Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen causing infections in a variety of plant and animal hosts. The gene mcpB, part of the chemosensory gene cluster II, encodes a soluble chemoreceptor whose function remains unknown. Previous studies show that the cheB2 gene, also located in the chemosensory cluster II, is involved in a specific response during infection and it is required for full pathogenicity of P. aeruginosa. To determine whether the McpB (or Aer2) chemoreceptor is involved in virulence processes, we generated a mcpB mutant and tested its phenotype using a virulence-measuring system. This system was developed by our group and is based on different bioassays using organisms living at different soil trophic levels, including microbial, nematode, arthropod, annelid, and plant model systems. The deletion of mcpB resulted in an attenuation of bacterial virulence in different infection models, and wild-type virulence was restored following genetic complementation of the mutant strain. Our study indicates that the McpB chemoreceptor is linked to virulence processes and may constitute the basis for the development of alternative strategies against this pathogen.
Subject terms: Environmental microbiology, Soil microbiology, Biotechnology
Introduction
The Gram-negative bacterium Pseudomonas aeruginosa is a widespread opportunistic human pathogen responsible for multiple hospital-acquired infections, mainly in immunocompromised and cystic fibrosis patients1,2. In addition, the host spectrum of P. aeruginosa is not restricted to humans since it was also shown to infect different animals and plants3–8.
To adapt efficiently to environmental changes, P. aeruginosa has evolved sophisticated regulatory networks that include one- and two-component systems as well as chemosensory signalling pathways9. The action of chemosensory pathways is initiated by sensing signal molecules by chemoreceptors10 (Fig. 1A). The molecular stimulus resulting from chemoreceptor activation is transmitted to the histidine kinase CheA homologue. The sensitivity of a chemosensory pathway is adjusted by chemoreceptor methylation or demethylation catalysed by the CheR methyltransferase and CheB methylesterase homologues, respectively. The signal output of chemosensory pathways is alterations in the level of phosphorylated CheY homologue (CheY-P). Most chemosensory pathways appear to be involved in chemotaxis11, in those cases CheY-P binds to the flagellar motor altering its activity. Other chemosensory pathways are associated with type IV pili-mediated taxis or have been shown to regulate alternative cell functions, such as the modulation of the intracellular levels of the bacterial second messengers cAMP and c-di-GMP11–13. Although chemotaxis chemoreceptors having been studied extensively, information on chemoreceptors carrying out alternative functions is scarce.
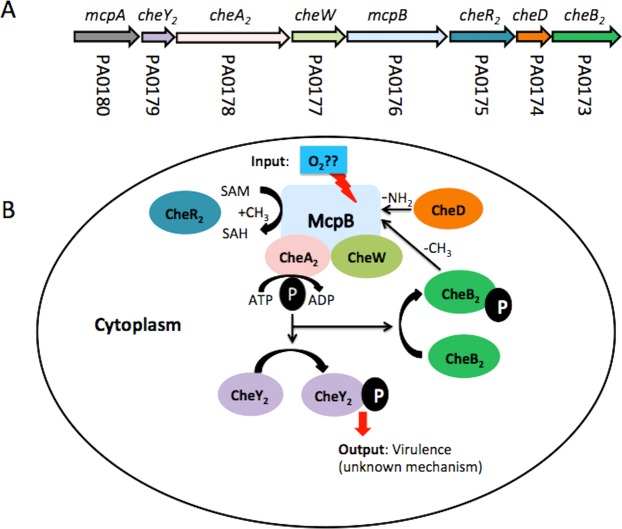
Figure 1.
Proposed model of the Che2 signaling pathway. Panel A shows the genes that form part of the gene cluster II encoding the signaling protein of the Che2 pathway. Panel B shows the hypothetical mechanism of the Che2 pathway.”P” phosphoryl group, SAM: S-adenosylmethionine, SAH: S-adenosylhomocysteine. Note: although the mcpA gene form part of gene cluster II multiple pieces of evidence suggests that this chemoreceptor may feed into the Che pathway24.
Proteins encoding chemosensory signalling proteins in P. aeruginosa PAO1 are encoded by five different gene clusters and form four different chemosensory pathways9,14. Gene cluster II encodes proteins of the Che2 pathway, which was originally associated with aerotaxis15 but its function remains controversial, since this role in aerotaxis could not be reproduced in subsequent studies14,16.
Cluster II consists of eight genes encoding the chemosensory proteins CheB2, CheD, CheR2, CheW2, CheA2 and CheY, as well as the chemoreceptors McpB (Aer2) and McpA14 (Fig. 1B). Proteins encoded by cluster II have been shown to form complexes that co-localize at cell poles, and the requirement of McpB for the formation of these complexes has been demonstrated16. The McpB lacks transmembrane domains and it has been shown to be a soluble chemoreceptor17. The McpB contains a C-terminal pentapeptide (GWEEF) that enables the specific binding of CheR2 and chemoreceptor methylation17. The heterologous expression of McpB in a chemoreceptor-deficient strain of Escherichia coli showed this chemoreceptor to be responsible for mediating repellent chemotactic responses to oxygen, nitric oxide, and carbon monoxide18. The PAS-type ligand binding domain of McpB contains bound heme18 and a recent study showed that oxygen is the native ligand of McpB19.
Chemotaxis has been shown to play an important role in the pathogenicity of a broad range of bacterial pathogens20 and several reports have correlated chemotaxis with virulence of P. aeruginosa4,21–23. One of these studies has linked the Che2 pathway of P. aeruginosa PA14 with pathogenicity because a mutant defective in cheB2 showed attenuated virulence in Caenorhabditis elegans and murine lung-infection models4. A recent bioinformatics study suggests that McpB is the sole chemoreceptor feeding into the Che2 pathway24. The notion that the Che2 pathway is involved in virulence stems from high-throughput screening experiments of bacterial mutants that has resulted in the identification of the cheB2 mutant as the most severely affected mutant; a finding that was verified by experimentation in mice and complementation studies4. Very little is known on the Che2 pathway nor on the specificity of the function of the four CheB paralogues of P. aeruginosa. No information is available on the nature of the pathway output, nor have the receptor(s) that feed into this pathway been identified experimentally.
In the present study we have used multiple bioassays to evaluate the involvement of McpB in bacterial virulence. These assays were based on a diverse range of model organisms recently described to determine the virulence levels of bacterial strains25. Our results show that McpB plays a critical role in the virulence and pathogenesis of P. aeruginosa PAO1 in a variety of hosts.
