Abstract
In this study, we have selected a series of a new family of molecules bearing Triazolo-benzodiazepines, an eleven membered heterocyclic ring has been studied for antidepression activity. Docking studies suggested that all the eleven ligands interacted well within active site of Drosophila melanogaster dopamine transporter (dDAT) (PDB ID: 4M48). Most ligands formed H-bond with amino acid Phe43, Asp46, Asp475, Tyr123, Ser421 and/or Gln316 and also exhibited Pi and Pi-Pi interactions with amino acid residues Tyr124, Phe319, Phe43, Phe325, Ala479 and Val120. In silico ADME evaluations of compounds showed more than 96% intestinal absorption for all compounds. During in vitro Toxicity properties prediction, the Triazolo-benzodiazepines derivatives: M1, M2, M3 and M11 showed less toxicity than the other studied molecules against algae, for daphnia the molecules M1, M2, M3, M8, M10 and M11 showed less toxicity than the reference molecule (Nortriptyline).
Keywords: Theoretical chemistry, Pharmaceutical chemistry, Bioinformatics, Biochemistry, Triazolo-benzodiazepine, Antidepressant activity, Molecular docking, ADMET properties, Nortriptyline
1. Introduction
Diazepines are a well-known class of heterocycles and they have gained importance since 1957, when the chlordiazepoxide (first benzodiazepine) was synthesized and studied in terms of psychotropic activity [1, 2, 3]. Actually, they possess a wide spectrum of biological activity including anxiolytic, hypnotic, sedative, anticonvulsant, skeletal, amnestic and muscle relaxant properties [4, 5, 6, 7].
Triazolo-benzodiazepines analogues are a key structural motif in numerous therapeutics that have sedative, muscle relaxant, and antitumor activities [8, 9]. Alprazolam, adinazolam and estazolam are commercially available chemical drugs based on triazolo-benzodiazepine scaffold that widely used as anxiolytic and sedative agents [10, 11, 12, 13]. Some triazolo-benzodiazepine derivatives have been reported to be weakly bound to the benzodiazepine receptor and prevent serine protease [14, 15].
So, due to the therapeutic and biological applications of this class of compounds, the study of type of interactions between these molecules and protein targetingby molecular docking methods for the prediction of the activity is definitely of great importance.
Molecular docking turns out to be a reliable method for preliminary evaluation of binding affinity and prediction of intermolecular interactions of novel compounds with receptors [16]. Nowadays, this method has become indispensable for studying protein-ligand interactions. Docking method can produce significant knowledge for complex systems, which complements experimentally achievable data. Molecular docking simulations have found widespread application for virtual screening and pose prediction of new or non-synthesized compounds [17, 18, 19, 20].
Molecular docking studies were focused on the dopamine trans-porter (DAT). This transmembrane protein is responsible for reup-take of dopamine from the synaptic cleft. DAT inhibitors are used in the treatment of depression due to the increased level of dopamine in the synaptic cleft as well as in adjuvant therapy of Parkinson's Disease (PD) [21].
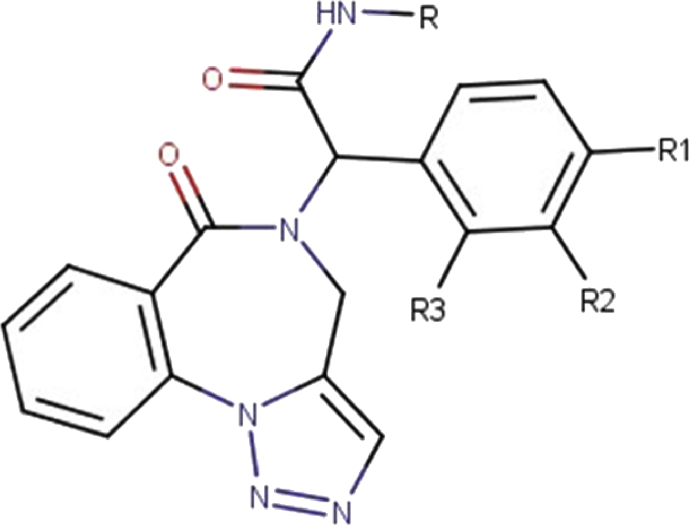
In this paper, a new family of Triazolo-benzodiazepines (Fig. 1) was docked to neurotransmitter trans-porter (DAT). We predict and interpret thebinding affinity and intermolecular interactions of complexes formed by docking of these molecules on DAT protein, so as to gain insight if those newly synthesized compounds could be of use as therapeutics in medicine.
