Abstract
Objective:
Abstinence outcomes are typically prioritized in the treatment of cocaine use disorder while ignoring patterns of low-frequency cocaine use. This study examined patterns of cocaine use frequency during treatment and evaluated how these patterns related to baseline characteristics and functioning outcomes 6 and 12 months after treatment.
Method:
We used a pooled dataset (N = 720) from seven randomized clinical trials for cocaine use disorder. The Addiction Severity Index (ASI) was used to assess functioning. Repeated-measures latent class analysis was used to derive patterns of cocaine use.
Results:
Three patterns were identified: abstinence (10.6%), low-frequency use (approximately 1 day/week; 66.3%), and persistent frequent use (approximately 4 days/week; 23.1%). The low-frequency group was associated with male gender, younger age, and a criminal justice referral. The abstinent group had the highest alcohol problem severity score at baseline. At Month 6, the low-frequency group reported lower problem severity than the persistent frequent use group across multiple ASI areas, including the cocaine use as well as psychological, family, employment, and legal domains. At Month 12, the low-frequency group did not differ from the abstinent group in problem severity on any ASI domain and, relative to the persistent frequent use group, had lower cocaine use and employment problem severity.
Conclusions:
These findings highlight the importance of adopting a harm reduction approach and recognizing the potential clinical benefits associated with nonabstinent outcomes.
Cocaine use disorder (CUD) is associated with significant negative costs and remains a critical public health issue (Whiteford et al., 2013). Similar to treatment for other illicit drug use disorders, treatment for CUD generally focuses on abstinence from cocaine use as the key indicator of a successful treatment outcome. Ongoing use of any cocaine, especially several weeks or months into outpatient treatment, is typically deemed as a sign of poor treatment response that may require more intensive treatment. However, regarding any continued cocaine use during treatment, even several weeks or months into treatment, as a sign of failure may be misguided given addiction is viewed as a chronic, relapsing/remitting condition (Kiluk et al., 2016; McCann et al., 2015; McLellan et al., 2000). Furthermore, patterns of ongoing cocaine use during active treatment may be remarkably different (e.g., daily use, several times per week, once a week, or occasional lapses).
Gaining a better understanding of patterns of cocaine use during active CUD treatment is critical for moving beyond an abstinence-only approach and providing information about patterns (e.g., persistent frequent use) that may signal a poor outcome versus those patterns (e.g., low frequency, occasional use) that may be associated with better outcomes. Yet, there is currently no empirical guidance about patterns of cocaine use frequency during treatment, how baseline client factors relate to patterns, and how patterns relate to long-term functioning following treatment.
It is also important to note that sustained abstinence from illicit drugs, such as cocaine, is currently the only primary endpoint accepted by regulatory agencies in the United States and Europe for approval of new pharmacotherapies for drug use disorders (Food and Drug Administration & the Psychopharmacologic Drugs Advisory Committee, 2013). Although abstinence is considered a clinically meaningful outcome, it is also regarded as an overly stringent criterion for evaluating treatment success for a chronic and relapsing/remitting condition (Kiluk et al., 2016; McCann et al., 2015; McLellan et al., 2000). For a nonabstinence cocaine use endpoint to be deemed clinically meaningful, it would need to be predictive of clinical benefit, such as improvements in functioning (Kiluk et al., 2016; Winchell et al., 2012).
The alcohol treatment field has established a low-risk drinking measure as a meaningful endpoint in clinical trials: the percentage of participants with no heavy drinking days. A heavy drinking day is defined as consuming more than three drinks for women or more than four drinks for men in any given day, although the validity of these cutoffs has been challenged more recently (Pearson et al., 2016, 2017; Witkiewitz et al., 2017b). The absence of heavy drinking days has been accepted as a primary endpoint in alcohol pharmacotherapy trials by the U.S. Food and Drug Administration and reduction in heavy drinking days accepted by the European Medicines Agency, based in part on findings from observational data (Breslow & Graubard, 2008; Dawson et al., 2007) and clinical trial data (Falk et al., 2010). Additional studies have found that low-risk drinkers are comparable to abstainers in terms of long-term drinking, psychosocial problems (Kline-Simon et al., 2013), and health care utilization and costs (Kline-Simon et al., 2014). However, because cocaine is an illicit substance and can be administered through different routes (e.g., smoking vs. intranasally), there is no standard unit of size or purity for measuring quantity in a given episode or day (e.g., individuals may report amounts in grams, lines, bags, or rocks), making it challenging to reliably assess “heavy” cocaine use. Although there is no analogue of a heavy drinking day for cocaine, it may be reasonable to explore “low-risk” patterns of cocaine use based on frequency (rather than quantity). The identification of such patterns might serve as a starting point for establishing a non–abstinence-based endpoint for cocaine clinical trials.
Some studies have evaluated trajectories of cocaine use. In an observational study following 430 crack cocaine users over 8 years, three latent trajectory groups were identified based on the probability of achieving periods of 6-months of consecutive abstinence: low probability of abstinence (59%), low/moderate probability of abstinence (23%), and high probability of abstinence (18%; Falck et al., 2007). Another observational study among 400 young adults not in treatment over a 1-year period used latent class growth modeling to identify four patterns of cocaine use: consistent use (48%), inconsistent use (14%), decreasing use (28%), and consistent nonuse (11%; Ramo et al., 2011). In a sample of 229 cocaine using military veterans (Siegal et al., 2002), three patterns of cocaine use during an 18-month period following discharge from primary treatment were identified: sustained abstinence (31%), lack of abstinence (29%), and inconsistent abstinence (40%). Those who maintained sustained abstinence demonstrated the best levels of psychosocial functioning at follow-up (Siegal et al., 2002). Despite some research on patterns of cocaine use, there are still no studies on patterns of cocaine use during active CUD treatment or on how these patterns relate to baseline characteristics or long-term outcomes.
Regarding client characteristics related to patterns of cocaine use among treatment-engaged individuals, there are limited data. In the abovementioned study on patterns during the 18 months following treatment, there were no differences among the groups in the following factors assessed at intake: age; education level; readiness for treatment; employment; legal status; and severity levels of the Addiction Severity Index subscales for alcohol use, drug use, or for legal, employment, family medical, and psychological problems (Siegal et al., 2002).
Importantly, in the alcohol treatment field there are several studies on distinct patterns of alcohol use over time, as well as baseline client factors and long-term outcomes associated with these patterns (Goodwin et al., 2017; Halonen et al., 2017; Kelso-Chichetto et al., 2018; Maisto et al., 2018; Witkiewitz et al., 2014a, 2017a, 2018). For example, seven drinking patterns across three alcohol clinical trials were identified using repeated measures latent class analysis (RMLCA); individuals with patterns of abstinence or low-risk drinking had the best long-term outcomes, including fewer drinks per drinking day, lower drinking consequences, and better mental health (Witkiewitz et al., 2017b). Further, low-risk patterns of drinking during treatment were predicted by lower alcohol dependence severity, less baseline drinking, fewer negative mood symptoms, and fewer heavy drinkers on one’s social network (Witkiewitz et al., 2017a). Studies on patterns of use in the alcohol treatment field have yielded important findings to guide clinical decision making and inform the identification of outcome indicators. Moreover, these studies have shown that “person-centered” latent variable modeling (e.g., latent class analysis) is a useful analytic tool for identifying distinct patterns of use.
