Abstract
Objective
Per-implantitis is one of the implant treatment complications. Dentists have failed to restore damaged periodontium by using conventional therapies. Tissue engineering (stem cells, scaffold and growth factors) aims to reconstruct natural tissues. The paper aimed to isolate both periodontal ligament stem cells (PDLSCs) and bone marrow mesenchymal stem cells (BMMSCs) and use them in a co-culture method to create three-layered cell sheets for reconstructing natural periodontal ligament (PDL) tissue.
Materials and methods
BMMSCs were isolated from rabbit tibia and femur, and PDLSC culture was established from the lower right incisor. The cells were co-cultured to induce BMMSC differentiation into PDL cells. Cell morphology, stem cells and PDL-specific markers (CD90, CD34, and periostin) were also detected using immunofluorescent assay. Co-cultured cell monolayers were detached using temperature-responsive tissue culture dishes and collagen graft to create the three-layer construct. The 3D-engineered tissue was examined histologically and by field emission scanning electron microscopy (FESEM).
Results
BMMSCs co-cultured with PDLSCs successfully induced more PDL cells. The newly induced PDL cells exhibited periostin and CD90 expression. Fluorescence green intensity was measured for the co-cultured cells that were stained with periostin, the mean fluorescence green intensity (periostin expression) was significantly higher for the newly induced PDL cells after 1, 2, and 3 weeks when compared with control (BM-MSCs), at 21 days non-significant difference was measured when compared with control (PDLSCs).
The results showed the successful formation of 3D multilayer PDL tissue. Histological cross-section showed cell sheets and the stable adhesion between them. FESEM examination was conducted for the cross-section, showing three-layered cell sheets with stable adhesion between cells.
Conclusions
The results of this paper report that the three layered-cell sheets were successfully constructed by the novel use of collagen graft as a scaffold to be used in treatment of periodontitis and to envelop the dental implants to create biohybrid implant.
Keywords: Collagen graft, Mesenchymal stem cells, Periostin, Isolation PDL, Scaffold, Three-dimension culture
1. Introduction
Periodontitis is a common infection of oral disease in adults and is the main reason for distraction of tooth support, leading to tooth loss. Dentists have failed to restore damaged periodontium by using conventional therapies. Tissue engineering aims to reconstruct natural tissues [1]. Periodontal ligament (PDL) “is a specialized soft connective tissue that connected the tooth root surface with alveolar bone socket,” and it consists of different cell populations, including endothelial cells, fibroblasts, epithelial cells, osteoblasts, the rest of Malassez and cementoblasts [2]. In addition, the PDL contains multipotent stem cells (undifferentiated mesenchymal cells) that can differentiate into mesenchyme linages, including PDL derived from the cranial neural crest [3]. Mesenchymal stem cells (MSCs) were isolated from PDL and named PDLSCs, which were identified and found to generate specific attachments of the tooth PDL-like complex in mice; after this discovery, numerous clinical attempts were performed on animals and humans [4]. PDLSCs play an essential role in periodontal tissue regeneration in animal and human models [5] and can use the PDL as stem cell source [6]. Compared with other MSC-like populations, PDLSCs possess unique properties, including their ability to form cementum, alveolar bone, Sharpey's fibers and to undergo self-renewal [7]. However, various problems are associated with isolation of PDLSCs; these problems include the risk of tumorigenesis, contamination and vulnerable of stem cell condition due to donor quality [8]. In addition, limitations in the number of stem cells; the small cell numbers (average 1250 cells) are yielded from the primary cultures of PDLSCs, that is not enough to generate cells sheet for periodontal ligament, which needs at least 4 × 106 cells [9], [10]. PDLSC transplantation was found to be an excellent solution to the limitations of PDLSC autologous transplantation [11]. Bone marrow MSCs (BMMSCs) can differentiate into numerous types of cells [12], [13]. These stem cells were used successfully for in vivo and in vitro studies, leading to their clinical use in primary studies for clinical trials and therapies [14]. BMMSCs, PDLSCs and alveolar periosteal cells can simulate periodontal regeneration. Complete regeneration is formed by tissue engineering, which is the combination of these stem cells and scaffold; other types of multipotent MSCs have been used in clinical trials for periodontal regeneration [15], [16]. The paper aimed to isolate both periodontal ligament stem cells (PDLSCs) and bone marrow mesenchymal stem cells (BMMSCs) and use them in a co-culture method to create three-layered cell sheets for reconstructing natural periodontal ligament (PDL) tissue.
2. Materials and methods
2.1. Animal and sample collection
This study was approved by the Iraqi Center for Cancer and Medical Genetics Research (ICCMGR)/Mustansiriyah University. The rabbits were housed in an animal house under standard conditions and with free access to a regular supply of soft food and water. MSCs were gathered from femur and tibia of New Zealand white male rabbits at age of 3–6 weeks, whereas PDLSCs were obtained from the periodontal ligament of lower incisor teeth of rabbits. All work was performed under sterilised conditions.
2.1.1. Isolation of BM-MSCs
The work was conducted under general anaesthesia by intramuscular injection according to the weight of each animal (ketamine 10%, 0.35 ml/kg; Kepro, Holland) and xylazine 20%, 0.5 ml/kg, Kepro, Holland) at the back of the leg. Less than 15 min was the needed time to achieve anesthesia [17]. The right limb of the rabbit was shaved, and the skin was cleaned with alcohol and iodine. Skin incision was performed by using a sharp blade to expose the muscle, which was then dissected to expose the femur and tibia. Handpiece and surgical drills (1 mm; SAEYANG/Korea) were used to create a hole in the tibia and femur with irrigation using normal saline. About 2–3 ml of bone marrow was aspirated immediately by using a syringe (20 ml with an 18-gauge cannula), as shown in Fig. 1.
Fig. 1.
