Abstract
Delayed wound healing particularly in difficult wounds and in elderly with co morbidities is a major concern. It leads to the pain, morbidity, prolonged treatment, and require major reconstructive surgery which imposes enormous social and financial burden. Vacuum-assisted closure (VAC) is an alternative method of wound management, which uses the negative pressure to prepare the wound for spontaneous healing or by lesser reconstructive options. Method of VAC application includes thorough debridement, adequate haemostasis and application of sterile foams dressing. A fenestrated tube is embedded in the foam and wound is sealed with adhesive tape to make it air tight. The fenestrate tube is connected to a vacuum pump with fluid collection container. The machine delivers continuous or intermittent suction, ranging from 50 to 125 mmHg. The VAC dressings are changed on 3rd day. Negative pressure therapy stabilizes the wound environment, reduces wound edema/bacterial load, improves tissue perfusion, and stimulates granulation tissue and angiogenesis. All this improves the possibility of primary closure of wounds and reduce the need for plastic procedures. VAC therapy appears to be a simple and more effective than conventional dressings for the management of difficult wound in terms of reduction in wound volume, depth, treatment duration and cost.
Keywords: Vacuum assisted closure, (VAC), Negative pressure wound therapy, (NPWT), Difficult wounds, Sub-atmospheric pressure dressing
1. Introduction
Delayed wound healing particularly in difficult wounds and in elderly with co morbidities is a major concern. It leads to the pain, morbidity, prolonged treatment, and require major reconstructive surgery which imposes enormous social and financial burden. Vacuum-assisted closure (VAC) can be used as an alternative to the conventional methods of wound management. Use of negative pressure optimizes the wound for spontaneous healing or by lesser reconstructive options. The vacuum-assisted closure is a non-pharmacologic/non surgical means for modulating wound healing; it was first proposed by Argenta and Morykwas in 1997.1,2 The application of vacuum reduces oedema, infection and increases local blood flow which promote healing.3 It is used as an adjunct or alternate to surgery for wide range of wounds with an aim to decrease morbidity, cost, duration of hospitalization and increase patient comfort.4, 5, 6
2. Material and methods
Authors did literature using keywords: ‘Vacuum assisted closure; (VAC); negative pressure wound therapy; (NPWT); difficult wounds' on Pub med, and Google Scholar. Relevant articles were chosen, and the review is based on them.
2.1. Method of VAC application7
Wound is thoroughly debrided, irrigated with normal saline, adequate haemostasis is achieved and peri-wound skin is made dry. Sterile foams are used for dressing as they provide an even distribution of negative pressure over the whole wound bed. Two types of foam are commonly used, black (Polyurethane ether, lighter, hydrophobic with a pore size of 400–600 mm) used for thoracic and abdominal cavity wounds. White (Polyvinyl alcohol, dense, hydrophilic with a pore size of 250 mm) used for superficial surface wounds.8 A fenestrated evacuation tube is fixed in the foam, which is connected to a vacuum pump. The wound is then sealed with an adhesive drape. Drapes should cover the foam and tubing and at least 3–5 cm of surrounding healthy tissue to ensure a watertight/airtight seal. The dressing is usually changed on 3rd day. The negative pressure mode can be either continuous or intermittent, ranging from 50 to 125 mmHg. Intermittent mode consists of a cycle of 5 min on and 2 min off phase. The pressure setting can be kept low (50–75 mmHg) particularly for painful chronic wounds. Higher pressures (150 mmHg plus) are used for large cavity and exhudative wounds.
3. Discussion
Initially negative pressure therapy was used to accelerate bedside preparation of wounds. The Morykwas and argents1,2 conducted series of animal experimentation to assess the effect of topical negative pressure therapy on local blood flow, formation of granulation tissue, bacterial clearance and flap survival. Subsequently they used foam dressing with a vacuum pump for management of wounds that allowed adjustment of vacuum pressure and selection of continuous or intermittent mode.
Indications of VAC includes diabetic foot ulcers, bed sores, skin graft fixation, flap salvage, burns, crush injuries, sternal/abdominal wound dehiscence, fasciotomy wounds, extravasation wounds and animal bites/frostbite. Vacuum therapy is contraindicated in patients with malignant wound, untreated osteomyelitis, fistulae to organs or body cavities, presence of necrotic tissue and those with exposed arteries/nerves/anastomotic site/organs. Relative contraindications include patients with blood dyscrasias, patients on anticoagulants or with actively bleeding wounds. During VAC therapy red flag signs include active or excessive bleeding, surrounding invasive sepsis, increased pain, signs of infection, such as fever, pus or foul-smelling drainage and allergic reaction to the adhesive. Complications of VAC therapy include failure of the VAC system (loss of seal, power failure, and blockage of the drainage system), wound infection, pain, bleeding, allergies to the adhesive drape, excoriation of the skin, restricted mobility, adherence of the tissues to the foam, lack of patient compliance and skin necrosis.9 VAC therapy leads to reduced rate of dressing changes, patient comfort, reduced hospital stay, reduced bacterial load, improved skin perfusion, reduction of oedema and provision of a closed moist wound healing environment.
