Abstract
The exact etiology of dementia is still unclear, but both genetic and lifestyle factors are thought to be key drivers of this complex disease. The recognition of familial patterns of dementia has led to the discovery of genetic factors that play a role in the pathogenesis of dementia, including the apolipoprotein E (APOE) genotype and a large and still growing number of genetic variants.1,2 Beyond the genetic architecture, several modifiable risk factors have been implicated in the development of dementia.3 Prevention trials to halt or delay cognitive decline are increasingly recruiting older individuals who are genetically predisposed to dementia. However, it remains unclear whether targeted health and lifestyle interventions can attenuate or even offset this increased genetic risk. Here, we leverage long-term data on both genetic and modifiable factors from 6352 individuals aged 55 years and older within the population-based Rotterdam Study. In this study, we demonstrate that among individuals at low- and intermediate genetic risk, favorable modifiable risk profiles are related to a lower risk of dementia compared to those with an unfavorable profile. In contrast, these protective associations were not found among those at high genetic risk.
Recent analyses have shown that if currently known modifiable risk factors were to be eliminated at a population level, over a third of all dementia cases could be prevented.3 In view of these findings, several dementia prevention trials have been conducted to investigate the efficacy of lifestyle interventions, but have yielded inconsistent results to date.4–6 Such interventions have been proposed to be more effective when targeted at individuals who have an increased risk of dementia, identified through their genetic or clinical profile, or a combination thereof.7
The genetic risk of developing dementia or Alzheimer’s disease (AD) may be detrimental for some individuals,8 yet this risk may be mitigated for most when adhering to a healthy lifestyle. A recent subgroup analysis from a 2-year multi-domain intervention trial found that healthy lifestyle changes had beneficial effects on cognitive performance, even in APOE-ε4 carriers.9 Evidence from randomized controlled trials is ideally required to determine whether these effects, beyond cognitive performance, are also true for dementia. However, since treatment effects from lifestyle interventions at an individual level are generally small, large trials with long follow-up are needed. Such trials are expensive and prone to high attrition rates. Instead, data from long-term prospective cohort studies can be leveraged to gain insights in the interplay between genetic and lifestyle factors, with the potential to inform the design of future clinical trials.
Prior studies mostly focused on one individual protective factor with respect to the risk of dementia,10,11 yet the combination of multiple factors may yield more beneficial effects than the sum of their parts.12 Combining such data is also important as it takes into account the multifactorial nature of late-life dementia.13 We used data from the Rotterdam Study to determine to what extent a favorable profile based on modifiable risk factors is associated with a lower risk of dementia among individuals at low, intermediate or high genetic risk.
In 6352 participants, we determined genetic risk based two approaches: (1) APOE genotype and (2) a weighted polygenic risk score including 27 genetic variants (excluding APOE) that showed genome-wide significant evidence for associations with AD (Supplementary Table 1). We grouped participants into high APOE-risk (ε2ε4, ε3ε4 or ε4ε4 genotypes), intermediate risk (ε3ε3 genotype) or low risk (ε2ε2 or ε2ε3 genotypes), or based on tertiles of the polygenic risk score. In these participants, we also measured several health and/or lifestyle factors that have been implicated to lower the risk of dementia.11,14 Among these are: (1) abstaining from smoking, (2) absence of depression, (3) absence of diabetes, (4) regular physical activity, (5) avoiding social isolation, and (6) adherence to a healthy dietary pattern, which included limited alcohol consumption (see Methods for additional information). Based on these six factors, we computed an overall score of modifiable risk factors ranging from zero to six. A higher score reflects a more favorable profile. Subsequently, we categorized participants into the following three groups. An unfavorable profile: a score of ≤2 factors; an intermediate profile: a score of 3-4 factors; and a favorable profile: a score of ≥5 protective factors. Alternatively, we determined modifiable risk based on the Ideal Cardiovascular Health score and 10-year predicted risk of fatal cardiovascular diseases.15 We subsequently stratified participants on both their genetic and modifiable risk. We calculated the risk of developing dementia for each stratum separately, on both a relative and absolute scale using Cox proportional hazards and competing risk models, respectively.
This study included more women (56.2%) than men. Baseline characteristics were roughly similar across categories of APOE-risk (Table 1). As expected, APOE ε4 carriers generally had a younger age of dementia diagnosis (P=1.38×10-12), more often had a parental history of dementia (P=4.55×10-4), and had higher total cholesterol levels (P=1.24×10-19), compared to non-carriers. During a median follow-up of 14.1 years among a total of 6352 participants, 915 participants were diagnosed with dementia, of whom 739 were diagnosed with AD, and 2644 participants died free from dementia.