The current study, a secondary analysis of a pooled sample of 720 individuals receiving CUD treatment, aimed to identify patterns of within-treatment cocaine use and evaluate how baseline client characteristics and long-term functioning were associated with these patterns.
Method
Overview
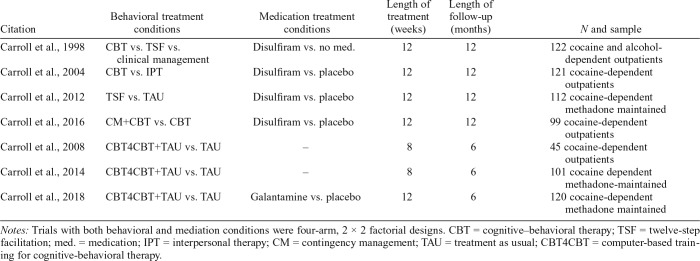
This study was a secondary data analysis using pooled data (N = 720) from seven independent randomized clinical trials evaluating behavioral treatment and/or pharmacotherapy for CUD among individuals in outpatient settings in the United States (Carroll et al., 1998, 2004, 2008, 2012, 2014, 2016, 2018). Table 1 provides an overview of the seven trials. These seven trials were chosen for the pooled dataset because they share key methodological features (i.e., study design, consistency in outcome measurements, and the length of posttreatment follow-up) and were conducted by the same research team, thereby facilitating integration of data into a pooled sample.
Table 1.
Overview of studies in pooled dataset
| Citation | Behavioral treatment conditions | Medication treatment conditions | Length of treatment (weeks) | Length of follow-up (months) | N and sample |
| Carroll et al., 1998 | CBT vs. TSF vs. clinical management | Disulfiram vs. no med. | 12 | 12 | 122 cocaine and alcohol-dependent outpatients |
| Carroll et al., 2004 | CBT vs. IPT | Disulfiram vs. placebo | 12 | 12 | 121 cocaine-dependent outpatients |
| Carroll et al., 2012 | TSF vs. TAU | Disulfiram vs. placebo | 12 | 12 | 112 cocaine-dependent methadone maintained |
| Carroll et al., 2016 | CM+CBT vs. CBT | Disulfiram vs. placebo | 12 | 12 | 99 cocaine-dependent outpatients |
| Carroll et al., 2008 | CBT4CBT+TAU vs. TAU | – | 8 | 6 | 45 cocaine-dependent outpatients |
| Carroll et al., 2014 | CBT4CBT+TAU vs. TAU | – | 8 | 6 | 101 cocaine dependent methadone-maintained |
| Carroll et al., 2018 | CBT4CBT+TAU vs. TAU | Galantamine vs. placebo | 12 | 6 | 120 cocaine-dependent methadone maintained |
Notes: Trials with both behavioral and mediation conditions were four-arm, 2 × 2 factorial designs. CBT = cognitive–behavioral therapy; TSF = twelve-step facilitation; med. = medication; IPT = interpersonal therapy; CM = contingency management; TAU = treatment as usual; CBT4CBT = computer-based training for cognitive-behavioral therapy.
Measures
Cocaine use.
All trials used the calendar-based Timeline Followback method (Robinson et al., 2014; Sobell & Sobell, 1992) to assess self-reported cocaine use on a day-by-day basis during the entire study period. Urine toxicology screens were also administered at each weekly study assessment visit.
Functioning.
The Addiction Severity Index (ASI; McLellan et al., 1992) was used to assess problem severity across multiple domains of functioning. The ASI is a multidimensional semi-structured interview that measures problem severity using a composite score for each of the following domains: psychological, medical, employment, social, legal, primary drug use, alcohol use, and other drug use. Higher scores indicate greater problem severity.
Psychiatric symptoms.
The Brief Symptoms Inventory (Derogatis & Melisaratos, 1983) was used to assess psychiatric symptoms. We examined the global severity index, the positive symptom total subscale, anxiety, and depression subscales.
Other client characteristics.
The ASI interview was used to assess age, gender, race/ethnicity, marital status, education level, employment, route of administration, chronic medical problems, and years of cocaine use in lifetime.
Statistical analyses
SPSS (IBM SPSS Statistics for Windows, Version 24; IBM Corp., Armonk, NY) was used for descriptive analyses and for conducting chi-square and analysis of variance models to compare identified patterns of cocaine use on baseline client characteristics. Mplus Version 7.4 (Muthén & Muthén, 2012) was used to estimate patterns of within-treatment cocaine use via repeated measures latent class analysis (RMLCA). The indicators of latent class included self-reported cocaine use days per week for Weeks 1 through 8 of active treatment (Week 8 was chosen as the last week included for consistency across trials). We conducted the RMLCA only with participants who reported at least 1 day of cocaine use during treatment. Those who reported cocaine abstinence during the entire period from Week 1 to Week 8 were considered an “observed” abstinent subgroup. We excluded those with observed abstinence from the RMLCA in order to compare individuals reporting abstinence to individuals reporting varying patterns of cocaine use. The RMLCA approach is based on probability and requires subjective decisions regarding the number of classes; therefore, we may not have been able to identify a class that consisted of only those with observed abstinence had we included this subgroup in the RMLCA. We discovered only five participants (0.7% of the full sample) who reported abstinence during the entire period yet submitted at least one cocaine positive urine sample. Results presented below were unchanged when excluding these 5 participants from analyses. Given that we are pooling data from seven studies, our data represents a clustered data structure. Hence, we used the sandwich estimator, which adjusts the standard errors to account for nonindependence of observations related to clustering.
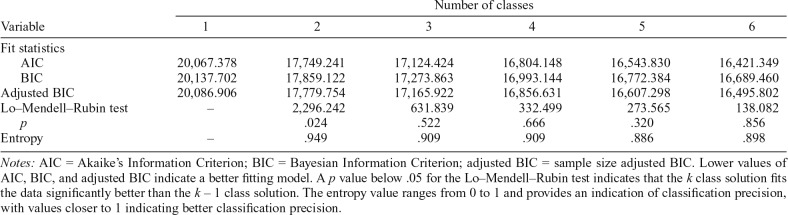
To determine the optimal class solution, we considered (a) model fit indices, including Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC), and sample size adjusted BIC (aBIC), with lower values indicating better fit; (c) the Lo–Mendell–Rubin adjusted likelihood ratio test (LRT; Lo et al., 2001), which compares whether a k class solution fits better than a k – 1 class solution (a p less than .05 indicates significantly better model fit for the k class solution than the k – 1 class solution); (c) entropy values, with higher values indicating better classification precision; and (d) parsimony and theoretical relevance of the class solution.
