy adults were vaccinated with seasonal influenza vaccine and PBMC were collected before and up to 30 days after vaccination. Myeloid and lymphoid cell cytokine secretion was measured after in vitro PBMC restimulation with low dose IL-15, alone or in combination with inactivated H3N2 virus; the associated NK cell response was assessed by flow cytometry. PBMCs collected 30 days post-vaccination showed heightened cytokine production in response to IL-15 compared with PBMCs collected at baseline, these responses were further enhanced when IL-15 was combined with H3N2. NK cell activation in response to IL-15 alone (CD25) and H3N2 plus IL-15 (CD25 and IFN-γ) was enhanced post-vaccination. We also observed proliferation of less differentiated NK cells with downregulation of cytokine receptors as early as 3 days after vaccination, suggesting cytokine stimulation in vivo. We conclude that vaccination-induced “training” of accessory cells combines with the generation of CIML NK cells to enhance the overall NK cell response post-vaccination.
Keywords: NK cells, influenza, vaccination, IL-15, cytokine
Introduction
NK cells are large, granular, lymphoid cells, classically categorised as cells of the innate immune system as they express activating and inhibitory receptors from germline encoded genes. The balance of activating and inhibitory signals transduced by these receptors controls the direct activation of NK cells, and this is fine-tuned by signals from innate cytokines such as IL-12, IL-15 and type I interferon (IFN) produced by myeloid accessory cells. NK cells can also augment and regulate adaptive immune responses by production of cytokines such as IFN-γ, and the adaptive response feeds back to support the NK cell response, for example via CD4+ T cell IL-2 secretion (1–3). Evaluation of this bi-directional relationship has contributed to our understanding of memory-like NK cell responses (reviewed in (4)).
Cytokine pre-activated or cytokine-induced memory like (CIML) NK cells have been described in mouse and human in vitro cell culture models. NK cells pre-activated with combinations of IL-12, IL-15 and IL-18 displayed enhanced proliferation and IFN-γ production after cytokine restimulation; this was maintained for up to 12 weeks and through several cycles of cell division (5–7). Vaccination with whole inactivated or live attenuated viral vaccines generate CIML NK cells in vivo, with heightened potential for IFN-γ secretion after re-encounter of pro-inflammatory cytokines (8–10). In particular, both seasonal influenza vaccination and in vitro pre-activation of human PBMC with inactivated influenza virus can enhance NK cell responses to cytokine restimulation for up to 4 weeks (9). In mice, murine cytomegalovirus (MCMV) infection induces both “antigen-specific” memory NK cells (Ly49H recognition of m157) and CIML NK cells, suggesting that diverse subsets of memory/memory-like NK cells can be induced by a single infection (11, 12). The pro-inflammatory cytokine IL-12 was critical in MCMV induced “antigen-specific” NK cell expansions and maintenance, the expansion of NKG2C+ NK cells in human cytomegalovirus (HCMV) infection and reactivation and in the generation of CIML NK cells, suggesting this cytokine may be a common requirement for the generation of memory-like NK cells (13–15).
In human PBMC, low concentrations of exogenous IL-15 (0.75ng/ml) enhance influenza virus-induced secretion of myeloid cell derived cytokines such as GM-CSF, IFN-α, IL-12 and IL-1β and this, in turn, enhances cytokine-dependent NK cell activation (16). Moreover, NK cell IFN-γ responses to low concentrations of IL-12 and IL-18 (12.5pg/ml and 10ng/ml respectively) are enhanced after influenza vaccination (9), suggesting that vaccine induced primed or co-stimulated NK cells have a lower threshold for cytokine-induced activation.
Whilst vaccine antigen driven NK cell recall responses are known to be dependent on vaccine specific T cell derived IL-2 and antibody (9, 10, 17, 18), the mechanisms of increased NK cell responses to innate cytokines after vaccination are not well understood. Our previous studies showed that IL-15 could indirectly promote NK cell responses by boosting the in vitro production of accessory cell derived cytokines in response to inactivated influenza H3N2 (16). We therefore hypothesised that IL-15 stimulation could also reveal whether a similar indirect effect could be promoted by active vaccination with seasonal trivalent or quadrivalent influenza vaccine (TIV/QIV). We examined IL-15 stimulated accessory cell derived cytokine production in vitro before and after vaccination and the associated impact on NK cell function. Restimulation of human PBMC cultures with low concentrations of IL-15 alone revealed enhanced production of both myeloid and lymphoid-cell derived cytokines four weeks after vaccine administration. Co-stimulation with IL-15 and influenza H3N2 resulted in further enhancement of cytokine production and in NK cell IFN-γ and CD25 upregulation in post-vaccination compared with baseline samples. These studies contribute to the understanding of vaccine induced pre-activation of innate immune cells, and of the in vivo mechanisms promoting the generation of CIML NK cells in humans.
Materials and Methods
Study participants and sample collection
Fifty-one healthy adult volunteers (median age 39y, age range 24-66y, 41% male) were recruited from amongst staff and students of the London School of Hygiene and Tropical Medicine (LSHTM). The study was approved by the Ethical Review Committee of the LSHTM (reference number 10336). All subjects received a single dose of 2015-2016 inactivated TIV (n=37) or 2017-2018 inactivated QIV (n=14) by the intramuscular route (Split Virion BP, Sanofi Pasteur). Heparinised blood was collected prior to vaccination (baseline; day 0), and 3 days, 7 days and 30 days post-vaccination. Whole blood was also collected from non-vaccinated volunteers for use in additional experiments. PBMC were isolated using Histopaque 1077 (Sigma-Aldrich, Gillingham, U.K.) gradient centrifugation and cryopreserved in liquid nitrogen. Plasma samples were stored at -80°C. Baseline plasmas samples were used to assess HCMV seropositivity; 41% were HCMV seropositive by IgG ELISA (Demeditec, Kassel, Germany).
Cell cultures
Before use, cryopreserved cells were thawed, washed in RPMI 1640 supplemented with 100U/ml penicillin/streptomycin and 20mM L-glutamine (Life Technologies, Thermo Fisher), and rested for 2 hours in the absence of exogenous cytokines. Cells were counted using a Countess II FL Automated Cell Counter (Invitrogen, Thermo Fisher); the median yield was 51% and median viability by trypan blue exclusion was 89%. PBMCs (4x105) were either stained immediately for ex vivo phenotyping or cultured for 6 or 18 hours with or without 2μg/ml inactivated whole H3N2 influenza virus (influenza A/Victoria/361/2011 H3N2 (IVR-165); formalin inactivated, in PBSA buffer plus 1% (w/v) sucrose, National Institute for Biological Standards and Control, Potters Bar, U.K.), with or without 0.75ng/ml recombinant human IL-15 (PeproTech, London, U.K.) in RPMI plus 5% pooled human AB serum (Sigma-Aldrich). Additional cultures were stimulated with a high concentration of cytokines (HCC) consisting of IL-12 (5ng/ml; PeproTech) and IL-18 (50ng/ml; R&D Systems, Oxford U.K.).
