Abstract
Background/purpose
Circadian rhythm is an endogenous daily variation observed in most physiological functions including salivary secretion. Irregular lifestyle causes many diseases such as obesity and sleep disorders. The aim of this study is to examine the effects of the timings of sleep and meal on the prevalence of dental caries.
Materials and methods
Study was conducted at university hospital in Japan. We asked 230 children (1–16 years old) to record the following life habits for 8 days: waking time, bedtime, mealtimes, snacking frequency, and tooth brushing frequency. We analyzed sleep habits from all data and compared dental caries and life habits using data from subjects with primary (2–7 years old) or permanent (11–16 years old) dentition period.
Results
The number of dental caries assessed using the decay or filled teeth (dft) index correlated with bedtime, supper time, regularity of supper time, and snacking frequency in subjects with primary dentition. Multiple regression analysis revealed that bedtime and snacking frequency were mutually independent risk factors for dental caries. No correlations were found between the prevalence of dental caries and other measurement items. The number of caries correlated with the regularity of supper time and age in subjects with permanent dentition.
Conclusion
Children with daily life habits associated with eveningness have a higher prevalence of dental caries.
Keywords: Dental caries, Oral health, Circadian rhythms, Life habits, Questionnaire
Introduction
Appropriate timing and duration of sleep are necessary for our health and productivity, and irregular lifestyle can lead to sleep disorders and other lifestyle-related health conditions. A recent study reported that disturbed daily rhythms induce obesity in adults.1 Regarding oral health and dentistry, daily variations of salivary flow and content are well known.2, 3, 4, 5 Salivary flow is low in the morning, increases in the afternoon or evening, and decreases thereafter. The daily variations in salivary flow and content are the results of circadian rhythms, which are endogenous rhythms with a period of approximately 24 h.6,7 Therefore, eating at late night, when salivary secretion is reduced, could easily cause dental caries.
Timing of life habits such as sleep and meal affects circadian rhythms,8,9 and circadian rhythms could affect oral functions including salivary secretion.2,5,10,11 Therefore, irregular sleep or mealtime disturbs internal circadian rhythms and this seems to cause irregular salivary secretion and dental caries. This will often apply for children, because circadian rhythms in developmental period could be affected more by external perturbations,12 those are irregular life habits in other words. In line with this hypothesis, correlations between these life habits and the number of dental caries should be analyzed. Although there are many reports which show correlations between the emergence of oral diseases such as dental caries and a variety of life habits,13 most of them focused on effects of sugar consumption, ethnics or rearing environment such as mother's education.14, 15, 16, 17, 18 In the present study, we asked children and their parents or guardians to record the children's daily life habits including waking time, bedtime, and mealtimes for 1 week at home. We then analyzed them with the number of dental caries obtained from the children's medical records.
Material and methods
Study population
This study was conducted at our university hospital, in accordance with the guidelines in the Declaration of Helsinki. Two hundred and thirty outpatients (age range, 1–16 years) of the Division of Dentistry for Children and Disabled Persons with no systemic diseases voluntarily participated in the study. This study was approved by our institutional review board.
Data collection
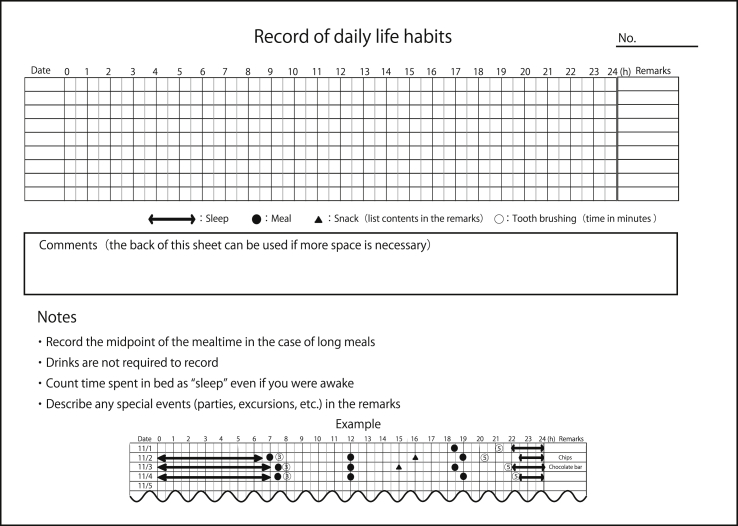
We explained the objectives of this study and recording procedures to the subjects and their parents or guardians. A sheet for recording daily life habits (Fig. 1) and an envelope were given to subjects after obtaining written informed consent from their parents or guardians. Subjects were asked to record five habits: waking time, bedtime, mealtimes, snacking time, and tooth brushing time for 8 days at home. Subjects were asked to avoid recording during any period in which they attended a special event such as parties, excursions, or athletic competitions. After 8 days of recording, the sheet was sent by mail to our university. All subjects filled out only one recording sheet. We analyzed responses from 140 children (77 male and 63 female, mean age ± standard deviation [SD], 7.2 ± 3.5 years) in which subjects recorded these five life habits over 7 days.