We examined conditional response means to interpret the identified classes. We used regression analyses (with saved class membership and dummy-coding) to test for differences among the cocaine use groups (the identified latent classes and the observed abstinent group) in functioning outcomes at the 6- and 12-month follow-up. All seven of the trials included a 6-month follow-up. Four out of seven trials included a 12-month follow-up. For models examining 12-month outcome, we therefore included only the four trials with 12-month follow-ups. For the regression models, we controlled for the baseline score of each ASI composite included in the outcome of a model. We used full information maximum likelihood estimation, which provides the variance-covariance matrix for all available data and is the preferred method when some data is missing (Hallgren & Witkiewitz, 2013; Witkiewitz et al., 2014b).
Results
Repeated measures latent class analyses (RMCLA)
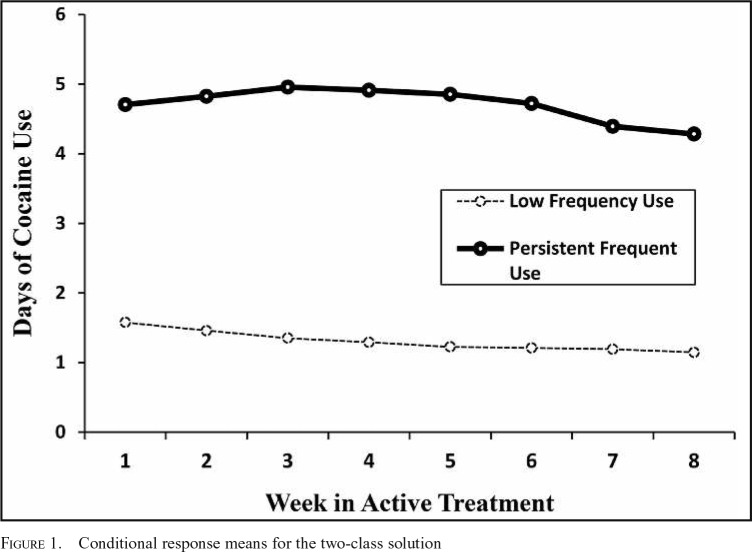
From the total sample of 720 participants, 71 participants (10.6% of the sample) reported abstinence from cocaine during Weeks 1–8. These participants were designated as an observed “abstinence group” and were not included in the RMLCA. Table 2 shows the model fit statistics and LRT results across the class solutions for the RMLCA conducted among individuals reporting at least some cocaine use during treatment. Based on the fit statistics, the LRT results, model parsimony, and theoretical relevance, we chose a two-class solution. Entropy of .949 indicated excellent classification precision. For clarity, we refer to the patterns of cocaine use as “groups” (including the abstinent group and the two patterns identified from the latent class analysis). Figure 1 shows the conditional response means for the two-class RMLCA solution. We labeled the first group derived from the RMLCA as the “low-frequency use group” (n = 444; 66.3% of the sample) as this group was characterized by a stable pattern of approximately 1 day of cocaine use per week, on average, during treatment. We labeled the other group “persistent frequent use group” (n = 155; 23.1% of the sample) because this group was characterized by a pattern of approximately 4–5 days of cocaine use per week, on average, during treatment.
Table 2.
Fit statistics for Class Solutions 1 through 6
| Variable | Number of classes |
|||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Fit statistics | ||||||
| AIC | 20,067.378 | 17,749.241 | 17,124.424 | 16,804.148 | 16,543.830 | 16,421.349 |
| BIC | 20,137.702 | 17,859.122 | 17,273.863 | 16,993.144 | 16,772.384 | 16,689.460 |
| Adjusted BIC | 20,086.906 | 17,779.754 | 17,165.922 | 16,856.631 | 16,607.298 | 16,495.802 |
| Lo–Mendell–Rubin test | – | 2,296.242 | 631.839 | 332.499 | 273.565 | 138.082 |
| p | .024 | .522 | .666 | .320 | .856 | |
| Entropy | – | .949 | .909 | .909 | .886 | .898 |
Notes: AIC = Akaike’s Information Criterion; BIC = Bayesian Information Criterion; adjusted BIC = sample size adjusted BIC. Lower values of AIC, BIC, and adjusted BIC indicate a better fitting model. A p value below .05 for the Lo–Mendell–Rubin test indicates that the k class solution fits the data significantly better than the k – 1 class solution. The entropy value ranges from 0 to 1 and provides an indication of classification precision, with values closer to 1 indicating better classification precision.
Figure 1.
Conditional response means for the two-class solution
Comparison of cocaine use groups on baseline characteristics
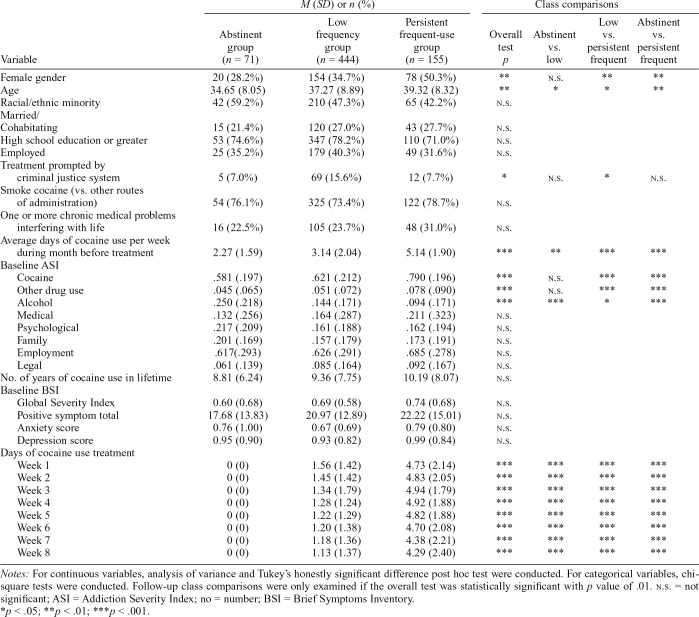
Table 3 provides a summary of the comparison of groups on baseline characteristics. Relative to the low-frequency group, the abstinent group was younger in age and reported less baseline cocaine use but more severe baseline alcohol problems. Relative to the persistent frequent use group, the low-frequency group had a greater proportion of men, was younger in age, had a greater proportion of those referred by the criminal justice system, and at baseline reported lower cocaine use, lower cocaine problem severity, and lower other drug problem severity but greater alcohol problem severity. Relative to the persistent frequent use group, the abstinent group had a greater proportion of men, was younger in age, and at baseline reported less cocaine use, lower cocaine problem severity, and lower other drug problem severity but greater alcohol problem severity. There were no significant differences among the cocaine use groups for race/ethnicity, marital status, education level, employment, cocaine route of administration, having one or more chronic medical problems, number of years of cocaine use in lifetime, baseline psychiatric symptoms, and baseline medical, psychological, family, employment, and legal problem severity.
