Abstract
Objectives
To compare percutaneous mitral valve repair (PMVR) with optimal medical therapy (OMT) in patients with heart failure (HF) and severe functional mitral regurgitation (FMR).
Background
Many patients with HF and FMR are not suitable for surgical valve replacement and remain symptomatic despite maximal OMT. PMVR has recently emerged as an alternative solution.
Methods
We performed a systematic review and a meta-analysis to address this question. Cochrane CENTRAL, MEDLINE, and Scopus were searched for randomized (RCT) and nonrandomized studies comparing PMVR with OMT in patients with HF and FMR. Primary endpoint was all-cause midterm mortality (at 1 and 2 years). Secondary endpoints were 30-day mortality and cardiovascular mortality and HF hospitalizations, at maximum follow-up. Studies including mixed cohort of degenerative and functional MR were allowed initially but were excluded in a secondary sensitivity analysis for each of the study's end points. This meta-analysis was performed following the publication of two RCTs (MITRA-FR and COAPT).
Results
Eight studies (six observational, two RCTs) comprising 3,009 patients were included in the meta-analysis. In comparison with OMT, PMVR significantly reduced 1-year mortality (RR: 0.70 [0.56, 0.87]; p=0.002; I2=47.6%), 2-year mortality (RR: 0.63 [0.55, 0.73]; p<0.001; I2=0%), and cardiovascular mortality (RR: 0.32 [0.23, 0.44]; p<0.001; I2=0%). No significant difference between PMVR+OMT and OMT was noted in HF hospitalization (HR: 0.69 [0.40, 1.20]; p=0.19; I2=85%) and 30-day mortality (RR: 1.13 [0.68, 1.87]; p=0.16; I2=0%).
Conclusions
In comparison with OMT, PMVR significantly reduces 1-year mortality, 2-year mortality, and cardiovascular mortality in patients with HF and severe MR.
1. Introduction
Functional mitral regurgitation (FMR) is seen in most patients with heart failure (HF) and is classified as moderate to severe in 30% of them [1, 2]. The presence of significant FMR in patients with left ventricular dysfunction is associated with adverse outcomes, including death and frequent hospitalization for HF [3–5]. Optimal medical therapy (OMT) may provide symptomatic relief in some patients but many remain symptomatic despite maximal OMT [6]. Contemporary surgical mitral repair and replacement operations are performed with excellent short-term outcomes. However, only a minority of patients with FMR are referred for isolated mitral valve repair or replacement due to the lack of compelling data proving the long-term efficacy of surgical interventions for FMR [7, 8]. The emergence of percutaneous mitral valve repair (PMVR) was accompanied with a wealth of clinical investigations aiming to assess its value in addressing the unmet need of treating severe symptomatic FMR in HF patients [9, 10]. Several studies have demonstrated the safety and efficacy of the MitraClip (Abbott Vascular, Lake Bluff, Illinois) in patients with FMR [11–13]. However, only a few studies compared the outcomes of PMVR with MitraClip to OMT. We hence performed a systematic review and a meta-analysis to address these questions.
2. Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines and the American Heart Association guidelines [14]. We utilized the relevant keywords “MitraClip”, “percutaneous mitral valve repair”, and “transcatheter mitral valve repair” in conjunction with MeSH terms to search MEDLINE, Cochrane, CENTRAL, and Scopus databases. The search was conducted from inception of the databases to September 25, 2018 (Supplemental Table 1). A supplementary search was done using citation chasing from relevant articles and hand searching of journals. Sources for the supplementary search included bibliographies of relevant reviews, editorials from major medical journals, websites of major journals, and conference proceedings for indexed abstracts. No language restrictions were placed.
All retrieved articles were transferred to EndNote X7 (Clarivate Analytics, Pennsylvania, United States) and duplicates were identified and removed. The remaining articles were screened by 2 reviewers (MUL and MSU) based on title and abstract. A third reviewer (MSK) was consulted to resolve discrepancies. Articles were selected based on the following eligibility criteria: (I) PMVR was compared with OMT in adult population (age ≥18 years) and at least 70% of the patients had heart failure complicated by functional MR. Study data were sought from the full texts of the included articles. Data were abstracted on study characteristics, baseline variables of patients, and outcomes of interest. In case cohorts of patients overlapped between studies, we included the study with the larger sample size in the analysis. When available, data from propensity-matched cohorts was preferred over unmatched data. The primary outcome was midterm all-cause mortality measured at 1- and 2-year intervals. The secondary outcomes were 30-day mortality, HF related hospitalizations, and cardiovascular death.