Figure 1.
Recording sheet for daily life habits used in this study (translated into English from Japanese).
The number of dental caries was obtained from subjects' medical records. Diagnosis of dental caries was performed by subject s' attending dentists, who were faculties of our university hospital. We unify the diagnosis criterion for dental caries which needs treatments. The first author, one of dentists in our department, confirmed all subjects.
Data analysis
We used the mean midpoint of sleep time (between bedtime and waking time) as the mean sleep time, and the mean sleep time from Friday night to Saturday morning and from Saturday night to Sunday morning as the mean sleep time on the weekend. Decayed, missing, or filled teeth (DMFT) index of children with the permanent dentition and decayed or filled teeth (dft) index of those with the primary dentition was used for the number of caries.
Differences between the two groups were analyzed by Student's t-test or the Mann–Whitney U test, and factors were compared using Pearson's correlation coefficient or Spearman's rank correlation coefficient. In order to analyze the interrelation among life habits, multiple regression analysis was performed. All statistics were performed using SPSS software (version 22.0; IBM, Armonk, NY, USA). P values < 0.05 were considered statistically significant.
Results
Both sleep duration and mean sleep time on the weekend correlated with age (sleep duration: r = −0.742, P < 0.0001, mean sleep time: r = 0.397, P < 0.0001) (Fig. 2). These results suggest that children sleep less and later as they age. No significant differences were found between sexes in all items recorded.
Figure 2.
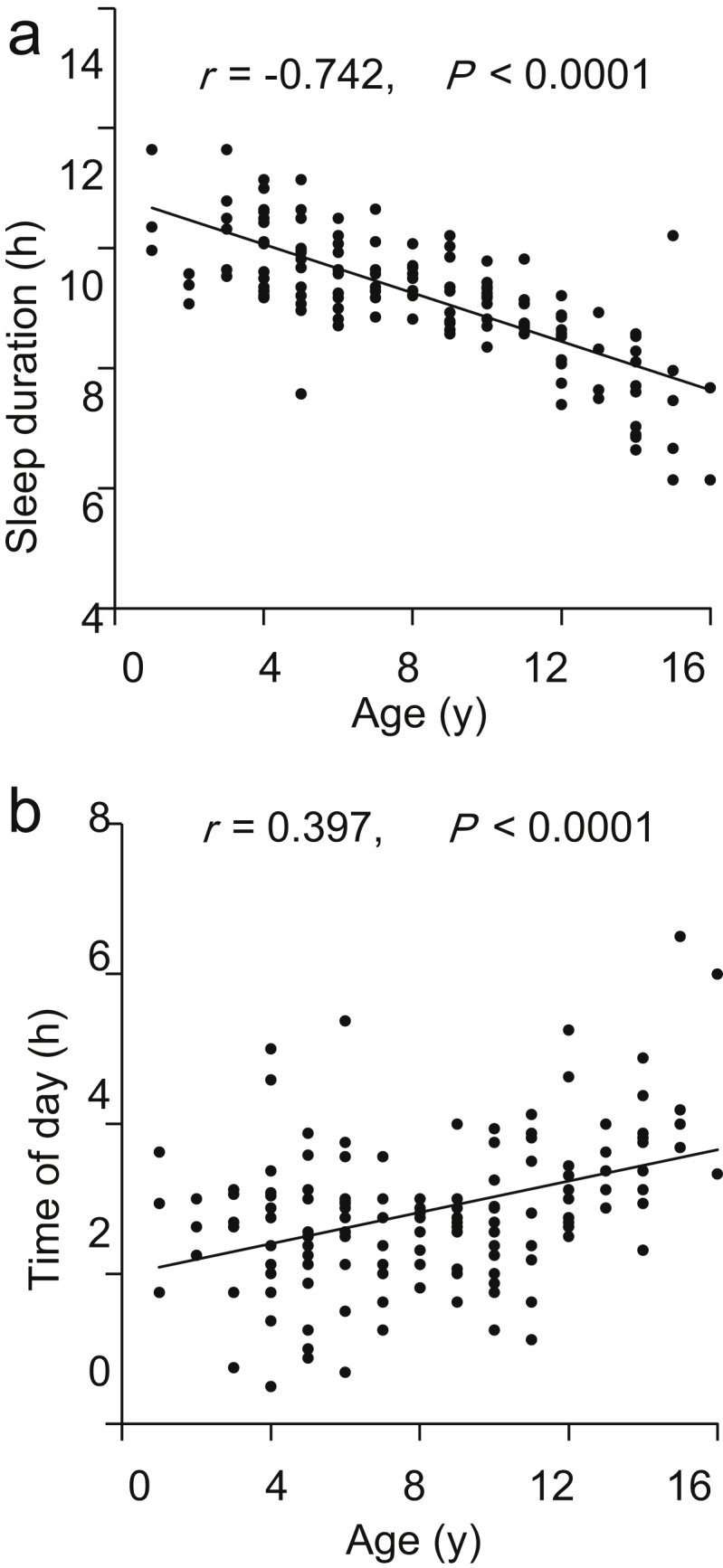
Correlations between age and sleep duration (a), and between age and sleep time (b). The abscissa indicates the age of the subjects. The ordinate indicates the weekly mean sleep duration (weekday and weekend) (a) and the midpoint of sleep time (weekend) (b), respectively.
