Abstract
The scavenging capacity of glycoprotein DMBT1 helps defend mucosal epithelia against microbes. DMBT1 binding to multiple bacterial species involves its conserved Scavenger Receptor Cysteine-Rich (SRCR) domains, localized to a 16-mer consensus sequence peptide, SRCRP2. Previously, we showed that DMBT1 bound Pseudomonas aeruginosa pili, and inhibited twitching motility, a pilus-mediated movement important for virulence. Here, we determined molecular characteristics required for twitching motility inhibition. Heat-denatured DMBT1 lost capacity to inhibit twitching motility and showed reduced pili binding (~40%). Size-exclusion chromatography of Lys-C-digested native DMBT1 showed that only high-Mw fractions retained activity, suggesting involvement of the N-terminal containing repeated SRCR domains with glycosylated SRCR-Interspersed Domains (SIDs). However, individual or pooled consensus sequence peptides (SRCRPs 1 to 7) showed no activity and did not bind P. aeruginosa pili; nor did recombinant DMBT1 (aa 1–220) or another SRCR-rich glycoprotein, CD163. Enzymatic de-N-glycosylation of DMBT1, but not de-O-glycosylation, reduced its capacity to inhibit twitching motility (~57%), without reducing pili binding. Therefore, DMBT1 inhibition of P. aeruginosa twitching motility involves its N-glycosylation, its pili-binding capacity is insufficient, and it cannot be conferred by the SRCR bacteria-binding peptide domain, either alone or mixed with other unlinked SRCRPs, suggesting an additional mechanism for DMBT1-mediated mucosal defense.
Subject terms: Cellular microbiology, Infectious diseases
Introduction
DMBT1 (Deleted in Malignant Brain Tumors 1) is a ~340 kDa glycoprotein that was first isolated from saliva, belongs to the highly conserved Scavenger Receptor Cysteine-Rich (SRCR) protein superfamily, and is involved in mucosal innate immunity1. From the N-terminal, DMBT1 contains 13 highly homologous SRCR domains separated by SIDs (SRCR-interspersed domains). A 14th SRCR domain is separated from the other 13 by a CUB (C1r/C1s Uegf Bmp1) domain, another of which separates the 14th domain from the ZP (zona pellucida) domain that forms the C-terminal2,3. DMBT1 salivary agglutinin (DMBT1SAG) has been shown to interact with, and agglutinate, several Gram-positive and Gram-negative bacteria, except for Pseudomonas aeruginosa4–8. Similarly, tear fluid DMBT1 was shown to bind Staphylococcus aureus, but not P. aeruginosa9. DMBT1 binding of Streptococcus mutans and many other bacteria, e.g. S. aureus, Escherichia coli, and Helicobacter pylori involves the SRCR domains, and was specifically localized to a 16 amino acid consensus sequence peptide of DMBT1 designated SRCRP23.
DMBT1 is expressed in multiple tissues and body fluids and can undergo modifications that affect its function at specific sites1,9. Indeed, there are different human DMBT1 alleles within the population and different isoforms in various tissues due to alternative splicing and post-translational modifications1,4,10–12. In salivary-derived DMBT1SAG, ~25% of the molecular mass is due to glycosylation (~10% for N-glycosylation, and ~15% for O-glycosylation)13,14.
Previously, we showed that tear fluid DMBT1, and DMBT1 purified from saliva, play a protective role for mucosal tissues by inhibiting twitching motility of P. aeruginosa15. Bacterial twitching motility is a surface-associated movement commonly used by Gram-negative bacteria driven by extension and retraction of Type IV pili (T4P)16. In P. aeruginosa, the T4P is a polymer predominately made up by the PilA subunit17. Extension and retraction, required for movement, is powered by ATPases PilB, PilU, and PilT18. In previous studies, we showed that P. aeruginosa twitching mutants were defective in host cell exit after cell invasion, exhibited impaired traversal of corneal epithelia in vitro19, and showed reduced virulence in the injured murine cornea20. Consistent with those results, in the study above15, purified DMBT1 inhibited P. aeruginosa from traversing multi-layered cultured epithelial cells in vitro, and reduced P. aeruginosa virulence in a murine model of corneal infection. It was also shown that DMBT1 could bind extracted pili from P. aeruginosa suggesting the involvement of pilus interaction in twitching inhibition.
Given the importance of twitching motility for P. aeruginosa virulence20, we sought to further understand mechanisms by which DMBT1 inhibits twitching motility, the relationship to pilus binding, and if the SRCR domains were involved. We report structure-function studies using purified salivary DMBT1 (DMBT1SAG), enzymatically-digested fragments, and synthetic DMBT1 consensus sequence peptides (SRCRP1–7) aimed at identifying the molecular domains and characteristics involved in mediating this host defense against P. aeruginosa.
Results
DMBT1 inhibition of P. aeruginosa twitching motility is lost and pili binding reduced after heat denaturation
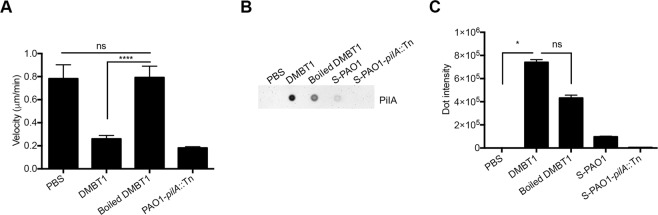
Previously, we showed that DMBT1 purified from human saliva inhibited P. aeruginosa (PAO1) twitching motility and could also bind to extracted pili15. To determine if these activities were heat-stable, purified salivary glycoprotein DMBT1 was boiled for 10 min at 95 °C. This protein-denaturing treatment abrogated DMBT1 inhibition of twitching motility (as measured by twitching velocity) (Fig. 1A) suggesting that glycoprotein conformation is important for this activity. Furthermore, the anti-PilA dot-blot and its quantification showed that boiled DMBT1 exhibited reduced ability to bind extracted pili (~40%) compared to untreated DMBT1 (Fig. 1B,C). Although the reduction of pili binding after heat denaturation was not statistically significant, the data suggest that DMBT1 binding to pilin is optimal in its native conformation.
Figure 1.
Boiled DMBT1 does not inhibit P. aeruginosa twitching motility. DMBT1 (100 ng/µL) was heated at 95 °C for 10 min to denature the protein. (A) Boiled DMBT1 lost inhibitory activity against P. aeruginosa PAO1 twitching velocity. Data are shown as the mean ± SEM per sample from three independent experiments. One-way ANOVA with Tukey’s post-hoc analysis, ****P < 0.0001; ns, not significant. (B) Dot-immunoblot assay using anti-PilA antibody to show binding of PAO1 pili to DMBT1 (500 ng) or boiled DMBT1 (500 ng) after 40 min incubation with a pili-containing extract from PAO1. Diluted pili extracts from PAO1 (S-PAO1) or similarly diluted extracts from its pilA mutant (S-PAO1-pilA::Tn) served as controls (see Methods), along with PBS alone. A representative experiment of two independent experiments is shown. (C) Quantification of dot-intensity from the dot-immunoblot assay shown in panel B. Data are shown as the mean ± SEM of triplicate measurements from each sample. Kruskal-Wallis test with Dunn’s multiple comparisons, *P < 0.05; ns, not significant.
