Abstract
Management of patients presenting to the Emergency Department with chest pain is continuously evolving. In the setting of acute coronary syndrome, the availability of high-sensitivity cardiac troponin assays (hs-cTn) has allowed for the development of algorithms aimed at rapidly assessing the risk of an ongoing myocardial infarction. However, concerns were raised about the massive application of such a simplified approach to heterogeneous real-world populations. As a result, there is a potential risk of underdiagnosis in several clusters of patients, including women, for whom a lower threshold for hs-cTn was suggested to be more appropriate. Implementation in clinical practice of sex-tailored cut-off values for hs-cTn represents a hot topic due to the need to reduce inequality and improve diagnostic performance in females. The aim of this review is to summarize current evidence on sex-specific cut-off values of hs-cTn and their application and usefulness in clinical practice. We also offer an extensive overview of thresholds reported in literature and of the mechanisms underlying such differences among sexes, suggesting possible explanations about debated issues.
1. Background
Chest pain is one of the most common symptoms and reasons for admission in patients who present to the Emergency Department (ED) [1, 2], setting a major challenge for emergency physicians due to the large number of conditions included in the differential diagnosis [3, 4]. These include cardiovascular diseases (e.g., stable angina, acute coronary syndrome (ACS), aortic dissection, and pulmonary embolism) as well as a broad spectrum of non-cardiovascular causes, such as pneumonia, pleuritis, gastrointestinal disease, and psychogenic causes [5, 6].
In this setting, the first and most important diagnosis to exclude is ACS, due to its high rates of morbidity and mortality [7, 8] and to the need for a prompt therapeutic intervention in case of a confirmed myocardial infarction (MI) [9, 10]. Cardiac troponin (cTn), a protein involved in cardiomyocyte contraction, is a reliable and widely used biomarker of cardiac injury. Its measurement plays an essential role in the diagnostics of ACS [11], to the point of its being included in the universal definition of MI [12]. The availability of a both sensitive and specific marker of myocardial injury, especially with the introduction of the newest high-sensitivity assays (hs-cTn), has revolutionized the workup of ACS in the ED. With this comes the newfound ability to rule out suspected ongoing ischemic heart disease in patients presenting with chest pain and no obvious electrocardiographic signs of MI [13].
To date, a concentration of hs-cTn above the assay-specific upper reference limit (derived from a reference population) is used as a cut-off point for the diagnosis of MI [12]. However, the application of one standard threshold value may not be appropriate for all patients. Sex is one of the several variables that could influence its concentration and interpretation, potentially leading to underdiagnosis and inequality in the treatment of acute MI in women. Coronary artery disease (CAD) and MI are primary causes of mortality in the female population [14]. This is partly due to the frequent atypical clinical presentation in this group, which complicates recognition of symptoms, and can delay following interventions. Moreover, a recent study has shown that women with MI suffer from higher excess mortality compared to men, a difference which is reduced after adjusting for the use of guideline-indicated care [15].
The aim of this review is to summarize the available evidence on the influence of sex on the diagnostic performance of hs-cTn and to present novel implications and applications of sex-specific cut-offs in the management of ACS. For this purpose, we searched for relevant articles on PubMed, combining the terms “troponin”, “hs-cTn”, “gender”, “sex”, “women”, “females”, “men” and “males”.
2. Cardiac Troponin: Silver Bullet in the Diagnostics of ACS and MI
The troponin complex is a well-known component of the skeletal and cardiac muscles and plays a key role in myocyte contraction. The complex is composed of three subunits (troponin C, troponin I, and troponin T), each with a peculiar function in the genesis of contraction [16]. Unlike the C subunit, troponins I and T are expressed in the heart in cardiac-specific isoforms (cTnI and cTnT, respectively), allowing them to be recognized as belonging to cardiomyocytes. Following ischemic and non-ischemic myocardial injury, plasmatic levels of both cTnI and cTnT begin to increase and become detectable [11], with kinetics that mostly depend on the type of damage and, in the case of ischemic injury, on the duration of the ischemia and the timing of reperfusion [17]. Usually, troponin levels begin to increase 2 to 4 hours after an ischemic event and remain high for as long as 14 days [18]. Because of these characteristics, cTnI and cTnT have established themselves as the main biomarkers used in the diagnostics of ACS and MI [11, 12]. The advent of hs-cTn has led to an improved ability in detecting slight increases or variations in troponin blood levels, thus resulting in a better chance of rapidly identifying a higher number of MI [19]. Simultaneously, hs-cTn have also increased the safety and reliability of ruling out those patients with stable, low concentrations of hs-cTn and an unlikely ongoing MI [20, 21].
One of the most important open issues regarding the use of hs-cTn is the biological variability in baseline troponin levels, and how this could impact their role in the diagnostics of ACS [19]: 99th percentile levels of hs-cTn are broadly used as the cut-off to rule in or rule out possible MI. These are obtained by studying reference populations composed of supposedly healthy people, but questions were raised about the suitability of using a single cut-off in a heterogeneous real-world population in which patients differ in age, sex, and comorbidities [19, 22]. Some authors argue that serial measurements of hs-cTn could lead to an enhanced prognostic value of this marker by detecting relevant changes in its levels [23–25], thus highlighting the importance of weighting intra-patient variability for the interpretation of hs-cTn values. Indeed, a growing number of studies suggest that the use of a single threshold for hs-cTn irrespective of age, sex, and other parameters may not be ideal [22, 26–28].
Furthermore, several concerns were raised about the definition of hs-cTn, with an ensuing struggle to state unequivocal criteria to identify the necessary standard to be met by an assay in order to be labelled as “high-sensitivity” [29–31]. Consensus of experts proposed a definition that identifies cTn assays as “high-sensitivity” if two criteria are met: (a) total imprecision (i.e., coefficient of variation) ≤10% at the value of the 99th percentile; (b) ability to measure levels of cTn between limit of detection and 99th percentile in at least 50% of healthy subjects [29, 32, 33].