We next analyzed correlations between the number of dental caries and daily life habits in the children with primary dentition, who had all 20 primary teeth and did not have any permanent teeth (age range, 2–7 years; n = 38). The number of caries was significantly correlated with the mean sleep onset time (r = 0.370, P = 0.022), mean supper time (r = 0.384, P = 0.017), and SD of supper time (r = 0.346, P = 0.034) (Table 1, Fig. 3). A significant correlation was also detected between the mean sleep onset time and mean supper time (r = 0.820, P < 0.001). Children went to bed about 3 h after supper on average. No correlation was found between the number of caries and the mean tooth brushing time or frequency (Fig. 4).
Table 1.
Correlations between the prevalence of caries and daily habits in the primary dentition period.
| Factor 1 | Factor 2 | n | r | t | P |
|---|---|---|---|---|---|
| Midpoint of sleep time (weekday) | dft | 38 | 0.256 | 1.589 | 0.121 |
| Midpoint of sleep time (weekend) | dft | 38 | 0.269 | 1.678 | 0.102 |
| Sleep onset | dft | 38 | 0.370 | 2.393 | 0.022a |
| SD of sleep onset | dft | 38 | 0.171 | 1.039 | 0.306 |
| Sleep offset | dft | 38 | 0.126 | 0.760 | 0.452 |
| SD of sleep offset | dft | 38 | 0.030 | 0.181 | 0.857 |
| Sleep duration | dft | 38 | −0.276 | 1.722 | 0.094 |
| Breakfast time | dft | 38 | 0.106 | 0.638 | 0.527 |
| SD of breakfast time | dft | 38 | −0.078 | 0.469 | 0.642 |
| Lunch time | dft | 38 | 0.206 | 1.260 | 0.216 |
| SD of lunch time | dft | 38 | −0.058 | 0.349 | 0.729 |
| Supper time | dft | 38 | 0.384 | 2.493 | 0.017a |
| SD of supper time | dft | 38 | 0.346 | 2.210 | 0.034a |
| Snacking frequency | dft | 38 | 0.403 | 2.640 | 0.012a |
| Tooth brushing frequency | dft | 36 | −0.012 | 0.069 | 0.945 |
| Tooth brushing time | dft | 35 | −0.131 | 0.759 | 0.453 |
| Age | dft | 38 | 0.044 | 0.262 | 0.795 |
| Sleep onset | Supper time | 38 | 0.820 | 8.594 | <0.001a |
| Snacking frequency | Supper time | 38 | 0.319 | 2.017 | 0.051 |
| Sleep onset | SD of supper time | 38 | 0.366 | 2.361 | 0.024a |
| Snacking frequency | SD of supper time | 38 | 0.039 | 0.233 | 0.817 |
| Sleep onset | Snacking frequency | 38 | 0.206 | 1.266 | 0.214 |
SD: standard deviation; dft: decay, filled teeth index.
