Abstract
Background/purpose
Mechanical stretch plays a key role in promoting proliferation and differentiation of bone marrow mesenchymal stem cells (BMSCs) in distraction osteogenesis (DO). A better understanding of how the extracellular biomechanical stimulation is transferred to intracellular signal expression will benefit DO. Focal adhesion kinase (FAK) is a key factor in integrin signaling pathway. However, little is known about the effect of integrin-FAK signaling during the process of stretch induced osteogenic differentiation of BMSCs.
Materials and methods
A specific short hairpin RNAs (shRNAs) lentiviral expression vector was used to silence Fak gene and a well-established in vitro uniaxial dynamic stretching device was applied to stimulate DO. Fak silencing was confirmed by fluorescence microscopy and the detection of Fak mRNA and FAK, p-FAK protein expression. Alkaline phosphatase (ALP) activity, expression of osteogenic differentiation markers - runt-related transcription factor 2 (RUNX2/Runx2) and alkaline phosphatase (Alp) together with integrin upstream signal transduction molecules integrin beta-1 (ITGB1/Itgb1) and downstream signal transduction molecules integrin-linked kinase (ILK) were detected after the stretch.
Results
The results showed that mechanical stretch in control groups significantly induced the osteogenic differentiation of BMSCs with increased ALP activity, expression of RUNX2/Runx2 and Alp, together with upregulated ITGB1/Itgb1 and ILK, which all vanished in Fak silencing group.
Conclusion
Silencing of the Fak gene inhibited the osteogenic differentiation of rat BMSCs induced by in vitro mechanical stretch through integrin signaling pathway.
Keywords: Distraction osteogenesis, Focal adhesion kinase, Integrin signaling pathway, Mesenchymal stem cells, Mechanical stretch
Introduction
Distraction osteogenesis (DO) is an endogenous tissue engineering technology used to repair skeletal including craniofacial deformities, in which mechanical stretch is applied to stimulate the formation of new bone.1 Nowadays, DO is still a long treatment course with high cost and infection risk that may be improved with a better understanding in cellular molecular biology.
Osteogenic differentiation of primitive mesenchymal cells stimulated by controlled mechanical stretch is the key step for new bone regeneration within the distraction gap.2 Studies have shown that BMSCs are so sensitive to mechanical stimulation that they may be the main functional cells to bone distraction.3
The previous study of Qi MC et al. has successfully demonstrated that mechanical stretch acts as a stimulator to induce osteogenic differentiation of rat BMSCs into osteoblasts which is vital for bone formation in distraction osteogenesis.4 However, the signal transduction pathways and molecular mechanisms which translate biomechanical signals into intracellular biochemical signals to induce cells proliferation, differentiation and mineralization during mechanical stretch are still not fully understood.5
Multiple studies have indicated that integrin signaling plays a critical role in mechanical stress transduction and is a bridge for two-way transmission between the extracellular matrix and the cellular cytoskeleton.6, 7, 8 And high expression level of integrin-related factors has been found in callus from distraction osteogenesis in vivo.9
Focal adhesion kinase (FAK) is a key factor that participate in the integrin signaling pathway by activating downstream signaling molecules and regulating the cytoskeletal structure and cell adhesion, migration, proliferation, and differentiation.10 However, to the best of our knowledge, little is known about the effect of integrin-FAK signaling during stretch induced osteogenic differentiation of BMSC.
In this study, we hypothesized that integrin-FAK signaling pathway is involved in osteogenic differentiation of BMSC stimulated by biomechanical stretch and Fak silence will impair osteogenic differentiation of BMSCs.
To test our hypothesis, an Fak-specific shRNA lentiviral expression vector was constructed to silence the FAK gene expression in BMSC. A well-established in vitro uniaxial dynamic stretching device was applied as a platform to stimulate DO, which could successfully induce BMSCs osteogenic differentiation in the previous study of Zhao C et al..11 Alkaline phosphatase (ALP) activity, expression of osteogenic differentiation markers - runt-related transcription factor 2 (RUNX2/Runx2) and alkaline phosphatase (Alp) together with integrin upstream signal transduction molecules integrin beta-1 (ITGB1/Itgb1) and downstream signal transduction molecules integrin-linked kinase (ILK) were detected after the stretch.
