Abstract
Background/purpose
Dysregulation of cell cycle checkpoint control may lead to the independence of growth regulating signals. Checkpoint protein such as the PD-1/PD-L1 immune checkpoint involving tumor cells and host immune defense lymphocytes is a well-studied therapeutic target in oncology. Acting at a cell surface receptor on plasma membrane integrin αvβ3, thyroxine stimulates intracellular accumulation of PD-L1 in cancer cells. Although resveratrol also binds to integrin αvβ3, it reduces PD-L1 expression.
Materials and methods
In current studies, we investigated the roles of resveratrol and thyroxine in regulating expression of proliferation-related genes and checkpoint genes, PD-L1, BTLA in two oral cancer cell lines.
Results
Thyroxine suppressed the expression of pro-apoptotic BAD but induced proliferative CCND1 expression in SSC-25 cells and OEC-M1 cells. It activated expression of PD-L1 and BTLA in both cell lines. On the other hand, resveratrol suppressed the expression of all. Alternatively, it activated BAD expression. Thus thyroxine induces checkpoint gene expression which may promote proliferation in cancer cells. Alternatively, resveratrol reverses the stimulatory effects of thyroid hormone to induce anti-proliferation.
Conclusion
These findings provide new insights into the antagonizing effect of resveratrol on the thyroxine-induced expression of checkpoint genes and proliferative genes in oral cancers.
Keywords: L-thyroxine, Resveratrol, Checkpoint genes, Oral cancer
Introduction
Negative checkpoint regulators down-regulate immune responses to prevent out-of-proportion immune activation, minimize collateral damage and maintain peripheral self-tolerance.1 The most actively studied two negative checkpoint regulators are cytotoxic T lymphocyte (CTL)-associated antigen 4 (CTLA-4, CD152) and programmed cell death protein 1 (PD-1, CD279).2 They regulate immune responses at completely different levels via different mechanisms.3 CTLA-4 primarily regulates the amplitude of the early stages of T cell activation. On the other hand, PD-1 predominantly regulates effector T cell activity within tissue and tumors where the immune response is ongoing. Receptor-mediated signaling pathways are involved in the induction of PD-L1.4 B- and T-lymphocyte attenuator (BTLA) is a checkpoint co-inhibitory receptor classified to CD28 superfamily (also known as the Immunoglobulin [Ig] superfamily). It presents in a wide range of immune cells, including T cells, B cells, and NK cells.5 BTLA is structurally and functionally related to CTLA-4 and PD-1.6 The increased BTLA level correlates with the development and poor prognosis of gastric cancer.7 Therefore, overexpression of checkpoint genes affects the cell cycle, cell proliferation, carcinogenesis, and apoptosis.
Resveratrol induces anti-cancer growth in different cancer cells. By binding to its receptor on integrin αvβ3, resveratrol inhibits cell proliferation in several types of human cancer cells. Extracellular signal-regulated kinase-1 and -2 (ERK1/2) activated by resveratrol is vital for resveratrol-induced nuclear accumulation of inducible cyclooxygenase (COX)-2. Nuclear phosphorylated ERK1/2 (pERK1/2) in conjunction with the phosphorylation of p53 at Ser-15 promotes anti-proliferation in cancer cells. Thyroid hormones, l-thyroxine (T4) and 3, 5, 3′-triiodo-l-thyronine (T3) are able to enhance cancer cell proliferation. Recent studies also indicate that thyroid hormone promotes growth of human lung and ovarian cancer cell through cross-talk between estrogen receptor α (ERα) and cell surface αvβ3 integrin receptors.8,9 Evidence indicates that thyroxine activates ERK1/2 induces PD-L1gene expression and PD-L1 protein abundance consequently in various cancers.10, 11, 12 In addition, thyroxine interferes with resveratrol-induced anti-proliferative effect in cancer cells by disrupting resveratrol-induced pERK1/2-dependent nuclear COX-2 complex.