Fig. 1.
Chemical structures of studied compounds.
2. Material and methods
2.1. Data collection
2.1.1. Ligands
In the present study, a series of 11 selected Triazolo-benzodiazepines derivatives were taken from literature (Table 1) [22], these molecules were considered to molecular docking study. The selected Triazolo-benzodiazepines were prepared by Asgari et al. from simple methods and substrates [22].
Table 1.
Chemical structures of the 11 selected Triazolo-benzodiazepines derivatives.
| N° | R | R1 | R2 | R3 |
|---|---|---|---|---|
| 1 | Cyclohexyl | O–CH3 | H | H |
| 2 | Cyclohexyl | O–CH3 | O–CH3 | H |
| 3 | Cyclohexyl | Cl | H | H |
| 4 | t-Butyl | CH3 | H | H |
| 5 | t-Butyl | H | H | CH3 |
| 6 | t-Butyl | O–CH3 | H | H |
| 7 | t-Butyl | O–CH3 | O–CH3 | H |
| 8 | t-Butyl | Cl | H | H |
| 9 | t-Butyl | H | H | F |
| 10 | Cyclohexyl | F | H | H |
| 11 | 1,1,3,3-tetramethyl-butyl | F | H | H |
2.1.2. Receptor: dopamine transporter DAT
The dopamine trans-porter (DAT) is transmembrane protein. Drosophila melanogaster dopamine transporter (dDAT) is a proven model of DAT, which is 50% similar to mammalian DAT sequence, and is used in research into the mechanism of action of many compounds. Thus, in our docking studies we made use of the X-ray structure of dDAT in complex with the tricycilcanitide-pressant nortriptyline, which indicate primary binding site (PDB code: 4M48) [16].
2.2. Molecular docking studies
Molecular docking turns out to be a reliable method for pre-liminary evaluation of binding affinity and prediction of intermolecular interactions of novel compounds with receptors. We decided to use neurotrans-mitter transporters (DAT) for docking study. In general, our research is based on crystal structures of receptors with bound ligand molecules. This structure has been obtained from X-ray crystal data of RCSB Protein Data Bank (PDB). In the majority of selected structures, co-crystallized ligand molecules are known drugs with proven action, and thus determine the binding site location in DAT as well as serve as references in our considerations.
In this research space, a docking process is launched for each studied molecule; the bioactive conformations were simulated using Autodock vina and Autodock tools 1.5.6 [23]. The results were analyzed using Discovery studio 2016 [24] and PyMol [25] softwares.
2.3. ADME and toxicity prediction
Absorption, distribution, metabolism, excretion and toxicity are predicted for the 11 selected Triazolo-benzodiazepines derivatives using Pre ADMET predictor server [26, 27].
3. Results and discussion
3.1. Molecular docking
The top-scoring pose of each molecule is selected according to the best interaction energy with the Drosophila melanogaster dopamine transporter (dDAT) (Table 2).
Table 2.
Comparison of Autodock score (kcal/mol) of the 9 best poses obtained by docking of 11 selected Triazolo-benzodiazepines derivatives and the re-docking of reference molecule (Nortriptyline) with dDAT.