Results and Discussion
P. aeruginosa PAO1 ΔmcpB shows attenuated virulence in C. elegans
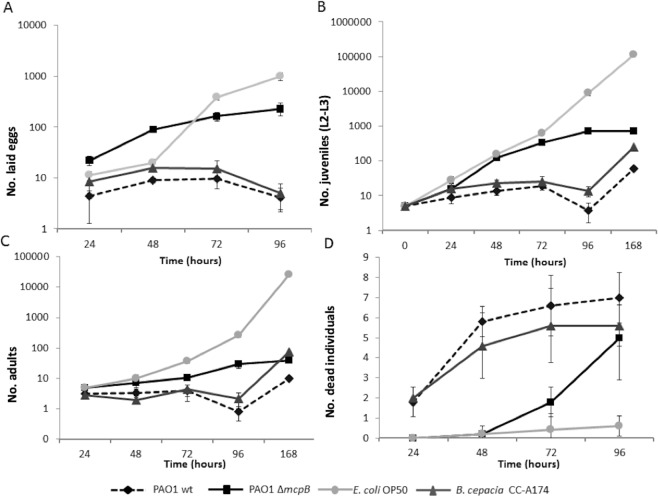
Virulence factors responsible for the killing of C. elegans have also been found relevant for virulence in mammalian hosts26,27. Previous studies have shown that P. aeruginosa is virulent in a C. elegans model4,28,29, a model commonly used to study bacterial virulence mechanisms29–31. To test the role of McpB in the virulence properties of P. aeruginosa PAO1, we constructed an in-frame deletion mutant mcpB-deficient (PAO1 ΔmcpB). Subsequently, we evaluated the effects of the wild-type P. aeruginosa PAO1 (PAO1 wt) and ΔmcpB on the number of eggs laid, number of juvenile and adult nematodes, and death rates25,32. Our results showed that the ΔmcpB exhibited lower nematicidal activities compared to the PAO1 wt strain (Fig. 2). The number of eggs laid by the nematodes in the presence of the ΔmcpB strain resulted in an approximately 10- and 60-fold increase after 24 h and 96 h, respectively, compared to the parental strain (PAO1 wt) using ANOVA (P ≤ 0.05; Fig. 2A). Furthermore, the number of juvenile and adult nematodes was significantly higher when PAO1 ΔmcpB was used to feed C. elegans compared to the PAO1 wt strain. Therefore, an increase of approximately 30- and 100-fold in the number of adult and juvenile nematodes, respectively, was observed when PAO1 ΔmcpB was used as the feeding source after 96 h compared to the those fed with the PAO1 wt strain (P ≤ 0.05, Fig. 2B,C). Additionally, the number of dead nematodes throughout the experiment was significantly lower (P ≤ 0.05) when worms were fed on saturated cultures of the ΔmcpB strain grown on potato dextrose agar (PDA) plates after 24 h than when they were fed with the PAO1 wt strain (Fig. 2D).
Figure 2.
Virulence of P. aeruginosa strains against C. elegans. The number of eggs laid (A), juveniles at the larval stage L2-L3 (B), adults (C) and dead C. elegans individuals (D) are shown when cultured with P. aeruginosa strains. B. cepacia CC-A174 and E. coli OP50 were used as pathogenic and non-pathogenic control strains, respectively. Means and standard deviations of three biological replicates are shown.
Then, we detected a 4- and 10-fold increase in the survival of the adult and juvenile nematodes, respectively, when they were fed with PAO1 ΔmcpB compared to those fed with the PAO1 wt strain (last sampling time, Fig. 2B,C). The virulence properties of P. aeruginosa PAO1 was similar to that observed in Burkholderia cepacia CC-A174, a strain with nematicidal properties used as an internal control.
Wild-type virulence is restored in PAO1 ΔmcpB by in trans expression of mcpB
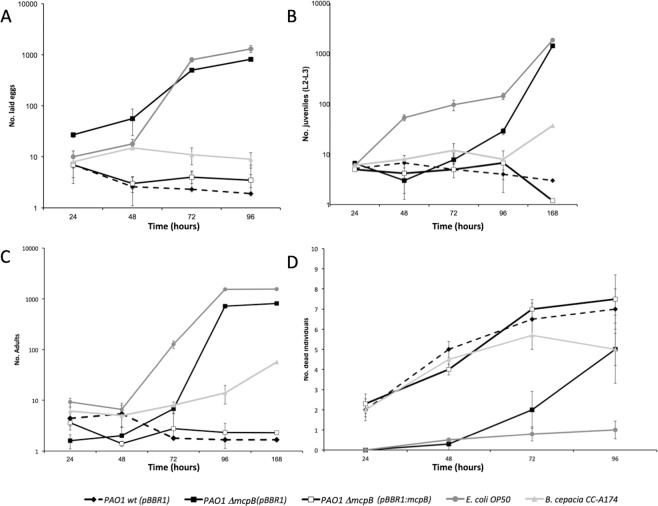
To confirm the role of McpB in the observed phenotypes, the mutation in mcpB was functionally complemented by the expression of the mutated gene in trans using the broad host range pBBR1MCS-based plasmid pBBR1:mcpB. Subsequently, the above reported experiments were repeated with the complemented mutant and compared with PAO1 wt and PAO1 ΔmcpB strains bearing the pBBR1MSC-2 backbone plasmid. The results showed that the in trans expression of mcpB restored wt nematicidal properties in the PAO1 ΔmcpB (pBBR1:mcpB) strain using ANOVA (P ≤ 0.05, Fig. 3). We analyzed plasmid stability in control experiments showing that the plasmid in all P. aeruginosa strains was highly stable and independent of the presence of antibiotic (data not shown).
Figure 3.
In trans expression of mcpB restores P. aeruginosa PAO1 ΔmcpB virulence in C. elegans. Shown are the number of eggs laid (A), juveniles (B), adults (C), and dead individuals (D). Both, P. aeruginosa wt strains PAO1 (solid diamonds and back dotted line) and P. aeruginosa PAO1 ΔmcpB (solid squares and black line) harbour pBBR1MCS-2 (named as pBBR1). McpB complemented P. aeruginosa PAO1 ΔmcpB (white squares and black line) harbours pBBR1:mcpB plasmid. E. coli OP50 (circles and dark grey lines) was used as non-pathogenic control and B. cepacia CCA-174 (triangles and light grey lines) was used as pathogenic control. Means and standard deviations of three biological replicates are shown. For statistic analysis ANOVA (P ≤ 0.05) was used for comparison of datasets obtained at the end of each experiment.
Despite the molecular mechanisms of signalling through the Che2 pathway have not been established, currently available data strongly suggest that oxygen is the natural ligand serving as input19. In addition to CheB2, we show here that McpB is involved in virulence. This strongly suggests that McpB feeds into this pathway and that it may be the target for CheB2 action. This interpretation is consistent with Garvis et al. (2009)4, showing a reduction in virulence of the cheB2 mutant. These authors have shown that a mutant defective in the methylesterase-encoding gene cheB2 results in reduced virulence in C. elegans and murine models. Non-mammalian models have been used to study infections caused by P. aeruginosa. One such model is based on the interaction and subsequent killing of the bacteria-feeding nematode C. elegans allowing to identify bacterial virulence factors and host responses to them28.