In the current report, we studied the action of T4 on the expression of checkpoint genes, PD-L1 and BTLA, in addition to proliferative gene, CCND1 in oral cancer cells. The promotive effects were inhibited by resveratrol. On the other hand, thyroxine suppressed pro-apoptotic gene, BAD expression was reversed by resveratrol co-treatment. In addition, resveratrol suppressed thyroxine-induced PD-L1 accumulation in nuclei. These results suggest that In addition to PD-L1, thyroxine activates other checkpoint gene expression which protects cancer to escape immune-surveillance. On the other, resveratrol is able to reduce the promotive effect of thyroid hormone.
Materials and methods
Cell cultures
Human oral epidermoid carcinoma cell line, OEC-M1 cells, was a gift from Dr. Hsien-Chung Chiu (Department of Periodontology, School of Dentistry, National Defense Medical Center and Tri-Service General Hospital, Taipei, Taiwan). Human squamous carcinoma of the tongue, SCC-25 cells (ATCC® CRL-1628™) were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). SCC-25 cells had been tested and authenticated by Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan) (isoenzyme analysis, Mycoplasma, cytogenetics, tumorigenesis, receptor expression testing). Cells were maintained in RPMI-1640 supplemented with 10% FBS in the incubator with 5% CO2 at 37 °C, and then used for experiments until passage 15. Before the study, cells were placed in 0.25% hormone-depleted serum-supplemented medium for 2 days.
Quantitative real-time PCR
As previous description,13, 14, 15, 16 total RNA was extracted by Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare Life Sciences, Buckinghamshire, UK) with eliminating genomic DNA. cDNA was prepared using one μg of DNase I-treated total RNA by RevertAid H Minus First Strand cDNA Synthesis Kit (Life Technologies Corp.). The cDNA was used as the template for real-time PCR reactions. The real-time PCR reactions were conducted using QuantiNovaTM SYBR® Green PCR Kit (QIAGEN, Hilden, Germany) on CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). This involved an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturing at 95 °C for 5 s and combined annealing/extension at 60 °C for 10 s, as detailed in the manufacturer's instructions. The primer sequences were shown as follows: Homo sapiens programmed death ligand 1 (PD-L1) (CD274), forward 5′-GTTGAAGGACCAGCTCTCCC-3′ and reverse 5′-ACCCCTGCATCCTGCAATTT-3’ (Accession No. AY254342.1); H. sapiens B and T lymphocyte associated (BTLA), forward 5′-GAGGAGAGTAGGAAGAGCCTG-3′ and reverse 5′- GCAAAAACGTGGTAGAGCGG-3’ (Accession No. NM_181780.3); H. sapiens cyclin D1 (CCND1), forward 5′- CAAGGCCTGAACCTGAGGAG-3′ and reverse 5′- GATCACTCTGGAGAGGAAGCG-3’ (Accession No. NM_053056); H. sapiens Bcl-2-associated death promoter (BAD), forward 5′-CTTTAAGAAGGGACTTCCTCGCC-3′ and reverse 5′-AAGTTCCGATCCCACCAGGA-3’ (accession no.: NM_004322), forward 5’-; H. sapiens 18S ribosomal RNA (18S), forward 5′- GTAACCCGTTGAACCCCATT-3′ and reverse 5′- CCATCCAATCGGTAGTAGCG-3’ (Accession No. NR_003286). Calculations of relative gene expression (normalized to 18S as reference gene) were performed according to the 2−ΔΔCT method. Fidelity of the PCR reaction was determined with melting temperature analysis.
Confocal microscopy
Exponentially growing oral cancer OEC-M1 cells and SCC-25 cells were seeded on sterilized cover glasses (Paul Marienfeld, Lauda-Königshofen, Germany). After exposure to 0.25% stripped FBS-containing medium for 2 days, oral cancer cells were treated with 40 μM resveratrol, 10−7 M T4, or their combination for 24 h. As previous description,17,18 cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min and then permeabilized in 0.06% Triton X-100 for 30 min. Cells were incubated with a monoclonal rabbit anti-PD-L1 antibody (1:100, GeneTex International Corporation, Hsinchu City, Taiwan), followed by an Alexa-647-labeled goat anti-rabbit antibody (1:300, GeneTex) and mounted in EverBrite Hardset mounting medium with DAPI (Biotium, Fremont, CA). The fluorescent signals from PD-L1 were recorded and analyzed with the TCS SP5 Confocal Spectral Microscope Imaging System (Leica Microsystems, Wetzlar, Germany). The figures shown are representative of at least four fields for each experimental condition.