Significant correlation.
Figure 3.
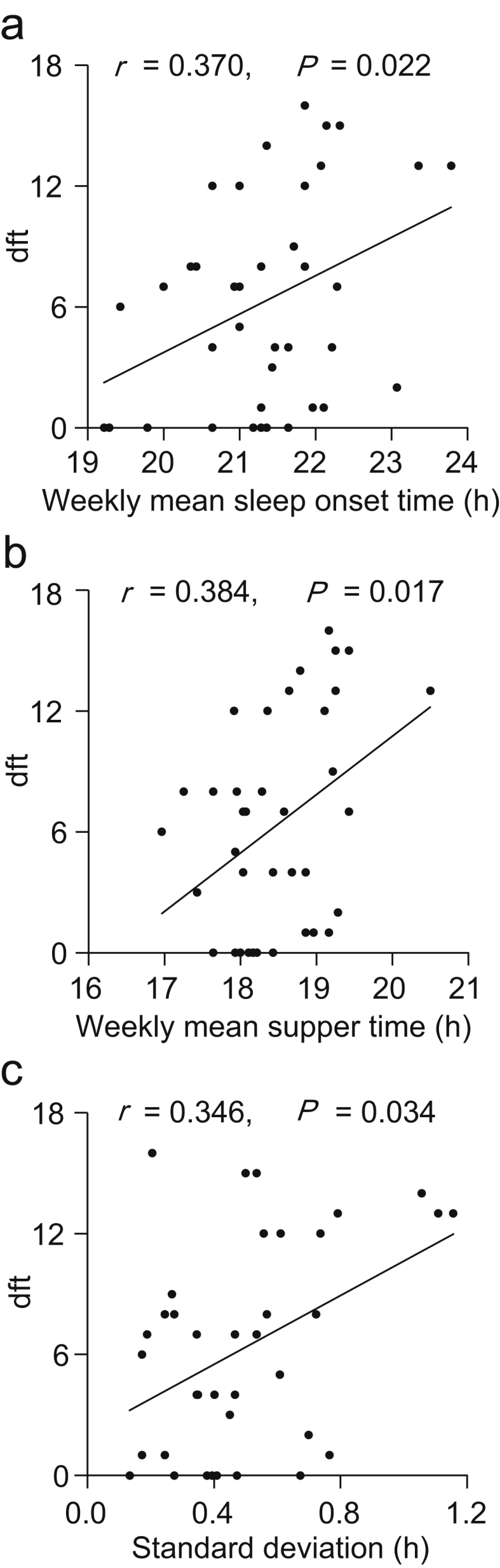
Correlations between daily life habits and the dft index score in the primary dentition. The ordinate indicates the dft index score. The abscissa indicates the weekly mean sleep onset time (a), the weekly mean supper time (b), and the standard deviation of the weekly mean supper time (c), respectively.
Figure 4.