Risk of bias was assessed by two independent reviewers (MSU and MAAK), and a third reviewer was consulted to solve disagreements. Cochrane Collaboration's risk of bias 2.0 (ROB 2.0) tool was used to ascertain the risk of bias of the RCTs while the “Risk of Bias In Nonrandomized Studies-of Interventions” (ROBINS-I) tool was used to assess the risk of bias of observational studies.
Review manager (v.5.3) and Open MetaAnalyst were used to perform the analysis. For the mortality outcomes, odd ratio and 95% confidence intervals (CIs) were calculated using raw, unadjusted data from each included study. For HF hospitalization, the hazard ratios (HRs) provided by the studies were converted to generic inverse variances and standard errors and used as the effect size. The ORs/HRs were pooled using a random-effects model because of anticipated heterogeneity. Subgroup analysis according to type of study (observational versus RCTs) was conducted, and the chi-squared test was used to evaluate subgroup differences. Leave-one-out sensitivity analysis was conducted for all outcomes to assess if any single study disproportionately influenced the results. In order to study a cohort exclusively composed of patients with functional MR, we conducted a sensitivity analysis by removing studies with both functional MR and degenerative MR patients. Furthermore, we carried out a cumulative meta-analysis on primary outcome to study temporal trends. This chronological meta-analysis reveals if there is a consistency in the results of consecutive studies and indicates the point at which no further studies are necessary because the results continually favor 1 intervention. A secondary analysis was conducted to estimate the pooled risk difference between the PMVR and OMT groups per 1000-patient years and subsequently calculate the Number Needed to Treat (NNT) to prevent mortality. Heterogeneity across studies was evaluated using the I2 index, and a value of I2=25%-50% was considered mild, 50%-75% moderate, and >75% severe. Visual inspection of the funnel plot and Egger's regression test were used to assess publication bias. A p value of <0.05 was considered significant in all cases.
3. Results
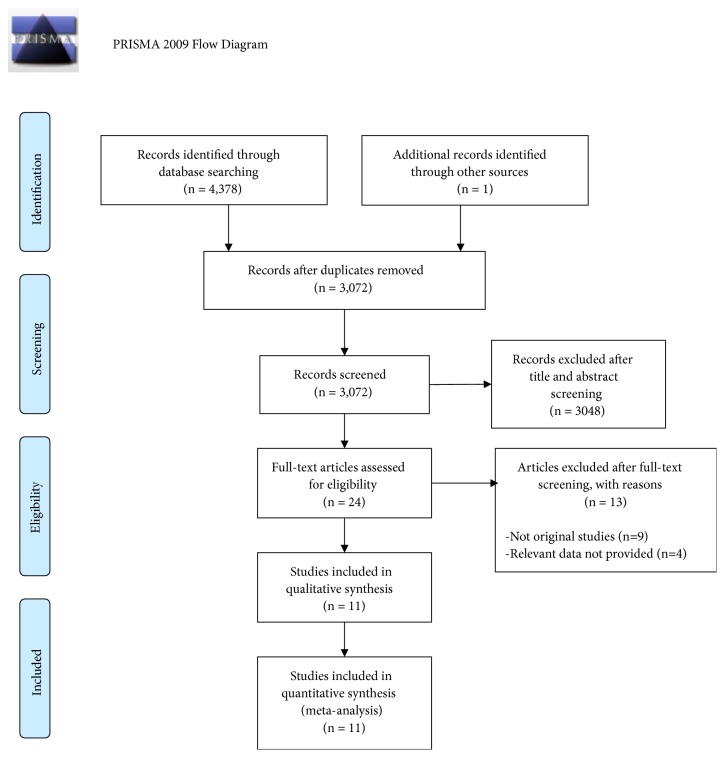
The initial search revealed 4,379 potentially relevant articles. After excluding duplicates and nonrelevant or incomplete publications (abstracts), 8 primary studies including 3,009 patients (1,689 in the PMVR arm, and 1320 in the OMT arm) were used in the synthetic analysis (Figure 1) [15–22].
Figure 1.
Study flow chart.
3.1. Quality Assessment
All included observational studies were of moderately good methodological quality (Supplementary Tables 2 and 3). Five of the observational studies used propensity-matched analysis. Although both included RCTs had a robust methodology, there was a risk of bias due to lack of allocation concealment and lack of blinding in these studies.
3.2. Patient and Study Characteristics
The average age of the included patients was 72 years, and 62% of them were male. The average LVEF of the population was 33%, and 69% of them were classified as New-York-Heart-Association class III or IV. More than half (53%) of the study population had been diagnosed with coronary artery disease, and 48% had a history of atrial fibrillation. Baseline characteristics are outlined in Table 1. A summary of the inclusion criteria and study characteristics are given in Table 2.
Table 1.