Statistical analysis
All of the collected data of immunoblot and nucleotide densities were analyzed by IBM®SPSS® Statistics software version 19.0 (SPSS Inc., Chicago, IL, USA). Two tails Student's t-test was conducted and considered significant at p-values < 0.05 (*, or #), 0.005 (** or ##) and 0.001 (*** or ###).
Results
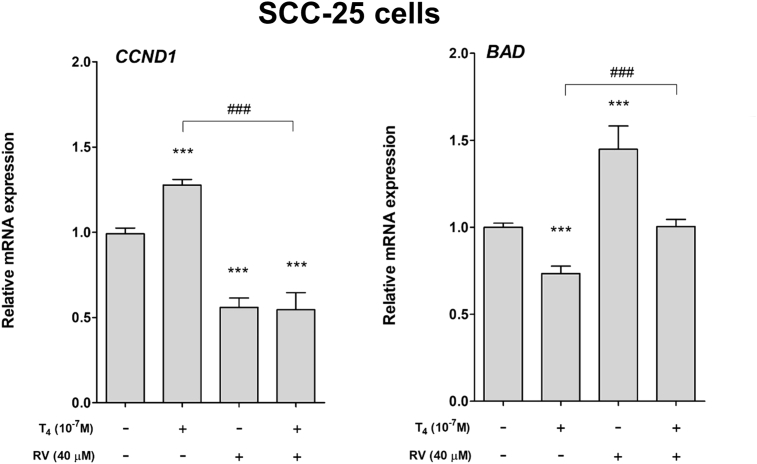
Effect of thyroid hormone and resveratrol on expression of proliferative gene and pro-apoptotic gene in human oral cancer cells. Human oral cancer SCC-25 cells were treated with 10−7 M T4, 40 μM resveratrol and their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for proliferative gene, CCND1 and pro-apoptotic gene, BAD. Thyroxine induced CCND1 expression but inhibited BAD expression (Fig. 1). On the other hand, resveratrol reversed thyroxine-induced effect in SCC-25 cells (Fig. 1).
Figure 1.
Effect of thyroid hormone and resveratrol on expression of proliferative gene and pro-apoptotic gene in human oral cancer SCC-25 cells. Human oral cancer SCC-25 cells were treated with 10−7 M T4, 40 μM resveratrol and their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for proliferative gene, CCND1 and pro-apoptotic gene, BAD. Number of independent experiments (N) = 3. (Data are expressed as mean ± SD; ***p < 0.001, compared with untreated control; ###p < 0.001, compared with T4 treatment.)
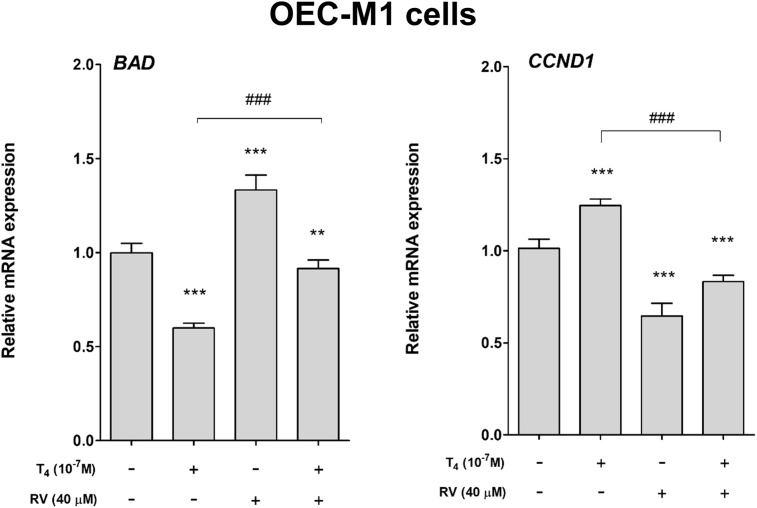
Parallel studies were conducted by using another human oral cancer OEC-M1 cell line. Cells were treated with 10−7 M T4, 40 μM resveratrol and their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for proliferative gene, CCND1 and pro-apoptotic gene, BAD. Thyroxine induced CCND1 expression but inhibited BAD expression (Fig. 2). On the other hand, resveratrol reversed thyroxine-induced effect in OEC-M1 cells (Fig. 2).