| Ligands | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Ref: Nortriptyline | -10.0 | -9.3 | -9.2 | -9.0 | -8.8 | -8.7 | -8.7 | -8.5 | -8.0 |
| M1 | -10.0 | -9.8 | -9.2 | -9.1 | -9.0 | -8.9 | -8.8 | -8.7 | -8.7 |
| M2 | -9.9 | -9.5 | -9.4 | -9.2 | -9.1 | -8.9 | -8.9 | -8.8 | -8.7 |
| M3 | -10.6 | -10.0 | -10.0 | -9.9 | -9.8 | -9.6 | -9.5 | -9.4 | -9.4 |
| M4 | -10.5 | -9.4 | -9.3 | -9.3 | -9.3 | -9.2 | -9.2 | -9.1 | -8.8 |
| M5 | -10.4 | -9.3 | -9.3 | -9.3 | -9.1 | -8.7 | -8.7 | -8.7 | -8.6 |
| M6 | -9.6 | -9.2 | -9.1 | -9.1 | -9.0 | -9.0 | -8.9 | -8.9 | -8.7 |
| M7 | -10.0 | -9.9 | -9.5 | -9.5 | -9.4 | -9.2 | -9.0 | -9.0 | -8.9 |
| M8 | -10.4 | -9.8 | -9.5 | -9.4 | -9.4 | -9.3 | -9.3 | -9.2 | -8.9 |
| M9 | -10.3 | -9.4 | -9.4 | -9.3 | -9.3 | -9.1 | -9.0 | -8.9 | -8.8 |
| M10 | -12.3 | -10.6 | -10.5 | -10.3 | -10.0 | -10.0 | -9.8 | -9.6 | -9.5 |
| M11 | -10.1 | -10.1 | -9.9 | -9.5 | -9.3 | -9.1 | -8.8 | -8.8 | -8.7 |
The best energies of interaction with the Drosophila melanogaster dopamine transporter (dDAT) (lowest energy level) are observed for the ligand M10, whereas the ligand M6 is the least stable ligand in the list of studied molecules (Table 2). We can also observe that all complex formed by studied compounds and dDAT are more stable than the complex formed with the reference molecule (Nortriptyline) except for two studied molecules: M2 and M6.
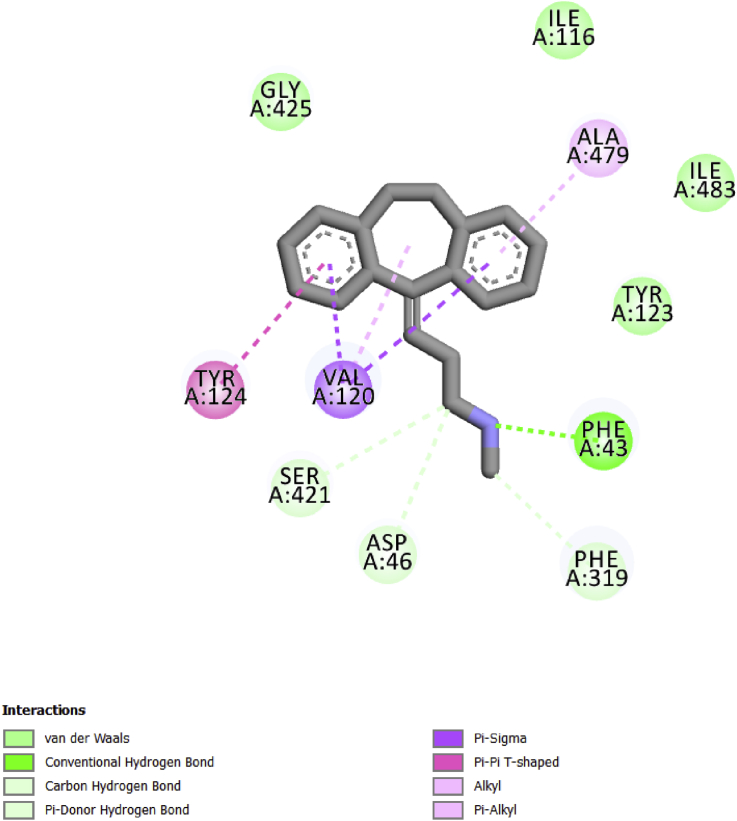
The result of the re-docked Nortriptyline molecule and its position in the PDB structure of protein dDAT is shown in Fig. 2.
Fig. 2.
Types of interactions between the dDAT (PDB code: 4M48) and Nortriptyline.
Nortriptyline is involved in Pi-Pi T-shaped interactions with Tyr124 as well as alkyl and Pi-alkyl interactions with Ala479, and Pi-sigma interactions with Val120. The N-methylpropanamine chain of nortriptyline enables formation of carbon hydrogen bonds with Asp46, Ser421 and Phe319, while conventional hydrogen bon and Pi-donor hydrogen bond are created only with Phe43 because nitrogen atom (N) is present in this position. The Van der Waals interactions are observed with Ile 483, Tyr 123 and Gly 425 amino acids.
