Abstract
Livestock losses due to rabies and health and the corresponding benefits of controlling the disease are not often considered when the cost-effectiveness of rabies control is evaluated. In this research, assessed the benefits of applying a One Health perspective that includes these losses to the case of canine rabies vaccination in Ethiopia. We constructed a dynamic epidemiological model of rabies transmission. The model was fit to district-specific data on human rabies exposures and canine demography for two districts with distinct agro-ecologies. The epidemiological model was coupled with human and livestock economic outcomes to predict the health and economic impacts under a range of vaccination scenarios. The model indicates that human exposures, human deaths, and rabies-related livestock losses would decrease monotonically with increasing vaccination coverage. In the rural district, all vaccination scenarios were found to be cost-saving compared to the status quo of no vaccination, as more money could be saved by preventing livestock losses than would be required to fund the vaccination campaigns. Vaccination coverages of 70% and 80% were identified as most likely to provide the greatest net health benefits at the WHO cost-effectiveness threshold over a period of 5 years, in urban and rural districts respectively. Shorter time frames led to recommendations for higher coverage in both districts, as did even a minor threat of rabies re-introduction. Exclusion of rabies-related livestock losses reduced the optimal vaccination coverage for the rural district to 50%. This study demonstrated the importance of including all economic consequences of zoonotic disease into control decisions. Analyses that include cattle and other rabies-susceptible livestock are likely better suited to many rural communities in Africa wishing to maximize the benefits of canine vaccination.
Keywords: Cost-effectiveness, Ethiopia, Modeling, One-health, Rabies
1. Background
Vaccines provide a benefit to society by eliminating, preventing or controlling the spread of disease in the population [1]. However, vaccine-based control programs can involve large investments [2,3]. In many resource-limited countries, funding such a project could be at the expense of other competing interests such as investing in basic infrastructure. Given these competing interests and high cost of implementation, it is important to identify the cost-effective level of vaccination coverage for a disease in order to avoid the drawbacks of over or under-vaccination and to help prioritize efforts to increase vaccination coverage [4,5].
Rabies control cost-effectiveness evaluations conducted in Africa and elsewhere traditionally focus on a relatively narrow set of benefits, such as averted deaths among humans [6]. Such approaches may substantially undervalue the benefit of vaccination [7], especially in settings where economically important animals are also afflicted. As demonstrated in an assessment of Brucellosis control in Mongolia [8], a more complete assessment would reflect vaccination's impact on disease in the source animal, other affected animal species, and related economic sectors.
Rabies claims the lives of 60,000 people annually worldwide and imposes an associated annual economic loss of 8.6 billion dollars accounting for direct and indirect expenditures related to post-exposure prophylaxis, dog vaccination expenditures, human productivity losses, and livestock losses [9]. However, efforts to control the disease are lacking, particularly in sub-Saharan African nations such as Ethiopia. As >99% of all human cases worldwide result from the bite of a domestic dog [10], the World Health Organization (WHO) recommends canine rabies vaccination to eliminate the disease in canine populations and consequently in humans. Many countries in the Western Hemisphere have significantly reduced the human rabies incidence through large-scale dog vaccination campaigns, controlling free-ranging dog populations and enforcing legislation for responsible pet ownership, as well as providing rabies post-exposure prophylaxis [11]. As a result, starting in the 1920s and 1930s, mass vaccination programs were largely responsible for the elimination of dog rabies and reducing the annual incidence of human rabies to almost zero in North America, while the number of cases in Central and South America as well as the Caribbean have decreased markedly [[12], [13], [14]].
In Ethiopia, canine vaccination has never been enforced as compulsory nor promoted as a public good, and as such very few dog owners vaccinate their dogs in the country [15,16]. In addition to threatening humans, rabies incidence in cattle in Ethiopia and associated economic losses are estimated to be one of the highest globally [9]. Almost all cattle rabies incidence in Ethiopia were also attributed to suspected rabid dog bite. Cattle are one of the most important and numerous livestock species in Ethiopia, representing a major source of animal protein and providing draft power to support crop farming, in addition to other socio-cultural roles [17]. Herd-level incidence has been shown to vary based on agroecology and cattle productions system [18]. The impact of rabies is most substantial for pastoral cattle-keeping households due to their complete dependency on livestock for their livelihoods.
To identify the economically optimal program for rabies control in Ethiopia, there is a need for an overarching One-Health oriented cost-effectiveness analysis that includes rabies-related livestock losses in cattle-keeping communities. We present the first cost-effectiveness model of rabies vaccination that combines human health outcomes and livestock losses. The model is tailored to two districts of Ethiopia with distinct agro-ecology, using parameters derived from case investigation of human and cattle exposures to rabid dogs in each setting.
2. Materials and methods
2.1. Epidemiological model
We adapted a previously developed canine rabies transmission model encompassing dogs and human with an extension to incorporate cattle in a One-Health concept [19]. We applied canine demographic parameters specific to Ethiopia, and fit epidemiological parameters to district-specific, retrospective data on human rabies exposures and deaths using a Bayesian Monte-Carlo Markov chain (MCMC) process.
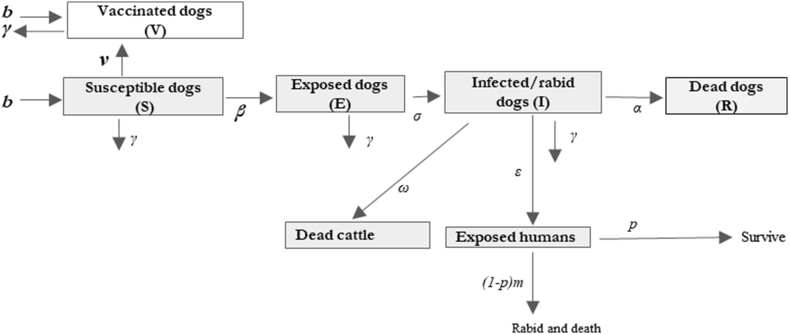
A stochastic compartmental rabies transmission model was constructed for domestic dogs (Fig. 1). The canine population (N) is divided into five classes: susceptible (S), exposed (E), infectious (I), and vaccinated (V). Dogs are born into the vaccinated class at rate bv(S + V + E), and into the susceptible class at a rate b(1-v)(S + V + E). Infectious and exposed dogs were excluded from the reproductive rate, as the virus typically has a short incubation period and puppies born to rabid animals are unlikely to survive [19]. Upon exposure to rabies, dogs move to the exposed class at rate βSI/K, where K is the carrying capacity and β is the dog-to-dog daily transmission rate. The dog-to-dog transmission rate approximates the daily number of new canine exposures resulting from a single infectious dog and does not have a geographically-linked component. Exposed dogs transition to the infectious class at rate σ. Death from rabies occurs at rate α. Death from all other causes, excluding rabies but including carrying capacity and resource constraints, removes animals from all compartments, proportional to compartment size, at a density-dependent death rate γ that increases as the population size approaches its carrying capacity, K, according to the Verhulst logistic equation [20].
Fig. 1.
Rabies transmission model schematic.
(b = birth rate; γ = death rate; β = dog-to-dog transmission rate; σ = transition rate to the infectious class, α = rate of death from rabies; ε = human exposure rate to rabid dogs; ω = cattle exposure rate to rabies; p = probability of exposed human receives sufficient doses of the post-exposure prophylaxis (PEP); v = proportion of susceptible dogs vaccinated, m = probability of death after exposure in non-recipients of the PEP)
Transmission dynamics model equations
Humans and cattle are dead-end hosts and are exposed to rabid dogs at rates ε and ω, respectively. We used the dog-to-human rabid biting rate calculated by empirical studies in Tanzania [19,21], assuming that rabid dog behavior is not markedly different across settings. Following human exposure, a person receives sufficient doses of post-exposure prophylaxis (PEP) with probability p. We assume all recipients of PEP survive, but that non-recipients have a probability m of death, which is conditional on the distribution of bite wounds across the body and the probability of death associated with exposure to each wound type. For the transmission to rate to cattle, ω, value was assumed. The prevalence of cattle under any dog rabies vaccination coverage is proportional to the reduction in dog rabies incidence, for coverage ranging from zero (status quo in Lemuna-bilibilo) to 90%. Thus, the economic loss in cattle was estimated accordingly. In other words, we assumed a relationship between rabid dogs and rabies-related livestock loss (ω), to be linear relationship across predictions for vaccination coverages assumed. Therefore, rabies-related livestock losses will rise or fall in concert with the incidence of canine rabies. Subsequent reduction of dog rabies by increasing dog vaccination coverage was assumed to reduce the incidence in cattle by the same proportion (estimated from Jibat and colleagues [22])and thus reduce the economic loss from cattle rabies.