Isolation of bone marrow: A) expose the femur, B) prepare a hole in the tibia, C) Bone marrow was aspirated by needle (20 ml with an 18-gauge cannula).
The aspirated bone marrow was placed in a sterile test tube (15 ml). Then, free minimum essential medium (MEM) (Capricorn Scientific, Germany) was added to the test tube, which was shaken until homogeneity of the solution. Then, the test tube was centrifuged (1500 rpm for 10 min at 18 °C). The supernatant was removed to separate the red blood cells (RBCs) and cell debris. The pellet was further purified from the remaining RBCs by adding RBC lysis buffer at room temperature and centrifuging again for 5 min. The supernatant was removed, and 8–10 ml of total culturing MEM supplemented with L-glutamine, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, 20% foetal bovine serum (FBS) (Capricorn Scientific, Germany), 100 U/ml ampicillin, 100 μg/ml amphotericin B and 100 μg/ml of streptomycin (Capricorn Scientific, Germany) was added to the test tube. The large bone marrow cores were dispersed in the cell suspension by pipetting [18].
Finally, the suspension was added to the tissue culture disk (falcon T-75 cm2) and incubated at 37 °C in humidified 95% air and 5% CO2 incubator. After 24 h, the cells adhered, and the MEM containing 20% FBS was changed to remove non-adherent cells [19]. The medium was replaced every 72 h, and the remaining non-adherent cells were removed until the cultures achieved semiconfluence of adherent cells to form a cell monolayer after 18–20 days. To reach passage 1 (P1) and P2, whose cells were used for experiments, on day 20, the MSCs were trypsinised and subcultured at about 90% confluence. In the next 5–6 days, P1 proliferated and showed semiconfluence of adherent cells forming cell monolayers.
2.1.2. Isolation of PDLSCs
Under sterilised condition, the lower right incisor from each animal was extracted; dental extraction was performed as follows:
Intramuscular and infiltration techniques were performed with induction by general and local anaesthesia by ketamine 10%, xylazine 20% and local anaesthetic (lidocaine HCL 2%, Septodont/France) to control the bleeding.
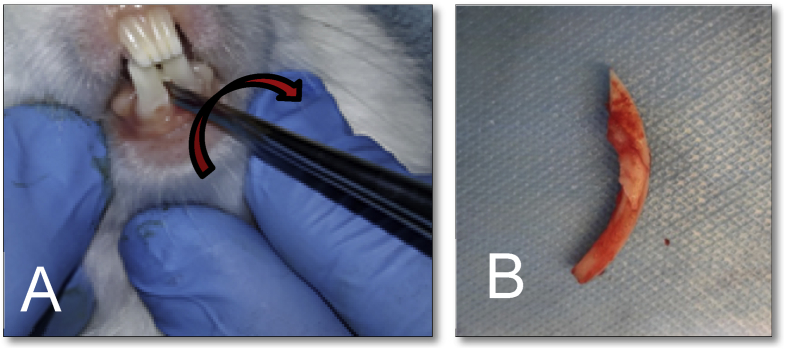
A small root-tip pick elevator was inserted carefully between the tooth and bone mesially to subluxate the tooth (Fig. 2). Then, a small mosquito forceps was used for extraction with rocking movement, with no rotational movement due to curvature and relative brittleness of the tooth as illustrated in Fig. 2. After dental extraction, the animal was injected with 0.5 ml/kg penicillin (Kepro, Holland) for 3 days, and dental care solution 1:50 (Zahnpflege Wasser, Germany) was added to the drinking water of rabbits 1 day before and 3 days after extraction to exclude any infection. The extracted teeth were subjected instantly to transport media at 4 °C.
Fig. 2.
Dental extraction of the lower central incisor: A) Extraction movements, B) Lower incisor after extraction.
Lower incisors teeth were rinsed thrice for 3 min with phosphate-buffered saline (PBS) containing 100 μg/ml amphotericin, 100 μg/ml streptomycin and 100 U/penicillin. Teeth roots were scraped using a surgical scalpel to collect the PDL inside the test tube. Then, the PDL was dispersed with 0.75 μg/ml collagenase I (USBiological, USA) in total culturing MEM for 8 h at 37 °C with shaking to obtain a single cell suspension (Fig. 3). The suspension was then centrifuged at 1000 rpm for 5 min at 18 °C. The medium was discarded and replaced with a new of 8–10 ml complete MEM. PDL cells were dispersed in the cell suspension by pipetting. Finally, the suspension was added to tissue culture T-75 cm2 flask and incubated at 37 °C in humidified 95% air and 5% CO2 incubator.
Fig. 3.
Surgical isolation of PDLSCs: A) Extracted teeth in transport media, B) Extracted teeth in petri dish, C) Teeth roots were scraped, D) PDL dispersed with 0.75 μg/ml collagenase I in free MEM. E) PDL cells.
After incubation of PDL, cells were allowed to adhere, and non-adherent cells were removed by changing the medium after five days. The medium used was MEM containing 20% FBS, 100 μg/ml ascorbic acid (USBiological, USA) [20] and 12.5 ng/ml fibroblast growth factor (FGF2; USBiological, USA), which were added freshly with each change. The medium was changed every 72 h, and the remaining non-adherent cells were removed until the cultures became semiconfluent with adherent cells to form cell monolayer after three weeks of culturing. On day 21, the PDLSCs were trypsinised and subcultured at about 80%–90% confluence. For the following week, P1 proliferated and achieved semiconfluence of adherent cells forming cell monolayers.