Role of McpB in the insecticidal properties against Chrysoperla carnea and Adalia bipunctata
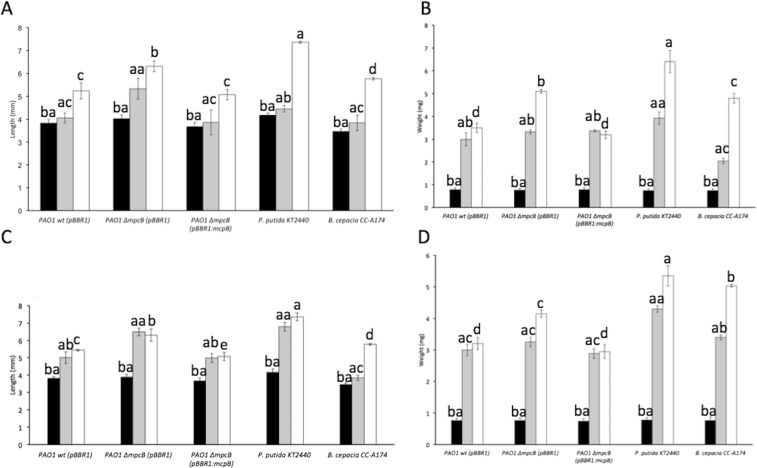
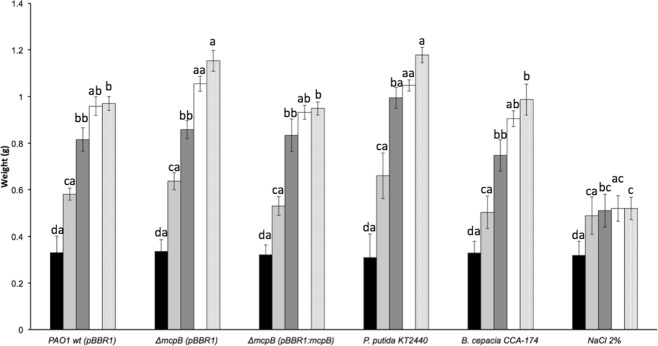
P. aeruginosa is an universal pathogen that, apart from humans, was also shown to infect for example plants, fungi or insects33. We used two insect models, green lacewings (C. carnea) and ladybirds (A. bipunctata), to test the implication of McpB in the insecticidal properties of P. aeruginosa. These insects are considered efficient biological control agents and have been previously used to validate the virulence features of different proteobacteria and bacterial pathogens in humans34,35. In this test, the bacterial strains were concentrated and supplied in a semidry format mixed with the insect feed. Bacterial virulence was evaluated by scoring the number of dead insects and changes in weight and length after feeding them with PAO1 wt or PAO1 ΔmcpB strains using approximately 15 insects per strain or condition. The insecticidal strain B. cepacia CC-A174 and the non-virulent strain P. putida KT2440 were included in the assay as controls. Our results have shown that when C. carnea and A. bipunctata were fed with P. putida KT2440, the weight and length of the insects showed similar values to those found when the insects were fed with trehalose (control) in the absence of bacteria. In contrast, the length and weight of the insects was reduced when they were fed with the PAO1 wt. Both, weight and length of the insects were statistically higher (P ≤ 0.05) when a PAO1 ΔmcpB was used to feed the insects than when PAO1 wt was used, despite not finding differences at time zero among the different treatments. This phenotype was complemented by the expression of the mcpB gene in trans in the PAO1 ΔmcpB (pBBR1:mcpB) (Fig. 4).
Figure 4.
Length and weight of A. bipunctata and C. carnea individuals fed with different bacterial strains. Virulence assays against A. bipunctata (A,B) and C. carnea (C,D) are shown. Measurements were made 0 (dark bars), 7 (grey bars), and 14 days (white bars) after feeding. Means and standard deviations of five biological replicates are shown. Data were submitted to an ANOVA test (posthoc Tukey) and significant differences (P ≤ 0.05) are highlighted by different single letters (after 14 days), double letters starting with a (after 7 days), and double letters starting with b (time zero). B. cepacia and P. putida KT2440 were used as pathogenic and non-pathogenic bacterial controls, respectively.
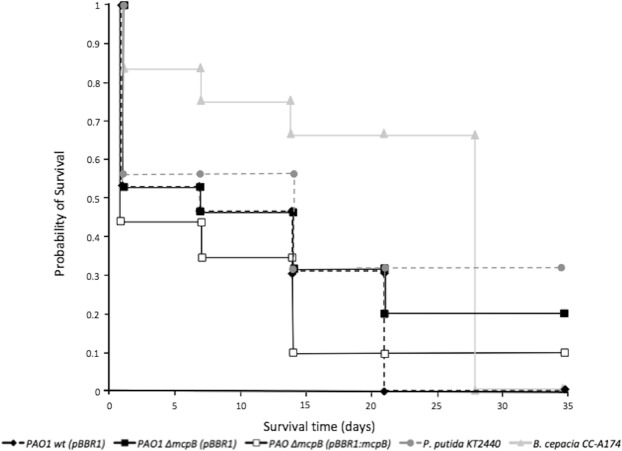
The survival of C. carnea individuals in the presence of P. aeruginosa PAO1 strains has been also analyzed. PAO1 wt was capable of killing over 80% (12 out of 15) of the individuals after 28 days, whereas PAO1 ΔmcpB was severely impaired in virulence and less than 46% of the insects (7 out of 15) were killed after 28 days. When the mcpB-restored strain (PAO1 ΔmcpB (pBBR1:mcpB)) was used, results were similar to those of the parental strain (13 killed out of 15 insects). This complemented strain showed higher virulence than B. cepacia for most of the sampling times. The Kaplan-Meier method was used following the description of Bewick et al. (2004) to estimate the survival. The comparison of the survival curves of PAO1 wt and PAO1 ΔmcpB strains using the log rank tests (Bewick et al., 2004) showed significant differences between both strains (Fig. 5). Statistically significant differences between the PAO1 wt (pBBR1) and PAO1 ΔmcpB (pBBR1:mcpB) and PAO1 ΔmcpB strains has been also observed when experiments were conducted using A. bipunctata as the model organism (Fig. S1). Burrowes et al. (2006) have reported that the post-transcriptional regulator RsmA exerts control over cheB2, since its expression was found to be reduced 10-fold in a rsmA mutant background36. RsmA has been reported to act in conjunction with small non-coding RNA to regulate the expression of multiple virulence genes, including the quorum-sensing lasI and rhlI genes37. The authors proposed that the Che2 signalling pathway may be regulated by RsmA, which constitutes further support to the suggestion that cluster II is involved in virulence36. Biofilm tests performed showed that the mcpB mutant strain did not alter the biofilm production despite of finding similar number of CFUs for all different strains (data not shown). The concentrated format for the supply of bacterial cells for these insect tests could in theory facilitate a quorum-sensing response, therefore we postulate that the involvement of McpB in virulence occurs in a different manner than the production of biofilms and do not discard a quorum-sensing depending response.
