Abstract
Introduction: Achieving maximum functional outcome in primary malignant bone sarcoma surgery (PMBS) patients, is challenging for both patients and clinicians. This study, aimed to evaluate different factors that affect postoperative functional outcome of the primary malignant bone sarcoma patients following upper limb (UL) and lower limb (LL) salvage surgery using Toronto Extremity Salvage Score (TESS).
Methods: 136 PMBS adult patients were identified and were grouped as lower limb (LL) and upper limb (UL). Each group then sub-grouped to major and minor surgeries. Their functional outcome was compared using TESS by demographic variables(gender and age), neoadjuvant/adjuvant therapy and tumour variables (anatomical sites). In the UL groups, TESS was also compared for major and minor surgery subgroups based on their dominant or non-dominant limb.
Results: The result of TESS has revealed that chemotherapy, radiotherapy, and gender have no effect on the functional outcome in PMBS patients. Functional outcome however was significantly affected by age in both LL and UL groups. The TESS was significantly different between major and minor subgroups in UL group with p= 0.0001. In patients with upper LSS on their dominant limb, no significant difference between major/ minor surgery subgroups was observed with p=0.077.
Conclusion: Our findings using TESS revealed that factors such as patient’s age, and type of surgery (major or minor) in PMBS patients will affect the patients’ functional outcome after LSS especially in those PMBS patients with upper LSS.
Keywords: Functional outcome, Primary malignant bone sarcoma, Limb salvage surgery, TESS
1. Introduction
Primary malignant bone tumours are rare forms of human neoplasms, and their incident rate is approximately 10 cases per 1 million population per year. 1 In fact, malignant bone tumours, account for only a small percentage for cancers diagnosed.2 Over the last decades, bone sarcoma patients have an improved survival rate3 and limb salvage surgery (LSS) procedures have become more available to sarcoma patients as alternatives to amputation.2 LSS are now well established and patients have better survival rates and quality of life.4,5, 6, 7, 8, 9 However, after LSS, many patients experience some functional difficulty; therefore success of LSS should not be assessed only by surgical result, but also by patient reported functional outcome.10
In this study, we evaluated the patient reported functional outcome of primary malignant bone sarcoma (PMBS) patients who had undergone both lower limb (LL) and upper limb (UL) surgery using Toronto Extremity Salvage Score (TESS) at all five bone sarcoma specialist centres in England, United Kingdom. The centres are Royal National Orthopaedic Hospital NHS Trust, The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust, Oxford University Hospitals NHS Trust, The Newcastle Upon Tyne Hospitals NHS Foundation Trust and Royal Orthopaedic Hospital NHS Trust.
The TESS is a patient-centred questionnaire and was selected as the main functional outcome measure in this study. It is a well-validated questionnaire which gives a disease-specific measure of functional ability and is proven to be the most receptive compared with other functional outcome measures.11 It was created based on definitions of disability, impairment and handicap as described by the World Health Organization (1980). 12 It includes 30 questions for the LL version and 29 for the UL version13 which place the emphasis on the patient’s ability to carry out activities of daily living.14 Each question measures the difficulty the individual has undertaking the task. The patient grades the overall difficulty of all activities undertaken in the previous week and overall level of disability using a similar scale.15 A total questionnaire score is a standardised score ranging from 0–100.12 Higher patient-reported function results in a higher TESS score. 15,16 Functional outcome scores in studies of PMBS are mostly derived from measurements such as TESS as it reports on the patients’ insight and satisfaction of their own physical activity and functional ability after an operation such as LSS.17 In this study capturing patient reported outcome measures and thus a questionnaire completed by the patient was key.
Van Egmond-van Dam et al have reported that scores resulting from functional measures such as TESS which consists of daily life activities, are more objective compared to clinicians questionnaires and interviews.17 Additionally, functional outcome measures such as TESS have excellent accuracy and low standard error of measurement (SEM) over a broad range of ability levels, which means it measures physical function accurately irrespective of how the patient’s physical function18. Additionally previous studies demonstrated that use of information from patient reported outcome measures leads to better communication and decision making between doctors and patients and improves satisfaction.19,20
Many studies have investigated LSS and functional outcome following surgery of either LL or UL and in literature bone sarcoma studies, prospective studies concerning the functional outcome after limb surgery for bone sarcoma of both UL and LL are scarce. However this study assessed the effect of both LL and UL salvage surgery on the functional outcome in PMBS patients with different minor and major surgeries (Table 1).