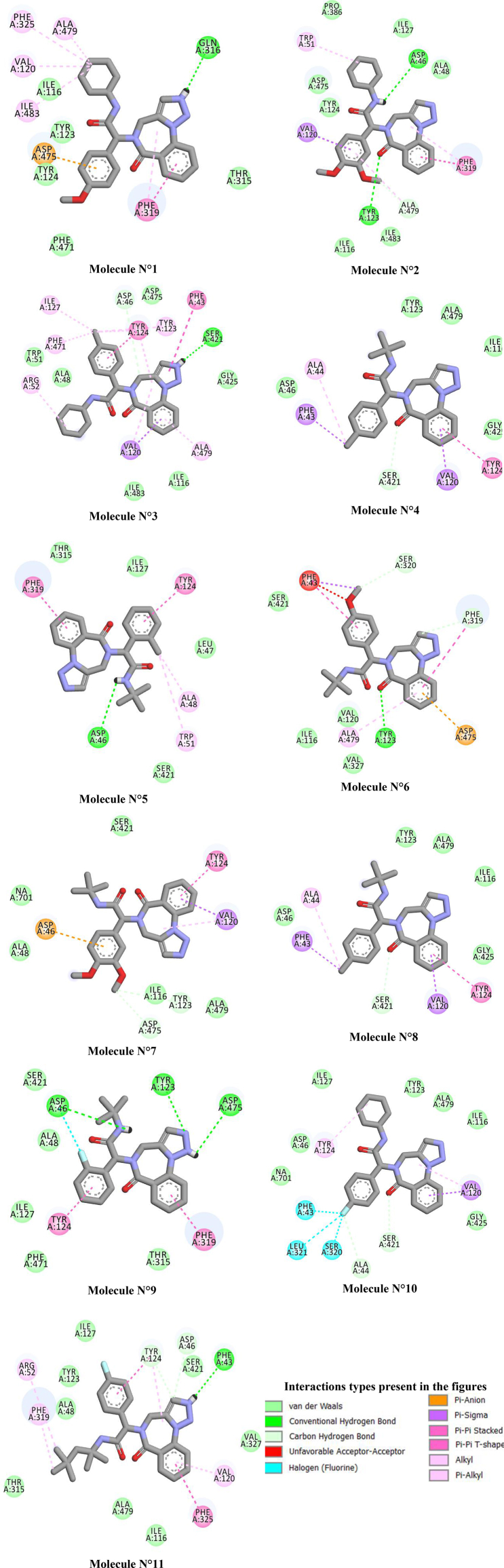
The docking result of 11 selected Triazolo-benzodiazepines derivatives and dDAT is shown in Fig. 3. And the comparison of these results and the result of the re-docked Nortriptyline molecule and its position in the PDB structure of protein dDAT is shown in Table 3.
Fig. 3.
Types of interactions between the dDAT (PDB code: 4M48) and the 11 selected Triazolo-benzodiazepines derivatives.
Table 3.
Comparison of interactions formed by docking of 11 selected Triazolo-benzodiazepines derivatives and the re-docking of reference molecule (Nortriptyline) with dDAT.
| Type of interactions | Residues | Molecules |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref: Nortriptyline | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | |||
| Hydrogen Bonds | Conventional H-Bond | PHE43 | X | X | ||||||||||
| GLN316 | X | |||||||||||||
| ASP46 | X | X | X | |||||||||||
| TYR123 | X | X | X | |||||||||||
| SER421 | X | |||||||||||||
| ASP475 | X | |||||||||||||
| Carbon -H-Bond | SER421 | X | X | X | X | |||||||||
| ASP46 | X | X | X | |||||||||||
| PHE319 | X | X | ||||||||||||
| ALA479 | X | |||||||||||||
| SER320 | X | |||||||||||||
| ASP475 | X | |||||||||||||
| TYR123 | X | |||||||||||||
| ALA44 | X | |||||||||||||
| SER124 | X | |||||||||||||
| Pi-Donor-H-Bond | PHE43 | X | ||||||||||||
| Hydrophobic interactions | Pi-Pi | TYR124 | X | X | X | X | X | X | X | X | ||||
| PHE319 | X | X | X | X | X | |||||||||
| PHE43 | X | X | ||||||||||||
| PHE325 | X | |||||||||||||
| Alkyl | VAL120 | X | X | X | X | X | ||||||||
| ALA479 | X | |||||||||||||
| ILE483 | X | |||||||||||||
| ILE127 | X | |||||||||||||
| ARG52 | X | X | ||||||||||||
| ALA44 | X | X | ||||||||||||
| ALA48 | X | |||||||||||||
| TYR124 | X | |||||||||||||
| Pi-Alkyl | ALA479 | X | X | X | X | |||||||||
| PHE319 | X | X | X | |||||||||||
| PHE325 | X | |||||||||||||
| TRP51 | X | X | ||||||||||||
| TYR123 | X | |||||||||||||
| TYR124 | X | X | X | |||||||||||
| PHE471 | X | |||||||||||||
| VAL120 | X | |||||||||||||
| Pi-Sigma | VAL120 | X | X | X | X | X | X | X | ||||||
| PHE43 | X | X | ||||||||||||
Visual inspection of the docked poses of molecule M11 clearly indicates similarity between binding modes and interactions of this molecule and the reference molecule (Nortriptyline) with dDAT. Both of them form carbon hydrogen bonds with Asp46, while conventional hydrogen hydrogen bond is formed with Phe43. Moreover, Tyr124 is bonded with M3, M4, M5, M7, M8, M9 and M9 by Pi-Pi interactions, which play a similar role in the binding of docked Nortriptyline molecule. All orientations of the discussed Triazolo-benzodiazepines derivatives are stabilized in the dDAT cavity by weak hydrophobic interactions with Val120 and Ala479 in a similar manner to docked Nortriptyline except the two molecules M5 and M9.