Figure 5.
Survival of C. carnea fed with different bacterial strains. The probability of survival of C. carnea fed with P. aeruginosa PAO1 (PAO1 wt (pBBR1)) (solid diamonds and black dotted lines), its mcpB mutant PAO1 ΔmcpB (pBBR1)) (solid squares and black lines) (both strains carrying the pBBR1MCS-2 plasmid), the complemented mcpB strain carrying the pBBR1:mcpB plasmid (PAO1 ΔmcpB (pBBR1:mcpB)) (white squares and black lines) as well as for B. cepacia CC-A174 (pathogenic control) (triangles and light grey lines), P. putida KT2440 (non-pathogenic control) (circles and dotted dark grey lines) are shown. The figure shows a representative experiment with three biological replicates. For statistic analysis ANOVA (P ≤ 0.05) was used for comparison of datasets obtained at the end of each experiment.
The deletion of mcpB gene causes a reduction in the ecotoxicity towards earthworms
Eisenia foetida earthworms present a highly developed immune system38. These worms have developed efficient defence mechanisms against microbes that they either ingest or enter their bodies after injury39–41. However, several soil-related bacterial pathogens were found to affect the developmental and reproductive capacity of E. foetida42. Since E. foetida lives underground we could simulate environments of different gradients of oxygen limitations.
To study the effect of P. aeruginosa strains on E. foetida development, we measured weight gain, length gain and the reproductive efficiency (number of juvenile worms and oothecas) of E. foetida after adding different freeze-dried bacterial strains in trehalose to their diet. Our results showed no difference in the length increase between the earthworms exposed to PAO1 wt and those exposed to PAO1 ΔmcpB (data not shown). However, the annelids gained more weight when they were fed with PAO1 ΔmcpB compared to the earthworms exposed to PAO1 wt. Furthermore, a similar weight gain was observed when these worms were fed with the mcpB-restored strain PAO1 ΔmcpB (pBBR1:mcpB), compared to the PAO1 wt (pBBR1), despite not finding differences at time zero among the different treatments (Fig. 6).
Figure 6.
Weight of E. foetida fed with different bacterial strains or in the presence of trehalose or NaCl. The weight of E. foetida individuals at 0 (black bars), 7 (light grey bars), 14 (dark grey bars), 21 (white bars), and 28 days (dotted bars) after feeding is shown. B. cepacia and NaCl 2% (w/v) were used as pathogenic and toxic controls, respectively. P. putida KT2440 was used as non-pathogenic control. The means and standard deviations of 5 individuals from three biological replicates are shown. Data were submitted to an ANOVA test and significant differences (P ≤ 0.05) and posthoc Tukey are highlighted by different single letters (after 28 days), double letters starting with a (after 21 days), double letters starting with b (after 14 days), double letters starting with c (after 7 days) and double letters starting with d (at time zero).
The number of eggs laid, and therefore the reproductive efficiency of E. foetida, significantly increased in the presence of PAO1 ΔmcpB compared to the number of eggs laid by PAO1 wt strain and PAO1 ΔmcpB (pBBR1:mcpB) strain despite not finding differences at time 7 days among the different bacterial treatments (supplementary Fig. S2A). In addition, the exposure of annelids to the PAO1 ΔmcpB strain resulted in more than 4-fold increase in the number of juveniles compared to PAO1 wt (ANOVA P ≤ 0.05) at 21 and 28 days (supplementary Fig. S2B), although the same number of juveniles were used at time zero for all different treatments.
A mutant in the methylesterase-encoding gene cheB1, which is part of the cluster I, showed no effect on virulence but chemotaxis and motility were significantly attenuated4. Altogether, these results suggest that the Che pathway plays the dominant role in P. aeruginosa chemotaxis and flagellar motility, whereas cluster II is associated with a chemosensory pathway activated during infection due to the sensing of an unknown signal4, most probably oxygen19. In fact, several studies have shown a link between oxygen levels and virulence of P. aeruginosa43,44. Bioinformatics studies have predicted that a single chemoreceptor, McpB, feeds into the Che2 pathway24, which is consistent with studies showing that the deletion of the mcpB and cheB2 genes reduce bacterial virulence4. These results together with previous studies conducted by different groups, suggest that the Che2 pathway plays a role in P. aeruginosa virulence through oxygen sensing19. Accordingly, studies performed on different bacteria have established a correlation between oxygen sensing and virulence20. For example, the plant pathogen Ralstonia solanacearum presents two aerotaxis receptors, termed Aer1 and Aer2. Mutant strains defective in aer2 or aer1/aer2 have a delayed development of wilt disease in tomato plants upon infection with the R. solanacearum mutant strains45.
Effect of metabolites secreted by P. aeruginosa strains on bacterial communities, and crustaceans
Bioactive metabolites produced and released into the environment can alter the composition of microbial communities and their interactions46–48. In the particular case of P. aeruginosa as an animal pathogen, these metabolites can affect host microbiomes, which may result in the development of diseases associated with microbiome alterations49. Accordingly, we first evaluated the effect of filter-sterilized supernatants of P. aeruginosa strains on the viability of E. coli MC4100, the bacterial metabolism using Microtox®, and on Daphnia magna as a representative of the aquatic ecosystems using DaphToxKit®. The bioassays showed no statistically significant differences in E. coli MC4100 survival, in bacterial metabolism using Microtox®, nor on D. magna survival using DaphToxKit®, when these organisms were exposed to the supernatants of PAO1 ΔmcpB (pBBR1) strain compared to the supernatants of PAO1 wt (data not shown).
Taken together, our results indicate that the deletion of mcpB did not cause any measurable changes in the pool of toxic bioactive metabolites of P. aeruginosa PAO1.
P. aeruginosa PAO1 ΔmcpB strain does not affect pepper plants
Some studies describe virulence pathways in P. aeruginosa that are required for the infection of human and plant hosts50. For this reason, virulence assays using pepper plants (Capsicum annuum) were conducted. However, no statistical differences were found regardless the strain used (data not shown).