2.2. Model parameterization and fitting
We used a Bayesian Monte-Carlo Markov chain (MCMC) process to fit the epidemiological and demographic parameters to district-specific empirical data on human rabies incidence and canine population size. Flat priors were specified for β, the dog-to-dog rabies transmission rate, and K, the canine population carrying capacity. For all other parameters, prior distributions were defined with empirical data, either collected specifically for this study or published in the literature (Table 1). As several parameters differ between districts, fitting was conducted separately for each district.
Table 1.
Rabies transmission dynamics model parameters, distribution, prior/initial input estimates.
| Parameters | Description | Distribution | Estimate |
Source | |
|---|---|---|---|---|---|
| Bishoftu/Urban | Lemuna-bilbilo | ||||
| β | Dog to dog bite rate/day | normal | 0.42 | 0.4 | [19] |
| K | Carrying capacity (dogs/km2) | logistic | 340 | 35.4 | Field survey |
| b | Birth rate/day | normal | 0.0015 | 0.0011 | Field survey; [19] |
| 1/σ | Incubation period (in days) | normal | 22.3 | 22.3 | [19] |
| 1/α | Infectious period (in days) | gamma | 3.1 | 3.1 | [19] |
| v | Vaccination coverage | 0 | 18% | Field survey | |
| Dog density/km2 | normal | 340 | 35.5 | Field survey | |
| ε | Human bite rate/rabid dog | normal | 0.51 | 0.51 | [19] |
| Human density/km2 | 3500 | 158 | [37] | ||
| Human exposure/year | 189 | 189 | [24] | ||
| Number of human deaths/year | 1 | 8 | [24] | ||
| p | Probability to receive sufficient doses of PEP* | beta | 0.77 | 0.5 | [24] |
| (1-p)m | Probability of death due to rabies | multinomial | 0.16 | 0.15 | [24,26] |
| District size (in km2) | 40 | 1184 | [37] | ||
| ω | Cattle bite rate/rabid dog | – | – | 1.38 | – |
PEP* = Post Exposure Prophylaxis.
The per capita birth rate of dogs (b) was assumed to be the product of the sex ratio (h), the average litter size (l) and litter frequency (f), and pup survival (s) [9]. A district-specific sex ratio was estimated from a field survey conducted in both districts in April and May 2015.
Canine density was estimated from the same survey which used techniques of capture-mark and resights [23]. Assuming similarities between the agro-ecologies, we derived estimates of litter size, litter frequency, and pup survival from studies in Tanzania. Prior distributions for the rabies incubation period, 1/σ, and the infectious period, 1/α, and the dog-to-human bite rate, δ, were also derived from Tanzania [21].
District-specific human rabies exposures and death cases were collected from health centers in both districts from Sept 2013 through August of 2014 [24]. Contact tracing further identified victims who did not report to health centers through “snowballing” exercise. Exposures and deaths from potentially rabid dogs were identified using six retrospective diagnostic criteria for the biting dogs [25]. In Bishoftu, 189 human exposures and 1 death, and in Lemuna-Bilbilo, 189 likely human exposures and 8 deaths were identified to be from potentially rabid dogs. With this survey data, we set the prior distribution for the post-exposure prophylaxis probability p as Beta (147, 42) for Bishoftu and Beta (95, 94) for Lemuna-Bilbilo. We drew from a multinomial distribution characterizing wound location and assumed Beta distributions for wound-specific probabilities of developing rabies and then death from our previous study [24] and from Tanzania [26].
We constructed a likelihood function to evaluate the probability of the data on human exposure, human death, and canine population size, using Poisson distributions around the human exposure and death data and a normal distribution around canine population size. For each district:
where L is the likelihood of x, d, n, and q given λ, μ, and ϕ. X is the number of human rabies exposures, d is the number of human deaths, n and q are the mean and standard error for canine density. The parameters λ, μ, and ϕ represent the model estimates for annual human rabies exposures, annual human rabies deaths, and canine density respectively.
The differential equations underlying the SEIVR model were solved in continuous time in R statistical program using the package “deSolve”. For each district, we used the Metropolis-Hasting algorithm [27] to generate four chains of 20,000 iterations each, initialized at dispersed values for β and K. We applied the Gelman-Rubin diagnostic [28] in the R package “coda” [29] to test for convergence. We thinned each chain by a factor of 15 and combined the final 250 parameter sets from each thinned chain to create each district-specific joint posterior distribution. The parameter set in the joint distribution with the highest likelihood was designated as the representative set and used for base-case analyses.
2.3. Human rabies exposure data collection and contact tracing
A retrospective case study was conducted to compile data on the incidence of human rabies exposure over the period of one year (September 2013 to August 2014) through an extensive bite case search in the two districts of Ethiopia. Animal bite victims were traced using data collected from the recorded cases at health centers as well as by information obtained from questioning the local community to trace unregistered bite cases. After the tracing, both registered and unregistered case, victims (and their family) were contacted and questioned about their use of PEP, if the victim died after the bite, the incurred costs and the behavioral manifestation of the biting animal. Based on these data the health burden of people who were bitten by potentially rabid dogs was assessed as well as the costs of the applied post-exposure prophylaxis. A detailed description of the human health burden and costs of treatment following rabies exposure is published elsewhere [24].
2.4. Economic model
We evaluated the total costs of rabies under each strategy consisting of the costs associated with the vaccination campaign, human post-exposure prophylaxis (PEP), and the rabies-related cattle losses.
2.4.1. Vaccination campaign costs
The costs of canine vaccination were based on a previously standardized economic model [30]. Accordingly, the costs of mass canine vaccination include the costs of the vaccine, consumables (needle and syringe, ice bar, disinfectant and swab, certificate and collar), temporary vaccinators and supervisors (per-diem, training, and transportation), advertising, and capital costs (refrigerator, muzzle, and cool bags). We assumed that a short-acting canine rabies vaccine currently produced by the National Veterinary Institute in Ethiopia would be purchased at its currently proposed price and would provide at least one year of full protection. Other costs of the vaccination campaign were established through a market survey. We incorporated increasing dog search costs for coverage rising above 50% in both districts. We also incorporated additional transportation costs at low coverage below 50% in Lemuna-bilbilo, as teams must travel longer distances between villages/dogs to ensure even geographic distribution of vaccinated dogs. Detailed procedures of costs estimation for dog vaccination campaigns and inputs are listed in Supplementary information.
2.4.2. Human health and economic burden
We quantified the human rabies burden in terms of both the costs of treating rabies-exposed people with PEP as well as the disability-adjusted life years (DALY) incurred from rabies mortality. PEP costs consist of health care costs for wound care, tetanus anti-toxin and post-exposure prophylaxis vaccine, as well as the non-healthcare costs of lost productivity for the victim and any accompanying family associated with seeking care [31]. Our previous study [32] estimated that the average PEP costs for individuals who completed the regimen recommended by the Ethiopian Public Health Institute [33] were $23.2 and $30.4 in urban and rural districts, respectively.
To estimate the health burden, we considered only years of life lost (YLL) by individuals who developed rabies. We did not consider morbidity (temporary disability) as the disease is acute and swiftly fatal [34]. We estimated the YLL per human rabies case from the primary data on age from rabies deaths in each district, assuming a multinomial distribution across 5-year age intervals. The average years of life lost incurred per human rabies death was calculated by multiplying the number of deaths within an age category multiplied by the life expectancy of the age group (i). The average life expectancy for Ethiopia is derived from the life table estimate of 2013 as published by WHO [35].
We calculated the PEP costs per rabid dog as the product of the rate at which a rabid dog bites a human (δ), the post-exposure prophylaxis probability (p), and PEP costs. Similarly, the health burden per rabid dog was estimated by multiplying the probability of a rabid dog biting a human (δ), a probability that the victim will not receive sufficient treatment (1-p) and the life-years lost per human rabies death. To estimate the total health and economic costs of rabies under each canine vaccination strategy, we multiplied these per-rabid-dog losses by the number of rabid dogs in the epidemiological model predictions.
2.4.3. Costs of cattle rabies
In rural Ethiopia, the economic impact of rabies is largely mediated by cattle exposure. Nearly all cattle rabies exposures are from rabid dogs and canine vaccination is currently non-existent [36]. Because cattle are dead-end hosts and not active members of the rabies transmission cycle, we assumed that cattle rabies is linked directly to canine rabies. Following the model fit, we calculate a relationship between rabid dogs and rabies-related livestock loss (omega), and then hold this linear relationship constant across predictions. Therefore, rabies-related livestock losses will rise or fall in concert with the incidence of canine rabies. Specifically, the cattle rabies-related economic loss under status quo (Lsq) equals the product of herd-level rabies incidence (Hinc) under status quo, the number of herds in the district (Nherd), and the economic loss per affected herd (Lpah). Under a specific canine vaccination strategy, this product is modified by the proportion of dogs which are rabid compared to the status quo (rabid ∝). Economic parameters and status quo cattle rabies incidence were extracted from our previous study on the economic impact of cattle rabies as described in the supplementary information.