Baseline characteristics of included patients.
| Author (year) | Average Age | Average MR Grade | Male Sex % | Previous Afib % | NYHA III-IV (%) | Average LVEF % |
|---|---|---|---|---|---|---|
| Overall (Treatment/Control) | Overall (Treatment/Control) | Overall (Treatment/Control) | Overall (Treatment/Control) | Overall (Treatment/Control) | ||
| Velazquez, 2015 | 73.7 (73.7) | 3-4+ | 57.1 (59.8/54.4) | 33 (33/33) | 33.21 (34/32) | |
| Asgar, 2016 | 71.8 (75/68) | 3-4+ | 75.37 (74/77) | 39 (35/43) | 74.5 (73/76) | 36 (37/35) |
| Armeni, 2016 | 71 (71/71) | 73.39 (73/74) | 49.48 (53/40.7) | 87.87 (88.5/86.4) | 36.11 (36.8/34.5) | |
| Giannini, 2016 | 75.5 (75/76) | 3-4+ | 66.5 (70/63) | 61.55 (64.9/58.2) | 79 (78.2/79.8) | 41.75 (41.5/42) |
| Geis, 2017 | 62.01 (68.2/54.3) | 41.57 (74.4/0.65) | 58.82 (61.6/52.8) | 54.65 (54.4/55.2) | ||
| Obadia, 2018 | 70.35 (70.1/70.6) | 3-4+ | 74.65 (78.9/70.4) | 51.29 (55.6/43) | 56.8 (86.3/61.9) | 36.07 (37.22/33.85) |
| Kortlandt, 2018 | 74.02 (73.96/74.15) | 3-4+ | 54.93 (56.5/51.9) | 46.3 (65.0/23) | 25.45 (25/26) | |
| Stone, 2018 | 72.26 (71.7/72.8) | 3-4+ | 64.01 (66.6/61.5) | 55.22 (57.3/53.2) | 60.86 (57/64.6) | 31.3 (31.3/31/3) |
MR: mitral regurgitation; Afib: atrial fibrillation; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction.
Table 2.
Characteristics of the included studies.
| Author (year) | Total Participants | Participants | Participants | Follow-up, weeks | Primary endpoints |
|---|---|---|---|---|---|
| (# of FMR) | Treatment Group | Control Group | |||
| Velazquez, 2015 | 478 (415 FMR) | 239 | 239 | 48 | All-cause mortality |
| Asgar, 2016 | 92 (45 FMR) | 50 | 42 | 144 | All-cause mortality; Cost-effectiveness metrics |
| Armeni, 2016 | 383 (383 FMR) | 232 | 151 | 48 | Cost-effectiveness metrics |
| Giannini, 2016 | 120 (NR) | 60 | 60 | 48 | All-cause mortality |
| Geis, 2017 | 155 (124 FMR) | 86 | 69 | 48 | Cardiac remodeling metrics (LVEF, LVESD) |
| Obaida, 2018 | 304 (304 FMR) | 152 | 152 | 240 | Composite: all-cause mortality and HF hospitalizations |
| Kortlandt, 2018 | 863 (593 FMR) | 568 | 295 | 96 | All cause mortality |
| Stone, 2018 | 614 (614 FMR) | 302 | 312 | 96 | HF hospitalizations at 24 months, device related complications at 12 months |
FMR: functional mitral regurgitation; LVEF: left ventricular ejection fraction; LVESD: left ventricular end systolic diameter
3.3. Meta-Analysis of 1-Year Mortality
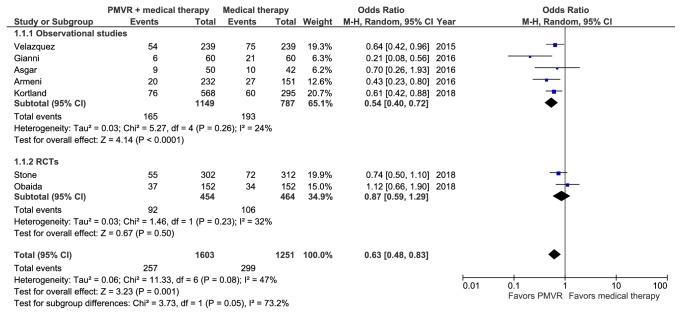
Seven studies representing 2,854 patients reported all-cause mortality at 1 year [15, 16, 18–21, 23]. A meta-analysis of these studies showed that PMVR significantly reduced 1-year all-cause mortality in comparison with OMT (RR: 0.70 [0.56, 0.87]; p=0.002; I2=47.6%) (Figure 2). Observational studies corroborated the overall finding, showing significant change in relative risk of 0.61 ([0.48, 0.78]; p<0.001; I2=26.3%). In contrast the results from RCTs were nonsignificant (RR: 0.90 [0.66, 1.23]; p=0.51; I2=33.3%). However, the difference between the two subgroups was nonsignificant (p interaction >0.05). Sensitivity analysis by removing studies including degenerative MR patients did not significantly change the results (RR: 0.76 [0.59, 0.99]; p=0.043; I2=48.8%).