Table 1. Baseline characteristics per genetic risk category based on APOE carrier status.
| Low risk (ε2ε2 or ε2ε3) N=887 |
Intermediate risk (ε3ε3) N=3718 |
High risk (ε2ε4, ε3ε4 or ε4ε4) N=1747 |
P for difference | |
|---|---|---|---|---|
| Age, years | 69.4 (8.5) | 69.2 (8.3) | 68.7 (7.9) | 0.042 |
| Women | 529 (59.6) | 2072 (55.8) | 971 (55.5) | 0.102 |
| Educational years, median (IQR) | 10 (7-13) | 10 (7-13) | 10 (7-13) | 0.325 |
| Parental history of dementia | 53 (8.1) | 219 (7.8) | 155 (11.6) | 4.55×10-4 |
| History of stroke | 33 (3.7) | 138 (3.7) | 61 (3.5) | 0.929 |
| Body mass index, kg/m2 | 27.4 (4.0) | 27.0 (4.0) | 26.9 (3.9) | 0.014 |
| Systolic blood pressure, mmHg | 145 (22) | 143 (21) | 143 (21) | 0.045 |
| Diastolic blood pressure, mmHg | 77 (11) | 77 (12) | 77 (11) | 0.239 |
| Total cholesterol, mmol/L | 5.6 (1.0) | 5.8 (1.0) | 5.9 (1.0) | 1.24×10-19 |
| High-density lipoprotein cholesterol, mmol/L | 1.43 (0.4) | 1.39 (0.4) | 1.35 (0.4) | 1.00×10-5 |
| Fasting glucose, mmol/L | 5.6 (1.5) | 6.0 (1.6) | 6.0 (1.6) | 0.902 |
| Baseline MMSE score, median (IQR) | 28 (27-29) | 28 (27-29) | 28 (27-29) | 0.049 |
| Age of dementia diagnosis | 85.5 (5.9) | 84.1 (6.3) | 81.3 (6.5) | 1.38×10-12 |
| Modifiable health and lifestyle factors | ||||
| No current smoking | 714 (81) | 2961 (80.1) | 1389 (79.9) | 0.801 |
| Absence of depression | 799 (90.1) | 3351 (90.1) | 1567 (89.7) | 0.881 |
| Absence of diabetes | 744 (88.3) | 3096 (88.3) | 1461 (89.0) | 0.750 |
| Regular physical activity | 484 (56) | 2109 (58.5) | 1005 (59.4) | 0.501 |
| Absence of social isolation | 588 (66.7) | 2664 (72.4) | 1256 (72.1) | 0.005 |
| Adherence to a healthy diet | 141 (15.9) | 559 (15.0) | 252 (14.4) | 0.649 |
| Modifiable risk profile category | ||||
| Favorable: 5-6 health and lifestyle factors |
568 (64.0) | 2453 (66.0) | 1132 (64.8) | 0.648 |
| Intermediate: 3-4 health and lifestyle factors |
224 (25.2) | 884 (23.8) | 443 (25.4) | |
| Unfavorable: 0-2 health and lifestyle factors |
95 (10.7) | 381 (10.2) | 172 (9.8) | |
Abbreviations: APOE=apolipoprotein E, N=number of people at risk, SD=standard deviation, IQR=interquartile range, MMSE=Mini-Mental State Examination. Data presented as frequency (percent) for categorical values and mean ± SD for continuous variables unless indicated otherwise. We compared baseline characteristics across APOE strata using analysis of variance (anova) tests. In the case of frequency distributions or when data were non-normally distributed, we compared variables between groups using non-parametric tests (chi-square, Mann-Whitney or Kruskal Wallis). Two-sided P values were uncorrected for multiple testing.
Dementia risk was significantly higher among participants with a high and intermediate APOE genetic risk status compared to those at low genetic risk (Fig. 1, Table 2). The risk of dementia also increased among participants who had fewer protective factors (P for trend: 0.0044), such that those with an unfavorable profile (≤2 out of 6 factors) had a 32% higher risk of dementia compared to participants with a favorable one (≥5 out of 6 factors) (Fig. 1, Table 2). The strength of this association remained nearly identical when adjusting for parental history of dementia and cardiovascular risk factors (hazard ratio [HR]: 1.29 [95% confidence interval (CI): 1.05;1.59], P for trend: 0.0087).
Fig. 1. Cumulative incidence of dementia during follow-up.
