Abstract
This study sought to identify potential therapeutic targets in herpes simplex keratitis (HSK) patients with active and inactive infection by investigating peripheral cytokine production. Peripheral blood mononuclear cells (PBMCs) and serum were prepared from healthy controls and HSK patients during active infection or following treatment (inactive infection). Serum antibody titres were determined by ELISA. Protein expression levels were analysed by Western blot. Cytokine levels were determined by multiplex ELISA. Active corneal herpes simplex virus type 1 (HSV-1) infection resulted in significantly elevated peripheral levels of IL-1β in HSK patients compared to healthy controls, and remained significantly increased following treatment. Elevated production of IL-1β in inactive patients was associated with significantly increased levels of IRF3 and STAT1, key proteins involved in promoting anti-viral immune responses. Our data suggest that inflammation persists beyond the period that it is clinically evident and that enhanced peripheral production of IL-1β may have implications for HSV-1 viral clearance in active and inactive HSK patients.
Keywords: herpes simplex virus type 1, herpes simplex keratitis, inflammation, peripheral immune response, pathogenesis
INTRODUCTION
Herpes simplex keratitis (HSK), caused by herpes simplex virus type 1 (HSV-1), is a sight-threatening infection and is the commonest cause of infectious blindness in the developed world, affecting up to 90% of adult populations in certain countries[1]. A hallmark of HSV-1 infection—as with all members of the Herpesvirus family—is that following primary infection, the virus remains latent for the life of the host. In the case of HSV-1, the site of latency is the trigeminal ganglion. The virus may then undergo cycles of reactivation from latency causing inflammation and scarring that can permanently damage the cornea. Recurrent episodes of HSK, can lead to corneal damage, visual morbidity and even corneal melting and perforation in necrotising stromal keratitis, one of the most severe manifestations of the disease. Corneal transplantation for visual or tectonic indications in HSK is associated with a high risk of HSK recurrence in the transplant as well as graft rejection and failure. Therefore, there is a need to develop better treatments that can both control the infection quickly to limit the damage caused by replicating virus and prevent reactivation of the disease by keeping it in its latent state. HSV-1 is a ubiquitous human pathogen, with remarkably high prevalence of HSV-1 infection that increases with age: autopsy studies have revealed HSV-1 DNA in the trigeminal ganglia in 93% of adults, and 92% of individuals with no reported history of herpes infection have been shown to periodically shed HSV-1 DNA in their tears[2]. Despite being highly prevalent among the general population less than 1% of people who are infected with HSV-1 develop ocular infection. Murine studies suggest that the elevated levels of cytokines including IL-6, TGF-β, IL-1β, TNF-α and more recently IL-17, detected within corneas following HSV-1 infection are important contributors to the development of HSK pathogenesis[3]. While current evidence supports a role for cytokines acting locally in the cornea during HSK pathogenesis, peripheral cytokines have not yet been characterised. To investigate the effect of HSV-1 infection peripherally, serum cytokines and expression of key signalling molecules were evaluated in healthy controls and patients with active and inactive HSV-1 infection.
SUBJECTS AND METHODS
Ethical Approval
This study was conducted in accordance with the Helsinki Declaration. The study was approved by the Research and Ethics Committee of the Royal Victoria Eye and Ear Hospital and written informed consent was obtained from all participants.