Table 1.
Patient Groups and Subgroups.
| Groups | Major Surgery Subgroups | Minor Surgery Subgroups |
|---|---|---|
| Lower Limb | Distal Femoral Replacement (DFR), Proximal Tibial Replacement (PTR), Major Hip/Pelvis Surgery, Sacrectomy |
|
| Upper Limb | Proximal Humeral Replacement (PHR), Total Scapulectomy and Partial Scapulectomy |
|
The objective of this study was to assess the PMBS patients' perspectives of their functional outcome after LSS for both LL and UL. We postulated whether different attributes such as type of surgery, patients’ age, gender, and receiving neoadjuvant/adjuvant therapy will affect the result of TESS. This study also evaluates if LSS on the dominant or non-dominant UL will have any significant changes on the TESS outcome. Our assessment was based on PMBS patients’ perspective rather than clinicians and healthcare professionals’ perspective.
2. Material and methods
Research and development department at all five participated bone sarcoma specialist centres gave their approval for this study. 220 patients diagnosed with PMBS were identified through retrospective sarcoma databases at participating centres. Inclusion criteria stipulated that adult patients (over 18 years old) must have been diagnosed with PMBS and had limb salvage surgery with a minimum follow up and survival of 12 months follow up. This time scale was chosen to ensure patients who were participating in the study had either completed their rehabilitation or had received a considerable proportion of it to be able to constructively input in the project. It was also felt that if a broader timescale was used, rehabilitation practices may have changed and therefore an unfair comparison could be made and thus influence the results.
The patients were grouped as LL and UL group (Table 1). In the LL group the major surgery subgroups were distal femoral replacement (DFR), proximal tibial replacement (PTR) and Hip/Pelvis. In the UL group the major surgery subgroups were proximal humeral replacement (PHR), total scapulectomy and partial scapulectomy. All other surgeries were categorised as minor surgery in both LL and UL groups. Table 1 includes the list of different minor surgeries, however in order to obtain statistically significant sample size, we put our study’s minor surgery's subjects in one group for each LL and UL.
For continuous variables, descriptive statistics such as average, standard deviations and statistical test such as t-test were calculated. Differences in mean values between two groups were compared using unpaired t-tests. The Mann–Whitney U test was used to detect any differences between TESS scores using GraphPad Prism software version 6. The assumption of each statistical test was met by the corresponding data set. The significance level was set at P ≤ 0.05, with a 95% confidence interval (CI), to assess reliability in the estimates.
Out of the 220 questionnaires sent, 136 questionnaires were returned (female n = 58 and male n = 78). At α = 0.05, this study’s sample size (n = 136) has ensured 90% power to detect this difference in the proportion of patients with PMBS for a two-sided test. The groups were compared according to TESS by gender, age group, neoadjuvant/adjuvant treatment, type of surgery, rating of daily activities and disabilities. In the UL groups, the TESS was compared between major and minor surgery subgroups as to whether surgery was undertaken on the dominant or non-dominant limb.
3. Results
TESS was compared in total for the LL and UL groups and then a comparison was made separately in the major and minor surgery subgroups between different genders and age groups.
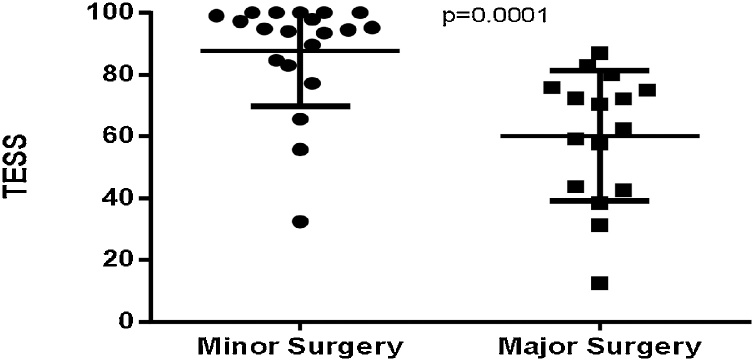
TESS was significantly different between major and minor subgroups in UL group (P = 0.0001, mean difference [MD] = 27.47 ± 6.479 and 95% confidence interval [CI] = 14.30–40.65) (Fig. 1), however there is no significant difference was observed in LL group (Table 2).