Materials and methods
All experiments were performed according to the Guidelines of the Institutional Animal Care & Use Committee of the authors' affiliated institutions, following the ARRIVE guidelines and the National Institutes of Health guide for the care and use of Laboratory animals. And This research was approved by the IRB of the authors’ affiliated institutions.
BMSC isolation
Three 40-day-old male Sprague–Dawley rats were obtained from West China Laboratories Animal Centre (Chengdu, China). Rat BMSCs were harvested and cultured using a modification of the method described by Haynesworth.12 After the rats were anesthetized with 2% pentobarbital sodium, femurs were harvested and placed in cell culture plates with low glucose-Dulbecco's Modified Eagle Medium (LG-DMEM, Gibco, New York, NY, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA). Bone marrow was flushed out and the adipose layer was discarded by centrifugation at 400 ×g for 10 min. Cells were seeded into 60 mL flasks and cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco) in a constant temperature incubator (37 °C and 5% CO2). The medium was exchanged 3 times a week. The cells were trypsinized and passaged at a rate of 1:2 or 1:3 when reached 80–90% confluency, using trypsin solution containing EDTA. Cells of the third generation were used for experiments after identified by flow cytometry (BD, Franklin Lakes, NJ, USA).
Construction of Fak-specific shRNA lentiviral expression vector and BMSC transfection
Both the interference target and the interference vector framework were determined and constructed based on the nucleotide sequence (AF020777) of the focal adhesion kinase (Fak) gene for rats in GenBank according to RNA interference sequence design principles. The interference vector framework and lentiviral vector pGCSIL-green fluorescence protein (GFP; Shanghai Genechem Co., Ltd., Shanghai, China) were double-digested by restriction enzymes AgeI (20,000 units/ml, New England BioLabs, Ipswich, MA, USA) and EcoRI (20,000 units/ml, New England BioLabs) and connected to construct the interference vector pGCSIL-Gfp-Fak. The interference vector pGCSIL-Gfp-Fak was used to transform competent Escherichia coli DH5α cells (Shanghai Genechem Co., Ltd.). Polymerase chain reaction (PCR) analysis was used to identify positive clones after 16 h of cell culture at 37 °C. Identification of positive clones indicated that the Fak-specific shRNA lentiviral expression vector had been successfully constructed. Using the transfection reagent Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA), 293T cells (ATCC) were co-transfected with the constructed interference vector and assistant vector (pHelper 2.0) to complete viral packaging. The 293T cells were lysed 48 h after co-transfection, and the supernatant containing the virus was collected and concentrated for use.
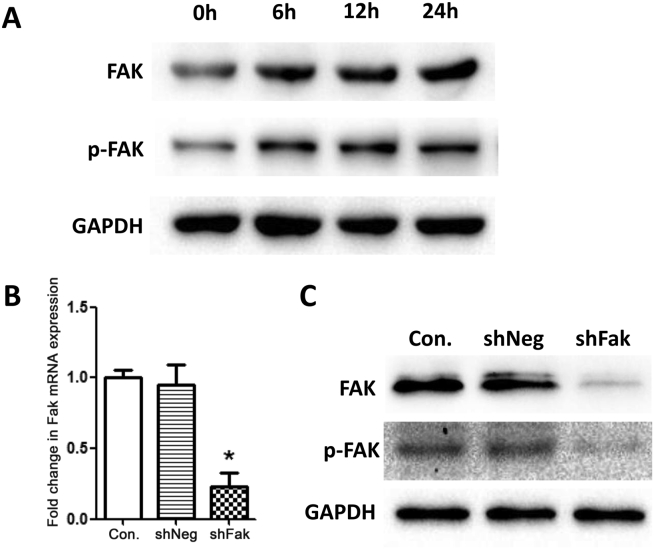
Three groups of third generation BMSCs were prepared using the following treatments: the untreated control group (Con.), the negative control group (shRNA-Neg) and the Fak silencing group (shRNA-Fak). The sequences were designed separately for shRNA-Fak (CTATGAAGTGTTAGAGAAA) and shRNA-Neg (TTCTCCGAACGTGTCACGT, scrambled). Expression of GFP was observed under an inverted fluorescence microscope, and 72 h after transfection, the expression of Fak mRNA was detected by real-time quantitative PCR and the protein expression of FAK and p-FAK were detected by Western immunoblotting respectively.
