Abstract
There is an increasing interest in the production of biodiesel as bio-renewable fuel source, with numerous biofuel byproducts becoming available. The annual productions of biodiesel and crude glycerol were 34.5 and 3.8 billion liters, respectively, in 2016 and that of biodiesel is expected to reach 41 billion liters in 2019. Glycerol is a sugar alcohol without a color or odor, but with a sweet taste and high solubility index in water. Experiments support the use of glycerol at low levels ranging from 5% to 8% of the diet dry matter as a transition cow therapy. Administration of glycerol increases serum glucose and decreases ketone bodies. Glycerol is very rapidly fermented in the rumen to propionate and butyrate, at the expense of acetate, resulting in a decreased milk fat. Because glycerol is highly fermented in the rumen, it requires an adaptation period at the beginning of feeding. Administration of glycerol in the diet of lactating animals was paralleled with a decreased or an unaffected feed intake in most experiments. Improved ruminal environment to enhance nutrient digestibility was observed in many experiments; however, others observed reduced digestion of dietary fiber with feeding glycerol. Enhanced, lowered, or unaffected milk production and composition were observed with the administration of glycerol in lactating animal diets; however, in most cases, glycerol decreased milk fat content. The inconsistencies between results of experiments are due to the level and the purity of glycerol, diets, production stage of the animals, and other factors. Therefore, further research should be conducted to establish the efficacy of different levels, purity and administration periods of glycerol, and production stage of dairy animals fed glycerol-based or supplemented diets.
Keywords: Biodiesel byproducts, Feed utilization, Glycerol, Milk production and composition, Ruminal fermentation
1. Introduction
About the calving time, dairy cows are confronted by peculiar nutritional challenges. Generally, 1 week before and 3 weeks after calving, feed consumption declines by about 30%, making a cow in a negative energy balance. Glucose is the most important nutrient required for milk synthesis. The liver is responsible for converting propionate from ruminal fermentation to glucose and starch, and also synthesizing glucose from glucogenic amino acids and glycerol from adipose triglycerides. Glucose is utilized fundamentally for milk production in the mammary gland. With low feed intake and glucose supply, the dairy cow mobilizes large amounts of body fat, causing fat accumulation in the liver which results in lowered blood glucose, enhanced ketone bodies production, and ultimately ketosis. The incapability of gestating dairy cows to circumvent the problem of feed intake reduction towards calving and during the first few weeks of calving compels animal nutritionists to explore methods to overcome feed depression around calving. Oral drenches of some glucose precursors, such as calcium-propionate and propylene glycol, were tested with good results for glycerol drenching as an effective treatment of lactation ketosis. The metabolic pathway of glycerol is much closer to that of glucose compared to other glucose precursors (Johnson, 1954, Fisher et al., 1973).
Recently, there is an increasing global demand for biofuels (ethanol and biodiesel), resulting in an increased demand for the feedstocks (corn, wheat, and oilseeds) and soaring prices of livestock feeds. The main byproduct during the production of biodiesel is glycerol, which can be used as livestock feeds. In 2016, the annual production of crude glycerol was 3.8 billion liters while the biodiesel production, which is expected to reach 41 billion liters in 2019 (Food and Agriculture Organization of the United Nations (FAO), 2016), was about 34.5 billion liters. Biodiesel production accounts for about 65% of world total production of glycerol. The main users of purified glycerol are the cosmetic and pharmaceutical industries, which utilize about 3% to 4% of the total glycerol production. This has compelled glycerol producers to explore new markets for this material, including its utilization as a feedstuff for animals, especially with the recent soaring prices of corn and concentrates (Andrade et al., 2018, van Cleef et al., 2018). The EU legislation approved glycerol as a feed additive with no restrictions as related to animal species or quantity that may be fed (FDA, 2007). Production of a ton of biodiesel has been reported to yield about 100 kg of crude glycerol (Cotrill et al., 2007). The composition of glycerol depends on manufacturing process, and different grades may be available (Cotrill et al., 2007).
Glycerol, with its high and rapidly available energy, has been used for many years as a supplement to alleviate ketosis in dairy animals (Cotrill et al., 2007, Donkin, 2008). It contains about 4.32 Mcal/kg gross energy and 2.27 Mcal/kg net energy for lactation (NEL) (Cotrill et al., 2007). There are a lot of experiments on the utilization of glycerol, as an alternative energy source, in the diets of dairy animals because of many unanswered questions regarding the handling, rates of administration, and feeding value compared to other energy-rich feeds. The effects of glycerol administration in the diet of lactating animals are not consistent. However, many experiments reported decreased feed consumption in cows (Ezequiel et al., 2015, Paiva et al., 2016), buffaloes (Saleem et al., 2018) and goats (Andrade et al., 2018), whereas enhanced dry matter (DM) and crude protein (CP) digestibility and decreased fiber digestibility (Donkin et al., 2009, Shin et al., 2012) were observed in many experiments. However, Wang et al. (2009a) and Saleem et al. (2018) observed increased fibre digestibility with glycerol supplementation. Increased blood glucose and decreased ketone bodies concentrations were observed in many experiments (Osman et al., 2006, Porcu et al., 2018). Improved (Omazic et al., 2013a, Saleem et al., 2018), lowered (Paiva et al., 2016, Porcu et al., 2018) or unaffected (Ezequiel et al., 2015, Thoh et al., 2017) milk production and composition were observed with the administration of glycerol in lactating animal diets; however, in most cases glycerol decreased milk fat content. The inconsistency between experiments reveals that more experiments are recommended to validate or refute the importance of including glycerol as a feed ingredient or feed supplement in the diet of lactating animals. Therefore, this review summarizes findings from researches conducted on the utilization of glycerol in the diet of dairy animals, provides information on its ruminal metabolism, and practical use in dairy animal diets.
2. Glycerol as a byproduct of biodiesel production
The term biodiesel refers to the main end product of the methyl or ethyl esters of fatty acids (FA) (Donkin, 2008). The rate of glycerol production is 10 L of crude glycerol every 100 L of biodiesel (Donkin, 2008).
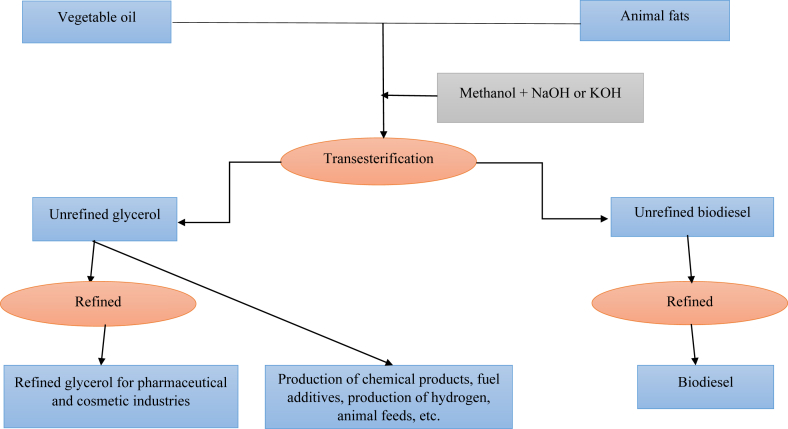
The main source of biodiesel is the oils through some trans-esterification reactions to split or hydrolyze fats for production of pure FA for human food, cosmetic and drug applications (Fig. 1). Briefly, in the presence sodium hydroxide or potassium hydroxide, plant oil (e.g. soybean oil) is reacted with methanol to produce biodiesel and crude glycerol. Biodiesel separation from glycerol is achieved by gravity separation or centrifugation, followed by the removal of methanol by flash evaporation or distillation. Addition of acid to the resulting crude glycerol neutralizes the unutilized catalysts and soaps to produce crude glycerol, which contains 80% to 88% glycerol. Crude glycerol is further purified to 99% or a higher purity by removing impurities such as catalysts, salts, and methanol, which may be toxic when glycerol is used as a livestock feed (Donkin, 2008). The unpurified glycerol has a low economic value, making it potentially competitive animal feed ingredient.