For blocking experiments, the following antibodies were used; anti-IL-12 at 3μg/ml (BD Biosciences, Oxford, U.K.) and mouse IgG1 isotype control at 3μg/ml final (eBiosciences, Thermo Fisher). There was no difference in NK cell activation in the presence or absence of isotype control antibody. GolgiStop (Monensin; 1/1500 concentration; BD Biosciences) and GolgiPlug (Brefeldin A; 1/1000 final concentration; BD Biosciences) were added for the final 3 hours of all cell cultures. For IL-12 intracellular staining experiments, 2x106 cells/well were cultured as above for 18 hours with GolgiStop and GolgiPlug for the final 5 hours. Culture supernatants were collected and stored at -80°C for Luminex analysis.
Antibody-dependent NK cell activation assay
96 well flat bottom tissue culture treated plates were coated overnight with 1μg/ml inactivated H3N2 virus at 4°C, washed and blocked with 5% FCS in RPMI 1640 supplemented as above for 30 minutes. PBMC from fresh blood of one previously tested (non-vaccinated) donor with pre and post-vaccination serum from each participant at a final concentration of 0.1% (plus 4.9% FCS) were plated on the coated plates and incubated for 5 hours at 37°C. Cells were harvested into round-bottom plates by pipetting up and down and soaking in cold PBS containing 0.5% FCS, 0.05% sodium azide and 2mM EDTA for staining.
Flow cytometry and Luminex
PBMCs were stained in 96-well round-bottomed plates as described previously (16). Briefly, cells were incubated for 5 minutes with Fc Receptor Blocking Reagent (Miltenyi Biotec) and then with a viability marker (Fixable Viability Stain 700; BD Biosciences) and antibodies to surface markers diluted in FACS buffer (PBS containing 0.5% FCS, 0.05% sodium azide and 2mM EDTA). Cells were then washed in FACS buffer, fixed and permeabilised using Cytofix/Cytoperm Kit (BD Biosciences) or Foxp3/Transcription Factor Fixation/Permeabilization Kit (eBiosciences) according to the manufacturer’s instructions. Cells were then stained for intracellular markers with FcR blocking and washed again and finally, cells were resuspended for acquisition on a BD LSRII flow cytometer.
Fluorophore-labelled antibodies used were: anti-CD3-V500 (clone UCHT1), anti-CD56-PE-Cy7 (clone NCAM16.2), anti-CD107a-FITC (clone H4A3) (all BD Biosciences), anti-CD57-eFlour450 (clone TB01), anti-CD25–PerCP-Cy5.5 (clone BC96), anti-IL-18Rα-PE (clone H44), anti-IL-12(p40)-e660 (clone C17.8), anti-CD19-PECy5 (clone HIB19) (all eBiosciences), anti-IL-12Rβ2-PerCP-Cy5.5 (clone 622509) (R&D systems), anti-CD25–FITC (clone BC96), anti-IFN-γ-APC (clone 4S.B3), anti-CD11c-PerCPCy5.5 (clone 3.1), anti-CD14-AF700 (clone 63D3) and anti-Ki67-PEDazzle (clone Ki67) (all Biolegend, London, U.K). Cells were acquired using FACSDiva software, data were analysed using FlowJo V10.4 (Tree Star, Oregon, U.S.A). FACS gates were set using unstimulated cells or FMO controls. Samples with less than 100 NK cell events were excluded from analysis.
Concentrations of GM-CSF, IFN-α2, IFN-γ, TNF-α, IP-10, IL-1β, IL-10, IL-12p70, IL-15 and IL-2 in cell culture supernatants were determined by Luminex technology, kit number HCYTOMAG-60K-09 (Merck Millipore, Watford, U.K) using Bio-Plex software (Bio-Rad, Watford, U.K.) for data acquisition.
RNA sequencing analysis
Gene expression was assessed in MACS separated NK cell enriched and NK cell depleted populations (NK Cell Isolation Kit, Miltenyi Biotec), cultured with 0.75ng/ml IL-15 as above for 18 hours by 3’ RNA-seq. (as detailed in Supplementary figure 4). Library preparation was performed using QuantSeq 3’ mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen GmbH, Austria), following the manufacturer’s instructions. 3’ RNA sequencing generates one read per transcript at the 3’ end of polyadenylated mRNA and maintains strand-specificity. Sample RNA concentration was measured using Qubit HS RNA kits (Thermo Fisher Scientific) and RNA quality was tested using Agilent RNA 6000 Pico chips (Agilent Technologies, USA). 5 μl RNA was used for library preparation from sample concentrations ranging between 600pg/μl to 70ng/μl. The PCR Add-on kit (Lexogen GmbH) was used during sample preparation to generate libraries that were not under or over-cycled. Final library concentration was assessed using Agilent DNA chips and Qubit before libraries were normalised to 6nM prior to pooling. Libraries were shipped to Lexogen GmbH for quality control and sequencing on two lanes of an Illumina HiSeq (Illumina). Bam files were converted to FastQ using Picard, FastQ files were uploaded to BlueBee Genomics Platform (BlueBee). FastQ files were trimmed and quality filtered using the default Integrated QuantSeq FWD workflow. In brief, Bbduk was used to trim low-quality tails, adapter contamination and polyA read-through, reads were aligned to human genome version GRCh38.77 using STAR Aligner and reads were counted using HTSeq-count with kit-specific options.
Read counts were normalised by variance-stabilising transformation (R package DESeq2). A linear mixed-effects model with normalised read counts as the outcome and including individual ID as a random effect was used to identify differentially expressed (DE) genes at 30 days post-vaccination vs baseline (R package lmer). A cut off P value of <0.05 and fold-change of >2/<-2 were used to define DE (a), the DE with a p value of <0.001 and fold-change of >2/<-2 are listed as gene symbol in a table (b). MINSEQE compliant raw and processed sequencing data are deposited in the NCBI GEO public database (https://www.ncbi.nlm.nih.gov/geo/ (GSE133478)).
Data analysis
Statistical analysis was performed using GraphPad Prism version 7.04 (GraphPad, California, U.S.A.). Differences in responses were analysed using Wilcoxon signed-rank test or one-way ANOVA Friedman test with Dunn’s correction for multiple comparisons. Correlation analysis was performed using linear regression. Significance levels are assigned as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 for all tests.