Fig. 1.
Comparison of TESS between major and minor surgery subgroups in the UL group.
Table 2.
Comparison of TESS between major and minor surgery subgroups (5 centres combined).
| LSS Site | Average TESS ± SD TESS Range (Major Surgery) |
Average TESS ± SD TESS Range (Minor Surgery) |
P value |
|---|---|---|---|
| Lower Limb | 67.82 ± 21.75 15.52-100 |
79.40 ± 20.85 33.33-100 |
0.073 |
| Upper Limb | 60.24 ± 21.07 12.50-87.06 |
87.71 ± 17.81 32.40-100 |
0.0001‡ |
There was no significant difference in TESS between genders in both LL and UL groups (p = 0.6 and p = 0.35 respectively) (Table 3). For females in the LL group TESS averaged 70.50 ± 22.65 compared to 68.66 ± 21.4 for males. Both female and male patients from minor surgery subgroups of the UL group have the highest TESS with average of 90.42 ± 13.58 and 88.28 ± 19.49 respectively. Analysis was not conducted in scapulectomy and partial scapulectomy surgery subgroup due to the small sample size.
Table 3.
TESS in Different Genders (LL and UL).
| Gender | Average TESS ± SD | P* |
|---|---|---|
| Lower Limb | ||
| Female Male |
70.50 ± 22.65 68.66 ± 21.40 |
0.6 |
| Upper Limb | ||
| Female Male |
71.02 ± 26.19 79.53 ± 21.36 |
0.35 |
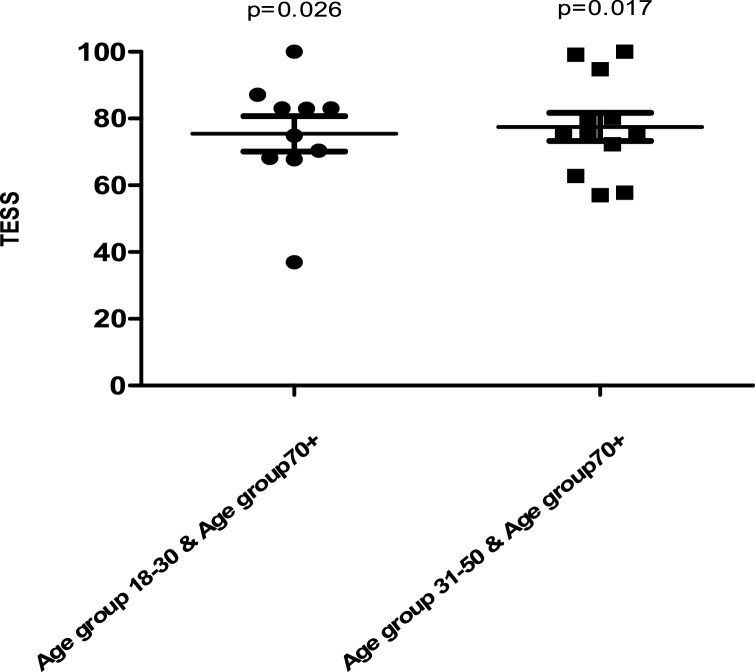
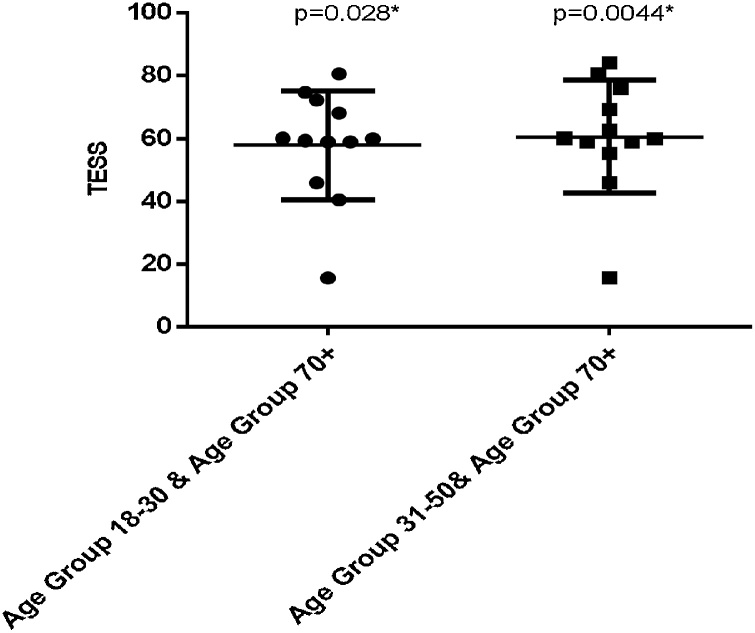
In both UL and LL groups, age did have an effect on the TESS. The TESS decreased with increasing age with an unexplained rise in 70+ group in DFR + PTR surgery subgroup with average TESS of 85.57 ± 19.11 higher than any other age group in this group. In the UL group, there were statistically significant differences between the age