BMSC biomechanical stretching model
A uniaxial dynamic stretching device (Chinese patent number: ZL 20091 0164248.0) was applied to establish an in vitro rat BMSC stretching model, which had been proved validated in our previous study. This device of multiple units could keep the same stretching frequency in all chambers and the stretch stimulation was reciprocating cycle along the single axis, which was similar to the mechanical stimulation in DO (Fig. 1A–D). And after stretch, cells showed a fibroblast-like spindle and paralleled along the axis of loading (Fig. 1E and F).
Figure 1.
Uniaxial dynamic stretching device. A. Control system; B. Stretching units; and C. Bone during distraction osteogenesis in vivo; D. Cells during biomechanical stretch in stretching unit; E. Primary rat bone mesenchymal stem cells. Scale bars, 100 μm; F. Cells after mechanical stretch. Scale bars, 100 μm.
BMSCs (1.5 × 105/ml) of all three groups were seeded onto the silica gel film (Dow Corning, Midland, MI) and cultured for 2–3 days until 80%–90% confluence under the same conditions and then subjected to mechanical stretch in the chambers with DMEM respectively.
The parameters of distraction were set as follows: dynamic mechanical stretch frequency of 1 Hz, displacement and shrinkage strains of 3 mm, and stretch applied along the long axis 2 h x 8 with a 6 h interval between each time.
Alkaline phosphatase activity assay
After stretch, cells were harvested at 0, 6, and 12 h then lysed by sonication in 0.5 mL of 10 nM Tris-HCl (pH 7.5) containing 0.1% Triton X-100. ALP activity was detected by measuring the absorbance in p-nitrophenyl phosphate (PNPP) substrate by microtiter plate reader at 520 nm.
Quantitative real-time PCR analysis
At 0, 6, 12, and 24 h after stretch, total RNA was extracted from BMSCs of each group and reverse-transcribed into cDNA with the PrimeScript RT Reagent Kit (Takara Biomedical Technology Co., Ltd., Beijing, China). The products of reverse transcription were prepared as templates for quantitative real-time PCR analysis on an ABI PRISM 7300 (Applied Biosystems, Foster City, CA, USA). The sequences of the primers used are listed in Table 1, and the PCR conditions were 95 °C for 35 s followed by 40 cycles between 95 °C and 56 °C for 30 s. Each sample was subjected to triplicate PCR cycles, and the threshold cycle value of samples was normalized by the expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an endogenous housekeeping gene. The standard curves were generated by serial dilutions of a 100 ng/mL sample cDNA in five 10-fold dilution steps and used for regression analyses. In addition, the subsequent PCR products were confirmed by agarose gel electrophoresis.
Table 1.
Primers used for quantitative real-time PCR analysis.
| Gene product | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Gapdh | TATGACTCTACCCACGGCAAGT | ATACTCAGCACCAGCATCACC |
| Alp | GACCCTGCCTTACCAACTCATT | GTGGAGACGCCCATACCATCT |
| Runx2 | CTTCGTCAGCGTCCTATCAGTTC | CAGCGTCAACACCATCATTCTG |
| Itgb1 | TCTCACCAAAGTAGAAAGCAGGGA | ACGATAGCTTCATTGTTGCCATTC |
Abbreviations:Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Alp, alkaline phosphatase; Runx2, Runt-related transcription factor 2; Itgb1, Integrin beta-1.
Western immunoblotting
At 0, 6, 12, and 24 h after stretch, whole-cell lysates were prepared from all three groups of cells, and the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) using standard methods. Blots were incubated with primary antibodies (diluted to working concentrations of 1:1000 using blocking buffer) including rat monoclonal antibodies to ITGB1, ILK, RUNX2, and GAPDH (Cell Signaling Technology), overnight at 4 °C. After washing in TBS/T, secondary antibody, anti-mouse or rabbit immunoglobulin G conjugated to horseradish peroxidase (Biolegend, San Diego, CA, USA), was added for incubation at room temperature for 1 h. Immunoblot bands were visualized with an enhanced chemiluminescence solution (Millipore).