Patient Recruitment
Six consecutive patients attending the emergency department who met the inclusion criteria for this study and were willing to participate were recruited. Of 1 female and 5 female patients with an average age of 36-66y (mean age 48.3±10.7y) were recruited. Inclusion criteria were recurrent acute epithelial or stromal HSK, age over 18y, and ability to provide informed consent. Exclusion criteria were ocular or systemic infection or inflammation other than HSK and a history of autoimmune disease. All patients recruited to this study had presented with a sore, red, watery eye and blurring of vision. Active HSK was defined by the presence of a distinctive (usually dendritic) ulcer or inflammation in the cornea which was clinically consistent with HSK. Upon diagnosis, suitable patients provided a blood sample on the day of the diagnosis. Patients commenced standard treatment with topical acyclovir +/- topical corticosteroids and oral acyclovir where clinically indicated and were followed up at regular intervals, i.e. every 2-6wk until the inflammation had resolved. Resolution of inflammation was determined by an ophthalmologist in the course of follow up appointments, following a clinical exam of the eye, skin, conjunctiva, anterior chamber, iris, retina, etc. Specifically for keratitis, resolution of inflammation and healing of the corneal wound was determined by clinical examination and negative fluorescein staining. A second blood sample was obtained when the disease was determined to be inactive or resolved based on clinical examination and for the purpose of the study these patients were designated inactive HSK patients. None of the active or inactive HSK patients recruited for the study suffered from recurrent orolabial herpes. Five healthy controls who did not have a history of, and were not suffering from, either HSK or recurrent orolabial herpes were recruited for comparison.
Sample Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using a Ficoll density gradient centrifugation and cultured in phenol red-free RPMI-1640 medium supplemented with 10% fetal calf serum and 100 µg/mL penicillin-streptomycin. Antibody titres were determined in serum samples from HSK patients and healthy controls using HerpeSelect® 1 ELISA IgG and HerpeSelect® 2 ELISA IgG ELISA kits (Focus Diagnostics). Index values were then calculated according to kit instructions. Samples with index values >1.1 were recorded as positive, index values <0.9 were recorded as negative. Values between 0.9 and 1.1 were recorded as equivocal. Serum samples of HSK patients and healthy controls were further analysed by multiplex ELISA (Meso Scale Dicovery) for the following cytokines: IL-1β (0.57-10 000 pg/mL), IL-12/p70 (0.23-10 000 pg/mL), IL-10 (0.32-10 000 pg/mL), TNF-α (0.69-10 000 pg/mL), IL-6 (0.13-10 000 pg/mL), IL-8 (0.15-10 000 pg/mL), and IFN-γ (3.2-10 000 pg/mL). Lysates were prepared and changes in the expression of STAT1 (Santa Cruz Biotechnology #sc-592), and IRF3 (Santa Cruz Biotechnology #sc-15991), were determined by Western blot followed by optical densitometry.
Statistics
Student's paired t-tests were performed to examine differences in antibody titres, cytokine levels and protein expression between active and inactive HSK patients. Differences in antibody titres, cytokine levels and protein expression between HSK patients and controls were examined using the non-parametric Mann-Whitney test.
RESULTS
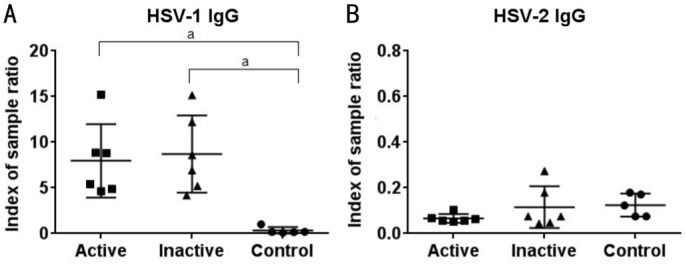
Levels of anti-HSV-1 and 2 antibodies were determined in serum samples for all study participants (Figure 1). All study participants were negative for HSV-2 antibodies. All HSK patients were positive for anti-HSV-1 IgG antibodies during the active and inactive stages of the disease and all healthy controls were negative for anti-HSV-1 antibodies. As expected, we observed no significant difference in antibody titres between patients with active or inactive infection (Figure 1).
Figure 1. Detection of anti-HSV-1 and 2 antibody titers in patients with active or inactive HSK and healthy controls.
Levels of anti-HSV-1 and 2 antibodies in serum samples for patients with active or inactive HSK and healthy controls were determined by ELISA as indicated. Each symbol represents individual samples where numerical values denote index of sample ratio. All analyses were performed using GraphPad Prism 6.0 for Windows (GraphPad Software, La Jolla, CA, USA). aP<0.005 versus healthy controls.