The similarities between interactions of 11 Triazolo-benzodiazepines derivatives and reference molecule are retained to usas therapeutics in medicine to treat the depression.
3.2. ADME, toxicity and drug likeness prediction
Absorption, distribution, metabolism, excretion, toxicity and drug likeness are predicted for the 11selected Triazolo-benzodiazepines derivatives using Pre ADMET predictor server, and the results are presented in Tables 4 and 5.
Table 4.
Predicted ADME properties of the 11 studied compounds in comparison with the reference drug.
| Ref | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBB | 13.406 | 0.318 | 0.320 | 0.340 | 0.122 | 0.159 | 0.245 | 0.197 | 0.277 | 0.180 | 0.331 | 0.246 |
| BS | 66.488 | 93.970 | 52.011 | 59.350 | 247.204 | 77.602 | 326.688 | 181.526 | 206.459 | 368.859 | 189.694 | 157.659 |
| CYP_2C19_inhibition | Inhibitor | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non |
| CYP_2C9_inhibition | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non |
| CYP_2D6_inhibition | Inhibitor | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non |
| CYP_2D6_substrate | Substrate | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non | Non |
| CYP_3A4_inhibition | Non | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor |
| CYP_3A4_substrate | Non | Weakly | Substrate | Weakly | Substrate | Substrate | Substrate | Substrate | Substrate | Substrate | Weakly | Substrate |
| HIA | 100 | 96.828 | 97.356 | 96.548 | 96.466 | 96.466 | 97.004 | 97.525 | 96.457 | 96.506 | 96.438 | 96.440 |
| MDCK | 96.879 | 1.121 | 0.143 | 3.675 | 24.933 | 112.593 | 6.772 | 1.476 | 9.663 | 27.802 | 2.223 | 0.043 |
| Pgp_I | Inhibitor | Non | Inhibitor | Inhibitor | Non | Non | Inhibitor | Inhibitor | Inhibitor | Non | Non | Inhibitor |
| PPB | 100 | 90.524 | 89.190 | 91.919 | 88.306 | 87.741 | 83.385 | 80.719 | 87.854 | 88.330 | 89.897 | 89.716 |
| PWS | 2.941 | 2.472 | 1.721 | 0.520 | 9.662 | 18.801 | 22.013 | 15.382 | 4.624 | 21.762 | 1.755 | 0.514 |
| SKlogD_value | 3.500 | 3.554 | 3.5489 | 4.269 | 3.407 | 3.3850 | 2.876 | 2.872 | 3.592 | 3.046 | 3.735 | 4.600 |
| SKlogP_value | 4.844 | 3.554 | 3.5489 | 4.269 | 3.407 | 3.3850 | 2.876 | 2.872 | 3.592 | 3.046 | 3.735 | 4.600 |
| SKlogS_buffer | -3.621 | -3.676 | -3.961 | -3.880 | -3.213 | -3.716 | -3.109 | -3.394 | -3.312 | -3.043 | -3.359 | -3.468 |
| SKlogS_pure | -4.975 | -5.256 | -5.441 | -5.938 | -4.621 | -4.332 | -4.280 | -4.466 | -4.962 | -4.272 | -5.393 | -5.955 |
BBB = in vivo blood-brain barrier penetration (C.brain/C.blood), BS = Buffer Solubility (mg/l), CYP2C19 = cytochrome P4502C19, CYP2C9 = cytochrome P4502C9, CYP3A4 = cytochrome P4503A4, CYP2D6 = cytochrome CYP2D6, PgP I = P-glycoprotein inhibition, PPB = Plasma Protein Binding %, PWS = Pure Water Solubility (mg/l), HIA = Human intestinal absorption %, MDCK = in vitro MDCKcellpermeability (Mandin Darby Canine Kidney (nm/sec)).