In our study we have used models based on the exposure to bacterial supernatants (such as the DaphToxKit®, Microtox®, or the test of viability of E. coli MC4100) and models based on ingestion of the strain (i.e. C. carnea, or A. bipunctata). Interestingly, changes were only observed when the strain was supplied as a component of the organisms’ diet, whereas mcpB deletion had no effect when assays evaluated the production of bioactive molecules or toxins secreted to the extracellular medium. This implies that Che2-mediated virulence requires the direct contact between the strain and host cells. Further research is needed to elucidate the output of the Che2 pathway and to understand the molecular mechanism underlying the role of McpB in virulence.
The deletion of mcpB increases the environmental and human safety index (EHSI) of P. aeruginosa
We have previously proposed the use of the EHSI, an index that combines a set of mortality, reproduction, and development tests, as a value indicative of the virulence potential of a bacterial strain25. This index ranges from 0 to 100, with higher values representing higher safety of the bacterial strain25. Therefore, the EHSI was used to quantify the differences between the pathogenic capabilities of PAO1 wt (pBBR1) and PAO1 ΔmcpB (pBBR1) (Table 1).
Table 1.
Summary of results obtained with different animal models for the wild-type (PAO1 wt (pBBR1)), mcpB mutant (ΔmcpB (pBBR1)) and complemented mutant (ΔmcpB (pBBR1:mcpB)) strains of P. aeruginosa PAO1.
| Bioassay | PAO1 wt (pBBR1) | ΔmcpB (pBBR1) | ΔmcpB (pBBR1:mcpB) |
|---|---|---|---|
| EHSI | |||
| E. coli MC4100 | 5.00 | 5.00 | 5.00 |
| Microtox® (V. fischeri) | 1.25 | 1.25 | 1.25 |
| C. elegans | 0 | 6.00 | 0 |
| C. carnea | 3.18 | 6.75 | 2.18 |
| A. bipunctata | 4.12 | 6.75 | 4.12 |
| E. foetida | 8.75 | 10.62 | 8.75 |
| D. magna | 1.87 | 1.87 | 1.87 |
| C. annuum | 4.00 | 4.00 | 4.00 |
| Final score | 28.17 | 42.24 | 27.17 |
Values were obtained using standard values proposed by Vilchez et al. (2016).
These data were used to calculate the Environmental and Human Safety Index (EHSI) following the protocol reported by25.
Although both PAO1 strains, wt and ΔmcpB, showed an EHSI indicative of pathogenicity, the PAO1 ΔmcpB showed a higher safety score than the PAO1 wt strain. This result indicates an attenuation in the bacterial virulence in the PAO1 ΔmcpB strain compared to the PAO1 wt strain. This analysis also revealed that complementation of the mutant strain using the pBBR1:mcpB plasmid reduced this index, indicative of a reduction in safety (Table 1). In this context, we established a link between the chemosensory cluster II of P. aeruginosa PAO1 and the virulence of the strain by using a panel of virulence assays employing different model organisms which can be used to calculate the EHSI25. In addition to the C. elegans and the pepper plant tests we have also included an assay based on E. foetida. The strong immune system of the earthworm allows us to discriminate between larger effects due to different pathogenic mechanisms. Furthermore, we have also included more sensitive systems, such as DaphToxKit®, due to the higher sensitivity of D. magna to toxins and virulence factors.
Conclusions
We have designed a method to numerically quantify the relevance of potential virulent factors involved in the process of pathogenesis of P. aeruginosa PAO1 based on a panel of different bioassays. The index called Environmental and Human Safety Index (EHSI) derived from PAO1 wt increased from 28 up to 42 when the mcpB gene was in-frame deleted, while the in trans complementation of the mutation reduced it back down to 27 showing that the role of McpB in pathogenesis is not specific to a given model but was observed in species as diverse as C. elegans, E. foetida or the insects A. bipunctata and C. carnae. The action of McpB requires the ingestion of the pathogen since exposure to bacterial supernatants had no effect on D. magna, E. coli or V. fisheri. The simplicity of this approach can be easily extended to test other proteins or genes involved in pathogenesis for comparison of the virulence relevance of each molecule and determine the most effective targets at fighting a relevant pathogen as P. aeruginosa PAO1, as well as to other pathogens.
Materials and Methods
Organisms, culture media, and growth conditions of bacteria
Organisms used in this study are listed in Table 2. Bacterial strains used in the bioassays were routinely grown on tryptic soy broth (TSB medium: 17 g tryptone L−1, 3 g phytone L−1, 5 g NaCl L−1, 2.5 g K2HPO4 L−1, and 2.5 g glucose L−1), except for nematode bioassays where potato dextrose medium (PDA: 4 g potato extract L−1, 20 g dextrose L−1 and 15 g agar powder L−1) was used. The growth temperature was 30 °C, except for P. aeruginosa PAO1 and its variants, which were cultured at 37 °C. For mutant construction, P. aeruginosa was grown on Luria-Bertani medium (5 g yeast extract L−1, 10 g bactotryptone L−1 and 5 g NaCl L−1) and in basal M9 medium supplied with 1 mM MgSO4, 6 mg L−1 Fe-citrate, as previously described51. For plasmid stability assays, the bacterial strains were plated on tryptone soy agar medium (TSA: 15 g agar L−1, 15 g casein peptone (pancreatic) L−1, 5 g NaCl L−1 and 5 g soy peptone (papainic) L−1). When appropriate, antibiotics were used at the following final concentrations (in µg mL−1): ampicillin, 100; kanamycin, 25 (E. coli strains) and 100 (P. aeruginosa strains); streptomycin, 50 (E. coli strains) and 400 (P. aeruginosa strains). Sucrose was added to a final concentration of 10% (w/v) when required to select derivatives that had undergone a second crossover event during marker-exchange mutagenesis.
Table 2.
