1. Introduction
Peripheral nerve injuries are frequently encountered in clinical practice and often result in functional disability. In the past, due to poor understanding of pathophysiology, the results of nerve repair were unpredictable. Sunderland1 in 1945 described the microsurgical techniques which improved the results of nerve repairs. In the past few decades an understanding of nerve regeneration, advances in microsurgical techniques, along with ongoing research in molecular biology has helped in improving the results in peripheral nerve surgery.
1.1. Classification of nerve injuries
Seddon2 classified nerve injuries into three broad categories; neurapraxia, axonotmesis, and neurotmesis. In neurapraxia, transient functional loss is observed without affecting loss of nerve continuity. A complete disruption of the nerve axon and surrounding myelin along with preservation of perineurium and epineurium is observed in axonotmesis. Neurotmesis causes complete functional loss because of disconnection of a nerve. Sunderland's classification system classifies nerve injuries to five categories according to severity. A first-degree injury is comparable to Seddon's neurapraxia. In second and third-degree there is disruption of the axon and is equivalent to axonotmesis. In fourth-degree injury there is disruption of axon, endoneurium and perineurium. There is a complete loss of continuity of nerve in fifth-degree corresponding to neurotmesis. While Seddon classification is simpler to follow, Sunderland grading is more often used by surgeons to take a decision on nerve repair. In 1989, Mackinnon3 has added a sixth-degree injury, which is a combination of various degrees of nerve injury.
1.2. Pathophysiology of nerve injury –
During first few days after axonal injury, local degenerative events are accompanied by both retrograde and anterograde degeneration of axon and myelin. During the intermediate phase (a few days to weeks), the anterograde pattern of Wallerian degeneration proceeds to completion with infiltrating macrophages contributing to the removal of tissue debris and Schwann cells undergoing mitosis.4 The axotomised neuronal cell body undergoes reactive, chromatolytic changes and the severed proximal end of the axon develops regenerative axonal sprouts. Of the numerous axonal sprouts that successfully traverse the injury site (during the first few weeks to months), some re-enter appropriate endoneurial tubes and continue to extend through the distal nerve stump. Failure of regenerating axonal sprouts to cross the injury site (possibly due to the formation of a physical scarring barrier or the loss of a large segment of nerve) results in neuroma formation. In the proximal stump, the shortening of axon and myelin occurs due to degradative action by Schwann cells. Either the proximal degradation can be negligible covering the injury site till the next node of Ranvier or it can entirely extend up to the cellular body.
1.3. Neurotrophic factor in nerve regeneration
Nerve growth factor (NGF) was the first neurotrophic factor to be identified. Other neurotrophic factor like brain derived neurotrophic factor, ciliary neurotrophic factor and glial derived neurotrophic factor (GDNF) are important factors in nerve repair. They are released from the target organs and Schwann cells which are believed to be transferred to cell body in a retrograde fashion by the axon.5 Any disruption of the transport of NGF is a trigger factor for the repair process. The neurotrophic factors provide continuous stimulus for growth as well guide for advancing axons. Whitworth et al. observed that administration of exogenous NGF resulted in sustained axon regeneration which has been related to reduction in the incidence of neuronal cell death.6 Similarly GDNF has a beneficial effect on axonal regeneration and improves the conduction velocity of motor neurons following regeneration and that of small diameter sensory neurons.7,8
1.4. Diagnosis of nerve injury
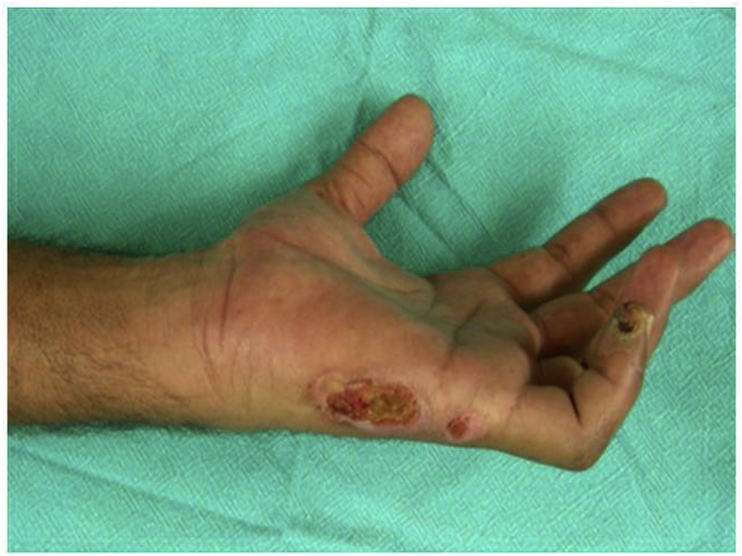
Nerve injury clinically manifests as loss of motor (Fig. 1), sensory (Fig. 2) and autonomic functions. These must be evaluated at the time of nerve injury and findings meticulously documented. It is important to distinguish between neuropraxia, axonotmesis and neurotmesis. While complete recovery is expected in neuropraxia and axonotmesis, neurotmesis will not recover without surgical intervention. This distinction may be difficult at times and hence there is a role for electrophysiological studies in the diagnosis of nerve injuries. They are also helpful in documenting recovery and in the diagnosis of compressive neuropathies.