Table 3.
Comparisons among cocaine pattern groups on baseline demographics, treatment-related variables, and observed cocaine use by treatment week
| Variable |
M (SD) or n (%) |
Class comparisons |
|||||
| Abstinent group (n = 71) | Low frequency group (n = 444) | Persistent frequent-use group (n = 155) | Overall test p | Abstinent vs. low | Low vs. persistent frequent | Abstinent vs. persistent frequent | |
| Female gender | 20 (28.2%) | 154 (34.7%) | 78 (50.3%) | ** | n.s. | ** | ** |
| Age | 34.65 (8.05) | 37.27 (8.89) | 39.32 (8.32) | ** | * | * | ** |
| Racial/ethnic minority | 42 (59.2%) | 210 (47.3%) | 65 (42.2%) | n.s. | |||
| Married/Cohabitating | 15 (21.4%) | 120 (27.0%) | 43 (27.7%) | n.s. | |||
| High school education or greater | 53 (74.6%) | 347 (78.2%) | 110 (71.0%) | n.s. | |||
| Employed | 25 (35.2%) | 179 (40.3%) | 49 (31.6%) | n.s. | |||
| Treatment prompted by criminal justice system | 5 (7.0%) | 69 (15.6%) | 12 (7.7%) | * | n.s. | * | n.s. |
| Smoke cocaine (vs. other routes of administration) | 54 (76.1%) | 325 (73.4%) | 122 (78.7%) | n.s. | |||
| One or more chronic medical problems interfering with life | 16 (22.5%) | 105 (23.7%) | 48 (31.0%) | n.s. | |||
| Average days of cocaine use per week during month before treatment | 2.27 (1.59) | 3.14 (2.04) | 5.14 (1.90) | *** | ** | *** | *** |
| Baseline ASI | |||||||
| Cocaine | .581 (.197) | .621 (.212) | .790 (.196) | *** | n.s. | *** | *** |
| Other drug use | .045 (.065) | .051 (.072) | .078 (.090) | *** | n.s. | *** | *** |
| Alcohol | .250 (.218) | .144 (.171) | .094 (.171) | *** | *** | * | *** |
| Medical | .132 (.256) | .164 (.287) | .211 (.323) | n.s. | |||
| Psychological | .217 (.209) | .161 (.188) | .162 (.194) | n.s. | |||
| Family | .201 (.169) | .157 (.179) | .173 (.191) | n.s. | |||
| Employment | .617 (.293) | .626 (.291) | .685 (.278) | n.s. | |||
| Legal | .061 (.139) | .085 (.164) | .092 (.167) | n.s. | |||
| No. of years of cocaine use in lifetime | 8.81 (6.24) | 9.36 (7.75) | 10.19 (8.07) | n.s. | |||
| Baseline BSI | |||||||
| Global Severity Index | 0.60 (0.68) | 0.69 (0.58) | 0.74 (0.68) | n.s. | |||
| Positive symptom total | 17.68 (13.83) | 20.97 (12.89) | 22.22 (15.01) | n.s. | |||
| Anxiety score | 0.76 (1.00) | 0.67 (0.69) | 0.79 (0.80) | n.s. | |||
| Depression score | 0.95 (0.90) | 0.93 (0.82) | 0.99 (0.84) | n.s. | |||
| Days of cocaine use treatment | |||||||
| Week 1 | 0 (0) | 1.56 (1.42) | 4.73 (2.14) | *** | *** | *** | *** |
| Week 2 | 0 (0) | 1.45 (1.42) | 4.83 (2.05) | *** | *** | *** | *** |
| Week 3 | 0 (0) | 1.34 (1.79) | 4.94 (1.79) | *** | *** | *** | *** |
| Week 4 | 0 (0) | 1.28 (1.24) | 4.92 (1.88) | *** | *** | *** | *** |
| Week 5 | 0 (0) | 1.22 (1.29) | 4.82 (1.88) | *** | *** | *** | *** |
| Week 6 | 0 (0) | 1.20 (1.38) | 4.70 (2.08) | *** | *** | *** | *** |
| Week 7 | 0 (0) | 1.18 (1.36) | 4.38 (2.21) | *** | *** | *** | *** |
| Week 8 | 0 (0) | 1.13 (1.37) | 4.29 (2.40) | *** | *** | *** | *** |
Notes: For continuous variables, analysis of variance and Tukey’s honestly significant difference post hoc test were conducted. For categorical variables, chisquare tests were conducted. Follow-up class comparisons were only examined if the overall test was statistically significant with p value of .01. n.s. = not significant; ASI = Addiction Severity Index; no = number; BSI = Brief Symptoms Inventory.
p < .05;
p < .01;
p < .001.
Comparison of cocaine use groups on follow-up functioning
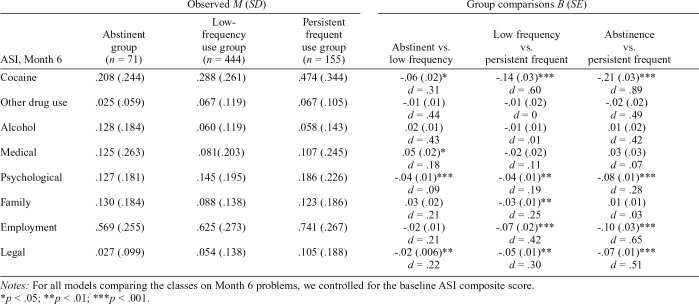
Table 4 provides a summary of the comparisons of groups on ASI composite scores at 6 months following treatment, with Cohen’s d effect sizes. Relative to the low-frequency group, the abstinent group had less problem severity (and small effect sizes) for the cocaine, psychological, and legal domains but greater medical problem severity (small effect size) at the Month 6 follow-up. Relative to the persistent frequent use group, the low-frequency group had less problem severity for the following domains at the Month 6 follow-up: cocaine (medium effect size), psychological (small effect size), family (small effect size), employment (medium effect size), and legal (small effect size). Relative to the persistent frequent use group, the abstinent group had less problem severity for the following domains at 6-month follow-up: cocaine (large effect size), psychological (small effect size), employment (medium effect size), and legal (medium effect size).
Table 4.