Table 5.
Toxicity predicted of the 11 studied compounds in comparison with the reference drug.
| ID | Ref | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| algae_at | 0.008 | 0.019 | 0.013 | 0.011 | 0.053 | 0.064 | 0.055 | 0.040 | 0.037 | 0.076 | 0.025 | 0.011 | |
| Ames_test | Mutagen | Mutagen | Non-mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | |
| Carcino_Mouse | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
| Carcino_Rat | Positive | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
| daphnia_at | 0.030 | 0.024 | 0.022 | 0.009 | 0.0434 | 0.044 | 0.078 | 0.070 | 0.029 | 0.059 | 0.020 | 0.012 | |
| hERG_inhibition | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | Medium_risk | |
| TA100_10RLI | Negative | Negative | Negative | Negative | Positive | Positive | Positive | Positive | Positive | Positive | Negative | Negative | |
| TA100_NA | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
| TA1535_10RLI | Positive | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
| TA1535_NA | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
The analysis of predicted ADME properties results (Table 4) shows that: the eleven molecules have different predicted in vivo blood-brain barrier penetration, the molecules M1, M2, M3 and M10 have highest penetration (0.318, 0.320, 0.340 and 0.331, respectively) in comparison with the other molecules, whereas the molecule M4 has a very low permeability (0.122). All these values are largely insufficient; in fact blood-brain barrier penetration of antidepressant molecules can reach for example in Nortriptyline 13.406.
All the molecules can't inhibit or be substrate for cytochromes CYP_2C19, CYP_2C9 and CYP_2D6 while they inhibit and substrate cytochrome CYP_3A4. These molecules have a high absorption which can exceed 96% for all the molecules, which is important for oral administration. A percent of plasma proteinbinding more than 80% is noted for all molecules which mean that 20% of the fraction of these molecules can actually give the pharmacological effect. This doesn't prevent that protein binding can influence the drug's biological half-life. The bound portion may act as a reservoir or depot from which the drug is slowly released as the unbound form.
The results of the prediction of the toxicity presented in Table 5 show that these molecules show a very low toxicity on the algae and daphnia, and a negative toxicity according to the four Ames tests (in vitro Ames test in TA100 strain (Metabolic activation by rat liver homogenate), in vitro Ames test in TA100 strain (No metabolic activation), in vitro Ames test in TA1535 strain (Metabolic activation by rat liver homogenate), in vitro Ames test in TA1535 strain (No metabolic activation)) except M4, M5, M6, M7, M8 and M9 whom has a positive toxicity on in vitro Ames test in TA100 strain (Metabolic activation by rat liver homogenate).
The Ames's mutagenicity test that uses several strains of the bacterium Salmonella typhimurium that carry mutations in genes involved in histidine synthesis, so that they require histidine for growth show that the molecule M2 can induce mutations, and none of these molecules present a risk of carcinogenicity neither in the rat nor in the mouse, and all present a medium risk to inhibit HERG (Human ether-a-go-go related gene channel). It should be noted that all these values are predicted.