Fig. 1.
Clawing of ring and little fingers in ulnar nerve injury.
Fig. 2.
Sensory impairment in ulnar nerve injury.
1.5. Electromyography (EMG)
The recording of muscle action potential can help in documenting the extent of denervation as well as its distribution.9 EMG studies should be done after 2–3 weeks of injury for the muscle to show denervation changes. Complete denervation is characterized by low amplitude sharp waves or fibrillation potential with muscle at rest and absent evoked muscle action potential (MUAP). With reinnervation these changes begin to reverse.
1.6. Nerve conduction studies (NCS)
They play an important role in identifying the type and age of peripheral nerve injury. In neuropraxia compound muscle action potential (CMAP) amplitude remains normal distal to the site of injury and drops to zero with proximal site stimulation. In axonal injury the CAMP is present in the first week and thereafter falls rapidly after Wallerian degeneration has occurred.10
1.7. Management of nerve injuries
When a nerve injury is identified, the dilemma is when to operate, and what kind of repair should be undertaken. If the nerve injury is due to penetrating trauma with neurological deficit, immediate exploration and repair of nerve is indicated. However, if the mechanism is due to blunt trauma the serial examination is necessary for allowing spontaneous recovery to occur. In these circumstances delayed repair can be done. Electrophysiological studies are helpful in deciding the timing of surgery in these patients.
1.8. Surgical repair
The core issue in a peripheral nerve surgery is tension free coaptation of nerve ends with minimum number of nonabsorbable monofilament sutures. The tension in the suture line is associated with increased fibrotic reaction and poor regeneration.11 Best results of nerve repair are possible with correct matching of motor and sensory component. Intraoperative motor-sensory differentiation is possible with immunohistochemical and electrophysiological methods.12 A divided nerve gap can be bridged by number of means which include direct repair, nerve grafts and nerve conduits. However, in cases where these are not feasible, nerve transfers and neurotization can be performed to achieve good functional outcome.
1.9. Basic principles in nerve repair
A meticulously performed nerve repair allows the growth of the regenerating axons into the endoneurial tubes of the distal nerve stump with minimal loss of regenerating axons.
Certain basic principles are followed in nerve repair-
-
1.
A good illumination is essential
-
2.
A bloodless field is achieved, either with a proximal tourniquet or local tumescent infiltration
-
3.
Nerve repairs are performed under magnification. Nerve dissection is performed under loupe magnification and coaptation is done under an operating microscope
-
4.
Appropriate microsurgical instrumentation with suture materials and/or tissue glue
-
5.
Nerve are manipulated with microinstruments and repaired with microsutures
-
6.
Keyhole surgery is avoided. Incision is extended both proximally and distally to get a good exposure of nerve ends (Fig. 3)
Fig. 3.
Skin flaps raised both proximally and distally to get a good exposure of nerve ends.
1.10. Direct nerve repair
A direct repair is recommended if two nerve ends can be held without tension by a single 8-0 suture.13 Three techniques are described for direct nerve repair viz epineural repair, perineural repair and group fascicular repair. The epineural repair is the commonest form of nerve repair and is associated with certain advantages in terms of short operating time, technical ease and avoids injury to intraneural tissues and fascicles. The perineural repair is indicated in nerve grafting and nerves with less than 5 fascicles. The group fascicular repair can be performed at a site where nerve has given branches and individual fascicles can be identified in the main trunk. Theoretically motor and sensory fascicles can be matched and hence motor-sensory cross innervations avoided.
