Abstract
Carotenoid production in some non-phototropic bacteria occurs in a light-dependent manner to protect cells from photo-oxidants. Knowledge regarding the transcriptional regulator involved in the light-dependent production of carotenoids of non-phototrophic bacteria has been mainly confined to coenzyme B12-based photo-sensitive regulator CarH/LitR family proteins belonging to a MerR family transcriptional regulator. In this study, we found that bacteria belonging to Micrococcales and Corynebacteriales exhibit light-dependent carotenoid-like pigment production including an amino acid-producer Corynebacterium glutamicum AJ1511. CrtR is a putative MarR family transcriptional regulator located in the divergent region of a carotenoid biosynthesis gene cluster in the genome of those bacteria. A null mutant for crtR of C. glutamicum AJ1511 exhibited constitutive production of carotenoids independent of light. A complemented strain of the crtR mutant produced carotenoids in a light-dependent manner. Transcriptional analysis revealed that the expression of carotenoid biosynthesis genes is regulated in a light-dependent manner in the wild type, while the transcription was upregulated in the crtR mutant irrespective of light. In vitro experiments demonstrated that a recombinant CrtR protein binds to the specific sequences within the intergenic region of crtR and crtE, which corresponds to −58 to −7 for crtE, and +26 to −28 for crtR with respect to the transcriptional start site, and serves as a repressor for crtE transcription directed by RNA polymerase containing SigA. Taken together, the results indicate that CrtR light-dependently controls the expression of the carotenoid gene cluster in C. glutamicum and probably closely related Actinobacteria.
Subject terms: Bacterial genetics, Bacterial genes
Introduction
Carotenoids are yellow to red colored pigments that are widely produced by plants, algae, and some fungi and bacteria1–3. They are tetraterpenoids that consist of a polyene hydrocarbon chain derived from eight isoprene units. Most carotenoids consist of 40 carbon atoms that are modified in several ways, such as cyclization and desaturation, to produce a variety of compounds with divergent chemical structures3. Recently, the C30 and C50 biosynthetic pathways were found in Micrococcus, Corynebacterium, and Flavobacterium4. Carotenoids can function as photoprotectors, light harvesting molecules, or membrane stabilizers5. In non-phototrophic bacteria, the main function of carotenoids is the protection of cells from photo-oxidative damage by scavenging harmful agents such as singlet and triplet molecular species produced upon illumination1,6.
In non-phototrophic bacteria, the control of carotenoid production is classified into three types: constitutive, light-inducible, and cryptic manner7. We and other groups have been studying the phenomena and molecular mechanism of light-inducible production of carotenoids of phylogenetically different bacteria including S. coelicolor A3(2)8, Thermus thermophilus HB279–14, Bacillus megaterium QM B155115,16, Mycobacterium marinum17, and Arthrobacter arilaitensis18. Our study has revealed that LitR/CarH family proteins, a MerR family transcriptional regulator retained by S. coelicolor, T. thermophilus, and B. megaterium, play a central role in light-dependent gene expression19. Other groups have also reported that LitR/CarH family proteins serve as a photosensor in the light-inducible production of carotenoids in Myxococcus xanthus20,21.
LitR/CarH family proteins commonly contain a DNA-binding domain in its N-terminus and an adenosyl-coenzyme B12 (AdoB12)-binding domain in its C-terminus, and serves as a negative regulator for light-inducible transcription13,19. The molecular mechanism of light-inducible transcription mediated by the LitR/CarH family involves the photolysis of AdoB12 associated with LitR/CarH due to light, which results in a large conformational change and inactivation of the DNA-binding activity of LitR/CarH11. LitR homologs are widely distributed in the phylogenetically diverged genera of non-phototrophic bacteria, including gram-negative and gram-positive bacteria, which indicate that LitR/CarH family proteins are general photosensors and have diverse roles in non-phototrophic bacteria. Recently, we have also reported the existence of a novel type of LitR/CarH family proteins in Burkholderia multivorans belonging to Beta-proteobacteria, which requires a different type chromophore from coenzyme B1222.
The wide distribution of LitR/CarH family protein among non-phototrophic bacteria leads to our assumption that other type of light-inducible transcriptional regulators is present in non-phototrophic bacteria. The finding of a novel type of photosensors would deepen insights on bacterial environmental responses. In this study, we performed a wide screen of bacteria exhibiting light-inducible production of carotenoids, which led us to the finding that some groups of gram-positive bacteria including C. glutamicum, an amino acid producer, exhibit photo-dependent production of carotenoids. The evidence indicates that a light-induced MarR family regulator CrtR, is distributed to some groups of Actinobacteria and is involved in the light-inducible expression of crt biosynthesis genes.
Results
Light-inducible yellow-color pigment production in bacteria belonging to the order Micrococcales
We previously reported that a number of Bacillus spp. isolated from soil showed light-dependent production of carotenoid on rich LB medium15. Our genetic and biochemical study of B. megaterium QM B1551 revealed that LitR in complex with B12 serves as a photosensitive transcriptional regulator to control the expression of crt biosynthesis genes in a light-dependent manner. Here, we used several media including not only LB but also 10-fold diluted LB medium (1/10 LB), 1/10 LB containing 1% glucose (1/10 LBG), and R2A medium to more widely screen bacteria exhibiting light-inducible yellow-color pigment production (see Materials and Methods). We isolated a number of bacteria from various environmental source including biotope, paddy field, forest soil, bran pickled, snow, residential land, and cattle manure in Japan. Of these, bacteria showing white light-dependent pigment production were subjected to the phylogenetic analysis based on DNA sequence of the 16S rRNA gene. As summarized in Table 1, a total of 24 strains out of approximately 1,100 isolates exhibited light-inducible yellow-colored pigment production. The number of the isolated photo-responsive bacteria was 1 strain out of 121 (0.8%) with LB medium, 7 out of 526 (1.3%) with 1/10 LB medium, 14 out of 361 (3.9%) with 1/10 LBG medium, and 2 out of 92 (2.2%) with R2A medium. The isolates mainly belonged to the order Micrococcales, a high G + C gram-positive Actinobacteria, including the genus Arthrobacter, Leifsonia, Microbacterium, Brevibacterium, and Agromyces. As minor groups, Bacillus, Sphingobacterium, and Simplicispira genus were also isolated. All isolates exhibited >99% sequence identity with the known species affiliating to those bacterial taxa. This result indicated that 1/10 LB and 1/10 LBG medium were suitable for the isolation of photo-responsive Micrococcales bacteria.
Table 1.
Phylogenetic characteristics of isolates based on the 16S rRNA gene sequence.
| Isolates No. | Source of isolation (Locality of source) | Isolation media | Closest taxon | Closest GeneBank relative | Response to lightb | ||
|---|---|---|---|---|---|---|---|
| Genus/Species | Order | Accession Noa | Similarity (%) | ||||
| 16 | Biotope (Kanagawa pref.) | LB | Bacillus megaterium | Bacillales | AY030338 | 99 | + |
| 199 | Paddy field (Ibaraki pref.) | 1/10 LB | Bacillus megaterium | EU880506 | 99 | + | |
| 295 | Forest soil (Kanagawa pref.) | 1/10 LB | Agromyces ulmi | Micrococcales | AY427830 | 98 | ++ |
| 296 | 1/10 LB | Bacillus subtilis | Bacillales | FJ483514 | 99 | ++ | |
| 423 | Bran pickled (Kanagawa pref.) | R2A | Brevibacterium linens | Micrococcales | DQ361016 | 98 | ++ |
| 459 | Snow (Yamanashi pref.) | 1/10 LBG | Sphingobacterium siyangense | Sphingobacteriales | EU646272 | 99 | ++ |
| 496 | Soil of residential land (Chiba pref.) | 1/10 LBG | Paeniglutamicibacter sulfureus | Micrococcales | AB046358 | 99 | ++ |
| 504 | 1/10 LBG | Paeniglutamicibacter sulfureus | X83409 | 99 | ++ | ||
| 514 | 1/10 LBG | Paeniglutamicibacter sulfureus | EF154245 | 99 | ++ | ||
| 516 | 1/10 LBG | Microbacterium phyllosphaerae | AJ277840 | 100 | + | ||
| 526 | 1/10 LBG | Microbacterium foliorum | EU714341 | 100 | + | ||
| 527 | 1/10 LBG | Paeniglutamicibacter sulfureus | AB046358 | 99 | ++ | ||
| 550 | 1/10 LBG | Paeniglutamicibacter sulfureus | X83409 | 99 | ++ | ||
| 647 | R2A | Paenibacillus alvei | Bacillales | AB377108 | 99 | + | |
| 732 | Biotope (Hokkaido) | 1/10 LB | Leifsonia shinshuensis | Micrococcales | DQ232614 | 99 | ++ |
| 742 | Paddy field (Ibaraki pref.) | 1/10 LB | Sinomonas atrocyanea | X80746 | 99 | ++ | |
| 905 | Cattle manure (Iwate pref.) | 1/10 LB | Glutamicibacter arilaitensis | EU834260 | 99 | ++ | |
| 919 | 1/10 LB | Simplicispira metamorpha | Burkholderiales | Y18618 | 96 | + | |
| 943 | 1/10 LBG | Glutamicibacter nicotianae | Micrococcales | EU857420 | 99 | ++ | |
| 951 | 1/10 LBG | Paenarthrobacter nicotinovorans | AB363933 | 99 | + | ||
| 1031 | 1/10 LBG | Simplicispira metamorpha | Burkholderiales | Y18618 | 96 | + | |
| 1039 | 1/10 LBG | Microbacterium natoriense | Micrococcales | AY566291 | 99 | ++ | |
| 1052 | 1/10 LBG | Microbacterium natoriense | AY566291 | 98 | ++ | ||
| 1077 | 1/10 LBG | Microbacterium natoriense | AY566291 | 99 | ++ | ||
aCorresponding number in the nucleotide database of NCBI (https://www.ncbi.nlm.nih.gov/nuccore/).
b+ and ++ indicate that carotenoid-like pigment production is weakly and strongly induced by illumination, respectively.