Figure 2.
Forest plot displaying the risk of 1-year mortality in the PMVR group compared to the OMT group.
3.4. Meta-Analysis of 2-Year Mortality
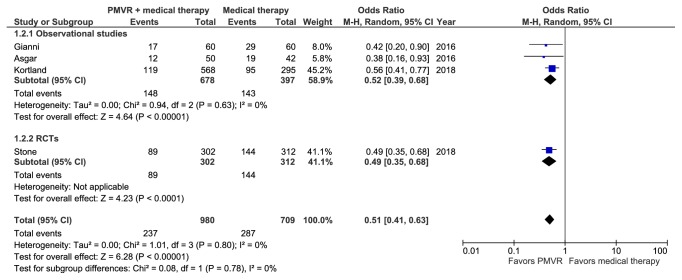
Four studies including 1,689 patients reported all-cause mortality rates at 2 years [16, 19, 21, 23]. A meta-analysis of these studies showed that PMVR was superior to OMT alone in reducing 2-year mortality (RR: 0.63 [0.55, 0.73]; p<0.001; I2=0%) (Figure 3). Both observational studies (RR: 0.63 [0.51, 0.76]; p<0.001; I2=0%) and the single RCT (RR: 0.64 [0.52, 0.79]; p<0.001) corroborated with the overall result (p interaction > 0.05). Sensitivity analysis removing studies that included patients with degenerative MR did not significantly change the results (RR: 0.47 [0.35, 0.62]; p<0.001; I2=0%).
Figure 3.
Forest plot displaying the risk of 2-year mortality in the PMVR group compared to the OMT group.
3.5. Meta-Analysis of 30-Day Mortality
Six studies comprising 2,064 patients reported all-cause mortality at 30 days [15, 17, 18, 20–22]. A meta-analysis of these studies showed no significant difference between the PMVR and OMT groups (RR: 1.13 [0.68, 1.87]; p=0.16; I2=0%) (Figure 4). Results from both observational studies (RR: 1.0 [0.49, 2.02]; p=0.42; I2=11.4%) and RCTs (RR: 1.72 [0.66, 4.36]; p=0.26; I2=0%) were nonsignificant (p interaction >0.05). Sensitivity analysis by removing studies comprising patients with degenerative MR did not significantly change the results (RR: 1.38 [0.62, 3.07]; p=0.43; I2=0%).
Figure 4.
Meta-analysis displaying the risk of 30-day mortality in the PMVR group compared to the OMT group.
3.6. Meta-Analysis of Cardiovascular Mortality
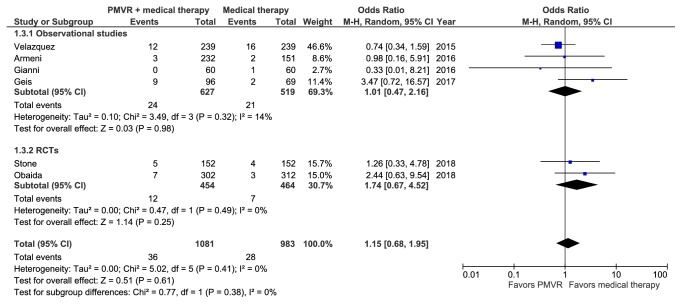
Four studies (representing 1,236 patients) reported cardiovascular mortality [15, 17, 20, 21]. During a mean follow-up of 1.54 years, PMVR significantly reduced cardiovascular mortality in comparison to OMT (RR: 0.53 [0.31, 0.91]; p=0.021; I2=85.6%) (Figure 5). Pooled observational studies also showed significant reduction in cardiovascular mortality (RR: 0.32 [0.23, 0.44]; p<0.001; I2=0%). Pooled RCTs, on the other hand, did not show significant reduction (RR: 0.81 [0.50, 1.31]; p=0.38; I2=71.5%) (p interaction < 0.05). The results became nonsignificant (RR: 0.65 [0.38, 1.09]; p=0.10; I2=76.7%) upon removing studies, which included patients with degenerative FMR.
Figure 5.
Forest plot displaying the risk of cardiovascular mortality in the PMVR group compared to the OMT group.