Results
Enhanced IL-15 stimulated cytokine production after influenza vaccination
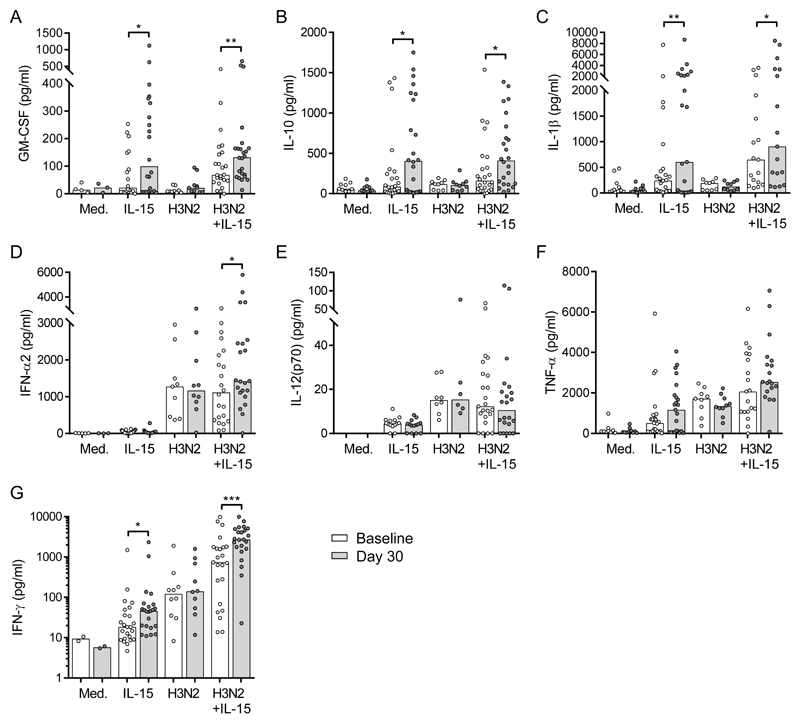
Our previous study (16) revealed that IL-15 co-stimulation enhanced the production of myeloid cell derived cytokines from influenza H3N2-stimulated PBMC. We therefore reasoned that influenza vaccination might also prime accessory cells for cytokine production which could be revealed on restimulation of PBMC with IL-15 in vitro. Baseline and day 30 post-vaccination PBMC from volunteers vaccinated with a single dose of 2015-2016 TIV were cultured in vitro for 18 hours with 0.75ng/ml exogenous IL-15, with or without inactivated H3N2 virus or medium alone. Cytokine concentrations in cell culture supernatants were determined by Luminex (Figure 1). In line with our previous data in unvaccinated individuals (16), baseline samples showed significant induction of IL-10 (p=0.0391) and IFN-γ (p=0.006) in response to IL-15 alone (Figure 1b, g) and influenza H3N2 stimulation increased levels of IFN-α2 (p=0.001), IL-12(p70) (p=0.002), TNF-α (p=0.008), and IFN-γ (p=0.009) compared with medium alone (Figure 1d-g). Co-stimulation of baseline, pre-vaccination samples with H3N2 and IL-15 further enhanced secretion of GM-CSF (p=0.0039), IL-1β (p=0.004), TNF-α (p=0.0039) and IFN-γ (p=0.005) compared with either stimulus alone (Figure 1a, c, f, g).
Figure 1. Influenza vaccination enhances IL-15 stimulated cytokine production.
Baseline (white bars) and 30 day post-vaccination (grey bars) PBMC samples were cultured with medium (n=10), 0.75ng/ml IL-15 alone (n=26), inactivated H3N2 (n=10) or H3N2 + IL-15 (n=26) for 18 hours and culture supernatants were collected. Concentrations (pg/ml) of GM-CSF (a), IL-10 (b), IL-1β (c), IFN-α2 (d), IL-12(p70) (e), TNFα (f), and IFN-γ (g) in supernatant were determined by Luminex. Graphs show one dot per donor, with a bar representing median value. Comparisons between vaccination time points were made using Wilcoxon signed-rank test. *p < 0.05, **p < 0.01, *** p < 0.001.
We then compared post-vaccination to pre-vaccination cytokine secretion in response to each stimulation condition alone or in combination. In vitro restimulation of PBMC with IL-15 alone significantly increased GM-CSF (3.6 fold), IL-10 (3.6 fold), IL-1β (1.4 fold) and IFN-γ (1.5 fold) secretion at 30 days post-vaccination compared with baseline (Figure 1a-c, g). An increase in median concentration of TNF-α (baseline, 509.4pg/ml; day 30, 1169pg/ml) was also observed in response to IL-15 alone after vaccination (Figure 1f). Vaccination did not result in increased levels of cytokines in unstimulated (medium alone) cultures, indicating that enhancement of cytokine responses after vaccination was an active process, reliant both on in vivo priming and in vitro restimulation, and not due to residual activated cells in the cultures. Although H3N2 stimulated significant levels of IFN-α2 and IL-12(p70) secretion, no enhancement post-vaccination was observed to this stimulation (Figure 1d-e). However, when cells were co-stimulated with IL-15 and H3N2, significant increases in GM-CSF, IL-10, IL-1β, IFN-α2 and IFN-γ were observed post-vaccination compared with baseline (Figure 1a-d, g). No enhancement in IL-2 was measured in cell culture supernatants in response to in vitro stimulation after vaccination (not shown). These data show that restimulation in vitro with low concentrations of exogenous IL-15 are sufficient to reveal enhanced cytokine secretion after vaccination in the absence of antigen specific stimuli. Whilst increased IFN-γ secretion is consistent with enhanced NK cell activation, GM-CSF, IL-10 and IL-1β are indicative of myeloid cell activation, suggesting that influenza vaccination primes both myeloid and lymphoid cells in vivo for increased cytokine production.
Enhanced NK cell responses to IL-15 post-vaccination
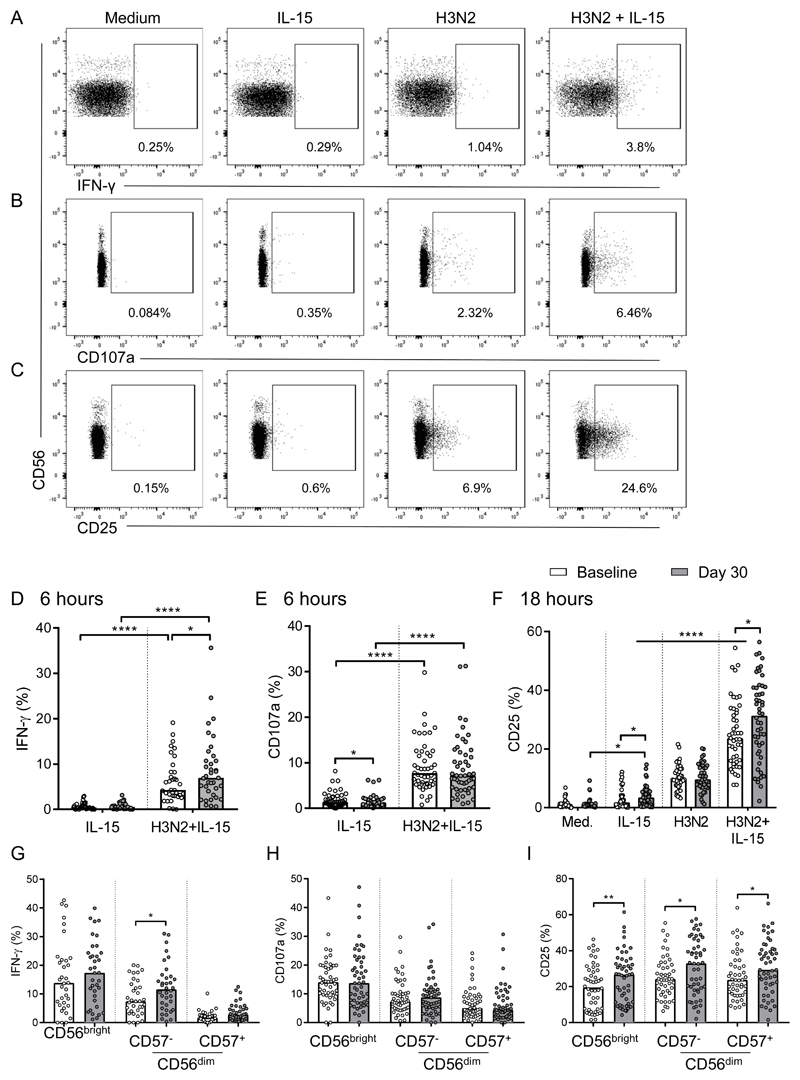
To examine the effect of influenza vaccine-induced priming of innate immune cells on NK cell responses, baseline and 30 day post-vaccination PBMC were cultured for 6 or 18 hours in vitro with medium alone, IL-15 alone, H3N2 or H3N2 + IL-15 and analysed for NK cell (CD56+CD3-) IFN-γ, CD25 and CD107a expression by flow cytometry; the gating strategy is shown in Figure 2a-c. There were no significant differences between responses of individuals vaccinated with 2015-2016 TIV or 2017-2018 QIV (not shown); all data were therefore pooled for analysis. NK cell IFN-γ and CD107a were induced after 6 hours stimulation with H3N2 (Figure 2a, b) and CD25 was upregulated after 18 hours (Figure 2c), NK cell activation was significantly higher after H3N2 + IL-15 co-stimulation compared with either H3N2 or IL-15 alone, consistent with our previous observations (Figure 2a-c) (16).