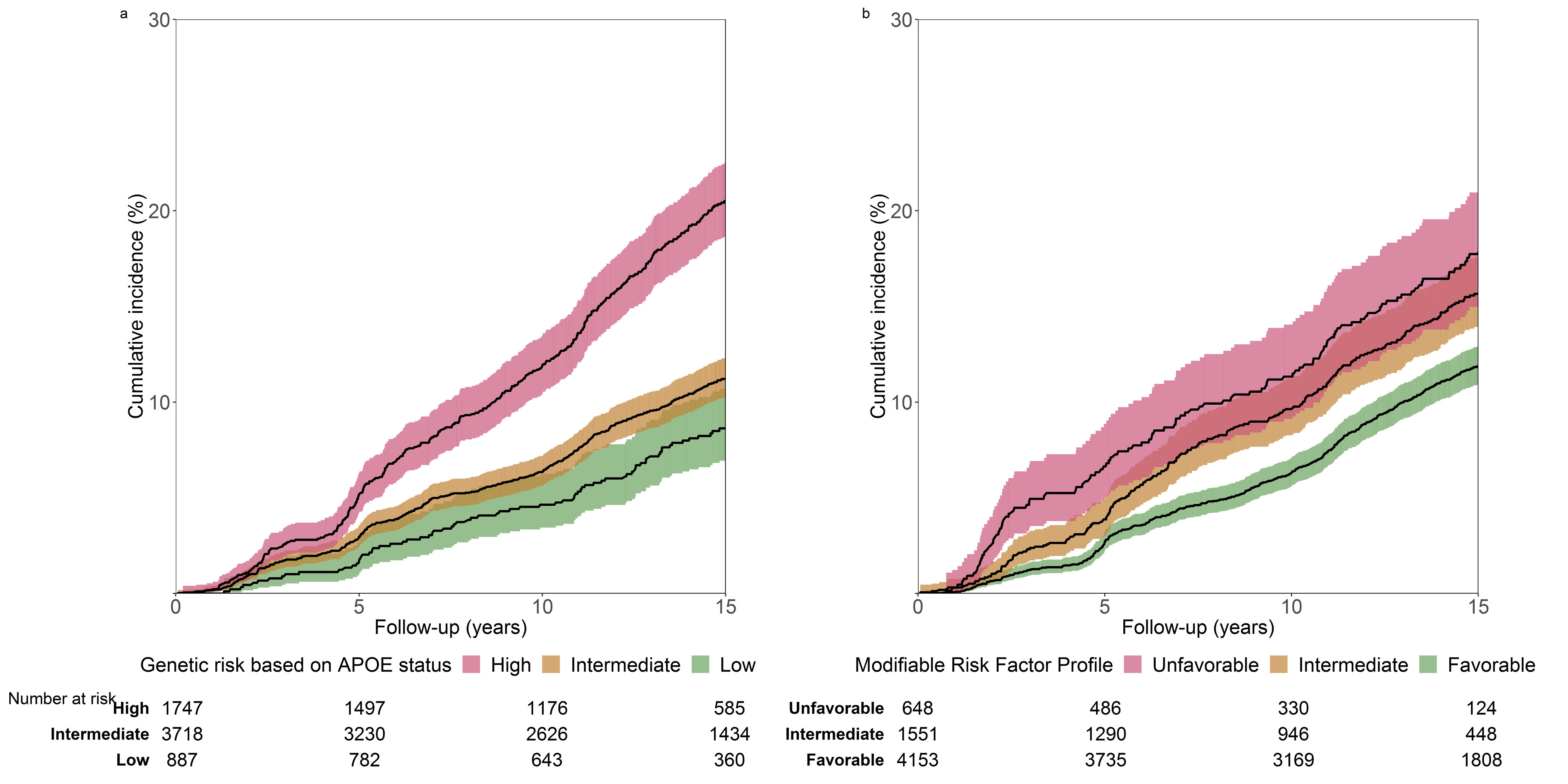
a, according to genetic risk based on APOE genotyping, and b, according to modifiable risk factor profiles. For a and b, shaded areas represent 95% confidence intervals.
Table 2. Risk of incident dementia according to APOE-related risk and lifestyle categories.
| APOE-related risk | N/n | Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|---|---|---|---|
| Low risk (ε2ε2/ε2ε3) | 887/85 | Reference | Reference |
| Intermediate risk (ε3ε3) | 3718/456 | 1.45 (1.15;1.83) | 1.45 (1.15;1.83) |
| High risk (ε2ε4/ε3ε4/ε4ε4) | 1747/374 | 3.02 (2.38;3.82) | 3.02 (2.38;3.83) |
| P for trend | 2.10×10-30 | 1.87×10-30 | |
| Lifestyle risk category | |||
| Favorable | 4153/538 | Reference | Reference |
| Intermediate | 1551/259 | 1.15 (0.98;1.34) | 1.14 (0.98;1.33) |
| Unfavorable | 648/118 | 1.32 (1.08;1.63) | 1.29 (1.05;1.59) |
| P for trend | 0.0044 | 0.0087 |
Model 1 - adjusted for: age, sex and education
Model 2 - additionally adjusted for: parental history of dementia, history of stroke, systolic blood pressure, total and high-density lipoprotein cholesterol
Abbreviations: N=number of individuals at risk, n=number of dementia cases during follow-up, HR=hazard ratio, CI=confidence interval.
APOE genotype significantly modified the association between protective factors and dementia (P for interaction=0.023). In those at low- or intermediate APOE-risk, the risk of dementia in participants with an unfavorable profile was higher compared to those with a favorable one (HR: 2.51, 95% CI: 1.40;4.48, and 1.39, 1.04;1.85 respectively, Table 3). In those at high APOE-risk, we did not find differences in risk between an unfavorable and intermediate compared to a favorable profile (HR: 1.00, 95%: 0.79;1.28, and 1.05, 95% CI: 0.73;1.50, respectively).
