Key Points
Question
Is mental stress–induced endothelial dysfunction associated with major adverse cardiovascular events?
Findings
This cohort study of patients with stable coronary artery disease found a graded positive association between transient endothelial dysfunction with mental stress and major adverse cardiovascular events.
Meaning
Impairment in endothelium-dependent relaxation induced by mental stress confers an increased hazard for adverse events and could be an important risk biomarker for patients with stable coronary artery disease.
This cohort study assesses the association of mental stress–induced impairment in endothelium-dependent relaxation with adverse cardiovascular outcomes among individuals with stable coronary artery disease.
Abstract
Importance
Acute mental stress can result in transient endothelial dysfunction, but the prognostic relevance of this phenomenon is unknown.
Objective
To determine the association between mental stress–induced impairment in endothelium-dependent relaxation as assessed by brachial artery flow-mediated vasodilation and adverse cardiovascular outcomes among individuals with stable coronary artery disease.
Design, Setting, and Participants
This cohort study was conducted at a university-affiliated hospital network between June 2011 and August 2014. A cohort of individuals with stable coronary artery disease were included. Data analysis took place from November 2018 to May 2019.
Exposures
Study participants were subjected to a laboratory mental stress task (public speaking).
Main Outcomes and Measures
Flow-mediated vasodilation was measured before and 30 minutes after a public-speaking mental stress task. We examined the association of the rest (prestress), poststress, and δ flow-mediated vasodilation (poststress minus prestress levels) with an adjudicated composite end point of adverse events, including cardiovascular death, myocardial infarction, unstable angina leading to revascularization, and heart failure hospitalization, after adjusting for sociodemographic factors, medical history, and depression.
Results
A total of 569 patients were included (mean [SD] age, 62.6 [9.3] years; 420 men [73.8%]). Flow-mediated vasodilation decreased from a mean (SD) of 4.8% (3.7%) before mental stress to 3.9% (3.6%) after mental stress (a 23% reduction; P < .001), and 360 participants (63.3%) developed transient endothelial dysfunction (a decrease in flow-mediated vasodilation). During a median (interquartile range) follow-up period of 3.0 (2.9-3.1) years, 74 patients experienced a major adverse cardiovascular event. The presence of transient endothelial dysfunction with mental stress was associated with a 78% increase (subdistribution hazard ratio [sHR], 1.78 [95% CI, 1.15-2.76]) in the incidence of major adverse cardiovascular event. Both the δ flow-mediated vasodilation (sHR, 1.15 [95% CI, 1.03-1.27] for each 1% decline) and poststress flow-mediated vasodilation (sHR, 1.14 [95% CI, 1.04-1.24] for each 1% decline) were associated with major adverse cardiovascular event. Risk discrimination statistics demonstrated a significant model improvement after addition of either poststress flow-mediated vasodilation (change in the area under the curve, 0.05 [95% CI, 0.01-0.09]) or prestress plus δ flow-mediated vasodilation (change in the area under the curve, 0.04 [95% CI, 0.00-0.08]) compared with conventional risk factors.
Conclusions and Relevance
In this study, transient endothelial dysfunction with mental stress was associated with adverse cardiovascular outcomes in patients with coronary artery disease. Endothelial responses to stress represent a possible mechanism through which psychological stress may affect outcomes in patients with coronary artery disease.
Introduction
Psychological stress is associated with increased cardiovascular morbidity and mortality.1 One postulated mechanism is that chronic or repeated exposure to psychological stress, through activation of the sympathoadrenal pathways, causes cumulative wear and tear of the endothelial lining of blood vessels, eventually leading to endothelial dysfunction, accelerated atherogenesis, and elevated incidence of cardiovascular events.2,3,4 However, there are few empirical data in humans in support of this theoretical model.
Prior work has shown that acute exposure to an emotional stressor can induce transient endothelial dysfunction.3,5,6 This was evidenced by a sustained decrease in brachial artery flow-mediated vasodilation (FMD) induced by mental stress that was apparent for up to 4 hours after the end of the mental stress task, long after resolution of stress-induced increases in blood pressure and heart rate.3,5,6 Numerous studies have also shown that lower FMD levels are associated with adverse cardiovascular outcomes in patients with and patients without coronary artery disease (CAD).7,8,9 However, the prognostic importance of a transient decline in FMD in response to mental stress remains untested. Mental stress in the laboratory is considered a proxy of stressful exposures during daily life.10,11 Thus, if a link between stress-induced endothelial compromise and clinical events can be demonstrated, this would uncover an important stress-associated risk pathway for cardiovascular disease.
In a large and well-characterized sample of patients with stable CAD, we sought to determine whether a transient decline in FMD induced by a brief episode of mental stress in the laboratory is associated with major adverse cardiovascular events (MACE). We hypothesized that a stress-induced transitory decline in FMD would be associated with worse outcomes, independent of prestress FMD levels and other prognostic factors.