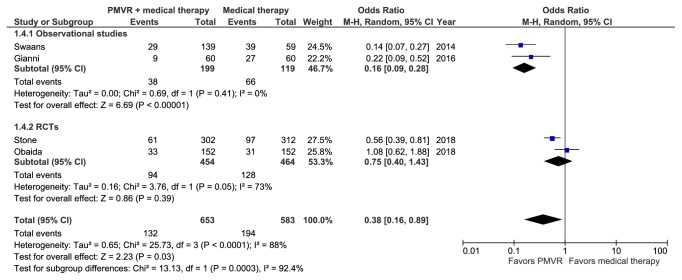
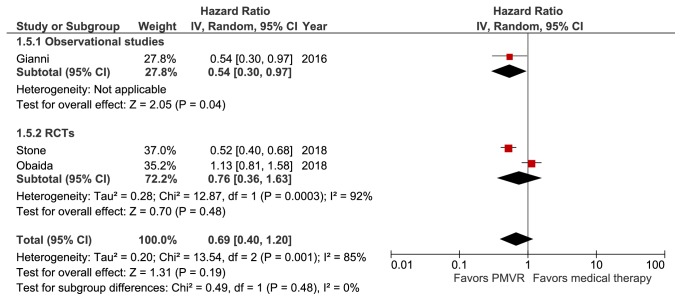
3.7. Meta-Analysis of HF Hospitalizations
Three studies (representing 1,038 patients) reported HF hospitalization at a mean follow-up of 1.64 years [20, 21, 23]. None of the studies included patients with degenerative MR. There was no significant difference in the incidence of HF hospitalization between patients treated with PMVR + OMT versus those who were treated with OMT alone (HR: 0.69 [0.40, 1.20]; p=0.19; I2=85%) (Figure 6). The difference between results from the observational study (HR: 0.54 [0.30, 0.97]; p=0.04) and RCTs (HR: 0.76 [0.36, 1.63]; p=0.48; I2=92%) was nonsignificant (p>0.05).
Figure 6.
Forest plot displaying the incidence of heart failure hospitalization in the PMVR group compared to the OMT group.
3.8. Pooled Risk Difference and Number Needed to Treat
The risk difference for all-cause mortality was -61.3 events per 1000-patient years and a Number Needed to Treat of nine to prevent one death per year. The risk difference for cardiovascular mortality was -53.7 events per 1000-patient years, with a Number Needed to Treat of five to prevent one cardiovascular death per year (Supplementary Table 4).
3.9. Leave-One-Out Meta-Analysis
The results for 30-day, 1-year, and 2-year mortality were robust, with no single study having a disproportionate effect on the results (Supplementary Figures 1-3). The cardiovascular mortality outcome became nonsignificant upon removal of all studies except one (Obadia, 2018) (Supplementary Figure 4).
3.10. Cumulative Meta-Analysis
A temporal trend towards higher mortality with PMVR (higher RRs) was seen for the 30-day mortality outcome. Lack of consistency for this outcome highlights the uncertainty of current evidence. For 1-year, 2-year, and cardiovascular mortality, no clear temporal shift in the results was seen (Supplementary Figures 5-8).
3.11. Publication Bias
The funnel plot (Supplementary Figure 9) suggested presence of publication bias. The vacant right, lower quadrant suggested that missing studies would have been of small size and could have possibly shown increased mortality with PMVR. Presence of publication bias was confirmed by Eggers regression test (p=0.009).
4. Discussion
This meta-analysis of more than 3000 patients with HF and severe MR shows that PMVR significantly reduces midterm all-cause and cardiovascular mortality compared with OMT alone. However, HF hospitalizations and 30-day mortality were not significantly different between the two groups. A previous meta-analysis has shown a similar reduction in all-cause mortality but also showed a reduction in HF hospitalizations with PMVR [23]. Nonetheless, this study was conducted prior to the publication of two recent RCTs (MITRA-FR and COAPT) [20, 21]. Our updated meta-analysis significantly enhances the evidence from the previous one, with an additional 888 patients included from one observational and two RCTs.
Functional mitral regurgitation results from left ventricular dilation and/or regional wall dyskinesis leading to dislocation of the papillary muscles and tethering of the leaflets. The differential negative impact of FMR on patient's symptomatology, progressive remodeling, and long-term outcomes of patients with HF has been long established [24, 25]. However, the ideal treatment for patients with FMR who remain symptomatic despite OMT has been an area of intense debate. Surgical mitral valve repair or replacement can successfully eliminate FMR, but neither intervention has been shown to reduce the morbidity and mortality associated with FMR [26, 27]. In addition, both approaches are associated with significant early risk of death and postoperative complications [27–29]. The emergence of transcatheter mitral valve repair and replacements systems led to a plethora of investigations assessing their utility specifically in patients with FMR.