Table 3. Risk of incident dementia while stratifying participants on both their APOE-related risk and modifiable risk factor profile.
| APOE-related risk | Risk factor profile | N/n | HR (95% CI) |
|---|---|---|---|
| Low (ε2ε2/ε2ε3) | Favorable | 568/44 | Reference |
| Intermediate | 224/23 | 1.14 (0.66;1.96) | |
| Unfavorable | 95/18 | 2.51 (1.40;4.48) | |
| P for trend | 0.0059 | ||
|
| |||
| Intermediate (ε3ε3) | Favorable | 2453/253 | Reference |
| Intermediate | 884/139 | 1.27 (1.02;1.57) | |
| Unfavorable | 381/64 | 1.39 (1.04;1.85) | |
| P for trend | 0.0087 | ||
|
| |||
| High (ε2ε4/ε3ε4/ε4ε4) | Favorable | 1132/241 | Reference |
| Intermediate | 443/97 | 1.00 (0.79;1.28) | |
| Unfavorable | 172/36 | 1.05 (0.73;1.50) | |
| P for trend | 0.8300 | ||
Adjusted for: age, sex and education
Abbreviations: N=number of people at risk, n=number of dementia cases during follow-up, HR=hazard ratio, CI=confidence interval.
Among those at low APOE-risk, mean anticipated absolute 15-year risk of dementia ranged from 32.1% (95% CI: 0.0;59.9) for those with an unfavorable profile to 12.6% (95% CI: 4.5;26.8) for those with a favorable one (supplementary Table 2). Individuals at intermediate APOE-risk with an unfavorable profile had a 22.0% (95% CI: 8.3;39.2) anticipated risk, which was 13.5% (95% CI: 8.9;15.6) for those with a favorable profile. Among participants at high APOE-risk, the anticipated 15-year risk of dementia remained largely unchanged across the different profiles (ranging from 18.2% for a favorable to 19.5% for an unfavorable profile).
Stratified analyses showed that protective associations of favorable risk profiles with dementia tended to be stronger in younger individuals compared to older individuals, and were most pronounced for younger individuals at low APOE-risk (Supplementary Tables 3-4). In all of these analyses, no significant protective associations were found among APOE-ε4 carriers. There was no effect modification of the association between risk profiles and dementia risk by sex (Supplementary Table 5).
In sensitivity analyses, using a different approach namely a polygenic risk score for AD (without APOE), we similarly found that associations between protective factors were modified (P for interaction=0.0003), with patterns across strata of polygenic risk that were attenuated yet largely comparable to those of APOE (Supplementary Tables 6-7).
These patterns also remained unchanged when we varied the composition of modifiable risk factors. For instance, similar results were found when we stratified participants on their Ideal Cardiovascular Health score (P for interaction=0.026, Supplementary Table 8), or when we stratified participants on their predicted absolute 10-year risk of fatal cardiovascular diseases using the SCORE equation (P for interaction=7.82×10-5 , Supplementary Table 9). All of the individual health and lifestyle factor-specific associations with dementia risk that were included in the different profiles are presented in Supplementary Tables 10, 11 and 12, respectively.
Most evidence on the interaction between specific genetic and modifiable factors with dementia comes from observational studies. These studies primarily examined the associations and interactions of single health or lifestyle factors, such as diabetes, physical activity, alcohol use, smoking, and dietary patterns, with different APOE alleles. Most of these studies reported associations of these factors with dementia,16–19 generally with more pronounced effects in APOE-ε4 carriers during midlife.16–19 In contrast, studies conducted in older individuals (aged ≥60 years), primarily found beneficial effects of these factors on risk of dementia among non-carriers,20–26 or reported no interaction.27,28
Further evidence comes from the Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) Study among middle-aged individuals that took multiple protective factors into consideration.29 APOE genotype modified the association between several lifestyle factors and the risk of dementia. The results from the CAIDE Study indicate that APOE-ε4 carriers are particularly prone to hazardous health and lifestyle factors during midlife. The CAIDE study was conducted in a younger population (mean age 50.6 years) compared to this study (mean age 69.1 years). This may have led to survival bias in the current study conducted among individuals aged 55 years and older. Since older APOE-ε4 carriers are potentially less prone to the effects of unfavorable risk factors on dementia risk later in life.