Methods
Study Design and Participants
Participants
The study design and methods have been published previously.12 Briefly, patients were enrolled into the Mental Stress Ischemia Prognosis Study, a prospective study that recruited 695 patients with stable CAD between June 2011 and August 2014 from Emory University–affiliated hospitals and clinics. The presence of CAD was defined by an abnormal coronary angiographic result demonstrating evidence of atherosclerosis with at least luminal irregularities, documented previous percutaneous or surgical coronary revascularization, documented myocardial infarction, or a positive nuclear stress test result. Patients with an acute coronary syndrome or decompensated heart failure (HF) during the previous 2 months, end-stage renal disease, or unstable psychiatric conditions were excluded. Clinical information, including previous CAD events, CAD risk factors, coronary angiography results, and current medications, was documented using standardized questionnaires and medical record reviews. A lifetime diagnosis of major depression was assessed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).13 Race was self-reported, and participants chose from predefined categories. We assessed race to describe the study population together with other demographic characteristics. The research protocol was approved by the institutional review board of Emory University, and all participants provided informed consent.
Follow-up and Assessment of Outcome Events
All participants were followed up for a median of 3 years. Outcome events included cardiovascular death, myocardial infarction, admission with unstable angina leading to revascularization, and hospitalization for HF. Outcome data were collected during follow-up clinic visits at 1 and 2 years and by telephone calls at 3 years, as well as by medical records reviews and queries to the Social Security Death Index. Cardiovascular death was defined as any death attributable to an ischemic cardiovascular cause (fatal myocardial infarction), cardiac arrhythmia (including cases in which resuscitation occurred), acute decompensated HF, or a cardiac procedure (angioplasty or coronary artery bypass grafting surgery). All events were independently adjudicated by study investigators (A.A.Q., A.S., and M.H.) who were blinded to other study data, following criteria previously described by the Multi-Ethnic Study of Atherosclerosis.14 The main outcome of the study was a combined end point of MACEs, including cardiovascular death, myocardial infarction, unstable angina leading to revascularization, and hospitalization for HF.
Mental Stress Procedure
Patients were tested in the morning after a 12-hour fast. In a quiet, dimly lit, temperature-controlled (21-23°C) room, after a 30-minute rest period, vital signs were measured and mental stress was induced by a standardized public-speaking task.12 A complete description of the vascular function measurements appears in eAppendix 1 in the Supplement.
Biomarker Measurements
High-Sensitivity C-Reactive Protein
High-sensitivity C-reactive protein levels were measured from serum samples collected prestress (n = 554) using the electrochemiluminescence system by Meso Scale (Meso Scale Diagnostics) and the SECTOR Imager 2400 (Meso Scale Diagnostics). The lower limit of detection was 1.33 × 10−6 mg/L. The interassay and intra-assay coefficients of variation were 3.06% and 2.33%, respectively.
Epinephrine
Plasma epinephrine levels were measured from samples obtained prestress and 5 minutes after the mental stress test (n = 540 and 519, respectively) using the enzyme immunoassay kit (2-CAT ELISA [2-catecholamine enzyme-linked immunosorbent assay] [Labor Diagnostika Nord]). This assay has an analytical sensitivity of 7 pg/mL (to convert to picomoles per liter, multiply by 5.459).
Vascular Function Measurements
Endothelium-dependent brachial artery FMD was measured to evaluate conductance artery endothelial function using ultrasonography (Acuson 10-mHz linear-array transducer [Acuson]), as described previously,15,16,17 before and 30 minutes after the mental stress test. Analyzable data were available for most patients before (n = 577) and after mental stress (n = 569). A complete description of the vascular function measurements appears in eAppendix 2 in the Supplement.
CAD Severity Scoring
Quantitative angiographic scoring was performed using the Gensini score for the 495 patients who had angiographic data, with a median time between the angiogram and enrollment of 2.1 years (interquartile range, 1.0-4.7 years). The Gensini score quantifies CAD severity by a nonlinear point system for degree of luminal narrowing, along with a multiplier for specific coronary tree locations. For example, 1 point is equivalent to a 25% lesion in the right coronary artery. The score has prognostic importance.18
Statistical Analyses
Transient endothelial dysfunction with mental stress, or the δ FMD level, was defined as any decrease in FMD level with mental stress (ie, a poststress minus prestress FMD value <0). To examine differences in patient characteristics between those with vs without stress-induced transient endothelial dysfunction, we used 2-sample t tests or Wilcoxon tests for continuous variables and χ2 tests for categorical variables. We examined the change in vascular function measurements (FMD level, brachial artery diameter, velocity-time integral, and shear rate), hemodynamic parameters (systolic blood pressure, heart rate, and rate-pressure product) and catecholamine (epinephrine) values before and after mental stress, using linear mixed models for repeated measures.