2.4.3.1. Interventions
For each district separately, we simulated annual rabies mass vaccination campaigns, at coverage varying from the status quo to 90% in increments of 10%. Annual canine vaccination campaigns were assumed to be delivered once a year under an intensive campaign executed within a few days or weeks per district. We also assumed previously vaccinated dogs would be revaccinated, and that no waning from an initial vaccine efficacy of 100% would occur within the year. We compared two districts in Ethiopia, Bishoftu, and Lemuna-bilbilo which were, representative of two quite different degrees of urbanization in Ethiopia. Bishoftu is an urban district with a human density of 3500 inhabitants/km2 an average canine density of 340 dogs/km2, and a human rabies exposure rate of around 135 rabid dog bites per 100,000 inhabitants. There are no cattle raised outdoor in this urban district and cattle exposure to rabies was assumed negligible in Bishoftu. Lemuna-bilbilo is a rural district, substantially less dense than Bishoftu at 158 inhabitants/km2 [37], an average dog density of 35.4 dogs/km2 and a human rabies exposure rate of around 100 per 100,000 inhabitants [32,38]. Cattle exposure to rabies was assumed mainly from domestic dogs with an average incidence of 2% and 21% at cattle and herd level respectively [18].
2.4.3.2. Simulation of cost-effectiveness
We conducted a cost-effectiveness evaluation to identify the most efficient strategies for rabies control in Ethiopia. To do so, the epidemiological model was integrated with an economic model that estimated human and cattle rabies-related costs as well as canine vaccination campaign costs. The integrated model was simulated over 5 years for two districts in Ethiopia: Bishoftu and Lemuna-bilbilo, tailoring the analyses to urban and rural settings. The canine vaccination campaigns varied from the status quo (i.e. current vaccination coverage of 18% in urban and no vaccination in rural districts) to 90% coverage, quantifying the impact of each level of coverage on human exposure, health burden (DALY), health costs, cattle related economic loses and campaign costs. The district-specific optimal strategy, i.e. the strategy with the highest net health benefit at a given willingness-to-pay threshold, was chosen based on cost-effectiveness thresholds recommended by WHO which is up to 3 times the GDP per capita [39]. All base case analyses were conducted on a time frame of 5 years with time value of money set at a 3% discount rate, as recommended by the World Bank Disease Control Priorities Study and the Global Burden of Disease project [40].
With the base-case predictions for each district, we first identified the non-dominated vaccination strategies or those for which greater health benefits could not be achieved at a lower cost per life-year. We then calculated the incremental cost-effectiveness ratio (ICER) of moving from one specific canine vaccination coverage to the next most costly non-dominated coverage. Those strategies with a cost-per-DALY falling at or below Ethiopia's per-capita GDP of $568 [41], or three times this value (i.e. $1704), were considered “very cost-effective” or “cost-effective,” respectively [39].
To incorporate parameter uncertainty into our identification of the optimal rabies control strategies, we also applied a net health benefit (NHB) framework [42]. Net benefits are calculated for a given strategy as the difference between the health benefit (in DALY) provided by that strategy and its associated campaign costs compared to status quo, divided by the societal willingness-to-pay for DALYs. This framework gives a single outcome measure that identifies the strategy which provides the largest overall benefit at the given willingness-to-pay. For each of 1000 parameter sets drawn, we identified the strategy that conferred the highest net benefits at a value of willingness-to-pay set to three times the GDP per capita. We then tabulated the probability that each strategy would confer the highest net benefits, with the strategy associated with the highest probability being considered “optimal” at that threshold [43]. We conducted this analysis across societal willingness-to-pay thresholds which is assumed to be a proxy of willingness to pay in a potentially realistic range from $1 to $5000 per DALY saved.
2.4.3.3. Sensitivity analyses
A series of one-way sensitivity analyses were performed using the net benefits framework, assessing the sensitivity of the optimal strategy choice to changes in dog rabies vaccine price, number of dogs vaccinated daily per vaccinator team, training and advertising costs during vaccination campaign, post-exposure prophylaxis (PEP) costs, and cattle rabies herd level incidence. For each, the specific input parameter was adjusted to its alternative point estimate, maintaining the base case for all other parameters. Two-way sensitivity analyses were similarly performed on the time frame and discount rate.
For the rabies transmission parameters, we also performed sensitivity analyses around the bite rate and the probability of rabies re-introduction through rabid dogs from neighboring villages. We assumed a lower (0.20) and a higher (0.99) rabid dog-human bite rate in cases where rabid dogs were killed or not killed and consequently had a lower or higher chance to bite humans, respectively. Considering the possibility of rabies reintroduction from neighboring villages, we assumed that an equivalent of 1% and 3% of the rabid dogs were reintroduced to the districts every year. For each scenario of re-introduction, the MCMC process was re-fitted and run with the new parameters, so that optimal coverage around the incremental cost-effectiveness ratios could be re-estimated.
3. Results
3.1. Estimated model parameters
3.1.1. Estimation of epidemiological parameters and model validation
Fitting the rabies epidemiological model to the data, the most likely carrying capacities for dogs (K) were estimated to be 361 dogs/km2 (95% CI: 346–367) and 42.6 dogs/km2 (95% CI: 41.8–45.2), for the Bishoftu and Lemuna-bilbilo districts respectively. The predicted dog-to-dog transmission rates (β) were estimated to be 0.46 (95% CI: 0.39–0.47) and 0.40 (95% CI: 0.37–0.44) daily transmission events per rabid dog, and the dog-to-human bite rate (δ) estimated as 0.50 (95% CI: 0.45–0.56) and 0.56 (95% CI: 0.46–0.57) transmission events per rabid animal, in the Bishoftu and Lemuna-bilbilo districts. The incubation period (1/σ) was estimated to be 22.5 (95% CI: 20.0–24.6) and 21.6 (95% CI: 19.8–24.8) days, while the infectious period (1/α) was 3.13 (95% CI: 2.85–3.40) and 3.08 (95% CI: 2.88–3.35), for Bishoftu and Lemuna-bilbilo respectively. The probability of human developing rabies following exposure and without treatment was 0.16 (95% CI: 0.14–0.17) in both districts. The post-exposure prophylaxis coverage was estimated to be 0.80 (95% CI: 0.74–0.84) and 0.59 (95% CI 0.45–0.59), for the Bishoftu and Lemuna-bilbilo districts respectively (Table 2).
Table 2.
Posterior distribution of epidemiological parameters.
| Parameters | Description | MLa estimates and 95% CI |
|
|---|---|---|---|
| Urban/Bishoftu | Rural/Lemuna-bilbilo | ||
| β | dog to dog transmission/day | 0.46 (0.39–0.47) | 0.40 (0.37–0.44) |
| K | Carrying capacity (dogs/km2) | 361 (346–367) | 42.6 (41.8–45.2) |
| 1/σ | Incubation period (days) | 22.5 (19.9–24.6) | 21.6 (19.8–24.8) |
| 1/α | Infectious period (days) | 3.13 (2.85–3.40) | 3.08 (2.88–3.35) |
| δ | Rabid dog-human bite rate | 0.50 (0.45–0.56) | 0.56 (0.46–0.57) |
| m | Probability of developing rabies | 0.16 (0.15–0.17) | 0.16 (0.15–0.17) |
| p | Post-exposure prophylaxis coverage | 0.80 (0.74–0.84) | 0.59 (0.45–0.59) |
ML = maximum likelihood.
The joint posterior distribution produced estimates of human rabies exposure consistent with the data from each district in terms of exposure but higher predicted deaths. In Bishoftu, the predicted a number of human exposures (95% CI: 172–187) 179 annual and 4.6 deaths (95% CI: 3.87–7.79 deaths), compared with the observed levels of 189 exposures and one death. In Lemuna-bilbilo the model predicted 184 annual human exposures (95% CI: 183–187) and 11.2 deaths (95% CI: 10.0–17.7), compared with the observation of 189 exposures, and 8 deaths. Under status quo coverage, the fitted epidemiological model estimated the annual number of rabid dogs to be 340 (95% CI: 336–345) and 331 (95% CI: 326–335) in the Bishoftu and Lemuna-bilbilo districts respectively.