4. Conclusion
In this study, the docking study was performed to elucidate the type of interactions between 11 selected Triazolo-benzodiazepines derivatives and Drosophila melanogaster dopamine transporter (dDAT), the results show that all the eleven ligands interacted well within active site of dDAT (PDB ID: 4M48); the molecules showed promising in silico results as indicated by their high protein–ligand interaction energy. The studied compounds are screened by ADME and Toxicity properties; these molecules are predicted to have more than 96% intestinal absorption for all compounds. During in vitro Toxicity properties prediction, the Triazolo-benzodiazepines derivatives: M1, M2, M3, M8, M10 and M11 showed less toxicity than the reference molecule (Nortriptyline) against daphnia.
A deep investigation of in vitro activity supported by docking results and in silico ADMET results clearly suggested that these molecules could be of use as therapeutics in medicine to treat the depression.
Declarations
Author contribution statement
Mohammed Bouachrine, Assia Belhassan, Hanane Zaki, Mohamed Benlyas, Tahar Lakhlifi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the “Association Marocaine des ChimistesThéoriciens” (AMCT) for its pertinent help concerning the programs.
References
- 1.Ban T.A. The role of serendipity in drug discovery. Dialogues Clin. Neurosci. 2006;8:335–344. doi: 10.31887/DCNS.2006.8.3/tban. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181823/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternbach L.H., Reeder E. Quinazolines and 1, 4-benzodiazepines. IV. 1, 2 transformations of 7-Chloro-2-methylamino-5-phenyl-3H-1, 4-benzodiazepine 4-oxide3. J. Org. Chem. 1961;26:4936–4941. [Google Scholar]
- 3.Zbinden G., Randall L.O. Adv. Pharmacol. Elsevier; 1967. Pharmacology of benzodiazepines: laboratory and clinical correlations; pp. 213–291. [DOI] [PubMed] [Google Scholar]
- 4.Mofakham H., Shaabani A., Mousavifaraz S., Hajishaabanha F., Shaabani S., Ng S.W. A novel one-pot pseudo-five-component condensation reaction towards bifunctional diazepine-tetrazole containing compounds: synthesis of 1H-tetrazolyl-1H-1,4-diazepine-2,3-dicarbonitriles and 1H-tetrazolyl-benzo[b][1,4]diazepines. Mol. Divers. 2012;16:351–356. doi: 10.1007/s11030-012-9371-4. [DOI] [PubMed] [Google Scholar]
- 5.Eleftheriadis N., Neochoritis C.G., Tsoleridis C.A., Stephanidou-Stephanatou J., Iakovidou-Kritsi Z. One-pot microwave assisted synthesis of new 2-alkoxycarbonylmethylene-4-oxo-1,5-benzo-, naphtho-, and pyridodiazepines and assessment of their cytogenetic activity. Eur. J. Med. Chem. 2013;67:302–309. doi: 10.1016/j.ejmech.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Huang S.-G., Mao H.-F., Zhou S.-F., Zou J.-P., Zhang W. Recyclable gallium(III) triflate-catalyzed [4+3] cycloaddition for synthesis of 2,4-disubstituted-3H-benzo[b][1,4]diazepines. Tetrahedron Lett. 2013;54:6178–6180. [Google Scholar]
- 7.Gerratana B. Biosynthesis, synthesis, and biological activities of pyrrolobenzodiazepines. Med. Res. Rev. 2012;32:254–293. doi: 10.1002/med.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midazolam and Other Benzodiazepines | SpringerLink, (n.d.). https://link.springer.com/chapter/10.1007/978-3-540-74806-9_16 (accessed April 28, 2019).