Fig. 1.
Generation of glycerol and its alternative routes.
Glycerol, a viscous liquid byproduct of biodiesel production (Donkin and Doane, 2007), is produced from the hydrolysis of FA. Glycerol is generally recognized as safe for human and ruminant use (FDA, 2007). Methanol levels in the crude glycerol should be less than 0.5%. According to FDA, methanol levels higher than 150 parts per million are considered unsafe for animal feed (FDA, 2007).
The economic use of glycerol in the diets of dairy animals depends on the supply, fuel demand, and also the price of other feeds and oilseeds or fats that are used for production. The production is increasing but this depends on government policies; however, the recent increases in the price of oilseeds have greatly diminished the profitability of biodiesel production. Moreover, the recent trends for more refining and purification of crude glycerol to higher value products will decrease supply to the animal feed market and increase its cost (Drackley, 2008).
3. Nutritional value of glycerol
The chemical composition and purity of glycerol determine its nutritional value. Though refined glycerol is available as an energy source, its use in dairy animal feeding is not recommended due to its high cost. The energy value of glycerol is unavailable for typical feeding scenarios. However, Schröder and Südekum (1999) estimated the caloric value of glycerol to range from 1.98 to 2.26 Mcal NEL/kg. This value is not completely true because the energy value of glycerol depends on the energy density of the diet, the level of glycerol feeding, and interactions with other ration components (Donkin, 2008). In another experiment, DeFrain et al. (2004) reported energy value of 1.91 Mcal NEL/kg for glycerol. Both glycerol and corn starch have similar energy value (Donkin, 2008). However, for the diet with a high starch content (55% of ration DM), the glycerol energy value as a replacement for corn decreases (Schröder and Südekum, 1999) due to decreased neutral detergent fiber (NDF) digestibility. On the other hand, the energy estimates for glycerol are higher with low starch diets (40% of ration DM). When replacing corn with glycerol as a sole energy source, attention should be given to the fact that glycerol does not supply protein or important minerals; therefore, these should be compensated (Drackley, 2008, Khattab et al., 2012). As mentioned before, glycerol contains impurities which should be considered before its use in dairy nutrition. In separate experiments, Schröder and Südekum (1999) used a low-purity glycerol containing 26.7% methanol, while DeFrain et al. (2004) used glycerol containing 1.3% methanol. Such issue would affect the nutritive value of glycerol, especially in preruminant calves and nonruminants which have less ability to detoxify methanol to some degree in the rumen (Drackley, 2008).
4. Ruminal metabolism of glycerol
Glycerol is completely fermented by ruminal fermentation to volatile fatty acids (VFA), especially propionate and butyrate (Rémond et al., 1993, Silva et al., 2014), which decrease ruminal pH and cause negative effects on ruminal microbial protein synthesis, cellulolytic activity of Ruminococcus flavefaciens and Fibrobacter succinogenes, ruminal fermentation, and feed digestion (Roger et al., 1992, Kijora et al., 1998). Kijora et al. (1998) reported a decreased ruminal pH and acetate to propionate ratio with the intraruminal administration of glycerol. The decreased acetate to propionate ratio is beneficial, as it, often times, increases milk production and depresses milk fat synthesis. Moreover, Linke et al. (2004) reported that glycerol administration at 1 kg/d decreased acetate and increased propionate and butyrate 4 h after feeding. Kristensen and Raun (2007) observed that glycerol infusion into the rumen decreased acetate and increased butyrate, without affecting propionate. In another experiment, Trabue et al. (2007) reported that glycerol increased concentrations of butyrate, valerate, and caproate in vitro. DeFrain et al. (2004) noted that drenching cows with glycerol increased proportion of ruminal butyrate. The varying effect of glycerol administration on ruminal VFA may be due to different doses, purity of the administered glycerol, and the nature of the fed diets.
The effect of glycerol on ruminal fermentation depends mainly on the dose and the rate of glycerol disappearance in the rumen. Actually, the rate of glycerol disappearance in the rumen is very fast due to the fast adaptation of ruminal microbes to it (Kijora et al., 1998, Porcu et al., 2018). Kijora et al. (1998) reported that 85% of the twice-daily infusion of 200 g glycerol disappeared in the rumen after 2 h of feeding and increased plasma glycerol in cow. Rémond et al. (1993) suggested that most of the glycerol can be absorbed directly in the rumen; however, it is difficult to determine the relative amount of absorption as glycerol vs. fermentation. Though the maximal rates of glycerol ruminal disappearance range from 1.2 to 2.4 g/h (Rémond et al., 1993), Kristensen and Raun (2007) reported that the net absorption of glycerol in the rumen is limited even with the administration of large doses. They reported that only about 10% of administered glycerol (925 g/d per cow) was recovered as glycerol in the portal vein and taken up by the liver for the synthesis of glucose. Further studies are advocated on how to improve the efficiency of absorption of administered glycerol for synthesis of glucose needed to produce energy for improved milk production in dairy animals.
5. In vitro evaluation of glycerol administration in diets of lactating animals
The in vitro technique evaluates of new feeds and ingredients before feeding them to animals. However, the doses of glycerol used in in vitro experiments differ from those used in the live animals. Avila et al. (2011) observed that barley grain replacement with increasing proportion of glycerol linearly increased in vitro propionate and reduced acetate concentrations. Abo El-Nor et al. (2010) did not find any changes in the in vitro DM degradability with glycerol administration at 10% or 20%. However, van Cleef et al. (2018) reported linear increases in vitro DM digestibility and a decreased in vitro NDF degradability with glycerol supplementation. Similar findings were observed by AbuGhazaleh et al. (2011) who attributed improved DM digestibility and depressed in vitro NDF degradability to the reduction in the numbers of microorganisms involved in fiber digestion. The dose and method of application may be responsible for the discrepancies between results.
Increased dietary glycerol concentration linearly decreased total gas and carbon dioxide production due to lowered ruminal fermentation and VFA production (Lee et al., 2011, van Cleef et al., 2018). Ferraro et al. (2016) evaluated the effect of glycerol combined with corn silage or alfalfa on in vitro ruminal fermentation and reported a lowered gas production and delayed onset of gas production. Moreover, glycerol produced the lowest proportion of propionate and the highest proportion of butyrate. Regarding the effect of glycerol on in vitro methane (CH4) production, van Cleef et al. (2018) noted a tendency for a linear reduction of CH4 production with glycerol administration due to the negative effects of glycerol on the growth and activity of fiber-fermenting bacteria (AbuGhazaleh et al., 2011). Additionally, Lee et al. (2011) reported that glycerol has the ability to reduce in vitro CH4 production, suggesting a positive impact of glycerol on dietary energy use efficiency. In another experiment, Avila et al. (2011) did not observe any reduction in CH4 production when glycerol was administered after 48 h incubation. As previously noted, the dose, the purity and application method of glycerol and nature of incubated substrates among other factors are responsible for the inconsistency of results of experiments on in vitro CH4 production.