Lys-C endoproteinase digestion of DMBT1 does not abolish inhibition of twitching motility
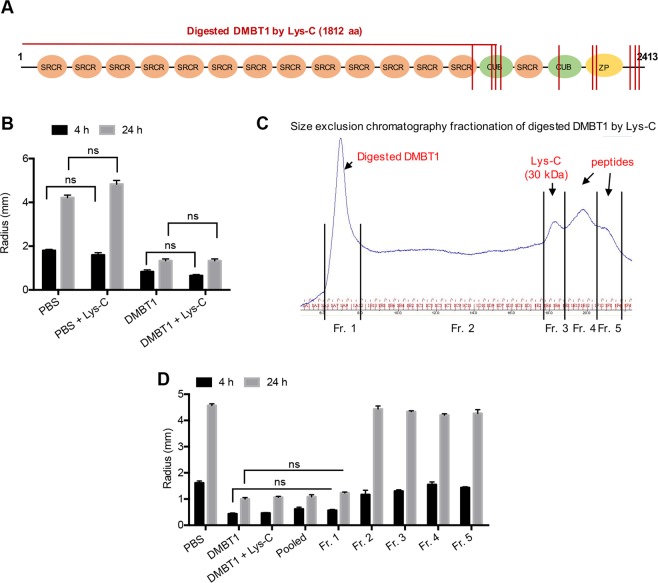
To further investigate the role of different DMBT1 domains in the inhibition of P. aeruginosa twitching motility, purified salivary DMBT1 was enzymatically digested with Lys-C, an endoproteinase. Others have shown that DMBT1 contains 10 lysine residues located in the C-terminal region3. As such, Lys-C digestion should produce a large fragment containing the majority of SRCR domains and SRCR Interspersed Domains (SIDs) in addition to several smaller fragments (Fig. 2A). Lys-C endoproteinase digested DMBT1 retained inhibition of P. aeruginosa twitching motility (Fig. 2B). Size exclusion chromatography of Lys-C digested DMBT1 under native buffer conditions showed an expected pattern of fractionation (Fig. 2C). Each Lys-C digested DMBT1 fraction obtained under native buffer conditions was tested for inhibition of P. aeruginosa twitching motility. Only Fraction 1, expected to contain fragments of combined high molecular-weight SRCR/SID domains (aa 1–1812), along with intact DMBT1, inhibited bacterial twitching motility (Fig. 2D), suggesting that the SRCR/SID components of DMBT1 were required, and showing that smaller fragments of the C-terminal region produced by Lys-C digestion were ineffective.
Figure 2.
DMBT1 digested by Lys-C under native conditions inhibits P. aeruginosa twitching motility. (A) Schematic representation of human salivary DMBT1 with 14 scavenger receptor cysteine-rich (SRCR) domains, 2 CUB domains, and the Zona Pellucida (ZP) domain, showing endoproteinase Lys-C cleavage sites (red lines). (B) Effect of Lys-C digested DMBT1 (100 ng/µL) versus a DMBT1 control on colony size of P. aeruginosa strain PAO1 after 4 h and 24 h incubation. Two-way ANOVA with Tukey’s multiple comparisons, ns, not significant. (C) Size exclusion chromatography separation of Lys-C digested DMBT1. Fraction 1 contains high molecular-weight fragments (including aa 1–1812). (D) Colony size of P. aeruginosa after 4 h and 24 h incubation in different fractions of Lys-C digested DMBT1. Only fraction 1 inhibited P. aeruginosa twitching motility, similar to DMBT1. Two-way ANOVA with Tukey’s multiple comparisons, ns, not significant.
Evaluation of DMBT1 SRCR domains, its N-terminal peptide, and CD163
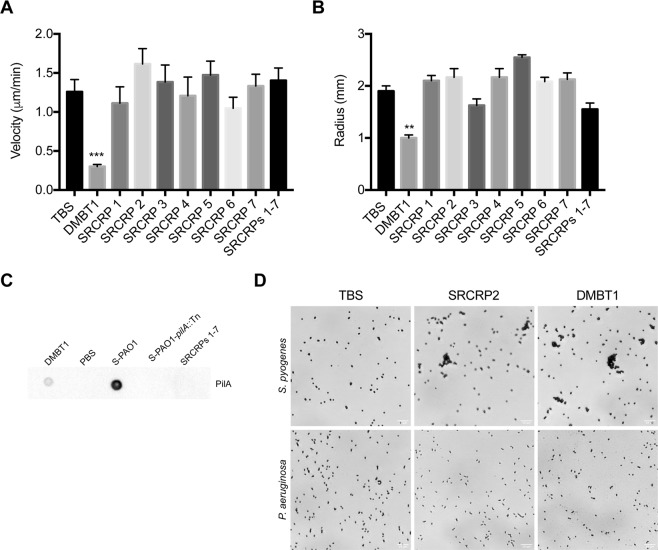
Fraction 1 of Lys-C digested DMBT1 should have contained high-molecular weight fragments consisting of combined multiple SRCR/SID domains (aa 1–1812). Thus, a series of custom-synthesized peptides (SRCRP) spanning the consensus sequence of the DMBT1 SRCR domains3 were tested. Indeed, SRCRP2 has previously been shown to be involved in bacterial binding3. However, each of the individual SRCR consensus-based peptides tested (SRCRPs 1–7) had no effect on P. aeruginosa twitching motility (Fig. 3A,B), and no inhibition was observed when these peptides were pooled (Fig. 3A,B). It was also confirmed that (pooled) SRCR peptides (1–7) did not bind pili (Fig. 3C). A control experiment confirmed previous findings that SRCRP2 caused agglutination/aggregation of Streptococcus spp. (shown here using S. pyogenes)3, but not P. aeruginosa (Fig. 3D). As we have shown previously15, purified salivary DMBT1 inhibited P. aeruginosa twitching motility (Fig. 3A,B), but did not cause bacterial agglutination/aggregation (Fig. 3D). Thus, exposing P. aeruginosa to the consensus sequence of the DMBT1 SRCR domains (albeit in fragments) was insufficient to inhibit twitching motility, or to bind extracted pili, supporting our previous hypothesis15 that DMBT1 inhibition of P. aeruginosa twitching motility involves a different type of bacterial cell interaction than shown for other bacterial species3.
Figure 3.
SRCR domain consensus sequence peptides do not inhibit twitching motility. Effect of custom-synthesized peptides SRCRPs 1–7 (200 ng/µL diluted in TBS with 10 mM CaCl2) on (A) twitching velocity, and (B) colony size, of P. aeruginosa strain PAO1 after 4 h. Data are shown as the mean ± SEM. Experiments were performed in triplicate and significance determined using one-way ANOVA with Dunnett’s multiple comparisons test. ***P < 0.001, **P < 0.01. None of the peptides showed a significant difference versus the TBS (with 10 mM CaCl2) control. (C) Dot-immunoblot assay using anti-PilA antibody shows the binding of PAO1 pili to purified DMBT1 (400 ng), but not to pooled SRCRPs 1–7 (400 ng). Diluted pili extracts from PAO1 (S-PAO1) or those from its pilA mutant (S-PAO1-pilA::Tn) and PBS were used as controls. (D) Agglutination assay showing that purified salivary DMBT1, and the consensus sequence peptide SRCRP2, agglutinated/aggregated S. pyogenes (upper panels) as previously shown19, but not P. aeruginosa (lower panels), versus TBS controls (see Methods).