groups 18–30 and 70+ (p = 0.026, MD =−26.08 ± 9.79 and 95% CI =−48.14 to −4.02) and between age groups 31–50 and 70+ (p = 0.017, MD = 27.25 ± 9.17 and 95% CI=−48.58 to −5.91) (Fig. 2). In the hip and pelvis surgery subgroup of the LL group there was also significant differences between the age group 18–30 and 70+age group (P = 0.028, MD= −22.42 ± 7.970 and 95% CI= -40.44 to -4.406) and between the age group 31–50 and 70+ age group (P = 0.0044, MD= −25.31 ± 7.241 and 95% CI= −40.73 to -9.900) (Fig. 3).
Fig. 2.
Comparison of TESS between different age groups in the UL group.
Fig. 3.
Comparison of TESS between different age groups in Hip and Pelvis surgery subgroups of the LL group.
The TESS results were not significantly different between patients who had chemotherapy and those who did not in both LL and UL groups with p = 0.59, and p = 0.66 respectively. Further analysis was also carried out on each surgery subgroup and no significant difference in the TESS was observed either. There was no significant difference observed in TESS between patients who had radiotherapy and those who did not in both LL and UL group with p = 0.89 and p = 0.53 respectively.
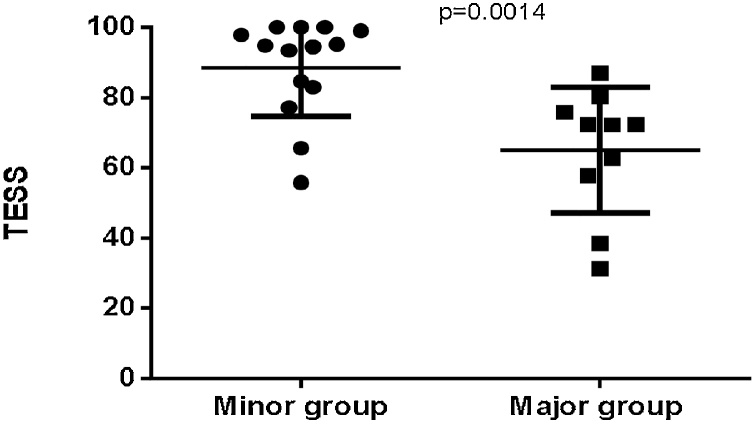
In the UL group, TESS did not differ between patients in the major and minor surgery groups who had surgery on their dominant limb (p = 0.077, MD = −32.63 ± 15.89 and 95% CI = −69.58 to 4.32). However there was a significant difference of TESS between major and minor surgery group of patients who had surgery on their non-dominant limb (p = 0.0014, MD = 23.63 ± 6.78 and 95% CI = −37.99 to −9.27) (Fig. 4).