Organisms used in this study.
| Bacteria/Nematodes/Arthropods/Annelids | Genotype, relevant characteristics and usesa | Reference or source |
|---|---|---|
| E. coli MC4100 | F- araDI39 Δ(argF-lac) U169 rpsL150 relAl flb5301 deoCI ptsF24 rbsR. Used to evaluate the presence of antibiotic compounds in supernatants of PAO1 strains. | 56 |
| E. coli OP50 | Uracil auxotroph. Used to feed C. elegans. | 57 |
| E. coli β2163 | KmREmR; F− RP4-2-Tc::Mu ΔdapA::(erm-pir). | 58 |
| E. coli DH5α | supE44 lacU169 (Ø80lacZΔ M15) hsdR17 (rk-mk-) recA1 endA1 gyrA96 thi-1 relA1. | 59 |
| B. cepacia CC-Al74 | Wild type. Risk Group 2 bacteria and proposed as plant growth promoting rhizobacteria (PGPR). Used as a pathogenic control in virulence assays. | 60 |
| P. putida KT2440 | Wild type; Risk Group 1 bacteria and PGPR. Used as negative control in virulence assays. | 61 |
| V. fischeri ATCC 49387 | Wild type; Used as bioluminescent strain in MicroTox® assays. | 62 |
| P. aeruginosa PAO1 | Wild type pathogenic strain. | 9 |
| P. aeruginosa ΔmcpB | In-frame deletion mutant in mcpB (1116 bp Δ). | This study |
| C. elegans Bristol strain N2 | Nematode used in virulence assays. Provided by the Laboratory of Nematology, National Museum of Nature Sciences-CSIC (Madrid, Spain) | Navas et al., 2007 |
| A. bipunctata | Arthropod used in virulence assays. Supplied by ControlBio Co. (Almería, Spain). | Ref. CBi K04884 |
| C. carnea | Arthropod used in virulence assays. Supplied by ControlBio Co. (Almería, Spain). | Ref. CBi 124 K04280 |
| E. foetida | Annelid used in virulence assays. Supplied by Lombriventa (Gerona, Spain). | 63 |
| D. magna | Crustacean used in virulence assays. Supplied by Daphtoxkit™ (Creasel, Belgium). | 64 |
Construction of in-frame deletion mutant of PAO1 ΔmcpB
Oligonucleotides and plasmids used in this study are listed in Tables S1, S2, respectively. A mutant defective in mcpB (PA0176) was constructed using homologous recombination. A derivative plasmid of the suicide vector pKNG101 was used for this purpose. The plasmid for the construction of the in-frame deletion mutant was constructed by amplifying the upstream and downstream flanking regions of mcpB. The resulting PCR products were digested with EcoRI and BamHI or BamHI and HindIII for the upstream and downstream regions of mcpB, respectively. These products underwent a three-way ligation into pUC18Not in order to generate plasmid pUC18:ΔmcpB, previously to be cloned into the NotI site of the marker exchange vector pKNG101. The sequence cloned into the resulting plasmid, called pKNG:ΔmcpB, was confirmed by DNA sequencing and it carried an in-frame deletion of mcpB gene for the replacement of wt gene in the chromosome. For the construction of the ΔmcpB mutant strain, biparental conjugations were performed as previously described52. Briefly, in a biparental mating, 100 µl of overnight cultures of P. aeruginosa PAO1 and E. coli β2163 harbouring pKNG:ΔmcpB were mixed, collected by centrifugation, resuspended in 30 µl of fresh Luria Broth (LB), and spotted on an LB agar plate supplemented with 300 µM 2,6-diaminopimelic acid (DAPA). After overnight incubation at 37 °C, cells were scraped off the plate and resuspended in 1 mL of LB. Serial dilutions were plated on LB agar medium containing 400 µg mL−1 streptomycin. DAPA was not added to the LB agar medium plates to avoid E. coli donor growth. We added sucrose to a final concentration of 10% (w/v) to select derivatives that had undergone a second crossover event during marker-exchange mutagenesis. Final mutants lacking the mcpB gene were confirmed using PCR and sequencing.
Plasmid construction for genetic complementation assays
For the construction of the complementing plasmid, a full copy of the mcpB gene was amplified by PCR using the primers mcpB-compl-NdeI-F and mcpB-compl-EcoRI-R listed in Table S1. The resulting fragment was digested with NdeI and EcoRI and cloned into the same sites in pBBR1MCS2_START53 to generate pBBR1:mcpB. The insert was confirmed by PCR and sequencing, and pBBR1:mcpB was used to transform the mcpB defective mutant by electroporation.
Stability assays of plasmids pBBR1 and pBBR1:mcpB in immobilized P. aeruginosa PAO1 strains
To perform bacterial virulence assays using C. elegans, the stability of the pBBR1 and pBBR1:mcpB plasmids in P. aeruginosa PAO1 strains were tested in the absence of antibiotic. Briefly, P. aeruginosa PAO1 strains were cultured overnight in TSB medium supplemented with kanamycin (50 µg mL−1) at 37 °C in an orbital shaker at 200 r.p.m. Subsequently, 200 µl from each culture medium were spread on PDA plates and incubated at 37 °C for 5 h until a thin growth film was visible. Plates were kept at 20 °C for one week to reproduce C. elegans biosafety test conditions. Biomass from each strain was resuspended in 1 mL of M9 salts medium and serial dilutions were plated onto TSA plates in the presence or absence of kanamycin (50 μg mL−1). Plasmid stability was determined by calculating the ratio between CFU mL−1 in the presence or absence of antibiotic.
Biofilm assays and quantification
Biofilm formation was quantified using a borosilicate glass tubes (hydrophilic surface) following the methodology described by O’Toole et al. with some modifications54. Briefly, the borosilicate glass tubes were inoculated with 2 mL aliquots of LB cultures of P. aeruginosa WT and ΔmcpB at A600 of 0.05 in triplicate. The tubes were incubated at 37 °C at 40 rpm for 5 h. Unattached cells were removed by rinsing the tubes thoroughly with water. Attached cells were subsequently stained with 0.3% crystal violet and incubated at 37 °C for 15 min. Then, tubes were washed twice with water to remove any unbound stain. Crystal violet was then solubilized with 5 mL of 10% (v/v) glacial acetic acid and spectrophotometrically quantified at 590 nm. Biofilm formation was normalized with the corresponding cell density.
Virulence assays
Virulence and ecotoxicity of P. aeruginosa strains were evaluated using a combination of bioassays that were performed as described previously by our group25. These assays included antibacterial activity against E. coli MC4100, microbial metabolism assays using Vibrio fischeri (Microtox®; Modern Water), pathogenicity bioassay against Caenorhabditis elegans, ecotoxicity tests using green lacewings (Chrysoperla carnea), ladybirds (Adalia bipunctata), earthworms (Eisenia foetida) and Daphnia magna (DaphToxKit®; Microbiotest), and bacterial effects on pepper (Capsicum annuum L. cv. Maor) plants. In each case, bioactivities were compared with those of non-pathogenic and pathogenic bacterial strains, or with trehalose as carrier of the dry formats as previously described25. The number of replicates is stated in the description of each experiment.
Bacterial effects in pepper (Capsicum annuum) plants
Virulence in pepper plants was tested according to Vílchez et al.25 with some modifications. Pots containing pepper plants of about 10 cm height were inoculated with a 4 mL of bacterial suspension (108-109 CFU/mL) in 0.5 X M9 sterile saline solution. Plants were irrigated twice a week with 4 mL water per plant. Fourteen days after inoculation, height, fresh weight, fully turgid weight and dry weight were recorded. As a control, pepper plants were amended with saline buffer.
