The N-methyl-d-aspartate receptor (NMDAR) antagonist (R,S)-ketamine has been hailed as the most important advance in the treatment of depression for the past 50 years. The rapid and sustained antidepressant effects of (R,S)-ketamine have spurred a great deal of research interest, with growing off-label use for the treatment of depression, although the concerns about the safety of repeated (R,S)-ketamine infusions persist [1].
(R,S)-ketamine (Ki = 0.53 µM for NMDAR) is a racemic mixture containing equal parts of (R)-ketamine (or arketamine) (Ki = 1.4 µM for NMDAR) and (S)-ketamine (or esketamine) (Ki = 0.30 µM for NMDAR) (Fig. 1). Previously, we reported that (R)-ketamine showed greater potency and longer lasting antidepressant effects than esketamine in animal models of depression [2]. Unlike esketamine, (R)-ketamine does not induce psychotomimetic side effects or exhibit abuse potential in rodents [3]. A positron emission tomography study using conscious monkeys demonstrated a marked reduction in dopamine D2/3 receptor binding in the monkey striatum after a single infusion of esketamine (0.5 mg/kg for 40 min), suggesting a marked release of dopamine from presynaptic terminal [4]. Interestingly, it is reported that a single infusion of esketamine (0.5 mg/kg for 40 min) caused psychotomimetic and dissociative symptoms (e.g., strange experiences like a feeling of floating in outer space or depersonalization/derealization) in patients with treatment-resistant depression [5]. Therefore, it is suggested that esketamine-induced dopamine release might be associated with the acute psychotomimetic and dissociative side effects in humans. Collectively, (R)-ketamine could be a safer antidepressant in humans than esketamine [2–4] .
Fig. 1.
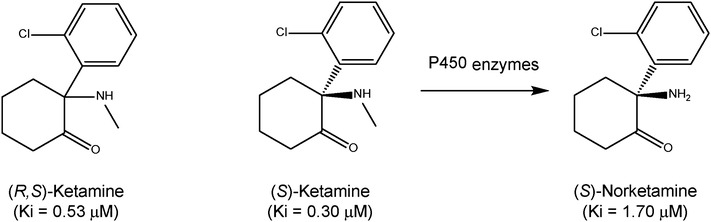
Chemical structure of (R,S)-ketamine, (S)-ketamine (or esketamine) and its metabolite (S)-norketamine. (S)-ketamine is metabolized into (S)-norketamine by the microsomal cytochrome P450 system. The values in parentheses are the inhibitor constant values for NMDAR (10)
The Janssen Pharmaceutical Companies of Johnson & Johnson has been developing esketamine nasal spray as the novel antidepressant. Two phase 2 randomized clinical trials of intranasal esketamine have been reported. Daly et al. [6] reported the efficacy of intranasal esketamine (28, 56, or 84 mg twice weekly) for the rapid reduction of symptoms of depression and suicidality in treatment-resistant patients. Change in Montgomery–Åsberg Depression Rating Scale (MADRS) total score in all three esketamine groups was superior to placebo, with a significant dose–response relationship [6]. Furthermore, Canuso et al. [7] reported the efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide. Esketamine (84 mg twice weekly for 4 weeks) along with antidepressant treatment significantly decreased suicidal symptoms and depression at both 4 and 24 h; however, these effects did not differ from those of placebo after 4 weeks of treatment. In contrast, differences in suicide risk scores, as determined by clinical global judgment, were not statistically significant between the groups at any time point [7]. Dissociative symptoms began shortly after each infusion and attenuated after repeated doses. The abuse potential of intranasal esketamine was not specifically examined within this short trial [6, 7]. It seems that the effects of intranasal esketamine may be less potent than intravenous (R,S)-ketamine. At the American Psychiatric Association annual meeting in May 2018, the company presented mixed findings of two phase 3 trials for intranasal esketamine [8]. Adult treatment-resistant patients who received esketamine had a significantly greater drop in MADRS score from baseline at day 28 compared with placebo group. In contrast, 65 and older patients with treatment-resistant depression who received esketamine plus a newly initiated oral antidepressant had no statistical difference in change in the MADRS score from baseline to day 28 compared with placebo group [8].
The potential for ketamine abuse is one of the most important drawbacks of repeated ketamine infusions for treating mood disorders [9]. Ketamine-induced rewarding effects are associated with the potent inhibition of the NMDAR in the brain. Esketamine is metabolized into (S)-norketamine (Ki = 1.70 µM for NMDAR) by the microsomal cytochrome P450 system (Fig. 1). Preclinical studies have revealed the abuse liability of esketamine in rodents, although its metabolite (S)-norketamine does not possess abuse liability in rodent model [10]. Given the role that NMDAR inhibition plays in the abuse potential of ketamine, clinicians should carefully monitor signs of abuse potential for (R,S)-ketamine and esketamine in their patients.
Very recently, we reported that (S)-norketamine exhibits rapid and sustained antidepressant effects in rodent models of depression, with a potency similar to that of the antidepressant actions of esketamine [10]. (S)-norketamine’s antidepressant effects are less potent than (R)-ketamine. Unlike esketamine, (S)-norketamine does not cause behavioral and biochemical abnormalities, such as prepulse inhibition deficits, abuse potential, loss of parvalbumin immunoreactivity in the medial prefrontal cortex, or increases in baseline γ-oscillations [10]. Given the lower affinity of (S)-norketamine for NMDAR, repeated infusions of (S)-norketamine may have fewer detrimental side effects in humans than its parent compound esketamine; however, additional long-term studies of repeated infusions of these compounds are warranted.
In conclusion, (S)-norketamine could be a safer alternative antidepressant than esketamine for use in humans.
Acknowledgements
This study was supported by the Grants from AMED, Japan (to K.H., JP18dm0107119) and National Natural Science Foundation of China (to C.Y., 81703482).
Conflict of interest
Dr. Hashimoto is an inventor on filed patent applications on “The use of R-ketamine in the treatment of psychiatric diseases” and “(S)-norketamine and salt thereof as pharmaceutical” by the Chiba University. Dr. Hashimoto has received research support from Dainippon Sumitomo, Otsuka, and Taisho.
References
- 1.Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med. 2016;46:2449–2451. doi: 10.1017/S0033291716000969. [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci. 2017;267:173–176. doi: 10.1007/s00406-016-0692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC. Efficacy and safety on intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175:620–630. doi: 10.1176/appi.ajp.2018.17060720. [DOI] [PubMed] [Google Scholar]
- 8.Psychiatric News (2018) J&J presents mixed findings for esketamine nasal spray at APA Annual Meeting. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2018.pp5a2
- 9.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systemic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Kobayashi S, Nakao K, Dong C, Han M, Qu Y, Ren Q, Zhang JC, Ma M, Toki H, Yamaguchi J, Chaki S, Shirayama Y, Nakazawa K, Manabe T, Hashimoto K. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry. 2018 doi: 10.1016/j.biopsych.2018.05.007. [DOI] [PubMed] [Google Scholar]