In addition, to confirm whether both FAK and FAK activation were involved in the stretch-mediated changes, we performed another group of western blot analysis to detect the protein expression of FAK, p-FAK and GAPDH (Cell Signaling Technology, Danvers, MA, USA) in BMSCs in the control group only at 0, 6, 12, and 24 h after exposed to mechanical stretch simulating distraction.
Statistical analysis
Results are presented as means ± standard deviation (SD) of at least three independent biological experiments. The experimental data were analyzed using SPSS, version 20.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was performed to identify significant differences among the results for the groups, and a p value < 0.05 was considered to indicate a statistically significant difference.
Results
Confirmation of BMSC transfection and depression of FAK activation
BMSCs of the third generation were used for experiments after identified by flow cytometry with a result that positive for CD29 (99.9%) and CD90 (99.1%) and negative for CD34 (0.3%) and CD45 (2.4%) (Fig. 2).
Figure 2.
Antigens expression on cell surface. Positive for CD29 and CD90, negative for CD34 and CD45.
72 h after transfection, BMSCs in the shRNA-Fak and shRNA-Neg groups displayed prominent expression of GFP, whereas no GFP expression was observed in cells of the control group (Fig. 3). The transfection efficiency for both shRNA-treated groups was 80%.
Figure 3.
Representative fluorescence images showing GFP expression in BMSCs of the three groups after transfection: A. Control group; B. shRNA-Neg group and C. shRNA-Fak group. Scale bar, 100 μm.
The protein expression results of FAK and p-FAK in BMSCs in the control group at 0, 6, 12, and 24 h after exposed to mechanical stretch simulating distraction showed that both FAK and p-FAK protein expression increased over time, indicating that FAK activation was involved in BMSCs differentiation mediated by uniaxial stretch (Fig. 4A). However, after transfection, Fak mRNA expression (Fig. 4B) and FAK, p-FAK protein expression (Fig. 4C) in the shRNA-Fak group was significantly decreased than in the control groups. These observations confirmed that both FAK expression and activation in shRNA-Fak group were successfully silenced after transfection before stretch which laid the foundation for subsequent experiments.
Figure 4.
A. Representative images of Western blots showing FAK, p-FAK and GAPDH protein expression in control group of BMSCs after exposed to mechanical stretch simulating distraction. B. Fak mRNA expression in three groups of BMSCs. C. FAK and p-FAK protein expression in three groups of BMSCs (*p < 0.05).
Alkaline phosphatase activity assay results
Alkaline phosphatase (ALP) activity was tested as an indicator for BMSCs osteoblastic differentiation. The ALP activity in shRNA-Fak group was significantly lower than control groups at 6 and 12 h (P < 0.05). No significant difference was found between control groups (Table 2).
Table 2.
Detection of activity of ALP (optical density value).
| Time | 0 h | 6 h | 12 h |
|---|---|---|---|
| Con. | 0.245 ± 0.021 | 0.702 ± 0.034 | 0.901 ± 0.033 |
| shNeg | 0.213 ± 0.033 | 0.665 ± 0.019 | 0.889 ± 0.015 |
| shFak | 0.205 ± 0.026 | 0.224 ± 0.043∗ | 0.253 ± 0.048∗ |
Abbreviations: ALP, alkaline phosphatase. *P < 0.05.