6. Glycerol in the diet of newborn animals and transition cows
There are 3 main stages in the life of dairy animals: growth, dry or non-lactating, and lactation stages. Therefore, the effect of glycerol feeding to dairy animals will depend on the life stage.
During the early stage of newborns, dehydration and energy deficiency, as a result of diarrhea, are important causes of mortality (Barrington et al., 2002). Oral rehydration solutions maintain the fluid and electrolyte balance. Information available in the literature on the effect of feeding glycerol to newborns is very scanty. Oral feeding of glycerol in rehydration solutions can play an important role in treating newborns suffering from metabolic disorders through affecting blood glucose. In their experiment, Omazic et al. (2013b) compared oral rehydration solution containing glycerol with oral rehydration solution containing glucose and reported that calves given glycerol oral rehydration solution had increased plasma glucose levels compared to calves given oral rehydration solution containing glucose. Since plasma glucose level is a good indicator of energy status of an animal, the result implies that glycerol enhances energy supply in oral rehydration solution relative to glucose. Additionally, the numbers of enterobacteria and lactobacilli were not affected by the inclusion of glycerol in the oral rehydration solution; however, the glycerol-utilizing Lactobacillus reuteri was detected in calf feces.
The transition period comprises the fetus final growth, calving, and commencement of lactation. During this period, there is an intense increase in energy requirement by the mammary organ for lactogenesis, resulting in cows suffering from low and high levels of blood glucose and insulin and blood non-esterified fatty acids (NEFA) and beta-hydroxybutyric acid (BHBA), respectively (Ingvartsen and Andersen, 2000). Glycerol, as a prophylaxis for metabolic problems in transition cows, was evaluated in many experiments (Goff and Horst, 2001, DeFrain et al., 2004). Glycerol supplementation in early lactation improves blood glucose and lowers NEFA and BHBA concentrations, causing enhanced metabolic status (Johnson, 1954, Fisher et al., 1973). Many years back, Johnson (1954) reported the use of glycerol to prevent ketosis. In assays to evaluate the potential value of glycerol in treating ketosis, Donkin (2008) recommended glycerol feeding rates ranging from 5% to 8% of the diet DM for transition cows, but Schröder and Südekum (1999) used 10% glycerol to replace 50% of the starch in the dairy cattle diet and observed no negative effects on nutrient intake and digestibility or ruminal microbial synthesis.
7. Application of glycerol in the dairy animal industry
7.1. Effect of glycerol administration on feed intake
Feed intake greatly affects the lactation performance of dairy animals. The effect of glycerol on feed intake is dose-dependent. Increasing the dose of glycerol was paralleled with lowering feed intake in most experiments that evaluated glycerol administration in ruminant diets (Ezequiel et al., 2015, Andrade et al., 2018, Saleem et al., 2018). The effective dose that increases or decreases feed intake is not defined in the literature because many factors including the purity of glycerol, the basal diets, the production stage of the dairy animal, etc. affect the dose.
Palatability of feed is the main factor that affects feed intake, and it is expected that glycerol inclusion in the diet can enhance the palatability of feed due to its sweet taste. However, the high energy concentration in glycerol and its effect on ruminal fermentation and alteration of ruminal VFA proportions can negatively affect feed consumption (Andrade et al., 2018). In an experiment on transition cows, DeFrain et al. (2004) top-dressed glycerol in the diet of the cows at 0, 430, or 860 g/d from 14 d prepartum to 21 d postpartum. They reported a lowered feed intake for the prepartum cows and an unaffected feed intake for the postpartum cows. In another experiment, Paiva et al. (2016) reported a decreased feed intake in lactating cows fed 210 g dietary crude glycerol/kg DM feed. Also, Ezequiel et al. (2015) observed that feeding glycerol to dairy cows up to 300 g/kg of diet DM reduced DM intake by 15% compared with those fed diet without glycerol. Donkin et al. (2009) and Ezequiel et al. (2015) noted reduced feed consumption in lactating cows administered glycerol at more than 10% and 30% of the diet DM, respectively. In lactating goats, Andrade et al. (2018) reported that feeding glycerol in large amounts (more than 10.9% crude glycerol) limits feed intake via the production of high amounts of propionate and acetate through glycerol fermentation in the rumen. In buffaloes, Saleem et al. (2018) evaluated increasing levels of glycerol in the diet of dairy buffaloes in early lactation. They reported a decreased feed intake with glycerol feeding at low (150 mL/d per buffalo) and high (300 mL/d per buffalo) levels, and the decrease in the feed intake was greater in the high glycerol feeding level than in the low feeding level.
The reduced feed intake when glycerol was fed to dairy animals is attributed to high energy production and satiety, resulting from improved ruminal VFA production and their increased flow to the liver (Trabue et al., 2007). This is one of the major constraints of using high crude glycerol dose in the diet of lactating animals. Three major reasons could explain the undesirable effects of crude glycerol on the metabolism and performance of animals: the concentration of impurities such as methanol (Thompson and He, 2006), the speed of ruminal fermentation of glycerol (Rémond et al., 1993, Wang et al., 2009a, Shin et al., 2012), and the absorption of glycerol by the rumen epithelium (Paiva et al., 2016). In addition, the high energy content of glycerol influences oxidation reactions and increases Krebs cycle in the liver, resulting in a stimulated satiety and reduced DM intake (Trabue et al., 2007, Allen et al., 2009). Reduction in feed intake can be explained by the hepatic oxidation theory. According to Allen et al. (2009), due to hepatic fuel oxidation, adenosine triphosphate (ATP) concentrations in hepatocytes increase, sending inhibitory signals through vagus nerve to nucleus tractus solitaries which inhibit hypothalamic satiety centers.
On the other hand, some experiments reported unaffected feed intake with glycerol administration in ruminant animals. The no negative effects on feed intake indicate that palatability was not compromised by glycerol. Khalili et al. (1997) and Wang et al. (2009b) evaluated glycerol feeding to mid- and early-lactation cows and reported unaffected DM intake. DeFrain et al., 2004, Kass et al., 2013, and Omazic et al. (2013a) reported unchanged feed intake when glycerol was fed to dairy cows during early lactation. Donkin et al. (2007) replaced corn grain with pure glycerol at 0, 5%, 10%, and 15% and reported unaffected DM intake, indicating that 15% of corn grain can be replaced with glycerol; however, feed consumption declined during the first 7 d of the assay. Using another livestock species, Porcu et al. (2018) evaluated the effect of intra-ruminal dosing of a glucogenic mixture (70% glycerol + 20% propylene glycol + 10% water) on late lactation Sarda ewes and reported decreased concentrate intake and unaffected hay and total feed intake. Additionally, Thoh et al. (2017) evaluated various levels of crude glycerol (0, 5% and 10% of diet) in the diet of dairy goat and observed unaffected daily intake.
As previously noted, the sweet taste of glycerol can enhance feed intake in animals. Ogborn (2006) and Shin et al. (2012) observed enhanced feed intake, when diets for dairy cows were supplemented with glycerol. Ariko et al. (2015) observed that inclusion of glycerol at 52, 104, and 156 g/kg DM in lactating cows diets linearly increased feed intake. Increased feed intake with glycerol suggests improved energy utilization efficiency by ruminal microbiota (Andrade et al., 2018). Moreover, the physical characteristics of glycerol, such as viscosity, could maintain feed particles aggregate and reduce ruminal fill (Castagnino et al., 2018). Recently, Bajramaj et al. (2017) observed that DM intake tended to increase when glycerol was fed to lactating cows compared with when corn was fed. In addition, Gaillard et al. (2018) fed lactating cows with a diet supplemented with glycerol at 6%, 12%, and 18% of dietary DM and reported increased intake by almost 1 kg with the 12% glycerol and decreased intake by approximately 1 kg when 12% glycerol was compared with 18% glycerol.