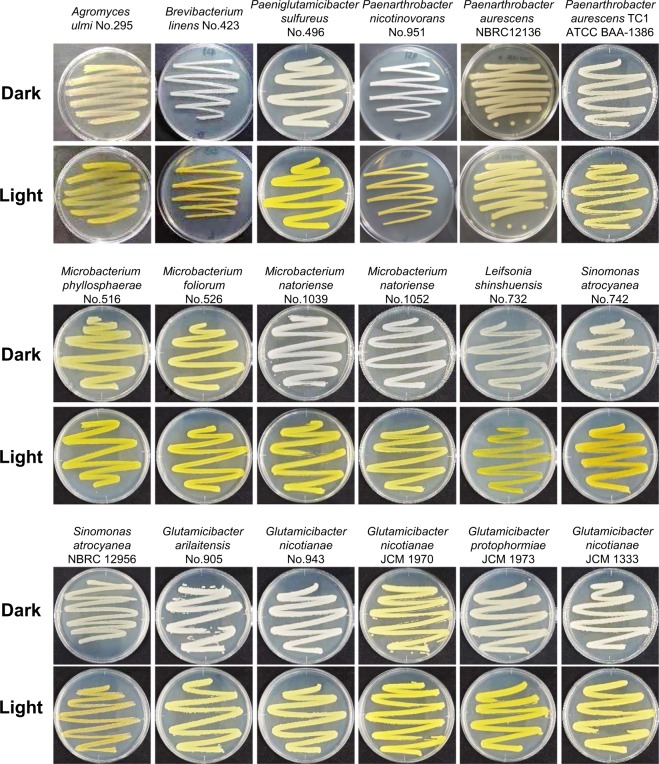
To confirm the light-inducible pigment production observed in the isolates, we characterized the phenotype of bacteria belonging to Micrococcales obtained from culture collections such as NBRC, JCM, and ATCC. As shown in Fig. 1 and summarized in Table 2, the bacterial genus belonging to Paenarthrobacter, Sinomonas, Glutamicibacter, Isoptericola, Jonesia, and Sanguibacter produced yellow pigments in response to light. To confirm that the pigments are carotenoid, we analyzed the UV-visible absorption spectrum of a methanol extract of the illuminated cells. As shown in Fig. S1, the following bacteria light-dependently produced a carotenoid-like pigment: Paenarthrobacter aurescens TC1 (ATCC BAA-1386), Sinomonas atrocyanea NBRC 12956, Isoptericola jiangsuensis JCM 17812, Isoptericola dokdonensis JCM 15137, Jonesia denitrificans DSM 20603, Sanguibacter keddieii DSM 10542. The light-responsive carotenoid production in Arthrobacter arilaitensis RE117 and Mycobacterium marinum M was also reported17,18. The pigment production in C. glutamicum AJ1511, C. glutamicum ATCC 13032, and C. callunae JCM 9489 was also induced by light (Table 2, Figs 2 and S1). The UV-visible absorption spectrum of a methanol extract of the illuminated cells of C. glutamicum AJ1511 and ATCC 13032 showed a typical carotenoid profile, exhibiting multiple absorption peaks at 418, 440, and 470 nm (Fig. S1). This profile was identical to that of the C50-terpene decaprenoxanthin and its glucosides, which are the predominant carotenoids in C. glutamicum ATCC 1303223. In C. glutamicum ATCC 13032, the carotenoid biosynthesis genes, crtI (NCgl0597), crtEb (NCgl0594), crtYe (NCgl0595), and crtYf (NCgl0596) are known to encode phytoene desaturase, a lycopene elongase, and a carotenoid C45/C50 ε-cyclase23. This result indicated that the yellow-color pigment is a carotenoid, and the ability to sense light was commonly spread in this group of bacteria.
Figure 1.
Light-dependent carotenoid-like pigment production of isolates and genome-sequenced bacteria. Light-dependent phenotype of strains grown at 28 °C under dark and blue light conditions for 24 h. The culture media used are shown in Table 1 (isolates) and Table 2 (strains obtained from culture collections). Colonies grown under light conditions appear vivid or pale yellow, whereas those grown under dark conditions appear white or cream.
Table 2.
Light-response of carotenoid-like pigment production in bacteria belonging to Micrococcales and Corynebacteriales.
| Strain No. | Isolation sourcea | Culture media | Taxon | Response to lightb | |
|---|---|---|---|---|---|
| Genus/Species | Order-Family | ||||
| ATCC BAA-1386 | Soil | LB | Paenarthrobacter aurescens TC1 | Micrococcales-Micrococcaceae | ++ |
| JCM 1333 | Air of tobacco warehouses | LB | Glutamicibacter nicotianae | ++ | |
| JCM 1970 | Sewage | LB | Glutamicibacter nicotianae | ++ | |
| JCM 1973 | Fly (Protophormia terrae-novae) | LB | Glutamicibacter protophormiae | ++ | |
| JCM 2522 | Soil | LB | Arthrobacter crystallopoietes | − | |
| NBRC 12136 | − | LB | Paenarthrobacter aurescens | ++ | |
| JCM 1338 | Oil-brine | LB | Paeniglutamicibacter sulfureus | − | |
| JCM 1335 | Pseudarthrobacter sp. | − | |||
| JCM 1336 | Pseudarthrobacter sp. | − | |||
| NBRC 12956 | Air | LB | Sinomonas atrocyanea | ++ | |
| NBRC 12708 | Soil | LB | Kocuria rhizophila | − | |
| JCM 15137 | Soil | LB | Isoptericola dokdonensis | Micrococcales-Promicromonosporaceae | + |
| JCM 17812 | Beach sand | LB | Isoptericola jiangsuensis | + | |
| JCM 15589 | Tufa from a burial chamber | LB | Isoptericola hypogeous | ++ | |
| JCM 19549 | Soil | LB | Isoptericola nanjingensis | ++ | |
| JCM 18063 | Mangrove soil from Chiayi County, Taiwan | LB | Isoptericola chiayiensis | + | |
| NBRC 104115 | Hindgut contents of Australian termite | LB | Isoptericola variabilis | ++ | |
| DSM 20603 | Boiled ox blood | LB | Jonesia denitrificans | Micrococcales-Jonesiaceae | + |
| DSM 10542 | Venous blood from healthy cow | LB | Sanguibacter keddieii | Micrococcales-Sanguibacteraceae | + |
| ATCC 13869 | − | LB | Corynebacterium glutamicum AJ1511 | Corynebacteriales-Corynebacteriaceae | ++ |
| JCM 1318 | Sewage | LB | Corynebacterium glutamicum ATCC 13032 | ++ | |
| JCM 9489 | − | LB | Corynebacterium callunae | + | |
| JCM 1305 | Stool of infant | LB | Corynebacterium ammoniagenes | − | |
| JCM 11950 | − | LB | Corynebacterium kroppenstedtii | − | |
a – indicates that isolation source is unknown.
b+ and ++ indicate that carotenoid-like pigment production is weakly and strongly induced by illumination, respectively. – shows no pigment production.
Figure 2.
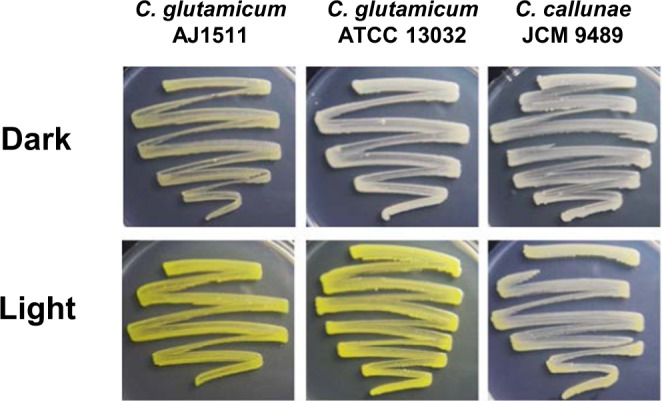
Light-dependent carotenoid-like pigment production of genome-sequenced Corynebacterium. C. glutamicum AJ1511, C. glutamicum ATCC 13032, and C. callunae JCM 9489 grown at 28 °C for 24 h on LB solid medium are shown.