1.11. Repair by nerve grafts
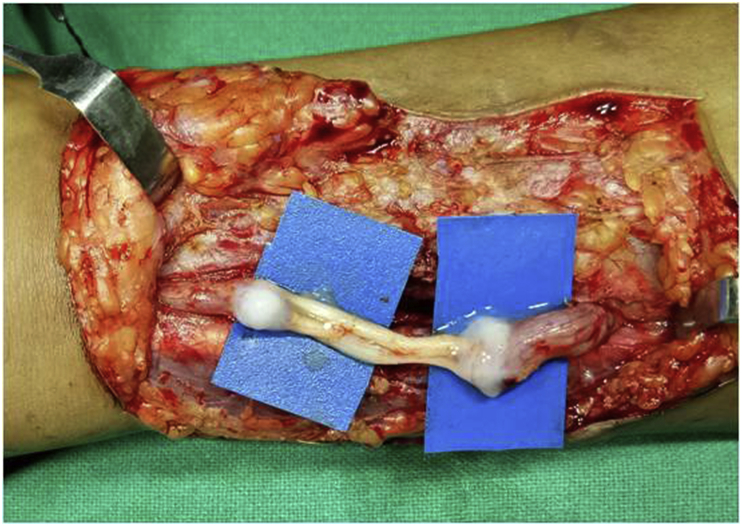
When nerve gap exceeds 2 cms and direct nerve repair is under tension nerve grafts are indicated to bridge the divided nerve ends.14,15 Three common types of nerve grafts are cable grafts, trunk grafts and vascularised nerve grafts. Cable grafts are multiple small caliber grafts from relatively dispensable nerves. The commonly used nerves are sural, superficial radial sensory nerve, anterior branch of median antebrachial cutaneous nerve and lateral femoral cutaneous nerve.16 The graft length should be 10–20% longer than the nerve gap to allow for shortening due to fibrosis (Fig. 4). The micro neural sutures or fibrin glue (Fig. 5) are used to co-apt the multiple cables to match the diameter of the nerve. Reversal of nerve grafts reduces the axonal disruption through the distal nerve branches.
Fig. 4.
Nerve gaps bridged with sural nerve grafts.
Fig. 5.
Graft coaptation with fibrin glue.
The trunk grafts are whole nerve grafts in a non functional nerve which can be used to reconstruct a nerve likely to function. However due to its thickness and ability to vascularise from the bed, they are associated with poor results. Vascularised nerve grafts consists of an entire nerve with its vascular pedicle used to restore nerve functions. Doi et al.17 in their series of vascularised vs non vascularised nerve grafts concluded that vascularised nerve grafts are indicated when the nerve gap is more than 6 cms and associated with massive skin loss and poor tissue bed vascularity.
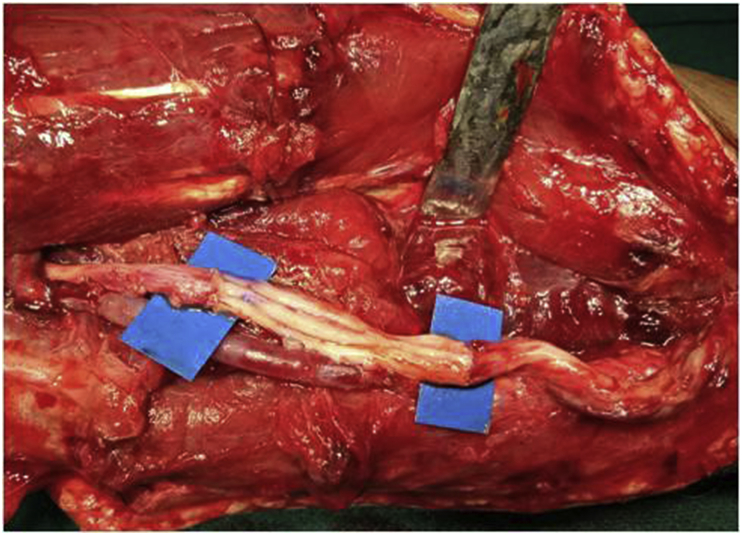
1.12. Nerve transfers
Neurotization involves use of proximal functioning nerve as donor nerve to repair the distal denervated nerve element (Fig. 6). The procedure therefore reinnervates the target organ from healthy functional nerve. The technique popularised by Narakas has been used extensively in brachial plexus injuries to reinnervate the distal muscles in cases of root avulsion or roots not found repairable.18
Fig. 6.