3. Sex and Gender: One Key Factor to Consider When Dealing with Troponins
In the context of cardiovascular disease, several differences between men and women have been described [34]. As for the diagnosis of cardiovascular disease, concentrations of several biomarkers were found to be influenced by sex [35–39], including hs-cTn [40–43], with men reportedly presenting higher concentrations than women. Accordingly, the need for sex-specific reference values has been pointed out by several authors [44–47], while other studies indicate that adopting sex-specific reference intervals for other biomarkers, such as total creatine kinase (CK) activity and MB fraction of CK [48], could also have potential benefits. The same applies to natriuretic peptides [36, 49–53], growth hormone [54], galectin-3 [55, 56], soluble ST2 [57, 58], and proneurotensin [59, 60], supporting the idea that sex differences should be taken into account when approaching laboratory tests.
The first cTn assays, however, required the use of a single, universal cut-off value [61]. The development of hs-cTn assays, in addition to increasing analytical sensitivity, has shown that men present significantly higher concentrations than women for both hs-cTnT and hs-cTnI, highlighting that the upper reference limit for the diagnosis of MI could be two-fold in men compared to women, regardless of the assay being used [29, 44, 61–63].
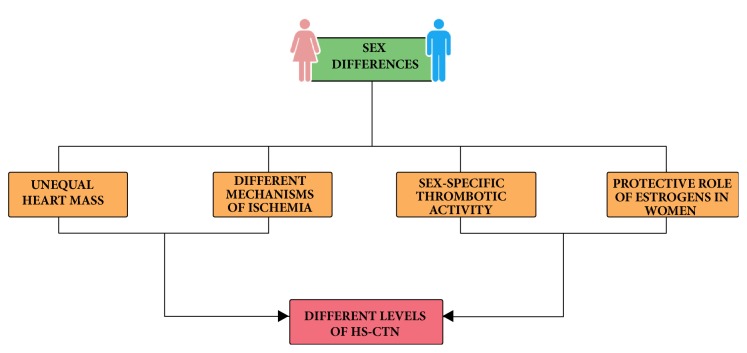
While still far from being comprehensively understood, several mechanisms may contribute to the aforementioned discrepancy between men and women (Figure 1). Based on the fact that troponin is a measure for the amount of damaged myocardium, some evidence suggests that differences in plasmatic levels of hs-cTn could be attributed to sex-specific variations in body composition [64], cardiac mass [65, 66], and rate of cardiomyocyte apoptosis due to cardiac remodeling [67]. Some insight was provided by authors who outlined potential mechanisms of troponin shedding in the absence of overt membrane injury: variations in the regulation of these events may partially explain the observed variability across healthy subjects [68]. Myocardial response to ischemia and reperfusion is assumed to be unequal in men and women, as well as the pathophysiological mechanism of cardiac ischemia, the grade of coronary atherosclerosis, and the presence of collateral blood flow [69–71]. Sexual hormones may also play a role in the differential expression of hs-cTn levels. Estrogens are thought to exert a protective role on the myocardium: their antioxidant properties and their ability to scavenge reactive oxygen species may contribute to limit cardiomyocyte injury [43, 72–74].
Figure 1.
Mechanisms contributing to the discrepancy in hs-cTn levels between men and women.
4. Sex-Related Cut-Offs: State of the Art
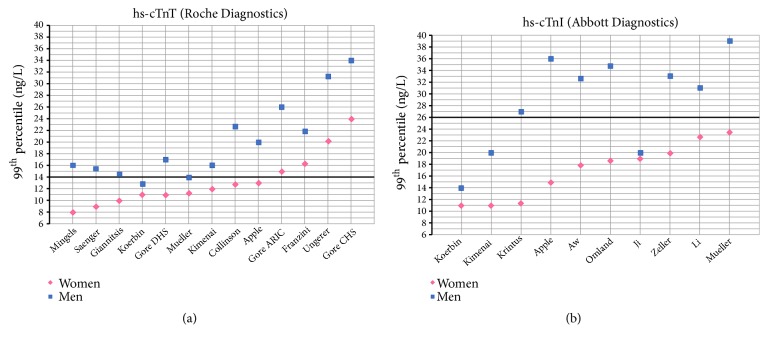
The 99th percentile reference limit (14 ng/L) for hs-cTnT assay (Roche Diagnostics) was set by a study of over 600 apparently healthy volunteers and blood donors [62] and subsequently restated in a multicenter cohort study [75]. In both studies, 50% of the population was composed of females and women showed significantly lower 99th percentile concentrations of hs-cTnT compared to men (10.0 versus 14.5 ng/L and 8.9 versus 15.5 ng/L, respectively). Several other studies support the existence of a discrepancy between 99th percentile values of hs-cTnT in men and women (Table 1 and Figure 2-panel a). Another large study, based on three wide cohorts, reports sex-related critical differences in reference values of hs-cTnT [44], and an Italian-based study of 1600 healthy subjects confirmed the lower threshold for the 99th percentiles in females, with the discrepancy consistent in each age-class [76]. This trend is strongly supported by several other studies, although reference values differ substantially between populations, thus highlighting the impact of the cohort's characteristics [40, 61, 77–82]. Criteria used for the identification of “healthy” individuals are among the most important matters of concern when recruiting a reference population for the purpose of identifying reference values. An elegant study sheds light on how these factors could affect the process of setting a standard reference limit: subsequent application of stricter selection criteria resulted in a progressive reduction of 99th percentile values in a cohort of supposedly healthy people [83], thus addressing the need to implement laboratory tests and clinical assessments in the process of identifying a reference population. These findings are consistent with those observed in other studies [40, 44] and highlight the importance of taking patients' variables into account when dealing with troponins.
Table 1.