3.1. Mechanism of action
Human and animal's studies have shown increased growth of granulation tissue, increased blood flow, diminution of the wound area, and regulation of inflammatory response with VAC therapy.1,10 VAC causes wound contraction, stabilization of the wound environment, decreased edema with removal of wound exudates, and microdeformation of cells. These effects allow VAC to accelerate wound healing by virtue of increase blood flow; reduced bacterial load; and improved wound bed preparation for subsequent coverage.11,12 The compression of tissue by negative pressure causes tissue hypoxia due to decreases perfusion beneath the foam which stimulates angioneogenesis, and local vasodilatation due to release of nitric oxide.13, 14, 15 This occurs during the “suction off” periods of VAC therapy. Therefore intermittent mode of VAC is more effective as compared to continuous mode.
Hypobaric interstitial pressure and increased permeability of vessels following injury leads to the formation of edema.16 VAC causes increased tissue pressure which leads to compression of vessels and increased velocity of the intravascular fluid (principle of continuity) which reduces the intravascular hydrostatic pressure (Bernoulli's principle). Both the factors cause less efflux of intravascular fluid and decreased edema. In addition higher blood velocity draw extracellular fluid into the vessel (Venturi principle). In addition the compressive forces of negative-pressure wound therapy physically force edema away from the injured tissues. All these mechanisms result in less interstitial hydrostatic pressure and improved oxygenation of cells. VAC therapy causes immobilization of wound which also aids in healing.17,18
Microdeformation/microstrain of cells due to VAC causes tissue expansion effect with release of growth factors.19, 20, 21 This tissue expansion effect is due to the differential pressure in the tissues after negative pressure application. The pressure within the cells is positive; while the pressure outside the cells and beneath the dressing is negative. This may lead to expansion of cells, growth of granulation tissue and pulling of wound edges closer to one another reducing wound size.
Recent study, however, has demonstrated that the pressure in the underlying wound is paradoxically increased (hyperbaric).22 The capillary perfusion pressure in the normal tissue is 10–35 mmHg.23 If the vascular tree is normal, hyperbaric pressure in tissues is unlikely to cause capillary occlusion. However, in ischemic tissue hyperbaric pressure may lead to ischemia and necrosis. Therefore negative-pressure wound therapy should be used with caution on ischemic tissues and especially when they are circumferential.
3.2. Optimum negative pressure
Controversy exists for the optimal application of negative pressure. Studies on animal model demonstrated increased granulation tissue formation with 125-mmHg vacuum compared with low (25 mmHg) or high (500 mmHg) vacuum suction. The low pressure suction (25 mmHg) results in decreases drainage of fluid from wound, decreased removal of toxins, and decrease deformation of cells. This results in reduced rate of granulation tissue formation. The high suction pressure (500 mmHg) causes increased mechanical deformation of tissues which leads to localized decrease in perfusion and reduced granulation tissue formation. Therefore negative pressure of 125 mm Hg is considered as an optimal pressure.24
Effects of different levels of negative pressure (10–175 mmHg) in different wounds showed that the level of negative pressure should be tailored according to the wound types. Acute traumatic wounds requires negative pressure of 125 mm Hg and for chronic non healing venous ulcer the optimum pressure is 50 mm Hg at intermittent cycles.25,26
3.3. Intermittent vs continuous VAC
Intermittent negative pressure is recommended as it generates more blood flow during vacuum “off” phase. Studies have shown that rate of granulation tissue formation is twice with intermittent negative pressure compared with continuous negative pressure. (103% with intermittent Vs 63% with continuous).27
Air leak in the dressing should be avoided as it leads to continual flow of air over the wound surface leading to desiccation of tissue and formation of eschar. This eschar seals the wound with retained exudate and leads to worsening of the wound.28 The pressure in VAC dressing gradually reduces over 2 days therefore, dressings should be changed after 48 h.29 One word of caution the VAC therapy should not be terminated abruptly after one session as it may result in a rebound phenomenon and worsening of the wound. Therefore 2–3 sessions of VAC should always be planned.