Figure 2. Enhanced NK cell responses to IL-15 post-vaccination.
Baseline (white bars) and 30 day post-vaccination (grey bars) PBMC samples from 2015-2016 TIV (n=37) or 2017-2018 QIV (n=14) vaccinated individuals (all donors were combined for analysis), were stimulated in vitro with medium alone, IL-15 alone, H3N2 or H3N2 + IL-15. IFN-γ, CD107a and CD25 expression of CD56+CD3- NK cells was measured after 6 (IFN-γ, CD107a) or 18 hours (CD25) by flow cytometry. Plots show the gating strategy for IFN-γ (a), CD107a (b) and CD25 expression (c) from one representative donor at baseline. Numbers shown are the percentage of total NK cells positive for each marker. IFN-γ (d), CD107a (e) in response to IL-15 and H3N2 + IL-15 and CD25 in response to all 4 conditions (f) is shown at baseline and 30 days post-vaccination. IFN-γ (g), CD107a (h) and CD25 (i) in response to H3N2 + IL-15 attributed to NK cell differentiation subsets defined by CD56 and CD57 expression is also shown. Plots show one dot per donor with a bar representing median percentage. Comparisons between vaccination time points were made using Wilcoxon signed-rank test and between culture conditions by one-way ANOVA with Dunn’s correction for multiple comparisons. *p < 0.05, **p < 0.01, ****p < 0.0001.
Importantly, when PBMCs were stimulated with H3N2 + IL-15, significantly higher percentages of NK cells expressing IFN-γ at 6 hours (median 2.6% increase; 34 of 49 donors), and CD25 at 18 hours (median 7.8% increase; 30 of 48 donors) were detected among PBMCs collected 30 days after vaccination compared with baseline (Figure 2d, f). Similarly, after IL-15 stimulation, a higher percentage of NK cells expressed CD25 in day 30 post-vaccination PBMCs than in baseline PBMCs (Figure 2f). Conversely, there were no significant differences in levels (mean fluorescence intensity; MFI) of CD25 expression on CD25+ cells after in vitro stimulation (IL-15 alone: baseline median MFI 165, interquartile range (IQR) 137-208; day 30 median 164, IQR 129-199; H3N2 + IL-15: baseline median 547, IQR 416-661; day 30 median 532, IQR 368-693; not shown) indicating that vaccination was increasing the frequencies of responsive NK cells rather than modulating the expression of CD25 expression per se. CD107a expression did not differ significantly between baseline and day 30 post-vaccination cells, irrespective of the restimulation conditions (Figure 2e). Finally, neither IFN-γ, CD107a (Supplementary Figure 1a, b) nor CD25 (Figure 2f) expression differed significantly between baseline and post-vaccination cells after restimulation with H3N2 alone.
Whilst higher frequencies of IFN-γ+ cells were observed among less differentiated (CD56bright and CD56dimCD57-) NK cells, both before and after vaccination, compared with more differentiated subsets (CD56dimCD57+) (Figure 2g), enhanced post-vaccination CD25 and IFN-γ (but not CD107a) responses to H3N2 + IL-15 were observed across all NK cell subsets indicating that vaccination influences NK cell function independently of differentiation state (Figure 2g, h, i). In line with previous studies (8, 9, 19), we observed small but significant increases in IFN-γ+ NK cells 30 days post-vaccination compared with baseline among cells restimulated with high concentrations of IL-12 and IL-18, consistent with an intrinsic vaccine-induced effect on NK cells (Supplementary Figure 1c-e). These data suggest that very low concentrations of IL-15 alone are sufficient to reveal vaccination mediated enhancement of total NK cell activation (CD25 expression) in post-vaccination cultures potentially through synergy with vaccine-primed cytokine secretion from accessory cells. A preliminary RNA sequencing analysis was performed to analyse the potential impact of vaccination on the IL-15 induced transcriptome. Baseline and 30 day post-vaccination PBMC samples were fractionated into NK cell enriched and NK cell depleted populations after 18 hours in vitro IL-15 stimulation. The data demonstrated a number of differentially expressed genes within the NK cell enriched and depleted fractions in paired samples 30 days post-vaccination compared with baseline (Supplementary Figure 4).
TIV and QIV vaccination induced significant levels of H3N2 specific antibodies (Supplementary figure 2a), therefore, we measured antibody-dependent NK cell activation (whole PBMC from one non-vaccinated control donor) to inactivated H3N2 using pre or post-vaccination plasma from each vaccinated individual. Indeed, we measured significantly higher NK cell CD107a and IFN-γ expression in response to inactivated virus with post-vaccination plasma compared with baseline plasma (Supplementary Figure 2b). There was a weak positive correlation (p=0.0006) between day 30 antibody concentration and NK cell IFN-γ secretion (Supplementary Figure 2c).