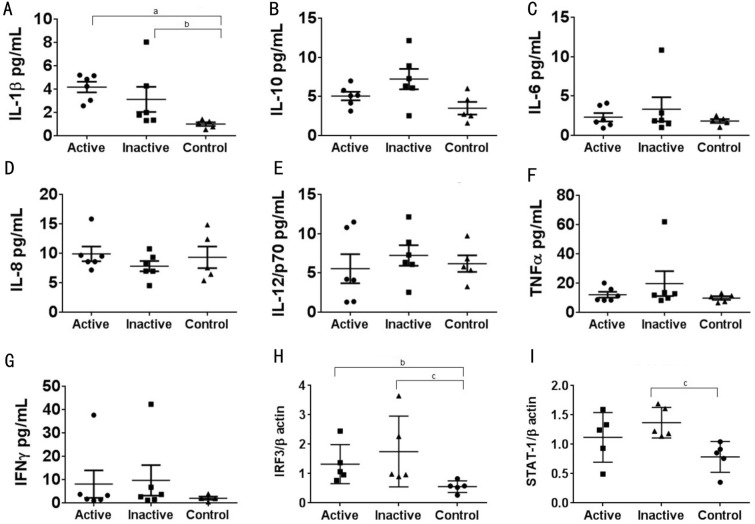
Serum samples of HSK patients and healthy controls were analysed by multiplex ELISA in order to determine whether peripheral cytokines are differentially expressed in HSK patients compared with healthy controls (Figure 2). We observed a significant increase in IL-1β levels between patients with active HSV-1 infection and healthy controls (P=0.004). Additionally, IL-1β levels remained significantly elevated (P=0.01) in these patients following treatment compared to healthy controls (Figure 2A). There was a trend towards increased levels of IL-1β in patients with active HSV-1 infection compared to inactive patients (Figure 2A). We observed no difference in peripheral levels of IL-10, IL-6, IL-8, TNF-α, IL-12 or IFN-γ in serum from HSK patients when compared to healthy controls (Figure 2B-2G). Interestingly, IL-1β binding to IL-1 receptor type 1 (IL-1R1) has been shown to activate certain IFN regulatory factors (IRFs), including IRF3 which functions downstream of TLR3 to initiate the production of both type I interferon and inflammatory cytokines, while TLR3 deficiency in humans increases the risk of HSV-1 infection[4]. In keeping with the importance of IRF3 for effective anti-viral responses, previous studies have shown that ICP0, a HSV-1 protein, inhibits IFNβ production by blocking IRF3 nuclear translocation[5]. Additionally in the context of immune evasion HSV-1 has been shown to suppress JAK phosphorylation, while HSV-1's immediate-early protein ICP27 decreases STAT1 phosphorylation and partially inhibits the translocation of STAT1 into the nucleus[5]. Additionally, IL-1β and type I interferon have been shown to reciprocally regulate each other, and thus the induction of an anti-viral state via STAT1[6]. To ascertain how HSV-1 affects the innate immune system in PBMCs, changes in the expression of STAT1 and IRF3 were determined by Western blot. We observed a significant increase in IRF3 expression in PBMCs from patients with either active or inactive HSK compared to healthy controls (P=0.0159 and P=0.0079 respectively; Figure 2H). STAT-1 expression was significantly increased in patients with inactive HSK compared to healthy controls (P=0.0079; Figure 2I). These results suggest that localised HSV-1 infection has direct consequences for peripheral expression of key proteins involved in promoting an anti-viral response.
Figure 2. Comparison of peripheral cytokine levels and transcription factor expression between patients with active or inactive HSK and healthy controls.