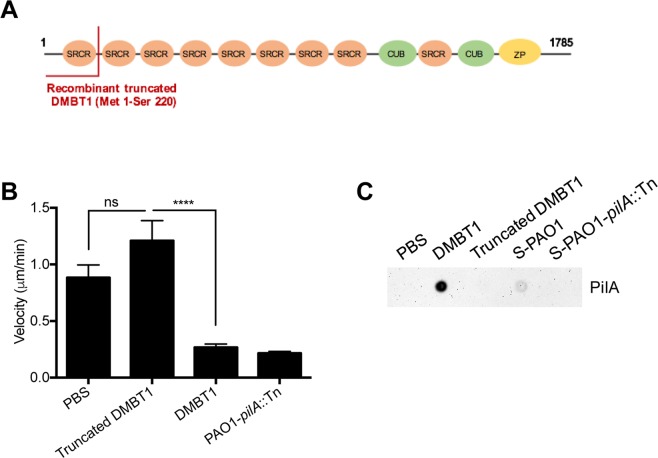
Since Fraction 1 of Lys-C digested DMBT1 would also contain the N-terminal region (albeit as part of a large fragment containing multiple SRCRs/SIDs), a recombinant truncated peptide consisting of the N-terminal domain of DMBT1 (Met1-Ser 220) (Fig. 4A) was tested. However, this recombinant N-terminal peptide had no effect on twitching motility (Fig. 4B), and did not bind pili (Fig. 4C).
Figure 4.
Recombinant DMBT1 (Met 1 - Ser 220) has no effect on P. aeruginosa twitching motility. (A) Schematic representation of recombinant truncated human DMBT1 (NP_004397.2) (Met 1-Ser 220) shown in the primary sequence of DMBT1 with 9 scavenger receptor cysteine-rich (SRCR) domains, 2 CUB domains and Zona pellucida (ZP) domain. (B) Effect of truncated DMBT1 (100 ng/µL) on twitching velocity of P. aeruginosa strain PAO1 after 4 h. PAO1-pilA::Tn served as a negative control. One-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001. (C) Dot-immunoblot assay using anti-PilA antibody shows binding of extracted PAO1 pili to DMBT1 (500 ng), but not truncated DMBT1 (500 ng). Pili extracts from PAO1 (S-PAO1), extracts from the pilA mutant (S-PAO1-pilA::Tn), both diluted 1 in 500 in PBS were used as controls, along with PBS alone.
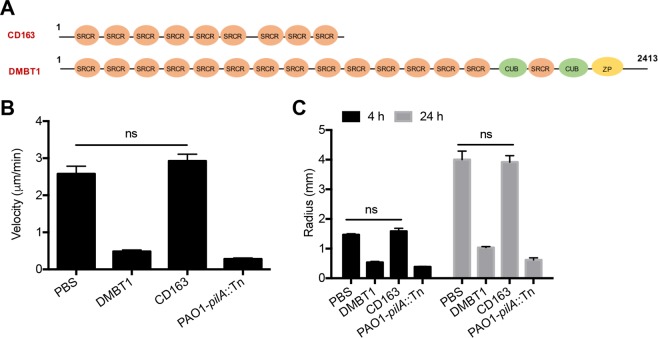
CD163 was also tested for inhibition since it is a member of the SRCR-superfamily (Fig. 5A). Moreover, this plasma membrane glycoprotein is expressed on monocytes and macrophages and functions as a receptor for bacteria, resulting in the expression of proinflammatory cytokines21. However, results showed that recombinant human CD163 had no effect on P. aeruginosa twitching motility (Fig. 5B,C), despite considerable similarities in structure, i.e. multiple SRCR/SID regions, to DMBT1.
Figure 5.
Glycoprotein CD163 does not inhibit P. aeruginosa PAO1 twitching motility. (A) Schematic representation of CD163 as expressed in HEK 293 cells (BioVision, Inc.) compared to human salivary DMBT1. (B) Effect of CD163 (200 ng/µL) on twitching velocity of P. aeruginosa PAO1 after 4 h treatment (One-way ANOVA with Tukey’s multiple comparisons, ns, not significant), and (C) on colony size after 4 h and 24 h. PAO1-pilA::Tn served as a negative control. Significance determined using two-way ANOVA with Tukey’s multiple comparisons, ns, not significant.
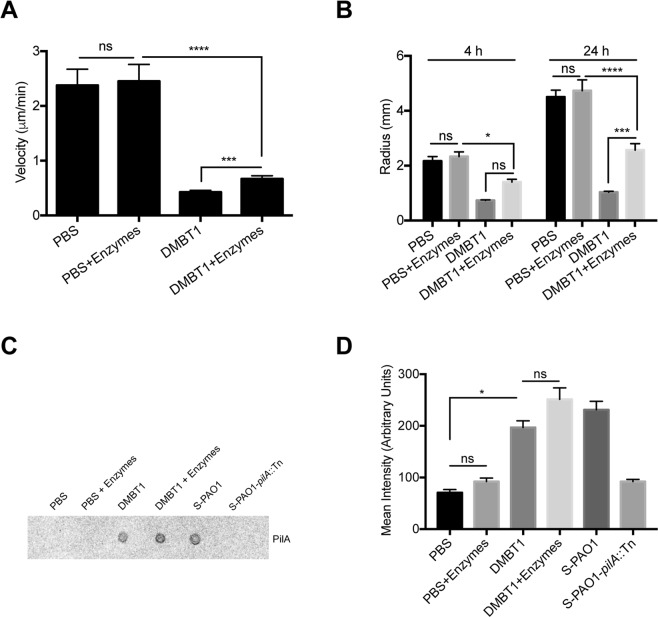
N-glycosylation of DMBT1 is important for its inhibition of P. aeruginosa twitching motility
Having established that inhibition of twitching motility resided in high molecular-weight fractions of Lys-C digested DMBT1 containing the N-terminal region and combined SRCR/SID domains (aa 1–1812), but without finding activity on individual SRCR peptides of DMBT1 or proteins with similar SRCR composition, we studied if post-translational modifications were involved. Since DMBT1 is a highly-glycosylated protein2, we postulated that glycosylation was involved in twitching inhibition. To test this, DMBT1 was digested by a deglycosylation enzyme mix containing N-glycosidase PNGase F, O-glycosidase, neuraminidase, exoglycosidase β1-4 galactosidase, and β-N-acetylglucosaminidase under native conditions to remove all N-linked and simple O-linked glycans, as well as some complex O-linked glycans (see Methods). After deglycosylation, DMBT1 showed significantly reduced inhibition activity of P. aeruginosa twitching motility compared to native DMBT1 controls, as measured by twitching velocity (P < 0.001, Fig. 6A) and colony size (P < 0.001 at 24 h, Fig. 6B). Examination of colony size data at 24 h indicated that full O- and N deglycosylation of DMBT1 reduced its ability to inhibit twitching motility by ~41% (Fig. 6B). However, deglycosylated DMBT1 still bound to pili as shown by dot-immunoblot assay (Fig. 6C,D). These results suggest that the glycosylation of DMBT1 is involved in the inhibition of P. aeruginosa twitching motility. However, observation that reduced DMBT1-mediated twitching inhibition did not correlate with any reduction in pili binding suggests that additional (or other) mechanisms may be involved.
Figure 6.
DMBT1 glycosylation is involved in inhibition of P. aeruginosa twitching motility. Effect of DMBT1 digested by deglycosylation enzymes on (A) twitching velocity of P. aeruginosa PAO1 after 4 h, and (B) colony size of P. aeruginosa PAO1 after 4 h and 24 h. Data are shown as the mean ± SEM per sample from three independent experiments. Significance was determined using one-way ANOVA (panel A) or two-way ANOVA (panel B) each with Tukey’s multiple comparisons, ****P < 0.0001; ***P < 0.001; *P < 0.05; ns, not significant. (C) Dot-immunoblot assay with anti-PilA antibody shows continued binding of PAO1 pili to purified DMBT1 (400 ng) after deglycosylation digestion. Pili extracts from PAO1 (S-PAO1), extracts from the pilA mutant (S-PAO1-pilA::Tn), both diluted 1 in 500 in PBS were used as controls, along with PBS alone. (D) Quantification of dot-intensity using ImageJ from the dot-immunoblot assay shown in panel C. Data are shown as the mean ± SEM of duplicate measurements from each sample. Significance determined used Kruskal-Wallis test with Dunn’s multiple comparisons, *P < 0.01; ns, not significant.