It is well known that the carotenoid-producing ability is widespread in groups of bacteria including Actinobacteria, especially Micrococcales18,24–26, Corynebacteriales27, and Streptomycetales7; however, the molecular mechanism underlying the regulation of carotenoid production has not been well characterized except in S. coelicolor A3(2) analyzed in our previous study8. Therefore, we assessed for the gene organization of crt biosynthesis clusters of the genome-sequenced bacteria affiliating with the taxonomic group.
Gene synteny of carotenoid biosynthesis gene cluster and its adjacent MarR-type regulator
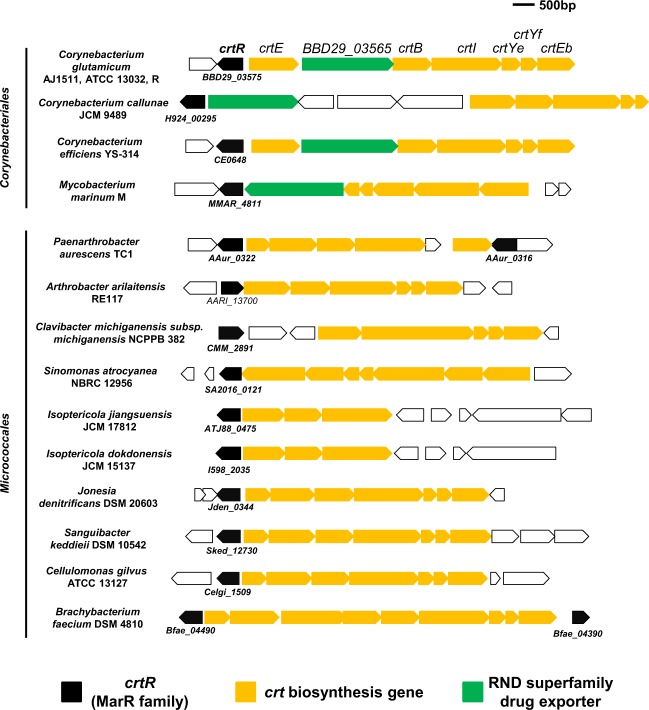
The genome of bacteria exhibiting a light-dependent carotenoid-like pigment production retained a putative crt biosynthesis gene cluster (Fig. 3), while any apparent photosensor homologous with LitR, light-oxygen-voltage (LOV) domain protein28, or blue-light receptor using flavin (BLUF) domain protein29 was not found. Alternatively, a MarR family regulator was encoded in the divergent region of the crt gene cluster (Fig. 3). Generally, members of the MarR family are preferentially negative regulators for the gene clusters adjacent to them30,31. Hereafter, the MarR family protein was designated as CrtR based on a previous study27.
Figure 3.
Schematic representation of the crt biosynthesis gene cluster and adjacent crtR gene in C. glutamicum and related bacteria. Positions and directions of open reading frames predicted by the genome sequences of C. glutamicum and related bacteria belonging to the order Corynebacteriales and Micrococcales are indicated by arrows. The crtR coding sequence numbers from the genome sequence database assigned to each sequence are shown. The crtR, crt biosynthesis genes, and RND superfamily drug exporter are shown by arrows colored with black, orange, and green, respectively.
The gene organization consisting of a crt cluster and crtR homolog was conserved in the genome of Micrococcales, including P. aurescens TC1 (ATCC BAA-1386), A. arilaitensis RE117, Clavibacter michiganensis subsp. michiganensis NCPPB 382, S. atrocyanea NBRC 12956, I. jiangsuensis JCM 17812, I. dokdonensis JCM 15137, J. denitrificans DSM 20603, S. keddieii DSM 10542, Cellulomonas gilvus ATCC 13127, and Brachybacterium faecium DSM 4810 (Fig. 3). Similar gene organization comprising the crt gene cluster and crtR homolog was also conserved in the genome of Corynebacteriales, including C. glutamicum AJ1511, C. glutamicum ATCC 13032, C. glutamicum R, C. callunae JCM 9489, C. efficiens YS-314, M. marinum M (Fig. 3), M. liflandii 128FXT, M. ulcerans Agy99, and M. avium 104 (data not shown). Therefore, 11 out of 16 strains harboring crtR-crt gene shown in Fig. 3 exhibited a light-dependent carotenoid-like pigment production. We therefore assumed that CrtR proteins might be related to the light-inducible expression of the crt gene cluster.
Phylogenetic analysis of CrtR family proteins
To classify the CrtR family proteins, phylogenetic analysis was carried out using the full-length amino acid sequences of CrtRs distributed to Micrococcales and Corynebacteriales. As shown in Fig. S2, CrtR family proteins were largely classified into two branches. A large clade is composed of CrtRs from the order Micrococcales including the genus Arthrobacter, Leifsonia, Microbacterium, Brevibacterium, and Agromyces, and the order Corynebacteriales including the genus Corynebacterium. The other small branched group includes Mycobacterium and Nocardia belonging to the order Corynebacteriales. CrtRs derived from Corynebacterium were included in the large clade of Micrococcales, which indicates that CrtRs of Corynebacterium and Mycobacterium independently evolved.
Gene disruption of crtR in Corynebacterium glutamicum AJ1511
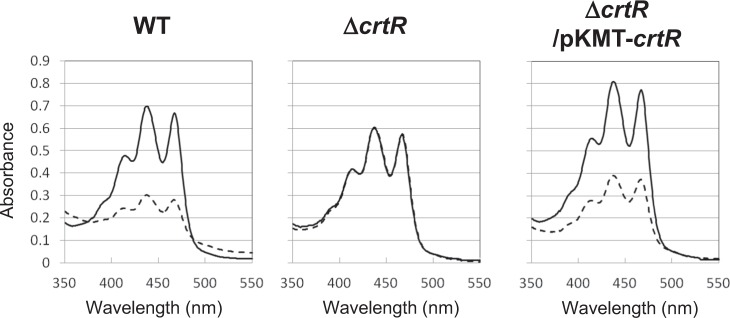
We selected a genome-sequenced C. glutamicum AJ1511 (ATCC 13869), an amino acid producer, to examine the light-inducible mechanism. This strain carries advantages of genetic analysis due to its high transformation efficiency as well as the association of carotenoids with unique colony morphologies as shown by our previous study32. In order to examine the physiological role of crtR in C. glutamicum AJ1511 retaining a 97% similarity with that of ATCC 1303227, we generated a crtR null mutant of this strain. In this mutant, a drug marker for selection was removed to exclude possible polar effects on the expression of the flanking coding sequences. The resultant crtR mutant (designated ΔcrtR) exhibited constitutive production of carotenoids under both dark and light conditions (Fig. 4). The amount of carotenoids produced by illuminated and non-illuminated ΔcrtR was almost the same with that of the wild type cultured under light condition. To confirm whether the phenotype was due to the inactivation of crtR, we performed a complementation experiment with a chromosome integration vector (see Material and Methods). The genetically complemented strain (ΔcrtR/pKMT + crtR) showed similar light-responsive carotenoid production as the wild type (Fig. 4). These results indicated that CrtR was involved in the regulation of light-inducible carotenoid production, and serves as a negative regulator for the transcriptional initiation of crt biosynthesis genes. These results are consistent with the fact that CrtR is a repressor27. Hereafter, we focused the study on CrtR and its target promoter.
Figure 4.
UV-visible absorption spectrum of carotenoid extracted from C. glutamicum AJ1511. UV-visible spectrum of the crude carotenoid fraction extracted from C. glutamicum AJ1511 wild-type cells, the crtR mutant (ΔcrtR), and the genetically complemented crtR mutant (ΔcrtR/pKMT-crtR) grown for 15 h under blue light (solid line) and dark (dash line) conditions are shown.
Determination of transcriptional start sites (TSSs) for crtE and crtR
Generally, members of the MarR family preferentially regulate the transcription of the gene cluster adjacent to them30,31. Therefore, we speculated that the intergenic region between crtE, a geranylgeranyl pyrophosphate synthase gene, and crtR is directly controlled by CrtR. We determined the TSS of crtE and crtR by 5′ RACE to identify the promoter structure (−10 and −35 regions). As shown in Fig. 5A, the TSS of crtE and crtR were assigned 85 and 41 nucleotides, respectively, upstream of the translational start codon (TTG for crtE and ATG for crtR). This result was consistent with the start site of crtE in C. glutamicum ATCC 13032 reported previously23. Comparison of each promoter to the consensus sequence recognized with sigma factor revealed that the crtE promoter exhibits a similarity with that of SigA of C. glutamicum, a house-keeping sigma factor. Namely, the potential -35 (5′-TTAAAA-3′) and -10 (5′-TATAAA-3′) sequences of the crtE promoter were similar to those (5′-TTGC/GCA-3′ and 5′-TANAAT-3′) recognized probably by SigA33 (Fig. 5B). On the other hand, the potential -35 (5′-CAGGAA-3′) and -10 (5′-TTAATA-3′) sequences of the crtR promoter were not similar to those recognized by sigma factors of C. glutamicum.