The MitraClip system is the first PMVR system to become commercially available. Although the MitraClip was initially approved to treat degenerative (primary) mitral regurgitation, many FMR patients were treated with the MitraClip on an off-label basis [12, 13]. This has led to a substantial body of evidence suggesting its safety and efficacy in this challenging group of patients. Nonetheless, data comparing PMVR to OMT remained limited. A previous meta-analysis addressing the same question found beneficial effects for PMVR in FMR patients but it only included observational data [23]. In light of the recent publication of the first landmark RCTs comparing OMT to PMVR, we sought to perform an updated systematic review and a meta-analysis to elucidate the best available evidence on the key question of whether PMVR carries an incremental benefit over OMT alone in patients with FMR. Our analysis revealed that PMVR with the MitraClip decreased all-cause mortality by 30% at 1 year and by 37% at 2 years. While the 1-year mortality benefit was mostly driven by observational data, the 2-year benefit was corroborated in both observational studies and the single RCT reporting 2-year mortality data. In addition, PMVR was associated with lower cardiovascular death and no excess short-term mortality (at 30 days).
The findings of this meta-analysis raise several important issues: (1) FMR is not a one-size-fits-all entity. This is best illustrated by the striking differences between the COAPT and MITRA-FR RCTs. Both of these trials were set to address the same questions (utility of PMVR in FMR) and reached strikingly different conclusions. This is likely because each study enrolled a different subset of FMR patients. The MITRA-FR trial enrolled patients with severely dilated ventricles (no limit of left ventricular dimensions) and less degrees of FMR (effective regurgitant orifice area>20 mm2, regurgitant volume >30 ml/beat), while COAPT only allowed patients with less dilated ventricles (left ventricular diastolic dimension <7 cm) and higher degrees of FMR (effective regurgitant orifice area>30 mm2, regurgitant volume >45 ml/beat). In other words, COAPT likely selected patients in whom the valvular disease was a large component of their pathology while the valve disease was likely a pure bystander in MITRA-FR. Hence, the substantial benefits of PMVR observed in COAPT versus MITRA-FR are not surprising. (2) The underlying mechanism by which PMVR substantially improved the outcomes of FMR patients remains a subject of study. However, this meta-analysis might confirm the earlier observations suggesting a major role of PMVR-induced reverse remodeling in improving long-term outcomes of patients with FMR [12, 30]. (3) The mortality benefit that was observed in our meta-analysis was robust but was more consistent at 2 years (I2=0), suggesting that longer-term follow-up might be needed in future PMVR investigations to elucidate the potential benefit of the therapy in FMR patients. (4) The lack of reduction in HF hospitalization should be interpreted with caution, due to the limited number of studies reporting this endpoint and the potential subjectivity of this endpoint itself.
Limitations: certain limitations must be taken into consideration when interpreting the results of this study. Firstly, the results of this analysis were partially based on observational studies, which are relatively more susceptible to bias due to confounding. It must be noted, however, that we found all observational studies to be of robust methodological quality, with most employing propensity-matched analysis. Second, the results for the cardiovascular mortality and HF hospitalization outcomes had significant heterogeneity, which could not be explained by subgroups according to study design. Third, using RRs stratified according to time (1 and 2 years) for the mortality outcome could potentially lead to an overestimation of the effect size when compared to time-to-event effect sizes. Fourth, variation in follow-up time was not accounted for in the cardiovascular mortality outcome, which could have led to some bias.
5. Conclusions
This meta-analysis suggests that, in comparison with OMT, PMVR is not associated with excess 30-day mortality and significantly reduce all-cause mortality at 1 and 2 years. Given the heterogeneity in the included FMR populations, further studies are needed to confirm the results of this meta-analysis and to identify to ideal candidate for PMVR.
Data Availability
All the data used in the analysis is presented within the manuscript.
Disclosure
Muhammad Uzair Lodhi and Muhammad Shariq Usman are co-first authors. All authors listed below confirm that each author listed here meets the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors. Each author has made a contribution to three essential elements for authorship: concept or design, data collection or analysis, and writing or editing. Each author has seen and approved this manuscript and confirms that the data that are presented in the figures and tables are accurate and the analyses and statistics applied are appropriate, all journal policies have been adhered to, and colleagues listed as coauthors have contributed and deserve the designation “author”.
Conflicts of Interest
None of the authors have any conflicts of interest to declare.