To our knowledge, only the Prevention of Dementia by Intensive Vascular care (preDIVA) trial has assessed the effect of health and lifestyle interventions on dementia.16 This trial showed no overall benefit on dementia incidence of intensive vascular care management in a primary care setting. In a subgroup analysis of this trial, no significant differences were observed between APOE-ε4 carriers and non-carriers. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial assessed the effect of multiple lifestyle interventions on cognitive performance among older individuals.4 A pre-specified subgroup analysis of this trial observed similar beneficial effects of the intervention on cognitive performance in both APOE-ε4 carriers and non-carriers after two years of follow-up.9
In the current study, we aimed to complement evidence from these clinical trials with long-term observational data. Such an approach has been undertaken previously to study potential interaction between genetic and modifiable factors for other chronic diseases, such as heart disease and stroke.30,31 Our results confirm that individuals with a favorable profile have a lower risk of dementia compared to those with intermediate or unfavorable profiles based on modifiable risk factors. In contrast with the FINGER subgroup analysis, a favorable profile could not offset a high APOE-risk in this study. The FINGER trial intervened on multiple lifestyle factors simultaneously, whereas in our observational study, data on health and lifestyle factors was used to establish risk factor profiles. Non-differential misclassification may, in part, have led to an underestimation of the benefits of a favorable risk factor profile in our study. This may for instance apply to physical activity and diet quality as we have chosen cut-offs points to depict ‘favorable’, ‘intermediate’ or ‘unfavorable’ for these variables based on guideline recommendations.32,33 Moreover, as we lacked validated questionnaires to measure social engagement, we may not have fully captured its beneficial effect on dementia. Misclassification may also have occurred in genetic risk stratifications, since we did not have enough numbers to divide all individuals into single strata on their specific genotype. We therefore had to collate some categories of genetic risk to estimate meaningful effect sizes. As an example, we grouped individuals who are either heterozygous or homozygous for APOE-ε4. This may have led to an underestimation of the benefits of a favorable profile for individuals who are heterozygous for APOE-ε4, as this group resembles an intermediate risk group between those who are homozygous for APOE-ε3 or APOE-ε4.1
Furthermore, we studied the interplay between genetic and lifestyle factors on the long-term risk of developing dementia, while the FINGER subgroup analysis assessed effects on cognitive performance after a 2-year follow-up. The multi-domain lifestyle intervention improved cognitive performance compared to the control group in the short-term, it however remains questionable whether such effects also hold in the long-term. For instance, participants of the FINGER trial may already have developed essential APOE-related brain changes earlier in life,34 making them vulnerable to develop dementia later in life, irrespective of their modifiable risk factor profile.
In this study, a high risk of developing dementia based on APOE carrier status was not offset by a favorable profile. These findings contrast with those from other large, observational population-based studies that examined the interaction between genetic and modifiable factors for other chronic diseases, including for instance heart disease and stroke.30,31 These studies found protective associations of favorable modifiable profiles across genetic risk, even for those at highest genetic risk. Several reasons may underlie this discrepancy.
First, the harmful effects of APOE-ε4 on cholesterol metabolism are apparent throughout life, with cumulative effects on dementia risk with advancing age.1 Second, APOE-ε4 alters neuronal functioning which may lead to irreversible neuronal cell loss.35 With advancing age, these effects build up and may, in absence of disease-modifying drugs and proven preventive strategies, ultimately have a more detrimental effect for the risk of dementia in older individuals. Third, the risk for competing diseases at older age, such as coronary heart disease and stroke, may be mitigated or even reversed, by having a favorable risk profile through the reduction of atherosclerotic disease.36,37 Fourth, potential epi-genetic changes, such as methylation effects of APOE or additional variants, may be age-dependent and exert their effects in midlife or even earlier, yet this notion deserves further study. The APOE-ε4 allele finally triggers cascades that may be more independent of the studied profiles, such as for example pathways in inflammatory response. Such a response may lead to blood-barrier breakdown, which in turn causes neurovascular dysfunction.38 In summary, the interaction between genetic and environmental factors plays an important role in the pathophysiology of dementia.
Our findings provide a less optimistic outlook for individuals at high genetically determined risk of dementia, yet may have important implications the design of future clinical trials. Considering the earlier age at onset of dementia among APOE-ε4 carriers compared to non-carriers, our results imply that these individuals need to be targeted earlier in the disease process (e.g., midlife or even earlier) to influence their risk.1 Additionally, other interventions beyond lifestyle improvements warrant further study. For instance, lipid lowering drugs may be considered to lower dementia risk in these individuals, yet evidence for such interventions is still inconclusive.39 On the positive side, results from this study show that avoiding an unhealthy lifestyle could potentially prevent or postpone the onset of dementia in most individuals in the population (73%), namely those at low and intermediate genetic risk. Among those, the majority was categorized as having a favorable profile (66%), yet room for improvement is still substantial since potential relative risk reductions up to 30% can be achieved when adherent to a favorable risk profile.
Several limitations of this study need to be addressed. First, we lacked data in these study waves on hearing impairment, a potentially important modifiable risk factor for dementia, since assessments to measure hearing were implemented in the study protocol from 2011 onwards. Second, the components to compute the modifiable risk factor profile were measured at baseline, which does not capture the possibility of shifting from a more adverse risk profile to a more optimal one during follow-up, or vice versa. Third, by stratifying on both genetic and environmental information, results are based on small samples in each stratum resulting in wide confidence intervals around point estimates. Results of this single cohort study therefore warrant replication in other population-based studies. Nevertheless, we were able to replicate our findings in several sensitivity analyses, rendering findings as a result of chance less likely. Finally, this older population is predominantly of European descent (97%), limiting the generalizability of these findings to younger populations and to other ethnicities. Strengths of this study include the availability of genetic data in combination with the meticulous assessment of several health and lifestyle factors, along with long-term and consistent dementia follow-up.