Figure 5.
Promoter sequence located in the intergenic region of crtR and crtE. (A) Nucleotide sequence of the intergenic region between crtR and crtE. Putative −10 and −35 hexamer sequences of the crtR promoter (PcrtR) and crtE promoter (PcrtE) are indicated by dotted lines. The transcriptional start sites determined by 5′ RACE are indicated by vent arrows. The binding sites of CrtR determined by a DNase I footprint analysis (Fig. 9) are underlined. The essential binding motif TTAA for CrtR27 is indicated by bold letters. (B) Comparison of putative −10 and −35 hexamer sequences of PcrtR and PcrtE with that of SigA consensus in C. glutamicum ATCC 13032. The putative −10 and −35 sequences, and the TSS are indicated by an underline and +1, respectively.
Transcriptional analysis of light-inducible genes
We performed a quantitative RT-PCR analysis to investigate whether the photo-dependent carotenoid production was regulated at the transcriptional level. For this analysis, total RNA was purified from the wild type, ΔcrtR, and its complemented strain (ΔcrtR/pKMT-crtR) cultured under dark and light conditions for 10 and 15 h. The house-keeping 16S rRNA gene was used as an internal control for RNA quality and quantity. As shown in Fig. 6, the transcription of crtR, BBD29_03565, crtE, and crtI in the wild type was markedly induced under light conditions, while the transcription of phrB encoding a DNA photolyase, and sigA was not largely affected by light. In the ΔcrtR, the transcriptional level of the light-inducible genes was almost identical between the dark and light conditions. The introduction of an intact crtR gene into the chromosome of the ΔcrtR restored the light-dependent transcription of crtR, BBD29_03565, crtE, and crtI. These results were consistent with that for the production of carotenoids, indicating that light-inducible carotenoid production is regulated at a transcriptional level, and CrtR is involved in the control of the crt gene expression. Further, to study the role of CrtR, we calculated the ratios of the transcripts in the ΔcrtR to that in the wild type. As shown in Fig. 7, the transcripts of BBD29_03565, crtE, and crtI were increased in ΔcrtR compared to the wild type. In contrast, the transcripts of phrB and sigA were similar amount in the wild type and ΔcrtR. These results indicate that CrtR serves as a transcriptional repressor to the crt cluster.
Figure 6.
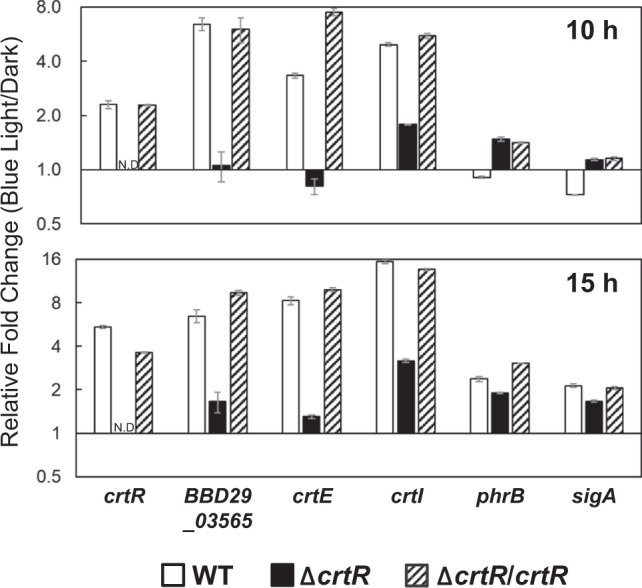
Transcriptional analysis of crtR and crt genes by quantitative RT-PCR. Vertical axes show the ratios of blue light condition/dark condition of the transcriptional intensity measured by quantitative RT-PCR analysis. The amounts of light-inducible genes and sigA transcripts in the wild-type strain, the crtR mutant (ΔcrtR), and the genetically complemented crtR mutant (ΔcrtR/pKMT-crtR) were analyzed. Total RNA was isolated from cells cultured in LB liquid medium at 28 °C under dark and blue light conditions for 10 h and 15 h. N.D., not detected due to the gene disruption eliminating the corresponding sequence. Errors bars represent the SD calculated from the results of quantitative RT-PCR runs performed in triplicates.
Figure 7.
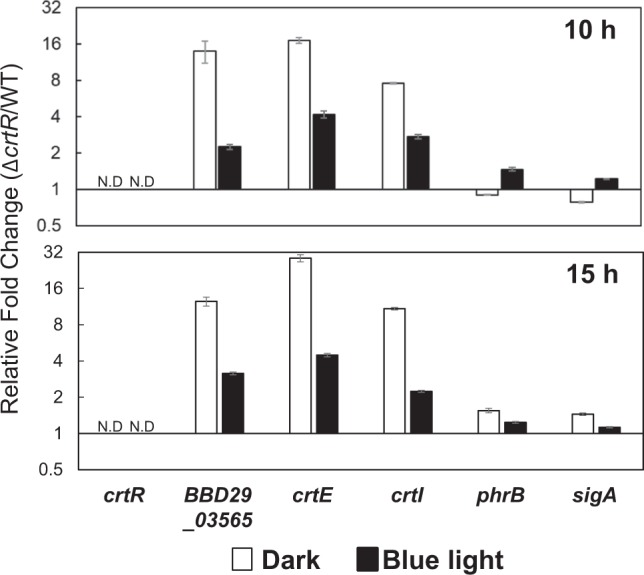
Transcriptional analysis of crtR and crt genes by quantitative RT-PCR. Vertical axes show the ratios of ΔcrtR/wild type of the transcriptional intensity measured by quantitative RT-PCR analysis.
Binding of CrtR to the intergenic region of crtE and crtR promoters
The above results suggest that the CrtR protein binds to the promoter region of crtR and crtE to control the light-inducible expression. Therefore, we then performed a gel shift assay to investigate the DNA-binding activity of CrtR to the intergenic region between crtE and crtR. A recombinant protein for CrtR was overexpressed in E. coli and purified to homogeneity by affinity chromatography (see Materials and Methods). As shown in Fig. 8, CrtR recombinant protein retarded the probe containing the intergenic region of crtE and crtR in a dose-dependent manner, while the retardation by the CrtR activity was not observed when the sigA promoter region was used as a control probe. The result indicated that CrtR protein specifically binds to the divergent promoter region of crtE and crtR.
Figure 8.
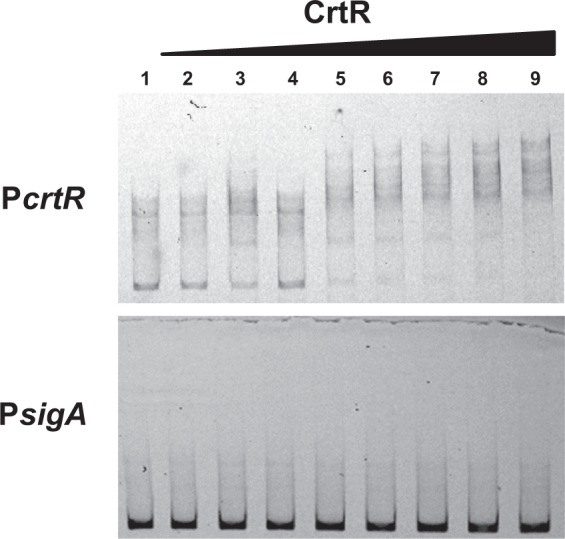
Gel shift assay of the intergenic region of crtR and crtE with purified CrtR. Various amounts of purified CrtR were mixed with the probe including the intergenic region of crtR and crtE and then applied to a non-denaturing polyacrylamide gel. As a control, sigA promoter (PsigA) was used. The amounts of CrtR used in lanes 1 to 9 were 0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, and 0.4 pmol, respectively. Approximately 1.6 pmol of probes were commonly used.