Studies reporting 99th percentile values for hs-cTnT in different reference populations. †=median [IQR]; a=only range provided; CI: confidence interval; cTn: cardiac troponin; eGFR: estimated glomerular filtration rate; UK: United Kingdom; US: United States; ARIC: Atherosclerosis Risk in Communities study; CHS: Cardiovascular Health Study; DHS: Dallas Heart Study.
| Study | Study objective | Year | Location | Study population | Age, mean ± SD |
Population, according to sex | 99th percentile (ng/L) [95% CI] | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Males (%) | Females (%) | Males | Females | |||||||
| hs-cTnT (Roche Diagnostics) | ||||||||||
|
| ||||||||||
| Collinson et al. [40] | To determine the effect of patient selection on the 99th reference percentile | 2012 | UK | 545 | 58 [51-67]† | 259 (47.5) | 286 (52.5) | 22.8 | 12.8 | Reference population selection based on: medical history, biomarkers and cardiac imaging |
|
| ||||||||||
| Apple et al. [61] | To systematically assess 99th percentiles of cTn concentrations in a single population for a large number of assays | 2012 | US | 524 | 18-64a | 272 (52) |
252 (48) | 20 | 13 | Reference population selection based only on health questionnaire interviews |
|
| ||||||||||
| Giannitsis et al. [62] | To validate the hs-cTnT assay | 2010 | US | 616 | 44 ± 13.8 | 309 (50.2) | 307 (49.8) | 14.5 | 10 | Reference population selection based only on medical records |
|
| ||||||||||
| Saenger et al. [75] | To evaluate the analytical performance of the hs-cTnT assay in a multicenter, international trial | 2011 | US, Europe | 533 | 37 | 268 (50.3) | 265 (49.7) | 15.5 | 9 | Reference population selection based only on medical records |
|
| ||||||||||
| 1600 (whole cohort) | 61 ± 14 | 872 (54.6) | 728 (45.4) | 21.8 [19.8-33.9] | 16.3 [12.4-18] | Reference population selection based on medical history, biomarkers and cardiac imaging; population stratified by age; Various subpopulations included: high heterogeneity and reference population unable to achieve the recommended statistical power to determine the 99th percentile for each subgroup |
||||
| Franzini et al. [76] | To determine the 99th upper reference limit for cTnT in Italian apparently healthy subjects | 2015 | Italy | 553 | <20 | 270 (48.8) | 283 (51.2) | 10.9 [6.7-20.4] | 6.8 [5.2-8.9] |
|
| 872 | 20-64 | 503 (57.7) | 369 (42.3) | 23.2 [17.3-34.1] | 10.2 [8.5-21.9] | |||||
| 175 | >65 | 99 (56.6) | 76 (43.4) | 36.8 [21.7-37] | 28.6 [17.6-28.6] | |||||
|
| ||||||||||
| Mingels et al. [77] | To study the improvements made by new hs-cTn assays in detecting exercise-induced cTn release | 2009 | US | 479 | 51 [26-71]† | 264 (55.1) | 215 (44.9) | 16 | 8 | Reference population selection based only on medical records |
|
| ||||||||||
| 1540 (whole cohort) | 57 ± 8 | 733 (47.6) | 807 (52.4) | 16 [15-17] | 12 [10-14] | Reference population selection based on: medical history and biomarkers; population stratified by age | ||||
| Kimenai et al. [78] | To assess sex-specific and age-specific 99th percentile upper reference limits of hs-cTnT and hs-cTnI in a single reference cohort | 2016 | Netherlands | 283 | 40-49 | 120 (42.4) | 163 (57.6) | 16 [10-17] | 12 [7-16] | |
| 946 | 50-64 | 443 (46.8) | 503 (53.2) | 14 [13-16] | 12 [9-15] | |||||
| 311 | 65-75 | 170 (54.7) | 141 (45.3) | 28 [19-40] | 27 [12-36] | |||||
|
| ||||||||||
| Koerbin et al. [79] | To evaluate the analytical characteristics of the hs-cTnT assay | 2010 | Australia | 111 | 25-74a | 62 (55.9) | 49 (44.1) | 12.9 | 11 | Reference population selection based on medical history, biomarkers and cardiac imaging |
|
| ||||||||||
| DHS: 1978 | 43.2 ± 9.6 | 873 (44.1) | 1105 (55.9) | 17 [13-50] | 11 [7-15] | Reference population selection based on: progressive cohorts restriction based on clinical history, imaging and/or laboratory tests | ||||
| Gore et al. [44] | To determine the 99th percentile values in three large community-based subcohorts, restricted by healtiness criteria | 2014 | US | ARIC: 7575 | 61 ± 9 | 2972 (39.2) | 4603 (60.8) | 26 [23-30] | 15 [14-17] | |
| CHS: 1374 | 72 ± 6 | 489 (35.6) | 885 (64.4) | 34 [26-42] | 24 [18-35] | |||||
|
| ||||||||||
| Mueller et al. [80] | To assess 99th percentile in a blood donors population | 2016 | Austria | 402 | 35 [25.9-45.1] |
259 (64.4) | 143 (35.6) | 13.9 | 11.3 | Reference population selection based on: no overt cardiovascular disease, eGFR>90 ml/min |
|
| ||||||||||
| Ungerer et al. [81] | Determine and compare 99th percentile cut-offs of 3 cTn assays in a cohort of blood donors | 2016 | Australia | 2004 | Male: 43.7 [30.7-54.3] | 1299 (64.8) | 705 (35.2) |
31.3 [90% CI: 25.0-57.5] | 20.2 [90% CI: 9.9-51.7] | Reference population selection based on: health questionnaire |
| Female: 33.2 [24.6-50.32] | ||||||||||
|
| ||||||||||
| Yang et al. [95] | Establish 99th percentile in a healthy Chinese population | 2016 | China | 1725 | Male: 54 ± 20 |
818 (47.4) | 907 (52.6) | Several according to age | Several according to age | Reference population selection based on clinical history, physical examination, lab tests |
| Female: 54 ± 19 | ||||||||||
|
| ||||||||||
| Monneret et al. [107] | Establish age and sex specific 99th percentile in patients without CKD | 2018 | France | 2707 | Male: 62 [52-70] |
1548 (57.2) | 1159 (42.8) |
Several according to age | Several according to age | Reference population selection based on age partitioning and outliers removal. Cut-off obtained with an analytical imprecision-based approach |
| Female: 63 [48-75] | ||||||||||
|
| ||||||||||
| Welsh et al. [108] | Evaluating the influence of several variables, including sex, on the 99th percentile levels of hs-cTnT and hs-cTnI | 2018 | Scotland | 19501 | 35-65a | 8126 (41.7) | 11375 (58.3) | Several according to age | Several according to age | Reference population selection based on general population; health questionnaire; lab tests |
Figure 2.