To investigate the association between FMD and cardiovascular events, prestress, poststress, and δ FMD levels were examined as continuous variables in Fine and Gray subdistribution hazard models with noncardiovascular death treated as the competing risk.19 Selection of factors to be included in the models was based on prior evidence of an association with FMD or cardiovascular disease events.20 These factors included demographic factors (age, sex, and race), lifestyle and clinical risk factors known to affect endothelial function (smoking, body mass index [calculated as weight in kilograms divided by height in meters squared], dyslipidemia, diabetes, hypertension, HF, and high-sensitivity C-reactive protein), medications (β-blockers, calcium-channel blockers, and statins), vascular factors (brachial-artery diameter and shear rate), CAD severity (Gensini score), prior revascularization, and left ventricular ejection fraction. Models for δ FMD levels were also adjusted for prestress FMD levels.21 These analyses were repeated for allometrically scaled FMD levels. We also performed a subgroup analysis stratified by demographic and medical history factors and medication use, adjusted for the same factors.
Prestress and poststress FMD levels were also analyzed as categorical variables after dichotomizing them into high (≥median) vs low (<median) FMD outcomes. Using the cumulative incidence function homogeneity test of Gray,19 the cumulative incidence of the study end points were compared between the following groups: (1) those with prestress FMD measurements greater than the median vs prestress FMD less than the median; (2) those with poststress FMD greater than the median vs poststress FMD less than the median; (3) those with transient endothelial dysfunction with mental stress present (δ FMD <0) vs absent (δ FMD ≥0); and (4) those in 4 categories that combined prestress FMD (greater or less than the median) and transient endothelial dysfunction status (δ FMD <0).
The C statistic and net reclassification improvement at the mean event rate were calculated as a measure of risk discrimination.22,23,24,25 The significance level for both main effects and interactions was set at P < .05. All statistical analyses were conducted using SAS version 9.4 (SAS Institute). Data analysis took place from November 2018 to May 2019.
Results
Of the 695 enrolled patients, 569 (81.9%) completed both the prestress and poststress FMD protocols. Among these, 6 patients (1.1%) were lost to follow-up and were not included in the analytical sample. The mean (SD) age was 62.6 (9.3) years; 420 were men (73.8%), and 168 individuals were black (29.5%). Overall, 360 patients (63.3%) developed mental stress–induced transient endothelial dysfunction with mental stress (reduction in FMD from prestress). The risk factor profile of the participants is described in Table 1. The clinical characteristics of patients with transient endothelial dysfunction (compared with those without transient endothelial dysfunction) was similar, with the exception of β-blocker use, which was more prevalent in patients without transient endothelial dysfunction (165 [79.3%] vs 252 [70.2%]; P = .03) (Table 1).
Table 1. Characteristics of the Study Population by Mental Stress–Induced Transient Endothelial Dysfunction Status.
| Characteristic | Patients, No. (%) | P Value | ||
|---|---|---|---|---|
| Total | Transient Endothelial Dysfunction With Mental Stressa | |||
| Absent | Present | |||
| Total, No. | 569 | 209 | 360 | |
| Age, mean (SD), y | 62.6 (9.3) | 62.4 (9.5) | 62.6 (9.2) | .89 |
| Female | 149 (26.2) | 50 (23.9) | 99 (27.5) | .38 |
| Black race | 168 (29.5) | 58 (27.7) | 110 (30.5) | .54 |
| Years of school, mean (SD) | 15.1 (4.2) | 14.9 (5.1) | 15.2 (4.5) | .62 |
| Medical history and CAD risk factors | ||||
| Current smoking | 75 (13.2) | 21 (10.0) | 54 (15.0) | .51 |
| Diabetes | 187 (32.9) | 64 (30.1) | 128 (35.6) | .13 |
| Hypertension | 437 (76.8) | 158 (75.5) | 279 (77.5) | .49 |
| Dyslipidemia | 464 (81.5) | 169 (80.9) | 295 (81.9) | .83 |
| BMI, mean (SD) | 29.8 (4.9) | 28.8 (5.5) | 30.4 (5.3) | .44 |
| Lifetime history of major depression | 153 (26.8) | 47 (22.5) | 106 (29.5) | .07 |
| Prior myocardial infarction | 224 (38.9) | 74 (35.6) | 150 (41.7) | .15 |
| Heart failure | 134 (23.5) | 42 (20.6) | 92 (26.6) | .09 |
| Prior revascularization | 311 (54.7) | 111 (53.9) | 200 (55.8) | .55 |
| CAD severity (Gensini), median | 24 (10-59) | 23 (8-59) | 26 (10-57) | .71 |
| Ejection fraction, mean (SD) | 51.9 (12.9) | 50.4 (13.7) | 52.8 (12.3) | .10 |
| High-sensitivity C-reactive protein level, mean (SD), mg/L | 3.9 (3.7) | 3.7 (4.1) | 4.2 (3.8) | .43 |
| Medications | ||||
| Aspirin | 493 (86.9) | 186 (89.4) | 307 (85.3) | .20 |
| β-Blocker | 417 (73.8) | 165 (79.3) | 252 (70.2) | .03 |
| Calcium-channel blocker | 122 (21.6) | 49 (23.4) | 73 (20.3) | .34 |
| Angiotensin-converting enzyme inhibitors | 263 (46.4) | 98 (47.1) | 165 (46.2) | .78 |
| Antidepressants | 129 (22.8) | 54 (26.1) | 75 (21.0) | .11 |
| Statin use | 489 (86.4) | 183 (88.4) | 306 (85.4) | .32 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAD, coronary artery disease.