Distal nerve transfer in isolated musculocutaneous nerve injury.
2. Emerging concepts in peripheral nerve surgery
2.1. End to side neurorraphy
This technique of nerve coaptation was described by Letrevant in 1893 in a case of nerves with loss of significant length. This was however abandoned due to poor results. In 1992 Viterbo et al.19 reintroduced this technique which consists of coaptation of distal stump of transected nerve to the trunk of adjacent donor nerve. This is considered as an alternative technique when the proximal stump is unavailable or the nerve gap is too long to be bridged by a nerve graft.20
The nerve regeneration in end to side neurorraphy takes place by collateral sprouting. The regenerated axons emerge from the most proximal node of Ranvier and travel in the epineurium of donor nerve.21,22 In 1993 Viterbo used cross facial nerve graft transplantation using end to side neurorraphy in facial palsy.23 Amr et al. reported satisfactory results in 11 cases of brachial plexus injury managed with end to side neurorraphy.24 Other authors25 have also reported promising results with this technique. Nevertheless this is an interesting technique and in the future it may be a viable option in peripheral nerve injuries.
2.2. Nerve conduits
Use of cylindrical tube to bridge a gap between nerve ends has been widely reported in current literature. Through neurotrophism the regeneration of axons occurs within this tube. Axons regenerating from proximal stump grow through the conduit and selectively find their original pathways in the distal stump by chemotactic attraction. This would negate the problem of cross innervation of motor and sensory bundles.
It avoids the morbidity associated with nerve graft. The semi rigid tube prevents the soft tissue coming in between the nerve ends. Several studies have indicated the comparable results to direct nerve repair and nerve grafts when nerve conduit was used to reconstruct a short segment of nerve26,27(Fig. 7). The limitation of nerve conduits is the distance between the divided ends which can be bridged. The 3–4 cms is the defining upper limit of nerve gap which can be bridged with comparable results.28,29 Conduits from various biological and synthetic sources have been used. The biological tubes include the use of arteries, veins, muscles and modified biological tissues such as laminin and collagen. The limitations of biological conduits in terms of early fibrosis, scar infiltration and tissue reaction have led to emergence of conduits made from synthetic materials. Commonly used synthetic conduits are polyester such as polyglycolic acid, polylactic acid and polygalactin.30 In order to enhance nerve regeneration in these conduits the use of exogenous growth factors and neurotrophic factors have been used.31 However for digital nerve repair excellent to good sensory function in 75% has been reported and for larger mixed nerves functional recovery was obtained in 75% of patients with 1–4 cms nerve gap reconstructed with conduits. Therefore the use of nerve conduits in selected patients can produce comparable results obviating the need for donor nerves and its resultant morbidity.
Fig. 7.
Use of a biodegradable nerve conduit to bridge a short nerve defect.
2.3. Nerve allografts
The use of nerve allografts have been reported in primates by Bain et al..32 The immunosuppressant FK 506 (Tacrolimus) has benefitted the experimental allografting results which are comparable with autografting in animal studies.33 However despite these advances there remains limited indications for its application which include insufficient nerve autografts,34 limb transplantation and pre-existing immunesuppression. The duration of immune suppression required for nerve allograft remains undetermined. Mackinnon reported return of motor and sensory functions in 6 out of 7 nerve allograft transplants to upper and lower limbs.35
2.4. Immune modulators in nerve repair
The use of immunosuppressant FK 506 (Tacrolimus) has been shown to accelerate the nerve regeneration and functional recovery. It acts via FK 506 binding protein (FKBP) receptors. The FKBP 12 in receptor is responsible for immune suppression.36 The current application of FK 506 is in enhancing the nerve regeneration after nerve repair but its role as an adjunct to nerve allografting is promising. Yan et al. in their experimental study on rat demonstrated significant therapeutic impact in short term use of FK 506 in nerve regeneration. In future, the use of FK 506 is likely to play an important role in nerve regeneration.37
2.5. Results after peripheral nerve repair
The outcomes of nerve reconstruction are influenced by multifactorial variables. In 1991, Sunderland made certain observations regarding nerve repair. He found that outcomes in younger patients, early repair, repair close to target muscle, repair of single function nerve and short nerve graft had better outcomes. Kallio et al.38 reported their results of 132 median nerve reconstruction which were managed with nerve grafting and secondary neurorraphy. They reported good to excellent results in 49%, fair results in 11% and poor results in 40% of patients. The poor results were associated with injury proximal to the elbow, age more than 54 years, graft length of more than 7 cms and delayed surgery of more than 23 months. Similar results were published by Vastamaki et al.39 in reconstruction of ulnar nerve with 52% of patients achieving useful recovery.