Fig. 4.
Comparison of TESS between major and minor surgery groups in patients with surgery on the non- dominant limb.
4. Discussion
LSS is a common treatment of primary bone sarcomas; however, this has not always been the case as amputation was previously the most common procedure of choice.21 Different considerations such as the efficacy of neo-adjuvant chemotherapy and the newer functional reconstructive modalities of treatment and imaging changed the trend towards LSS21 and as a result PMBS patients may live longer and have a better functional outcome. Improvement of functional ability, that is, the ability to fully perform one’s activities of daily life is one of the main objectives of LSS, therefore, assessments of functional outcome after LSS are now common practice among clinicians and generally focus on patients’ level of activity and functional measures.
This study revealed that limitation to functional ability is dependent on patients’ age and surgery type.
Our results demonstrate that in the UL groups, major LSS had a strong impact on TESS and having major surgery significantly affects patient’s functional outcome. Additionally, it has been reported before, 22 that this patient group also suffer from common symptoms of pain, stiffness, fatigue and weakness suggesting that they are experiencing greater limitation in their functional activities. This result confirmed those of Renard et al23 and Wright et al16 who reported that LL function was satisfactorily preserved in patients with lower LSS and this patient group has less functional complexity than the patients with upper LSS.
Our study also revealed that having chemotherapy or radiotherapy has no effect on functional ability. This finding is consistent with the previous study.16 In fact in some surgical subgroups, patients who had chemotherapy or radiotherapy have a higher average TESS. For example, in the PHR group, patients who had chemotherapy treatment had a higher average TESS 63.25 ± 27.58 compared to 54.89 ± 24.70 of those who did not. The same was correct for hip/pelvis surgery group; those who had radiotherapy had a higher average TESS 92.94 ± 2.91 vs. those who didn’t with average TESS 85.16 ± 24.14. According to Schreiber et al24 this result is perhaps due to 1 year follow- up which is too early for manifestation of the late toxicity effects. This result might be a useful prognostic marker for PMBS patients who require neoadjuvant/adjuvant therapy and indeed previous authors25,26,27 have reported that LSS combined with adjuvant therapy can be beneficial for improving patients’ quality of life.
Our finding also confirmed that gender, as another patient’s demographic factor, has no effect on functional outcome. This finding is similar to a previous study28 which reported that differences in gender were not associated with a lower functional outcome or significant difference in TESS. In fact, in the LL group female patients have a higher average TESS (70.50 ± 22.65) compared to average TESS (68.66 ± 21.40) in male patients. However, in PHR surgery subgroup female patients scored lower than any other surgical subgroup with average TESS of 47.16 ± 28.74 reiterating our finding those patients who underwent upper LSS had more functional complexity.
Our finding also extend those of Clayer et al 28 who demonstrated that age has an effect on the TESS in patients with LSS. However we did not observe a decrease in TESS in older female patients as was observed by Clayer et al.28 Our older female patients in the LL group had an average TESS 75.58 ± 22.53 compared to our older male patients with an average TESS 65.53 ± 24.83.
We also sought to determine whether there was any effect on functional outcome of those patients who had LSS on their dominant limb. Our result was surprising as it revealed that TESS was not significantly different between major and minor surgery groups of the UL group who had surgery on their dominant limb. This was an unexpected finding and we postulate that the heterogeneity of this patient group with regard to age, and type of surgery has affected the result of TESS so any difference in the functional outcome may have been overlooked. Additionally, the number of patients in this patient group who completed the TESS was low compared to other patient groups.
Our study is unique in its attempt to analyse patient-reported functional scores using TESS in a diverse patient population of UL and LL salvage surgery. Our statistically significant results of TESS between different age groups will be clinically relevant as patient reported functional outcome can easily be obtained and carry little cost. By incorporating the results of patient reported functional outcome measures as a standard procedure especially within the older patient groups, will be beneficial for clinicians to help them strive to achieve the highest possible functional outcome for all patients. Additionally, by carefully planning rehabilitation and regularly assessing the patients and incorporating functional outcome measures will not only ensure that treatment is appropriate and effective but will also help to promote activity which is recommended for optimal health in all age groups.