Statistical analyses
The analysis of variance (ANOVA) test was used to determine the effects of treatments and errors associated with the experiment with replicates and treatments as random effects. The Statistical Analysis Software (SAS) version 9.1 was used (SAS Institute Inc., Cary, NC). Means were compared to identify significant differences among treatments. The protected least significance difference (LSD) (P ≤ 0.05) test was used for this purpose; mean square error obtained in this test was used to estimate the standard error of differences between means. The survival function S(t) was calculated as the probability of surviving at least to time t to analyse the survival data with C. carnea and A. bipunctata. The survival curve was represented using S(t) against t. The Kaplan-Meier method was used to generate survival curves from the observed survival times without the assumption of an underlying probability distribution. This analysis was based on the assumption that the probability of surviving k or more periods from entering the study is a product of the k observed survival rates for each period, following the formula:
where p1 is the proportion surviving the first period, p2 is the proportion surviving the second period, and so on. Therefore, for a specific period i the proportion of survival was calculated as:
where ri is the number of alive insects at the beginning of the period and di the number of dead insects within the period55. Comparison of the survival curves obtained with different bacterial strains was performed using the log rank test as statistical hypothesis.
Supplementary information
The involvement of McpB chemoreceptor from Pseudomonas aeruginosa PAO1 in virulence
Acknowledgements
Work in Dr. Manzanera’s laboratory was funded by the Spanish Ministry for Economy and Competitiveness, within the context of the research projects CTM2017-84332-R, and CGL2017-91737-EXP. Dr. Krell’s laboratory was supported by FEDER funds and Fondo Social Europeo through grants from the Junta de Andalucía (grant CVI-7335) and the Spanish Ministry for Economy and Competitiveness (grants BIO2013-42297 and BIO2016-76779-P). We thank Prof. Caroline Harwood (University of Washington) for providing a P. aeruginosa PAO1 wt strain and the mcpB mutant, that were used for initial experiments not reported here.
Author Contributions
C.G.-F., J.I.V., M.G.-R., M.A.M. and M.M. performed the experimental procedures and interpreted results. J.G.L., T.K. and M.M. designed experiments, analysed data and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49697-7.
References
- 1.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rada, B. Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis. Pathog. Basel Switz. 6 (2017). [DOI] [PMC free article] [PubMed]
- 3.Walker TS, et al. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol. 2004;134:320–331. doi: 10.1104/pp.103.027888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvis S, et al. Caenorhabditis elegans semi-automated liquid screen reveals a specialized role for the chemotaxis gene cheB2 in Pseudomonas aeruginosa virulence. PLoS Pathog. 2009;5:e1000540. doi: 10.1371/journal.ppat.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starkey M, Rahme LG. Modeling Pseudomonas aeruginosa pathogenesis in plant hosts. Nat. Protoc. 2009;4:117–124. doi: 10.1038/nprot.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breidenstein EBM, de la Fuente-Núñez C, Hancock REW. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Pereira SG, et al. Virulence factors and infection ability of Pseudomonas aeruginosa isolates from a hydropathic facility and respiratory infections. J. Appl. Microbiol. 2014;116:1359–1368. doi: 10.1111/jam.12463. [DOI] [PubMed] [Google Scholar]
- 9.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 10.Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signalling in networked arrays. Trends Biochem. Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuichet K, Zhulin IB. Origins and diversification of a complex signal transduction system in prokaryotes. Sci. Signal. 2010;3:ra50. doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zusman DR, Scott AE, Yang Z, Kirby JR. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 14.Ferrández A, Hawkins AC, Summerfield DT, Harwood CS. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J. Bacteriol. 2002;184:4374–4383. doi: 10.1128/JB.184.16.4374-4383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong CS, et al. Chemotaxis proteins and transducers for aerotaxis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2004;231:247–252. doi: 10.1016/S0378-1097(04)00009-6. [DOI] [PubMed] [Google Scholar]
- 16.Güvener ZT, Tifrea DF, Harwood CS. Two different Pseudomonas aeruginosa chemosensory signal transduction complexes localize to cell poles and form and remould in stationary phase. Mol. Microbiol. 2006;61:106–118. doi: 10.1111/j.1365-2958.2006.05218.x. [DOI] [PubMed] [Google Scholar]
- 17.García-Fontana C, Corral Lugo A, Krell T. Specificity of the CheR2 methyltransferase in Pseudomonas aeruginosa is directed by a C-terminal pentapeptide in the McpB chemoreceptor. Sci. Signal. 2014;7:ra34. doi: 10.1126/scisignal.2004849. [DOI] [PubMed] [Google Scholar]
- 18.Watts KJ, Johnson MS, Taylor BL. Different conformations of the kinase-on and kinase-off signalling states in the Aer HAMP domain. J. Bacteriol. 2011;193:4095–4103. doi: 10.1128/JB.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia, D., Orillard, E., Johnson, M. S. & Watts, K. J. Gas Sensing and Signalling in the PAS-Heme Domain of the Pseudomonas aeruginosa Aer2 Receptor. J. Bacteriol. 199 (2017). [DOI] [PMC free article] [PubMed]
- 20.Matilla, M. A. & Krell, T. The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol. Rev. 42 (2018). [DOI] [PubMed]
- 21.McLaughlin HP, Caly DL, McCarthy Y, Ryan RP, Dow JM. An orphan chemotaxis sensor regulates virulence and antibiotic tolerance in the human pathogen Pseudomonas aeruginosa. PloS One. 2012;7:e42205. doi: 10.1371/journal.pone.0042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath KS, et al. Pseudomonas aeruginosa Cell Membrane Protein Expression from Phenotypically Diverse Cystic Fibrosis Isolates Demonstrates Host-Specific Adaptations. J. Proteome Res. 2016;15:2152–2163. doi: 10.1021/acs.jproteome.6b00058. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer C, Fischer H, Machen TE. Chemotaxis and Binding of Pseudomonas aeruginosa to Scratch-Wounded Human Cystic Fibrosis Airway Epithelial Cells. PloS One. 2016;11:e0150109. doi: 10.1371/journal.pone.0150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega DR, et al. Assigning chemoreceptors to chemosensory pathways in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2017;114:12809–12814. doi: 10.1073/pnas.1708842114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vílchez JI, Navas A, González-López J, Arcos SC, Manzanera M. Biosafety Test for Plant Growth-Promoting Bacteria: Proposed Environmental and Human Safety Index (EHSI) Protocol. Front. Microbiol. 2015;6:1514. doi: 10.3389/fmicb.2015.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan-Miklos S, Rahme LG, Ausubel FM. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 2000;37:981–988. doi: 10.1046/j.1365-2958.2000.02056.x. [DOI] [PubMed] [Google Scholar]