Effects of mechanical stretch on gene expression in BMSCs
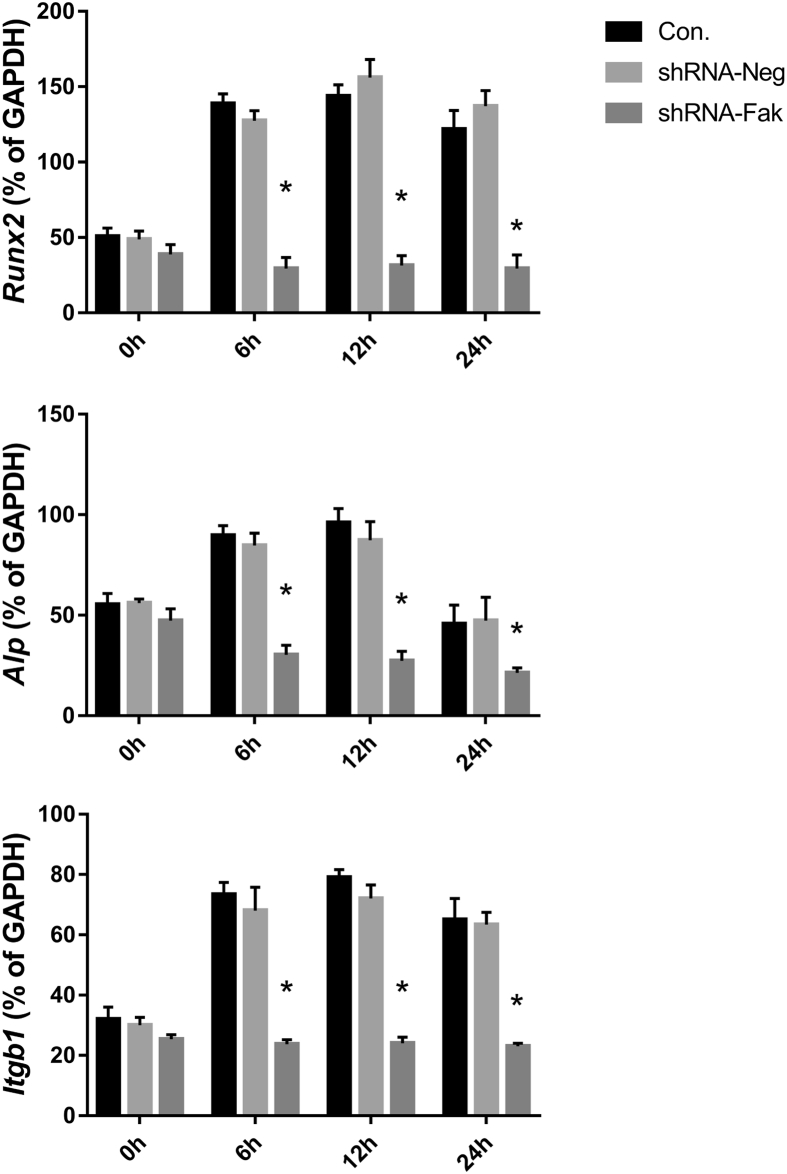
After mechanical stretch, the osteogenic mRNA expression level of Runx2, Alp together with integrin upstream Itgb1 in both control groups and shRNA-Neg group showed a similar trend that they all significantly increased and reached the peak at 12 h post stretch which started to decrease at 24 h. The mRNA expression reacting to the mechanical stretch disappeared in shRNA-Fak group (Fig. 5).
Figure 5.
mRNA expression level of Itgb1, Alp, and Runx2 in three groups of BMSCs after exposed to mechanical stretch simulating distraction (*p < 0.05).
Effects of mechanical stretch on protein expression in BMSCs
Protein expression of osteogenesis related protein RUNX2, integrin upstream ITGB1 and downstream ILK was detected by Western blotting in all three groups. BMSCs in the control and shRNA-Neg groups showed increasing expression of RUNX2, ITGB1 and ILK over time, which indicated that biomechanical stretch had successful osteogenic induction as expected. However, the shRNA-Fak group showed much lower expression levels for those proteins which was consistent with qPCR results (Fig. 6).
Figure 6.
A. Representative images and B. Quantitative analysis of Western blots showing ITGB1, ILK, RUNX2, and GAPDH protein expression in three groups of BMSCs after exposed to mechanical stretch simulating distraction (*p < 0.05).
Overall, the obviously increased ALP activity together with expression of RUNX2/Runx2 and Alp in both control groups showed a significant induction of BMSCs osteogenic differentiation, which vanished in shRNA-Fak group after Fak silencing. The integrin signaling upstream ITGB1/Itgb1 and downstream ILK expression changes in control groups were not observed in shRNA-Fak group neither.
Discussion
Mechanical stretch plays a key role in promoting proliferation, differentiation and maturation of BMSCs in distraction osteogenesis. A better understanding of how the extracellular biomechanical stimulation is transferred to intracellular signal expression will benefit DO. In our study, to imitate the biomechanical stretch in clinical DO, we applied the in vitro uniaxial dynamic stretching device and validated culture system which is similar to the devices and systems used previously by Haasper et al.,13 Jagodzinski et al.,14 and Diederichs et al.15 This system had already been demonstrated to successfully induce BMSC osteogenic differentiation in the previous study of Zhao C et al. In this system, cells in different stretch units are subjected to the same uniaxial dynamic stretching conditions at the same time, which is more efficient and reduces within-group error.