In conclusion, this large population-based study demonstrates that among those at low- and intermediate genetic risk, a favorable modifiable risk profile is related to a lower risk of dementia compared to those with an unfavorable one. In contrast, these protective associations were not found among those at high genetic risk. These results may inform clinical trial design, since dementia prevention trials increasingly recruit individuals genetically predisposed to dementia.
Methods
Study population
We used data from participants of the Rotterdam Study, a prospective population-based cohort study. In 1990, all residents aged 55 and older living in Ommoord, a district of Rotterdam, the Netherlands, were invited. Of 10215 invited inhabitants, 7983 (78%) agreed to participate in the baseline examination. In 2000, the cohort was extended: all residents who turned 55 or moved into the research area. Of the 4472 invitees, 3011 (67%) agreed to participate. Follow-up examinations take place every 3 to 4 years.40
Analyses of this study are based on data obtained from the third examination of the original cohort in 1997-1999 (N=4797) and the first examination of the extended cohort in 2000-2001 (N=3011). These two cohorts were similar in design and examinations were identical. After excluding participants who did not complete the interview and research center visit in these rounds (N=873), had dementia or insufficient screening for dementia at baseline (N=170), did not undergo genotyping (N=365), did not provide informed consent to access medical records and hospital discharge letters (N=33), or if there was no follow-up due to logistic reasons (N=15), 6352 participants were included for analysis in this study (study flowchart displayed in Supplementary Fig. 1. A comparison of baseline characteristics for in- and excluded participants is presented in Supplementary table 13).
Ethics statement
The Rotterdam Study has medical ethics committee approval per the Population Study Act: Rotterdam Study, executed by the Ministry of Health, Welfare and Sport of the Netherlands. Written informed consent was obtained from all participants.
APOE genotyping
DNA was extracted from blood samples drawn at baseline. APOE genotype was determined using a polymerase chain reaction in the original cohort and was determined with a bi-allelic TaqMan assay (rs7412 and rs429358) in the extended cohort on coded DNA samples. APOE-ε4 carrier status was defined as carrier of one or two ε4 alleles.
Calculation of a polygenic risk score
The majority of samples (81.1%) were further genotyped with the Illumina 610K and 660K chips and imputed to the Haplotype Reference Consortium reference panel (version 1.0) with Minimac 3. We included 27 genetic variants that showed genome-wide significant evidence for associations with AD to calculate a weighted polygenic risk score. Supplementary Table 1 provides an overview of the included variants. The polygenic risk score was calculated as the sum of the products of single nucleotide polymorphism dosages of the 27 genetic variants (excluding APOE) and their respective reported effect estimates. All 27 variants selected for the calculation of the polygenic risk score were well imputed (imputation score R2 > 0.6, median 0.99).
Modifiable risk factor profile
We adapted six health and/or lifestyle factors shown to be important during later life to lower dementia risk, as set out by a recent meta-analysis and endorsed by the World Health Organization (WHO).11,14 Among these are: (1) abstaining from smoking, (2) absence of depression, (3) absence of diabetes, (4) regular physical activity, (5) avoiding social isolation, and (6) adherence to a healthy dietary pattern, which included limited alcohol consumption. Based on these six factors, we computed an overall profile of modifiable risk factors ranging from zero to six, and we subsequently grouped participants into three categories of modifiable risk (unfavorable-: ≤2, intermediate-: 3-4, and favorable profiles: ≥5 protective factors)
Assessment of individual health and lifestyle factors
During a structured home interview, participants were enquired about their smoking habits. Participants were classified as never, former or current smokers. In addition, participants were screened with the Center for Epidemiologic Studies Depression Scale during the interview. Presence of depressive symptoms was defined as a score of >16 points on scale of 0 to 60. Diabetes was defined as fasting serum glucose levels ≥7.0 mmol/L, non-fasting serum glucose levels ≥11.0 mmol/L (if fasting samples were unavailable), and/or the use of blood glucose-lowering medication. Physical activity levels were assessed using a validated adapted version of Zutphen Physical Activity Questionnaire and expressed in Metabolic Equivalent of Task hours (METhours) per week. The METhours/week are the product of MET-values of specific activities (walking, cycling, domestic work, sports and gardening) with time in hours per week spent in that activity. Categories were calculated based on International Physical Activity Questionnaire and also expressed in METhours/week. We defined being physically active based on a minimum of ≥40 minutes of exercise per week with a MET intensity of ≥4.33 Social engagement was constructed using three domains based on various questionnaires. We included marital status, living arrangements (living alone, with spouse or with others) and asked if the participant felt lonely during the past week. If a participant lived alone and felt lonely during the past week – we considered them as being less socially engaged. A validated food frequency questionnaire was used to measure the dietary pattern of participants.32 A healthy dietary pattern was ascertained on the basis of adherence to at least half of the following dietary guidelines: consumption of an sufficient amount of fruits, nuts, vegetables, whole grains, fish, and dairy products and a limited amount of refined grains, processed meats, unprocessed red meats, sugar-sweetened beverages, trans fats, sodium and alcohol, for which further details and cut-off values are described elsewhere. 32
Other covariates
Participants were questioned about parental history of dementia during the interview. During the center visit, blood pressure was assessed at the right upper arm with the participant in sitting position. The mean of two measurements was used in the analyses. Serum total cholesterol-, and high-density lipoprotein cholesterol were acquired by an automated enzymatic procedure (Boehringer Mannheim System). Glucose levels were measured after overnight fasting (8–14 h). The history of clinical stroke was assessed by both self-report and continuous monitoring of medical records through digitized linkage of files from general practitioners with the study database. All strokes were adjudicated by a panel of study physicians.