3.2. Human rabies exposure predictions
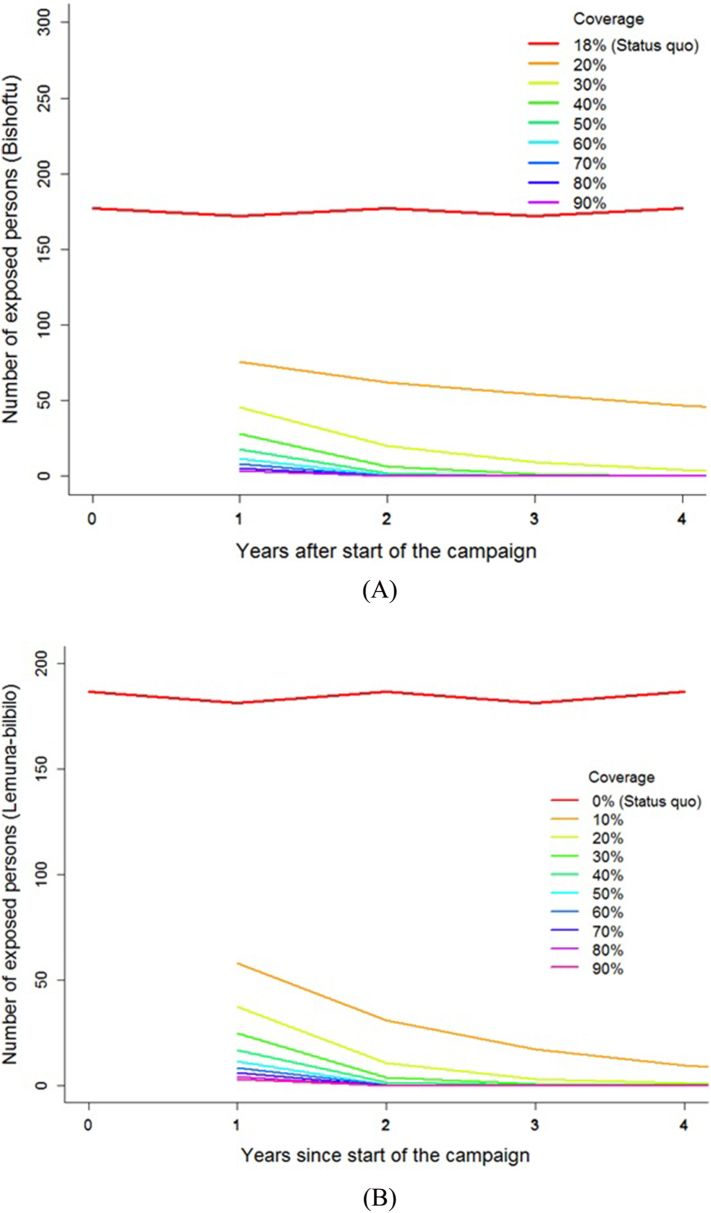
The model predicted the number of rabid dog cases and consequently, predicted the human exposure to decrease monotonically as vaccination coverage increased. In Bishoftu, the mean annual number of rabid dogs predicted to decline to 1.5 (95% CI: 0–6.89) at 90% coverage. In Lemuna-bilbilo, the mean annual number of rabid dogs dropped to 0.84 (95% CI: 0–3.78) at 90% coverage. In Bishoftu, all vaccination campaigns above 40% coverage lead to fewer than 1 rabid dog annually by the end of 4 years, while in Lemuna-bilbilo coverage above 20% leads to fewer than 1 rabid dogs annually by the end of 4 years. In both districts, high coverage achieves faster reductions in rabies than low coverage (see Supplementary information). In urban Bishoftu, for the status quo (18%) and 90% coverage, the cumulative number of human exposures over 5 years were 876 and 4.0. Similarly, in the rural district, the cumulative number of human exposures over 5 years were 922 and 3, under the status quo (0%) and 90% coverage conditions, respectively (Fig. 2a–b).
Fig. 2.
Total number of human rabies exposures over 5 years for each vaccination coverage in a) Bishoftu and b) Lemuna-bilbilo under different canine vaccination coverage strategies.
3.3. Human health burden in DALY lost
As human deaths are the consequence of delay or failure to receive an appropriate PEP, these deaths were predicted to decrease proportionately with reduced canine rabies incidence as canine vaccination coverage increased. Adjusting for district-specific data on the age of death, a single human death corresponded to 46.7 (95% CI: 46.7–46.7) and 50.6 (95% CI: 46.2–54.9) DALYs in Bishoftu and Lemuna-bilbilo, respectively. Adjusting further for PEP access, each rabid dog was associated with estimated values of 0.74 and 1.67 DALYs. The cumulative number of DALYs over 5 years under the status quo and 90% coverages were 911 and 4 for Bishoftu and 7402.6 and 2.72 for Lemuna-bilbilo districts respectively.
3.4. Economic burden of rabies and costs of intervention strategies
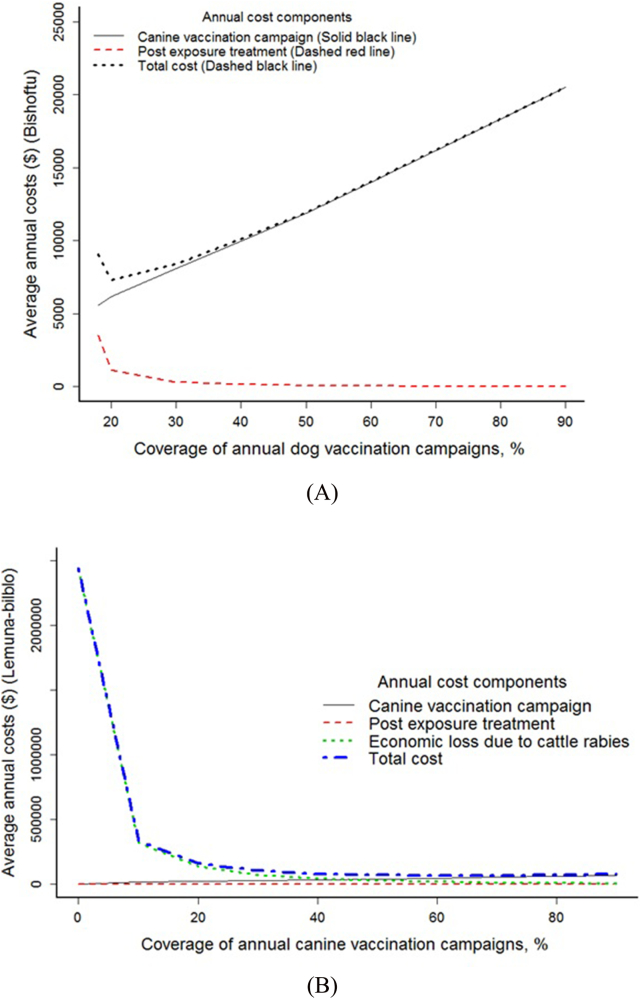
Under the status quo, the mean annual costs for rabies control in Bishoftu (including canine vaccination and PEP) was estimated to be $8562 ($214/km2). This mean annual costs reached a maximum of $19,332 ($483/km2) under 90% coverage and a minimum of $6902($172/km2) under 20% coverage. With the increase in vaccination coverage, the cost of PEP decreased whereas the costs of dog vaccination increased. The total annual costs increased monotonically when coverage rose above 20%, as vaccination campaign costs outstrip any savings from reduced PEP use (Fig. 3a). In Bishoftu, the urban district, the vaccination coverage of 20% was the cheapest of all scenarios and the total cost was found to be majorly function of the dog vaccination campaign.
Fig. 3.
Cost components and average annual (undiscounted) costs of rabies across different vaccination coverage in a) Bishoftu and b) Lemuna-bilbilo. NB: The scales on the y-axis are different as the average annual costs of rabies in Bishoftu has a maximum of about $20,000 whereas Lemuna-bilbilo has a maximum of $2,500,000.
In Lemuna-bilbilo, besides the costs of providing PEP, the economic losses due to cattle rabies declined with an increase in vaccination coverage as a result of a declining incidence in canine rabies (Fig. 3b). The mean annual costs of rabies under status quo (no vaccination) was estimated to be $2,443,572 ($2055/km2), consisting of $3331 ($2.8/km2) PEP-related expenditures, and $2,440,241 (2052/km2) of economic losses due to cattle rabies. The lowest cost strategy which includes the sum of losses and expenditures was shown to be at 60% coverage. All vaccination strategies had lower total costs compared to the cost of the status quo situation with no vaccination.
3.5. Incremental cost-effectiveness ratios
Canine vaccination coverage rates of 20% in Bishoftu and 60% in Lemuna-bilbilo resulted in a higher reduction in DALYs for lower costs than campaigns that adopted alternate levels of vaccination at lower coverages. Accordingly, coverage less than these levels in each respective districts is considered dominated. A dominated strategy is one which provides fewer health benefits than other strategies of equal or lesser cost. Each coverage also represents the minimum cost strategy for each district. WHO-recommended thresholds for “very cost-effective” and “cost-effective” strategies in Ethiopia to be equal to $568 and $1710 per DALY saved, respectively. According to these thresholds, vaccination coverages of below 50% and 70% are very cost-effective in Bishoftu and Lemuna-bilbilo respectively. In Lemuna-bilbilo, vaccination coverage between 60% and 70% would be the only cost-effective strategy, as the ICER associated with moving from 70% to 80% coverage exceeds the WHO-recommended threshold (Table 3).