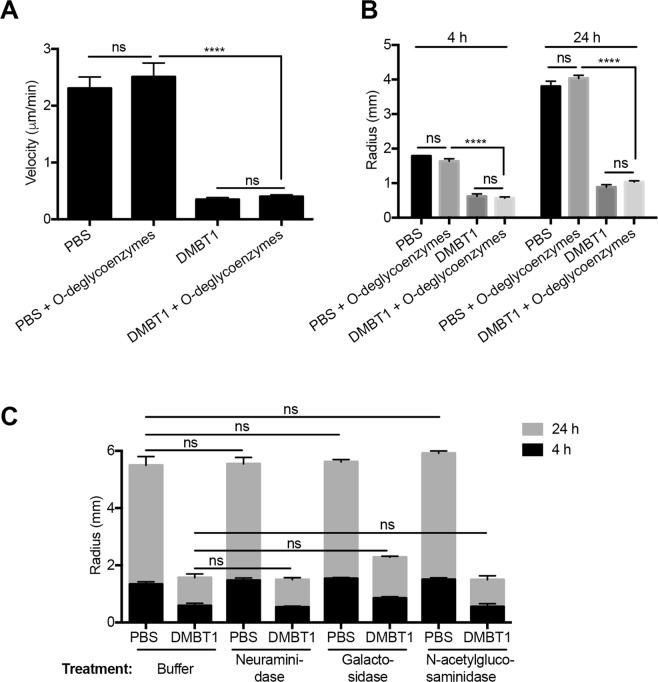
We next explored if the type of DMBT1 glycosylation linkage was important by selectively targeting O-linked versus N-linked moieties. The involvement of O-glycosylation in DMBT1 inhibition of twitching activity was studied with an O-glycan targeted enzyme mix (O-glycosidase, neuraminidase, exoglycosidase β1-4 galactosidase, and β-N-acetylglucosaminidase) under native conditions. No significant difference was found between DMBT1 and de-O-glycosylated DMBT1 on twitching inhibition (Fig. 7A,B). The same results were obtained using longer incubation times with bacteria (from 24 to 48 h), and/or higher concentrations of deglycosylation enzymes (data not shown). According to the manufacturer’s (Biolabs) documentation, this combination of enzymes may not remove all O-linked oligosaccharides, but should remove many common oligosaccharide structures under native conditions. Since denatured DMBT1 lost inhibition of twitching motility (Fig. 1A), denatured de-O-glycosylation could not be used to completely remove O-linked glycans. Nevertheless, the results suggest that simple O-glycosylation is not involved in DMBT1 inhibition of P. aeruginosa twitching motility.
Figure 7.
O-Glycosylation of DMBT1 does not contribute to inhibition of bacterial twitching motility. (A) Effect of DMBT1 digested by an O-deglycosylation enzyme mix on twitching velocity of P. aeruginosa PAO1 after 4 h. DMBT1 (100 ng/µL) was digested by a mixture of O-deglycosylation enzymes (O-glycosidase, Neuraminidase, Galactosidase and N-acetyl-glucosaminidase) at 37 °C for 2 days in native glycobuffer (50 mM sodium phosphate, pH 7.5) to remove complex O-glycosylation of DMBT1. PBS and DMBT1 diluted with same volume of glycobuffer served as controls. (B) Effects of DMBT1 digested by the O-deglycosylation enzyme mix on colony size of P. aeruginosa PAO1 after 4 h and 24 h. (C) Effects of differently digested DMBT1 on colony size of P. aeruginosa PAO1 after 4 h and 24 h. DMBT1 (100 ng/µL) was digested by Neuraminidase (desialylation glycosidase), Galactosidase or N-acetylglucosaminidase (both exoglycosidases) at 37 °C for 2 h in glycobuffer (50 mM sodium acetate, 0.5 mM CaCl2, pH 5.5) to remove sialic acid residues and other complex glycosylation of DMBT1. PBS and DMBT1 diluted with same volume of glycobuffer served as controls. Data shown as the mean ± SEM per sample from three independent experiments. Significance was determined using one-way ANOVA (panel A) or two-way ANOVA (panel B) each with Tukey’s multiple comparisons, ****P < 0.0001; ns, not significant.
To study if complex DMBT1 O-glycosylation could be involved in twitching motility inhibition, DMBT1 was digested with neuraminidase, β1-4 galactosidase, or β-N-acetylglucosaminidase in a different (more optimal) digestion buffer (50 mM sodium acetate, 0.5 mM CaCl2, pH 5.5). However, none of these complex de-O-glycosylation treatments of DMBT1 had a significant effect on its ability to inhibit twitching motility, although β1-4 galactosidase appeared to have some effect (Fig. 7C). Thus, the data suggest that complex O-glycosylation of DMBT1 was also not required for inhibition of twitching motility.
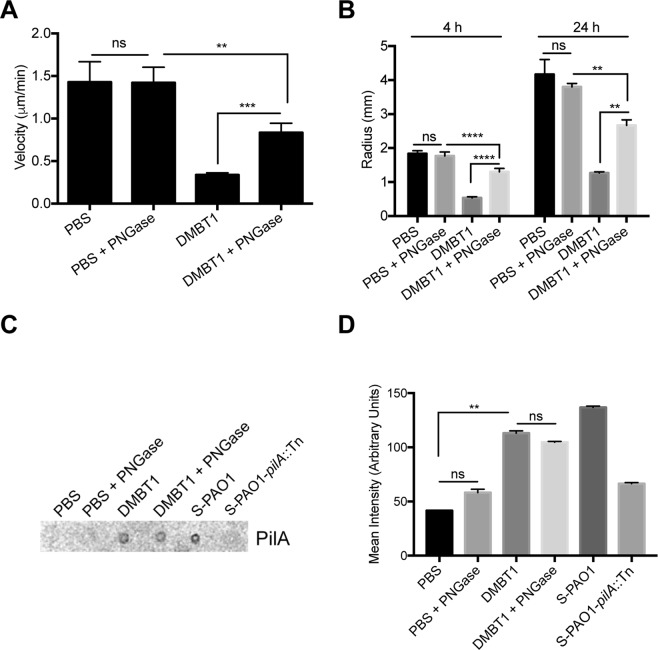
Purified DMBT1 was digested with PNGase F as well in order to remove N-linked glycans in native buffer. De-N-glycosylation enzyme treatment of DMBT1 significantly reduced its ability to inhibit P. aeruginosa twitching motility compared to DMBT1 (Fig. 8A,B). Examination of colony size data at 24 h indicated that de-N-glycosylation of DMBT1 reduced its ability to inhibit twitching motility by ~57% (Fig. 6B). Thus, the extent to which DMBT1 de-N-glycosylation reduced twitching motility inhibitory activity versus untreated DMBT1 was similar to, if not greater than, that observed with non-specific deglycosylation (Fig. 6A,B). Thus, these data suggest that N-glycosylation of DMBT1 is involved in the inhibition of twitching motility. However, de-N-glycosylated DMBT1 still showed a significant inhibition of twitching motility compared to PBS-enzyme controls (Fig. 8A,B), suggesting that N-glycosylation of DMBT1 is only partly involved in twitching inhibitory activity, and other molecular characteristics are also required. Furthermore, de-N-glycosylated DMBT1 still bound to pili similarly to native DMBT1 (Fig. 8C,D), suggesting that factors additional to, or instead of, direct pilus interaction are needed.