Chart showing different 99th percentile values for hs-cTnT (panel a) and hs-cTnI (panel b) assays, derived from selected population studies as reported in Tables 1 and 2. Bold lines represent non-sex-specific, standard cut-offs for hs-cTnT and hs-cTnI (14 ng/L and 26 ng/L, respectively).
Unlike hs-cTnT, several hs-cTnI assays have been developed [84]. The 99th percentile reference values, limits of detection and variance coefficients all vary between assays [19]. Despite these major differences, and consistently with data on hs-cTnT, several studies identified sex-related differences in reference limits of hs-cTnI (Table 2 and Figure 2-panel b). 99th percentile reference values of hs-cTnI were found to be systematically lower in females, regardless of the assay used, ethnicity of the population, or criteria used to identify healthy cohorts. Still, these factors heavily affect the point estimates of the 99th percentile, which differ across the studies [27, 61, 63, 78, 80, 85–90].
Table 2.
Studies reporting 99th percentile values for hs-cTnI in different reference populations. †=median [IQR]; a=only range provided; BMI: body mass index; BNP: brain natriuretic peptide; CI: confidence interval; cTn: cardiac troponin; eGFR: estimated glomerular filtration rate; HbA1c: glycated hemoglobin; US: United States.
| Study | Study objective | Year | Location | Study population | Age, mean ± SD | Population, according to sex | 99th percentile (ng/L) [95% CI] | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Males (%) | Females (%) | Males | Females | |||||||
| hs-cTnI (Abbott Diagnostics) | ||||||||||
|
| ||||||||||
| Apple et al. [61] | To systematically assess 99th percentiles of cTn concentrations in a single population for a large number of assays | 2012 | US | 524 | 18-64a | 272 (52) | 252 (48) | 36 | 15 | Reference population selection based only on health questionnaire interviews |
|
| ||||||||||
| Koerbin et al. [63] | To assess analytical characteristics and to apply the assay to a population of apparently cardiovascular disease-free people | 2012 | Australia | 497 | 20-84a | 231 (46.5) | 266 (53.5) | 14 | 11 | Reference population selection based on medical history and biomarkers |
|
| ||||||||||
| Aw et al. [85] | To determine 99th percentile reference values in a large Asian cohort | 2013 | Asia | 1120 | 50.4 ± 8.2 | 597 (53.3) | 523 (46.7) | 32.7 | 17.9 | Reference population selection based on: medical history |
|
| ||||||||||
| Krintus et al. [27] | To assess 99th percentile for hs-cTnI in a large multicenter European cohort | 2015 | Europe | 1769 | 49 [18-60]† | 776 (43.9) | 993 (56.1) | 27 | 11.4 | Reference population selection based on blood donors, health questionnaires and no overt cardiovascular disease |
|
| ||||||||||
| Omland et al. [86] | To assess sex-related differences in hs-cTnI distribution across sexes | 2015 | Norway | 8099 | Males: 50.2 ± 17.1 | 3670 (45.3) | 4429 (54.7) | 34.8 [26.3-49.4] | 18.7 [14.8-23.1] | Reference intervals are reported for women and men without history of major cardiovascular disease or risk factor |
| Females: 49.7 ± 16.4 | ||||||||||
|
| ||||||||||
| Zeller et al. [87] | To assess sex-specific 99th percentile reference values in a large German-based cohort | 2015 | Germany | 4138 | 50 [42 − 61]† | 2098 (50.7) | 2040 (49.3) | 33.1 [28.3-45.8] | 19.9 [16.1-23.9] | Reference population selection based on different criteria with several subgroups reported (here the overall) |
|
| ||||||||||
| 1535 (whole cohort) | 57 ± 8 | 733 (47.6) | 807 (52.4) | 20 [14-22] | 11 [8-13] | Reference population selection based on: medical history and biomarkers; population stratified by age | ||||
| Kimenai et al. [78] | To assess sex-specific and age-specific 99th percentile upper reference limits of hs-cTnT and hs-cTnI in a single reference cohort | 2016 | Netherlands | 283 | 40-49 | 120 (42.4) | 163 (57.6) | 13 [5-15] | 12 [10-14] | |
| 944 | 50-64 | 441 (46.7) | 503 (53.3) | 22 [13-23] | 9 [6-14] | |||||
| 308 | 65-75 | 168 (54.5) | 140 (45.6) | 20 [13-25] | 13 [10-13] | |||||
|
| ||||||||||
| Mueller et al. [80] | To assess 99th percentile in a blood donors population | 2016 | Austria | 402 | 35 [25.9-45.1]† |
259 (64.4) | 143 (35.6) | 39.0 | 23.5 | Reference population selection based on: no overt cardiovascular disease, eGFR > 90 ml/min |
|
| ||||||||||
| Ji et al. [88] | To assess 99th percentile values in a Korean cohort | 2016 | South Korea | 854 | 49.8 ± 10.2 | 426 (49.9) | 428 (50.1) | 20 | 19 | Reference population selection based on clinical history and laboratory tests (eGFR, HbA1c, BNP) |
|
| ||||||||||
| Li et al. [89] | To assess 99th percentile for hs-cTnI in a Chinese-based population | 2017 | China | 1485 | 36 ± 13 | 731 (49.2) | 754 (50.8) | 31.1 | 22.7 | Reference population selection based on: clinical history, BMI, renal function |
|
| ||||||||||
| Welsh et al. [108] | Evaluating the influence of several variables, including sex, on the 99th percentile levels of hs-cTnT and hs-cTnI | 2018 | Scotland | 19501 | 35-65a | 8126 (41.7) | 11375 (58.3) | Several according to age | Several according to age | Reference population selection based on general population; health questionnaire; lab tests |
|
| ||||||||||
| hs-cTnI (Beckman Coulter) | ||||||||||
|
| ||||||||||
| Apple et al. [61] | To systematically assess 99th percentiles of cTn concentrations in a single population for a large number of assays | 2012 | US | 524 | 18-64a | 272 (52) | 252 (48) | 52 | 23 | Reference population selection based only on health questionnaire interviews |
|
| ||||||||||
| hs-cTnI (Singulex) | ||||||||||
|
| ||||||||||
| Apple et al. [41] | To determine 99th percentile reference value for hs-cTnI assay | 2010 | US | 348 | 18-76a | 147 (42.2) | 201 (57.8) | 16.6 | 9.4 | Reference population selection based only on health questionnaire interviews |