Table 3.
Cumulative costs over 5 years, and discounted to present value (3% discount rate) in a) Bishoftu and b) Lemuna-bilbilo districts.
| Vaccination coverage | Cost of vaccination ($) | Cost of PET ($) | Cattle rabies related loss ($) | DALY saved | Total economic burden ($) | ICER ($/DALY)a |
|---|---|---|---|---|---|---|
| a) Bishoftu | ||||||
| 0.18 | 26314 | 16500 | 0 | NA | 42814 | NA |
| 0.2 | 29149 | 5363 | 0 | 580 | 34512 | Minimum cost |
| 0.3 | 38111 | 1589 | 0 | 777 | 39700 | 26 |
| 0.4 | 47033 | 719 | 0 | 822 | 47752 | 178 |
| 0.5 | 55939 | 401 | 0 | 839 | 56340 | 519 |
| 0.6 | 66104 | 248 | 0 | 846 | 66352 | 1254 |
| 0.7 | 76265 | 163 | 0 | 851 | 76428 | 2260 |
| 0.8 | 86425 | 110 | 0 | 854 | 86535 | 3717 |
| 0.9 | 96584 | 77 | 0 | 855 | 96661 | 5764 |
| b) Lemuna-bilbilo | ||||||
| 0 | 0 | 15715 | 11510855 | NA | 11526570 | NA |
| 0.1 | 82010 | 2133 | 1559457 | 2601 | 1643601 | Dominated |
| 0.2 | 109311 | 930 | 677651 | 2832 | 787891 | Dominated |
| 0.3 | 136622 | 517 | 375654 | 2911 | 512793 | Dominated |
| 0.4 | 160792 | 322 | 234043 | 2948 | 395157 | Dominated |
| 0.5 | 187845 | 215 | 155583 | 2969 | 343643 | Dominated |
| 0.6 | 222181 | 148 | 107638 | 2981 | 329968 | Minimum cost |
| 0.7 | 256517 | 105 | 76419 | 2990 | 333042 | 373 |
| 0.8 | 290853 | 76 | 55187 | 2995 | 346117 | 2331 |
| 0.9 | 325189 | 56 | 40300 | 2999 | 365545 | 4938 |
ICER value for dominated strategies is not displayed.
3.6. Net Health Benefit uncertainty analysis
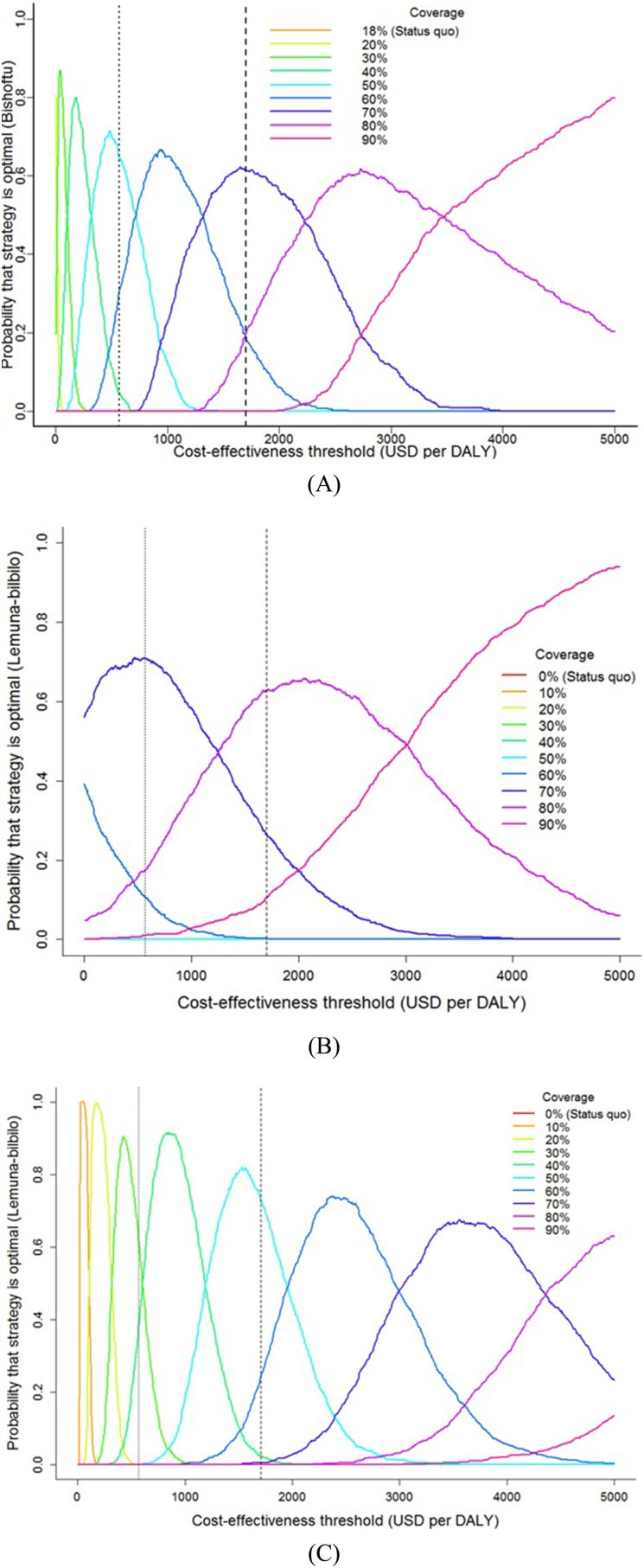
Incorporating parameter uncertainty, we assessed the probability that each strategy would be optimal (i.e. provide the highest net health benefits) across a range of willingness-to-pay values (Fig. 4). We found that coverages of 70% and 80% were the most likely to be optimal at the “cost-effective” threshold in Bishoftu and Lemuna-bilbilo districts, respectively (Fig. 4a and b). At the “very cost-effective” threshold, the optimal coverage drops to 50% for Bishoftu and to 70% in Lemuna-bilbilo. We found zero probability that the status quo would be optimal/recommended at all willingness-to-pay values in both districts. In Bishoftu and Lemuna-bilbilo districts, 70% and 80% vaccination coverages were the recommended/optimal choice at a likelihood of about 0.62 and 0.59, respectively.
Fig. 4.
Acceptability curves, showing the probability that each strategy would be optimal across a range of cost-effectiveness threshold for a) Bishoftu b) Lemuna-bilbilo district including cattle-related losses c) Lemuna-bilbilo district excluding cattle-related losses. (Dashed line indicates WTP based on 1× Ethiopia's per-capita GDP of 568$ (left) and 3× (i.e. $1704) (right).
To allow direct comparisons with Bishoftu, we analyzed a scenario for Lemuna-bilbilo where we considered only human health and economic benefits, excluding the economic benefit of cattle rabies. We found that the recommended/optimal coverage would drop to 50% at a cost-effective threshold (Fig. 4c).
3.7. Sensitivity and scenario analyses
We investigated how the recommended strategy, i.e., the strategy most likely to be optimal at the cost-effectiveness threshold, would shift in response to changes in economic parameters. For both districts, the recommended strategy was found to be sensitive to vaccine price and a number of dogs vaccinated per team but not to PEP costs, or vaccination campaign training and advertising costs. If the price of a canine vaccine increases from the baseline of $0.55 to beyond $1 per dog, the recommended coverage declines and if the number of dogs vaccinated per day per team increases, the recommended coverage will increase. The recommended strategy in Lemuna-bilbilo was also sensitive to the herd-level cattle rabies incidence and required higher coverage at increased annual cattle herd level incidence levels beyond the 20% estimated baseline incidence (see supplementary information: Fig. 3:a-d).
The two-way scenario analyses indicated that using a shorter time frame for analysis (3 yrs) coupled with a higher discounting rate (5%) would shift the recommended strategy towards 90% coverage in both districts. A longer time frame (10 yrs) and a lower discount rate (1%) altered the recommended coverage to 50% (see supplementary information: Fig. 4a–d).
The scenario analysis revealed that the recommended coverage for Bishoftu remains 70% across rabid dog biting rates ranging from 0.2 to 0.99 humans per rabid dog. For Lemuna-bilbilo, optimal coverage drops to 70% at the lower biting rate (see supplementary information: Fig. 5: a-d). Similarly, the recommended coverage shifts to 80% and 90% in Bishoftu when rabid dogs are introduced at a percentage equivalent to 1% and 3% of current canine rabies respectively. For Lemuna-bilbilo district, the recommended coverage increased to 90% and remained the same for reintroduction equivalent to 1% and 3% of current canine rabies (see supplementary information: Fig. 6: a–d).