Figure 8.
DMBT1 inhibition of P. aeruginosa twitching motility involves its N-Glycosylation. DMBT1 (100 ng/µL) was digested by PNGase F (glycerol-free) at 37 °C for 2 days in native glycobuffer (50 mM sodium phosphate, pH 7.5) to remove N-glycosylation of DMBT1. PBS and DMBT1 diluted with same volume of native glycobuffer, and PBS treated with same volume of PNGase served as controls. (A) Effect of DMBT1 digested by PNGase F on twitching velocity of P. aeruginosa PAO1 after 4 h. (B) Effects of DMBT1 digested by PNGase F on colony size of P. aeruginosa PAO1 after 4 h and 24 h. Data are shown as the mean ± SEM per sample from three independent experiments. Significance was determined using one-way ANOVA (panel A) or two-way ANOVA (panel B) each with Tukey’s multiple comparisons, ****P < 0.0001; ***P < 0.001; **P < 0.01; ns, not significant. (C) Dot-immunoblot assay using anti-PilA antibody showing the binding of PAO1 pili to purified DMBT1 (400 ng) even after PNGase F digestion. Pili extracts from PAO1 (S-PAO1), extracts from the pilA mutant (S-PAO1-pilA::Tn), both diluted 1 in 500 in PBS were used as controls, along with PBS alone. (D) Quantification of dot-intensity using ImageJ from the dot-immunoblot assay shown in panel C. Data are shown as the mean ± SEM of duplicate measurements from each sample. Significance determined used Kruskal-Wallis test with Dunn’s multiple comparisons, **P < 0.01; ns, not significant.
Discussion
Previously, we showed that the glycoprotein DMBT1 in human tear fluid, and purified from human saliva, inhibited twitching motility of P. aeruginosa and consequently prevented bacterial traversal through human corneal epithelial cells in vitro, and reduced disease in a murine model of corneal infection15. Here, we focused on determining how salivary DMBT1 (DMBT1SAG) inhibited twitching motility using structure-function studies. Results showed that the ability of salivary DMBT1 to inhibit twitching motility was removed by heat denaturation, correlating with a reduction in binding to purified pili. Results also showed that Lys-C digestion of DMBT1 had no effect on twitching inhibition, the activity residing in high molecular-weight fractions after Lys-C digestion. Moreover, no effect on twitching inhibition was observed for custom-synthesized individual, or pooled, SRCR-consensus-sequence peptides, recombinant N-terminal DMBT1, nor the SRCR-SID rich glycoprotein CD163. However, experiments using enzymatic deglycosylation of DMBT1 showed that the inhibition of twitching motility was partially, but significantly, associated with N-glycosylation, but not O-glycosylation, and that loss of inhibitory activity after de-N-glycosylation did not correlate with a reduction in binding to purified pili.
The finding that heat denatured salivary DMBT lost its ability to inhibit P. aeruginosa twitching motility suggests that structural conformation is important for this activity. Indeed, the result is consistent with our previous results with tear fluid containing DMBT1, which also lost its inhibitory effects on twitching motility after heat denaturation15. In addition to loss of structural conformation, loss of inhibitory activity after heat denaturation could include the compromise of DMBT1 moieties that normally bind bacterial ligands. Indeed, heat denaturation did cause reduced DMBT1 binding to pili extracts from P. aeruginosa. Heat denaturation may also cause a loss of DMBT1-associated molecules that mediate, or help-mediate, twitching inhibition. However, in our previous study, mass spectrometry analysis of purified salivary DMBT1 did not reveal any known DMBT1-associated innate defense molecules15, suggesting this possibility is less likely.
Digestion of saliva-purified DMBT1 with Lys-C followed by size exclusion chromatography, suggested that the SRCR/SID domains of salivary DMBT1 were essential for the inhibition of P. aeruginosa twitching motility, i.e. high-Mw fragments of the N-terminal (aa 1–1812). That result would be consistent with a previous study in which Lys-C digestion of salivary DMBT1 revealed that binding of S. mutans was associated with SRCR/SID domains3. In that study, and a subsequent study, a series of custom-synthesized peptides that together span the 109 amino acid consensus sequence of the SRCR domains of salivary DMBT1 were used to identify specific bacterial binding regions. SRCR-mediated DMBT1 binding of S. mutans and many other bacteria, e.g. Staphylococcus aureus, Escherichia coli, and Helicobacter pylori, was specifically localized to a 16 amino acid peptide designated SRCRP2, and even more specifically to a minimal bacterial binding region of 11 amino acids (the DMBT1 pathogen-binding site)3,22. In our study, however, SRCRP2 did not inhibit P. aeruginosa twitching motility, nor did it bind pili extracts. Controls also confirmed that SRCRP2 could agglutinate Streptococcus spp. as previously shown3, but not P. aeruginosa. Moreover, none of the other SRCR peptides representing other regions of the SRCR domain consensus sequence had any effect either individually or pooled. Of course, these findings do not preclude involvement of the SRCR domains in DMBT1-mediated inhibition of P. aeruginosa twitching motility, e.g. use of peptides with a different sequence from the SRCR domain consensus sequence may be needed. Alternatively, SID domains may be required by themselves, or to bridge a series of SRCR domains. For example, a region of the N-terminal domain of DMBT1 containing one SRCR and half of its neighboring SID binds HIV-1 gp-120 to exert antiviral effects23. Moreover, similar molecules to DMBT1 with multiple, connected SRCR domains, e.g. CD163 expressed on tissue macrophages, also bind multiple different bacteria triggering release of proinflammatory mediators21. However, neither recombinant N-terminal DMBT1 (aa 1–220) nor recombinant CD163 had any effect on P. aeruginosa twitching, nor did they bind pili extracts. Again, this does not preclude an involvement of the N-terminal domain of DMBT1 with multiple SRCR domains and their respective SIDs, in the inhibition of P. aeruginosa twitching motility. However, these collective findings do suggest that the mechanism involves DMBT1-bacterial cell interaction(s) that differ from those reported for numerous other Gram-positive and Gram-negative bacteria, at least it cannot be conferred by the SRCR bacteria-binding peptide domain alone, or when mixed (albeit unlinked) with other consensus sequence peptides.
It is possible that the activity of high-Mw fractions of Lys-C digested DMBT1 reflect the presence of residual undigested DMBT1, since it is very difficult to separate undigested native DMBT1 from other high-Mw fragments, and heat denaturation removed its twitching inhibitory activity. Even if residual DMBT1 were present, however, that would not change the above conclusion that the mechanism(s) of twitching motility inhibition likely involve a different type of DMBT1 interaction with P. aeruginosa than reported for other bacteria. Moreover, lack of activity of smaller-Mw fractions of Lys-C digested DMBT1 does show that the C-terminal fragments generated by Lys-C digestion were, by themselves, insufficient for twitching inhibition. That finding, in turn, suggests that twitching inhibition does not involve the two CUB domains and ZP domain of DMBT1.