2.2. Immunophenotypic analysis
2.2.1. Immunophenotypic analysis of BM-MSCs
Cultured MSCs were seeded at 5 × 105 cells into the tissue culture slide chamber in MEM with 20% FBS and incubated at 37 °C for 1–2 days. Then, the cells were tested for the presence of CD90 as positive marker and CD34 as negative marker to examine the mesenchymal phenotype by immunofluorescence assay according to manufacturer's instructions:
Cold acetone was used to fix the cells for 2 min and left to dry. Then, the cells were washed thrice with PBS for 5 min. The slides were incubated with 4% blocking reagent (Santacruz Biotechnology, USA) for 1 h to block nonspecific binding and then washed thrice with PBS for 5 min. Afterward, the cells were stained with primary antibodies diluted at 1:100 by CD90 Thy1.1 (USBiological, USA) and CD 34 (Santacruz Biotechnology, USA) and incubated for 2 h at room temperature. Then, the cells were washed thrice with PBS for 5 min each wash. A fluorophore-conjugated secondary antibody (fluorescein isothiocyanate) (Santacruz, Biotechnology, USA) was incubated for 2 h at room temperature in the dark chamber. The conjugated antibody was prepared by diluting at 1:50 (6 μg secondary antibody with 300 μl blocking reagent). The secondary antibody was washed thrice with PBS for 5 min. Control cells were stained with secondary antibody only. Cover slips were mounted on a microscopic slide using glycerine 50% with PBS 50% and then examined using an inverted fluorescence microscope (Leica, Germany) in a dark room. The results were calculated by measuring the mean of fluorescence intensity (green colour of expression) by using program imageJ version 1.47(National institutes of health, Bethesda, MD, USA). In this program the images were converted to histograms by showing the number of pixels of each value, regardless of location, and the mean and maximum value [21], [22].
2.2.2. Immunophenotypic analysis of PDLSCs
PDLSCs were seeded at 5 × 105 cells in four tissue culture chambers (control cells stained only with secondary antibody (periostin (Santacruz Biotechnology, USA) as positive marker, CD90 as positive marker and CD34 as negative marker) in MEM with 20% FBS and incubated at 37 °C for 1–2 days. Then, the cells were prepared as described in section 2.2.1.
2.3. Morphological study
Haematoxylin and eosin (H&E) staining was used for morphological study. Each BM-MSC and PDLSC was cultured on the cover slide in six-well tissue culture plate at 5 × 105 cells each plate. The medium was aspirated after three days, and 10% formaldehyde (diluted in PBS) was added for 30 min to fix the cells. Cells were washed twice with distilled water (D.W.). Then, the cells were dehydrated in absolute ethanol with different (100%, 90% and 70%) concentrations, with each treatment lasting for 2 min. Next, cells were washed twice with D.W. Then, the slide was stained with haematoxylin (as a nuclear stain) for 20 min. Afterward, cells were washed twice with D.W. The slide was subsequently stained with eosin (as a cytoplasmic stain) for 2 min, and cells were washed twice with D.W. The cells were then dehydrated in with absolute 100% ethanol for 5 min. Finally, the cells were mounted with DPX and photographed by a light microscope with a camera (Micros, Austria).
2.4. Co-culturing method for BMMSC differentiation into PDL cells
Both of PDLSCs and BMMSCs at the 7th day of P2, were cultured together in 75 cm2 cell culture dishes, at a density of 5 × 105 cells/ml for each cell type (Fig. 4A), with MEM containing 20% FBS, 100 μg/ml ascorbic acid and FGF2 (12.5 ng/ml), which was added freshly with each change. The medium was changed every 3 days. Then, the co-cultured cells were examined for phenotype identification after 1, 2 and 3 weeks.
Fig. 4.
Summarize co-culture method and creating the 3D layers. A) co-culture of BMMSCs and PDLSCs. B) The method of creating the three-layered construct. C) co-cultured monolayer cells before the removal. D) the dish is empty from cells as all the monolayer were removed to form the 3D construct.
2.5. Cell sheet engineering (transfer of layered cell sheets)
Cell monolayers were detached using temperature-responsive tissue culture dishes (Nunc UpCell Surface, USA) with collagen graft (GENOSS, Korea CSD2030) to form 3D tissue models. At P3 to P7, the co-cultured cells were seeded on 3.5 mm diameter temperature-responsive dishes (3 dishes) at a cell density of 5 × 104 cells per dish for 1 week at 37 °C in humidified 95% air and 5% CO2 incubator. After the confluent layer was obtained in each dish (Fig. 4C), the medium was aspirated from the first dish; to support the cell layer, the collagen graft membrane (1 mm thickness) was placed on the top of the cell layer, thus gently avoiding air bubbles and creases between the cell layer and collagen graft membrane. A total of 50 μl medium was added to prevent drying of cells, and the dish was placed at 20 °C–25 °C for 40 min (detachment time for first co-culture layer from the dish) to allow harvesting. Afterward, the collagen graft membrane was gently grasped with sterilised tweezers and withdrawn (with first layered cell sheet) from the dish (Fig. 4D). Medium was aspirated from the second dish, and the collagen graft membrane (with first layered cell sheet facing downward) was transferred to the second temperature-responsive tissue culture dish, gently placed on the top of the second cell layer and left undisturbed at 37 °C for 1 h to allow stable adhesion between cells. Then, the second dish was placed at 20 °C–25 °C for 40 min (detachment time of two-layered cell sheets from the dish). The collagen graft membrane was gently grasped with sterilised tweezers and withdrawn (with two-layered cell sheets) from the dish. The collagen graft membrane was transferred (with two-layered cell sheets facing downward) to the third temperature-response tissue culture dish and was placed on the top of the third cell layer gently and left undisturbed at 37 °C for 1 h to allow for good adhesion between cell layers. Afterward, the third dish was placed at 20 °C–25 °C for 40 min (detachment time for three-layered cell sheets from the dish), and the constructs comprising collagen graft and three-layered cell sheets were harvested from the dishes, as illustrated in Fig. 4B. Cross-section of the construct was processed for histological assessment of the sheets and cell adhesion. Topography was assessed using field emission scanning electron microscopy (FESEM).