4. Discussion
We used the example of canine vaccination against rabies to evaluate the broader benefits which stem from controlling vaccine-preventable zoonotic diseases. Using the One Health paradigm, we created a unified framework that integrated the cross-sectoral burden of rabies, including human disease, medical costs, and cattle rabies-related economic losses in Ethiopia. This cost-effectiveness analysis is the first study to include cattle rabies-related losses, a component which has particular importance for livestock-dependent communities. Previous canine vaccination evaluations in Africa and elsewhere have focused primarily on the prevention of human rabies [6,19,[44], [45], [46], [47], [48]]. These studies indicated that high canine vaccination coverage would be cost-effective on the basis of the human health burden alone. These studies indicated that high canine vaccination coverage would be cost-effective on the basis of the human health burden alone. By contrast, we found that the inclusion of livestock costs is required in order for high coverage to be cost-effective in rural Ethiopia. A recognition of these broader benefits justifies campaigns at 80% coverage, compared to 50% coverage when only human health outcomes are considered.
Under an ideal situation without re-introduction, our base case analysis recommends canine vaccination campaigns at coverage of 70 and 80% for the Bishoftu (urban) and Lemuna-bilbilo (rural) districts, respectively. These levels are consistent with the WHO recommendation of 70% coverage, which aims to eliminate rabies without considering the costs of control [49]. For the rural districts, Lemunabilbilo, the required coverage was higher than that needed in the urban district. This was primarily due to the fact that livestock rabies-related costs serve as an additional incentive to increase the vaccination coverage by 10% in rural districts. While a rabies control program helps to control the disease in the dog population and thus in humans, it would not eliminate rabies from the population. Thus, there is a need for the program to sustainably run for a long time as rabies otherwise could be reintroduced from the adjacent uncontrolled neighbors i.e. both vaccination in dogs and PET would need to be maintained over a prolonged time period because of rabies in adjacent districts. Hence, our sensitivity analysis highlights the fact that when the optimal coverage was simulated over a longer period (of 10 years) coverage level lower than that recommended by the WHO could be adopted. This result suggests that rabies control could be efficiently achieved by adopting an intensive program over a shorter period of time, possibly followed by a less intensive (i.e. lower coverage) program sustained over a longer period of time, as has been suggested elsewhere [50]. A fuller analytical exploration and optimization exercise involving a range of these combinations in Ethiopia or other resource-constrained contexts would be a valuable contribution to rabies control policy.
Despite the public health burden of rabies and availability of an effective vaccine, surveys throughout Ethiopia have indicated that the current canine vaccination practices are far below anything close to these optimal coverage levels [15,51]. In rural Lemuna-bilbilo, the current status quo of non-existent vaccination is costly than any of the vaccination strategies evaluated, due to the high cattle rabies-related losses. One of the reasons for such low coverage could be the perceived cost of vaccination campaigns and lack of evidence on the cost-effective level of control. This economic analysis would provide a strong incentive for immediate action on canine rabies vaccination in rural Ethiopia, as the entire financial investment will be recouped. In urban Bishoftu, canine vaccination represents a financial outlay, but with a very efficient return on investment in terms of human health. In comparison with cost-effectiveness estimates per DALY for providing PEP in sub-Saharan Africa, the ICERs presented here may seem high. However, as canine vaccination is being added to the existing PEP infrastructure, rather than replacing it, these relatively higher cost-effectiveness ratios are not unexpected. In addition, as PEP is less accessible for most parts of rural Ethiopia, canine vaccination is critical to save human lives. Canine vaccination is a one-time (or at most periodically scheduled) campaign-based operation, while PEP services require continuous year-round activity and the cold chain for the post-exposure prophylaxis vaccine as exposure may happen at any time.
Although we incorporated parameter uncertainty into our analysis whenever possible, it was necessary to make some parameter assumptions. Currently, there are no canine vaccination campaigns in Ethiopia from which we could extract epidemiological or economic data. This meant that our cost estimates were uncertain and potentially biased. Further, survey-based human rabies case investigation can never be complete, as not all cases are reported to health centers and thus human exposures are likely to be underestimated. Higher estimates of human exposure could justify greater vaccination coverage. Of particular relevance, the Ethiopian government plans to replace the nervous tissue vaccine widely used as a post-exposure prophylactic vaccine with a safer cell culture vaccine, and there is speculation that the cost of PEP per case would increase dramatically as a result. Nevertheless, our analysis found that the recommended coverage remained insensitive to PEP costs in both districts, which implies that the intended switch should not impact plans for canine vaccination coverage. Similarly, the time and resources required to replicate the Tanzanian study aimed to collect rabies transmission parameters in Ethiopia would be substantial. Therefore, we conducted a one-way scenario analysis for this parameter. The results of the scenario analysis show that high vaccination coverage is recommended for both districts across a wide range of values for the biting rate. The recommended vaccination coverage did not change as changes in the dog-to-human bite rates. The robustness of recommendations in response to these two parameters is largely due to the fact that PEP costs are relatively low when compared to the costs of vaccination programs. On the other hand, the potential improvement in the level of awareness and use of medical services and a decline in the use of traditional healers would contribute to an increase in demand of PEP might lead to expansion of PEP delivery health centres ultimately leading to lowering the cost of indirect PEP expenditures like transportation to health centers. The impact of opening additional health centers was not assessed in our sensitivity analysis.
The current practice of killing rabid dogs in Ethiopia was supposed to reduce the public health burden [16,52]. Killing rabid dogs is expected to decrease human exposure by preventing dog bites. However, the lower (0.2) and higher (0.99) rabid dog–to-human bite rates simulated in this study did not appear to alter the recommended vaccination coverage. This reduced sensitivity could be due to the facts that PEP costs, which are indirectly determined by the bite rate, represent an insignificant part of the total costs (<3%). Consequently, killing rabid dogs might reduce the number of rabid dogs as well as the number of human exposures but does not affect the optimal canine vaccination coverage.
We assumed a concerted vaccination campaign applied to all possible adjacent districts with no risk of reintroduction which would require a vaccination campaign of larger coverage. In reality reintroduction of rabies from neighboring areas is one of the key potential challenges of rabies elimination [53,54]. Accordingly, sensitivity analysis around the optimal coverage revealed that assuming a reintroduction rate at an equivalent of 1–2% of rabid cases, increased the optimal vaccination coverage up to 90%. This reintroduction could be from neighboring districts and or wild animals. Though we did not consider wild animals explicitly in the transmission dynamics of rabies as we had no data on the transmission of wildlife rabies in Ethiopia, the assumption would not compromise the optimal coverage estimated in this study as it is unlikely that the wildlife contribution to change the dynamics significantly, given that as <1% of human rabies exposures in Ethiopia are from wild animals in Ethiopia [32,55]. However, there could be a potential re-introduction from dogs human-mediated dog transport from neighboring districts if the campaigns are not consistent among adjacent districts and or regions. In addition, although intensive dog vaccination with higher coverage would reduce the rabies incidence in a shorter time, the sustainability of vaccinating dogs should be ensured which otherwise could lead to re-emergence of the disease. Based on personal communication with elders in the study areas, people welcome vaccination initiatives for other reason than the cost of vaccination and hence refusal was considered negligible.
The recommended annual coverage of 70% in Bishoftu would require an average annual budget of $5789 over 5 years of which 99.8% would go to canine vaccination campaign costs and the rest to PEP costs. Similarly, in the rural district (Lemuna-bilbilo), an optimal annual coverage of 80% would require on average $20,079 annually over 5 years, of which $19,050 would be for canine vaccination and the rest for PEP. Knowing that Ethiopia has 529 districts, of which 423 (80%) are rural districts, the estimate for a national annual canine vaccination budget would be in the order of $17.5 million; far less than the national annual loss due to cattle rabies, which is estimated to be $209 million [18].
A concerted effort to implement our recommended annual vaccination strategy would significantly minimize, if not eliminate, rabid dogs, and consequently human exposure, as well as a livestock-related loss within the first few years. One of the reasons for such a low coverage could be the perceived cost of vaccination campaigns and lack of evidence on the cost-effective level of control. Given the Ethiopian government determination to meet 2030 WHO plan to eliminate dog mediated human rabies [56] such economic evaluation would provide a strong incentive for the immediate action on canine rabies vaccination in rural Ethiopia, as the entire financial investment will be cost saving. However, such efforts from a resource-limited country like Ethiopia with various competing interests would not be possible without international support. Thus, rabies elimination would require international and regional collaboration, without which it will not be possible to eliminate dog-mediated human rabies by 2030 [10]. Studies such as the one reported here on optimal vaccination coverage such as that reported here are very useful in a resource-scarce setting as higher vaccination coverage does not necessarily lead to the best outcome. For instance, in rural areas of Tanzania, vaccination coverages as high as 90% have been shown to have similar epidemiologic impacts as coverages of 70% [48], implying that vaccination campaigns which aim at very high levels of coverage may not provide the anticipated payback. Although, the use of dogs in Ethiopia is predominantly for guarding and no financial value of dogs were included in our analysis, vaccinating all dogs would be beneficial for individual dog health and welfare.