Enzymatic deglycosylation of salivary DMBT1 did result in a partial reduction in twitching motility, which appeared to be specifically associated with the removal of N-glycosylated residues (~46% reduction in DMBT1 effects on bacterial twitching velocity, and a ~57% reduction in effects on bacterial colony size). While only a partial reduction in twitching inhibitory effects, the data show that N-glycosylation of DMBT1 is important for inhibition of P. aeruginosa twitching motility. N-linked glycosylation is known to be critical for general protein stability and dynamics24–26. Indeed, the precise location of N-glycans are significant for activity24. This could help to explain why CD163, a SRCR/SID containing bacterial receptor protein very similar to DMBT1 with many N-glycans, did not inhibit twitching of P. aeruginosa. For respiratory mucosal fluid-derived DMBT1 (gp-340) there are 14 potential sites of N-linked glycosylation2, only 4 of which reside within the N-terminal fragment of DMBT1 formed after Lys-C digestion. However, despite our data implicating SRCR/SID domains of DMBT1 (aa 1–1812) in twitching motility inhibition, and the absence of effects of C-terminal DMBT1 fractions obtained after Lys-C digestion (Fig. 2), it remains possible that C-terminal N-glycosylation (9 sites between aa 1813–2413) could also play a role. Interestingly, while not specific to DMBT1, N-glycans of tear proteins have been implicated in mediating their binding to clinical ocular isolates of P. aeruginosa27. As such, further investigation is warranted to determine which DMBT1 N-glycosylation site(s) is/are important for the twitching inhibitory phenotype.
While glycosylation differences (e.g. in sialyl-Le [Lewis] antigens) have been noted between different isoforms of DMBT1 (gp-304) from tear fluid, saliva, and respiratory fluid28,29, we previously noted that sialyl-Le (Lewis) antigen differences between DMBT1 isoforms would unlikely be involved since both tear fluid and salivary DMBT1 inhibit twitching motility, but express different sialyl-Le antigens15. Moreover, sialyl-Le antigens are attached to tear fluid and salivary DMBT1 by O-glycosylation29,30, and in the present study, enzymatic de-O-glycosylation of DMBT1 did not impact its inhibition of P. aeruginosa twitching motility.
While de-N-glycosylation reduced DMBT1’s ability to inhibit twitching, there remained a significant inhibition versus PBS indicating that other components of DMBT1 also contribute to twitching inhibition. Since heat denaturation of DMBT1 resulted in a complete loss of activity (Fig. 1), these other mechanisms are also heat-sensitive. However, given the similarity of inhibition levels between de-N-glycosylation and general deglycosylation (Fig. 8B versus Fig. 6B), and the lack of effect of specific de-O-glycosylation (Fig. 7B), suggest that these other mechanisms of twitching inhibition relate to other aspects of DMBT1 molecular structure.
In our previous study, the ability of DMBT1 to inhibit twitching motility correlated with binding to extracted bacterial pili suggesting involvement in the inhibitory mechanism15. In the present study, however, this correlation was weakened by two observations; (1) that heat-denatured DMBT1 retained pili binding (~60% after denaturation) despite the complete loss of activity, and (2) that de-N-glycosylation had no effect on pili binding, yet inhibitory activity was reduced by ~57%. Thus, DMBT1 can bind P. aeruginosa pili without inhibiting twitching motility. Previous studies have shown that DMBT1 (gp-340) can bind bacterial pili with various outcomes. For example, pili-mediated binding of Group A Streptococci to gp-340 was associated with bacterial aggregation and defense against bacterial adhesion31. In contrast, pili-mediated binding of Group B Streptococci to surface immobilized gp-340 was proposed as a host colonization mechanism, while bacterial aggregation by fluid-phase gp-340, a likely host defense, was pili independent5. Thus, our findings do not preclude an involvement of pili binding in DMBT1-mediated inhibition of twitching motility as a host defense, but suggest that this activity cannot be accomplished by binding to pili alone. Alternatively, DMBT1 binding to P. aeruginosa pili may serve an entirely separate function from twitching motility inhibition, either contributing to host defense via an additional mechanism or even to bacterial pathogenesis if circumstances allow, e.g. under a contact lens. Other potential mechanisms for DMBT1 inhibition of P. aeruginosa twitching motility could involve the deregulation of gene expression in the Pil-Chp pathway that controls type IV pilus production and twitching motility32,33. While our previous study showed that genes cyaB, chpB, and pilK were not necessary for DMBT1-mediated twitching inhibition15, other genes in this pathway could still be affected. DMBT1 could also interact with other proteins involved in pilus extension or retraction (e.g. ATPases PilB, PilT and PilU or chemosensory protein PilJ)34–36 which could compromise twitching motility. Thus, the role of DMBT1-pili interactions in the inhibition of P. aeruginosa twitching motility may be complex, and understanding them may require significant further study.
In conclusion, the results of this study add to our understanding of DMBT1-mediated inhibition of P. aeruginosa twitching motility, a contribution to mucosal fluid defense of surface epithelia that would help prevent bacteria from traversing mucosal epithelia, forming surface-associated biofilms, and causing disease pathology. The data show that the inhibitory mechanism of human salivary DMBT1 (DMBT1SAG) is heat-sensitive, suggesting that molecular conformation is important, and that DMBT1 N-glycosylation, but not O-glycosylation, contributes to ~50% or more of twitching inhibitory activity. The results also show that salivary DMBT1 can bind P. aeruginosa pili without inhibiting twitching motility, suggesting that if pili binding is involved, additional mechanisms are likely to play a role. Nevertheless, the lack of twitching inhibitory activity of consensus sequence-based peptides of salivary DMBT1, and presence of twitching inhibitory activity despite enzymatic de-O-glycosylation, suggest that the mechanism(s) involved differ from those demonstrated for DMBT1-mediated defense against other bacterial pathogens.
Materials and Methods
Bacterial strains and culture conditions
P. aeruginosa strain PAO1 was used for this study. Bacteria were grown on tryptic soy agar (TSA) plates (37 °C, 16 h) to obtain “lawn” cultures. The pilin mutant PAO1-pilA::Tn37 that lacks pili and thus twitching motility, was used as a negative control, and grown on TSA with tetracycline (60 µg/mL). We have previously verified this mutant15. Twitching motility assays involved growing bacteria on Twitching Motility Gellan Gum (TMGG) medium (0.8 g gellan gum, 0.4 g tryptone, 0.2 g yeast extract, 0.2 g NaCl, 0.1 g MgSO4·7H2O, in 100 mL H2O) at 37 °C in a humidified chamber for different times as specified, and as previously described15. For DMBT1 purification and agglutination assays (see below), we used S. pyogenes (ATCC19615) grown in Brain and Heart Infusion broth at 37 °C (overnight).
Purification of DMBT1 from human saliva
Human saliva was obtained from healthy volunteers (4 subjects) using a protocol approved by the Committee for the Protection of Human Subjects, University of California, Berkeley. This research followed the tenets of the Declaration of Helsinki, and methods were performed in accordance with United States Federal guidelines and regulations. Informed consent was obtained from all subjects, and each was informed of the nature of the study and potential consequences. Collected saliva was first clarified by centrifugation (3,800 × g, 10 min). To purify DMBT1 we used methods described previously7,38. Firstly clarified saliva was diluted 50% in PBS. S. pyogenes grown as above, was also collected by centrifugation (3,800 × g, 5 min), then washed with PBS 3 times. After adjusting the bacterial concentration to ~5 × 109 CFU/mL, equal volumes of bacterial suspension and diluted saliva were mixed and incubated (37 °C, 1 h). Bacteria were the collected by centrifugation (3,800 × g, 5 min), and washed with PBS 3 times. PBS (1.5 mL) with EDTA (5 mM) was used to release bound protein at room temperature (RT) (5 min). After centrifugation of the bacterial culture (15,000 × g, 5 min), the supernatant was passed through a 0.22 µm filter, and dialyzed (Slide-A-Lyzer dialysis cassettes, Thermo Fisher, NY) against PBS (4 °C, overnight). Dialyzed eluate was subjected to gel filtration chromatography on a Superose 6 10/300 GL column (GE Healthcare, CA) equilibrated in PBS (pH 7.4). Eluate at void volume was collected and used as purified DMBT1 (from saliva). DMBT1 concentration was measured with a micro BCA protein assay kit (Thermo Scientific, IL, USA).