3. Results
3.1. Isolation of BMMSCs
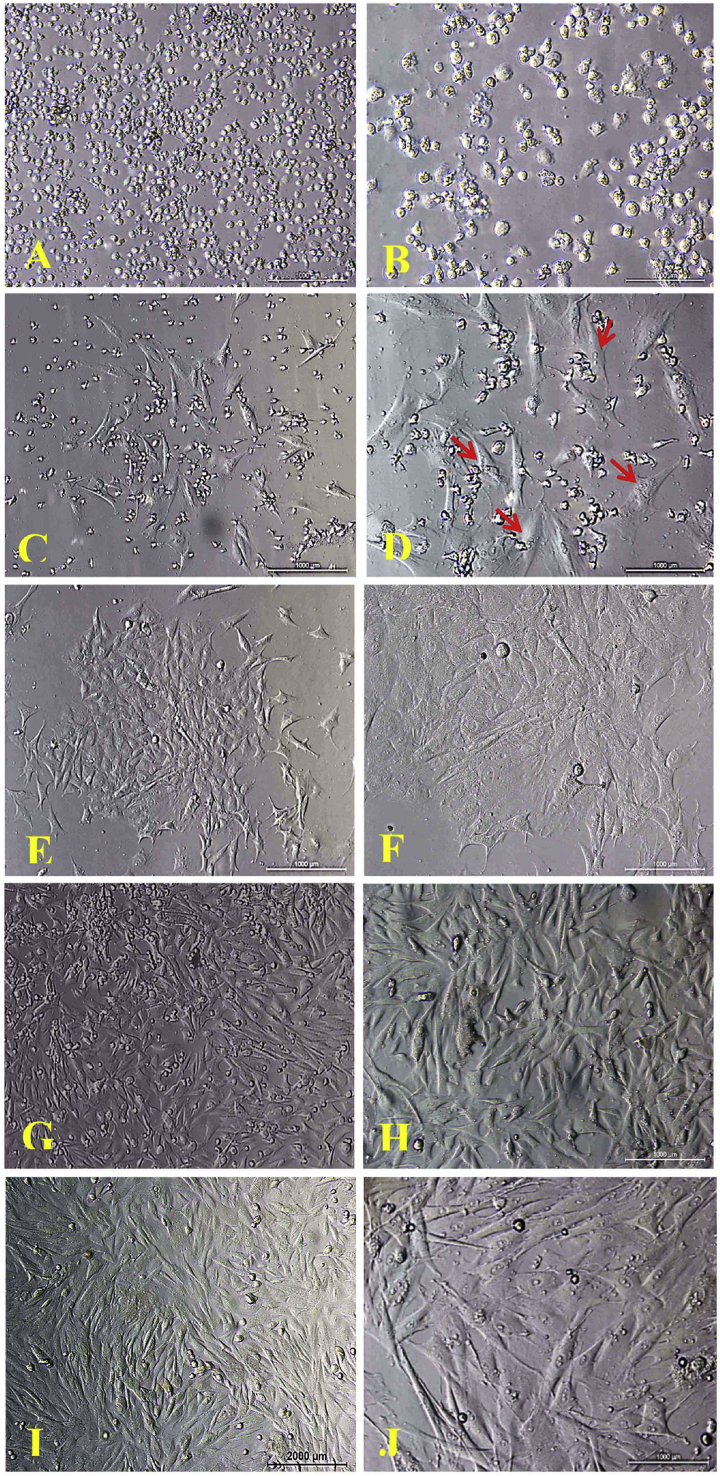
The primary culture of BMMSCs showed that cells sparsely attached to the plastic culture flasks after 24 h and formed adherent cells that were round and oval-shaped (representing MSCs) (Fig. 5A and B). Non-adherent cells (which represent hematopoietic cells, erythrocyte cells and cells debris) were discarded by changing the first medium after 24 h. Starting from the 2nd until the 9th day, the adherent cells proliferated, forming a small polygonal, star and spindle-shaped cells (Fig. 5C–F). As the cells continually grew and became elongated spindle-shaped, colonies gradually expanded in size with adjacent ones interconnecting with each other (Fig. 5G and H) at 15 and 18 days (Fig. 5I and J), at which the monolayer was formed.
Fig. 5.
Inverted microscope images, morphological characteristics of rabbit MSCs cultured in MEM+20% FBS showed a typical spindle shaped, P0 monolayer A) after 24 h 10X, B) after 24 h 20X; C) after 6 days 10X, D) after 6 days 20X; E) after 9 days 10X, F) after 9 days 20X; G) after 15 days 10X, H) after 15 days 20X; I) 18 days confluence culture 10X, J) 18 days 20X. Scale bare indicates 1000 μm.
3.2. Isolation of PDLSCs
The primary culture showed small, rounded, single-cluster cells (Fig. 6A and B). After five days of culture, small colonies of adherent, spindle-shaped cells (which represent PDLSCs) were observed (Fig. 6C and D). The medium was changed to remove nonadherent cells. After 8–20 days, the adherent cells proliferated and migrated, showing a fibroblast-like a spindle shape (Fig. 6E–H). Colony size ranged from 0.25 mm to 4 mm in diameter, reaching 80% confluency by 20–22 days (Fig. 6I and J).
Fig. 6.
Morphology of rabbit PDLSCs in primary culture under light inverted microscope, cell cultured in MEM+20% FBS. A) after removing the collagenase 8 h, 10X, B) 20X; C) spindle-shaped after 7 days 10X, D) 20X; E) after 15 days 10X, F) 20X; G) after 18 days 10X, H) 20X; I) after 21 days 10X, J) 20X. Scale bare indicates 1000 μm.