Integrins are heterodimeric glycoproteins that recognize components of the ECM and mediate cell adhesion, and they are widely distributed over the cell surface.16 These transmembrane glycoproteins are heterodimers composed of noncovalently linked alpha and beta subunits.17 The beta subunit is involved in the formation of focal adhesion plaques and transmits extracellular signals into the cell that then influence gene expression.18 As the main cell membrane receptor of ECM, integrin beta-1 (ITGB1) likely plays a vital role as an upstream signaling molecule in regulating stem cell differentiation in response to specific extracellular cues.19
Integrin engagement triggers activation of several signaling pathway including Fak, which is recognized as the key signal transduction factor in the integrin signaling pathway that functions by activating downstream signaling in focal adhesion plaques.20 Whether changes in integrin-FAK signaling pathway would influence osteogenic differentiation in osteoblast-like cell line or not was still not known clearly, which was our main interest.21
ILK is a protein kinase linked to downstream signaling of integrins that mediates response to actin changes by interacting with cytoplasmic domains of Integrin beta subunits.22 Yamaji S et al. found that the stimulation of ILK is dependent on Pi3 kinase action which was blocked by the lack of FAK.23 In our study, the increased expression levels of ITGB1 and ILK in control groups was attenuated in shRNA-Fak group during mechanical stretch, which suggested that both upstream and downstream expression of integrin signaling pathway was inhibited after Fak silencing. The inhibition of Fak induced the decrease of ILK probably in a PI3-kinase-dependent manner. And ILK is crucial for both focal adhesions and activation of the ITGB1 that the absence of ILK activation inhibits ITGB1 function,24 which is consistent with the decreased Itgb1 expression after Fak silencing in our study.
The increase of ALP activity and RUNX2/Runx2, Alp expression in both control groups demonstrated successful osteogenic differentiation of BMSCs after mechanical stretch. Alp expression is a well-established marker of BMSC transformation into osteoblasts which is triggered by cell contact with extracellular matrix (ECM).25 The dramatically decreased gene expression of Alp in the shRNA-Fak group was mostly associated with that the inhibition of Fak prevented osteogenic differentiation of BMSCs by decreasing the expression of extracellular signal-related kinase (ERK) dependent ALP activity.26 BMSCs osteogenesis in bone formation is mainly regulated by transcription of Runx2/Cbfa-1.27 The change of Runx2 vanished in the shRNA-Fak group was considered to be influenced via FAK-ERK1/2 downregulation.28 The decrease of Alp and Runx2 expression after Fak silencing indicated that the integrin-FAK signaling pathway is involved in the regulation of osteogenic differentiation of BMSC responding to mechanical stretch. The integrin signaling pathway influences this response as one pathway in a series of signaling pathways that together lead to osteogenesis.29 Signaling pathways such as Ras-Raf-MAPK/ERK, FAK-PI3K-Akt/Rac-JNK, and FAK-Rho-GTPase might be further activated to regulate and control cell functions after integrin-mediated activation of FAK.30
In conclusion, silencing of the Fak gene inhibited the osteogenic differentiation of rat BMSCs induced by in vitro mechanical stretching model through integrin signaling pathway.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81100779 and 81621062) and Sichuan Province Science and Technology Innovation Team Program (2017TD0016).
Contributor Information
En Luo, Email: luoen521125@sina.com.
Bin Ye, Email: yb6560@126.com.