This study provides an analysis of the functional outcome in PMBS patients after LSS for upper and lower surgery. We found that age and type of surgery (major and minor) were significantly associated with the functional outcome after LSS.
Our study was limited by some important factors; first our analysis was limited due to the retrospective nature of this study. The data that we collected was in the context of patient’s rehabilitation, therefore routine follow ups for obtaining constant data collection were not available. Furthermore, while we observed no significant difference in TESS between major and minor surgery groups who had surgery on their dominant UL compared to those who had surgery on their non-dominant UL, this group’s relatively small sample size does not represent the whole population of this surgery group, therefore, further research is needed to more fully explore the functional outcome of these patients. Nevertheless to the best of the authors’ knowledge this is the first study that has investigated the functional outcome of patients who underwent surgery on their dominant UL. Our finding also may be helpful in careful clinical planning and rehabilitation to improve functional outcome in PMBS patients with LSS. Furthermore, in our study we demonstrate the evaluation of patient reported functional outcome using validated measures such as TESS is essential to achieve the highest possible functional outcome and quality of life for this patient group.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgements
Financial support for this study was provided by the National Specialised Commissioning Group (NSCG) in England. The authors give thanks to Consultant Orthopaedic Surgeons Mr André Olivier, Mr Will Aston and Mr. Jake Jagiello for critical reviewing of this manuscript. The authors also give thanks to all members of staff at the participated five centres who were involved in this project, without them this study would not have been possible.
References
- 1.Von Eisenhart-Rothe R., Toepfer A. Primary malignant bone tumours. Orthopade. 2011;40(11):1121–1142. doi: 10.1007/s00132-011-1866-7. [DOI] [PubMed] [Google Scholar]
- 2.Yong-Jian S., Yan-Jun H., Dan J., Jian-Wei L., Bin Y. Health related quality of life after treatment for malignant bone tumors: a follow-up study in China. Asian Pac J Cancer Prev. 2012;13:3099–3102. doi: 10.7314/apjcp.2012.13.7.3099. [DOI] [PubMed] [Google Scholar]
- 3.Damron T.A., Ward W.G., Stewart A. Ostesarcoma, chondrosarcoma and ewing sarcoma: national cancer data Base report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez J.C., Perez E.A., Moffat F.L., Livingstone A.S., Franceschi D., Koniaris L.G. Should soft tissue sarcomas be treated at high-volume centres? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray P.W., Bell R.S., Bowen C.V., Davis A., O’Sullivan B. Limb salvage surgery and adjuvant radiotherapy for soft tissue sarcomas of the forearm and hand. J Hand Surg [Am] 1997;22:495–503. doi: 10.1016/S0363-5023(97)80019-6. [DOI] [PubMed] [Google Scholar]
- 6.Delaney T.F., Kepka L., Goldberg S.I. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Gerrand C.H., Wunder J.S., Kandel R.A. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma. Ann Surg Oncol. 2004;11:476–482. doi: 10.1245/ASO.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Karakousis C.P., De Young C., Driscoll D.L. Soft tissue sarcomas of the hand and foot: management and survival. Ann Surg Oncol. 1998;5:238–240. doi: 10.1007/BF02303779. [DOI] [PubMed] [Google Scholar]
- 9.Lehnhardt M., Kuhnen C., Dru¨cke D. Liposarcoma of the extremities: recent developments in surgical therapy—analysis of 167 patients. Chirurg. 2004;75:1182–1190. doi: 10.1007/s00104-004-0900-2. [DOI] [PubMed] [Google Scholar]
- 10.Pisters P.W., Pollock R.E., Lewis V.O. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–681. doi: 10.1097/SLA.0b013e318155a9ae. discussion 681–682. [DOI] [PubMed] [Google Scholar]