One of the limitations of this study relates to the parameter estimates used for transmission from rabid dogs to humans and cattle. Rabies transmission dynamics were modeled within the domestic dog population alone, while human and cattle populations were not considered as contributing to transmission. Other studies have taken a different approach, making transmission to humans dependent on human density as well [44]. In our study, the base case transmission rate from rabid dogs to humans was adopted from empirical observation in Tanzania [19]. Although we found that the recommendation for high vaccination coverage is robust to variation in this parameter, further study into the biting behavior of rabid dogs would improve the precision of the recommendations. Similarly, cattle rabies was assumed to be proportionately with canine rabies, such that control of canine rabies would lead to control of cattle rabies. It is possible that alternative hosts are also transmitting rabies to cattle, although none were observed or anecdotally reported to the field team.
Observational field studies could validate and improve model output in additional ways. For instance, canine vaccination does not guarantee the development of protective immunity, and the degree of protective immunity varies based on the health and nutritional status of the vaccinated dogs. In addition, diagnostic confirmation of canine rabies cases would give a more accurate number when compared to the six diagnostic criteria for biting dogs using retrospectively obtained dog history [25]. This approach has a number of limitations and laboratory diagnosis remains the gold standard. However, this method has a sensitivity of 90.2%, a specificity of 96.2% and accuracy of 94.6% for the clinical diagnosis of rabies, and, therefore, is an appropriate substitute in settings such as Ethiopia where rabies laboratories are very scarce and highly centralized. As an additional limitation, we still assumed homogenous distributions of dogs within each district. More realism could be achieved by collecting and incorporating data on canine contact networks, particularly to understand dynamics under high coverage [57]. Some comparable studies have also shown the break-even point for costs between mass canine vaccination and human PEP options [58]. It is likely that the inclusion of livestock costs leads to an earlier breakeven point for Ethiopia than calculated for a context using PEP costs alone.
In conclusion, results from this study indicate that a broader evaluation of vaccine-preventable diseases, especially zoonotic diseases, may result in a higher benefit than perceived benefit from saving human life alone. This has already been demonstrated for other diseases like brucellosis and Rift Valley Fever elsewhere [59]. In this study, we demonstrated that canine mass rabies vaccination in Ethiopia will be a cost-effective means of combating rabies by reducing the human health burden and by saving costs compared to the status quo of 18% canine vaccination coverage in urban districts and no vaccination in rural districts. With a primary focus on human health benefits (preventing human cases), the cost-effective level of canine vaccination coverages with minimal cost per DALY averted for the urban and rural district differed; with the model indicating 70% for the urban district and 50% for rural the district. With the inclusion of the livestock rabies-related losses in the rural district, the optimal level of canine vaccination coverage increased to 80%. This highlights the relevance of applying a broader perspective when considering rabies burden and the benefits of canine mass vaccination. This type of cost-effectiveness study is very useful in resource-scarce settings as higher vaccination coverages do not necessarily lead to the best result. Vaccination coverage rates of 70% and 80% were identified as the most likely scenarios to provide the greatest net health benefits at the WHO-recommended willingness-to-pay threshold over a time frame of 5 years for the Bishoftu and Lemuna-bilbilo districts respectively.
Contributions
TJB conceived the study, analyzed the data, interpreted the results, and prepared the first draft of the manuscript and revised the manuscript. MCF, MCMM, APG, and HH contributed to the study design. MCF contributed to model development, analysis, data interpretation, and revision of the manuscript. MCMM, APG, CWR, NC, MWS, and HH contributed to data interpretation, reviewed the manuscript and edited the manuscript. All authors have approved the final version of the manuscript.
Declaration of Competing Interest
No conflict of interest.
Acknowledgments
The authors would like to thank The Netherlands Organization for International Cooperation in Higher Education (NUFFIC) for funding this research. The authors also would like to thank Dr. Abraham Haile Kidane and Zoonosis research team at Ethiopian Public Health Institute (EPHI), the team at Ethiopian Ministry of Livestock and Fisheries Veterinary Services and Abishek Pandey at Yale School of Public Health for their contribution to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2019.100103.
Appendix A. Supplementary data
Supplementary material
References
- 1.Barrett S. Eradication versus control: the economics of global infectious disease policies. Bull. World Health Organ. 2004;82:683–688. [PMC free article] [PubMed] [Google Scholar]
- 2.Müller J. Optimal vaccination patterns in age-structured populations: endemic case. Math. Comput. Model. 2000;31(4–5):149–160. [Google Scholar]
- 3.Andre F.E., Booy R., Bock H.L., Clemens J., Datta S.K., John T.J. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008;86(2):140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller J. Optimal vaccination strategies—for whom? Math. Biosci. 1997;139(2):133–154. doi: 10.1016/s0025-5564(96)00140-x. [DOI] [PubMed] [Google Scholar]
- 5.Harvey M.J., Prosser L.A., Messonnier M.L., Hutton D.W. Hitting the optimal vaccination percentage and the risks of error: why to miss right. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick M.C., Shah H.A., Pandey A., Bilinski A.M., Kakkar M., Clark A.D. One health approach to cost-effective rabies control in India. Proc. Natl. Acad. Sci. 2016;113(51):14574–14581. doi: 10.1073/pnas.1604975113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom D.E., Brenzel L., Cadarette D., Sullivan J. Moving beyond traditional valuation of vaccination: needs and opportunities. Vaccine. 2017;35:A29–A35. doi: 10.1016/j.vaccine.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Roth F., Zinsstag J., Orkhon D., Chimed-Ochir G., Hutton G., Cosivi O. Human health benefits from livestock vaccination for brucellosis: case study. Bull. World Health Organ. 2003;81(12):867–876. [PMC free article] [PubMed] [Google Scholar]
- 9.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M. Correction: estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9(5) doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . 2015. Global Elimination of Dog-Mediated Human Rabies: Report of the Rabies Global Conference, 10–11 December 2015, Geneva, Switzerland. [Google Scholar]
- 11.Schneider M.C., Belotto A., Adé M.P., Hendrickx S., Leanes L.F., MJdF Rodrigues. Current status of human rabies transmitted by dogs in Latin America. Cadernos de Saúde Pública. 2007;23:2049–2063. doi: 10.1590/s0102-311x2007000900013. [DOI] [PubMed] [Google Scholar]
- 12.Rupprecht C.E., Hanlon C.A., Hemachudha T. Rabies re-examined. Lancet Infect. Dis. 2002;2(6):327–343. doi: 10.1016/s1473-3099(02)00287-6. [DOI] [PubMed] [Google Scholar]
- 13.Vigilato M.A., Cosivi O., Knöbl T., Clavijo A., Silva H.M. Rabies update for Latin America and the Caribbean. Emerg. Infect. Dis. 2013;19(4):678. doi: 10.3201/eid1904.121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco-Villa A., Escobar L.E., Sanchez A., Shi M., Streicker D.G., Gallardo-Romero N.F. Successful strategies implemented towards the elimination of canine rabies in the Western Hemisphere. Antivir. Res. 2017;143:1–12. doi: 10.1016/j.antiviral.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali A., Mengistu F., Hussen K., Getahun G., Deressa A., Yimer E. Overview of Rabies in and around Addis Ababa, in Animals Examined in EHNRI Zoonoses Laboratory Between, 2003 and 2009. Ethiopian Vet. J. 2010;14(2):91–101. [Google Scholar]
- 16.Kabeta T., Deresa B., Tigre W., Ward M.P., Mor S.M. Knowledge, attitudes and practices of animal bite victims attending an anti-rabies health center in Jimma Town, Ethiopia. PLoS Negl. Trop. Dis. 2015;9(6) doi: 10.1371/journal.pntd.0003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwai O., Hanotte O., Kwon Y.-J., Cho S. African indigenous cattle: unique genetic resources in a rapidly changing world. Asian Austral J. Anim. 2015;28(7):911–921. doi: 10.5713/ajas.15.0002R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jibat T., Mourits M.C., Hogeveen H. Incidence and economic impact of rabies in the cattle population of Ethiopia. Prev Vet Med. 2016;130:67–76. doi: 10.1016/j.prevetmed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick M.C., Hampson K., Cleaveland S., Mzimbiri I., Lankester F., Lembo T. Cost-effectiveness of canine vaccination to prevent human rabies in rural Tanzania. Ann. Intern. Med. 2014;160(2):91–100. doi: 10.7326/M13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacaër N. Springer Science & Business Media; 2011. A Short History of Mathematical Population Dynamics. [Google Scholar]
- 21.Hampson K., Dushoff J., Cleaveland S., Haydon D.T., Kaare M., Packer C. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7(3) doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jibat T., Mourits M.C.M., Hogeveen H. Incidence and economic impact of rabies in the cattle population of Ethiopia. Prevent. Vet. Med. 2016;130:67–76. doi: 10.1016/j.prevetmed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Hiby L.R., Reece J.F., Wright R., Jaisinghani R., Singh B., Hiby E.F. A mark-resight survey method to estimate the roaming dog population in three cities in Rajasthan, India. BMC Vet. Res. 2011;7(1):46. doi: 10.1186/1746-6148-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyene T.J., Mourits M.C.M., Kidane A.H., Hogeveen H. Estimating the burden of rabies in Ethiopia by tracing dog bite victims. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepsumethanon V., Wilde H., Meslin F.X. Six criteria for rabies diagnosis in living dogs. J. Med. Assoc. Thai. 2005;88(3):419–422. [PubMed] [Google Scholar]
- 26.Shim E., Hampson K., Cleaveland S., Galvani A.P. Evaluating the cost-effectiveness of rabies post-exposure prophylaxis: a case study in Tanzania. Vaccine. 2009;27(51):7167–7172. doi: 10.1016/j.vaccine.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlo C.M. 2004. Markov Chain Monte Carlo and Gibbs Sampling. Notes, (April) [Google Scholar]
- 28.Gelman A., Rubin D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992:457–472. [Google Scholar]
- 29.Plummer M., Best N., Cowles K., coda Vines K. Output Analysis and Diagnostics for MCMC. R Package Version 013-3. http://CRANR-projectorg/package=coda.2008 URL.