Comparison of cocaine use groups on Month 6 Addiction Severity Index (ASI) composites
| Observed M (SD) |
Group comparisons B (SE) |
|||||
| ASI, Month 6 | Abstinent group (n = 71) | Low-frequency use group (n = 444) | Persistent frequent use group (n = 155) | Abstinent vs. low frequency | Low frequency vs. `persistent frequent | Abstinence vs. persistent frequent |
| Cocaine | .208 (.244) | .288 (.261) | .474 (.344) | -.06 (.02)* | -.14 (.03)*** | -.21 (.03)*** |
| d = .31 | d = .60 | d = .89 | ||||
| Other drug use | .025 (.059) | .067 (.119) | .067 (.105) | -.01 (.01) | -.01 (.02) | -.02 (.02) |
| d = .44 | d = 0 | d = .49 | ||||
| Alcohol | .128 (.184) | .060 (.119) | .058 (.143) | .02 (.01) | -.01 (.01) | .01 (.02) |
| d = .43 | d = .01 | d = .42 | ||||
| Medical | .125 (.263) | .081 (.203) | .107 (.245) | .05 (.02)* | -.02 (.02) | .03 (.03) |
| d = .18 | d = .11 | d = .07 | ||||
| Psychological | .127 (.181) | .145 (.195) | .186 (.226) | -.04 (.01)*** | -.04 (.01)** | -.08 (.01)*** |
| d = .09 | d = .19 | d = .28 | ||||
| Family | .130 (.184) | .088 (.138) | .123 (.186) | .03 (.02) | -.03 (.01)** | .01 (.01) |
| d = .21 | d = .25 | d = .03 | ||||
| Employment | .569 (.255) | .625 (.273) | .741 (.267) | -.02 (.01) | -.07 (.02)*** | -.10 (.03)*** |
| d = .21 | d = .42 | d = .65 | ||||
| Legal | .027 (.099) | .054 (.138) | .105 (.188) | -.02 (.006)** | -.05 (.01)** | -.07 (.01)*** |
| d = .22 | d = .30 | d = .51 | ||||
Notes: For all models comparing the classes on Month 6 problems, we controlled for the baseline ASI composite score.
p < .05;
p < .01;
p < .001.
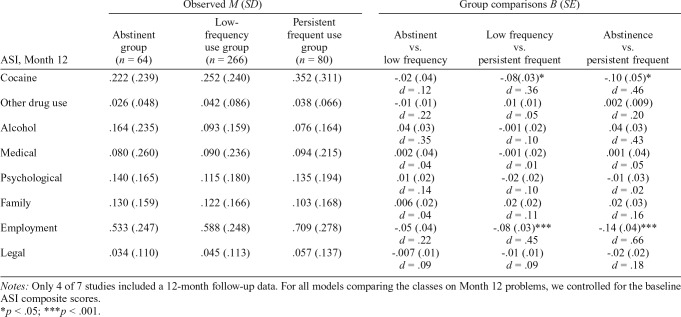
Table 5 provides a summary of the comparisons of groups on ASI composite scores at 12 months following treatment. Relative to the low-frequency group, the abstinent group was not significantly different on any domain of the ASI, including cocaine problem severity. Relative to the persistent frequent use group, the low-frequency group had less cocaine problem severity (small effect size) and employment problem severity (medium effect size). Last, relative to the persistent frequent use group, the abstinent group had less cocaine problem severity and employment problem severity (both medium effect size).
Table 5.
Comparison of cocaine use groups on Month 12 Addiction Severity Index (ASI) composites
| ASI, Month 12 | Observed M (SD) |
Group comparisons B (SE) |
||||
| Abstinent group (n = 64) | Low-frequency use group (n = 266) | Persistent frequent use group (n = 80) | Abstinent vs. low frequency | Low frequency vs. persistent frequent | Abstinence vs. persistent frequent | |
| Cocaine | .222 (.239) | .252 (.240) | .352 (.311) | -.02 (.04) | -.08 (.03)* | -.10 (.05)* |
| d = .12 | d = .36 | d = .46 | ||||
| Other drug use | .026 (.048) | .042 (.086) | .038 (.066) | -.01 (.01) | .01 (.01) | .002 (.009) |
| d = .22 | d = .05 | d = .20 | ||||
| Alcohol | .164 (.235) | .093 (.159) | .076 (.164) | .04 (.03) | -.001 (.02) | .04 (.03) |
| d = .35 | d = .10 | d = .43 | ||||
| Medical | .080 (.260) | .090 (.236) | .094 (.215) | .002 (.04) | -.001 (.02) | .001 (.04) |
| d = .04 | d = .01 | d = .05 | ||||
| Psychological | .140 (.165) | .115 (.180) | .135 (.194) | .01 (.02) | -.02 (.02) | -.01 (.03) |
| d = .14 | d = .10 | d = .02 | ||||
| Family | .130 (.159) | .122 (.166) | .103 (.168) | .006 (.02) | .02 (.02) | .02 (.03) |
| d = .04 | d = .11 | d = .16 | ||||
| Employment | .533 (.247) | .588 (.248) | .709 (.278) | -.05 (.04) | -.08 (.03)*** | -.14 (.04)*** |
| d = .22 | d = .45 | d = .66 | ||||
| Legal | .034 (.110) | .045 (.113) | .057 (.137) | -.007 (.01) | -.01 (.01) | -.02 (.02) |
| d = .09 | d = .09 | d = .18 | ||||
Notes: Only 4 of 7 studies included a 12-month follow-up data. For all models comparing the classes on Month 12 problems, we controlled for the baseline ASI composite scores.
p < .05;
p < .001.
Sensitivity analyses
When conducting the RMLCA models in two random split-half samples, the results remained unchanged. In both split-half samples the 2-class solution fit the data best based on the LRT test (ps < .05), the entropy was high (Sample 1: .943; Sample 2: .957), and the conditional response means revealed similar patterns with one class showing about 1 day of cocaine use during treatment and the other class showing about 4–5 days of cocaine use during treatment.
When conducting additional sensitivity analyses for the models comparing the cocaine use groups on Month 6 and Month 12 outcomes, there were no substantive changes in the pattern of findings. Specifically, the pattern of findings remained unchanged when we (a) incorporated the urine data to biochemically confirm self-reported abstinence among those in the abstinent group (those with conflicting urine data were included as “missing” in the regression models), and (b) controlled for baseline cocaine use, age, gender, and criminal justice referral.
Discussion
We conducted secondary analyses in a pooled sample of 720 individuals receiving treatment for CUD to identify patterns of within-treatment cocaine use and to evaluate how these patterns were associated with baseline client characteristics and long-term functioning. Our findings indicated three distinct patterns of within-treatment cocaine use: (a) abstinence (10.6% of the sample), (b) low-frequency use (approximately 1 day of cocaine use per week; 66.3% of the sample), and (c) persistent frequent use (approximately 4 days of cocaine use per week; 23.1% of the sample). One of the key findings from this study is that clients who were able to sustain a pattern of low-frequency use during treatment had levels of problem severity across multiple domains of functioning following treatment comparable to those who were completely abstinent from cocaine during treatment, and significantly better than clients who continued using at frequent levels during treatment. Specifically, at the 6-month follow-up, the low-frequency group demonstrated less problem severity for cocaine use than the persistent frequent use group, as well as less problem severity across multiple domains of functioning, including psychological, family, employment, and legal. Furthermore, at the 12-month follow-up, the low-frequency group did not significantly differ from the abstinent group in terms of problem severity across multiple domains of functioning and, again, showed better outcomes than the persistent frequent use group (with less problem severity in the areas of cocaine use and employment).