3.2.1. Morphological study
BMMSCs feature a round and large nucleus (25–30 μm in diameter) with a prominent nucleolus that is contoured by sublimely chromatin particles, allowing the nucleus to achieve a clear appearance. The cells are long and thin (80–100 μ), as shown in Fig. 7A–D. The PDL cells showed a more elongated spindle-shaped (90–140 μ) compared with BMMSCs (Fig. 7E–H).
Fig. 7.
Morphological characteristics of rabbit MSCs and PDL cells stained with H&E. A) BMMSCs after 15 days 10X, B) BMMSCs after 15 days 20X, C) BM-MSCs after 20 days 10X, D) BM-MSCs after 20 days 20X; E) PDLSCs after 15 days 10X, F) PDLSCs after 15 days 20X, G) PDLSCs after 20 days 10X, H) PDLSCs after 20 days 20X. Scale bare indicates 1000 μm.
3.3. Expression of stem cell markers in BM-MSCs
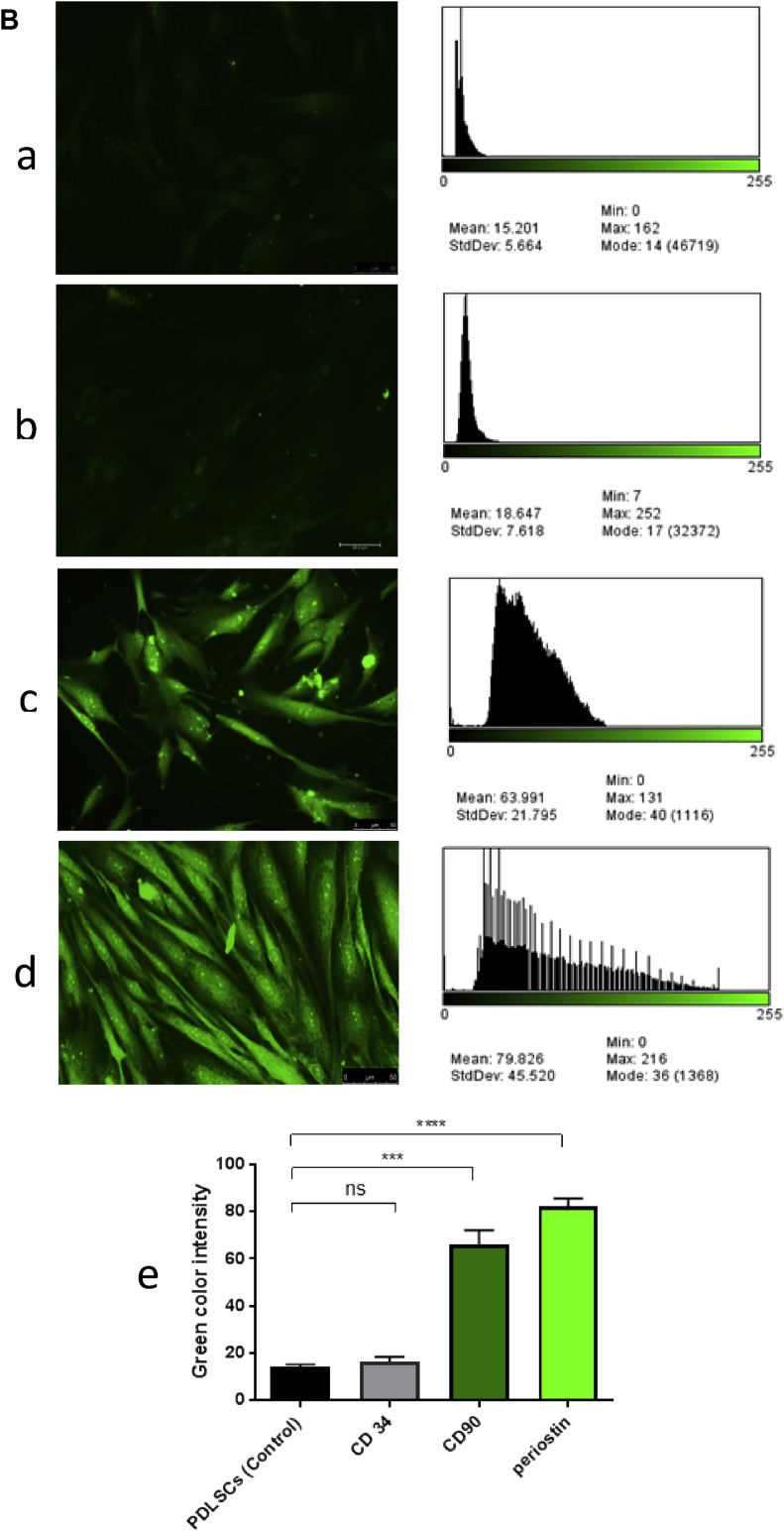
Immunofluorescence analysis revealed that more than 90% of BM-MSC-adherent cells were strongly stained with green fluorescence and were positive for CD90. On the other hand, the majority of BM-MSC-adherent cells were negative and showed no expression for CD34 (Fig. 8A–C).
Fig. 8.
A) Fluorescence microscopic images and their analysis to histogram using program imageJ at 20X, a) control of BM-MSCs, b)−CD34 of BM-MSCs, c) +CD90 (expression green) of BM-MSCs, d) Chart compare the mean of fluorescence (green color) intensity between different groups. Scale bare indicates 50 μm. B) Fluorescence microscopic images and their analysis to histogram using program imageJ at 20X, a) Control of PDLSCs, b)−CD34 of PDLSCs, c) +CD90 PDLSCs (expression green), d) + periostin of PDLSCs (expression green), e) Chart compare the mean of fluorescence (green color) intensity between different groups. Scale bare indicates 50 μm.
3.4. Expression of stem cell markers in PDLSCs
Immunofluorescence phenotyping of cultured PDLSCs showed that 80% and 95% of cells were stained positively for CD90 and periostin, respectively. By contrast, the majority of the PDLSC-adherent cells showed a negative expression for CD34 (Fig. 8D–G).