SI conversion factor: To convert high-sensitivity C-reactive protein to nmol/L, multiply by 9.524.
Transient endothelial dysfunction induced by mental stress was defined as any decrement in flow-mediated vasodilation with mental stress.
The mean (SD) prestress brachial artery diameter and FMD were 3.7 (0.9) mm and 4.8% (3.7%), respectively. Mental stress was associated with a significant decline in the prestress brachial artery diameter (by 0.2 [0.1] mm; 5% decline; P < .001) and FMD (by 0.9% [0.3%]; 23% decline; P < .001), but velocity-time integral and shear rate were unchanged (Table 2). During mental stress testing, there were significant increases in systolic blood pressure (mean [SD]: prestress level, 128 [18] mm Hg; poststress level, 169 [24] mm Hg; P < .001), heart rate (mean [SD]: prestress level, 61 [11] beats per minute; poststress level, 78 [15] beats per minute; P < .001), rate-pressure product (mean [SD]: prestress level, 7990 [1843] beats per minute × mm Hg; poststress level, 12 953 [3398] beats per minute × mm Hg; P < .001), and circulating epinephrine levels (mean [SD]: prestress level, 18.9 [21] pg/mL; poststress level, 32.2 [44] pg/mL; P < .001) (eTable 1 in the Supplement). In the bivariate analysis, there was no significant association between these changes and the changes in FMD levels, although prestress systolic blood pressure was significantly associated with lower FMD levels both at rest (β, −0.02 [SE, 0.008]; P = .01) and after stress (β, −0.01 [SE, 0.007]; P = .02) (eTable 2 in the Supplement).
Table 2. Hazard Ratios for Prestress Flow-Mediated Vasodilation, Poststress Flow-Mediated Vasodilation, and δ Flow-Mediated Vasodilation as Factors Associated With Major Adverse Cardiovascular Events in Unadjusted and Adjusted Fine and Gray Proportional Subdistribution Hazard Modelsa.
| Flow-Mediated Vasodilation (per 1% Decrement) | Prestress Flow-Mediated Vasodilation | Poststress Flow-Mediated Vasodilation | δ Flow-Mediated Vasodilation (Poststress Minus Prestress Level)b | |||
|---|---|---|---|---|---|---|
| sHR (95% CI)c | P Value | sHR (95% CI)c | P Value | sHR (95% CI)c | P Value | |
| Unadjusted | 1.00 (0.94-1.06) | .88 | 1.07 (1.01-1.13) | .04 | 1.11 (1.01-1.21) | .04 |
| Adjusted modeld | ||||||
| 1 | 1.00 (0.94-1.06) | .81 | 1.07 (1.01-1.13) | .04 | 1.12 (1.01-1.23) | .04 |
| 2 | 1.00 (0.95-1.05) | .92 | 1.11 (1.01-1.22) | .04 | 1.14 (1.02-1.27) | .03 |
| 3 | 1.02 (0.96-1.08) | .63 | 1.14 (1.01-1.28) | .04 | 1.14 (1.01-1.28) | .04 |
| 4e | 1.06 (0.98-1.14) | .18 | 1.16 (1.04-1.30) | .01 | 1.20 (1.06-1.37) | .006 |
Abbreviation: sHR, subdistribution hazard ratio.
A major adverse cardiovascular event was defined as any of the following: cardiovascular death, myocardial infarction, unstable angina with revascularization, and decompensated heart failure.
All the analyses with δ flow-mediated vasodilation were adjusted for prestress flow-mediated vasodilation.
Subdistribution hazard ratios represent the risk of end points per 1% decrement in flow-mediated vasodilation while treating noncardiovascular death as a competing risk.
Model 1 was adjusted for sex, African American race, and age. Model 2 was adjusted for model 1 covariates, plus hypertension, diabetes, dyslipidemia, prior myocardial infarction, heart failure, body mass index (continuous; calculated as weight in kilograms divided by height in meters squared), current smoking, high-sensitivity C-reactive protein, and medication use (β-blockers, calcium-channel blockers, and statins). Model 3 was adjusted for the covariates in models 1 and 2, plus vascular factors (baseline brachial artery diameter and shear rate). Model 4 was adjusted for the covariates in models 1, 2, and 3, plus coronary artery disease severity score (Gensini), prior revascularization, and ejection fraction.
Coronary artery disease severity (Gensini) score was only available for 495 patients.