The inconstancy between experiments reveals that more experiments with different doses of glycerol and different feeds offered to different species of ruminants are required; however, the purity of glycerol, as a prime factor that affects the response to glycerol administration, cannot be ignored.
7.2. Effect of glycerol administration on nutrient digestibility
As in feed intake, there are also some inconstancies between experiments regarding the effect of glycerol administration in dairy diets on nutrient digestibility. Almost the same factors responsible for the inconstancy in intake are responsible for inconstancy in feed digestion. In his review, Südekum (2008) reported that glycerol administration to ruminants had no effect on apparent digestibility of organic matter (OM), NDF, and starch. Wang et al. (2009a) observed increased DM, NDF, and acid detergent fibre digestibility with glycerol supplementation at 100, 200, and 300 g/d per cow, implying enhanced ruminal microbial activity (Andrade et al., 2018). Moreover, Paiva et al. (2016) observed greater digestibility of DM, CP, and ether extract with glycerol feeding to lactating cows at 70, 140, or 210 g/kg of crude glycerol of diet DM. Schröder and Südekum (2008) reported that glycerol promotes rumen environment in a similar manner as corn and can largely enhance nutrient digestibility. In buffaloes, feeding glycerol at 150 and 300 mL/d to lactating buffaloes did not affect the digestibility of OM and non-structural carbohydrates but the digestibility of DM, CP, and fiber increased, while there were no differences between the 2 levels for the digestibility of all nutrients (Saleem et al., 2018).
Researchers who observed positive effects with feeding glycerol on nutrient digestion hypothesized that glycerol supplied sufficient energy to rumen microbes to lyse N sources for the biosynthesize of microbial proteins. In addition, glycerol at levels up to 18% diet DM may promote soluble carbohydrates utilization (Andrade et al., 2018). Glycerol can be utilized by Selenomonas ruminantium, Megasphaera elsdenii and Streptococcus bovis to reduce the formation of nicotinamide-adenine dinucleotide (NADH) and promote the generation of propionate, acetate, and butyrate to provide other ruminal microorganisms with ATP needed to ferment feeds and enhance the energy efficiency in the rumen (Lee et al., 2011).
On the other hand, Shin et al. (2012) observed that concentrate replacement with crude glycerol at 10% of intake reduced digestion of dietary NDF in dairy cows by 30%. Similarly, Donkin et al. (2009) observed lowered fiber digestibility, when maize was replaced with glycerol at 5%, 10%, or 15% of DM intake in dairy cows. The negative effects of glycerol administration on ruminal microflora and cellulolytic activity are responsible for such effects. Crude glycerol administration reduced DNA concentration and enzymatic activity of Butyrivibrio fibrisolvens bacteria (Abo El-Nor et al., 2010). AbuGhazaleh et al. (2011) noted a reduction in cellulolytic activity in Ruminoccocus flavefaciens and F. succinogenes with crude glycerol administration in vitro. Such issues may be responsible for the reduction in nutrient digestibility, especially fibers. The discrepancies between results may be related to the level of administered glycerol, the diet fed to animals, and also the condition of the experiments (in vitro vs. in vivo experiments).
7.3. Effect of glycerol administration on ruminal fermentation
Part of this section has been discussed in a previous section (4. Ruminal metabolism of glycerol) in the present review. The ruminal and post ruminal metabolism effects of feeding glycerol to lactating cows are still under investigation. Ruminal pH and individual VFA proportions are the main parameters that are highly affected by the inclusion of glycerol in ruminant diet. Khalili et al., 1997, DeFrain et al., 2004, Wang et al., 2009a, Shin et al., 2012, Boyd et al., 2013, and Ariko et al. (2015) reported unaffected ruminal pH with glycerol administration to lactating animals. In other experiments, Kijora et al. (1998) reported declined ruminal pH with feeding glycerol. In sheep, van Cleef et al. (2018) evaluated the inclusion of crude glycerol up to 30% in the diets and observed a tendency for increasing ruminal pH with the increasing inclusion of glycerol in the diets. The inconsistency between results may due to the amounts of dietary glycerol used in different experiments (Ariko et al., 2015). Whereas Khalili et al. (1997) and Kijora et al. (1998) used small doses of glycerol, 252 and 400 g/d, respectively, Wang et al. (2009a) used 300 g/d glycerol in the diets. The method of glycerol administration is another probable reason for variation in results. Ariko et al. (2015) incorporated glycerol into a total mixed ration, while Wang et al. (2009a) included it in concentrate pellets. Kijora et al. (1998), however, used the drenching method for administering the glycerol. Such different administration methods result in more rapid or slow changes in the ruminal environment. Kijora et al. (1998) suggested that the administration method has more effect on ruminal environment than the amount of glycerol.
Regarding the effect of glycerol administration in diets of lactating animals on ruminal total and individual VFA, van Cleef et al. (2018) evaluated the inclusion of crude glycerol in diets of sheep and observed decreased ruminal total and individual VFA with the increasing inclusion of glycerol in the diets. Paiva et al. (2016) reported that feeding glycerol to lactating cows at 70, 140, or 210 g/kg of diet DM altered the ruminal VFA proportions, with a decrease in acetate and an increase in propionate, butyrate, valerate, isovalerate, and isobutyrate. The increased propionate concentration is attributed to the ruminal fermentation of 30% to 69% of the consumed glycerol to propionate (Rémond et al., 1993).
The results of effect of glycerol administration on ruminal ammonia-N and ruminal microbial protein synthesis in dairy animals show some inconstancies. Ariko et al. (2015) observed a linear increase in ruminal ammonia-N concentration with glycerol administration, which opposes the previous observations (DeFrain et al., 2004, Wang et al., 2009a, Shin et al., 2012, Boyd et al., 2013, Paiva et al., 2016). van Cleef et al. (2018) evaluated increasing levels of glycerol in diets of sheep and reported decreased ruminal ammonia–N with the increasing inclusion of glycerol in the diets. The different responses may be related to diet composition, DM intake, quality of the dietary protein, and glycerol feeding level. For instance, decreased dietary content and intake of CP with feeding glycerol implies less protein availability for ruminal degradation to ammonia-N. Donkin et al. (2009) and Shin et al. (2012) observed unimpaired microbial protein synthesis when glycerol was fed up to 150 g/kg of the diet, implying unaffected ruminal N supply.
7.4. Effect of glycerol administration on blood metabolites and ketosis
Treating and preventing the accumulation of ketone bodies (ketosis syndrome) and increasing the levels of blood glucose are the main objectives of glycerol administration in the diet of dairy animals. Osman et al. (2006) drenched lactating cows with glycerol at 500 mL/d for 14 d after calving and observed increased levels of blood glucose on d 7 and 13 and decreased concentration of NEFA. It is well documented that administration of glycerol promotes serum glucose (Johnson, 1954, Fisher et al., 1973, Rémond et al., 1993, Silva et al., 2014) in the livers of animals. Over 90% of total glucose production emanates from hepatic gluconeogenesis, which correlates strongly with digestible energy intake, absorption of propionate, and synthesis of glucose in growing ruminant liver (Kozloski, 2017). Most of the dietary glycerol is directly absorbed in the rumen epithelium or small intestine and conveyed to the liver where glycerol kinase enzyme converts it to glycerol-3-phosphate used to drive gluconeogenesis (Rojek et al., 2008).