3.5. BMMSC differentiation into PDL cells through co-culturing method
BMMSCs and PDLSCs were co-cultured at a constant ratio. Bone-marrow stem cells alone and PDLSCs alone were used as controls (Fig. 9A and B, respectively). The BMMSCs proliferated and dominated at day 5 of co-culturing, with fewer PDLSCs being observed (Fig. 9C). After 10 days, the PDL cells became more prominent (Fig. 9D). By day 15, the spindle-like shape PDL cells were the most observed cell type with few BMSCs in the field of vision (Fig. 9E). After 20 days of co-culturing, nearly all cells featured the PDL cell morphology with hardly detectable BMMSCs (Fig. 9F). The differentiated co-cultured cells were further analysed by immunofluorescence assay for PDL cell markers, revealing that BMMSCs showed no expression for periostin before the co-culture (Fig. 10A), whereas PDL cells indicated 100% expression (Fig. 10B). After 1 week of co-culturing, more than 45% of adherent cells were stained positive for periostin (Fig. 10C) which is significantly different when compared with the control. The percentage of stained cells increased with time, reaching about 65% in the second week (Fig. 10D) which is significantly different when compared with the control, and continually rising until day 21; the final expression reached more than 90% (Fig. 10E) statistically, a non-significant different when compared with the control PDLSCs. This finding implies that the BMMSCs differentiated into PDL cells under the effect of PDLSCs in MEM containing 20% FBS, ascorbic acid and FGF2. Co-culture method was successful in inducing BMMSCs into PDL cells as most of the adherent cells were stained positive for periostin and the cells morphology resembled the PDLSCs at the end of the culture period, This might be due to the soluble factors secreted by certain type of cells such as PDL cells, could diffuse into the medium and induce differentiation on other cell types, such as BMMSCs this result in agreement with [23], [24] who found that the co-culture technique is a novel method for clinical periodontium regeneration.
Fig. 9.
Inverted microscope images, BMMSCs and PDLSCs Co-cultured in MEM +20% FBS, monolayer after 10 days. A) BMMSCs, B) PDLSCs, C) Co-cultured at 5th day, D) Co-cultured at 10th day, E) Co-cultured at 15th day, F) Co-cultured at 20th day. Scale bare indicates 1000 μm.
Fig. 10.
A) Fluorescence microscopic images and their analysis to histogram using program imageJ at 20X, A) BMMSCs, B) PDLSCs, Co-culture positive by periostin (expression green) C) after 7 days, D) 14 days, E) 21 days. Scale bare indicates 50 μm. B) Chart compare the mean of fluorescence (green color) intensity between different groups.
3.6. Evaluation of three cell layers
Histologically, photomicrographs of H&E-stained cross-section showed Three-layered constituted a 3D construct (cell sheets) and stable adhesion between them (Fig. 11A and B, respectively). FESEM was performed to examine the cross section of collagen graft at different magnifications, indicating a porous scaffold that can support cells for tissue-like construct (Fig. 11C and D). Engineered tissue composed of layered cell sheets showed the three layers with stable adhesion between sheets (Fig. 11E). At higher magnifications, the cells attached to each other between sheets (Fig. 11F).
Fig. 11.
3D engineered tissue, A) photomicrographs of H&E stained cross-section showing three layered-cell sheets 20X, B) higher magnification to the three-layer sheets 40X. C) FESEM images, cross section of collagen graft at 60X, D) cross section of collagen graft at 150X, E) Topography view of layered-cell sheets showing the three layers marked with arrows (yellow arrow is for the first layer, red for the second and blue for the third layer, F) showing the adhesion between the sheets.
4. Discussion
Tissue-engineering methods using biomaterial scaffolds, in combination with in vitro-cultured cells that can replace damaged tissues and inserted to affected sites, have been suggested as novel substitutions to current therapies [25]. Rabbit BMMSCs were successfully isolated and characterised and then expanded to induce differentiation into PDL cells through co-culturing method. The morphological characteristics of MSCs were previously described by other researchers [19], [26]. BMMSCs feature numerous advantages, such as availabilty with easy manipulation, low risk of tumorigenicity and ability to differentiate into numerous types of cells and be used successfully in applications [14]. Furthermore, the successful rate of primary culture is greater with type I collagenase than with using trypsin as described by [10]. BM-MSC-adherent cells were strongly positive for CD90, whereas Zomer et al. [27] observed that CD90 expression was lower in rabbit MSCs when compared with human MSCs. In addition, the majority of BM-MSC-adherent cells showed no expression for CD34, which is one of the most important characteristics of MSCs [28], [29]. These results indicate that these cells were MSCs; they were undifferentiated cells or of hematopoietic origin [30]. MSCs (BMMSCs and PDLSCs) must be plastic-adherent and express specific surface antigens. Cells are considered MSCs when they are >95% positive for CD73, D90 (Thy-1), CD105 and CD44; at the same time, they show >95% negative expression for CD14, CD34 and CD45 [31]. Any alterations to surface marker expression of cells indicating phenotypic changes, including loss of multipotency, must be determined. MSC marker expressions and their function act as an indicative tool for cell behavior whether in vitro or clinics [32]. Periostin is an extracellular matrix protein related to the function of PDL [9], [33]; it is predominantly expressed in PDL rather than other tissues [34]. Loss of this protein causes severe destruction of PDL [35]. Periostin acts as a potential marker for PDLSC identification [33]. PDLSCs were cultured and propagated efficiently, and cells from P2 were used in this study. The morphological features of PDLSCs were reported earlier [36], [37]. According to surface antigen, the phenotypic features of PDLSCs were determined positive for CD90 and periostin and negative for CD34; this result agrees with those of other studies [6], [10]. Co-culturing method was successfully used in inducing BMMSCs into PDL cells in most adherent cells, which were stained positively for periostin and whose morphology resembled that of PDLSCs at the end of culture period. Bai et al. [24] reported co-culturing technique as a novel method for clinical periodontium regeneration. For the co-culturing method, we can hypothesise that soluble factors secreted by certain type of cells, such as PDL cells, could diffuse into the medium and induce differentiation into other cell types, such as BMMSCs [23]. The 3D tissue engineering using co-cultured cells successfully induced a multilayer tissue that can be used to restore the damaged periodontium for future applications. In this study, fabrication of construct composing three-layered cell sheets and collagen graft as a supporter was done successfully by using temperature responsive culture. This method results in higher cells density without damage cell to cell connection and superior transplantation efficiency more than those isolated by an enzymatic method, similar finding was described by [38], [39].