Association of Prestress, Poststress, and δ FMD Levels With MACE
Patients were followed up for a median (interquartile range) of 3.0 (2.9-3.1) years. A total of 74 patients had adverse events, including 13 cardiovascular deaths, 15 myocardial infarctions, 34 unstable angina events followed by revascularization, and 12 hospitalizations for HF.
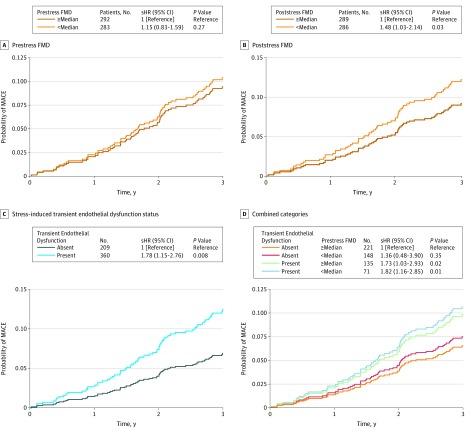
Prestress FMD levels were not associated with the composite MACE end point, before and after multivariable analysis, either as a continuous variable or when dichotomized into 2 groups by the median value (Table 2, Figure 1A). In contrast, poststress FMD levels were associated with MACE; for each 1% decrement in poststress FMD level, the MACE rate was 14% higher after full adjustment (sHR, 1.14 [95% CI, 1.01-1.28]; P = .04; Table 2). When dichotomized at the median, a low poststress FMD level (<median) was associated with an adjusted sHR of 1.48 (95% CI, 1.03-2.14; Figure 1B). The change in FMD level with stress (δ FMD) was also associated with MACE; for each 1% decline in FMD level with stress, the incidence of MACE was 17% higher after full adjustment (sHR, 1.17 [95% CI, 1.06-1.30]; Table 2). When examined as a categorical variable, presence of stress-induced transient endothelial dysfunction (any reduction of FMD level with stress) was associated with an adjusted 78% increased hazard of MACE (sHR, 1.78 [95% CI, 1.15-2.76]; Figure 1C). Furthermore, when transient endothelial dysfunction was evaluated jointly with prestress FMD level, there was an additive effect between transient endothelial dysfunction and a low prestress FMD level (sHR, 1.82 [95% CI, 1.16-2.85]; P = .01); this group had the highest risk of MACE (Figure 1D). These results remained unchanged when using allometrically scaled FMD measurements (prestress FMD: sHR, 1.03 [95% CI, 0.94-1.12]; P = .55; δ FMD: sHR, 1.18 [95% CI, 1.02-1.38]; P = .03; poststress FMD: sHR, 1.23 [95% CI, 1.04-1.45]; P = .01; eTable 3 in the Supplement). The sensitivity analysis showed that the association between δ FMD and the primary end point was generally similar across subgroups stratified by baseline demographics and clinical characteristics, although these results should be considered with caution, given the small number of events in some strata (eTable 4 in the Supplement).
Figure 1. Adjusted Cumulative Incidence of Major Adverse Cardiovascular Events (MACEs) According to Flow-Mediated Vasodilation (FMD) Levels.
Data stratified by prestress FMD (A), poststress FMD (B), stress-induced transient endothelial dysfunction status (C), and 4 categories combining information on prestress FMD and stress-induced transient endothelial dysfunction status (D). Major adverse cardiovascular events are defined as a combination of cardiovascular death, myocardial infarction, unstable angina with revascularization, and decompensated heart failure. Subdistribution hazard ratios (sHR) represent the risk of end points for the comparison vs the reference groups while treating noncardiovascular death as competing risks. P values were generated from the cumulative-incidence function homogeneity test of Gray.
Association of Prestress, Poststress, and δ FMD Values With Cardiovascular Death
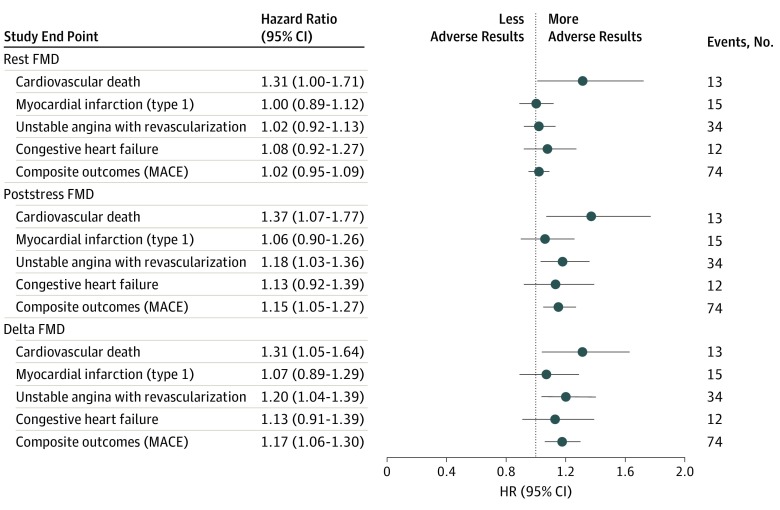
Prestress, poststress, and δ FMD levels were all significantly associated with cardiovascular death during follow-up. An adjusted 1% lower prestress (HR, 1.31 [95% CI, 1.00-1.71]), poststress (HR, 1.37 [95% CI, 1.07-1.77]), and δ FMD level (HR, 1.31 [95% CI, 1.05-1.64]) were all associated with more than a 30% increase in the hazard of cardiovascular death (Figure 2).