Goff and Horst (2001) drenched transition dairy cows with 1, 2, or 3 L of glycerol containing 80% glycerol and reported greater plasma glucose by 16%, 20%, and 25%, respectively. Moreover, Porcu et al. (2018) evaluated the effect of intra-ruminal dosing of a glucogenic mixture on lactating Sarda ewes and observed sharply increased glycerol circulating concentrations, glycaemia and insulinemia, and decreased NEFA, total protein and urea circulating concentrations. In their work on the effects of drenching with glycerol on blood plasma concentrations of glucose and insulin in ewes, Ferraro et al. (2016) observed a significant increase in glucose concentrations at 30 and 60 min after dosing, and the concentration of glucose remained elevated for 180 min after dosing. Moreover, they observed increased plasma concentrations of insulin within 60 min after drenching.
On the other hand, DeFrain et al. (2004) reported unaffected prepartum concentrations of glucose, insulin, NEFA, and BHBA; however, glycerol administration at 0, 430, or 860 g/d from 14 d prepartum to 21 d postpartum tended to increase postpartum concentrations of plasma glucose. It is well documented that plasma NEFA and BHBA concentrations reflect the energy status in dairy cows during early lactation and indicate the risk of metabolic diseases such as displaced abomasum and clinical ketosis. Saleem et al. (2018) noted that feeding glycerol to lactating buffaloes did not affect serum total protein, globulin, albumin, and glucose concentrations, but the concentrations of BHBA and NEFA decreased. Ogborn et al. (2004) and Ogborn (2006) reported that drenching cows from 21 d prepartum to 21 d post calving at 5% of diet DM did not affect prepartum or postpartum plasma glucose, NEFA, BHBA, triglycerides, and glycogen. Kass et al. (2013) and Omazic et al. (2013a) reported unaffected plasma levels of NEFA, BHBA, insulin, and glucose with glycerol supplementation during the first 4 weeks of lactation. However, DeFrain et al. (2004) reported that supplementing lactating cows with 860 g crude glycerol (80.2%) daily decreased blood glucose concentration between d 14 and 21 post calving. In contrast, Wang et al. (2009b) observed that feeding glycerol at 100, 200 and 300 g/d to lactating cows increased blood glucose.
7.5. Effect of glycerol administration on milk production and composition
Increasing the density of energy in the diet of lactating animal is expected to enhance the lactational performance of animal (Lomander et al., 2012, Bajramaj et al., 2017) due to its effect on blood insulin and glucose levels. Saleem et al. (2018) evaluated the effect of feeding glycerol at 150 and 300 mL/d to lactating buffaloes and observed improved milk production and 3.5% fat corrected milk for the buffaloes that received the higher glycerol level while milk composition was not affected by glycerol level. Additionally, Omazic et al. (2013a) observed that glycerol (>99%) supplementation to dairy cows for 4 weeks tended to increase milk yield (kg energy corrected milk/d); however, the administration of crude glycerol (88.1%) showed no response to feeding of glycerol. Kass et al. (2013) observed an enhanced milk production in cows drenched orally with crude glycerol (82.6%). Also, Lomander et al. (2012) noted increased milk yield in cows fed 450 g glycerol/d during the first 90 d of lactation. Increased energy intake with glycerol feeding may be responsible for the improved milk production (Porcu et al., 2018). Porcu et al. (2018) observed that the administration of glucogenics did not affect milk fat percentage, decreased milk production, milk contents of lactose and urea, and increased milk protein and casein percentages.
Contrary to the improved milk production with glycerol administration in some studies, Paiva et al. (2016) observed decreased milk yield in dairy cows fed crude glycerol at 21% for long periods. They attributed the lowered milk yield to the decreased water availability for the dilution of the solid milk components. This causes low water accessibility, which decreases water availability in the mammary gland, with resultant decreased milk yield (Porcu et al., 2018).
Other studies reported unchanged (Shin et al., 2012, Ezequiel et al., 2015, Thoh et al., 2017) daily milk production in cows and goats fed diets supplemented with glycerol. Khalili et al. (1997) fed glycerol at 3.6% to mid-lactation cows and reported unchanged milk production or milk composition. Further, Donkin et al. (2007) replaced corn grain with pure glycerol at 0, 5%, 10%, and 15% of total diet DM and observed no effect on milk yield or milk fat or protein content, but they noted decreased milk urea-N content. Moreover, Thoh et al. (2017) observed unchanged daily milk production of goats fed crude glycerol at 5% and 10% of diet DM.
The inconsistency in results may be due to the glycerol purity, duration of supplementation, lactation stage, and diet (Porcu et al., 2018). Paiva et al. (2016) reported 3 main reasons for the inconsistency among results: the quality of crude glycerol due to impurities, the speed with which glycerol is fermented in the rumen, and the absorption of glycerol, which is metabolized in the liver, in the rumen epithelium.
As previously noted, glycerol increases blood insulin concentrations which has a positive effect on milk protein synthesis. This shows that amino acids from sources other than the diet can be captured by the mammary gland (Mackle et al., 2000). Moreover, Donkin et al. (2009) suggested that glycerol improves N utilization efficiency, which could increase milk protein content and yield. This phenomenon works well when cows are confronted by energy deficiency, independent of increase in glycemia (Bodarski et al., 2005).
In many experiments, the administration of glycerol was paralleled with a depression in milk fat concentration and yield (Bajramaj et al., 2017); however, unchanged milk fat concentration has also been reported (Khalili et al., 1997, DeFrain et al., 2004, Carvalho et al., 2011). Others reported increased milk fat content with feeding glycerol at 5% of diet DM, while increasing the level of glycerol to 10% of diet DM decreased milk fat content (Thoh et al., 2017). Such results revealed the effect of glycerol feeding level on the inconsistency between experiments. As previously mentioned, glycerol feeding causes increased ruminal propionate production at the expense of acetate, which may be the main reason for milk fat depression (Maxin et al., 2011, Bajramaj et al., 2017). Furthermore, increased energy intake, with feeding glycerol, has been also observed to decrease milk fat yield and concentration (Sutton, 1989). This can be through conjugated linoleic acid formation in the rumen. The conjugated linoleic acid decreases mammary expression of genes associated with lipogenesis (Harvatine and Allen, 2006).
Few experiments evaluated the effect of glycerol administration on the profile of milk FA. Gaillard et al. (2018) observed that supplementing lactating cows diet at mid and late lactation with glycerol at 6%, 12%, and 18% of dietary DM linearly decreased C16:0, C18:1n9c (C18:1 cis-9), C18:2n6c (C18:2 cis-9-12), C18:3t, and conjugated linoleic acid proportions and increased the proportions of majority of the milk short- and medium-chain FA. Additionally, Thoh et al. (2017) observed that feeding lactating goats on diet containing 5% glycerol increased the medium-chain triglycerides of milk, without affecting conjugated linoleic acids.