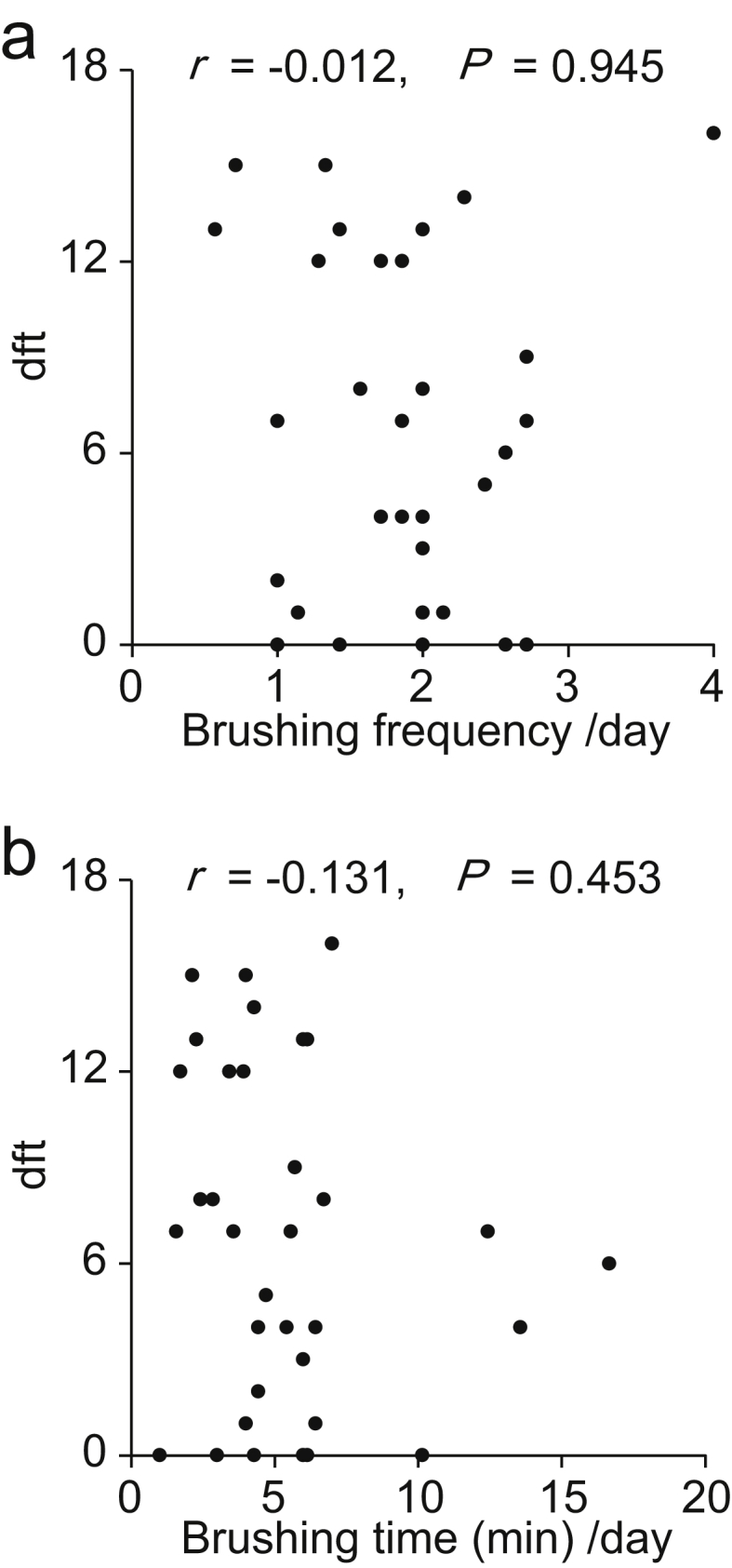
dft index plotted against tooth brushing habits. The ordinate indicates the dft index score. The abscissa indicates the frequency (a) or the total time (b) of tooth brushing per day, respectively.
Because snacking frequency was also correlated with the number of caries (r = 0.403, P = 0.012), we performed multiple regression analysis between dft (as the dependent variable) and the following independent variables: mean sleep onset time and snacking frequency. We excluded the mean and SD of supper time from the independent variables because there was a correlation with the mean sleep onset time (Table 1). Using mean sleep onset time and snacking frequency as independent values, the regression was significantly fitted (R2 = 0.307, P = 0.002), suggesting that these two items were correlated with dft mutually independently. In this regression, standard partial regression coefficients (beta values) were 0.346 for the mean sleep onset time and 0.408 for snacking frequency. In children with the permanent dentition, who did not have any primary teeth (age range, 11–16 years; n = 33), the number of caries correlated with SD of supper time (r = 0.368, P = 0.045) and age (r = 0.352, P = 0.045) (Table 2).