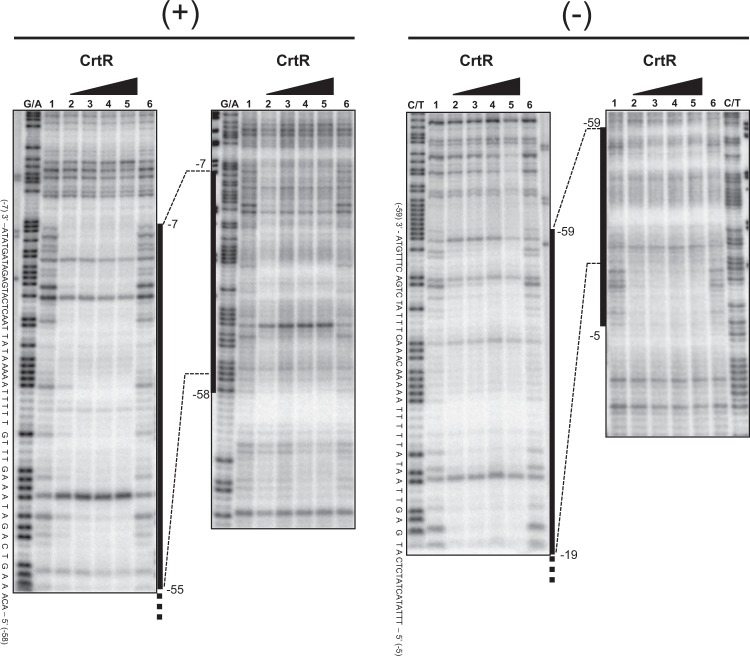
We carried out a DNase I footprint analysis to determine the binding sequence of CrtR protein in the intergenic region between crtE and crtR. As shown in Fig. 9, the protected regions corresponding to −58 to −7 in the sense strand and −59 to −5 in the antisense strand with respect to the TSS of crtE. These regions corresponded to crtR, +26 to −28 in the sense strand and +25 to −26 in the antisense strand with respect to the TSS, respectively. The determined binding sequence of CrtR overlapped with the −10 and −35 regions of crtE as well as the −10 region of crtR (Fig. 5A).
Figure 9.
DNase I footprint analysis for determining CrtR-binding site. The assay was performed on the sense (+) and antisense (−) strands. The amounts of recombinant CrtR used were 0 pmol (lanes 1 and 6), 10 pmol (lane 2), 20 pmol (lane 3), 40 pmol (lane 4), and 80 pmol (lane 5). The position number was based on that for the TSS of crtE promoter. The DNase I digests were run with the same probes that were chemically cleaved (G + A lanes for sense strand, C + T lanes for antisense strand).
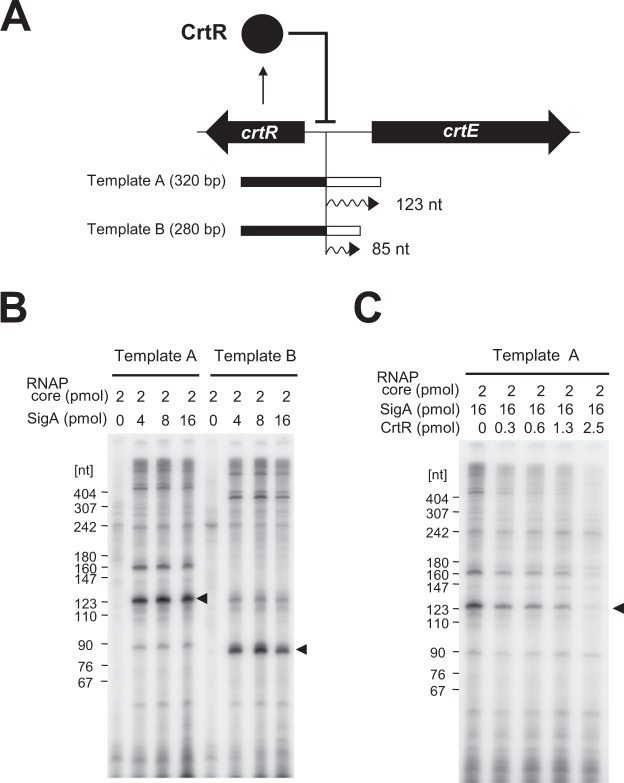
RNA polymerase SigA-directed crt transcription and its repression by CrtR
To verify the function of CrtR and to identify the sigma factor directing the transcriptional initiation of crtE and crtR, we performed an in vitro run-off transcriptional assay using recombinant proteins. Based on the existence of SigA-recognition sequence in the promoter region for crtE (Fig. 5B), a recombinant protein of SigA of C. glutamicum was overexpressed in E. coli and purified to near homogeneity (see Materials and Methods). To examine the specificity of SigA, two DNA fragments with different length were used as templates (Fig. 10A). As shown in Fig. 10B, an RNA polymerase containing SigA was used to synthesized mRNAs with the predicted length from crtE promoter. This indicated that SigA specifically recognized the −10 and −35 sequences preceding crtE. We also carried out an in vitro run-off assay in the presence of the CrtR recombinant protein. As shown in Fig. 10C, CrtR inhibited the generation of mRNA in a dose-dependent manner. This result clearly demonstrated that CrtR functions as a repressor.
Figure 10.
In vitro run-off transcriptional assay. (A) Two different length DNA templates including the crtE-crtR intergenic promoter region were used for the assay. The DNA length of Template A and Template B were 320 bp and 280 bp, respectively. The predicted length of the crtE transcript is 123 nt for Template A, and 85 nt for Template B. (B,C) In vitro run-off transcriptional assay. The indicated amounts of the RNA polymerase core enzyme of E. coli, SigA, and CrtR were used for the assay. The crtE transcripts with predicted lengths are shown by the closed triangles. Marker 10 (pBR322/MspI digest) was used as a size marker.
Discussion
In this study, we found that bacteria belonging to Corynebacteriales and Micrococcales exhibit light-inducible carotenoid production. The photochromogenicity in this group of bacteria has been known with Mycobacterium and used as an indicator for the classification of this genus34,35. Mycobacterium spp. belonging to the photochromogenic class include M. kansasii, M. marinum, M. asiaticum, M. simiae, M. parafortuitum, M. phelei, and M. vaccae. In a previous study on an opportunistic human pathogen M. marinum, the insertion of transposon into a gene encoding a MarR family regulator located in the proximal region of the crt biosynthesis gene cluster led to the constitutive production of carotenoids17. The MarR family regulator of M. marinum designated as CrtR (corresponding to MMAR_4811 product; Fig. 3) shares a 21.8% amino acid similarity with CrtR of C. glutamicum AJ1511. Although the similarity score is not high, CrtR was also located in the divergent region of the crt biosynthesis gene cluster. The synteny of the crtR and crt cluster was widely found in the genome of Mycobacterium including M. liflandii 128FXT, M. ulcerans Agy99, and M. avium 104. This suggests that CrtR may play a central role in the photochromogenicity of Mycobacterium spp.
The gene organization of crt biosynthesis genes and its regulator crtR is conserved in the bacteria affiliating with Corynebacteriales and Micrococcales. This implies that these bacteria show light-inducible carotenogenesis, and that a CrtR homolog is involved in the transcriptional regulation to prevent the biosynthesis under non-required condition. Furthermore, our present study showed that CrtR was expressed in a light dependent manner, suggesting that a MarR family regulator plays a central role in light-inducible carotenoid production. We assume that the CrtR expressed under light condition quickly switches off the expression of the target gene when it changes to dark condition, which contributes to save energy. Thus far, studies on light-inducible carotenoid production have been limited to a MerR family regulator, to which LitR/CarH family belongs, in non-phototrophic bacteria; however, our study suggested that the MarR family regulator also plays a role in light-inducible transcription in some bacteria.
C. glutamicum is an amino acid producer that has been studied as a model organism of primary metabolism; however, the environmental-factor-dependent control of secondary metabolite production has not been fully studied. Based on our results obtained in this study, we assume the following molecular mechanism of light-inducible transcription of carotenoid production in C. glutamicum (Fig. 10A). Under dark conditions, CrtR binds to the intergenic region of crtE and crtR, and serves as a negative regulator for the divergent transcription. Upon illumination, the DNA-binding activity of CrtR is diminished, probably due to the association of unidentified compounds as discussed below, and then SigA-RNA polymerase initiates transcription, which allows the expression of carotenoid biosynthesis genes. As a result, the expressed enzymes biosynthesize carotenoid to protect cells from photo-oxidants such as reactive oxygen species.
Recently, Henke et al.27 revealed that the transcription of crt biosynthesis gene cluster is controlled by CrtR in C. glutamicum ATCC 13032. It has been reported that the TTAA motif found in the 24–27 bases upstream from the TSS of crtE was essential for the binding of CrtR27. On the other hand, a similar motif, AAATTT in the 3–8 bases upstream of crtE was not essential for CrtR-binding. The TTAA motif was located in the center of the CrtR-binding sequence of C. glutamicum AJ1511 determined by DNase I footprinting (Figs 5A and 9). Generally, the binding site of MarR family proteins associate with 16–20 bp inverted repeats which may or may not be completely palindromic30. On the other hand, the length of CrtR-binding site determined by the DNase I footprint was 52 bp (+strand for crtE), which was longer than that of the typical MarR family protein. This suggests that CrtR may bind the multiple sites located in the intergenic region of crtE and crtR, although we could not find inverted repeats. Similar cases with this kind of transcriptional regulator have been known. For example, the length of the binding site of Sulfolobus solfataricus BldR is 40 bp36. PecS of Erwinia chrysanthemi is supposed to different operator sequences as a single or multiple dimers37 within its regulon, thereby demonstrating varying spans of protection from 20 to 100 bp.