There is one RCT which provides objective evidence regarding the use of VAC for different indications. There is Grade “C” evidence for traumatic wounds while the use of VAC as a bridging therapy between several debridements graded as Grade “B”. Strong recommendation (Grade “A”) is proposed only for management of skin grafting procedures.30
3.4. Cost
Many studies on different wounds suggests that VAC may be more economical as compare to conventional wound care methods as it requires fewer dressing changes and lesser reconstructive options for wound healing. Wound healing is faster and overall short duration of treatment and hospitalization. Even though VAC dressing is more expensive than conventional dressings, but in long term the overall cost of treatment is less with VAC.31,32
The KCI Wound VAC system (Kinetic Concepts, Inc, San Antonio, TX) and other commercial vendors providing negative-pressure therapy for wounds are expensive and may not be available everywhere. We have used the off-the-shelf components to provide cost effective negative pressure therapy without purchasing dressing material or renting the KCI system, which can cost more than Rs 7000/day. We have used locally available material like Ortho cling drape (Surgiware), abdominal drain (Romsons), Bactigras (Smith &Nephew) and foam sponges to assemble a dressing (Fig. 1).This is connected to wall mounted suction to create negative pressure (75–125 mm Hg). This indigenously made dressing is very cost effective. (Components cost is only Rs 500). This indigenously made low pressure dressing system was used in many cases without complications and with results that are comparable to the commercial system (Fig. 2, Fig. 3, Fig. 4). The limitations of indigenous dressing include the inability to use intermittent suction as wall mounted suction has only continuous mode and to treat patients on an inpatient basis.
Fig. 1.
Locally available material to assemble VAC dressing.
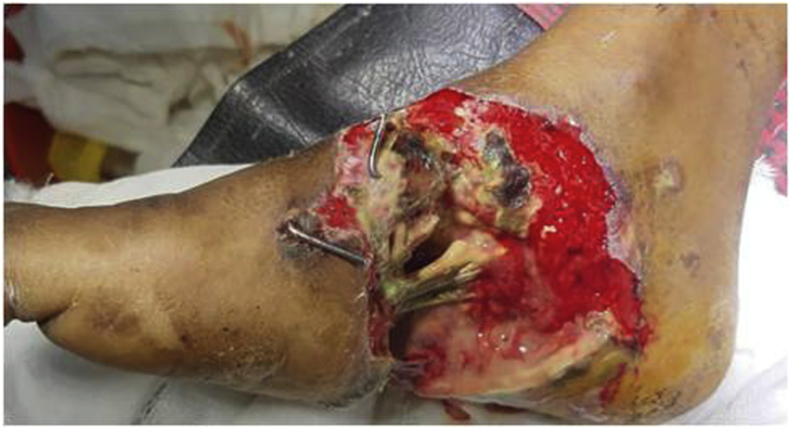
Fig. 2.
Pre operative wound following crush injury to foot.
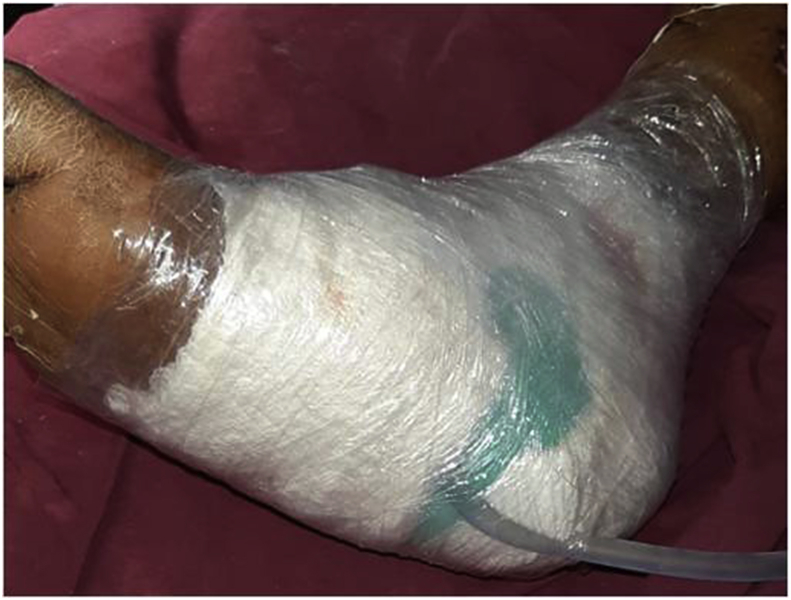
Fig. 3.