Figure 2. Multivariate Survival Analysis of the Associations of Prestress Measurements, Poststress Measurements, and δ Flow-Mediated Vasodilation with Study End Points.
δ Flow-mediated vasodilation (FMD) was defined as poststress minus prestress FMD. Subdistribution hazard ratios (HRs) represent the risk of end points per 1% decrement in FMD, while treating noncardiovascular death as competing risk. The δ FMD models were also adjusted for resting FMD. MACE indicates major adverse cardiovascular events.
Risk Discrimination
We tested the incremental value of adding prestress, poststress, or δ FMD levels to a model with traditional prognostic factors. The C statistic for incident MACE increased significantly when we added to the model poststress FMD (change in the area under the curve, 0.05 [95% CI, 0.01-0.09]) or prestress plus δ FMD (change in the area under the curve, 0.04 [95% CI, 0.00-0.08]; eTable 5 in the Supplement). The net reclassification improvement at the mean event rate showed significant reclassification of participant risk by addition of δ FMD (net reclassification improvement, 0.17 [95% CI, 0.05-0.29], Table 3).
Table 3. Net Reclassification Improvement at Event Rate of Anticipated Risk With the Addition of δ Flow-Mediated Vasodilationa.
| Anticipated Risk (Without δ Flow-Mediated Vasodilation)b | Reclassified Anticipated Risk (With δ Flow-Mediated Vasodilation) | Participants Reclassified, No. (%) | Net Correctly Reclassified, % | ||
|---|---|---|---|---|---|
| <13% | ≥13% | With Increased Risk | With Decreased Risk | ||
| Participants who experienced a MACE (n = 74) | |||||
| Low risk (<13%) | 49 | 17 | 17 (23) | 2 (3) | 20 |
| High risk (≥13%) | 2 | 6 | NA | NA | NA |
| Participants who did not experience a MACE (n = 465) | |||||
| Low risk (<13%) | 434 | 19 | 19 (4) | 6 (1) | 3 |
| High risk (≥13%) | 6 | 6 | NA | NA | NA |
| Net reclassification improvement (95% CI) | NA | NA | NA | NA | 0.17 (0.05-0.29) |
| P value | NA | NA | NA | NA | .008 |
Abbreviations: MACE, major adverse cardiovascular event; NA, not applicable.
A MACE was defined as any of the following: cardiovascular death, myocardial infarction, and unstable angina with revascularization and decompensated heart failure.
Anticipated risk based on traditional risk factors: sex, race, age (continuous), hypertension, dyslipidemia, prior myocardial infarction, heart failure, body mass index (continuous; calculated as weight in kilograms divided by height in meters squared), diabetes, smoking history, and high-sensitivity C-reactive protein level.
Discussion
To our knowledge, this is the first study to investigate the prognostic value of transient endothelial dysfunction induced by mental stress in patients with CAD. We show that a greater decrease in endothelium-dependent FMD provoked by mental stress is associated with higher rates of incident cardiovascular death and major adverse cardiovascular disease outcomes, independent of other patient characteristics and prestress FMD levels. These results point to endothelial responses to psychological stress as an important risk marker linking stress to adverse outcomes and disease progression in CAD patients.
Endothelial function assessment using FMD levels has emerged as a useful biomarker of cardiovascular risk both in populations with and without known CAD.7,8,9 However, endothelial function is a dynamic phenomenon, and, to our knowledge, prior prognostic studies have focused only on resting FMD measurements.7,8,9 In this study, we demonstrate that the FMD response to mental stress in patients with CAD is an even more important marker of long-term cardiovascular risk than the prestress value alone. The main end point was a composite MACE outcome including cardiovascular death, myocardial infarction, unstable angina with revascularization, and heart failure hospitalization. The decline in FMD level with mental stress, as well as poststress FMD, but not prestress FMD, were significantly associated with increased hazard of MACEs. When cardiovascular death alone was the outcome, however, prestress FMD levels, changes in FMD levels with stress, and poststress FMD levels were all associated with cardiac death. However, the total number of cardiovascular deaths was small.