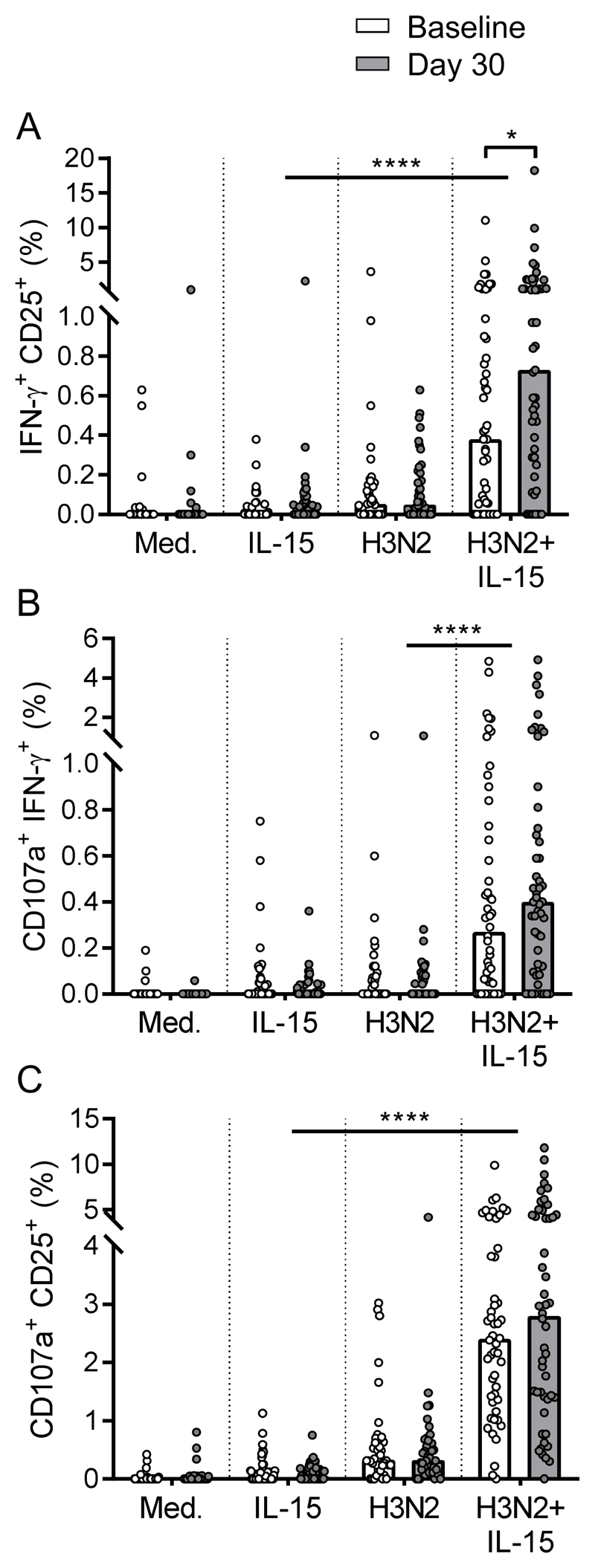
Vaccine induced enhancement of polyfunctional NK cell responses requires IL-15 co-stimulation
IL-15 is a major contributor to CIML NK cell function and synergises with IL-12 and IL-18 for polyfunctionality (20), suggesting that, in vivo, IL-15 may play an important role in maximising anti-viral NK cell activity. Here we examined whether vaccination would promote polyfunctional NK cell responses after restimulation with IL-15 alone or in combination with H3N2 (Figure 3). Frequencies of IFN-γ+CD25+, CD107a+IFN-γ+, and CD107a+CD25+ polyfunctional NK cells were significantly higher in response to H3N2 + IL-15 than H3N2 alone or IL-15 alone both pre and post-vaccination (Figure 3a-c). Enhanced polyfunctional NK cell responses were observed after vaccination; significantly higher percentages of IFN-γ+CD25+ NK cells (baseline median 0.38%; day 30 median 0.73%) and an increase in median percentage of CD107a+IFN-γ+ (baseline median 0.27%; day 30 median 0.40%) and CD107a+CD25+ (baseline median 2.4%; day 30 median 2.8%) were measured in response to H3N2 + IL-15 but not to either stimulus alone (Figure 3a-c). These data suggest that, consistent with viral induction of IFN-α2 and IL-12(p70) shown in Figure 1, enhancement of polyfunctional NK cell frequencies requires viral induced signals.
Figure 3. Vaccine induced enhancement of polyfunctional NK cell responses requires IL-15 co-stimulation.
Percentages of double positive IFN-γ+CD25+ (a), CD107a+IFN-γ+ (b) and CD107a+CD25+ (c) NK cells at baseline (white bars) and 30 days post vaccination (grey bars) were determined by flow cytometry after 18 hour stimulation with medium alone, IL-15 alone, H3N2 or H3N2 + IL-15 (n=51). Plots are one dot per donor with a bar representing median percentage. Comparisons between vaccination time points were made using Wilcoxon signed-rank test and between culture conditions by one-way ANOVA with Dunn’s correction for multiple comparisons. *p < 0.05, ****p < 0.0001.
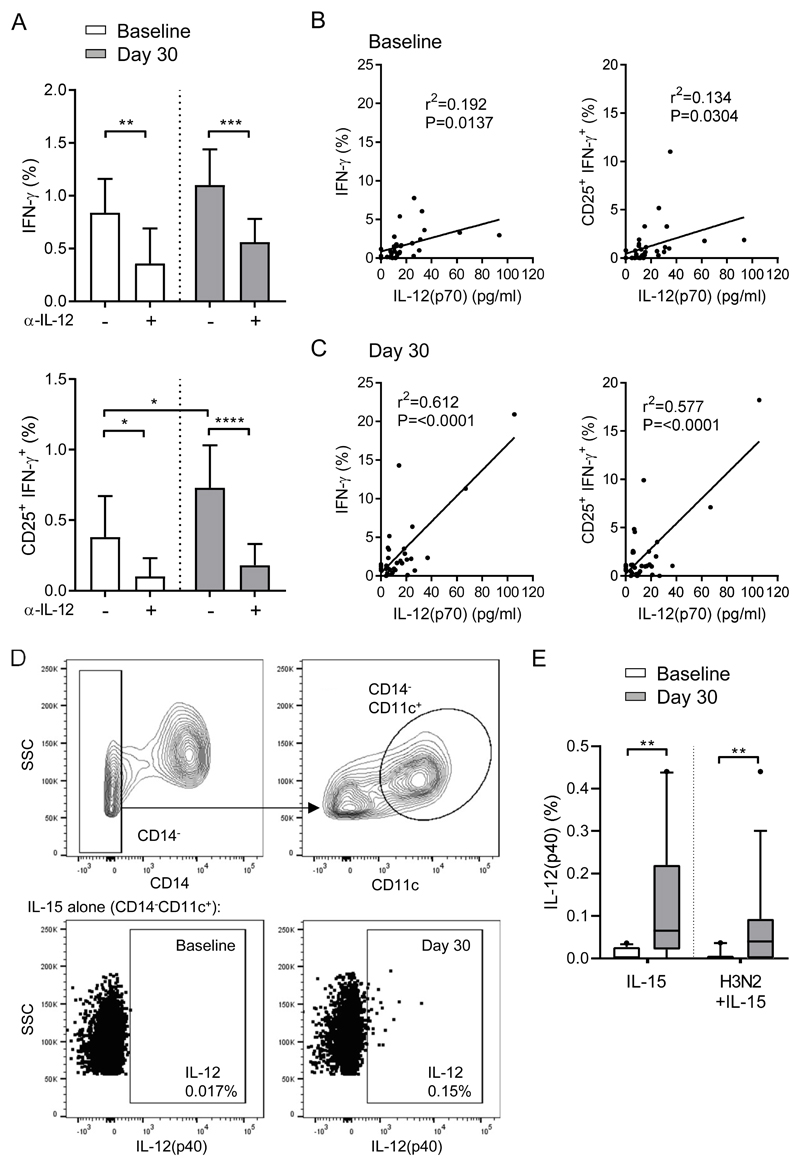
Post-vaccination NK cell responses are dependent on IL-12
Synergy between innate cytokines such as IL-12, and CD4+ T cell derived IL-2 are required for enhanced NK cell responses after vaccination (9, 16, 17, 19). Despite measuring no direct enhancement of IL-12 production compared with baseline samples by Luminex (Figure 1), we assessed whether the enhancement of NK cell responses after vaccination was nonetheless dependent on IL-12. Pre and post-vaccination PBMCs were stimulated in vitro with H3N2 + IL-15 in the presence of anti-IL-12 blocking antibody or isotype control antibody and NK cell function was assessed by flow cytometry. IL-12 blockade significantly reduced the percentages of IFN-γ+ positive and CD25+IFN-γ+ double-positive NK cells at baseline and ablated the vaccination-induced enhancement of CD25+IFN-γ+ double-positive NK cells (Figure 4a). The relationship between NK cell function and IL-12(p70) secretion in 18 hour in vitro cultures was assessed by linear regression. Both NK cell IFN-γ expression and CD25+IFN-γ+ polyfunctionality were loosely but significantly correlated with IL-12(p70) concentration at baseline (r2=0.19; p=0.014 and r2=0.13; p=0.03, respectively) (Figure 4b) but were more strongly correlated at 30 days post-vaccination (r2=0.61, p=<0.0001 and r2=0.58, p=<0.0001, respectively) (Figure 4c). Finally, pre and post-vaccination PBMCs were stimulated in vitro with IL-15 alone or H3N2 + IL-15 for 18 hours and intracellular IL-12(p40) expression in CD14-CD11c+ myeloid DCs (mDC) was measured by flow cytometry (gating strategy shown in Figure 4d). There was a significantly higher percentage of IL-12+ mDCs post-vaccination compared with pre-vaccination when simulated with IL-15 alone or H3N2 + IL-15 (Figure 4e). These data indicate that the enhancement of NK cell responses post-vaccination was dependent on IL-12 but that the intrinsic ability of NK cells to respond to this cytokine may also be influenced by vaccination.