A-G: Levels of cytokines in serum samples were simultaneously measured using a multiplex electrochemiluminescence assay (Meso Scale Discovery, Gaithersburg, MD, USA) and read by an Imager2400 plate reader (Meso Scale Discovery, Gaithersburg, MD, USA; n=5-6). H-I: PBMCs were isolated from HSK patients with active and inactive disease and healthy controls. Endogenous expression levels of indicated proteins were determined by Western blot (n=5). Results are presented as mean±SD. Data were deemed to be significantly different at P values less than 0.05. All analyses were performed using GraphPad Prism 6.0 for Windows (GraphPad Software, La Jolla, CA, USA). aP<0.00, bP<0.05 and cP<0.008 versus healthy controls.
DISCUSSION
Overall our data suggests that localised HSV-1 infection in the cornea results in potent IL-1β production and increased STAT-1 and IRF-3 expression in peripheral cells. These increases were shown to persist beyond the period that was clinically evident, suggesting that enhanced peripheral production of the pro-inflammatory cytokine IL-1β may have implications for HSV-1 viral clearance. Our findings are consistent with studies showing that prolonged use of topical steroids is required in the treatment of HSK to promote resolution and reduce recurrence[7]. Murine studies suggest that cytokines may be important contributors to the development of HSK pathogenesis. These studies have shown that following HSV-1 infection of the cornea the most prominent cytokines produced are IL-1β, IL-6, IL-10, IL-12, and IFN-γ[3], typically several days after the development of stromal keratitis. Elevated levels of IL-1β and TNF-α are associated with corneal inflammation, while IL-6 and TGF-β are thought to exert antiviral and inflammation regulatory activities in HSV-1 corneal infection[8]. Recent studies have confirmed that elevated levels of IL-1β and TNF-α are not important for inhibiting viral replication, but instead play a role in pathogenesis of HSV-1 infection[3]. IL-1β promotes the production of IL-17 and recent studies found that following HSV-1 infection IL-17 was detected in infected corneas and its suppression reduced the severity of the HSK[9]. Our study suggests that in addition to acting locally in the cornea during HSV infection, peripheral cytokines may also contribute to disease pathogenesis. In support of corneal HSV infection effecting peripheral cytokine production previous studies have demonstrated a role for the innate system in the pathogenesis of HSK, finding that prior to T cell mediated responses viral infection leads to the production of pro-inflammatory cytokines and chemokines and invasion of the cornea by polymorphonuclear leukocyte (PMN) initially thought to promote viral clearance[10]. However, subsequent studies have demonstrated that PMN invasion contributes to the pathology of HSK as these cells are a major source of angiogenesis and tissue damaging factors, including nitric oxide[11]. Thus, events occurring at the ocular surface during HSV infection can potentially contribute to the altered peripheral levels of IL-1β observed in this study, potentially resulting in increased numbers of lymphocytes being recruited to the cornea, while reduced induction of pro-inflammatory cytokines and anti-viral factors might fail to limit viral replication. Investigations into autoimmune conditions like Systemic Lupus Erythematosus have shown the utility of measuring cytokines as indicators of potential flares in these patients[12]. Thus, monitoring serum samples of patients with a history of HSV infection might prove to be a useful diagnostic tool as increased levels of IL-1β (and consequently IL-17) in the periphery may predict relapses of HSK. Current treatments for HSK include topical and systemic antiviral drugs such as acyclovir and trifluorothymidine[7]. Thus, in addition to current treatment options for HSK targeting IL-1β in the periphery may have a beneficial outcome for localised keratitis induced by HSV-1, as a means to reduce neutrophil and Th17 cell infiltration. Of note mice transgenic for the IL-1 receptor antagonist protein are resistant to HSK[13]. Further studies are required to determine if therapies targeting overproduction of IL-1β, such as anakinra, a recombinant IL1-Ra antibody, and canakinumab, an anti-IL-1β monoclonal antibody hold potential for the treatment of HSK[14].