Measuring FMD levels is a noninvasive method to quantify the degree of endothelial dysfunction during mental stress, which could be potentially used clinically in identifying patients with CAD at high risk of developing major adverse outcomes. It is believed that endothelial function assessments, such as FMD level, provide an index of the net composite injury to the vascular wall from exposure to risk factors, including their severity and lifetime exposure.17 Thus, in population studies,26 it appears to be a superior risk marker than the mere measurement of risk factors and can be considered as a barometer of total risk burden. However, previous studies20,27 have measured FMD levels in the resting state only and have largely focused on samples without overt CAD. In patients with CAD, the literature has been more mixed. These results demonstrate that the FMD response to mental stress is a powerful prognostic indicator for MACE in patients with CAD, and potentially more informative than resting FMD alone. The combination of prestress FMD and change in FMD with stress, but not prestress FMD alone, was an independent marker of risk of MACE and improved risk discrimination. These data suggest that dynamic changes in vascular function attributable to system perturbation with psychological stress can provide incremental prognostic information than FMD level at the steady state alone.
Transient impairment in endothelial function with mental stress is a known phenomenon,3,5,6 but the exact mechanisms through which it may influence the risk of adverse events in patients with CAD are unknown. A decrease in FMD levels with mental stress has been attributed to vasoconstriction and disruption of nitric oxide activity induced by sympathetic nervous system activation with emotional stress.28 This was evidenced in this study by the significant increases in heart rate, systolic blood pressure, and epinephrine release with the public-speaking task. Furthermore, patients taking β-blockers were less likely to display transient endothelial dysfunction with mental stress. This is in accordance with a recent meta-analysis that showed that β-blockers could blunt sympathetic effects on the vascular system and improve endothelial function compared with placebo.29 Other factors, such as immune response, oxidative stress, and/or endothelin release, may also contribute to the prolonged stress-induced endothelial dysfunction and, at the same time, accelerate cardiovascular risk.5,30,31
Clinical Implications
These findings have important clinical implications. In this study, we demonstrate that poststress FMD levels are more robustly associated with MACE than prestress measurements are. In addition, we have recently shown that mental stress promotes endothelium-dependent coronary vasoconstriction.32 Combined with prior observations that brachial arterial FMD levels reflect coronary vascular endothelial function,33 the current findings imply that the stress-induced transient-endothelial dysfunction in the peripheral circulation is reflected in the coronary vascular bed.34 Thus, to the extent that mental stress testing in the laboratory captures the physiological changes of mental stress in daily life, coronary endothelial function could represent an important mechanism linking daily emotional stress to cardiovascular outcomes. These results could lead to the development and validation of mental stress–testing methods in conjunction with vascular assessments that could be applied in the clinical care environment, and the assessment of future interventions to ameliorate endothelial responses to stress or their adverse consequences.
Strengths
This study has several strengths, including its large size, its prospective design, and the independent adjudication of outcome events, which allowed a rigorous investigation of the effects of changes in vascular function induced by mental stress on disease prognosis. Additionally, the experimental manipulation of the exposure (mental stress) allows a controlled assessment of the outcomes of stress on peripheral vascular changes.
Limitations
This study focused on patients with CAD, and thus the results cannot be generalized to people without CAD. In addition, the protocol of repeated FMD assessment before and after stress did not allow for the measurement of endothelium-independent vasodilation. Furthermore, because of the short half-life of plasma epinephrine, a time-associated curve, instead of a single point, may have better described its association with the hemodynamic parameters. Finally, since we used a laboratory-based mental stress test, further study is needed to determine whether the changes we observed during the laboratory protocol reflect endothelial function changes with stressors in everyday life.
Conclusions
In individuals with CAD, impairment of endothelium-dependent relaxation in conduit arteries provoked by mental stress, as assessed by FMD, is associated with adverse cardiovascular outcomes beyond traditional cardiovascular risk indicators and prestress FMD. These results highlight the role of endothelial dysfunction as a central player linking emotional stress to atherosclerosis progression and worse clinical outcomes in patients with CAD.