|
| ||||||||||
| Bossard et al. [90] | To assess factors related to hs-cTnI levels in a healthy young population without overt cardiovascular diseases | 2016 | Liechtenstein | 2077 | 36.7 [31.1-40.2]† | 975 (46.9) | 1102 (53.1) | 15.8 | 5.1 | Reference population selection based on: clinical records and absence of comorbidities |
|
| ||||||||||
| hs-cTnI (Siemens) | ||||||||||
|
| ||||||||||
| Apple et al. [61] | To systematically assess 99th percentiles of cTn troponin concentrations in a single population for a large number of assays | 2012 | US | 524 | 18-64a | 272 (52) | 252 (48) | 81 | 42 | Reference population selection based only on health questionnaire interviews |
|
| ||||||||||
| McKie et al. [42] | To define hs-cTnI reference values and determinants in the general community, in a healthy reference cohort, and in subsets with diseases | 2013 | US | 565 | 54 [50-61]† | 260 (45) | 305 (54) | 55 [32-124] | 33 [22-155] | Reference population selection based on medical history, biomarkers and cardiac imaging |
5. Application of Sex-Specific Cut-Offs in Clinical Practice
While there is a considerable body of evidence to support the role of sex in influencing reference levels of troponin, no definitive data are available on how this discrepancy could affect the diagnostic and prognostic value of hs-cTn in the work-up of ACS. A synopsis of the studies assessing the prognostic performance of sex-specific cut-offs is reported in Table 3. Specifically, the impacts of three sex-specific cut-offs for hs-cTnT, as reported by Saenger et al. [75], Gore et al. [44], and Kimenai et al. [78], were evaluated in a cohort of patients recruited in an ongoing trial (n=2734, 32% women), each presenting with suspected acute MI. Women were significantly older than men (median age [IQR]: 68 [55-77] versus 59 [48-71]) and showed lower estimated glomerular filtration rate values, whilst higher rates of CAD history and smoking were reported in men. With the application of sex-specific cut-offs instead of the universal one, reclassification from unstable angina (UA) to non-ST elevation MI (NSTEMI) occurred in two women, while only one man was downgraded to UA from NSTEMI. Similar findings were reported with all three sex-based cut-off values analyzed. Reclassification was not shown to impact short-term or long-term prognosis in this cohort, thus not providing evidence in favor of the application of sex-specific thresholds in the diagnostics of ACS [91]. These findings are supported by a subanalysis of the TRAPID-AMI (The High Sensitivity Cardiac Troponin T Assay for Rapid Rule-out of Acute Myocardial Infarction) study, which enrolled over 1200 patients (37% women) with chest pain to assess whether the application of Saenger's sex-oriented cut-offs for hs-cTnT would lead to a better reclassification of MI and an improvement in prognosis. While the use of different cut-offs resulted in an increase of acute MI rates in females (from 16.6% to 22.6%) and a decrease in males, this did not produce any benefit in terms of outcomes [92]. Furthermore, a large retrospective study showed slightly higher rates of diagnostic reclassification (8,4%) and an increase (+3.3%) in MI prevalence in women when using sex-specific cut-offs. Although this study confirmed no advantage in risk prediction when using sex-specific reference values, the risk in women was increased at levels of 10-12 ng/L, which is below the set standard point of 14 ng/L [93]. A recent observational study, focused on the diagnostic performance of several sex-specific hs-cTnT cut-offs for the rule-out of MI, showed an improved specificity with the adoption of different threshold levels [94]. These findings were also consistent with a recent Chinese study in which sex-specific cut-offs were calculated in an original reference population and then further stratified according to age. This study reports an increased specificity for sex-related hs-cTnT thresholds in the diagnostics of AMI, as well as higher negative and positive predictive values [95]. However, the impact of age-stratification probably played a decisive role in this study, still highlighting a possible interplay between these two variables.
Table 3.
Studies reporting performance and prognostic impact of sex-specific cut-offs in different populations. MACE: major adverse cardiovascular events; MI: myocardial infarction.
| Study | Year | Patients | Women (%) | Cut-off applied (ng/L) | Comments | |
|---|---|---|---|---|---|---|
| Men | Women | |||||
| hs-cTnT (Roche Diagnostics) | ||||||
|
| ||||||
| Mueller-Hennessen et al. [92] | 2016 | 1282 | 477 (37%) | 15.5 | 9.0 | Sex-specific cut-offs increased MI diagnosis in women (from 17% to 23%) but this did not affect outcomes |
|
| ||||||
| 15.5 | 9.0 | Reclassification occurred in only 3 patients; no effects on outcomes. Tested three different sets of sex-specific cut-offs | ||||
| Rubini Gimenez et al. [91] | 2016 | 2734 | 876 (32%) | 17.0 | 9.0 | |
| 12.0 | 16.0 | |||||
|
| ||||||
| 16.0 | 9.0 | Using sex-specific cut-offs, the prevalence of MI would increase by 3.3% in women. Sex-specific cut-offs did not improve risk prediction, but the study identified an increase of risk in women starting at 10-12 ng/L instead of 14 ng/L. | ||||
| Eggers et al. [93] | 2016 | 57556 | 22027 (38%) | 26.0 | 15.0 | |
| 34.0 | 24.0 | |||||
|
| ||||||
| Mueller et al. [99] | 2018 | 3588 | 1643 (46%) | 16 | 9 | Sex-specific cut-offs increased myocardial injury diagnosis in 11% of women compared to a 4% decrease in men |
|
| ||||||
| McRae et al. [94] | 2018 | 7130 | 3199 (45%) | Several combinations according to sex | Implementation of sex-specific cut-offs improved specificity of hs-cTnT in the diagnostic approach of ACS | |
|
| ||||||