Figure 4. Post-vaccination NK cell responses are dependent on IL-12.
Baseline (white bars) and 30 day post-vaccination (grey bars) PBMC were stimulated in vitro with IL-15 alone or H3N2 + IL-15 in the presence of an anti-IL-12 blocking antibody for 18 hours, (a) percentage of IFN-γ+ and CD25+IFN-γ+ double positive NK cells were determined by flow cytometry (n=51). Linear regression analysis showing correlation between IFN-γ+ and CD25+IFN-γ+ double positive NK cells and IL-12(p70) concentration measured by Luminex in response to H3N2 + IL-15 (no IL-12 blocking) at 18 hours both before (b) and after (c) vaccination. Percentage of lineage negative (CD3-CD56-CD19-) CD14-CD11c+ mDCs expressing IL-12(p40) was determined by flow cytometry (gating strategy shown in d) in response to IL-15 alone or H3N2+IL-15 (e). Graphs show median percentage with 95% confidence interval or box and whisker plots with 10th-90th percentile. Goodness of fit was determined using r2 and significance was determined by Pearson coefficient as p value below 0.05. Comparisons between culture conditions and vaccination time points were made using Wilcoxon signed-rank test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Altered ex vivo NK cell phenotype and cytokine receptor expression early after TIV vaccination
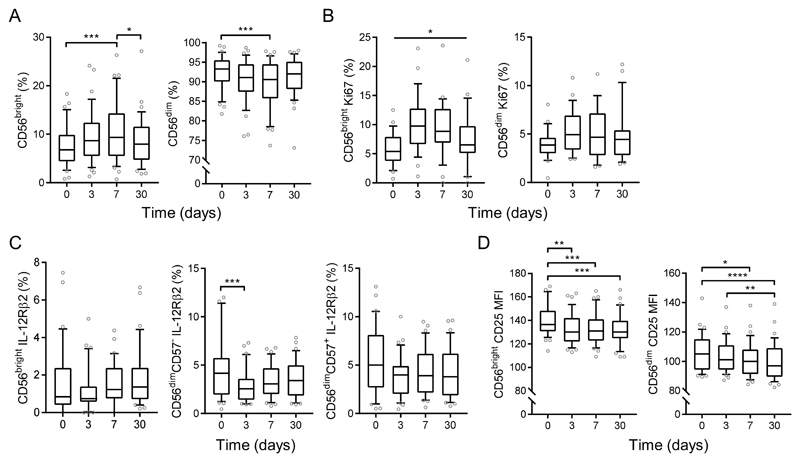
Enhanced cytokine-dependent NK cell responses observed after vaccination are restricted to less differentiated, cytokine responsive subsets (8–10, 19, 21). To determine how vaccination increases the functional potential of these less differentiated NK cells, including IL-12 responsiveness, we examined the effects of vaccination on NK cell phenotype at day 3, 7 and 30 post-vaccination by ex vivo analysis of expression of CD56, CD57, the proliferation marker Ki67, and cytokine receptors IL-12Rβ2, IL-18Rα and CD25 (gating strategy is shown in Supplementary Figure 3a). We observed a transient expansion of the CD56bright NK cell subset, along with concomitant contraction of the CD56dim subset within the total NK cell population, at 7 days post-vaccination when compared with baseline or day 30 (Figure 5a). This seems to be due to proliferation of CD56bright cells as demonstrated by a transient increase in the proportion of Ki67+CD56bright NK cells (Figure 5b) with no parallel effect on cell death (as monitored by viability dye) within both CD56bright and CD56dim NK cells across vaccination visits (Supplementary Figure 3b).
Figure 5. Altered ex-vivo NK cell phenotype and cytokine receptor expression early after TIV vaccination.
NK cell phenotype was measured after 2015-2016 TIV vaccination (n=37) at baseline, day 3, day 7 and day 30 post-vaccination. Percentage of CD56bright and CD56dim NK cells (a), Ki67 (b), IL-12Rβ2 (c) and CD25 MFI (d) were determined and attributed to NK cell differentiation subsets defined by CD56 and CD57 expression. Graphs show box and whisker plots with 10th-90th percentile, comparison across vaccination visits was determined by one-way ANOVA with Dunn’s correction for multiple comparisons. *p < 0.05, **p < 0.01, ***p< 0.001, **** p < 0.0001.
We observed significant downregulation of surface IL-12 receptor (IL-12Rβ2) expression on CD56dimCD57- cells 3 days after vaccination, although we did not detect any significant vaccination-induced changes in IL-12Rβ2 expression in CD56bright or CD56dimCD57+ NK cells (Figure 5c). No changes in surface IL-18 receptor (IL-18Rα) in any subset was observed (Supplementary Figure 3c). There was a small yet highly significant decline in the MFI of CD25 expression on both CD56bright and CD56dim NK cells post-vaccination (Figure 5d). Taken together, these data show transient proliferation of less differentiated NK cells, and a small but significant downregulation of cytokine receptors (IL-12Rβ2 and CD25) early after vaccination, consistent with vaccine induced responses to the cognate cytokines in vivo.