Reduction in milk fat content when glycerol was given to dairy animals may be adduced to decreased ruminal acetate concentration and mobilization of FA from body depots. Osman et al. (2006), however, attributed decreased milk fat content to reduced level of plasma NEFA. Ariko et al. (2015) observed increased proportions of saturated FA and odd-chain free FA (OCFA) in milk fat of dairy cows supplemented with glycerol at 5.2%, 10.4%, and 15.6% on DM basis in the diets. The altered milk FA profile coincides with the changes in ruminal VFA. According to French et al. (2012), OCFA synthesis in the rumen and the mammary gland increased as the ruminal propionate linearly increased. Glycerol, on direct absorption through the wall of the rumen, enters into the liver for gluconeogenesis, reducing the need for propionate for glucose synthesis, and becomes available as a precursor for short chain fatty acids (SCFA) production in mammary gland with decreased de novo synthesis of SCFA (Maxin et al., 2011). Moreover, reduced ruminal acetate and BHBA levels in blood were hypothesized to decrease SCFA (Maxin et al., 2011).
Scarce information is available on the effect of glycerol administration on milk physicochemical properties which greatly affects the palatability and heat stability of milk subjected to heat treatment during sterilization. In their experiment, Thoh et al. (2017) observed a decreased milk pH with feeding glycerol. Moreover, milk of goats fed diet containing 5% glycerol had lower L* (value corresponds to lightness) and b* (denotes blue/yellow) values. However, the authors related this effect to the fat content in the milk not glycerol.
7.6. Effect of glycerol administration on growth performance
The effects of feeding glycerol on body weight (BW) and body condition score (BCS) differ between experiments. Some reported no effects (DeFrain et al., 2004, Kass et al., 2013, Omazic et al., 2013a, Porcu et al., 2018, Saleem et al., 2018), while others reported positive effects (Donkin et al., 2007, Wang et al., 2009b) depending on the amount of glycerol fed to animal, the purity of glycerol, the basal diets, and other factors. In sheep (Porcu et al., 2018) and buffaloes (Saleem et al., 2018) unaffected BW and BCS with feeding glycerol were observed. However, cows fed glycerol at 0, 5%, 10%, and 15% of total diet DM showed greater BW changes (Donkin et al., 2007). Moreover, Wang et al. (2009b) reported a tendency toward increasing BW in dairy cows fed glycerol at 100, 200, and 300 g/d per cow. Researchers who found positive effects of feeding glycerol on BW gain explained their results based on the enhanced feed intake. Moreover, enhanced ruminal production of propionate, butyrate, and acetate increases energy availability for the maintenance and production in ruminants fed glycerol (Trabue et al., 2007). Almeida et al. (2018) reported that feeding glycerol increases BW of animal due to increased weight of stomach compartments. They attributed these results to the stimulation effect of glycerol on ruminal fermentation due to the improved total rumen VFA production (Trabue et al., 2007). Ruminal VFA promote the growth and proliferation of ruminal lumen, increase absorption area, improve the removal capacity of these acids, and provide greater energy absorption for the animal (Gorka et al., 2009). It appears that improved feed intake, VFA production and energy supply were the major factors responsible for improved BW and BCS in studies where positive effect of feeding glycerol on BW and BCS were observed in dairy animals.
8. Conclusions
The current chapter reviews utilization of glycerol as a byproduct from biodiesel production. Based on the body of research conducted in recent years, glycerol inclusion level should not exceed 15% of the diet DM in dairy cow ration. Such level does not cause negative effects in lactating cows. Glycerol could replace energy-rich grains, such as corn, with the same efficiency, considering the economic benefits. However, some important issues should be considered before feeding glycerol to dairy cows, which include the removal of methanol, replacement of glycerol for only grains with rapidly fermentable carbohydrates, and the need for more research to elucidate conditions that allow and maximize the beneficial use of glycerol in dairy cows diet due to the conflicting results of its feeding.
Addition of glycerol to pelleted feed mixtures for dairy animal seems promising. Though glycerol can be incorporated into manufactured feeds or total mixed rations, it may work well in pelleted concentrates. Mixing glycerol at 5% with protein feeds and grains as well as vitamin/mineral mixture before pelleting was effective in preserving high-moisture pellets as indicated by suppression of fungal growth. The quality of pellet and integrity is unchanged or improved by glycerol addition. This is a good practice, which, however, may produce high-moisture pellets with probable fungal growth. Mixing glycerol in pellets has a good advantage, especially with crude glycerol containing high methanol. Heating during pelleting causes the methanol to volatilize, making this method of feeding glycerol more promising.
Conflict of interest
Author declares that there are no present or potential conflicts of interest among him and other people or organizations that could inappropriately bias his work.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abo El-Nor S., AbuGhazaleh A.A., Potu R.B., Hastings D., Khattab M.S.A. Effects of differing levels of glycerol on rumen fermentation and bacteria. Anim Feed Sci Technol. 2010;162(3):99–105. [Google Scholar]
- AbuGhazaleh A.A., Abo, El-Nor S., Ibrahim S.A. The effect of replacing corn with glycerol on ruminal bacteria in continuous culture fermenters. J Anim Physiol Anim Nutr (Berl) 2011;95:313–319. doi: 10.1111/j.1439-0396.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- Allen M.S., Bradford B.J., Oba M. Board-invited review: the hepatic oxidation theory of the control of feed intake and its application to ruminants. J Anim Sci. 2009;87(10):3317–3334. doi: 10.2527/jas.2009-1779. [DOI] [PubMed] [Google Scholar]
- Almeida M.T.C., Ezequiel J.M.B., Paschoaloto J.R., Perez H.L., Carvalho V.B., Filho E.S.C. Rumen and liver measurements of lambs fed with high inclusions of crude glycerin in adaptation and finishing period of feedlot. Small Rumin Res. 2018;167:1–5. [Google Scholar]
- Andrade GP de, Carvalho FFR de, Batista Â.M.V., Pessoa R.A.S., da Costa C.A., Cardoso D.B. Evaluation of crude glycerin as a partial substitute of corn grain in growing diets for lambs. Small Rumin Res. 2018;165:41–47. [Google Scholar]
- Ariko T., Kass M., Henno M., Fievez V., Kärt O., Kaart T. The effect of replacing barley with glycerol in the diet of dairy cows on rumen parameters and milk fatty acid profile. Anim Feed Sci Technol. 2015;209:69–78. [Google Scholar]