Using allogeneic PDLSC is an effective method for periodontal regeneration, whereas PDLSC transplantation is an excellent solution to the limitations of PDLSC autologous transplantation [11].
Cross-section of 3D construct showed cells sheets and stable adhesion between them. Three layers were chosen to avoid necrosis, this agreed with the work of [20], and thickness of the 3D construct was set to avoid necrosis. The cross section of collagen graft at different magnifications, indicating a porous scaffold that support cells penetration with surface enhances cells attachment, proliferation, and penetration for tissue-like construct Future work will focus on using this construct in experimental treatment. FESEM images showed sheets and the stable adhesion between them. Under the growth factors and vitamin C, PDLSCs can yield layered cell sheet as the result of increased cell matrix production; the PDLSC sheets showed significant improvement in tissue engineering [20]. The 3D construct can be used to coat titanium dental implants with sintered β-tricalcium phosphate to simulate the normal PDL tissue [40].
5. Conclusion
The results of this paper report that the bone marrow and PDL of adult rabbits are ideal sources for isolation and expansion of BM-MSCs and PDLSCs and their use in co-culture to increase PDL cells and to produce 3D PDL cell layered sheets by the novel use of collagen graft as a scaffold. Co-culture is a method to overcome the limitations in the number of PDLSCs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None declared.
Ethical approval
The study complies with current ethical consideration.
Acknowledgements
The authors thank the Experimental Therapy Department at Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University for the support during the study.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.08.002.
Contributor Information
Ihab Nabeel Safi, Email: ihab202066@gmail.com.
Basima Mohammed Ali Hussein, Email: yasbasima@yahoo.com.
Ahmed Majeed Al-Shammari, Email: ahmed.alshammari@iccmgr.org.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Reich E., Hiller K.A. Reasons for tooth extraction in the western states of Germany. Community Dent Oral Epidemiol. 1993;21(6):379–383. doi: 10.1111/j.1600-0528.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 2.Lekic P., McCulloch C.A.G. Periodontal ligament cell populations: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996;245(2):327–341. doi: 10.1002/(SICI)1097-0185(199606)245:2<327::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Huang C.Y., Pelaez D., Dominguez-Bendala J., Garcia-Godoy F., Cheung H.S. Plasticity of stem cells derived from adult periodontal ligament. Regen Med. 2009;4(6):809–821. doi: 10.2217/rme.09.55. [DOI] [PubMed] [Google Scholar]
- 4.Chen F.-M., Gao B.-M., Tian X.-Y., Zhang Y.-J., Zhang G.-Y., Dong H. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng F., Akiyama K., Liu Y., Yamaza T., Wang T.M., Chen J.H. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16(1):20–28. doi: 10.1111/j.1601-0825.2009.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trejo Iriarte C.G., Ramírez Ramírez O., Muñoz García A., Verdín Terán S.L., Gómez Clavel J.F. Isolation of periodontal ligament stem cells from extracted premolars. Simplified method. Rev Odontol Mex. 2017;21(1):e12–e20. [Google Scholar]
- 7.Menicanin D., Mrozik K.M., Wada N., Marino V., Shi S., Bartold P.M. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014;23(9):1001–1011. doi: 10.1089/scd.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata M., Iwasaki K., Akazawa K., Komaki M., Yokoyama N., Izumi Y. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng A. 2017;23(9–10):367–377. doi: 10.1089/ten.tea.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata T., Yamato M., Zhang Z., Mukobata S., Washio K., Ando T. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–1099. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W., Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cell Int. 2015;2015:11. doi: 10.1155/2015/972313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsumanuma Y., Iwata T., Kinoshita A., Washio K., Yoshida T., Yamada A. Allogeneic transplantation of periodontal ligament-derived multipotent mesenchymal stromal cell sheets in canine critical-size supra-alveolar periodontal defect model. Biores Open Access. 2016;5(1):22–36. doi: 10.1089/biores.2015.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alshammari A.M., Salman M.I., Umran M.A. IN VITRO effect of differentiation factors on accumulation of COL1A1, COL2A1 and CRTAC1 for chondrogenesis of mice bone marrow mesenchymal stem cells. IJRSB. 2015;3(4):45–56. [Google Scholar]
- 13.Mohammad M., Yaseen N., Al-Joubory A., Abdullah R., Mahmood N., Ahmed A.A. Production of neural progenitors from bone marrow mesenchymal stem cells. Stem Cell Discov. 2016;6(1):1–12. [Google Scholar]
- 14.Seyed Foroutan K., Khodarahmi A., Alavi H., Pedram S., Baghaban Eslaminejad M.R., Bordbar S. Bone marrow mesenchymal stem cell and vein conduit on sciatic nerve repair in rats. Trauma Mon. 2015;20(1) doi: 10.5812/traumamon.23325. e23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata T., Washio K., Yoshida T., Ishikawa I., Ando T., Yamato M. Cell sheet engineering and its application for periodontal regeneration. J Tissue Eng Regenerat Med. 2015;9(4):343–356. doi: 10.1002/term.1785. [DOI] [PubMed] [Google Scholar]
- 16.Jabur A.R., Al-Hassani E.S., Al-Shammari A.M., Najim M.A., Hassan A.A., Ahmed A.A. Evaluation of stem cells' growth on electrospun polycaprolactone (PCL) scaffolds used for soft tissue applications. Energy Procedia. 2017;119:61–71. [Google Scholar]