Table 2.
Correlations between the prevalence of caries and daily habits in the permanent dentition period.
| Factor 1 | Factor 2 | n | r | t | P |
|---|---|---|---|---|---|
| Midpoint of sleep time (weekday) | DMFT | 33 | 0.302 | 1.763 | 0.088 |
| Midpoint of sleep time (weekend) | DMFT | 32 | 0.108 | 0.596 | 0.556 |
| Sleep onset | DMFT | 33 | 0.277 | 1.608 | 0.118 |
| SD of sleep onset | DMFT | 33 | −0.112 | 0.628 | 0.535 |
| Sleep offset | DMFT | 33 | 0.032 | 0.176 | 0.861 |
| SD of sleep offset | DMFT | 33 | −0.048 | 0.270 | 0.789 |
| Sleep duration | DMFT | 33 | −0.290 | 1.690 | 0.101 |
| Breakfast time | DMFT | 33 | 0.104 | 0.579 | 0.566 |
| SD of breakfast time | DMFT | 33 | −0.168 | 0.950 | 0.350 |
| Lunch time | DMFT | 33 | −0.096 | 0.536 | 0.596 |
| SD of lunch time | DMFT | 33 | −0.096 | 0.536 | 0.596 |
| Supper time | DMFT | 30 | 0.200 | 1.080 | 0.289 |
| SD of supper time | DMFT | 30 | 0.368 | 2.097 | 0.045a |
| Snacking frequency | DMFT | 33 | −0.234 | 1.342 | 0.189 |
| Tooth brushing frequency | DMFT | 33 | 0.112 | 0.628 | 0.535 |
| Tooth brushing time | DMFT | 33 | −0.071 | 0.395 | 0.695 |
| Age | DMFT | 33 | 0.352 | 2.093 | 0.045a |
| Sleep onset | Supper time | 30 | 0.223 | 1.211 | 0.236 |
| Snacking frequency | Supper time | 30 | −0.267 | 1.464 | 0.154 |
| Sleep onset | SD of supper time | 30 | 0.210 | 1.139 | 0.264 |
| Snacking frequency | SD of supper time | 30 | −0.127 | 0.679 | 0.503 |
| Sleep onset | Snacking frequency | 33 | 0.033 | 0.185 | 0.855 |
SD: standard deviation; DMFT: decayed, missing, or filled teeth index.
Significant correlation.
Discussion
Circadian rhythms regulate a variety of physiological functions including salivary flow, and are affected by environmental and habitual factors such as light or meals. Being awake late at night often leads to nighttime exposure to light and meals, which can affect circadian rhythms. Dental caries seems to emerge by eating when oral resistance to cariogenic microbes is low (e.g., at nighttime when salivary flow is decreased).
The number of caries was significantly correlated with several daily life habits in the primary dentition period, however, the same correlations were not observed in the permanent dentition period (Table 1, Table 2). This finding seems to be because the effect of irregular daily life habits on the emergence of dental caries is relatively greater in younger children than in older children. During the developmental period, circadian rhythms show high sensitivity and can be easily affected by irregular life habits.12,19
Multiple regression analysis revealed snacking frequency as the most influential factor on the dft index among the life habits investigated in the primary dentition period. This result confirms the effects of sugar consumption reported previously.14,20, 21, 22, 23 To our knowledge, this is the first report to demonstrate correlations between factors that directly affect circadian rhythms, such as sleep time and mealtime, and caries prevalence, although there is a few study in which the correlation is implied.24,25 Life habits associated with circadian rhythms did not correlate with snacking frequency (Table 1, Table 2), suggesting that these life habits were risk factors for caries emergence independent from sugar consumption. Additionally, we did not observe a statistically significant correlation between caries prevalence and tooth brushing, unlike other studies.14,26,27 Although the reason for this uncorrelation was not clear, it was thought that life habits such as sleep and snacking affected on the emergence of caries, at least in this population.