- Avila J.S., Chaves A.V., Hernandez-Calva M., Beauchemin K.A., McGinn S.M., Wang Y. Effects of replacing barley grain in feedlot diets with increasing levels of glycerol on in vitro fermentation and methane production. Anim Feed Sci Technol. 2011;166–167:265–268. [Google Scholar]
- Bajramaj D.L., Curtis R.V., Kim J.J.M., Corredig M., Doelman J., Wright T.C. Addition of glycerol to lactating cow diets stimulates dry matter intake and milk protein yield to a greater extent than addition of corn grain. J Dairy Sci. 2017;100(8):6139–6150. doi: 10.3168/jds.2016-12380. [DOI] [PubMed] [Google Scholar]
- Barrington G.M., Gay J.M., Evermann J.F. Biosecurity for neonatal gastrointestinal diseases. Vet Clin North Am Food Anim Pract. 2002;18:7–34. doi: 10.1016/S0749-0720(02)00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodarski R., Wertelecki T., Bommer F., Gosiewski S. The changes of metabolic status and lactation performance in dairy cows under feeding TMR with glycerin (glycerol) supplement at periparturient period. Electron J Pol Agric Univ Anim Husb. 2005;8:1–9. [Google Scholar]
- Boyd J., Bernard J.K., West J.W. Effects of feeding different amounts of supplemental glycerol on ruminal environment and digestibility of lactating dairy cows. J Dairy Sci. 2013;96(1):470–476. doi: 10.3168/jds.2012-5760. [DOI] [PubMed] [Google Scholar]
- Carvalho E.R., Schmelz-Roberts N.S., White H.M., Doane P.H., Donkin S.S. Replacing corn with glycerol in diets for transition dairy cows. J Dairy Sci. 2011;94(2):908–916. doi: 10.3168/jds.2010-3581. [DOI] [PubMed] [Google Scholar]
- Castagnino P.S., Dallantonia E.E., Fiorentini G., Vito E.S., Messana J.D., Lima L.O. Changes in ruminal fermentation and microbial population of feedlot Nellore cattle fed crude glycerin and virginiamycin. Anim Feed Sci Technol. 2018;242:69–76. [Google Scholar]
- Cotrill B.R., Smith T.C., Berry P., Weiseman R.W.J., White G., Temple M. Stoneleigh Park; Warwickshire, UK: 2007. Opportunities and implications of using the co- products from biofuel production as feeds for livestock by. [Google Scholar]
- DeFrain J.M., Hippen A.R., Kalscheur K.F., Jardon P.W. Feeding glycerol to transition dairy cows: effects on blood metabolites and lactation performance. J Dairy Sci. 2004 Dec;87(12):4195–4206. doi: 10.3168/jds.S0022-0302(04)73564-X. [DOI] [PubMed] [Google Scholar]
- Donkin S.S. Glycerol from biodiesel production: the new corn for dairy cattle. Rev Bras Zootec. 2008;37:280–286. [Google Scholar]
- Donkin S., Doane P. Tri-state state dairy nutrition conference. Fort Wayne, IN, USA. 2007. Glycerol as a feed ingredient for dairy cows. [Google Scholar]
- Donkin S.S., Pallatin M.R., Doane P.H., Cecava M.J., White H.M., Barnes E. Performance of dairy cows fed glycerol as a primary feed ingredient. J Dairy Sci. 2007;90:350. [Google Scholar]
- Donkin S.S., Koser S.L., White H.M., Doane P.H., Cecava M.J. Feeding value of glycerol as a replacement for corn grain in rations fed to lactating dairy cows. J Dairy Sci. 2009;92(10):5111–5119. doi: 10.3168/jds.2009-2201. [DOI] [PubMed] [Google Scholar]
- Drackley J.K. Opportunities for glycerol use in dairy diets. Urbana. 2008;51:113–118. [Google Scholar]
- Ezequiel J.M.B., Sancanari J.B.D., Machado Neto O.R., da Silva Z.F., Almeida M.T.C., Silva D.A.V. Effects of high concentrations of dietary crude glycerin on dairy cow productivity and milk quality. J Dairy Sci. 2015;98(11):8009–8017. doi: 10.3168/jds.2015-9448. [DOI] [PubMed] [Google Scholar]
- FDA . 2007. Department of health and human services, subchapter E – animal drugs, feeds, and related products: Part 582 – substances generally recognized as safe, subpart B – General purpose food additives code of federal regulations. 21CFR582.1320. [Google Scholar]
- Ferraro S.M., Mendoza G.D., Miranda L.A., Gutiérrez C.G. In vitro ruminal fermentation of glycerol, propylene glycol and molasses combined with forages and their effect on glucose and insulin blood plasma concentrations after an oral drench in sheep. Anim Feed Sci Technol. 2016;213:74–80. [Google Scholar]
- Fisher L.J., Erfle J.D., Lodge G.A., Sauer F.D. Effects of propylene glycol or glycerol supplementation of the diet of dairy cows on feed intake, milk yield and composition, and incidence of ketosis. Can J Anim Sci. 1973;53:289–296. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) 2016. OECD-FAO agricultural outlook 2016–2025. Paris, France. [Google Scholar]
- French E.A., Bertics S.J., Armentano L.E. Rumen and milk odd- and branched-chain fatty acid proportions are minimally influenced by ruminal volatile fatty acid infusions. J Dairy Sci. 2012;95(4):2015–2026. doi: 10.3168/jds.2011-4827. [DOI] [PubMed] [Google Scholar]
- Gaillard C., Sørensen M.T., Vestergaard M., Weisbjerg M.R., Larsen M.K., Martinussen H. Effect of substituting barley with glycerol as energy feed on feed intake, milk production and milk quality in dairy cows in mid or late lactation. Livest Sci. 2018;209:25–31. [Google Scholar]
- Goff J.P., Horst R.L. Oral glycerol as an aid in the treatment of ketosis/fatty liver complex. J Dairy Sci. 2001;84:153. [Google Scholar]
- Gorka P., Kowalski Z.M., Pietrzak P., Kotunia A., Kiljanczyk R., Flaga J. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J Physiol Pharmacol. 2009;60:47–53. [PubMed] [Google Scholar]
- Harvatine K.J., Allen M.S. Effects of fatty acid supplements on feed intake, and feeding and chewing behavior of lactating dairy cows. J Dairy Sci. 2006;89(3):1104–1112. doi: 10.3168/jds.S0022-0302(06)72178-6. [DOI] [PubMed] [Google Scholar]
- Ingvartsen K.L., Andersen J.B. Integration of metabolism and intake regulation: a review focusing on periparturient animals. J Dairy Sci. 2000;83(7):1573–1597. doi: 10.3168/jds.S0022-0302(00)75029-6. [DOI] [PubMed] [Google Scholar]
- Johnson R.B. The treatment of ketosis with glycerol and propylene glycol. Cornell Vet. 1954;44:6–21. [PubMed] [Google Scholar]
- Kass M., Ariko T., Samarütel J., Ling K., Jaakson H., Kaart T. Long-term oral drenching of crude glycerol to primiparous dairy cows in early lactation. Anim Feed Sci Technol. 2013;184:58–66. [Google Scholar]
- Khalili H., Varvikko T., Toivonen V., Hissa K., Suvitie M. The effects of added glycerol or unprotected free fatty acids or a combination of the two on silage intake, milk production, rumen fermentation and diet digestibility in cows given grass silage based diets. Agric Food Sci Finl. 1997;6:349–362. [Google Scholar]
- Khattab M.S., El-Nor S.A.H.A., El-Sayed H.M.A., El-Bordeny N.E., Abdou M.M., Matloup O.H. The effect of replacing corn with glycerol and fibrinolytic enzymes on the productive performance of lactating goats. Int J Dairy Sci. 2012;7(4):95–102. [Google Scholar]
- Kijora C., Bergner H., Götz K.-P., Bartelt J., Szakács J., Sommer A. Research note: investigation on the metabolism of glycerol in the rumen of bulls. Arch Tierernaehrung. 1998;51(4):341–348. doi: 10.1080/17450399809381931. [DOI] [PubMed] [Google Scholar]
- Kozloski G.V. 3rd ed. UFSM Publishing; 2017. Bioquímica dos ruminantes. [Google Scholar]