Ascertainment of dementia
Participants were screened for dementia at baseline and subsequent center visits with the Mini-Mental State Examination and the Geriatric Mental Schedule organic level.3 Those with a Mini-Mental State Examination score <26 or Geriatric Mental Schedule score >0 underwent further investigation and informant interview, including the Cambridge Examination for Mental Disorders of the Elderly. All participants also underwent routine cognitive assessment. In addition, the entire cohort was continuously under surveillance for dementia through electronic linkage of the study database with medical records from general practitioners and the regional institute for outpatient mental health care. Available information on cognitive testing and clinical neuroimaging was used when required for diagnosis of dementia subtype. An event adjudication panel led by a consultant neurologist established the final diagnosis according to standard criteria for dementia (DSM-III-R) and AD (NINCDS–ADRDA). Follow-up until 1st of January 2016 was virtually complete (95.5% of potential person-years). Within this period, participants were followed until the date of dementia diagnosis, death, loss to follow-up, or 1st of January 2016, whichever came first.
Statistical analysis
We used Cox proportional hazard models to assess the association of APOE-risk and the health and lifestyle factors with incident dementia. We verified the proportionality assumption with use of Schoenfeld residuals. We tested for interaction between APOE carrier status and the level of the modifiable risk factor profiles on a multiplicative scale. We subsequently evaluated the hazard ratios (HRs) for participants with a high APOE risk status (ε2ε4, ε3ε4 or ε4ε4 genotypes), and compared those with hazard ratios from those with an intermediate risk (ε3ε3 genotype) or low risk status (ε2ε2 or ε2ε3 genotypes). Similarly, we calculated hazard ratios for participants with a favorable profile (which was defined as the presence of at least five of the six health and/or lifestyle factors) with an intermediate profile (three or four factors) or an unfavorable profile (two or less factors). Models were adjusted for age, sex and level of attained education. In extended models, we additionally adjusted for parental history of dementia and cardiovascular risk factors. Finally, we used a competing risk framework based on the Fine & Gray model to calculate the anticipated 15-year absolute dementia risk for participants within each category of genetic risk and modifiable risk profile separately. Confidence intervals were computed based on 1000 bootstrap samples.
In stratified analyses, we explored whether associations differed among younger and older participants by stratifying on the median age of this study (68.2 years) and additionally on the age of 70 years as this age range is often used as an eligibility criterion to recruit individuals for preventative trials that assess multi-domain lifestyle interventions.4–6 Finally, we stratified on sex.
In sensitivity analyses, we studied the robustness of our findings by varying the definitions and compositions of both modifiable risk factors and genetic risk factors that were used in the main analyses. This is in particular important since some of the included modifiable risk factors, such as depression and social isolation, may have been altered by pre-clinical dementia. Similar to the main analyses, we first explored statistical interaction on a multiplicative scale between the studied genetic component (APOE or the polygenic score), and the risk factor profile under study. We subsequently repeated the main analyses while categorizing participants on the Ideal Cardiovascular Health metric (favorable, intermediate and unfavorable), instead of the currently employed modifiable risk factors. Similarly, we replaced the current modifiable risk factors by the 10-year predicted absolute risk of fatal cardiovascular disease and subsequently categorized participants into low-to-moderate <5%, high risk 5-10%, and very high risk > 10%, based on the European Coronary Risk Equation (SCORE), that includes age, sex, and several adverse risk factors namely current smoking, level of cholesterol and systolic blood pressure.15
Regarding genetic risk factors, we repeated the main analyses stratified for an AD polygenic risk score that included 27 genome-wide significant variants (excluding APOE), comparing participants at high genetic risk (i.e. highest tertile of the polygenic score) with those at intermediate risk (middle tertile), or low risk (lowest tertile).
We compared baseline characteristics across APOE strata using analysis of variance (anova) tests. In the case of frequency distributions or when data were non-normally distributed, we compared variables between groups using non-parametric tests (chi-square, Mann-Whitney or Kruskal Wallis).
Data were handled and analyzed with SPSS Statistics version 24.0.0.1 (IBM Corp., Armonk, NY) and R, CRAN version 3.5.1, with packages survival, rms and cmprsk. All analyses were performed at the significance level of 0.05 (2-tailed). P values were uncorrected for multiple testing.