eAppendix 1. Mental stress procedure
eAppendix 2. Vascular function measurements
eTable 1. Vascular and Hemodynamic Responses to Mental Stress
eTable 2. Age and Sex Adjusted Analysis of the Relationships Between Flow-Mediated Vasodilation (FMD) and the Hemodynamic Measurements Pre- and Post-Mental Stress
eTable 3. Hazards Ratios for Allometrically-Scaled Pre-stress FMD, Post-stress FMD and Delta FMD as Predictors of Major Adverse Cardiovascular Events (MACE) in Unadjusted and Adjusted Fine & Gray’s Proportional Sub-Distribution Hazards Models
eTable 4. Subgroup Analysis for the Association Between Delta FMD (Post minus Pre-Stress) and Major Adverse Cardiac Events (MACE) at 3-Years Follow-up
eTable 5. Discrimination Improvement of Statistical Models Associated with Major Adverse Cardiovascular Events (MACE) Including Vascular Parameters in Combination with Traditional Prognostic Factors*
References
- 1.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026-1032. doi: 10.1161/HYPERTENSIONAHA.109.146621 [DOI] [PubMed] [Google Scholar]
- 2.Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA, Liu K. Chronic stress and endothelial dysfunction: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2017;30(1):75-80. doi: 10.1093/ajh/hpw103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammadah M, Alkhoder A, Al Mheid I, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47-53. doi: 10.1016/j.ijcard.2017.05.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiya E, Watanabe M, Komuro I. The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 2014;7(2):109-119. doi: 10.3400/avd.ra.14-00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spieker LE, Hürlimann D, Ruschitzka F, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105(24):2817-2820. doi: 10.1161/01.CIR.0000021598.15895.34 [DOI] [PubMed] [Google Scholar]
- 6.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473-2478. doi: 10.1161/01.CIR.102.20.2473 [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15(7):736-746. doi: 10.1093/ehjci/jet256 [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4(11):e002270. doi: 10.1161/JAHA.115.002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344-351. doi: 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- 10.Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15(4):215-229. doi: 10.1038/nrcardio.2017.189 [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life: association and hemodynamic features. Circulation. 1995;92(8):2102-2108. doi: 10.1161/01.CIR.92.8.2102 [DOI] [PubMed] [Google Scholar]
- 12.Hammadah M, Al Mheid I, Wilmot K, et al. The mental stress ischemia prognosis study: objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79(3):311-317. doi: 10.1097/PSY.0000000000000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First M, Spitzer R, Williams J, Gibbons M. Structured Clinical Interview for DSM-IV—Patient Version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 14.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643-1653. doi: 10.1016/j.jacc.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrigan FE III, Al Mheid I, Eapen DJ, et al. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94-99. doi: 10.1016/j.ijcard.2015.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Mheid I, Corrigan F, Shirazi F, et al. Circadian variation in vascular function and regenerative capacity in healthy humans. J Am Heart Assoc. 2014;3(3):e000845. doi: 10.1161/JAHA.114.000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, et al. ; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257-265. doi: 10.1016/S0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 18.Ramadan R, Sheps D, Esteves F, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2(5):e000321. doi: 10.1161/JAHA.113.000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;18(3):1141-1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 20.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502-509. doi: 10.1161/CIRCULATIONAHA.109.864801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu R, Chen L. The need to control for regression to the mean in social psychology studies. Front Psychol. 2015;5:1574. doi: 10.3389/fpsyg.2014.01574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32(14):2430-2442. doi: 10.1002/sim.5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11-21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753-767. doi: 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45(12):1987-1993. doi: 10.1016/j.jacc.2005.02.073 [DOI] [PubMed] [Google Scholar]
- 28.Santos AC, Alves MJ, Rondon MU, Barretto AC, Middlekauff HR, Negrão CE. Sympathetic activation restrains endothelium-mediated muscle vasodilatation in heart failure patients. Am J Physiol Heart Circ Physiol. 2005;289(2):H593-H599. doi: 10.1152/ajpheart.01240.2004 [DOI] [PubMed] [Google Scholar]
- 29.Peller M, Ozierański K, Balsam P, Grabowski M, Filipiak KJ, Opolski G. Influence of beta-blockers on endothelial function: a meta-analysis of randomized controlled trials. Cardiol J. 2015;22(6):708-716. doi: 10.5603/CJ.a2015.0042 [DOI] [PubMed] [Google Scholar]
- 30.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO III, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol. 1997;80(8):1070-1074. doi: 10.1016/S0002-9149(97)00605-X [DOI] [PubMed] [Google Scholar]
- 31.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017;64:208-219. doi: 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadah M, Kim JH, Al Mheid I, et al. Coronary and peripheral vasomotor responses to mental stress. J Am Heart Assoc. 2018;7(10):e008532. doi: 10.1161/JAHA.118.008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuo S, Matsumoto T, Takashima H, et al. The relationship between flow-mediated brachial artery vasodilation and coronary vasomotor responses to bradykinin: comparison with those to acetylcholine. J Cardiovasc Pharmacol. 2004;44(2):164-170. doi: 10.1097/00005344-200408000-00004 [DOI] [PubMed] [Google Scholar]
- 34.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235-1241. doi: 10.1016/0735-1097(95)00327-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Mental stress procedure
eAppendix 2. Vascular function measurements
eTable 1. Vascular and Hemodynamic Responses to Mental Stress
eTable 2. Age and Sex Adjusted Analysis of the Relationships Between Flow-Mediated Vasodilation (FMD) and the Hemodynamic Measurements Pre- and Post-Mental Stress
eTable 3. Hazards Ratios for Allometrically-Scaled Pre-stress FMD, Post-stress FMD and Delta FMD as Predictors of Major Adverse Cardiovascular Events (MACE) in Unadjusted and Adjusted Fine & Gray’s Proportional Sub-Distribution Hazards Models
eTable 4. Subgroup Analysis for the Association Between Delta FMD (Post minus Pre-Stress) and Major Adverse Cardiac Events (MACE) at 3-Years Follow-up
eTable 5. Discrimination Improvement of Statistical Models Associated with Major Adverse Cardiovascular Events (MACE) Including Vascular Parameters in Combination with Traditional Prognostic Factors*