References
- 1.McCarthy J.G., Stelnicki E.J., Mehrara B.J. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:1812–1827. doi: 10.1097/00006534-200106000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Li G., Simpson A.H., Kenwright J. Assessment of cell proliferation in regenerating bone during distraction osteogenesis at different distraction rates. J Orthop Res. 1997;15:765–772. doi: 10.1002/jor.1100150520. [DOI] [PubMed] [Google Scholar]
- 3.Aronson J., Shen X.C., Gao G.G. Sustained proliferation accompanies distraction osteogenesis in the rat. J Orthop Res. 1997;15:563–569. doi: 10.1002/jor.1100150412. [DOI] [PubMed] [Google Scholar]
- 4.Qi M.C., Hu J., Zou S.J. Mechanical strain induces osteogenic differentiation: Cbfa1 and Ets-1 expression in stretched rat mesenchymal stem cells. Int J Oral Maxillofac Surg. 2008;37:453–458. doi: 10.1016/j.ijom.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Feng X., Tuo X., Chen F. Ultrastructural cell response to tension stress during mandibular distraction osteogenesis. Br J Oral Maxillofac Surg. 2008;46:527–532. doi: 10.1016/j.bjoms.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., McNamara L.M., Schaffler M.B. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA. 2007;104:15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiteri C., Raizman I., Pilliar R.M. Matrix accumulation by articular chondrocytes during mechanical stimulation is influenced by integrin-mediated cell spreading. J Biomed Mater Res A. 2010;94:122–129. doi: 10.1002/jbm.a.32706. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Zhao Z., Wang J. The role of extracellular matrix, integrins, and cytoskeleton in mechanotransduction of centrifugal loading. Mol Cell Biochem. 2008;309:41–48. doi: 10.1007/s11010-007-9641-0. [DOI] [PubMed] [Google Scholar]
- 9.Lai Q.G., Yuan K.F., Xu X. Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2011;111:412–419. doi: 10.1016/j.tripleo.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Tong L., Buchman S.R., Ignelzi M.A., Jr. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211–222. doi: 10.1097/01.PRS.0000033180.01581.9A. Discussion 23-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C., Li Y., Wang X. The effect of uniaxial mechanical stretch on Wnt/beta-Catenin pathway in bone mesenchymal stem cells. J Craniofac Surg. 2017;28:113–117. doi: 10.1097/SCS.0000000000003252. [DOI] [PubMed] [Google Scholar]
- 12.Haynesworth S.E., Baber M.A., Caplan A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Haasper C., Jagodzinski M., Drescher M. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol. 2008;59:355–363. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Jagodzinski M., Drescher M., Zeichen J. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cells Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. Discussion 41. [DOI] [PubMed] [Google Scholar]
- 15.Diederichs S., Bohm S., Peterbauer A. Application of different strain regimes in two-dimensional and three-dimensional adipose tissue-derived stem cell cultures induces osteogenesis: implications for bone tissue engineering. J Biomed Mater Res A. 2010;94:927–936. doi: 10.1002/jbm.a.32772. [DOI] [PubMed] [Google Scholar]
- 16.Schneider J.G., Amend S.R., Weilbaecher K.N. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48:54–65. doi: 10.1016/j.bone.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner M., Jurdic P., Tuckerman J.P. New insights into adhesion signaling in bone formation. Int Rev Cell Mol Bio. 2013;305:1–68. doi: 10.1016/B978-0-12-407695-2.00001-9. [DOI] [PubMed] [Google Scholar]
- 18.Prowse A.B., Chong F., Gray P.P. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Takada Y., Ye X., Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson W.R., Rubin C.T., Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons J.T., Martin K.H., Slack J.K. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 22.Canel M., Serrels A., Frame M.C. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 23.Yamaji S., Suzuki A., Kanamori H. Possible role of ILK-affixin complex in integrin-cytoskeleton linkage during platelet aggregation. Biochem Bioph Res Co. 2002;297:1324–1331. doi: 10.1016/s0006-291x(02)02381-1. [DOI] [PubMed] [Google Scholar]
- 24.Attwell S., Mills J., Troussard A. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;14:4813–4825. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balcerzak M., Hamade E., Zhang L. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol. 2003;50:1019–1038. [PubMed] [Google Scholar]
- 26.Takeuchi Y., Suzawa M., Kikuchi T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997;272:29309–29316. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- 27.Komori T. Runx2, a multifunctional transcription factor in skeletal development. J Cell Biochem. 2002;87:1–8. doi: 10.1002/jcb.10276. [DOI] [PubMed] [Google Scholar]
- 28.Salasznyk R.M., Klees R.F., Williams W.A. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haugh M.G., Vaughan T.J., McNamara L.M. The role of integrin alpha(V)beta(3) in osteocyte mechanotransduction. J Mech Behav Biomed. 2015;42:67–75. doi: 10.1016/j.jmbbm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Docheva D., Popov C., Alberton P. Integrin signaling in skeletal development and function. Birth Defects Res C. 2014;102:13–36. doi: 10.1002/bdrc.21059. [DOI] [PubMed] [Google Scholar]