| Yang et al. [95] | 2016 | 812 | 376 (46%) | Several according to age and sex | Sex-specific cut-offs were calculated in a healthy Chinese cohort and further stratified for age | |
|
| ||||||
| hs-cTnI (Abbott Diagnostics) | ||||||
|
| ||||||
| Shah et al. [96] | 2018 | 48282 | 22562 (47%) | 34 | 16 | Sex-specific cut-offs for an hs-cTnI assay, compared to a contemporary cTnI assay, led to a two-fold myocardial injury reclassification rate in women; no difference in 1-year outcomes among reclassified patients treated according to cTnI vs hs-cTnI levels |
|
| ||||||
| Shah et al. [98] | 2015 | 1126 | 504 (45%) | 34 | 16 | Sex-specific cut-offs increase MI diagnosis in women (from 16 to 22%) while having small effects on men |
|
| ||||||
| Mueller et al. [99] | 2018 | 3588 | 1643 (46%) | 34 | 16 | Sex-specific cut-offs increased myocardial injury diagnosis in 6% of women compared to a 3% decrease in men |
|
| ||||||
| Cullen et al. [97] | 2016 | 2841 | 1180 (41%) | 34 | 16 | Small amount of women and men reclassified using sex-specific thresholds, thus improving identification of women at long-term (1 year) risk for MACE |
|
| ||||||
| Eggers et al. [100] | 2014 | 2750 | 1073 (39%) | 24.8 | 16.6 | Sex-specific cut-offs were derived from a reference population recruited for the purposes of the study. Sex-specific cut-offs did not show improvement in the identification of more at-risk patients; however higher concentrations of troponins show stronger predictive value in women than men |
|
| ||||||
| Bohula May et al. [101] | 2014 | 4695 | 1460 (31%) | 34 | 16 | Population presenting with typical ischemic symptoms. Using sex-specific thresholds, only 6 patients were reclassified; no improvement in prognostic performance. |
The recently published High-STEACS (The High Sensitivity Cardiac Troponin T Assay for Rapid Rule-out of Acute Myocardial Infarction) study reports some of the most interesting findings to date on the topic of sex-specific cut-offs for hs-cTn and on the potential magnitude of the impact which their implementation could have in the management of patients with suspected ACS. In this multicenter, randomized control trial a high sensitivity (hs-cTnI) and a contemporary (cTnI) assay were compared in the diagnosis of suspected ACS. In the first phase of the study, clinical decisions were made according to the cTnI values, while the hs-cTnI concentration was masked. In the second phase, clinicians were provided with the hs-cTnI levels, while cTnI values were masked. The 99th percentiles for hs-cTnI were set to 34 ng/mL and 16 ng/mL in men and women, respectively. Compared with the contemporary assay, reclassification occurred in a significant part (17%) of the myocardial injuries identified by the hs-cTnI, with twofold frequency in women compared to men. However, no significant differences were observed in 1-year outcomes among reclassified patients treated according to cTnI versus hs-cTnI levels [96]. These findings are consistent with a multicenter observational study by Cullen et al., the first large investigation reporting the effects of sex-specific cut-offs (34 ng/L for males and 16 ng/L for females) on prediction of Major Adverse Cardiac Events (MACE) in ED patients. This study suggests that the use of sex-specific reference values for hs-cTnI improves the identification of women at high risk for cardiovascular events within 1 year. Even so, the authors conclude that the net effect across the whole ED population with chest pain symptoms would be minimal and there may be an increased risk of nonidentification of males at high risk for cardiovascular events. The limitation of the study, however, was the use of an overall cut-off to adjudicate endpoints. Overcoming this limitation would require additional testing in a prospective trial reporting outcomes following the clinical use of sex-specific thresholds [97].
Interesting data come from a prospective cohort of 1126 patients with suspected ACS. Classification according to sex-specific threshold levels for hs-cTnI (34 ng/L in men, 16 ng/L in women versus 26 ng/L as standard reference value) led to an increase in the number of MI diagnosed in women (from 16% to 22%) whereas the effect on men was less relevant. Furthermore, female patients with levels of hs-cTnI of 17-26 ng/L presented sixfold rates of death or recurrent MI at 1 year when compared to women with hs-cTnI ≤16 ng/L (23% versus 4%). Similar rates of 1-year outcomes were observed when comparing women in the 17-26 ng/L group with women with hs-cTnI above the standard reference value, suggesting that a sex-specific approach improved the identification of high-risk females in this cohort [98]. While there is further evidence in support of the higher reclassification rate observed in women when using this approach [99], a subanalysis of the GUSTO-IV trial failed to identify an improved risk prediction. Notably, in this study females accounted for less than 40% of the main cohort [100]. Likewise, in a study which pooled cohorts from two randomized controlled trials, small reclassification rates occurred when using sex-specific cut-offs, thus leading to no-impact on the prognostic performance of hs-cTnI. However, the small ratio of females enrolled (31%) and the population selection criteria (patients presenting with typical ischemic symptoms) represent important biases to keep into account when translating these findings to the real world [101].
6. Conclusions
The influence of patients' characteristics on biomarkers and their application to clinical decisions are gaining increasing importance and consideration in modern medicine. Sex, among others, represents one of the most important factors to consider when dealing with markers such as hs-cTn, whose concentrations can overturn clinical approaches and workups.