Discussion
CIML NK cells have been described in a number of different in vitro models and after infection and vaccination (reviewed in (22) and (23)). Similarly to in vitro priming with cytokines, in vitro priming with inactivated H1N1 virus and TIV vaccination led to enhanced NK cell responses to cytokines alone (9), suggesting that anti-viral cytokine stimulation is sufficient to generate CIML NK cells. Here, in line with previous data, we demonstrate the generation of CIML NK cells by vaccination but furthermore, for the first time, we show that vaccination also primes for heightened secretion of myeloid cell derived cytokines that can be induced by extremely low concentrations of IL-15 in vitro. Overall, our data suggest that influenza vaccination primes both myeloid cell cytokine secretion and intrinsic NK cell responsiveness, which together lead to the enhanced NK cell responses observed after influenza vaccination.
These observations are in line with systems level analyses of the PBMC response to TIV influenza vaccination in which NK cell activation, type I IFN, serum IP-10 (IFN-γ-induced protein) and DC activation signatures are seen within 48 hours (24, 25) and with innate cytokine secretion from PBMCs stimulated in vitro with inactivated influenza virus (16). Our study represents the first demonstration of enhanced myeloid cell derived cytokine production after viral vaccination and is reminiscent of the “trained immunity” described in human monocytes after Candida albicans infection or bacille Calmette-Guérin (BCG) vaccination, where increased TNF-α, IL-1β and IL-6 secretion occurred after secondary stimulation with the same or distinct stimuli (26, 27). Enhanced pro-inflammatory function in “trained” monocytes is associated with activation induced epigenetic modifications (28, 29). Jegaskanda et al recently reported that accessory cell IFN-α induced in response to influenza infection primed NK cells for enhanced responsiveness to antibody-dependent signals after a 12 hour rest period (without continued exposure). Consistent with our study, the authors measured increased Ki67 expression on the pre-exposed NK cells (30).
Although measurement of cytokines in supernatant after stimulation does not identify a specific cellular source, it is likely that the high levels of GM-CSF, IL-10, IL-1β and IFN-α2 measured here were derived from myeloid accessory cells. In agreement and in line with our previous data, we show IL-12 induction in CD14-CD11c+ mDCs in response to H3N2 +/- IL-15 (16). In contrast, IL-10 and TNF-α are produced by CD14+ monocytes and IFN-α and IFN-β are produced by conventional and plasmacytoid DCs within hours of TIV vaccination (31, 32). This vaccine induced enhancement of myeloid cell cytokine secretion may therefore demonstrate priming (or “training”) of monocytes, and/or other innate myeloid cell populations in response to viral vaccination.
Vaccine-induced enhanced cytokine secretion was revealed with low dose IL-15 stimulation, without the need for vaccine antigen-specific stimulation. This same concentration of IL-15 also enhanced secretion of myeloid cell derived cytokines, in particular IL-12 and IFN-α in combination with H3N2 (16). This suggests that pre-activation of these cells by the inactivated H3N2 virus within the vaccine leaves them with enhanced ability to respond to IL-15 and this effect is maintained for at least 30 days after initial stimulation. This enhanced cytokine secretion capacity has a knock-on effect on NK cell activation, with significant upregulation of CD25 in post-vaccination cultures compared with baseline in response to IL-15. In contrast to our previous study, where vaccination promoted limited enhancement of NK cells in response to H1N1 virus alone (9), enhanced responsiveness of H3N2 vaccination-primed NK cells required IL-15. This may be due to previous exposure to influenza by infection or vaccination leading to different levels of T cell help as suggested by comparing our previous study cohorts, lower overall induction of NK cell activation markers with H3N2 compared with other influenza viruses (such as H1N1) (9) or differences in virus specific cytokine secretion profiles. Such differences in prior exposure may also explain individual level differences in NK cell response within the current study. Production and trans-presentation of IL-15 is of particular significance in the lung during influenza infection, where local accumulation impacts T cell apoptosis, formation of IL-2-independent tissue resident memory T cells and recruitment of NK cells (33–36). Our previous study observed similar CIML NK cell generation with intranasal administration of live attenuated influenza vaccine (LAIV) (9), and thus the potential impacts of locally induced IL-15 in the lung after influenza vaccination or indeed infection merits further investigation.
Frequencies of IFN-γ+ and IFN-γ+CD25+ NK cells were correlated with IL-12 concentrations in cell supernatants, suggesting this cytokine is important for post-vaccination NK cell activation. Although, there was no evidence of enhanced IL-12(p70) in culture supernatant after vaccination measured by Luminex, which may be due to immediate utilisation of secreted IL-12 in vitro, enhanced IL-12 secretion post-vaccination was demonstrated by intracellular cytokine staining. Similarly, we could not detect IL-2 in supernatants even though IL-2 plays an essential role in NK cell responses to vaccination, supporting the suggestion that differential T cell help is playing a role in the post-vaccination response to H3N2 (9, 17, 19). NK cells required both viral stimuli and IL-15 for enhanced polyfunctionality after vaccination, which is consistent with the need for combinations of two or more of IL-12, IL-15 and IL-18 in order to induce polyfunctionality in non-pre-activated and in vitro generated CIML NK cells, and IL-15 playing a major role in CIML NK cell mediated function (20). Vaccine induced changes in frequencies of polyfunctional NK cell cells ranged from 0.13% to 0.4%, representing up to 1/2500 of total lymphocytes. Similar frequencies are observed for typical antigen specific T cell responses to whole antigens, indicating that the NK cell response could contribute meaningfully to vaccine induced immunity.
CIML NK cells retain their characteristics through subsequent cell divisions, suggesting that their induction is not simply a short term priming effect but rather reflects an intrinsic change or differentiation state (37). Proliferation of less differentiated (CD56bright and CD56dimCD57-) NK cell subsets after vaccination is consistent with the previously demonstrated effects of vaccination on NK cells and with other routes for generation of CIML NK cells (5, 8, 9, 19). In addition, we measured significant down regulation of IL-12 receptor at day 3 after vaccination suggesting cytokine mediated NK cell activation in vivo; down-regulation of IL-12Rβ2 expression after vaccination would be consistent with IL-12-mediated receptor turnover, supporting a critical role for this cytokine in CIML NK cell generation. There was no significant association between study participant age and NK cell response or cytokine production post-vaccination in this study, a weak but significant trend for decreasing cytokine-induced NK cell function with age was observed in a previous study with a larger sample size (38).
In summary, we show for the first time that influenza vaccination primes PBMCs for heightened innate cytokine production, that this can be revealed by very low concentrations of IL-15, and that this – in combination with the generation of CIML NK cells - leads to enhanced NK cell responses after vaccination. Further work is required to determine the significance of this pathway in post-vaccination immunity to influenza virus.
Supplementary Material
Key Points.
-
1)
Influenza vaccination primes myeloid cells for enhanced cytokine secretion.
-
2)
Vaccine-enhanced myeloid cytokines boost NK cell responses to influenza virus.
Acknowledgements
We thank Carolynne Stanley for recruiting and obtaining consent from study subjects and for blood sample collection.