Supplementary Materials
Supplementary Table S1: search strategy used in each database searched. Supplementary Table S2: Quality Assessment of the Observational Studies using the Robins-1 tool. Supplementary Table S3: Quality Assessment of the Randomized Studies using the ROB 2.0 scale. Supplementary Table S4: Pooled Analysis for the Number Needed to Treat. Supplementary Figure-1: leave-one-out meta-analysis for the 1-year mortality outcome. Supplementary Figure-2: leave-one-out meta-analysis for the 2-year mortality outcome. Supplementary Figure-3: leave-one-out meta-analysis for the 30-day mortality outcome. Supplementary Figure-4: leave-one-out meta-analysis for the cardiovascular mortality outcome. Supplementary Figure-5: cumulative meta-analysis for the 1-year mortality outcome. Supplementary Figure-6: cumulative meta-analysis for the 2-year mortality outcome. Supplementary Figure-7: Cumulative meta-analysis for the 30-day mortality outcome. Supplementary Figure-8: Cumulative meta-analysis for the cardiovascular mortality outcome. Supplementary Figure-9: funnel plot for the heterogeneity of the included studies.
References
- 1.Di Salvo T. G., Acker M. A., Dec G. W., Byrne J. G. Mitral valve surgery in advanced heart failure. Journal of the American College of Cardiology. 2010;55(4):271–282. doi: 10.1016/j.jacc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 2.Trichon B. H., Felker G. M., Shaw L. K., Cabell C. H., O'Connor C. M. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. American Journal of Cardiology. 2003;91(5):538–543. doi: 10.1016/S0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 3.Baskett R. J. F., Exner D. V., Hirsch G. M., Ghali W. A. Mitral insufficiency and morbidity and mortality in left ventricular dysfunction. Canadian Journal of Cardiology. 2007;23(10):797–800. doi: 10.1016/S0828-282X(07)70830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mowakeaa S., Dwivedi A., Grossman J. R., et al. Prognosis of patients with secondary mitral regurgitation and reduced ejection fraction. Open Heart. 2018;5(1) doi: 10.1136/openhrt-2017-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cioffi G., Tarantini L., De Feo S., et al. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. European Journal of Heart Failure. 2005;7(7):1112–1117. doi: 10.1016/j.ejheart.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Stone G. W., Vahanian A. S., Adams D. H., et al. Mitral valve academic research C. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 1: clinical trial design principles a consensus document from the mitral valve academic research consortium. Journal of the American College of Cardiology. 2015;66(3):278–307. doi: 10.1016/j.jacc.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Goel S. S., Bajaj N., Aggarwal B., et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: Comprehensive analysis to determine the potential role of mitraclip for this unmet need. Journal of the American College of Cardiology. 2014;63(2):185–186. doi: 10.1016/j.jacc.2013.08.723. [DOI] [PubMed] [Google Scholar]
- 8.Rankin J. S., Grau-Sepulveda M., Shahian D. M., et al. The impact of mitral disease etiology on operative mortality after mitral valve operations. The Annals of Thoracic Surgery. 2018;106(5):1406–1413. doi: 10.1016/j.athoracsur.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 9.Badhwar V., Thourani V. H., Ailawadi G., Mack M. Transcatheter mitral valve therapy: The event horizon. The Journal of Thoracic and Cardiovascular Surgery. 2016;152(2):330–336. doi: 10.1016/j.jtcvs.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Testa L., Latib A., Montone R. A., Bedogni F. Transcatheter mitral valve regurgitation treatment: State of the art and a glimpse to the future. The Journal of Thoracic and Cardiovascular Surgery. 2016;152(2):319–327. doi: 10.1016/j.jtcvs.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Godino C., Scotti A., Munafò A., et al. Observed versus predicted mortality after MitraClip treatment in patients with symptomatic heart failure and significant functional mitral regurgitation. European Journal of Heart Failure. 2018;20(10):1495–1496. doi: 10.1002/ejhf.1291. [DOI] [PubMed] [Google Scholar]
- 12.De Rosa R., Silverio A., Baldi C., et al. Transcatheter repair of functional mitral regurgitation in heart failure patients ― a meta-analysis of 23 studies on mitraclip implantation. Circulation Journal. 2018;82(11):2800–2810. doi: 10.1253/circj.CJ-18-0571. [DOI] [PubMed] [Google Scholar]
- 13.Ailawadi G., Lim D. S., Mack M. J., et al. On behalf of the EVEREST II investigators. One year outcomes following mitraclip® for functional mitral regurgitation. Circulation. 2018;139(1):37–47. doi: 10.1161/CIRCULATIONAHA.117.031733. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. British Medical Journal. 2009;339(b2700) doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez E. J., Samad Z., Al-Khalidi H. R., et al. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: a propensity-matched comparison. American Heart Journal. 2015;170(5):1050–1059. doi: 10.1016/j.ahj.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Asgar A. W., Khairy P., Guertin M.-C., et al. Clinical outcomes and economic impact of transcatheter mitral leaflet repair in heart failure patients. Journal of Medical Economics. 2017;20(1):82–90. doi: 10.1080/13696998.2016.1227828. [DOI] [PubMed] [Google Scholar]