3. Conclusion
Despite advances in understanding of pathophysiology of nerve injuries and advent of microsurgical techniques, the outcomes of repair have still not reached its zenith, with about 50% of patients achieving useful nerve function. Current research in nerve injuries is challenging and newer modalities are under evaluation to further improve the results of nerve reconstruction.
Conflicts of interest
The author reports no conflict of interest. The author is responsible for the content and writing of the paper.
References
- 1.Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- 2.Seddon H.J. Three types of nerve injury. Brain. 1943;66:237–288. [Google Scholar]
- 3.Mackinnon S.E. New directions in peripheral nerve surgery. Ann Plast Surg. 1989;22:257–273. doi: 10.1097/00000637-198903000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Menorca R.M., Fussell T.S., Elfar J.C. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29:317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16(5):1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 6.Taniuchi M., Clarke H.B., Schweiter J.B. Expression of nerve growth factors receptors by Schwann cells of axotomised peripheral nerves: ultra structure location, suppression by axonal contact and binding providing properties. Neuroscience. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitworth I.H., Terenghiti G., Green C.J., Brown R.A., Stevens E., Tomlinson D.R. Targeted delivery of nerve growth factor via fibronectin conduits assists nerve regeneration in control and diabetic rats. Eur J Neurosci. 1995;7:2220–2225. doi: 10.1111/j.1460-9568.1995.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 8.Munson J.B., McMahon S.B. Effects of GDNF on axotomised sensory and motor neurons in adult rats. Eur J Neurosci. 1997;9:1126–1129. doi: 10.1111/j.1460-9568.1997.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennett D.L.H., Micheal G.J., Ramachandran N. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauwens P. Electrodiagnostic definition of the site and nature of peripheral nerve lesions. Ann Phys Med. 1960;5:149–152. doi: 10.1093/rheumatology/v.5.149. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary V., Gornblath D.R. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve. 1992;15:687–693. doi: 10.1002/mus.880150610. [DOI] [PubMed] [Google Scholar]
- 12.Terzis J.K., Faibisoff B.A., Williams H.B. The nerve gap: suture under tension vs graft. Plast Reconstr Surg. 1975;56:166–170. [PubMed] [Google Scholar]
- 13.Ramachandran S., Midha R. Recent advances in nerve repair. Neurol India. 2019;67:106–114. doi: 10.4103/0028-3886.250702. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon S.E. Surgical management of peripheral nerve gap. Clin Plast Surg. 1989;16(3):587–603. [PubMed] [Google Scholar]
- 15.Millesi H., Meissl G., Berger A. The interfasicular nerve grafting of median and ulnar nerve. J Bone Joint Surg Am. 1972;54(4):727–750. [PubMed] [Google Scholar]
- 16.Berger A., Millesi H. Nerve grafting. Clin Orthop Relat Res. 1978;133:49–55. [PubMed] [Google Scholar]
- 17.Doi K., Tamaru K., Sakai K., Kuwata N., Kurafuji Y., Kawai S. A comparison of vascularised and conventional sural nerve grafts. J Hand Surg. 1992;17A:670–676. doi: 10.1016/0363-5023(92)90315-g. [DOI] [PubMed] [Google Scholar]
- 18.Narakas A.O., Hentz V.R. Neurotization in brachial plexus injuries: indications and results. Clin Orthop Relat Res. 1988;237:43–56. [PubMed] [Google Scholar]
- 19.Viterbo F., Trindade J.C., Hoshino K., Mazzoni N.A. Latero-terminal neurorraphy without removal of epineural sheath. Experimental study in rats. Rev Paul Med. 1992;110:267–275. [PubMed] [Google Scholar]