- 27.Ewbank JJ. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans. Microbes Infect. Inst. Pasteur. 2002;4:247–256. doi: 10.1016/S1286-4579(01)01531-3. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 1999;96:47–56. doi: 10.1016/S0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 29.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurz CL, Ewbank JJ. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat. Rev. Genet. 2003;4:380–390. doi: 10.1038/nrg1067. [DOI] [PubMed] [Google Scholar]
- 31.Hellberg JEEU, Matilla MA, Salmond GPC. The broad-spectrum antibiotic, zeamine, kills the nematode worm Caenorhabditis elegans. Front. Microbiol. 2015;6:137. doi: 10.3389/fmicb.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navas A, et al. Experimental validation of Haldane’s hypothesis on the role of infection as an evolutionary force for Metazoans. Proc. Natl. Acad. Sci. USA. 2007;104:13728–13731. doi: 10.1073/pnas.0704497104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen AS, et al. Quorum-sensing-regulated virulence factors in Pseudomonas aeruginosa are toxic to Lucilia sericata maggots. Microbiol. Read. Engl. 2010;156:400–407. doi: 10.1099/mic.0.032730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.v d Schulenburg JHG, et al. History of infection with different male-killing bacteria in the two-spot ladybird beetle Adalia bipunctata revealed through mitochondrial DNA sequence analysis. Genetics. 2002;160:1075–1086. doi: 10.1093/genetics/160.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernández M, et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015;6:871. doi: 10.3389/fmicb.2015.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burrowes E, Baysse C, Adams C, O’Gara F. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiol. Read. Engl. 2006;152:405–418. doi: 10.1099/mic.0.28324-0. [DOI] [PubMed] [Google Scholar]
- 37.Pessi G, et al. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 2001;183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulenburg H, Boehnisch C, Michiels NK. How do invertebrates generate a highly specific innate immune response? Mol. Immunol. 2007;44:3338–3344. doi: 10.1016/j.molimm.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Jarosz J, Gliński Z. Earthworm immune responses. Folia Biol. (Praha) 1997;45:1–9. [PubMed] [Google Scholar]
- 40.Bilej M, De Baetselier P, Beschin A. Antimicrobial defense of the earthworm. Folia Microbiol. (Praha) 2000;45:283–300. doi: 10.1007/BF02817549. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Sun Z, Chong W, Sun Z, He C. Growth and stress responses of the earthworm Eisenia fetida to Escherichia coli O157:H7 in an artificial soil. Microb. Pathog. 2009;46:266–272. doi: 10.1016/j.micpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Neuhauser EF, Callahan CA. Growth and reproduction of the earthworm Eisenia fetida exposed to sublethal concentrations of organic chemicals. Soil Biol. Biochem. 1990;22:175–179. doi: 10.1016/0038-0717(90)90083-C. [DOI] [Google Scholar]
- 43.O’Callaghan J, Reen FJ, Adams C, O’Gara F. Low oxygen induces the type III secretion system in Pseudomonas aeruginosa via modulation of the small RNAs rsmZ and rsmY. Microbiol. Read. Engl. 2011;157:3417–3428. doi: 10.1099/mic.0.052050-0. [DOI] [PubMed] [Google Scholar]
- 44.Schaible B, et al. Hypoxia Reduces the Pathogenicity of Pseudomonas aeruginosa by Decreasing the Expression of Multiple Virulence Factors. J. Infect. Dis. 2017;215:1459–1467. doi: 10.1093/infdis/jix139. [DOI] [PubMed] [Google Scholar]
- 45.Yao J, Allen C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 2007;189:6415–6424. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross H, Loper JE. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 2009;26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 47.Chowdhury SP, Hartmann A, Gao X, Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 - a review. Front. Microbiol. 2015;6:780. doi: 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mousa WK, Raizada MN. Biodiversity of genes encoding anti-microbial traits within plant associated microbes. Front. Plant Sci. 2015;6:231. doi: 10.3389/fpls.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers GB, van der Gast CJ, Serisier DJ. Predominant pathogen competition and core microbiota divergence in chronic airway infection. ISME J. 2015;9:217–225. doi: 10.1038/ismej.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aragon IM, et al. Diguanylate cyclase DgcP is involved in plant and human Pseudomonas spp. infections. Environ. Microbiol. 2015;17:4332–4351. doi: 10.1111/1462-2920.12856. [DOI] [PubMed] [Google Scholar]
- 51.Abril MA, Michan C, Timmis KN, Ramos JL. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J. Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matilla MA, Stöckmann H, Leeper FJ, Salmond GPC. Bacterial biosynthetic gene clusters encoding the anti-cancer haterumalide class of molecules: biogenesis of the broad spectrum antifungal and anti-oomycete compound, oocydin A. J. Biol. Chem. 2012;287:39125–39138. doi: 10.1074/jbc.M112.401026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obranić S, Babić F, Maravić-Vlahoviček G. Improvement of pBBR1MCS plasmids, a very useful series of broad-host-range cloning vectors. Plasmid. 2013;70:263–267. doi: 10.1016/j.plasmid.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 54.O’Toole GA, et al. Genetic approaches to study of biofilms. Methods Enzymol. 1999;310:91–109. doi: 10.1016/S0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 55.Swinscow, T. D. V. & Campbell, M. J. Statistics at square one. Stat. Sq. One (2002).
- 56.Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 1972;36:525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brenner S. The Genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demarre G, et al. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 2005;156:245–255. doi: 10.1016/j.resmic.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Woodcock DM, et al. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eberl L, Tümmler B. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: genome evolution, interactions and adaptation. Int. J. Med. Microbiol. IJMM. 2004;294:123–131. doi: 10.1016/j.ijmm.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Nakazawa T. Travels of a Pseudomonas, from Japan around the world. Environ. Microbiol. 2002;4:782–786. doi: 10.1046/j.1462-2920.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 62.Onorati F, Mecozzi M. Effects of two diluents in the Microtox toxicity bioassay with marine sediments. Chemosphere. 2004;54:679–687. doi: 10.1016/j.chemosphere.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Dorn PB, Vipond TE, Salanitro JP, Wisniewski HL. Assessment of the acute toxicity of crude oils in soils using earthworms, microtox®, and plants. Chemosphere. 1998;37:845–860. doi: 10.1016/S0045-6535(98)00089-7. [DOI] [Google Scholar]
- 64.Hernando MD, Ejerhoon M, Fernández-Alba AR, Chisti Y. Combined toxicity effects of MTBE and pesticides measured with Vibrio fischeri and Daphnia magna bioassays. Water Res. 2003;37:4091–4098. doi: 10.1016/S0043-1354(03)00348-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The involvement of McpB chemoreceptor from Pseudomonas aeruginosa PAO1 in virulence