Circadian rhythms in peripheral organs such as the salivary glands are important for maintaining physiological functions which increase at specific times. Sudden changes in sleep time can shift central and peripheral clocks in an organ-specific manner and induce conditions such as ‘jet lag’.28 Desynchronization among or within organs can lead to a decline in physiological functions. The results of the present study suggest that eveningness and irregular mealtime lead to the emergence of dental caries in children (Fig. 3, Table 1). Daily variations of the activities of cariogenic bacteria seem to the variation of caries prevalence. The variation of oral bacteria could be caused by two different possibilities, that is, variation of salivary flow or variation of bacterial activity itself. Although further study is needed to test these hypotheses, the activity of some bacteria has demonstrated circadian rhythms in human intestine.29
Sleep habits and their association with diseases have previously been investigated using questionnaires.30,31 The correlation between age and sleep time found in the present study (Fig. 2b) is consistent with a previous study in which it was observed that sleep time for children becomes increasingly later during development and peaks at around 20 years of age.32
In conclusion, children with daily life habits associated with eveningness have a higher prevalence of dental caries. Children in urban areas seem to be exposed to substantial light in the evening, which disrupts circadian rhythms and prevents sleep. To avoid the adverse effects of abundant evening light and late meals, children should be encouraged to go to bed early. Personalized counseling for oral health care, in which individuals' circadian rhythms are considered, should be promoted in the future.
Declaration of interest statement
The authors have no conflicts of interest.
Acknowledgements
We are grateful to all staff members of the Department of Pediatric Dentistry at Hokkaido University Hospital for introducing this project to their patients and providing clinical information.
This work was supported by JSPS KAKENHI grants (Nos. 24792267 and 26670878 to S.N., and No. 16K11799 to T.Y.) and The Akiyama Life Science Foundation (to S.N.).
References
- 1.Roenneberg T., Allebrandt K.V., Merrow M., Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson D.B., Fort A. Circadian variations in human resting submandibular saliva flow rate and composition. Arch Oral Biol. 1974;19:47–55. doi: 10.1016/0003-9969(74)90224-6. [DOI] [PubMed] [Google Scholar]
- 3.Seppa L., Pollanen L., Hausen H. Streptococcus mutans counts obtained by a dip-slide method in relation to caries frequency, sucrose intake and flow rate of saliva. Caries Res. 1988;22:226–229. doi: 10.1159/000261110. [DOI] [PubMed] [Google Scholar]
- 4.Jenzano J.W., Brown C.K., Mauriello S.M. Temporal variations of glandular kallikrein, protein and amylase in mixed human saliva. Arch Oral Biol. 1987;32:757–759. doi: 10.1016/0003-9969(87)90123-3. [DOI] [PubMed] [Google Scholar]
- 5.Shirakawa T., Mitome M., Oguchi H. Circadian rhythms of S-IgA and cortisol in whole saliva-Compensatory mechanism of oral immune systemfor nocturnal fall of saliva secretion- Pediatr Dent J. 2004;14:115–120. [Google Scholar]
- 6.Furukawa M., Kwamoto T., Noshiro M. Clock gene expression in the submandibular glands. J Dent Res. 2005;84:1193–1197. doi: 10.1177/154405910508401219. [DOI] [PubMed] [Google Scholar]
- 7.Vujovic N., Davidson A.J., Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol. 2008;295:R355–R360. doi: 10.1152/ajpregu.00498.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigeyoshi Y., Taguchi K., Yamamoto S. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 9.Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nirvani M., Khuu C., Utheim T.P. Circadian rhythms and gene expression during mouse molar tooth development. Acta Odontol Scand. 2017;75:144–153. doi: 10.1080/00016357.2016.1271999. [DOI] [PubMed] [Google Scholar]