Generally, apo-MarR-type regulator, a free ligand protein, represses the expression of the target gene by its binding to the operator, while in the presence of ligand, its activity as a repressor is diminished due to the interaction with the ligand30. Therefore, we hypothesize that CrtR binds to the promoter region in the absence of an unidentified ligand, and that the binding of its ligand inhibits the DNA-binding activity leading to the expression of crtE. There is a simple hypothesis that blue-light absorption by an unidentified ligand causes its binding to CrtR, which results in the aforementioned derepression and the activation of the crtE promoter. Based on this hypothesis, we examined whether known light-absorbing molecules such as FAD, FMN, para-coumaric acid, retinol, AdoB12 had an effect on the DNA-binding activity of CrtR, but the addition of the light-absorbing molecules did not affect effect on the DNA-binding activity of CrtR (our unpublished data). Recently, it has been reported that GGPP, a precursor of carotenoids, interfered with the in vitro DNA-binding activity of CrtR of C. glutamicum ATCC 1303227, but we have not yet succeeded in reproducing the effect of GGPP on CrtR activity.
The BBD29_03565 product belongs to a family of multidrug resistance pumps termed RND (resistance, nodulation, and division) proteins that recognize and mediate the transport of a great diversity of compounds38–40. In P. aeruginosa, the expression of the multidrug transporter MexAB-OprM, an RND family member, is regulated by MexR, a MarR-type regulator41. Currently, we do not think that BBD29_03565 is involved in carotenoid biosynthesis, since the inactivation of BBD29_03565 did not affect the production of carotenoids and its transcriptional level (our unpublished data). Possibly, it may play a role in the elimination of an unidentified ligand for CrtR, which becomes toxic if illuminated. However, the inactivation of BBD29_03565 did not affect its growth both under light and dark conditions, which suggests that the accumulation of the ligand is not toxic for C. glutamicum. The conservation of CrtR and RND family members in close proximity to each other was also found in the genome of Mycobacterium marinum M (Fig. 3), Clavibacter michiganensis, and Jonesia denitrificans (data not shown).
Although the exact mechanism therein is not yet known, the identification and characterization of the ligand of CrtR will help in understanding the exact mechanism of light-dependent transcriptional control in C. glutamicum and related bacteria. We anticipate that further studies focusing on the ligand for CrtR will elucidate the signaling pathway based on the unique light-responsive regulatory system in C. glutamicum. As evidenced by the previous report that an engineered C. glutamicum exhibited the high productivity of lycopene23, C. glutamicum harbors high potency of this bacterium as a production host of terpenoids. Our findings regarding the light-inducible gene expression may eventually reveal the ability of C. glutamicum and related bacteria to produce novel types of carotenoids and expand the possibility of their industrial application.
Methods
Bacterial strains, plasmids, and culture media
C. glutamicum AJ1511 (ATCC 13869) was obtained from Ajinomoto (Kanagawa, Japan). Other bacterial strains (Table 2) were obtained from Japan Collection of Microorganisms (JCM, Japan), NITE (NBRC, Japan), and American Type Culture Collection (ATCC, USA). The Escherichia coli strains HST08 (Takara Bio; Shiga, Japan) and BL21(DE3)pLysS (Merck KGaA, Darmstadt, Germany) were used as hosts for DNA manipulation and protein expression, respectively. The pUC118 and pMD19 (Takara Bio) plasmids were used as general cloning vectors in E. coli. The pK18mobsacB plasmid42 obtained from National BioResource Project (NIG, Japan), a kanamycin-resistance E. coli vector, was used for gene disruption in C. glutamicum. The pET26b( + ) plasmid (Merck) was used for the overexpression of CrtR and SigA in BL21(DE3)pLysS. The chemicals and enzymes used for DNA manipulation were purchased from Wako (Osaka, Japan), Kokusan (Tokyo, Japan), and Takara Bio unless otherwise indicated. For the screening media, Luria-Bertani (LB) containing 1.0% Bacto Tryptone (Becton, Dickinson and Co., Sparks, MD), 1.0% yeast extract (Becton, Dickinson), 0.5% NaCl (pH 7.2 by using NaOH), 10-fold diluted LB (pH 7.2) (designated 1/10 LB), 1/10 LB with 1.0% glucose (pH 7.2) (designated 1/10 LBG), and R2A (Becton, Dickinson) were used. To prepare the solid medium, 1.5% agar was added. The conditions for the culture and genetic manipulation of E. coli were performed as described previously43. C. glutamicum was grown on CM2B medium containing 1.0% polypeptone (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 1.0% yeast extract, and 0.5% NaCl (pH 7.0 by using KOH) or LB medium. To prepare the CM2B solid medium, 2.0% agar was added. Liquid culture was performed in a 500-mL baffled flask containing 100 mL medium in a rotary shaker at 135 rpm. Light irradiation was performed by an illuminating incubator (BR-180LF; Taitech; Saitama, Japan) equipped with a white or blue light fluorescent lamp (20 W; Toshiba; Tokyo, Japan). Light intensity was measured using a Model Li-250A Light Meter (LI-COR Inc., Lincoln, NE). To select for transformants of E. coli and C. glutamicum, ampicillin and kanamycin were added at 50 μg/ml.
Isolation of bacteria exhibiting light-dependent pigment production
To isolate bacteria, 1.0 g soil was suspended in 10 mL sterile distilled water, and incubated at room temperature for 1 h. The supernatant was diluted by distilled water, and spread on solid media including LB (pH 7.2), 1/10 LB (pH 7.2), 1/10 LBG (pH 7.2), and R2A. The single colonies were inoculated to two plates with tooth picks, and cultured under dark and light conditions at 28 °C for 2 or 3 d. The isolates exhibiting light-dependent pigment production were purified by single colony isolation method. A total of 24 strains out of 1,100 isolates exhibited photo-dependent yellow pigment production.
Taxonomic characterization of light-dependent isolates
To taxonomically characterize the isolates, total DNA was extracted from the isolates by the PurElute Bacterial Genomic Kit (Edge BioSystems; Gaithersburg, MD). The PCR amplicons of the 16S rRNA gene using the Eubacterium universal primer pair B8F/B1492R (the oligonucleotide primers used are summarized in Table S1) were inserted into pMD19 by TA-cloning. To determine the nucleotide sequence of the 16S rRNA gene clone, an ABI3100 automated DNA sequencer with a BigDye Terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific Inc., Waltham, MA) was used. The obtained nucleotide sequences of the 16S rRNA gene was compared with those in the GenBank/EMBL/DDBJ nucleotide sequence databases by using the BLASTN program (http://www.ncbi.nlm.nih.gov/BLAST/) and the SEQUENCE_MATCH program from the Ribosomal Database Project database44.
Molecular phylogenetic analysis
A neighbor-joining phylogenetic tree based on the full length amino acid sequence of CrtR homologs was constructed. The CrtR amino acid sequences, which are located in crt gene cluster of Micrococcales and Corynebacteriales, were obtained from the KEGG database (http://www.genome.jp/kegg/). The amino acid sequences were analyzed using ClustalW45 and MEGA 546. Molecular phylogenetic trees were reconstructed by the neighbor-joining method47. The distance matrix was calculated using Kimura’s two-parameter model48.
Carotenoid production
C. glutamicum AJ1511 (ATCC 13869) and C. glutamicum ATCC 13032 (JCM 1318) were cultured at 28 °C for 2 days on solid LB medium under dark and light conditions using an illuminating incubator equipped with white-light fluorescent lamps. Under light conditions, the solid culture was illuminated with white light at approximately 2.4 µmol s–1 m–2. The same lamp, covered with a blue-light filter, was used to generate blue (400–460 nm) light. The method of extracting carotenoids was described previously15. The absorption spectrum of the carotenoid fraction was recorded by using a UV spectrometer (UVmini-1240; Shimadzu, Kyoto, Japan).
Gene disruption of crtR
Construction of a markerless mutant of crtR was carried out using pK18mobsacB, which has a sacB gene to use as a counter-selective suicide marker42. To delete the crtR gene from the AJ1511 strain, regions approximately 1 kb upstream and downstream of crtR were amplified by PCR using the genomic DNA as a template with the two primer sets DL-F/DL-MR and DL-MF/DL-R (Table S1). The two amplified fragments were digested by BamHI, purified, and then ligated. The ligated products were then amplified by PCR using DL-F and DL-R, and the amplified fragment was digested with EcoRI and SphI, and then cloned between the same sites in pK18mobsacB in order to generate the disruption plasmid (pDIS). The pDIS plasmid was introduced into C. glutamicum by electroporation32, and the single crossover strains were able to grow on a LB agar plate containing kanamycin. Subsequently, the kanamycin-sensitive derivatives of the single crossover strains that were able to grow on LB agar plates containing 10% sucrose were selected. The expected double crossover-mediated homologous recombination in such derivatives was confirmed by PCR. The resulting kanamycin-sensitive recombinants were assessed to identify true recombinants by performing appropriate PCR and hybridization experiments, and one of the true recombinants was described to be a crtR-null mutant.