- 17.Giannini C., Fiorelli F., De Carlo M., et al. Comparison of percutaneous mitral valve repair versus conservative treatment in severe functional mitral regurgitation. American Journal of Cardiology. 2016;117(2):271–277. doi: 10.1016/j.amjcard.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Armeni P., Boscolo P. R., Tarricone R., et al. Real-world cost effectiveness of MitraClip combined with Medical Therapy Versus Medical therapy alone in patients with moderate or severe mitral regurgitation. International Journal of Cardiology. 2016;209:153–160. doi: 10.1016/j.ijcard.2016.01.212. [DOI] [PubMed] [Google Scholar]
- 19.Kortlandt F., Velu J., Schurer R., et al. Survival after mitraclip treatment compared to surgical and conservative treatment for high-surgical-risk patients with mitral regurgitation. Circulation: Cardiovascular Interventions. 2018;11(6) doi: 10.1161/CIRCINTERVENTIONS.117.005985. [DOI] [PubMed] [Google Scholar]
- 20.Obadia J., Messika-Zeitoun D., Leurent G., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. The New England Journal of Medicine. 2018;379(24):2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 21.Stone G. W., Lindenfeld J., Abraham W. T., et al. Transcatheter mitral-valve repair in patients with heart failure. The New England Journal of Medicine. 2018;379(24):2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 22.Geis N., Raake P., Lewening M., et al. Percutaneous repair of mitral valve regurgitation in patients with severe heart failure: comparison with optimal medical treatment. Acta Cardiologica. 2018;73(4):378–386. doi: 10.1080/00015385.2017.1401275. [DOI] [PubMed] [Google Scholar]
- 23.Giannini C., D'ascenzo F., Fiorelli F., et al. A meta-analysis of MitraClip combined with medical therapy vs. medical therapy alone for treatment of mitral regurgitation in heart failure patients. ESC Heart Failure. 2018 doi: 10.1002/ehf2.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goliasch G., Bartko P. E., Pavo N., et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. European Heart Journal. 2018;39(1):39–46. doi: 10.1093/eurheartj/ehx402. [DOI] [PubMed] [Google Scholar]
- 25.Rossi A., Dini F. L., Faggiano P., et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97(20):1675–1680. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 26.Acker M. A., Parides M. K., Perrault L. P., et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. The New England Journal of Medicine. 2014;370(1):23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein D., Moskowitz A. J., Gelijns A. C., et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. The New England Journal of Medicine. 2016;374(4):344–353. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crestanello J. A. Mitral valve surgery for congestive heart failure. Heart Failure Clinics. 2018;14(4):585–600. doi: 10.1016/j.hfc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Onorati F., Santini F., Dandale R., et al. Functional mitral regurgitation: A 30-year unresolved surgical journey from valve replacement to complex valve repairs. Heart Failure Reviews. 2014;19(3):341–358. doi: 10.1007/s10741-013-9392-9. [DOI] [PubMed] [Google Scholar]
- 30.Megaly M., Khalil C., Abraham B., et al. Impact of transcatheter mitral valve repair on left ventricular remodeling in secondary mitral regurgitation: a meta-analysis. Structural Heart. 2018;2(6):541–547. doi: 10.1080/24748706.2018.1516912. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: search strategy used in each database searched. Supplementary Table S2: Quality Assessment of the Observational Studies using the Robins-1 tool. Supplementary Table S3: Quality Assessment of the Randomized Studies using the ROB 2.0 scale. Supplementary Table S4: Pooled Analysis for the Number Needed to Treat. Supplementary Figure-1: leave-one-out meta-analysis for the 1-year mortality outcome. Supplementary Figure-2: leave-one-out meta-analysis for the 2-year mortality outcome. Supplementary Figure-3: leave-one-out meta-analysis for the 30-day mortality outcome. Supplementary Figure-4: leave-one-out meta-analysis for the cardiovascular mortality outcome. Supplementary Figure-5: cumulative meta-analysis for the 1-year mortality outcome. Supplementary Figure-6: cumulative meta-analysis for the 2-year mortality outcome. Supplementary Figure-7: Cumulative meta-analysis for the 30-day mortality outcome. Supplementary Figure-8: Cumulative meta-analysis for the cardiovascular mortality outcome. Supplementary Figure-9: funnel plot for the heterogeneity of the included studies.
Data Availability Statement
All the data used in the analysis is presented within the manuscript.