- Kristensen N.B., Raun B.M.L. Ruminal fermentation, portal absorption, and hepatic metabolism of glycerol infused into the rumen of lactating dairy cows. In: Ortigues- Marty I., editor. Energy and protein metabolism and nutrition – Proceedings of the 2nd International symposium on energy and protein metabolism and nutrition. EAAP Publication No. 124. Wageningen Academic Publishers; The Netherlands: 2007. pp. 355–356. [Google Scholar]
- Lee S.-Y., Lee S.-M., Cho Y.-B., Kam D.-K., Lee S.-C., Kim C.-H. Glycerol as a feed supplement for ruminants: in vitro fermentation characteristics and methane production. Anim Feed Sci Technol. 2011;166–167:269–274. [Google Scholar]
- Linke P.L., DeFrain J.M., Hippen A.R., Jardon P.W. Ruminal and plasma responses in dairy cows to drenching or feeding glycerol. J Dairy Sci. 2004;87:343. (Abstract) [Google Scholar]
- Lomander H., Frössling J., Ingvartsen K.L., Gustafsson H., Svensson C. Supplemental feeding with glycerol or propylene glycol of dairy cows in early lactation—effects on metabolic status, body condition, and milk yield. J Dairy Sci. 2012;95(5):2397–2408. doi: 10.3168/jds.2011-4535. [DOI] [PubMed] [Google Scholar]
- Mackle T.R., Dwyer D.A., Ingvartsen K.L., Chouinard P.Y., Ross D.A., Bauman D.E. Effects of insulin and postruminal supply of protein on use of amino acids by the mammary gland for milk protein synthesis. J Dairy Sci. 2000;83(1):93–105. doi: 10.3168/jds.S0022-0302(00)74860-0. [DOI] [PubMed] [Google Scholar]
- Maxin G., Glasser F., Hurtaud C., Peyraud J.L., Rulquin H. Combined effects of trans-10,cis-12 conjugated linoleic acid, propionate, and acetate on milk fat yield and composition in dairy cows. J Dairy Sci. 2011;94(4):2051–2059. doi: 10.3168/jds.2010-3844. [DOI] [PubMed] [Google Scholar]
- Ogborn K.L. Cornell Univ.; Ithaca, NY: 2006. Effects of method of delivery of glycerol on performance and metabolism of dairy cows during the transition period. [Google Scholar]
- Ogborn K.L., Paratte R., Smith K.L., Jardon P.W., Overton T.R. Effects of method of delivery of glycerol on performance of dairy cows during the transition period. J Dairy Sci. 2004;87:440. [Google Scholar]
- Omazic A.W., Tråvén M., Bertilsson J., Holtenius K. High- and low-purity glycerine supplementation to dairy cows in early lactation: effects on silage intake, milk production and metabolism. Animal. 2013;7(09):1479–1485. doi: 10.1017/S1751731113001110. [DOI] [PubMed] [Google Scholar]
- Omazic A.W., Tråvén M., Roos S., Mellgren E., Holtenius K. Oral rehydration solution with glycerol to dairy calves: effects on fluid balance, metabolism, and intestinal microbiota. Acta Agric Scand Sect A Anim Sci. 2013;63(1):47–56. [Google Scholar]
- Osman M., Mehyer N., Bobe G., Coetzee J., Beitz D. 2006. Acute effects of subcutaneous injection of glucagon and/or oral administration of glycerol on blood metabolites and hormones of holstein dairy cows affected with fatty liver disease. Iowa State Univ Anim Ind Report A S Leafl R2090. [Google Scholar]
- Paiva P.G., Del Valle T.A., Jesus E.F., Bettero V.P., Almeida G., Bueno I.C.S. Effects of crude glycerin on milk composition, nutrient digestibility and ruminal fermentation of dairy cows fed corn silage-based diets. Anim Feed Sci Technol. 2016;212:136–142. [Google Scholar]
- Porcu C., Manca C., Cabiddu A., Dattena M., Gallus M., Pasciu V. Effects of short-term administration of a glucogenic mixture at mating on feed intake, metabolism, milk yield and reproductive performance of lactating dairy ewes. Anim Feed Sci Technol. 2018;243:10–21. [Google Scholar]
- Rémond B., Souday E., Ouany J.P. In vitro and in vivo fermentation of glycerol by rumen microbes. Anim Feed Sci Technol. 1993;41:121–132. [Google Scholar]
- Roger V., Fonty G., Andre C., Gouet P. Effects of glycerol on the growth, adhesion, and cellulolytic activity of rumen cellulolytic bacteria and anaerobic fungi. Curr Microbiol. 1992;25:197–201. doi: 10.1007/BF01570719. [DOI] [PubMed] [Google Scholar]
- Rojek A., Praetorius J., Frokiaer J., Nielsen S., Fenton R.A. A current view of the mammalian aqua glyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- Saleem A.M., Zanouny A.I., Singar A.M. Effect of glycerol supplementation during early lactation on milk yield, milk composition, nutrient digestibility and blood metabolites of dairy buffaloes. Animal. 2018;12(4):757–763. doi: 10.1017/S175173111700180X. [DOI] [PubMed] [Google Scholar]
- Schröder A., Südekum K.H. Glycerol as a by-product of biodiesel production in diets for ruminants. In: Wratten N., Salisbury P.A., editors. International rapeseed congress. Canberra, Australia. 1999. p. 241. [Google Scholar]
- Schröder A., Südekum K.H. Glycerol as a by-product of biodiesel production in Science. J Anim Sci. 2008;86:392. [Google Scholar]
- Shin J.H., Wang D., Kim S.C., Adesogan A.T., Staples C.R. Effects of feeding crude glycerin on performance and ruminal kinetics of lactating Holstein cows fed corn silage- or cottonseed hull-based, low-fiber diets. J Dairy Sci. 2012;95(7):4006–4016. doi: 10.3168/jds.2011-5121. [DOI] [PubMed] [Google Scholar]
- Silva L.G., Torrecilhas J.A., Ornaghi M.G., Eiras C.E., Prado R.M., Prado I.N. Glycerin and essential oils in the diet of nellore bulls finished in feedlot: animal performance and apparent digestibility. Acta Sci Anim Sci. 2014;36:177–184. [Google Scholar]
- Südekum K.-H. Co-products from biodiesel production. In: Garnsworthy P.C., Weisman J., editors. Recent advances in animal nutrition 2007. Nottingham, UK. Nottingham University Press; 2008. pp. 201–219. [Google Scholar]
- Sutton J.D. Altering milk composition by feeding. J Dairy Sci. 1989;72(10):2801–2814. doi: 10.3168/jds.S0022-0302(89)79427-3. [DOI] [PubMed] [Google Scholar]
- Thoh D., Pakdeechanuan P., Chanjula P. Effect of supplementary glycerin on milk composition and heat stability in dairy goats. Asian-Australas J Anim Sci. 2017;30(12):1711–1717. doi: 10.5713/ajas.17.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.C., He B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agric. 2006;22:261–265. [Google Scholar]
- Trabue S., Scoggin K., Tjandrakusuma S., Rasmussen M.A., Reilly P.J. Ruminal fermentation of propylene glycol and glycerol. J Agric Food Chem. 2007;55:7043–7051. doi: 10.1021/jf071076i. [DOI] [PubMed] [Google Scholar]
- van Cleef E.H.C.B., Almeida M.T.C., Perez H.L., Paschoaloto J.R., Filho E.S.C., Ezequiel J.M.B. Effects of partial or total replacement of corn cracked grain with high concentrations of crude glycerin on rumen metabolism of crossbred sheep. Small Rumin Res. 2018;159:45–51. July 2017. [Google Scholar]
- Wang C., Liu Q., Huo W.J., Yang W.Z., Dong K.H., Huang Y.X. Effects of glycerol on rumen fermentation, urinary excretion of purine derivatives and feed digestibility in steers. Livest Sci. 2009;121(1):15–20. doi: 10.1017/S1751731108003364. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu Q., Yang W.Z.Z., Huo W.J.J., Dong K.H.H., Huang Y.X.X. Effects of glycerol on lactation performance, energy balance and metabolites in early lactation Holstein dairy cows. Anim Feed Sci Technol. 2009;151:12–20. [Google Scholar]