- 9.Hurley L.H., Reck T., Thurston D.E., Langley D.R., Holden K.G., Hertzberg R.P., Hoover J.R., Gallagher G., Jr., Faucette L.F. Pyrrolo [1, 4] benzodiazepine antitumor antibiotics: relationship of DNA alkylation and sequence specificity to the biological activity of natural and synthetic compounds. Chem. Res. Toxicol. 1988;1:258–268. doi: 10.1021/tx00005a002. [DOI] [PubMed] [Google Scholar]
- 10.Hanson S.M., Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABAA receptor. J. Neurosci. 2008;28:3490–3499. doi: 10.1523/JNEUROSCI.5727-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder P.J., Werth J., Giordani B., Caveney A.F., Feltner D., Maruff P. A method for determining the magnitude of change across different cognitive functions in clinical trials: the effects of acute administration of two different doses alprazolam. Hum. Psychopharmacol. Clin. Exp. 2005;20:263–273. doi: 10.1002/hup.692. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M., Maemura K., Kanbara K., Tamayama T., Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. In: Jeon K.W., editor. Int. Rev. Cytol. Academic Press; 2002. pp. 1–47. [DOI] [PubMed] [Google Scholar]
- 13.Levine J., Cole D.P., Chengappa K.N.R. Anxiety disorders and major depression, together or apart. Depress. Anxiety. 2001;14:94–104. doi: 10.1002/da.1051. [DOI] [PubMed] [Google Scholar]
- 14.Bertelli L., Biagi G., Giorgi I., Livi O., Manera C., Scartoni V., Martini C., Giannaccini G., Trincavelli L., Barili P.L. 1,2,3-Triazolo[1,5-a][1,4]- and 1,2,3-triazolo[1,5-a][1,5]benzodiazepine derivatives: synthesis and benzodiazepine receptor binding. Il Farmaco. 1998;53:305–311. doi: 10.1016/s0014-827x(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 15.Mohapatra D.K., Maity P.K., Shabab M., Khan M.I. Click chemistry based rapid one-pot synthesis and evaluation for protease inhibition of new tetracyclic triazole fused benzodiazepine derivatives. Bioorg. Med. Chem. Lett. 2009;19:5241–5245. doi: 10.1016/j.bmcl.2009.06.107. [DOI] [PubMed] [Google Scholar]
- 16.Adamski A., Kruszka D., Dutkiewicz Z., Kubicki M., Gorczyński A., Patroniak V. Novel family of fused tricyclic [1, 4] diazepines: design, synthesis, crystal structures and molecular docking studies. Tetrahedron. 2017;73:3377–3386. [Google Scholar]
- 17.Mobaraki N., Hemmateenejad B., Weikl T.R., Sakhteman A. On the relationship between docking scores and protein conformational changes in HIV-1 protease. J. Mol. Graph. Model. 2019;91:186–193. doi: 10.1016/j.jmgm.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2004;3:935. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 19.Gaieb Z., Liu S., Gathiaka S., Chiu M., Yang H., Shao C., Feher V.A., Walters W.P., Kuhn B., Rudolph M.G. D3R Grand Challenge 2: blind prediction of protein–ligand poses, affinity rankings, and relative binding free energies. J. Comput. Aided Mol. Des. 2018;32:1–20. doi: 10.1007/s10822-017-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gathiaka S., Liu S., Chiu M., Yang H., Stuckey J.A., Kang Y.N., Delproposto J., Kubish G., Dunbar J.B., Carlson H.A. D3R grand challenge 2015: evaluation of protein–ligand pose and affinity predictions. J. Comput. Aided Mol. Des. 2016;30:651–668. doi: 10.1007/s10822-016-9946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huot P., Fox S.H., Brotchie J.M. Dopamine reuptake inhibitors in Parkinson’s disease: a review of nonhuman primate studies and clinical trials. J. Pharmacol. Exp. Ther. 2016;357:562–569. doi: 10.1124/jpet.116.232371. [DOI] [PubMed] [Google Scholar]
- 22.Asgari M.S., Soheilizad M., Ranjbar P.R., Larijani B., Rahimi R., Mahdavi M. Novel and efficient synthesis of triazolobenzodiazepine analogues through the sequential Ugi 4CR-click-N-arylation reactions. Tetrahedron Lett. 2019;60:583–585. [Google Scholar]
- 23.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dassault Systèmes BIOVIA Discovery Studio Modeling Environment, Release 2017 Dassault Systèmes. 2016. http://accelrys.com/products/collaborative-science/biovia-discovery-studio/ [Google Scholar]
- 25.DeLano W.L. 2002. The PyMOL Molecular Graphics System, Httppymol Org. [Google Scholar]
- 26.Lee S.K., Chang G.S., Lee I.H., Chung J.E., Sung K.Y., No K.T. The PreADME: pc-based program for batch prediction of adme properties. EuroQSAR. 2004;9:5–10. [Google Scholar]
- 27.Lee S.K., Lee I.H., Kim H.J., Chang G.S., Chung J.E., No K.T. The PreADME Approach: web-based program for rapid prediction of physico-chemical, drug absorption and drug-like properties, EuroQSAR 2002 Des. Drugs Crop Prot. Process. Probl. Solut. 2003:418–420. [Google Scholar]