Twitching motility assays
Twitching motility was measured using a microscope slide assay39 with modifications previously described15. Bacteria were grown as described above, and TMGG medium dried for 20 min in a sterile airflow (BSL2 Biosafety Cabinet). Then, 5 µL of DMBT1, PBS or other solution was dropped onto the TMGG medium until absorbed. Bacteria were then inoculated onto the TMGG medium using a sterile toothpick. A glass coverslip was placed onto the medium to create an “interstitial” space. After incubating the slides for 4 h (37 °C), time-lapse videos (5 min) were captured at 10 s intervals via differential interference contrast (DIC) microscopy using a Nikon ECLIPS Ti microscope with a 60× oil-immersion objective at 37 °C.
Quantification of twitching motility
To calculate twitching velocity, the twitching distance of a colony leading edge was divided by time as previously described15. Image J was used to measure the distance traveled by bacteria from frames 1–31 of the 5 min video. Ten bacteria were tracked in each video, and each group performed in triplicate.
Dot-immunoblotting to test pili binding to DMBT1 or other solutions
Pili extracts were prepared as follows: P. aeruginosa PAO1 was suspended in PBS (OD600 of ~10), mixed by vortex (3 min), centrifuged (15,000 × g, 20 min) and the supernatant collected. After adding MgCl2 to the supernatant (to a final concentration of 100 mM), the supernatant was stored overnight at 4 °C. The next day, the superantant was centrifuged (15,000 × g, 20 min), and the pellet resuspended in PBS (500 μL) forming the pili-containing extract. PAO1-pilA::Tn was taken through the same process to make a negative control extract. Dot-immunoblot assays were performed as previously described15. Firstly, 2 μL of DMBT1 in PBS (250 ng/μL or 200 ng/μL, the latter concentration being equally effective) was spotted onto a nitrocellulose membrane (0.2 μm pore-size, BioRad). A PBS control was included along with pili extracts from PAO1 (positive control) or pilA mutant (negative control). The latter controls were prepared by diluting extracts in PBS (1 in 500). After drying, the membrane was blocked with BSA (5%) for 1 h at RT, washed for 5 min with PBS, then incubated with undiluted PAO1 pili extracts of PAO1 for 40 min at RT. After washing 5 times with PBS, membranes were probed with anti-PilA primary antibody (1:5000), then Goat anti-Rabbit HRP-conjugated secondary antibody (1:5000). Dot intensity was measured with AlphaView FluoChem HD2 software.
Digestion of DMBT1 by Lys-C
Endoproteinase Lys-C (New England BioLabs) was used to digest the carboxyl side of lysine residues of DMBT1 under native conditions according to the manufacturer’s instructions. Then, 50 µL of purified DMBT1 (200 ng/µL) was incubated with 10 µL of the Lys-C digestion solution (100 ng/µL), 10 µL of PBS, and 2 µL of Lys-C solution (20 mM Tris-HCL buffer, pH 8.0) at 37 °C for 16 h. The sample was separated by size exclusion chromatography. An AKTAmicro system was used with a Superose 6 10/300 GL column (GE Healthcare) with elution in PBS (pH 7.4). Peak broadening was minimized by using short lengths of 0.15 mm i.d. tubing between the injection valve and fraction collector. Digested DMBT1 was injected onto the column, and fractions (250 μL) collected. Protein was detected by UV absorbance at 280 nm. Eluted fractions were pooled according to protein peaks and concentrated using a ~3 kDa cut-off filter (Millipore). Fraction effects on twitching motility were then assessed.
SRCR peptides
Based on the amino acid sequence of DMBT1, a series of peptides spanning the consensus sequence of the DMBT1 SRCR domains (SRCRPs 1 to 7) were custom-synthesized as previously described3. Peptides were diluted to 200 ng/µL in Tris buffered Saline (TBS), pH = 7.5 supplemented with 10 mM calcium chloride and effects on bacterial twitching motility were tested as described above. For dot-immunoblots, 2 µL (400 ng) of SRCRPs 1–7 were spotted onto nitrocellulose membranes as described above.
Agglutination assay
Agglutination assays were performed as previously described3. S. pyogenes and P. aeruginosa (PAO1) were suspended in TTC (Tris-Buffered Saline containing 0.1% Tween 20 and 1 mM Ca2+) to a concentration of ~5 × 108 CFU/mL. Then, 150 µL of bacteria were mixed with 150 µL of SRCRP2 solution (200 ng/µL in Tris-Buffered Saline [TBS] with 10 mM Ca2+) in a 96-well plate then incubated for 2 h at 37 °C. After agglutination, 10 µL of the bacteria were transferred on a microscope slide. Bacteria were heat-fixed then stained with crystal violet solution and examined with light microscopy. Buffer alone (TBS with 10 mM Ca2+) and purified DMBT1 (200 ng/µL) were used as controls.
Recombinant CD163
Human recombinant CellExp™ CD163 was obtained from BioVision, Inc. (Milpitas, CA, USA). The recombinant protein has a reported Mw of 135–140 kDa due to glycosylation. For use in experiments, CD163 was resuspended in PBS to a final concentration of 200 ng/µL. Effects on P. aeruginosa twitching motility were tested as above.
Enzymatic deglycosylation
A deglycosylation enzyme mix (Biolabs) was used to digest DMBT1 under native conditions. For each enzyme (PNGase F, O-Glycosidase, Neuraminidase, β1-4 Galactosidase, β-N-acetylglycosaminidase), 5 µL was used to make the enzyme mix and incubated with 20 µL of purified DMBT1 (250 ng/µL) at 37 °C for 48 h. For complex de-O-glycosylation, 25 µL of purified DMBT1 (200 ng/µL) was incubated with 10X glycobuffer 2 (2 µL), enzyme mix (Neuraminidase, β1-4 Galactosidase, β-N-acetylglucosaminidase, and O-Glycosidase) (5 µL each) at 37 °C for 2 h. To remove N-linked glycans, 15 µL of purified DMBT1 (200 ng/µL) was incubated with 10X glycobuffer 2 (3 µL), PNGase F (5 µL) and H2O (7 µL) at 37 °C for 48 h.
Statistical analysis
Data were expressed as a mean ± standard error of mean (SEM) unless otherwise stated. The significance of differences between multiple groups was assessed using a two-way ANOVA with Tukey’s multiple comparisons for post-hoc analysis, or using a one-way ANOVA with Tukey’s or Dunnett’s multiple comparisons. The Kruskal-Wallis test with Dunn’s multiple comparisons was used for nonparametric data. P values of less than 0.05 were considered significant.
Acknowledgements
This work was supported by the National Institutes of Health (EY024060, SMJF), EY007043 (UC Berkeley Vision Science Training Grant), and an International Postdoctoral Exchange Program Fellowship from the China Postdoctoral Council (20140085, JL). P. aeruginosa PAO1 and its mutants were obtained from the University of Washington P. aeruginosa mutant collection supported by the National Institutes of Health (P30 DK089507). Many thanks to Dr. Joanne Engel (University of California, San Francisco) for providing the antibody to PilA, and to Dr. Chris Jeans (University of California, Berkeley) for help with protein purification.
Author Contributions
J.L., S.W., M.M., F.B., D.E. and S.F. designed the experiments. J.L., S.W., M.M., K.N. and S.M. performed the experiments. J.L., S.W., F.B., D.E. and S.F. analyzed the data and wrote the manuscript. J.L. and S.W. contributed equally to this work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianfang Li and Stephanie J. Wan contributed equally.