The current study provides empirical evidence that sustained low-frequency cocaine use during treatment may be a clinically meaningful outcome, as it is associated with similar levels of functioning following treatment as found among those who achieve sustained abstinence. To our knowledge, this is the first empirical study on patterns of cocaine use frequency during active outpatient treatment. Collectively, our findings are consistent with recent harm reduction research in the alcohol treatment field. For example, a recent analysis of three large alcohol use disorder clinical trials (Witkiewitz et al., 2017b) found that clients engaging in low-risk patterns of alcohol use during treatment did not statistically differ from those who were abstinent in regard to long-term functioning, and clients engaging in low-risk patterns had significantly better long-term functioning than those engaging in persistent and frequent alcohol use. Hence, patterns of low-frequency or low-risk substance use may be clinically meaningful when it comes to both alcohol and cocaine use during treatment.
Of note, in general the abstinent group still had the most favorable pattern of outcomes following treatment. Although the abstinent group did not differ from the low-frequency group at the 12-month follow-up in terms of problem severity across ASI domains, the abstinent group did show less problem severity for the cocaine, psychological, and legal domains than the low-frequency group at the 6-month follow-up. Although reductions to low-frequency patterns may confer clinical benefit, abstinence from cocaine use still represents the ideal outcome, especially when considering the potential harmful medical and legal consequences of any continued use. Nevertheless, although acknowledging that abstinence is ideal, it is essential to also fully recognize that sustained abstinence during treatment is rare. Only 10.6% of the sample reported abstinence throughout the treatment period across different treatment modalities. This highlights that sustained abstinence from cocaine during treatment is relatively rare and suggests that any continued cocaine use is not automatically a sign of poor progress. This study provides empirical evidence that continued low-frequency cocaine use during treatment, even 2 months into treatment, is not necessarily an indicator of poor prognosis. An alternative approach to relying on sustained abstinence as the only accepted endpoint is to acknowledge that slips can happen and that reductions in cocaine use frequency can be associated with meaningful improvement. Working toward reductions in use may also be more desirable for some clients (McKeganey, 2004).
Our study also provides general descriptive information on weekly patterns of cocaine use during outpatient treatment among a diverse sample of individuals with CUD. Empirical guidelines on patterns of cocaine use during treatment can guide clinicians on matters related to prognosis and referral to higher levels of treatment. Our findings suggest that clients in outpatient care who continue to use cocaine an average of 4 days per week during the first 2 months of treatment have a relatively poor prognosis and may need higher levels of treatment.
This study builds on our prior work in several important ways. In one study (Kiluk et al., 2017), when pre-selecting frequency levels based on the final month of treatment, we found that not exceeding 4 days of cocaine use in the final month of treatment predicted a greater likelihood of reporting zero problems across all ASI domains at follow-up (labeled problem-free functioning). The current study is distinct by using a person-centered latent variable modeling approach to empirically derive patterns of cocaine use by week over the initial 2-month treatment period, rather than evaluating pre-selected levels of cocaine use during the final month of treatment. Here we employed a data-driven approach for identifying the number and type of frequency patterns over time that provided the best model fit to the data. We also used the ASI composite scores, rather than a dichotomous indicator of whether someone reported any problems across all domains (Kiluk et al., 2017), which might be viewed as too stringent. Interestingly, when using a data-driven approach and evaluating the ASI composites, results of the present study are consistent with prior findings (Kiluk et al. 2017). These converging findings from different analytic approaches suggest that 1 cocaine use day per week, or 4 cocaine use days per month, may be a clinically meaningful threshold. The current study findings also advance those from another of our prior studies that indicated greater cocaine abstinence during treatment was associated with fewer problems following treatment (Kiluk et al., 2014). In the 2014 study, which included a smaller pooled sample of randomized controlled trial participants with CUD (n = 434), we examined within-treatment abstinence from cocaine use as a latent factor (indicated by percentage days abstinent, maximum days abstinent, and percentage positive urine samples) as well as a latent factor of “global problems” indicated by the days of problems reported across all ASI domains (Kiluk et al., 2014). Although the findings presented here are consistent with those reported in 2014 and 2017 overall, the current study advances this line of research by empirically identifying and characterizing patterns of cocaine use during treatment that are more clinically interpretable, which may ultimately contribute to defining a clinically meaningful non–abstinence-based endpoint.
The current study also examined client characteristics related to the patterns of cocaine use. We found those in the low-frequency group, relative to the persistent frequent use group, were more likely to be male, younger, have a criminal justice referral, and to report lower baseline cocaine use, and less cocaine and other drug problem severity. Criminal justice involvement has been found to be associated with better cocaine treatment outcomes compared with those not involved with the criminal justice system (Kiluk et al., 2015) and may serve as a protective factor from engaging in patterns of frequent use during outpatient treatment. The other differences in client characteristics, including gender, age, and levels of cocaine use at treatment entry, may reflect a link between dependence severity and ability to sustain patterns of low-frequency cocaine use during treatment. Prior research suggests that women present with more severe cocaine use disorder symptoms (Kosten et al. 1993; McCance-Katz et al., 1999). Research in the alcohol treatment field has shown that individuals with lower alcohol dependence severity are more likely to sustain patterns of low-risk drinking during treatment (Witkiewitz et al., 2017a). Interestingly, there were no differences in baseline psychiatric symptoms among the three patterns, suggesting individuals may be able to sustain patterns of low-frequency cocaine use during treatment regardless of psychiatric symptomology and psychological distress.
An additional finding of interest in this study relates to the interplay between cocaine use and alcohol use. Relative to the each of the other groups, the abstinent group reported significantly greater alcohol problem severity at baseline. At both the 6- and 12-month follow-up, the abstinent group also reported greater alcohol problem severity than the other groups, although these differences fell short of statistical significance. It is surprising that those in the abstinent group would report greater problems with alcohol compared with the other groups at multiple time points, as alcohol use has been found to increase the likelihood that an individual will relapse to their primary drug (Staiger et al., 2013). However, this finding should be interpreted with caution, as the ASI composite is a measure of alcohol problem severity rather than a direct measure of the frequency and quantity of alcohol use, and the lack of consistent measurement of alcohol use across studies in these data prohibited systematic evaluation of the pattern and timing of alcohol use with respect to cocaine use. Nevertheless, these findings suggest that alcohol use may be a concern among some clients entering treatment for cocaine use and that future research should examine patterns of use over time across different substances, which is an understudied area.