Indigenously made VAC in place.
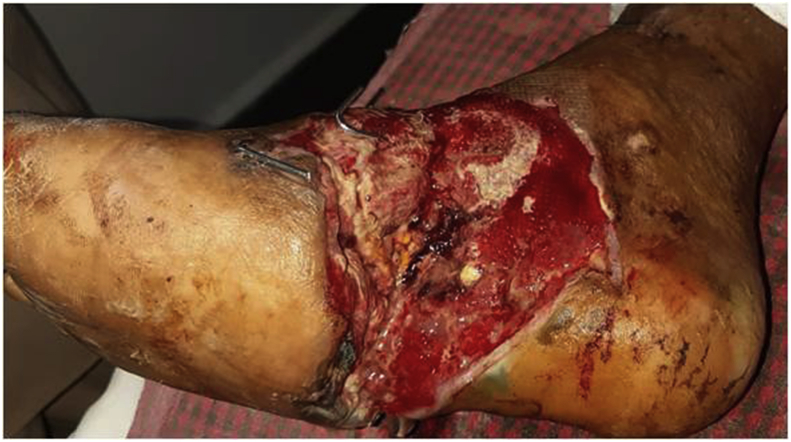
Fig. 4.
Post VAC wound after two sessions.
3.5. Key points
-
1.
VAC is a good alternative/adjunct to standard wound care especially for difficult wounds.
-
2.
It reduces the extent of reconstructive procedures.
-
3.
The optimum pressure setting is 125 mm of Hg.
-
4.
Intermittent suction is better than continuous suction.
-
5.
There are logistic benefits of VAC over conventional wound care methods.
-
6.
Cost of VAC is comparable to standard wound care methods and in long term it has a cost benefits.
4. Conclusions
VAC/NPWT stabilize the wound, reduce edema, reduces the bacterial load, improve tissue perfusion, and stimulate granulation tissue. It will improve the possibility of spontaneous wound healing and reduce the need for major plastic surgical procedures. VAC therapy is simple and effective substitute for the management of various wounds than conventional dressings in terms of reduction in wound size, treatment duration and cost.
Author's contribution
Prof. Pawan Agarwal- Conceptualization; Formal analysis; Investigation; Methodology; Roles/Writing - original draft; Writing - review & editing.
Rajeev Kukrele- Formal analysis; Investigation; Methodology; Roles/Writing - original draft; Writing - review & editing.
Prof. Dhananjaya Sharma- Formal analysis; Investigation; Methodology; review & editing.
Conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment. Animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment. Clinical experience. Ann Plast Surg. 1997;38:563–577. [PubMed] [Google Scholar]
- 3.Genecov D.G., Schneider A.M., Morykwas M.J., Parker D., White W.L., Argenta L.C. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219–225. doi: 10.1097/00000637-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D.G., Lavery L.A., Abu-Rumman P. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy/Wound Manag. 2002;48:64–68. [PubMed] [Google Scholar]
- 5.Orgill D.P. Utilizing negative pressure wound therapy on open chest/sternotomy wounds. Ostomy/Wound Manag. 2004;50(11A Suppl):15S–17S. [PubMed] [Google Scholar]
- 6.Orgill D.W., Austen W.G., Butler C.E. Guidelines for treatment of complex chest wounds with negative pressure wound therapy. Wounds (Suppl.) 2004;16:1–23. [Google Scholar]
- 7.Ellis G. How to apply vacuum-assisted closure therapy. Nurs Stand. 2016;30:36–39. doi: 10.7748/ns.30.27.36.s44. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann W., Becker U., Bischoff M., Hoekstra H. Vacuum sealing: indication, technique and results. Eur J Orthop Surg Traumatol. 1995;5:37–40. doi: 10.1007/BF02716212. [DOI] [PubMed] [Google Scholar]
- 9.Collinge C., Reddix R. The incidence of wound complications related to negative pressure wound therapy power outage and interruption of treatment in orthopaedic trauma patients. J Orthop Trauma. 2011;25:96–100. doi: 10.1097/BOT.0b013e3181de0134. [DOI] [PubMed] [Google Scholar]
- 10.Marks M.W., Argenta L.C., Thornton J.W. Rapid expansion: experimental and clinical experience. Clin Plast Surg. 1987;14:455–463. [PubMed] [Google Scholar]
- 11.Orgill D.P., Manders E.K., Sumpio B.E. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009;146:40–51. doi: 10.1016/j.surg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Mouës C.M., Vos M.C., van den Bemd G.J., Stijnen T., Hovius S.E. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 13.Kairinos N., Voogd A.M., Botha P.H. Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg. 2009;123:601–612. doi: 10.1097/PRS.0b013e318196b97b. [DOI] [PubMed] [Google Scholar]
- 14.Hsu S., Thakar R., Li S. Haptotaxis of endothelial cell migration under flow. Methods Mol Med. 2007;139:237–250. doi: 10.1007/978-1-59745-571-8_15. [DOI] [PubMed] [Google Scholar]