- 11.Janjić K., Kurzmann C., Moritz A., Agis H. Expression of circadian core clock genes in fibroblasts of human gingiva and periodontal ligament is modulated by L-Mimosine and hypoxia in monolayer and spheroid cultures. Arch Oral Biol. 2017;79:95–99. doi: 10.1016/j.archoralbio.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Nishide S.Y., Honma S., Honma K. The circadian pacemaker in the cultured suprachiasmatic nucleus from pup mice is highly sensitive to external perturbation. Eur J Neurosci. 2008;27:2686–2690. doi: 10.1111/j.1460-9568.2008.06231.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris R., Nicoll A.D., Adair P.M., Pine C.M. Risk factors for dental caries in young children: a systematic review of the literature. Community Dent Health. 2004;21:71–85. [PubMed] [Google Scholar]
- 14.Grindefjord M., Dahllof G., Nilsson B., Modeer T. Prediction of dental caries development in 1-year-old children. Caries Res. 1995;29:343–348. doi: 10.1159/000262090. [DOI] [PubMed] [Google Scholar]
- 15.Thibodeau E.A., O'Sullivan D.M. Salivary mutans streptococci and caries development in the primary and mixed dentitions of children. Community Dent Oral Epidemiol. 1999;27:406–412. doi: 10.1111/j.1600-0528.1999.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 16.Hooley M., Skouteris H., Boganin C., Satur J., Kilpatrick N. Parental influence and the development of dental caries in children aged 0-6 years: a systematic review of the literature. J Dent. 2012;40:873–885. doi: 10.1016/j.jdent.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Scorzetti L., Marcattili D., Pasini M., Mattei A., Marchetti E., Marzo G. Association between obesity and periodontal disease in children. Eur J Paediatr Dent. 2013;14:181–184. [PubMed] [Google Scholar]
- 18.Cianetti S., Lombardo G., Lupatelli E. Dental caries, parents educational level, family income and dental service attendance among children in Italy. Eur J Paediatr Dent. 2017;18:15–18. doi: 10.23804/ejpd.2017.18.01.03. [DOI] [PubMed] [Google Scholar]
- 19.Nishide S.Y., Hashimoto K., Nishio T., Honma K., Honma S. Organ-specific development characterizes circadian clock gene Per2 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2014;306:R67–R74. doi: 10.1152/ajpregu.00063.2013. [DOI] [PubMed] [Google Scholar]
- 20.Beighton D., Adamson A., Rugg-Gunn A. Associations between dietary intake, dental caries experience and salivary bacterial levels in 12-year-Old English schoolchildren. Arch Oral Biol. 1996;41:271–280. doi: 10.1016/0003-9969(96)84555-9. [DOI] [PubMed] [Google Scholar]
- 21.Holbrook W.P., Arnadottir I.B., Takazoe I., Birkhed D., Frostell G. Longitudinal study of caries, cariogenic bacteria and diet in children just before and after starting school. Eur J Oral Sci. 1995;103:42–45. doi: 10.1111/j.1600-0722.1995.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook W.P., de Soet J.J., de Graaff J. Prediction of dental caries in pre-school children. Caries Res. 1993;27:424–430. doi: 10.1159/000261574. [DOI] [PubMed] [Google Scholar]
- 23.Schroder U., Edwardsson S. Dietary habits, gingival status and occurrence of Streptococcus mutans and lactobacilli as predictors of caries in 3-year-olds in Sweden. Commun Dent Oral Epidemiol. 1987;15:320–324. doi: 10.1111/j.1600-0528.1987.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 24.Bahuguna R., Younis Khan S., Jain A. Influence of feeding practices on dental caries. A case-control study. Eur J Paediatr Dent. 2013;14:55–58. [PubMed] [Google Scholar]
- 25.Lundgren A.M., Ohrn K., Jonsson B. Do adolescents who are night owls have a higher risk of dental caries? - a case-control study. Int J Dent Hyg. 2016;14:220–225. doi: 10.1111/idh.12165. [DOI] [PubMed] [Google Scholar]
- 26.ElSalhy M., Honkala S., Soderling E., Varghese A., Honkala E. Relationship between daily habits, Streptococcus mutans, and caries among schoolboys. J Dent. 2013;41:1000–1006. doi: 10.1016/j.jdent.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Guido J.A., Martinez Mier E.A., Soto A. Caries prevalence and its association with brushing habits, water availability, and the intake of sugared beverages. Int J Paediatr Dent. 2011;21:432–440. doi: 10.1111/j.1365-263X.2011.01146.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki S., Numano R., Abe M. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 29.Thaiss C.A., Levy M., Korem T. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 31.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythm. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 32.Roenneberg T., Kuehnle T., Pramstaller P.P. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]