Figure 2.
Effect of thyroid hormone and resveratrol on expression of proliferative gene and pro-apoptotic gene in human oral cancer OEC-M1 cells. Human oral cancer OEC-M1 cells were treated with 10−7 M T4, 40 μM resveratrol and their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for proliferative gene, CCND1 and pro-apoptotic gene, BAD. N = 3. (Data are expressed as mean ± SD; **p < 0.01, ***p < 0.001, compared with untreated control; ###p < 0.001, compared with T4 treatment.)
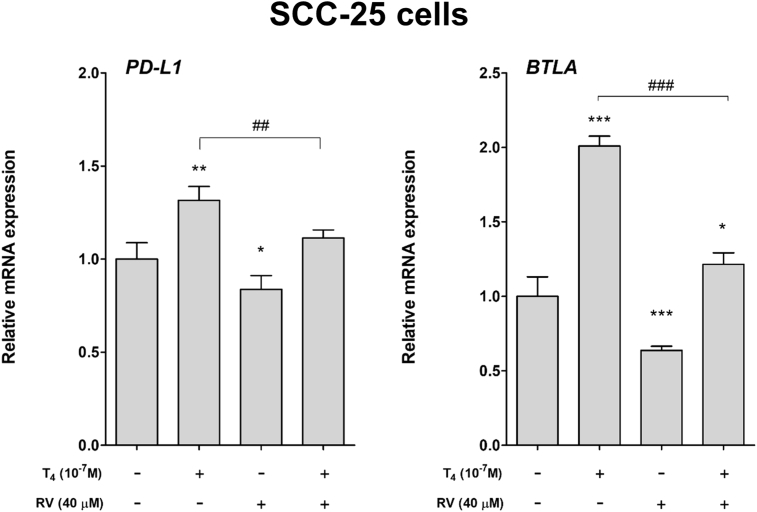
Effect of thyroid hormone and resveratrol on expression of checkpoint genes in human oral cancer cells. Human oral cancer SCC-25 cells were treated with thyroid hormone (10−7 M), resveratrol or their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for two checkpoint genes, PD-L1 and BLTA. Expression of PD-L1 and BLTA was significantly induced by thyroid hormone (T4) (Fig. 3).
Figure 3.
Effect of thyroid hormone and resveratrol on expression of checkpoint genes in human oral cancer SCC-25 cells. Human oral cancer SCC-25 cells were treated with thyroid hormone (10−7 M), resveratrol or their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for two checkpoint genes, PD-L1 and BLTA. N = 3. (Data are expressed as mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, compared with untreated control; ##p < 0.01, ###p < 0.001, compared with T4 treatment.)
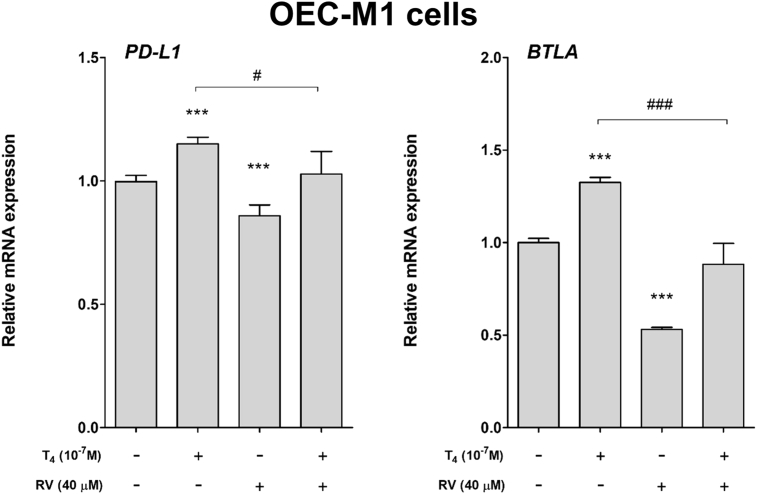
Parallel studies were conducted by using another human oral cancer OEC-M1 cell line. Cells were treated with 10−7 M T4, 40 μM resveratrol and their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for PD-L1 and BLTA. Thyroxine induced PD-L1 and BLTA expression (Fig. 4). On the other hand, resveratrol reversed thyroxine-induced effect in OEC-M1 cells (Fig. 4).