Plasmid for genetic complementation
For the complementation experiment in C. glutamicum, we first constructed a chromosome integration vector, pKMT1, for homologous recombination, which retains a partial gene sequence of thrC (NCgl2046), a threonine dehydratase. The internal region corresponding to 113 to 1261 nt of thrC was amplified by PCR using the primer pair thrCF and thrCR (Table S1). The DNA fragment containing thrC was digested with MfeI and inserted between the EcoRI sites of pK18mob to generate pKMT1. The intact crtR with its own promoter was generated by PCR using the primer sets limF/limR (Table S1), and cloned between the BamHI and SphI site of pKMT1 to generate pKMT-crtR. The resulting plasmid was introduced into the ΔcrtR strain by electroporation, and a single crossover recombination gave rise to strain ΔcrtR/pKMT-crtR. In all cases, proper integration was verified by PCR with the appropriate primer pairs.
RNA preparation and quantitative RT-PCR analysis
C. glutamicum strains were cultured in LB liquid medium under dark and blue light conditions at 28 °C for 10 h and 15 h by using an illuminating shaker at an intensity of 15.03 μmol·s–1·m–2. The total RNA of the C. glutamicum strains was extracted with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instruction. The concentration of total RNA was measured with the NanoDrop Lite (Thermo Fisher Scientific, Rockford, IL, USA). The following cDNA synthesis and its quantification with PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) and an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific) was performed according to manufacturer’s manual and our previous study22. For sample normalization, 16S rRNA gene was used as an internal control. The cDNA of sigA, crtE, crtI, BBD29_03565, crtR, and phrB were detected by the primer pairs, sigA-F/sigA-R, crtE-F/crtE-R, 3565-F/3565-R, limR-F/limR-R, and phrB-F/phrB-R, respectively (Table S1). Quantification of relative gene expression was calculated by the relative quantitative 2−ΔΔCt method49 using the signals of the 16S rRNA gene as internal references. All reactions were performed in triplicate.
5′ RACE
The TSSs of crtR and crtE were determined using a 5′-Full RACE Core Set (Takara Bio) and by Directed Amplification of TSSs (DMTSS)50, respectively. The 5′-Full RACE Core Set was used according to the manufacturer’s instructions, and DMTSS was performed as described15,50. In both experiments, 2 µg of total RNA and gene-specific primers (RT-RACE) were used (Table S1). The resulting PCR products were cloned into a pMD19 vector based on the TA cloning technique. The inserted DNA sequences were sequenced using an ABI 3100 Genetic analyzer or the sequencing was performed by Eurofins Genomics K.K. (Tokyo, Japan).
Preparation of recombinant proteins
For the expression of CrtR in E. coli, the corresponding coding sequences were amplified by PCR using the primers LimRex-F/LimRex-R (Table S1). The resultant amplicon was digested with NdeI and XhoI and then inserted between the same sites of pET-26b(+). The resulting plasmid directed the expression of CrtR fused to a C-terminal 6× His-tag in E. coli BL21(DE3)pLysS. The expression and purification of the recombinant followed the standard protocol for the His-tagged protein recommended by the manufacturer. To prepare the recombinant protein SigA (NCgl1836) with a His-tag at its C-terminus, a housekeeping major sigma factor, a protein expression vector for sigA from C. glutamicum ATCC 13032 similar to CrtR, was constructed. The primer set used was SigAex-F/SigAex-R (Table S1). The SigA protein was over-expressed in E. coli BL21(DE3)pLysS and purified to near homogeneity by affinity chromatography according to manufacturer’s instruction. The absorption spectra of the resultant recombinants were recorded by using a Cary 60 UV-Vis spectrophotometer (Agilent Technologies) or Multiskan GO spectrophotometer (Thermo Fisher Scientific). The protein concentration was measured with a protein assay kit (Bio-rad, Laboratories, Hercules, CA), and the absorbance was measured with the NanoDrop Lite (Thermo Fisher Scientific).
Gel shift assay
DNA-binding was determined by a gel shift assay. The DNA fragments containing the promoter region were amplified by PCR with primer sets PCL-F/PCL-R for PcrtR-PcrtE, and PA-F/PA-R for PsigA (Table S1). The resultant PCR amplicons were cloned into the pMD19 vector by TA cloning. To prepare the probes that were labelled with Cy-5 on the 5′-end, the cloned DNA fragment was amplified with a primer set consisting of Cy-5-labelled pMD19F(Cy5) and a non-labelled pMD19R (Table S1), both of which anneal to the pMD19 vector. A total of 1.6 pmol of Cy-5-labeled probe was mixed with 0–0.4 pmol of recombinant CrtR, and then incubated at 30 °C for 30 min in 50 μl of binding buffer containing 10 mM Tris-HCl (pH 7.2), 50 mM NaCl, 1 mM EDTA, 10% glycerol, and 0.5 μg poly(dI-dC). The reaction mixes were incubated at 30 °C for 30 min, and specific DNA–protein complexes were separated from the free probe on a non-denaturing polyacrylamide gel containing 6% acrylamide. The gels were imaged with a Typhoon 9410 image analyzer (GE healthcare).
DNase I footprint
To determine the binding site of CrtR, a DNase I footprint analysis was carried out as described previously15. The 32P-labeled DNA fragments were amplified by PCR using the primer pair DFP-F1*/DFP-R1 and DFP-F2*/DFP-R1 for the sense strand and DFP-F2/DFP-R2* and DFP-F2/DFP-R1* for the antisense strand. The 50-µl reaction mixture contained 10 kcpm 32P-labeled DNA probe, 10 to 80 pmol CrtR, 25 mM HEPES-KOH (pH 7.9), 0.5 mM EDTA-NaOH (pH 8.0), 50 mM KCl, and 10% glycerol. After incubation at 30 °C for 30 min, DNase I was added at a final concentration of 20 µg/ml, and the mixture was further incubated for 1 min at 25 °C. The reaction was terminated by the addition of 100 µl of the stop solution (containing 100 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1% sodium N-lauroyl sarcosinate, 10 mM EDTA-NaOH [pH 8.0], and 25 mg/ml salmon sperm DNA) and 300 µl of phenol-chloroform (1:1). After ethanol precipitation, the pellet was washed with 80% ethanol, and dissolved in a 6-µl formamide dye mixture. The samples ware applied to a 6% urea-polyacrylamide gel. Maxam-Gilbert sequencing ladders (G + A and T + C reactions) generated from the 32P-labeled probe DNA fragment were used as a reference. The gels were imaged with a Typhoon 9410 image analyzer.
In vitro run-off transcriptional assay
To analyze the function of SigA and CrtR, an in vitro run-off transcriptional assay was performed as described previously15. To prepare two DNA templates with different lengths, DNA fragments including the intergenic region for crtE-crtR were prepared by PCR with the primer pairs Runoff-F/Runoff-RA (Template A; 320 bp) and Runoff-F/Runoff-RB (Template B; 280 bp). A commercially available RNA polymerase core enzyme of E. coli (AR Brown, Tokyo, Japan) was used because this enzyme has been frequently used in experiments in Streptomyces. The [γ-32P]ATP-labeled Marker 10 (pBR322 cut with MspI) (NIPPON GENE CO., LTD., Tokyo, Japan) was used as a size marker. The reaction products were separated by denaturing polyacrylamide gel containing 6% acrylamide and 8 M urea. The radioactive signals detected were similar to those for the DNase I footprint.
Supplementary information
Supplementary Table S1, Figure S1, and Figure S2
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (C) for the support of scientists (no. 16K07676), Japan, the Noda Institute for Scientific Research, the Foundation NAGASE Science Technology Development and the Charitable Trust of the Araki Medical and Biochemical Research Memorial Fund.
Author Contributions
H.T. and K.U. designed the experiments. M.T., S.W., T.M., Y.T., T.K., Y.I., Y.S., and H.(S).T. performed isolation and taxonomic characterization of bacteria. Y.S., K.M., and E.W. performed quantification of carotenoids. S.S. and H.(S).T. performed molecular phylogenetic analysis. H.T. and T.M. performed gene disruption and genetic complementation. S.S. performed transcriptional analysis. Y.S. and H.T. performed gel-shift assay. H.T. performed DNase I footprint analysis and in vitro run-off transcriptional assay. H.T. and K.U. wrote the manuscript. H.T. and K.U. integrated the overall research project. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Satoru Sumi and Yuto Suzuki contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49384-7.
References
- 1.Armstrong GA. Genetics of eubacterial carsotenoid biosynthesis: a colorful tale. Annu Rev Microbiol. 1997;51:629–659. doi: 10.1146/annurev.micro.51.1.629. [DOI] [PubMed] [Google Scholar]
- 2.Bonet, M. L., Canas, J. A., Ribot, J. & Palou, A. Carotenoids in nature: biosynthesis, regulation, and function. Springer International Publishing, Cham. (2016).
- 3.Baranski, R. & Cazzonelli, C. I. Carotenoid Biosynthesis and Regulation in Plants 159–189. Carotenoids. John Wiley & Sons, Ltd, Chichester, UK. (2016).