- 11.Davis A.M., Bell R.S., Bradley E.M., Yoshida K., Williams J.I. Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res. 1999;358 90e100. [PubMed] [Google Scholar]
- 12.World Health Organisation (WHO) World Health Organisation; Geneva, Switzerland: 1980. International classification of impairments, disabilities, and handicaps. [Google Scholar]
- 13.Ruggieri P., Mavrogenis A.F., Mercuri M. Quality of life following limb salvage surgery for bone sarcomas. Expert Rev Pharmacoecon Outcomes Res. 2011;11(1):59–73. doi: 10.1586/erp.10.91. [DOI] [PubMed] [Google Scholar]
- 14.Davis A.M., Wright J.G., Williams J.I., Bombardier C., Griffin A., Bell R.S. Development of a measure of physical function for patients with bone and soft-tissue sarcoma. Qual Life Res. 1996;5:508e16. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 15.Payne C.E., Hofer S.O., Zhong T., Griffin A.C., Ferguson P.C., Wunder J.S. Functional outcome following upper limb soft tissue sarcoma resection with flap reconstruction. J Plastic Reconstr Aesthetic Surg: JPRAS. 2013;66(5):601–607. doi: 10.1016/j.bjps.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Wright E.H., Gwilym S., Gibbons C.L., Critchley P., Giele H.P. Functional and oncological outcomes after limb-salvage surgery for primary sarcomas of the upper limb. J Plastic Reconstr Aesthetic Surg: JPRAS. 2008;61(4):382–387. doi: 10.1016/j.bjps.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 17.Van Egmond ‐van Dam J.C., Bekkering W.P., Bramer J.A.M., Beishuizen A., Fiocco M., Sander Dijkstra P.D. Functional outcome after surgery in patients with bone sarcoma around the knee; Results from a long‐term prospective study. J Surg Oncol. 2017;115(8):1028–1032. doi: 10.1002/jso.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen S.J.N.R., Paulino Pereira N.R.K.A., Raskin K.A. A comparison of questionnaires for assessing physical function in patients with lower extremity bone metastases. J Surg Oncol. 2016;114:691–696. doi: 10.1002/jso.24400. [DOI] [PubMed] [Google Scholar]
- 19.Nelson E.C., Eftimovska E., Lind C., Hager A., Wasson J.H., Lindblad S. Patient reported outcome measures in practice. BMJ. 2015;350 doi: 10.1136/bmj.g7818. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Ou L., Hollis S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13(no. 1) doi: 10.1186/1472-6963-13-211. article 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilton T.L., Hosking K. Endoprosthetic treatment of primary bone sarcomas with pathological fractures. SA Orthop J. 2016;15(2):43–48. [Google Scholar]
- 22.Aileen M.D., Sajeevan P., Anthony M.G., Jay S.W., Robert S.B. Symptoms and their relationship to disability following lower extremity tumour. Sarcoma. 1999;3(2) doi: 10.1080/13577149977677. 73-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renard A.J., Veth P.V., Schreuder H.W.B. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73:198e205. doi: 10.1002/(sici)1096-9098(200004)73:4<198::aid-jso3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber D., Bell R.S., Wunder J.S., O’Sullivan B., Turcotte R., Masri B.A., Davis A.M. Evaluating function and health related quality of life in patients treated for extremity soft tissue. Sarcoma. 2006;15(9):1439–1446. doi: 10.1007/s11136-006-0001-4. [DOI] [PubMed] [Google Scholar]
- 25.Orlic D., Smerdelj M., Kolundzic R., Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop (SICOT) 2006;30:458–464. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X., Cai Z.D., Chen Z.R., Yao Z.J., Zhang G.J. A preliminary evaluation of limb salvage surgery for Osteosarcoma around knee joint. PLoS One. 2012;7(3):e33492. doi: 10.1371/journal.pone.0033492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rastogi S.H., Trikha V., Alam Khan S.H. Limb salvage in osteogenic Sarcoma: an Indian perspective. Indian J Med Paediatr Oncol. 2004;25(2):46–50. [Google Scholar]
- 28.Clayer M., Doyle S., Sangha N., Grimer R. The Toronto extremity salvage score in unoperated controls: an age, gender, and country comparison. Sarcoma. 2012 doi: 10.1155/2012/717213. [DOI] [PMC free article] [PubMed] [Google Scholar]