Our review highlights some key aspects. Firstly, algorithms proposed for the work-up of ACS in the ED do not consider personal characteristics, thus potentially leading to underdiagnosis and inequality of care. Concerns were raised regarding the possible impact of sex on this issue, yet no definitive evidence is available. Secondly, current evidence clearly shows a significant difference in hs-cTn concentrations and reference limits between men and women. Among healthy people 99th percentile values were found to be consistently lower in females, even if point values broadly fluctuate across studies and seem to be closely related to their reference population. Thirdly, data on the real-world performance of these sex-specific cut-offs is far more unclear. While some evidence points to potential benefits in the classification of high-risk women, several studies failed to demonstrate an advantage in terms of prognosis and clinical management [91–93, 102], thus not supporting their implementation in clinical practice. Some remarks, however, are mandatory: most of these studies investigated a single set of sex-related cut-offs, making it difficult to establish which set (if any) has the better performance in terms of risk-prediction and prognosis. Moreover, rates of reclassification (i.e.: patients with a diagnosis upgraded from UA to NSTEMI) are generally low, partly due to the narrow gap between the standard cut-off and the threshold applied to women, thus leading to a scarce impact on the overall prognosis. This is also confirmed by a recent meta-analysis, which reported the mean between-sex differences for hs-cTn in several large populations, as well as showing that the gap between standard and sex-specific thresholds is narrower for hs-cTnT, for which the mean difference of sex-specific cut-offs is close to the limit of detection [103]. In our opinion, according to the data observed and the slight differences observed between sexes in terms of hs-cTn upper reference limits [103], definitive conclusions could only be drawn on the basis of larger studies involving a higher number of patients and a more representative proportion of females, who now account for roughly 35-40% in most studies. Furthermore, in the context of MI, it is conceivable that most patients will present high levels of hs-cTn. The application of sex-tailored cut-offs then, despite the slight reclassification rate, could still improve the management of a sizeable cluster of patients. Fourthly, mechanisms underlying this discrepancy have not yet been fully explained: although some hypotheses have been reported and several factors outlined, a more thorough comprehension is required to understand if sex-related cut-offs could really impact the management of ACS in the ED, and why. For example, women exhibit higher rates of type-2 MI [104, 105] and microvascular CAD [106], and the extent to which these differences could impact hs-cTn diagnostic performance (e.g.: affecting its release kinetics or its peak values) is still a matter of concern. Further investigations are required to explore and shed some light on these open issues.
In conclusion, current literature strongly identifies the existence of sex-driven differences in hs-cTn levels in reference populations. The adoption of sex-specific cut-offs is still debated and knowledge on the potential positive effect than this could have on the prognosis of ACS in women is partial. Caution is mandatory due to lacking data on pathophysiology and further studies are required to clarify whether and why the adoption of sex-oriented cut-offs could lead to better management of ACS in women.
Acknowledgments
The authors thank Prof. Alessandro Pierucci for his support and guidance. VR is funded for her research activity by the Scientific Independence of Young Researchers Program (RBSI14HNVT) promoted by the Italian Ministry of Education, University and Research (MIUR).
Abbreviations
- ACS:
Acute coronary syndrome
- CAD:
Coronary artery disease
- CK:
Creatine Kinase
- cTn:
Cardiac Troponin
- ECG:
Electrocardiography
- ED:
Emergency Department
- ESC:
European Society of Cardiology
- hs-cTnI:
High sensitivity cardiac troponin I
- hs-cTnT:
High sensitivity cardiac troponin T
- MACE:
Major adverse cardiac events
- MI:
Myocardial infarction
- NSTE-ACS:
Non-ST-elevation acute coronary syndrome
- NSTEMI:
Non-ST-elevation myocardial infarction
- UA:
Unstable angina.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Giulio Francesco Romiti and Roberto Cangemi equally contributed to this paper.
References
- 1.Goodacre S. The health care burden of acute chest pain. Heart. 2005;91(2):229–230. doi: 10.1136/hrt.2003.027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesh A. K., Dai Y., Ross J. S., Schuur J. D., Capp R., Krumholz H. M. Variation in US hospital emergency department admission rates by clinical condition. Medical Care. 2015;53(3):237–244. doi: 10.1097/MLR.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knockaert D. C., Buntinx F., Stoens N., Bruyninckx R., Delooz H. Chest pain in the emergency department: The broad spectrum of causes. European Journal of Emergency Medicine. 2002;9(1):25–30. doi: 10.1097/00063110-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Haasenritter J., Biroga T., Keunecke C., et al. Causes of chest pain in primary care – a systematic review and meta-analysis. Croatian Medical Journal. 2015;56(5):422–430. doi: 10.3325/cmj.2015.56.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thang N. D., Karlson B. W., Bergman B., et al. Patients admitted to hospital with chest pain — Changes in a 20-year perspective. International Journal of Cardiology. 2013;166(1):141–146. doi: 10.1016/j.ijcard.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Kontos M. C., Diercks D. B., Kirk J. D. Emergency department and office-based evaluation of patients with chest pain. Mayo Clinic Proceedings. 2010;85(3):284–299. doi: 10.4065/mcp.2009.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolansky D. M. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. American Journal of Managed Care. 2009;15(2):S36–S41. [PubMed] [Google Scholar]
- 8.Sanchis-Gomar F., Perez-Quilis C., Leischik R., Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Annals of Translational Medicine. 2016;4(13):256–256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roffi M., Patrono C., Collet J.-P., Mueller C., Valgimigli M., Andreotti F., et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 10.Steg P. G., James S. K., Atar D., et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European Heart Journal. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K., Mair J., Katus H., et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. European Heart Journal. 2010;31(18):2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K., Alpert J. S., Jaffe A. S., et al. Fourth universal definition of myocardial infarction (2018) European Heart Journal. 2018;60:1581–1598. doi: 10.1093/eurheartj/ehy462. [DOI] [Google Scholar]