This study has several limitations. First, latent class analyses classify samples based on probability and some individuals may have been misclassified. Nevertheless, the entropy value of .949 indicates high classification precision. Although patterns of cocaine use during Week 1 to 8 were examined, the full treatment period was 12 weeks for four of the seven trials. We chose to use data from Weeks 1 to 8 to establish consistency across all seven trials. Another limitation is that functioning was indicated by a self-reported measure of problem severity, rather than objective indices or collateral data to support positive levels of functioning or clinical benefit. Also, in this study, we were able to assess only cocaine use frequency (in terms of days per week) rather than quantity of use in a given day or episode (given the lack of accepted standardized measurements of cocaine sizing and purity for measuring quantity). Therefore, we were not able to detect whether some patients may have decreased the frequency of use but also potentially increased the quantity of cocaine use per day. Last, although urine samples were collected at least weekly across trials, the frequency categories were based entirely on self-report because of the challenge in calculating frequency of use from urine results and the multiple assumptions regarding handling missing urine data (Kiluk et al., 2016). The rates of discrepancy between self-report and urine result were fairly low in the total sample (7%–16% across trials), and only 5 of the 71 participants who reported abstinence during the entire period submitted a cocaine-positive urine (7%), offering general support for the accuracy of self-reported cocaine use in these data.
Overall, this study suggests that individuals who sustain a low-frequency pattern of cocaine use during treatment (approximately 1 day per week) have comparable levels of problem severity across multiple functional domains following treatment as those reporting sustained abstinence. Through empirical research, we may be better able to understand the nuances of continued cocaine use during treatment, including which clients can achieve certain patterns and how these patterns relate to long-term outcomes. This might inform not only future clinical trials investigating pharmacological and behavioral treatments for CUD but also potential development of guidelines for clinical decision making.
Footnotes
This research was supported by National Institute on Drug Abuse (NIDA) Grants R21DA041661, P50DA009241, and T32DA007238-27 and by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant R01 AA022328. NIDA and NIAAA had had no further role in study design; in collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- Breslow R. A., Graubard B. I. Prospective study of alcohol consumption in the United States: Quantity, frequency, and cause-specific mortality. Alcoholism: Clinical and Experimental Research. 2008;32:513–521. doi: 10.1111/j.1530-0277.2007.00595.x. doi:10.1111/j.1530-0277.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Carroll K. M., Ball S. A., Martino S., Nich C., Babuscio T., Gordon M. A., Rounsaville B. J. Computer-assisted cognitive-behavioral therapy for addiction. A randomized clinical trial of CBT4CBT. American Journal of Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. doi:10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. M., Fenton L. R., Ball S. A., Nich C., Frankforter T. L., Shi J., Rounsaville B. J. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized placebo-controlled trial. Archives of General Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. doi:10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. M., Kiluk B. D., Nich C., Gordon M. A., Portnoy G. A., Marino D. R., Ball S. A. Computer-assisted delivery of cognitive-behavioral therapy: Efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. American Journal of Psychiatry. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. doi:10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. M., Nich C., Ball S. A., McCance E., Rounsaville B. J. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. doi:10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll K. M., Nich C., DeVito E. E., Shi J. M., Sofuoglu M. Galantamine and computerized cognitive behavioral therapy for cocaine dependence: A randomized clinical trial. Journal of Clinical Psychiatry. 2018;79(1):17m11669. doi: 10.4088/JCP.17m11669. doi:10.4088/JCP.17m11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. M., Nich C., Petry N. M., Eagan D. A., Shi J. M., Ball S. A. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug and Alcohol Dependence. 2016;160:135–142. doi: 10.1016/j.drugalcdep.2015.12.036. doi:10.1016/j.drugalcdep.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. M., Nich C., Shi J. M., Eagan D., Ball S. A. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: A randomized placebo-controlled trial. Drug and Alcohol Dependence. 2012;126:224–231. doi: 10.1016/j.drugalcdep.2012.05.019. doi:10.1016/j.drugalcdep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. A., Goldstein R. B., Grant B. F. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: A 3-year follow-up. Alcoholism: Clinical and Experimental Research. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. doi:10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Derogatis L. R., Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychological Medicine. 1983;13:595–605. doi:10.1017/S0033291700048017. [PubMed] [Google Scholar]
- Falck R. S., Wang J., Carlson R. G. Crack cocaine trajectories among users in a midwestern American city. Addiction. 2007;102:1421–1431. doi: 10.1111/j.1360-0443.2007.01915.x. doi:10.1111/j.1360-0443.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- Falk D., Wang X. Q., Liu L., Fertig J., Mattson M., Ryan M., Litten R. Z. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcoholism: Clinical and Experimental Research. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. doi:10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration & the Psychopharmacologic Drugs Advisory Committee. Probuphine (buprenorphine hydrochloride subdermal implant) for maintenance treatment of opioid dependence. Silver Spring; MD: 2013. [Google Scholar]
- Goodwin L., Norton S., Fear N. T., Jones M., Hull L., Wessely S., Rona R. J. Trajectories of alcohol use in the UK military and associations with mental health. Addictive Behaviors. 2017;75:130–137. doi: 10.1016/j.addbeh.2017.07.010. doi:10.1016/j.addbeh.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Hallgren K. A., Witkiewitz K. Missing data in alcohol clinical trials: A comparison of methods. Alcoholism: Clinical and Experimental Research. 2013;37:2152–2160. doi: 10.1111/acer.12205. doi:10.1111/acer.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen J. I., Stenholm S., Pulakka A., Kawachi I., Aalto V., Pentti J., Kivimäki M. Trajectories of risky drinking around the time of statutory retirement: A longitudinal latent class analysis. Addiction. 2017;112:1163–1170. doi: 10.1111/add.13811. doi:10.1111/add.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso-Chichetto N. E., Plankey M., Abraham A. G., Ennis N., Chen X., Bolan R., Cook R. L. Association between alcohol consumption trajectories and clinical profiles among women and men living with HIV. American Journal of Drug and Alcohol Abuse. 2018;44:85–94. doi: 10.1080/00952990.2017.1335317. doi:10.1 080/00952990.2017.1335317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk B. D., Babuscio T. A., Nich C., Carroll K. M. Initial validation of a proxy indicator of functioning as a potential tool for establishing a clinically meaningful cocaine use outcome. Drug and Alcohol Dependence. 