Given the central role of IL-1β to the pathology of HSV-1 infection recent studies have investigated the role of inflammasome activation following HSV-1 infection. In human fibroblasts HSV-1 was shown to induce the activation of the IFI16 and NLRP3 inflammasomes and promote the maturation of IL-1β during the early phase of infection. Furthermore ICP0 was shown to target IFI16 for rapid proteasomal degradation at later times postinfection[5]. It has been suggested that IL-1β is secreted in a continuum which is dependent upon the extracellular requirement for IL-1β[15]. Given that IL-1β has a very short half-live in plasma[15], our data supports the view that peripheral IL-1β levels observed in our study are as a result of increased production in response to ocular HSK infection. However further studies in PBMCs are required to determine if HSV-1 proteins (including ICP0) play a role in modulating the expression of IRF3 and STAT1 in addition to regulating NLRP3 inflammasome activity and thus levels of IL-1β.
Acknowledgments
Foundation: Supported by the Health Research Board and the Royal Victoria Eye and Ear Hospital Research Foundation through the Medical Research Charities Group (No.1409).
Conflicts of Interest: Ní Gabhann-Dromgoole J, None; De Chaumont C, None; Shahnazaryan D, None; Smith S, None; Malone C, None; Hassan J, None; De Gascun CF, None; Jefferies CA, None; Murphy CC, None.
REFERENCES
- 1.Bhatt UK, Abdul Karim MN, Prydal JI, Maharajan SV, Fares U. Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts. Cochrane Database Syst Rev. 2016;11:CD007824. doi: 10.1002/14651858.CD007824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azher TN, Yin XT, Stuart PM. Understanding the role of chemokines and cytokines in experimental models of herpes simplex keratitis. J Immunol Res. 2017;2017:7261980. doi: 10.1155/2017/7261980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma R, Bharti K. Toll like receptor 3 and viral infections of nervous system. J Neurol Sci. 2017;372:40–48. doi: 10.1016/j.jns.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Suazo PA, Ibañez FJ, Retamal-Díaz AR, Paz-Fiblas MV, Bueno SM, Kalergis AM, González PA. Evasion of early antiviral responses by herpes simplex viruses. Mediators Inflamm. 2015;2015:593757. doi: 10.1155/2015/593757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer-Barber KD, Yan B. Clash of the Cytokine Titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol. 2017;14(1):22–35. doi: 10.1038/cmi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sykakis E, Karim R, Parmar DN. Management of patients with herpes simplex virus eye disease having cataract surgery in the United Kingdom. J Cataract Refract Surg. 2013;39(8):1254–1259. doi: 10.1016/j.jcrs.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Hu M, Dutt J, Arrunategui-Correa V, Baltatzis S, Foster CS. Cytokine mRNA in BALB/c mouse corneas infected with herpes simplex virus. Eye (Lond) 1999;13(Pt 3a):309–313. doi: 10.1038/eye.1999.80. [DOI] [PubMed] [Google Scholar]
- 9.Rolinski J, Hus I. Immunological aspects of acute and recurrent herpes simplex keratitis. J Immunol Res. 2014;2014:513560. doi: 10.1155/2014/513560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70(2):898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67(6):619–624. doi: 10.1006/exer.1998.0565. [DOI] [PubMed] [Google Scholar]
- 12.Gensous N, Marti A, Barnetche T, Blanco P, Lazaro E, Seneschal J, Truchetet ME, Duffau P, Richez C, FHU ACRONIM Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis Res Ther. 2017;19(1):238. doi: 10.1186/s13075-017-1442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas PS, Banerjee K, Kim B, Rouse BT. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: possible role for IL-1 in herpetic stromal keratitis pathogenesis. J Immunol. 2004;172(6):3736–3744. doi: 10.4049/jimmunol.172.6.3736. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger N, De Meulemeester M, Pikhlak A, Yücel AE, Richard D, Murphy V, Arulmani U, Sallstig P, So A. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13(2):R53. doi: 10.1186/ar3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]