Figure 4.
Effect of thyroid hormone and resveratrol on expression of checkpoint genes in human oral cancer OEC-M1 cells. OEC-M1 cells were treated with thyroid hormone (10−7 M), resveratrol or their combination for 24 h. Cells were harvested and total RNA was extracted. qPCR was conducted for two checkpoint genes, PD-L1 and BLTA. N = 3. (Data are expressed as mean ± SD; ***p < 0.001, compared with untreated control; #p < 0.05, ###p < 0.001, compared with T4 treatment.)
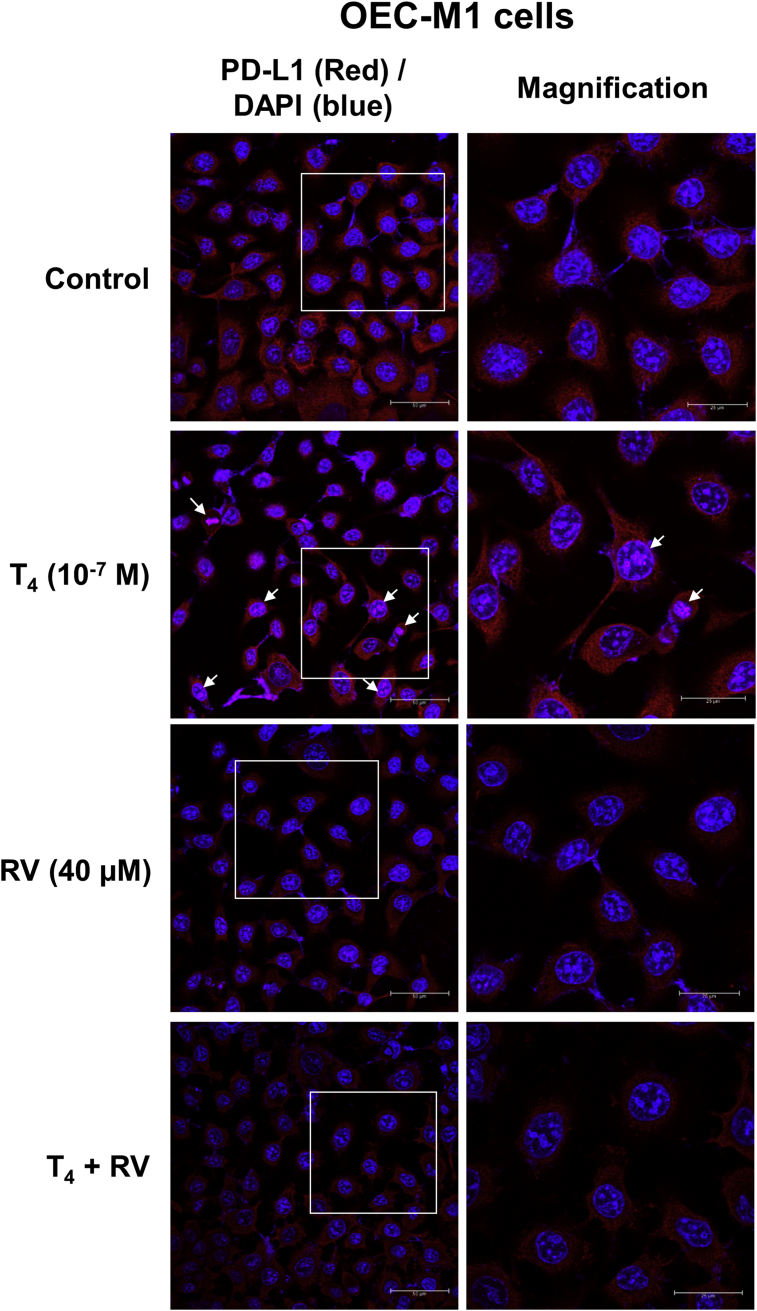
Resveratrol inhibits thyroxine-induced PD-L1 gene expression and nuclear accumulation in oral cancer cells. Thyroxine induces PD-L1 gene expression and PD-L1 protein accumulation. Thyroxine-induced PD-L1 can be blocked by co-treatment of resveratrol.19 To examine the effects of thyroxine and resveratrol on PD-L1 expression in oral cancer, OEC-M1 cells were treated with 10−7 M T4, 40 μM resveratrol or their combination for 24 h. Thyroxine increased PD-L1 nuclear accumulation. Resveratrol reduced constitutive expression of PD-L1 (Fig. 5). Interestingly, the upregulated PD-L1's nuclear accumulation by thyroxine was diminished in the presence of resveratrol (Fig. 5). These results indicated that resveratrol can not only inhibit thyroxine-induced PD-L1 expression but also block its nuclear accumulation when cells were co-incubated with thyroxine and resveratrol.