- 20.Lykissas M.G. Current concepts in the end to side neurorraphy. World J Orthop. 2011;2(11):102–106. doi: 10.5312/wjo.v2.i11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tham S.K., Morrison W.A. Motor collateral sprouting through an end to side nerve repair. J Hand Surg. Am. 1998;23:844–845. doi: 10.1016/S0363-5023(98)80161-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z., Soucacos P.N., Beris A.E., Bo J., Iaachim E., Johnson E.O. Long term evaluation of rat peripheral nerve repair with end to side neurorraphy. J Reconstr Microsurg. 2000;16:303–311. doi: 10.1055/s-2000-7338. [DOI] [PubMed] [Google Scholar]
- 23.Viterbo P. A new method for treatment of facial palsy: the cross face nerve transplantation with end to side neurorraphy. Rev Soc Bras Cir Plast Estet Reconstr. 1993;8:29–38. [Google Scholar]
- 24.Amr S.M., Moharram A.N. Repair of brachial plexus lesion by end to side grafting neurorraphy: experience based on 11 cases. Microsurgery. 2005;25:126–146. doi: 10.1002/micr.20036. [DOI] [PubMed] [Google Scholar]
- 25.Menner U. Side to side nerve suture in clinical practice. Hand Surg. 2003;8:33–42. doi: 10.1142/s0218810403001352. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg G., Rosen B., Dahlin L., Holmberger J., Rosen I. Tubular repair of median or ulnar nerve in human forearm: a 5 year follow up. J Hand Surg. 2004;29(3):100–107. doi: 10.1016/j.jhsb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Weber R.A., Breindenbach W.C., Brown R.E., Jabaley M.E., Mass D.P. A randomized prospective study of polyglycolic conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1035–1045. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Dellon A.L., Mackinnon S.E. An alternative to classical nerve graft for the management of short nerve gap. Plast Reconstr Surg. 1988;82:849–856. doi: 10.1097/00006534-198811000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Hung V., Dellon A.L. Reconstruction of 4 cms human median nerve gap by including an autogenous nerve slice in a bioabsorbable nerve conduit: a case report. J Hand Surg. 2008;33(A):313–315. doi: 10.1016/j.jhsa.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Belkas J.S., Schoichet M.S., Midha R. Peripheral nerve regeneration through guidance tube. Neurol Res. 2004;26(2):151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 31.Daly W., Yao L., Zeugolis D., Windebank A., Pandit A. A biological approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bain J.R., Mackinnon S.E., Hudson A.R., Wade J., Evans P. The peripheral nerve allografts in primate immunosuppressed with cyclosporine A. Histologic and electrophysiologic assessment. Plast Reconstr Surg. 1992;90:1036–1046. doi: 10.1097/00006534-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Weizwerg N., Grindel S., Gonzalez M., Kky D., Fang J., Sahani B. Peripheral nerve allotransplantation in rats immunosuppressed with transient or long term FK 506. J Reconstr Microsurg. 1996;12:451–459. doi: 10.1055/s-2007-1006618. [DOI] [PubMed] [Google Scholar]
- 34.Siemionow M., Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23(8):511–520. doi: 10.1055/s-2007-1022694. [DOI] [PubMed] [Google Scholar]
- 35.Mackinnon S.E., Doolabh V.B., Novak C.B., Trulock E.P. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Gold B. Neuroimmunophilin ligands: evaluation of their therapeutic potential for the treatment of neurological disorder. Expert Opin Investig Drugs. 2000;9:2331–2342. doi: 10.1517/13543784.9.10.2331. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y., Sun H.H., Hunter D.A., Mackinnon S.E., Johnson P.J. Efficacy of short term FK 506 administration on accelerating nerve regeneration. Neurorehabilitation Neural Repair. 2012;26(6):570–580. doi: 10.1177/1545968311431965. [DOI] [PubMed] [Google Scholar]
- 38.Kallio P.K., Vadtamaki M. An analysis of the results of late reconstruction of 132 median nerve. J Hand Surg. 1993;18:97–105. doi: 10.1016/0266-7681(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 39.Vastamaki P.K., Kallio P.K., Solomem K.A. The results of ulnar nerve repair. J Hand Surg. 1993;18:323–326. doi: 10.1016/0266-7681(93)90053-i. [DOI] [PubMed] [Google Scholar]