- 15.Breen E., Tang K., Olfert M., Knapp A., Wagner P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt Med Biol. 2008;9:158–166. doi: 10.1089/ham.2008.1010. [DOI] [PubMed] [Google Scholar]
- 16.Lund T., Wiig H., Reed R.K. Acute post burn edema: role of strongly negative interstitial fluid pressure. Am J Physiol. 1988;255:H1069–H1074. doi: 10.1152/ajpheart.1988.255.5.H1069. [DOI] [PubMed] [Google Scholar]
- 17.Argenta L.C., Morykwas M.J., Marks M.W., DeFranzo A.J., Molnar J.A., David L.R. Vacuum-assisted closure: state of clinic art. Plast Reconstr Surg. 2006;117:127S–142S. doi: 10.1097/01.prs.0000222551.10793.51. [DOI] [PubMed] [Google Scholar]
- 18.Venturi M.L., Attinger C.E., Mesbahi A.N., Hess Cl, Graw K.S. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6:185–194. doi: 10.2165/00128071-200506030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Saxena V., Hwang C.W., Huang S., Eichbaum Q., Ingber D., Orgill D.P. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–1098. doi: 10.1097/01.prs.0000135330.51408.97. [DOI] [PubMed] [Google Scholar]
- 20.Wilkes R.P., McNulty A.K., Feeley T.D., Schmidt M.A., Kieswetter K. Bioreactor for application of subatmospheric pressure to three-dimensional cell culture. Tissue Eng. 2007;13:3003–3010. doi: 10.1089/ten.2007.0036. [DOI] [PubMed] [Google Scholar]
- 21.Greene A.K., Puder M., Roy R. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006;56:418–422. doi: 10.1097/01.sap.0000202831.43294.02. [DOI] [PubMed] [Google Scholar]
- 22.Kairinos N., Solomons M., Hudson D.A. The paradox of negative pressure wound therapy --in vitro studies. J Plast Reconstr Aesthet Surg. 2010;63:174–179. doi: 10.1016/j.bjps.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Guyton A.C. eighth ed. Saunders; Philadelphia: 1991. Textbook of Medical Physiology; p. 1014. [Google Scholar]
- 24.Timmers M.S., LeCessie S., Banwell P., Jukema G.N. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665–671. doi: 10.1097/01.sap.0000187182.90907.3d. [DOI] [PubMed] [Google Scholar]
- 25.Fang R., Dorlac W.C., Flaherty S.F. Feasibility of negative pressure wound therapy during intercontinental aero medical evacuation of combat casualties. J Trauma. 2010;69(suppl 1):S140–S145. doi: 10.1097/TA.0b013e3181e452a2. [DOI] [PubMed] [Google Scholar]
- 26.Borgquist O., Ingemansson R., Malmsjö M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127:551–559. doi: 10.1097/PRS.0b013e3181fed52a. [DOI] [PubMed] [Google Scholar]
- 27.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment. Animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Morykwas M.J., Faler B.J., Pearce D.J., Argenta L.C. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Kairinos N., Solomons M., Hudson D.A. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg. 2009;123:589–598. doi: 10.1097/PRS.0b013e3181956551. discussion 599-600. [DOI] [PubMed] [Google Scholar]
- 30.Streubel P.N., Stinner D.J., Obremskey W.T. Use of negative-pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg. 2012;20:564–574. doi: 10.5435/JAAOS-20-09-564. [DOI] [PubMed] [Google Scholar]
- 31.Apelqvist J., Armstrong D.G., Lavery L.A., Boulton A.J. Resource utilization and economic costs of care based on a randomized trial of vacuum assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008;195:782–788. doi: 10.1016/j.amjsurg.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Philbeck T.E., Jr., Whittington K.T., Millsap M.H., Briones R.B., Wight D.G., Schroeder W.J. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare medicare patients. Ostomy/Wound Manag. 1999;45:41–50. [PubMed] [Google Scholar]