Figure 5.
Resveratrol inhibits thyroxine-induced PD-L1 nuclear accumulation in oral cancer cells. OEC-M1 cells were seeded in glass cover slide and were treated with 10−7 M T4, 40 μM resveratrol or their combination for 24 h. Cells were fixed for confocal microscopic analysis of PD-L1 expression (red color) and its nuclear accumulation (purple color, indicated by arrow marker). The nuclei were stained by DAPI as counter staining (blue color).
Discussion
Recently, we have shown that thyroxine induces PD-L1 expression and its protein accumulation in colorectal cancer, breast cancer, and ovarian cancer cells,8,12 Thyroid hormone has been shown to induce cancer cell growth in various types of cancers. From clinical view, the thyroid hormone axis is usually normal and thus activity of the PD-1/PD-L1 tumor cell self-defense system is in part due to endogenous thyroid hormone. In addition, thyroxine also induced the expression of PD-L1 and BTLA in oral cancer cells (Figure 2, Figure 4).
BTLA contains an immune-receptor tyrosine-based inhibitory motif (ITIM) and an immune-receptor tyrosine-based switch motif (ITSM), structurally similar to PD-1 and CTLA-4.20 Its ligand HVEM (also known as TNFRSF14) belongs to the tumor necrosis factor receptor (TNFR) superfamily. HVEM presents commonly on hematopoietic cells and on a variety of parenchymal cells such as breast, melanoma, esophageal, colorectal, and ovarian cancer cells.21, 22, 23, 24, 25 The combination of BTLA to cysteine-rich domains 1 (CRD1) of HVEM makes this pathway an important cross-talk between Ig and TNF superfamily.26,27 Furthermore, the BTLA/HVEM pathway appears to be a new possible approach of immune escape and is considered to be a critical factor in the physiological process of inflammation and tumorigenesis.
Thyroxine-induced expression of PD-L1 and BTLA was coincidental with increased expression of proliferative gene CCND1 (Figure 3, Figure 4) and down-regulated pro-apoptotic gene, BAD expression (Figure 3, Figure 4) in two oral cancer cell lines examined.
