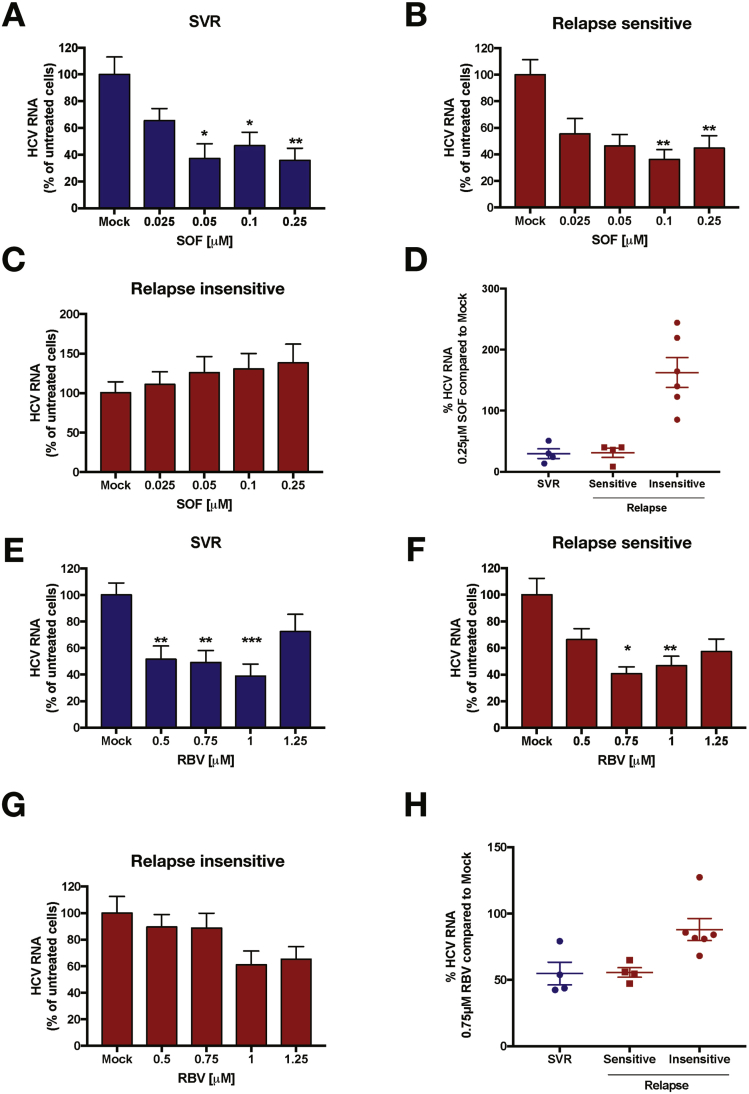
Figure 1.
SOF sensitivity in HCV genotype 3 treatment non-responders to direct-acting antiviral (DAA) therapy was assessed using the capture-fusion assay. Sera from patients with HCV genotype 3 (n = 14) who achieved SVR (blue) or relapsed (red) were used to assess sensitivity to SOF and RBV. Changes in HCV RNA for SOF and RBV in patients who achieved SVR (n = 4) (A, E) or relapsed are shown. Samples from patients who relapsed were further divided into 2 groups, depending on the assay outcome, those who were SOF- and RBV-sensitive (n = 4) (B, F) and insensitive (n = 6) (C, G). Data were summarized in (D; SOF) and (G; RBV) to show HCV RNA as a percentage of no drug treatment for a single dose of drug (0.25 μM of SOF and 0.75 μM of RBV). Graphs show mean ± SEM. P values were calculated using Kruskal-Wallis test. Drug sensitivity of each sample was assessed in quadruplicate for each concentration.