- 30.Wera E., Velthuis A.G.J., Geong M., Hogeveen H. Costs of rabies control: an economic calculation method applied to Flores Island. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO WHO Guide for Rabies Pre and Post Exposure Prophylaxis in Humans. 2014. http://www.who.int/rabies/PEP_Prophylaxis_guideline_15_12_2014.pdf?ua=1
- 32.Beyene T.J., Mourits M.C.M., Revie C.W., Hogeveen H. Determinants of health seeking behaviour following rabies exposure in Ethiopia. Zoonoses Public Health. 2018;0(0) doi: 10.1111/zph.12458. [DOI] [PubMed] [Google Scholar]
- 33.EHNRI . 2012. Proceding of the National Workshop on Rabies Prevention and Control in Ethiopia, Oct 18-19, 2012, Addis Ababa. [Google Scholar]
- 34.Hotez P.J., Alvarado M., Basáñez M.-G., Bolliger I., Bourne R., Boussinesq M. 2014. The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO WHO expert consultation on rabies. Second report. World Health Organ. Tech. Rep. Ser. 2013;(982):1. [PubMed] [Google Scholar]
- 36.Okell C.N., Pinchbeck G.P., Stringer A.P., Tefera G., Christley R.M. A community-based participatory study investigating the epidemiology and effects of rabies to livestock owners in rural Ethiopia. Prevent. Vet. Med. 2013;108(1):1–9. doi: 10.1016/j.prevetmed.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 37.CSA . Federal Democratic Republic of Ethiopia Central Statistical Agency; Addis Ababa, Ethiopia: 2013. Population Projection of Ethiopia for All Regions At Wereda Level from 2014–2017. [Google Scholar]
- 38.Beyene T.J., Mourits M.C., Kidane A.H., Hogeveen H. Estimating the burden of rabies in Ethiopia by tracing dog bite victims. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. Choosing Interventions That Are Cost-Effective (WHO-CHOICE). Geneva:World Health Organization. Accessed at www.who.int/choice/enon29/5/2017. 2010.
- 40.WHO . World Health Organization; 2001. National Burden of Disease Studies: A Practical Guide. Global Program on Evidence for Health Policy Geneva. [Google Scholar]
- 41.World Bank GDP Per Capita for Ethiopia. 2015. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/countries/ET?display=graph (access date-3/9/2015)
- 42.Briggs A.H., Goeree R., Blackhouse G., O'Brien B.J. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med. Decis. Mak. 2002;22(4):290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- 43.Barton G.R., Briggs A.H., Fenwick E.A. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI0) Value Health. 2008;11(5):886–897. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 44.Zinsstag J., Durr S., Penny M.A., Mindekem R., Roth F., Gonzalez S.M. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc. Natl. Acad. Sci. U. S. A. 2009;106(35):14996–15001. doi: 10.1073/pnas.0904740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mindekem R., Lechenne M.S., Naissengar K.S., Oussiguere A., Kebkiba B., Moto D.D. Cost description and comparative cost efficiency of post-exposure prophylaxis and canine mass vaccination against rabies in N'Djamena, Chad. Front. Vet. Sci. 2017;4:38. doi: 10.3389/fvets.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shim E., Hampson K., Cleaveland S., Galvani A.P. Evaluating the cost-effectiveness of rabies post-exposure prophylaxis: a case study in Tanzania. Vaccine. 2009;27(51):7167–7172. doi: 10.1016/j.vaccine.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wera E., Mourits M., Siko M., Hogeveen H. Cost-effectiveness of mass dog vaccination campaigns against rabies in Flores Island, Indonesia. Transbound. Emerg. Dis. 2016 doi: 10.1111/tbed.12590. [DOI] [PubMed] [Google Scholar]
- 48.Bilinski A.M., Fitzpatrick M.C., Rupprecht C.E., Paltiel A.D., Galvani A.P. Optimal frequency of rabies vaccination campaigns in Sub-Saharan Africa. Proc. R. Soc. B Biol. Sci. 2016;283(1842) doi: 10.1098/rspb.2016.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO . World Health Organization; 2013. WHO Expert Consultation on Rabies: Second Report. [PubMed] [Google Scholar]
- 50.Bogel K., Meslin F.X. Economics of human and canine rabies elimination: guidelines for programme orientation. Bull. World Health Organ. 1990;68(3):281–291. [PMC free article] [PubMed] [Google Scholar]
- 51.Jemberu W.T., Molla W., Almaw G., Alemu S. Incidence of rabies in humans and domestic animals and people's awareness in North Gondar zone, Ethiopia. PLoS Negl. Trop. Dis. 2013;7(5) doi: 10.1371/journal.pntd.0002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deressa A., Ali A., Bayene M., Selassie B.N., Yimer E., Hussen K. The status of rabies in Ethiopia: a retrospective record review. Ethiop. J. Health Dev. 2010;24(2) [Google Scholar]
- 53.Zinsstag J., Lechenne M., Laager M., Mindekem R., Naissengar S., Oussiguere A. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci. Transl. Med. 2017;9(421) doi: 10.1126/scitranslmed.aaf6984. [DOI] [PubMed] [Google Scholar]
- 54.Fitzpatrick M.C., Hampson K., Cleaveland S., Meyers L.A., Townsend J.P., Galvani A.P. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania. PLoS Negl. Trop. Dis. 2012;6(8) doi: 10.1371/journal.pntd.0001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos J.M., Melendez N., Reyes F., Gudiso G., Biru D., Fano G. Epidemiology of animal bites and other potential rabies exposures and anti-rabies vaccine utilization in a rural area in southern Ethiopia. Ann Agric Environ Med. 2015;22(1) doi: 10.5604/12321966.1141372. [DOI] [PubMed] [Google Scholar]
- 56.Coetzer A., Kidane A.H., Bekele M., Hundera A.D., Pieracci E.G., Shiferaw M.L. The SARE tool for rabies control: current experience in Ethiopia. Antivir. Res. 2016;135:74–80. doi: 10.1016/j.antiviral.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Laager M., Mbilo C., Madaye E.A., Naminou A., Léchenne M., Tschopp A. The importance of dog population contact network structures in rabies transmission. PLoS Negl. Trop. Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mindekem R., Lechenne M.S., Oussiguéré A., Kebkiba B., Moto D.D., Alfaroukh I.O. Cost description and comparative cost efficiency of post-exposure prophylaxis and canine mass vaccination against rabies in N'Djamena, Chad. Front. Vet. Sci. 2017;4:38. doi: 10.3389/fvets.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rostal M.K., Ross N., Machalaba C., Cordel C., Paweska J.T., Karesh W.B. Benefits of a one health approach: an example using Rift Valley fever. One Health. 2018;5:34–36. doi: 10.1016/j.onehlt.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material