PD-1 and PD-L1 may have additional functions within tumor cells that are independent of the checkpoint are indicated by actions of a thyroid hormone analogue, l-thyroxine, on these checkpoint components.12 Estrogen has been shown to upregulate PD-L1 protein expression in ERα-positive endometrial and breast cancers cells to suppress immune functions of T cells in the tumor microenvironment.28 Another example of hormone-driven cancer progression via PD-L1 pathway is that 1,25-dihydroxyvitamin D (1,25D) has shown to be a direct transcriptional inducer of the human genes encoding PD-L1 and PD-L2 through the vitamin D receptor and suggests elevated vitamin D signaling in humans could suppress anti-tumor immunity.29
Interestingly, 17β-Estradiol does not increase PD-L1 mRNA transcription, but stabilized PD-L1 mRNA.28 On the other hand, vitamin D29 and thyroxine8,12,16 are able to induce PD-L1 expression. In addition, 17β-Estradiol's effects were only observed in estrogen receptor α (ERα)-positive Ishikawa and MCF-7 cells, but not in ERα-negative MDA-MB-231 cells.28 1,25-dihydroxyvitamin D (1,25D) has shown to induce PD-L1 via the vitamin D receptor.29 On the other hand, thyroxine-induced PD-L1 expression may be integrin αvβ3-dependent.12,16
Tumor cell-induced PD-L1 expression involves several intracellular signaling pathways linked to include nuclear factor (NF)-κB, ERK1/2, phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin (mTOR), and Janus kinase/signal transducers and activators of transcription (JAK/STAT). Estrogen increases expression of PD-L1 protein via activation of phosphoinositide 3-kinase (PI3K)/Akt pathway in Ishikawa and Michigan Cancer Foundation-7 (MCF-7) cells.28 Phosphoinositide 3-kinase and Akt inhibitors could block estrogen's effects. Thyroxine-induced PD-L1 expression is ERK1/2-dependent.12
Resveratrol has been shown to antagonize thyroid hormone-induced proliferation.16 Both thyroid hormone and resveratrol activate ERK1/2 by binding to cell surface integrin αvβ3.9,19 Via ERK1/2 activation resveratrol induces nuclear COX-2 accumulation, p53 phosphorylation leading to COX-2-phosphorylated p53-dependent apoptosis.11 On the other hand, thyroxine induces ERK1/2 activation to activate β-catenin-HMGA2-dependent proliferation.10 In addition, thyroid hormone induces PD-L1 expression and its protein PD-L1 traps inducible COX-2 in the cytosol in resveratrol-treated cells.19 Under physiological condition, resveratrol was able to retain PD-L1 in the cytosol (Fig. 5) or reduce thyroxine-induced PD-L1 nuclear accumulation (Fig. 5).
In summary, thyroxine induces expression of proliferative genes such as CCND1 and checkpoint genes such as PD-L1 and BTLA in oral cancer cells. On the other hand, resveratrol reduces expression of PD-L1, BTLA, and CCND1 but increase expression of pro-apoptotic genes such as BAD. How to manage the resveratrol concentration to overcome the stimulatory effect of thyroid hormone concentration in physiological micro-environment will be a big task for future therapeutic concern by using resveratrol in oral cancers.
Conflicts of interest
All co-authors declare no competing financial interests.
Acknowledgments
This work was supported by a grant from Shin Kong Wu Ho-Su Memorial Hospital Collaborating Program (Dr. Sheng-Yang Lee, SKH-TMU-102-09). It was also in part supported in part by Chair Professor Research Fund to Dr. Jacqueline Whang-Peng, by Chair Professor Research Fund to Dr. Kuan Wang, by the “TMU Research Center of Cancer Translational Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, and by general grant of Ministry of Science and Technology, Taiwan (Hung-Yun Lin, MOST107-2314-B-038-017).