Grant support: This work was funded by the UK MRC and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (grant no. G1000808, EMR). H.R.W is supported by a UK Medical Research Council (MRC) Studentship in Vaccine Research (Grant no. MR/J003999/1). M.R.G is supported by the Innovative Medicines Initiative 2 Joint Undertaking (Grant no. 115861). This joint undertaking receives support from the Europeans Union’s Horizon 2020 Research and Innovation Programme and Association. H.P is supported by TRACVAC (Europeans Union’s Horizon 2020, Grant no. 2017–733373).
Abbreviations
- CIML
cytokine-induced memory-like
- H3N2
inactivated whole influenza virus (A/Victoria/361/2011)
- MFI
mean fluorescence intensity
- mDC
myeloid dendritic cell
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, Frascaroli G, Bayer C, Schmal T, Mertens T. Interleukin-2 from Adaptive T Cells Enhances Natural Killer Cell Activity against Human Cytomegalovirus-Infected Macrophages. Journal of virology. 2015;89:6435–6441. doi: 10.1128/JVI.00435-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jost S, Tomezsko PJ, Rands K, Toth I, Lichterfeld M, Gandhi RT, Altfeld M. CD4+ T-cell help enhances NK cell function following therapeutic HIV-1 vaccination. Journal of virology. 2014;88:8349–8354. doi: 10.1128/JVI.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nature reviews Immunology. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 5.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keppel MP, Yang L, Cooper MA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. Journal of immunology (Baltimore, Md. : 1950) 2013;190:4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquardt N, Ivarsson MA, Blom K, Gonzalez VD, Braun M, Falconer K, Gustafsson R, Fogdell-Hahn A, Sandberg JK, Michaelsson J. The Human NK Cell Response to Yellow Fever Virus 17D Is Primarily Governed by NK Cell Differentiation Independently of NK Cell Education. Journal of immunology (Baltimore, Md. : 1950) 2015;195:3262–3272. doi: 10.4049/jimmunol.1401811. [DOI] [PubMed] [Google Scholar]
- 9.Goodier MR, Rodriguez-Galan A, Lusa C, Nielsen CM, Darboe A, Moldoveanu AL, White MJ, Behrens R, Riley EM. Influenza Vaccination Generates Cytokine-Induced Memory-like NK Cells: Impact of Human Cytomegalovirus Infection. Journal of immunology (Baltimore, Md. : 1950) 2016;197:313–325. doi: 10.4049/jimmunol.1502049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, Toefy A, Lerumo L, Hopley C, Pienaar B, Chheng P, et al. Bacillus Calmette-Guerin (BCG) Revaccination of Adults with Latent Mycobacterium tuberculosis Infection Induces Long-Lived BCG-Reactive NK Cell Responses. Journal of immunology (Baltimore, Md. : 1950) 2016;197:1100–1110. doi: 10.4049/jimmunol.1501996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabekura T, Lanier LL. Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. The Journal of experimental medicine. 2016;213:2745–2758. doi: 10.1084/jem.20160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, Cerwenka A. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. The Journal of clinical investigation. 2014;124:5305–5316. doi: 10.1172/JCI77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. The Journal of experimental medicine. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagstaffe HR, Nielsen CM, Riley EM, Goodier MR. IL-15 Promotes Polyfunctional NK Cell Responses to Influenza by Boosting IL-12 Production. Journal of immunology (Baltimore, Md. : 1950) 2018;200:2738–2747. doi: 10.4049/jimmunol.1701614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. Journal of immunology (Baltimore, Md. : 1950) 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, et al. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. Journal of immunology (Baltimore, Md. : 1950) 2012;188:5054–5062. doi: 10.4049/jimmunol.1102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darboe A, Danso E, Clarke E, Umesi A, Touray E, Wegmuller R, Moore SE, Riley E, Goodier MR. Enhancement of cytokine-driven NK cell IFN-gamma production after vaccination of HCMV infected Africans. European journal of immunology. 2017 doi: 10.1002/eji.201746974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrén I, Mikelez I, Odriozola I, Gredilla A, González J, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. Implication of Interleukin-12/15/18 and Ruxolitinib in the Phenotype, Proliferation, and Polyfunctionality of Human Cytokine-Preactivated Natural Killer Cells. 2018;9 doi: 10.3389/fimmu.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jost S, Quillay H, Reardon J, Peterson E, Simmons RP, Parry BA, Bryant NN, Binder WD, Altfeld M. Changes in cytokine levels and NK cell activation associated with influenza. PloS one. 2011;6:e25060. doi: 10.1371/journal.pone.0025060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagstaffe HR, Mooney JP, Riley EM, Goodier MR. Vaccinating for natural killer cell effector functions. Clinical & translational immunology. 2018;7:e1010. doi: 10.1002/cti2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrien-Elliott MM, Wagner JA, Fehniger TA. Human Cytokine-Induced Memory-Like Natural Killer Cells. Journal of innate immunity. 2015;7:563–571. doi: 10.1159/000382019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, McCausland M, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoek KL, Samir P, Howard LM, Niu X, Prasad N, Galassie A, Liu Q, Allos TM, Floyd KA, Guo Y, Shyr Y, et al. A cell-based systems biology assessment of human blood to monitor immune responses after influenza vaccination. PloS one. 2015;10:e0118528. doi: 10.1371/journal.pone.0118528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell host & microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science (New York, N.Y.) 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner CM, Mills KH. The cells that mediate innate immune memory and their functional significance in inflammatory and infectious diseases. Seminars in immunology. 2016;28:343–350. doi: 10.1016/j.smim.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Jegaskanda S, Vanderven HA, Tan HX, Alcantara S, Wragg K, Parsons MS, Chung A, Juno JA, Kent SJ. Influenza infection enhances antibody-mediated NK cell functions via Type I interferon dependent pathways. Journal of virology. 2018 doi: 10.1128/JVI.02090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanty S, Joshi SR, Ueda I, Wilson J, Blevins TP, Siconolfi B, Meng H, Devine L, Raddassi K, Tsang S, Belshe RB, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. The Journal of infectious diseases. 2015;211:1174–1184. doi: 10.1093/infdis/jiu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Athale S, Banchereau R, Thompson-Snipes L, Wang Y, Palucka K, Pascual V, Banchereau J. Influenza vaccines differentially regulate the interferon response in human dendritic cell subsets. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aaf9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. The Journal of experimental medicine. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strutt TM, Dhume K, Finn CM, Hwang JH, Castonguay C, Swain SL, McKinstry KK. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal immunology. 2018;11:668–680. doi: 10.1038/mi.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbist KC, Cole CJ, Field MB, Klonowski KD. A role for IL-15 in the migration of effector CD8 T cells to the lung airways following influenza infection. Journal of immunology (Baltimore, Md. : 1950) 2011;186:174–182. doi: 10.4049/jimmunol.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbist KC, Rose DL, Cole CJ, Field MB, Klonowski KD. IL-15 participates in the respiratory innate immune response to influenza virus infection. PloS one. 2012;7:e37539. doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen CM, White MJ, Bottomley C, Lusa C, Rodriguez-Galan A, Turner SE, Goodier MR, Riley EM. Impaired NK Cell Responses to Pertussis and H1N1 Influenza Vaccine Antigens in Human Cytomegalovirus-Infected Individuals. Journal of immunology (Baltimore, Md. : 1950) 2015;194:4657–4667. doi: 10.4049/jimmunol.1403080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.