- 17.Al-Shammari A.M., Syhood Y., Al-Khafaji A.S. Use of low-power He-Ne laser therapy to accelerate regeneration processes of injured sciatic nerve in rabbit. Egyp J Neurol Psych Neurosurg. 2019;55(1):1. doi: 10.1186/s41983-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AL-Qaisy B.A., Alwachi S.N., Yaseen N.Y., AL-Shammari A.M. Isolationand Identification of mouse bone marrow derived mesenchymal stem cells. Iraqi J Cancer Med Gen. 2014;7(1):49–55. [Google Scholar]
- 19.Abdullah R., Yaseen N., Saleh S., Mohamed M., Al-Shammari A. Direct and simple method for mesenchymal stem cells isolation, culturing and detection. Int J Stem Cell Res Transplant. 2018;5:054. [Google Scholar]
- 20.Wei F., Qu C., Song T., Ding G., Fan Z., Liu D. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227(9):3216–3224. doi: 10.1002/jcp.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hand A.J., Sun T., Barber D.C., Hose D.R., Macneil S. Automated tracking of migrating cells in phase-contrast video microscopy sequences using image registration. J Microsc. 2009;234(1):62–79. doi: 10.1111/j.1365-2818.2009.03144.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shammari A.M., Ismaeel F.E., Salih S.M., Yaseen N.Y. Live attenuated measles virus vaccine therapy for locally established malignant glioblastoma tumor cells. Oncolytic Virotherapy. 2014;3:57–68. doi: 10.2147/OV.S59037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagi M. Cell processing engineering for ex-vivo expansion of hematopoietic cells. J Biosci Bioeng. 2005;99(3):189–196. doi: 10.1263/jbb.99.189. [DOI] [PubMed] [Google Scholar]
- 24.Bai Y., Matsuzaka K., Hashimoto S., Fukuyama T., Wu L., Miwa T. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig's epithelial root sheath cells. Bone. 2011;48(6):1417–1426. doi: 10.1016/j.bone.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Honda M.J., Imaizumi M., Tsuchiya S., Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52(4):541–552. doi: 10.2334/josnusd.52.541. [DOI] [PubMed] [Google Scholar]
- 26.Mohammad M.H., Al-Shammari A.M., Al-Juboory A.A., Yaseen N.Y. Characterization of neural stemness status through the neurogenesis process for bone marrow mesenchymal stem cells. Stem Cells Cloning Adv Appl. 2016;9:1. doi: 10.2147/SCCAA.S94545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zomer H.D., Roballo K.C., Lessa T.B., Bressan F.F., Gonçalves N.N., Meirelles F.V. Distinct features of rabbit and human adipose-derived mesenchymal stem cells: implications for biotechnology and translational research. Stem Cells Cloning Adv Appl. 2018;11:43–54. doi: 10.2147/SCCAA.S175749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calloni R., Cordero E.A.A., Henriques J.A.P., Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22(9):1455–1476. doi: 10.1089/scd.2012.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allakhverdi Z., Comeau M.R., Armant M., Agrawal R., Woodfolk J.A., Sehmi R. Mast cell-activated bone marrow mesenchymal stromal cells regulate proliferation and lineage commitment of CD34(+) progenitor cells. Front Immunol. 2013;4:461. doi: 10.3389/fimmu.2013.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhtina A., Tohfafarosh M., Lichtler A., Arinzeh T.L. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine. In Vitro Cell Dev Biol Anim. 2014;50(3):251–260. doi: 10.1007/s11626-013-9702-5. [DOI] [PubMed] [Google Scholar]
- 31.Reis M., McDonald D., Nicholson L., Godthardt K., Knobel S., Dickinson A.M. Global phenotypic characterisation of human platelet lysate expanded MSCs by high-throughput flow cytometry. Sci Rep. 2018;8(1):3907. doi: 10.1038/s41598-018-22326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bara J.J., Richards R.G., Alini M., Stoddart M.J. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima H., Kawanabe N., Murata S., Ishihara Y., Yanagita T., Balam T.A. SSEA-4 is a marker of human deciduous periodontal ligament stem cells. J Dent Res. 2012;91(10):955–960. doi: 10.1177/0022034512458123. [DOI] [PubMed] [Google Scholar]
- 34.Yamada S., Tauchi T., Awata T., Maeda K., Kajikawa T., Yanagita M. Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res. 2014;93(9):891–897. doi: 10.1177/0022034514543015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rios H., Koushik S.V., Wang H., Wang J., Zhou H.M., Lindsley A. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25(24):11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleuterio E., Trubiani O., Sulpizio M., Di Giuseppe F., Pierdomenico L., Marchisio M. Proteome of human stem cells from periodontal ligament and dental pulp. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071101. e71101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cen L.P., Ng T.K. Human periodontal ligament-derived stem cells promote retinal ganglion cell survival and axon regeneration after optic nerve injury. 2018;36(6):844–855. doi: 10.1002/stem.2812. [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi K., Shimizu T., Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release. 2015;205:83–88. doi: 10.1016/j.jconrel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Iwata T., Yamato M., Tsuchioka H., Takagi R., Mukobata S., Washio K. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30(14):2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Safi I.N., Hussein B.M.A., Al Shammari A.M., Tawfiq T.A. Implementation and characterization of coating pure titanium dental implant with sintered β-TCP by using Nd:YAG laser. Saudi Dent J. 2019;31(2):242–250. doi: 10.1016/j.sdentj.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.