References
- 1.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Canc. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder E.I., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin H.Y., Chin Y.T., Yang Y.C. Thyroid hormone, cancer, and apoptosis. Comp Physiol. 2016;6:1221–1237. doi: 10.1002/cphy.c150035. [DOI] [PubMed] [Google Scholar]
- 5.Murphy T.L., Murphy K.M. Slow down and survive: enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 6.Paulos C.M., June C.H. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Investig. 2010;120:76–80. doi: 10.1172/JCI41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan X., Li S., Gao H. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. OncoTargets Ther. 2017;10:919–926. doi: 10.2147/OTT.S128825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh M.T., Wang L.M., Changou C.A. Crosstalk between integrin alphavbeta3 and ERalpha contributes to thyroid hormone-induced proliferation of ovarian cancer cells. OncoTarget. 2017;8:24237–24249. doi: 10.18632/oncotarget.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H.Y., Hsieh M.T., Cheng G.Y. Mechanisms of action of nonpeptide hormones on resveratrol-induced antiproliferation of cancer cells. Ann N Y Acad Sci. 2017;1403:92–100. doi: 10.1111/nyas.13423. [DOI] [PubMed] [Google Scholar]
- 10.Nana A.W., Chin Y.T., Lin C.Y. Tetrac downregulates beta-catenin and HMGA2 to promote the effect of resveratrol in colon cancer. Endocr Relat Cancer. 2018;25:279–293. doi: 10.1530/ERC-17-0450. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T.M., Chin Y.T., Ho Y. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem Toxicol. 2018;112:67–75. doi: 10.1016/j.fct.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Lin H.Y., Chin Y.T., Nana A.W. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids. 2016;114:59–67. doi: 10.1016/j.steroids.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Lin H.Y., Tey S.L., Ho Y. Heteronemin induces anti-proliferation in cholangiocarcinoma cells via inhibiting TGF-beta pathway. Mar Drugs. 2018;16 doi: 10.3390/md16120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho Y., Sh Yang Y.C., Chin Y.T. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin alphavbeta3 and IGF-1R. Food Chem Toxicol. 2018;120:346–355. doi: 10.1016/j.fct.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Ho Y., Chen Y.F., Wang L.H. Inhibitory effect of anoectochilus formosanus extract on hyperglycemia-related PD-L1 expression and cancer proliferation. Front Pharmacol. 2018;9:807. doi: 10.3389/fphar.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S.J., Chin Y.T., Ho Y. Nano-diamino-tetrac (NDAT) inhibits PD-L1 expression which is essential for proliferation in oral cancer cells. Food Chem Toxicol. 2018;120:1–11. doi: 10.1016/j.fct.2018.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Nana A.W., Wu S.Y., Yang Y.S. Nano-Diamino-Tetrac (NDAT) enhances resveratrol-induced antiproliferation by action on the RRM2 pathway in colorectal cancers. Horm Cancer. 2018;9:349–360. doi: 10.1007/s12672-018-0334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin Y.T., Yang S.H., Chang T.C. Mechanisms of dihydrotestosterone action on resveratrol-induced anti-proliferation in breast cancer cells with different ERalpha status. OncoTarget. 2015;6:35866–35879. doi: 10.18632/oncotarget.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin Y.T., Wei P.L., Ho Y. Thyroxine inhibits resveratrol-caused apoptosis by PD-L1 in ovarian cancer cells. Endocr Relat Cancer. 2018;25:533–545. doi: 10.1530/ERC-17-0376. [DOI] [PubMed] [Google Scholar]
- 20.Haymaker C.L., Wu R.C., Ritthipichai K. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2015.1014246. e1014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T., Ye L., Han L., He Q., Zhu J. Knockdown of HVEM, a lymphocyte regulator gene, in ovarian cancer cells increases sensitivity to activated T cells. Oncol Res. 2016;24:189–196. doi: 10.3727/096504016X14641336229602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T., Sho M., Yasuda S. HVEM expression contributes to tumor progression and prognosis in human colorectal cancer. Anticancer Res. 2015;35:1361–1367. [PubMed] [Google Scholar]
- 23.Migita K., Sho M., Shimada K. Significant involvement of herpesvirus entry mediator in human esophageal squamous cell carcinoma. Cancer. 2014;120:808–817. doi: 10.1002/cncr.28491. [DOI] [PubMed] [Google Scholar]
- 24.Li D., Fu Z., Chen S. HVEM gene polymorphisms are associated with sporadic breast cancer in Chinese women. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasero C., Speiser D.E., Derre L., Olive D. The HVEM network: new directions in targeting novel costimulatory/co-inhibitory molecules for cancer therapy. Curr Opin Pharmacol. 2012;12:478–485. doi: 10.1016/j.coph.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Sedy J.R., Gavrieli M., Potter K.G. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez L.C., Loyet K.M., Calemine-Fenaux J. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Huang F., Mei J. Posttranscriptional control of PD-L1 expression by 17beta-estradiol via PI3K/Akt signaling pathway in eralpha-positive cancer cell lines. Int J Gynecol Cancer. 2017;27:196–205. doi: 10.1097/IGC.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrov V., Bouttier M., Boukhaled G. Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J Biol Chem. 2017;292:20657–20668. doi: 10.1074/jbc.M117.793885. [DOI] [PMC free article] [PubMed] [Google Scholar]