References
- 1.Madsen J, Mollenhauer J, Holmskov U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun. 2010;16:160–167. doi: 10.1177/1753425910368447. [DOI] [PubMed] [Google Scholar]
- 2.Holmskov U, et al. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci USA. 1999;96:10794–10799. doi: 10.1073/pnas.96.19.10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikker FJ, et al. Identification of the bacteria-binding peptide domain on salivary agglutinin (gp-340/DMBT1), a member of the scavenger receptor cysteine-rich superfamily. J. Biol. Chem. 2002;277:32109–32115. doi: 10.1074/jbc.M203788200. [DOI] [PubMed] [Google Scholar]
- 4.Ligtenberg AJM, Veerman ECI, Nieuw Amerongen AV, Mollenhauer J. Salivary agglutinin/glycoprotein-340/DMBT1: a single molecule with variable composition and with different functions in infection, inflammation and cancer. Biological Chemistry. 2007;388:35. doi: 10.1515/BC.2007.158. [DOI] [PubMed] [Google Scholar]
- 5.Brittan JL, Nobbs AH. Group B Streptococcus pili mediate adherence to salivary glycoproteins. Microbes Infect. 2015;17:360–368. doi: 10.1016/j.micinf.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Prakobphol A, et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J. Biol. Chem. 2000;275:39860–39866. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 7.Kukita K, et al. Staphylococcus aureus SasA is responsible for binding to the salivary agglutinin gp340, derived from human saliva. Infection and Immunity. 2013;81:1870–1879. doi: 10.1128/IAI.00011-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartshorn KL, et al. Salivary agglutinin and lung scavenger receptor cysteine-rich glycoprotein 340 have broad anti-influenza activities and interactions with surfactant protein D that vary according to donor source and sialylation. Biochemical Journal. 2006;393:545–553. doi: 10.1042/BJ20050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jumblatt MM, et al. Glycoprotein 340 in normal human ocular surface tissues and tear film. Infection and Immunity. 2006;74:4058–4063. doi: 10.1128/IAI.01951-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligtenberg AJM, Karlsson NG, Veerman ECI. Deleted in malignant brain tumors-1 protein (DMBT1): A pattern recognition receptor with multiple binding sites. International Journal of Molecular Sciences 2010, Vol. 11, Pages 5212-5233. 2010;11:5212–5233. doi: 10.3390/ijms1112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polley S, et al. Evolution of the rapidly mutating human salivary agglutinin gene (DMBT1) and population subsistence strategy. Proc Natl Acad Sci USA. 2015;112:5105–5110. doi: 10.1073/pnas.1416531112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikker FJ, et al. The scavenging capacity of DMBT1 is impaired by germline deletions. Immunogenetics. 2017;69:401–407. doi: 10.1007/s00251-017-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oho T, Yu H, Yamashita Y, Koga T. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infection and Immunity. 1998;66:115–121. doi: 10.1128/iai.66.1.115-121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmskov U, et al. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J. Biol. Chem. 1997;272:13743–13749. doi: 10.1074/jbc.272.21.13743. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Metruccio MME, Smith BE, Evans DJ, Fleiszig SMJ. Mucosal fluid glycoprotein DMBT1 suppresses twitching motility and virulence of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Pathog. 2017;13:e1006392. doi: 10.1371/journal.ppat.1006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattick John S. Type IV Pili and Twitching Motility. Annual Review of Microbiology. 2002;56(1):289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 17.Leighton TL, Buensuceso RNC, Howell PL, Burrows LL. Biogenesis of Pseudomonas aeruginosa type IV pili and regulation of their function. Environmental Microbiology. 2015;17:4148–4163. doi: 10.1111/1462-2920.12849. [DOI] [PubMed] [Google Scholar]
- 18.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112:201502025–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alarcon I, Evans DJ, Fleiszig SMJ. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2009;50:2237–2244. doi: 10.1167/iovs.08-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolfaghar I, Evans DJ, Fleiszig SMJ. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infection and Immunity. 2003;71:5389–5393. doi: 10.1128/IAI.71.9.5389-5393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabriek BO, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 22.Bikker FJ, et al. Bacteria binding by DMBT1/SAG/gp-340 is confined to the VEVLXXXXW motif in its scavenger receptor cysteine-rich domains. J. Biol. Chem. 2004;279:47699–47703. doi: 10.1074/jbc.M406095200. [DOI] [PubMed] [Google Scholar]
- 23.Wu, Z., Lee, S., Abrams, W., Weissman, D. & Malamud, D. The N-terminal SRCR-SID domain of gp-340 interacts with HIV type 1 gp120 sequences and inhibits viral infection, https://home.liebertpub.com/aid22, 508–515 (2006). [DOI] [PubMed]
- 24.Li J-H, et al. N-linked glycosylation at Asn152 on CD147 affects protein folding and stability: promoting tumour metastasis in hepatocellular carcinoma. Sci. Rep. 2016;6:35210. doi: 10.1038/srep35210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels R, Kurowski B, Johnson AE, Hebert DN. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol. Cell. 2003;11:79–90. doi: 10.1016/S1097-2765(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 26.Petrescu A-J, Milac A-L, Petrescu SM, Dwek RA, Wormald MR. Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology. 2004;14:103–114. doi: 10.1093/glycob/cwh008. [DOI] [PubMed] [Google Scholar]
- 27.Kautto L, et al. Glycan involvement in the adhesion of Pseudomonas aeruginosa to tears. Experimental Eye Research. 2016;145:278–288. doi: 10.1016/j.exer.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson C, et al. Variant size- and glycoforms of the scavenger receptor cysteine-rich protein gp-340 with differential bacterial aggregation. Glycoconj J. 2007;24:131–142. doi: 10.1007/s10719-006-9020-1. [DOI] [PubMed] [Google Scholar]
- 29.Schulz BL, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochemical Journal. 2002;366:511–520. doi: 10.1042/bj20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issa S, et al. O-linked oligosaccharides from salivary agglutinin: Helicobacter pylori binding sialyl-Lewis x and Lewis b are terminating moieties on hyperfucosylated oligo-N-acetyllactosamine. Glycobiology. 2010;20:1046–1057. doi: 10.1093/glycob/cwq066. [DOI] [PubMed] [Google Scholar]
- 31.Edwards AM, et al. Scavenger receptor gp340 aggregates group A Streptococci by binding pili. Molecular Microbiology. 2008;68:1378–1394. doi: 10.1111/j.1365-2958.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 32.Moulton RC, Montie TC. Chemotaxis by Pseudomonas aeruginosa. Journal of Bacteriology. 1979;137:274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulcher NB, Holliday PM, Klem E, Cann MJ, Wolfgang MC. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Molecular Microbiology. 2010;76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang P, et al. Functional role of conserved residues in the characteristic secretion NTPase motifs of the Pseudomonas aeruginosa type IV pilus motor proteins PilB, PilT and PilU. Microbiology. 2008;154:114–126. doi: 10.1099/mic.0.2007/011320-0. [DOI] [PubMed] [Google Scholar]
- 35.Bertrand JJ, West JT, Engel JN. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. Journal of Bacteriology. 2010;192:994–1010. doi: 10.1128/JB.01390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLange PA, Collins TL, Pierce GE, Robinson JB. PilJ localizes to cell poles and is required for type IV pilus extension in Pseudomonas aeruginosa. Curr Microbiol. 2007;55:389–395. doi: 10.1007/s00284-007-9008-5. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loimaranta V, et al. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infection and Immunity. 2005;73:2245–2252. doi: 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbull L, Whitchurch CB. Motility assay: twitching motility. Methods Mol. Biol. 2014;1149:73–86. doi: 10.1007/978-1-4939-0473-0_9. [DOI] [PubMed] [Google Scholar]