2017;179:400–407. doi: 10.1016/j.drugalcdep.2017.07.020. doi:10.1016/j.drugalcdep.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk B. D., Carroll K. M., Duhig A., Falk D. E., Kampman K., Lai S., Strain E. C. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug and Alcohol Dependence. 2016;158:1–7. doi: 10.1016/j.drugalcdep.2015.11.004. doi:10.1016/j.drugalcdep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk B. D., Nich C., Witkiewitz K., Babuscio T. A., Carroll K. M. What happens in treatment doesn’t stay in treatment: Cocaine abstinence during treatment is associated with fewer problems at follow-up. Journal of Consulting and Clinical Psychology. 2014;82:619–627. doi: 10.1037/a0036245. doi:10.1037/a0036245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk B. D., Serafini K., Malin-Mayor B., Babuscio T. A., Nich C., Carroll K. M. Prompted to treatment by the criminal justice system: Relationships with treatment retention and outcome among cocaine users. American Journal on Addictions. 2015;24:225–232. doi: 10.1111/ajad.12208. doi:10.1111/ajad.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon A. H., Falk D. E., Litten R. Z., Mertens J. R., Fertig J., Ryan M., Weisner C. M. Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2013;37(Supplement 1):E373–E380. doi: 10.1111/j.1530-0277.2012.01908.x. doi:10.1111/j.1530-0277.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon A. H., Weisner C. M., Parthasarathy S., Falk D. E., Litten R. Z., Mertens J. R. Five-year healthcare utilization and costs among lower-risk drinkers following alcohol treatment. Alcoholism: Clinical and Experimental Research. 2014;38:579–586. doi: 10.1111/acer.12273. doi:10.1111/acer.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T. A., Gawin F. H., Kosten T. R., Rounsaville B. J. Gender differences in cocaine use and treatment response. Journal of Substance Abuse Treatment. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. doi:10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- Lo Y., Mendell N. R., Rubin D. B. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. doi:10.1093/biomet/88.3.767. [Google Scholar]
- Maisto S. A., Hallgren K. A., Roos C. R., Witkiewitz K. Course of remission from and relapse to heavy drinking following outpatient treatment of alcohol use disorder. Drug and Alcohol Dependence. 2018;187:319–326. doi: 10.1016/j.drugalcdep.2018.03.011. doi:10.1016/j.drugalcdep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz E. F., Carroll K. M., Rounsaville B. J. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. American Journal on Addictions. 1999;8:300–311. doi: 10.1080/105504999305703. doi:10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McCann D., Ramey T., Skolnick P. Outcome measures in medication trials for substance use disorders. Current Treatment Options in Psychiatry. 2015;2:113–121. doi:10.1007/s40501-015-0038-5. [Google Scholar]
- McKeganey N., Morris Z., Neale J., Robertson M. What are drug users looking for when they contact drug services: Abstinence or harm reduction? Drugs: Education, Prevention, & Policy. 2004;11:423–435. doi:10.1080/09687630410001723229. [Google Scholar]
- McLellan A. T., Kushner H., Metzger D., Peters R., Smith I., Grissom G., Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Lewis D. C., O’Brien C. P., Kleber H. D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. doi:10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. MPlus user’s guide (7th ed.) Los Angeles, CA: Authors; 2012. [Google Scholar]
- Pearson M. R., Bravo A. J., Kirouac M., Witkiewitz K. The search for an elusive cutoff remains: Problems of binary classification of heavy drinking as an endpoint for alcohol clinical trials. Drug and Alcohol Dependence. 2017;171:91–96. doi: 10.1016/j.drugalcdep.2016.11.015. doi:10.1016/j.drugalcdep.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. R., Kirouac M., Witkiewitz K. Questioning the validity of the 4+/5+ binge or heavy drinking criterion in college and clinical populations. Addiction. 2016;111:1720–1726. doi: 10.1111/add.13210. doi:10.1111/add.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo D. E., Grov C., Delucchi K. L., Kelly B. C., Parsons J. T. Cocaine use trajectories of club drug-using young adults recruited using time-space sampling. Addictive Behaviors. 2011;36:1292–1300. doi: 10.1016/j.addbeh.2011.08.003. doi:10.1016/j.addbeh.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. M., Sobell L. C., Sobell M. B., Leo G. I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors. 2014;28:154–162. doi: 10.1037/a0030992. doi:10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Siegal H. A., Li L., Rapp R. C. Abstinence trajectories among treated crack cocaine users. Addictive Behaviors. 2002;27:437–449. doi: 10.1016/s0306-4603(01)00184-8. doi:10.1016/S0306-4603(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Timeline Followback: A technique for assessing self-reported alcohol consumption. In: Litten R. Z., Allen J., editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Staiger P. K., Richardson B., Long C. M., Carr V., Marlatt G. A. Overlooked and underestimated? Problematic alcohol use in clients recovering from drug dependence. Addiction. 2013;108:1188–1193. doi: 10.1111/j.1360-0443.2012.04075.x. doi:10.1111/j.1360-0443.2012.04075.x. [DOI] [PubMed] [Google Scholar]
- Whiteford H. A., Degenhardt L., Rehm J., Baxter A. J., Ferrari A. J., Erskine H. E., Vos T. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. doi:10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Winchell C., Rappaport B. A., Roca R., Rosebraugh C. J. Reanalysis of methamphetamine dependence treatment trial. CNS Neuroscience & Therapeutics. 2012;18:367–368. doi: 10.1111/j.1755-5949.2011.00288.x. doi:10.1111/j.1755-5949.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Dearing R. L., Maisto S. A. Alcohol use trajectories among non–treatment-seeking heavy drinkers. Journal of Studies on Alcohol and Drugs. 2014a;75:415–422. doi: 10.15288/jsad.2014.75.415. doi:10.15288/jsad.2014.75.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Falk D. E., Kranzler H. R., Litten R. Z., Hallgren K. A., O’Malley S. S., Anton R. F. & the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup. Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcoholism: Clinical and Experimental Research. 2014b;38:2826–2834. doi: 10.1111/acer.12543. doi:10.1111/acer.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Kirouac M., Roos C. R., Wilson A. D., Hallgren K. A., Bravo A. J., Maisto S. A. Abstinence and low risk drinking during treatment: Association with psychosocial functioning, alcohol use, and alcohol problems 3 years following treatment. Psychology of Addictive Behaviors. 2018;32:639–646. doi: 10.1037/adb0000381. doi:10.1037/adb0000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Pearson M. R., Hallgren K. A., Maisto S. A., Roos C. R., Kirouac M., Heather N. Who achieves low risk drinking during alcohol treatment? An analysis of patients in three alcohol clinical trials. Addiction. 2017a;112:2112–2121. doi: 10.1111/add.13870. doi:10.1111/add.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Roos C. R., Pearson M. R., Hallgren K. A., Maisto S. A., Kirouac M., Heather N. How much is too much? Patterns of drinking during alcohol treatment and associations with post-treatment outcomes across three alcohol clinical trials. Journal of Studies on Alcohol and Drugs. 2017b;78:59–69. doi: 10.15288/jsad.2017.78.59. doi:10.15288/jsad.2017.78.59. [DOI] [PMC free article] [PubMed] [Google Scholar]