- 4.Krubasik P, Kobayashi M, Sandmann G. Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem. 2001;268:3702–3708. doi: 10.1046/j.1432-1327.2001.02275.x. [DOI] [PubMed] [Google Scholar]
- 5.Gruszecki WI, Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta. 2005;1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants - A review. J Photochem Photobiol B Biol. 1997;41:189–200. doi: 10.1016/S1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 7.Takano H, Asker D, Beppu T, Ueda K. Genetic control for light-induced carotenoid production in non-phototrophic bacteria. J Ind Microbiol Biotechnol. 2006;33:88–93. doi: 10.1007/s10295-005-0005-z. [DOI] [PubMed] [Google Scholar]
- 8.Takano H, Obitsu S, Beppu T, Ueda K. Light-induced carotenogenesis in Streptomyces coelicolor A3(2): Identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J Bacteriol. 2005;187:1825–1832. doi: 10.1128/JB.187.5.1825-1832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano H, et al. Involvement of CarA/LitR and CRP/FNR family transcriptional regulators in light-induced carotenoid production in Thermus thermophilus. J Bacteriol. 2011;193:2451–2459. doi: 10.1128/JB.01125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takano H, et al. LdrP, a cAMP receptor protein/FNR family transcriptional regulator, serves as a positive regulator for the light-inducible gene cluster in the megaplasmid of Thermus thermophilus. Microbiology. 2014;160:2650–2660. doi: 10.1099/mic.0.082263-0. [DOI] [PubMed] [Google Scholar]
- 11.Jost M, et al. Structural basis for gene regulation by a B12-dependent photoreceptor. Nature. 2015;526:536–541. doi: 10.1038/nature14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutta RJ, et al. The photochemical mechanism of a B12-dependent photoreceptor protein. Nat Commun. 2015;6:7907. doi: 10.1038/ncomms8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padmanabhan S, Jost M, Drennan CL, Elías-Arnanz M. A New Facet of Vitamin B12: Gene Regulation by Cobalamin-Based Photoreceptors. Annu Rev Biochem. 2017;86:485–514. doi: 10.1146/annurev-biochem-061516-044500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jost M, Simpson JH, Drennan CL. The Transcription Factor CarH Safeguards Use of Adenosylcobalamin as a Light Sensor by Altering the Photolysis Products. Biochemistry. 2015;54:3231–3234. doi: 10.1021/acs.biochem.5b00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano H, et al. Role and Function of LitR, an Adenosyl B12-Bound Light-Sensitive Regulator of Bacillus megaterium QM B1551, in Regulation of Carotenoid Production. J Bacteriol. 2015;197:2301–2315. doi: 10.1128/JB.02528-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández-Zapata J, et al. Plasticity in oligomerization, operator architecture, and DNA binding in the mode of action of a bacterial B12-based photoreceptor. J Biol Chem. 2018;293:17888–17905. doi: 10.1074/jbc.RA118.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao LY, et al. Transposon mutagenesis of Mycobacterium marinum identifies a locus linking pigmentation and intracellular survival. Infect Immun. 2003;71:922–929. doi: 10.1128/IAI.71.2.922-929.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutthiwong N, Dufossé L. Production of carotenoids by Arthrobacter arilaitensis strains isolated from smear-ripened cheeses. FEMS Microbiol Lett. 2014;360:174–181. doi: 10.1111/1574-6968.12603. [DOI] [PubMed] [Google Scholar]
- 19.Takano H. The regulatory mechanism underlying light-inducible production of carotenoids in nonphototrophic bacteria. Biosci Biotechnol Biochem. 2016;8451:1–10.. doi: 10.1080/09168451.2016.1156478. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz-Guerrero JM, Polanco MC, Murillo FJ, Padmanabhan S, Elías-Arnanz M. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc Natl Acad Sci USA. 2011;108:7565–7570. doi: 10.1073/pnas.1018972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Marín MC, Padmanabhan S, Polanco MC, Murillo FJ, Elías-Arnanz M. Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol Microbiol. 2008;67:804–819. doi: 10.1111/j.1365-2958.2007.06086.x. [DOI] [PubMed] [Google Scholar]
- 22.Sumi, S., Shiratori-Takano, H., Ueda, K & Takano, H. Role and Function of Class III LitR, a Photosensor Homolog from Burkholderia multivorans. J Bacteriol 200 (2018). [DOI] [PMC free article] [PubMed]
- 23.Heider SAE, Peters-Wendisch P, Wendisch VF. Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol. 2012;12:198. doi: 10.1186/1471-2180-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meddeb-Mouelhi F, Moisan JK, Bergeron J, Daoust B, Beauregard M. Structural Characterization of a Novel Antioxidant Pigment Produced by a Photochromogenic Microbacterium oxydans Strain. Appl Biochem Biotechnol. 2016;180:1286–1300. doi: 10.1007/s12010-016-2167-8. [DOI] [PubMed] [Google Scholar]
- 25.Han S-R, Kim K-H, Ahn D-H, Park H, Oh T-J. Complete genome sequence of carotenoid-producing Microbacterium sp. strain PAMC28756 isolated from an Antarctic lichen. J Biotechnol. 2016;226:18–19. doi: 10.1016/j.jbiotec.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Krubasik P, Sandmann G. A carotenogenic gene cluster from Brevibacterium linens with novel lycopene cyclase genes involved in the synthesis of aromatic carotenoids. Mol Gen Genet. 2000;263:423–432. doi: 10.1007/s004380051186. [DOI] [PubMed] [Google Scholar]
- 27.Henke NA, Heider SAE, Hannibal S, Wendisch VF, Peters-Wendisch P. Isoprenoid Pyrophosphate-Dependent Transcriptional Regulation of Carotenogenesis in Corynebacterium glutamicum. Front Microbiol. 2017;8:633. doi: 10.3389/fmicb.2017.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrou J, Crosson S. Function, structure and mechanism of bacterial photosensory LOV proteins. Nat Rev Microbiol. 2011;9:713–723. doi: 10.1038/nrmicro2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Losi A, Gärtner W. Solving Blue Light Riddles: New Lessons from Flavin-binding LOV Photoreceptors. Photochem Photobiol. 2017;93:141–158. doi: 10.1111/php.12674. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson SP, Grove A. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr Issues Mol Biol. 2006;8:51–62. [PubMed] [Google Scholar]
- 31.Deochand DK, Grove A. MarR family transcription factors: dynamic variations on a common scaffold. Crit Rev Biochem Mol Biol. 2017;52:595–613. doi: 10.1080/10409238.2017.1344612. [DOI] [PubMed] [Google Scholar]
- 32.Takano H, et al. Characterization of developmental colony formation in Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2008;81:127–134. doi: 10.1007/s00253-008-1622-z. [DOI] [PubMed] [Google Scholar]
- 33.Pátek M, Nešvera J. Sigma factors and promoters in Corynebacterium glutamicum. J Biotechnol. 2011;154:101–113. doi: 10.1016/j.jbiotec.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Wayne LG, et al. Highly reproducible techniques for use in systematic bacteriology in the genus Mycobacterium: tests for pigment, urease, resistance to sodium chloride, hydrolysis of Tween 80, and β-galactosidase. Int J Syst Bacteriol. 1974;24:412–419. doi: 10.1099/00207713-24-4-412. [DOI] [Google Scholar]
- 35.Tsukamura M. Relationship Between Photochromogenicity and Test Temperature in Mycobacteria. J Clin Microbiol. 1981;14:225–226. doi: 10.1128/jcm.14.2.225-226.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiorentino G, Ronca R, Cannio R, Rossi M, Bartolucci S. MarR-like transcriptional regulator involved in detoxification of aromatic compounds in Sulfolobus solfataricus. J Bacteriol. 2007;189:7351–7360. doi: 10.1128/JB.00885-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hommais F, et al. PecS is a global regulator of the symptomatic phase in the phytopathogenic bacterium Erwinia chrysanthemi 3937. J Bacteriol. 2008;190:7508–7522. doi: 10.1128/JB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandhu P, Akhter Y. Evolution of structural fitness and multifunctional aspects of mycobacterial RND family transporters. Arch Microbiol. 2018;200:19–31. doi: 10.1007/s00203-017-1434-6. [DOI] [PubMed] [Google Scholar]
- 39.Blair JMA, Piddock LJV. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr Opin Microbiol. 2009;12:512–9. doi: 10.1016/j.mib.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Putman M, van Veen HW, Konings WN. Molecular Properties of Bacterial Multidrug Transporters. Microbiol Mol Biol Rev. 2000;64:672–693. doi: 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, et al. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc Natl Acad Sci USA. 2008;105:13586–13591. doi: 10.1073/pnas.0803391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J. & Russell, W. D. Molecular Cloning: A Laboratory Manual. Cold Spring Harb Lab Press Cold Spring Harb NY. (2001)
- 44.Cole JR, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–42. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 48.Givens GD, Seidemann MF. Middle ear measurements in a difficult to test mentally retarded population. Ment Retard. 1977;15:40–42. [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Mendoza-Vargas A, et al. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS One. 2009;4:e7526. doi: 10.1371/journal.pone.0007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1, Figure S1, and Figure S2