Supplementary Material
Acknowledgements
We acknowledge the dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district who took part in the Rotterdam Study. We acknowledge Frank J.A. van Rooij as data manager, and Brenda C.T. Leening-Kieboom as study coordinator. We thank Jolande Verkroost-van Heemst, for her invaluable contribution to the collection of the data. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Further support was obtained from the Netherlands Consortium for Healthy Ageing and the Dutch Heart Foundation (2012T008) and the Dutch Cancer Society (NKI-20157737). This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (project: ORACLE, grant agreement No: 678543).
Footnotes
Reporting Summary
Further information on research design of this study is available in the Nature Research Life Sciences Reporting Summary linked to this article.
Author Contributions:
S.L. contributed to study conceptualization, data curation, formal analysis, investigation, methodology and writing of the original draft of the manuscript.
S.A. contributed to investigation, analysis and writing & reviewing of the manuscript.
H.K.-C. contributed to investigation and writing & reviewing of the manuscript.
T.V. contributed to data curation, investigation and writing and reviewing of the manuscript.
M.J.G.L. contributed to investigation, methodology and writing & reviewing of the manuscript.
M.A.I. contributed to study conceptualization, funding acquisition, investigation, methodology, writing & reviewing of the manuscript and supervision of the study.
M.K.I. contributed to study conceptualization, investigation, methodology, writing & reviewing of the manuscript and supervision of the study.
Competing Interests Statement
The authors declare no competing interests.
Data Availability Statement
Rotterdam Study data can be made available to interested researchers upon request. Requests can be directed to data manager Frank J.A. van Rooij (f.vanrooij@erasmusmc.nl). We are unable to place data in a public repository due to legal and ethical restraints. Sharing of individual participant data was not included in the informed consent of the study, and there is potential risk of revealing participants’ identities as it is not possible to completely anonymize the data. This is of particular concern given the sensitive personal nature of much of the data collected as part of the Rotterdam Study.
References
- 1.van der Lee SJ, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community-based cohort study. Lancet Neurol. 2018;17:434–444. doi: 10.1016/S1474-4422(18)30053-X. [DOI] [PubMed] [Google Scholar]
- 2.Kunkle BW, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bruijn RF, et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 2015;13:132. doi: 10.1186/s12916-015-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngandu T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 5.Andrieu S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;6:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 6.Moll van Charante EP, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 7.Licher S, et al. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. Am J Psychiatry. 2019;176(7):543–551. doi: 10.1176/appi.ajp.2018.18050566. [DOI] [PubMed] [Google Scholar]
- 8.Guerreiro R, Bras J, Hardy J. SnapShot: genetics of Alzheimer's disease. Cell. 2013;155:968–968 e961. doi: 10.1016/j.cell.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Solomon A, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75(4):462–470. doi: 10.1001/jamaneurol.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart M, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Livingston G, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 12.Peters R, et al. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. 2019;9:e022846. doi: 10.1136/bmjopen-2018-022846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle PA, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85:114–124. doi: 10.1002/ana.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization, W.H. Risk reduction of cognitive decline and dementia: WHO guidelines. WHO; Geneva: 2019. Vol. CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 15.Conroy RM, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 16.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 17.Anttila T, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laitinen MH, et al. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson IK, et al. Apolipoprotein E epsilon4 genotype and the temporal relationship between depression and dementia. Neurobiol Aging. 2015;36:1751–1756. doi: 10.1016/j.neurobiolaging.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podewils LJ, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 21.Huang TL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 22.Barberger-Gateau P, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 23.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 24.Merchant C, et al. The influence of smoking on the risk of Alzheimer's disease. Neurology. 1999;52:1408–1412. doi: 10.1212/wnl.52.7.1408. [DOI] [PubMed] [Google Scholar]
- 25.Ott A, et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay J, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie K, et al. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samieri C, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–664. doi: 10.1001/jama.2018.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivipelto M, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera AV, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutten-Jacobs LC, et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. BMJ. 2018;363:k4168. doi: 10.1136/bmj.k4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voortman T, et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32:993–1005. doi: 10.1007/s10654-017-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 34.Chang L, et al. Gray matter maturation and cognition in children with different APOE epsilon genotypes. Neurology. 2016;87:585–594. doi: 10.1212/WNL.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornish D, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 37.Licher S, et al. Lifetime risk and multimorbidity of non-communicable diseases and disease-free life expectancy in the general population: A population-based cohort study. PLoS Med. 2019;16:e1002741. doi: 10.1371/journal.pmed.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016:CD003160. doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikram MA, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. doi: 10.1007/s10654-017-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Rotterdam Study data can be made available to interested researchers upon request. Requests can be directed to data manager Frank J.A. van Rooij (f.vanrooij@erasmusmc.nl). We are unable to place data in a public repository due to legal and ethical restraints. Sharing of individual participant data was not included in the informed consent of the study, and there is potential risk of revealing participants’ identities as it is not possible to completely anonymize the data. This is of particular concern given the sensitive personal nature of much of the data collected as part of the Rotterdam Study.