- 13.Bandstein N., Ljung R., Johansson M., Holzmann M. J. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. Journal of the American College of Cardiology. 2014;63(23):2569–2578. doi: 10.1016/j.jacc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Yahagi K., Davis H. R., Arbustini E., Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239(1):260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Wong J. A., Rexrode K. M., Sandhu R. K., Moorthy M. V., Conen D., Albert C. M. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. 2017;103 doi: 10.1136/heartjnl-2016-311002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmacek M. S., Solaro R. Biology of the troponin complex in cardiac myocytes. Progress in Cardiovascular Diseases. 2004;47(3):159–176. doi: 10.1016/j.pcad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Katus H. A., Remppis A., Scheffold T., Diederich K. W., Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. American Journal of Cardiology. 1991;67(16):1360–1367. doi: 10.1016/0002-9149(91)90466-X. [DOI] [PubMed] [Google Scholar]
- 18.Daubert M. A., Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vascular Health and Risk Management. 2010;6:691–699. doi: 10.2147/VHRM.S5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwood M. W., Kristin Newby L. High‐sensitivity troponin assays: Evidence, indications, and reasonable use. Journal of the American Heart Association. 2014;3(1) doi: 10.1161/JAHA.113.000403.e000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah A. S., Anand A., Sandoval Y., et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. The Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westwood M., van Asselt T., Ramaekers B., et al. High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis. Health Technology Assessment. 2015;19(44):1–234. doi: 10.3310/hta19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myint P. K., Kwok C. S., Bachmann M. O., Stirling S., Shepstone L., Zaman M. J. Prognostic value of troponins in acute coronary syndrome depends upon patient age. Heart. 2014;100(20):1583–1590. doi: 10.1136/heartjnl-2014-305533. [DOI] [PubMed] [Google Scholar]
- 23.Mueller M., Biener M., Vafaie M., et al. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clinical Chemistry. 2011;58(1):209–218. doi: 10.1373/clinchem.2011.171827. [DOI] [PubMed] [Google Scholar]
- 24.Keller T., Zeller T., Ojeda F., et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. Journal of the American Medical Association. 2011;306(24):p. 2684. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 25.Reichlin T., Irfan A., Twerenbold R., et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124(2):136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 26.Fan L. Y., Yu P., Yu S. S., et al. Age-specific 99th percentile cutoff of high-sensitivity cardiac troponin T for early prediction of non-st-segment elevation myocardial infarction (NSTEMI) in middle-aged patients. Journal of Clinical Laboratory Analysis. 2014;28(1):10–15. doi: 10.1002/jcla.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krintus M., Kozinski M., Boudry P., et al. Defining normality in a European multinational cohort: Critical factors influencing the 99th percentile upper reference limit for high sensitivity cardiac troponin I. International Journal of Cardiology. 2015;187:256–263. doi: 10.1016/j.ijcard.2015.03.282. [DOI] [PubMed] [Google Scholar]
- 28.Cardinaels E. P., Mingels A. M., Jacobs L. H., Meex S. J., Bekers O., van Dieijen-Visser M. P. A comprehensive review of upper reference limits reported for (high-)sensitivity cardiac troponin assays: the challenges that lie ahead. Clinical Chemistry and Laboratory Medicine. 2012;50(5):791–806. doi: 10.1515/cclm-2011-0895. [DOI] [PubMed] [Google Scholar]
- 29.Apple F. S., Collinson P. O., IFCC Task Force on Clinical Applications of Cardiac Biomarkers Analytical characteristics of high-sensitivity cardiac troponin assays. Clinical Chemistry. 2012;58(1):54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen K., Mair J., Giannitsis E., et al. How to use high-sensitivity cardiac troponins in acute cardiac care. European Heart Journal. 2012;33(18):2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 31.Clerico A., Lippi G. The state-of-the-art of “high-sensitivity” immunoassay for measuring cardiac troponin I and T. Journal of Laboratory and Precision Medicine. 2018;3:p. 53. doi: 10.21037/jlpm.2018.05.04. [DOI] [Google Scholar]
- 32.Wu A. H., Christenson R. H., Greene D. N., et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the academy of the american association for clinical chemistry and the task force on clinical applications of cardiac bio-markers of the international federation of clinical chemistry and laboratory medicine. Clinical Chemistry. 2018;64(4):645–655. doi: 10.1373/clinchem.2017.277186. [DOI] [PubMed] [Google Scholar]
- 33.Apple F. S., Jaffe A. S., Collinson P., et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clinical Biochemistry. 2015;48:201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Marzona I., Proietti M., Farcomeni A., et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: A systematic review and meta-analysis of 993,600 patients. International Journal of Cardiology. 2018;269:182–191. doi: 10.1016/j.ijcard.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Daniels L. B., Maisel A. S. Cardiovascular biomarkers and sex: the case for women. Nature Reviews Cardiology. 2015;12(10):588–596. doi: 10.1038/nrcardio.2015.105. [DOI] [PubMed] [Google Scholar]
- 36.Raymond I., Groenning B. A., Hildebrandt P. R., Nilsson J. C., Baumann M., Trawinski J., et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89(7):745–751. doi: 10.1136/heart.89.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada M., Shigematsu Y., Takezaki M., Ikeda S., Ogimoto A. Plasma levels of atrial and brain natriuretic peptides in apparently healthy subjects: Effects of sex, age, and hemoglobin concentration. International Journal of Cardiology. 2017;228:599–604. doi: 10.1016/j.ijcard.2016.11.197. [DOI] [PubMed] [Google Scholar]
- 38.Franconi F., Campesi I. Sex impact on biomarkers, pharmacokinetics and pharmacodynamics. Current Medicinal Chemistry. 2017;24(24) doi: 10.2174/0929867323666161003124616. [DOI] [PubMed] [Google Scholar]
- 39.Manson J. E., Bassuk S. S. Biomarkers of cardiovascular disease risk in women. Metabolism. 2015;64(3, supplement 1):S33–S39. doi: 10.1016/j.metabol.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collinson P. O., Heung Y. M., Gaze D., et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clinical Chemistry. 2012;58(1):219–225. doi: 10.1373/clinchem.2011.171082. [DOI] [PubMed] [Google Scholar]
- 41.Apple F. S., Simpson P. A., Murakami M. M. Defining the serum 99th percentile in a normal reference population measured by a high-sensitivity cardiac troponin I assay. Clinical Biochemistry. 2010;43(12):1034–1036. doi: 10.1016/j.clinbiochem.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 42.McKie P. M., Heublein D. M., Scott C. G., et al. Defining high-sensitivity cardiac troponin concentrations in the community. Clinical Chemistry. 2013;59(7):1099–1107. doi: 10.1373/clinchem.2012.198614. [DOI] [PubMed] [Google Scholar]
- 43.Kong Z., Nie J., Lin H., et al. Sex differences in release of cardiac troponin T after endurance exercise. Biomarkers. 2016;22:345–350. doi: 10.1080/1354750X.2016.1265007. [DOI] [PubMed] [Google Scholar]
- 44.Gore M. O., Seliger S. L., deFilippi C. R., et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. Journal of the American College of Cardiology. 2014;63(14):1441–1448. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apple F. S., Jaffe A. S. Men are different than women: It's true for cardiac troponin too. Clinical Biochemistry. 2014;47(10-11):867–868. doi: 10.1016/j.clinbiochem.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Slagman A., Searle J., Vollert J. O., et al. Sex differences of troponin test performance in chest pain patients. International Journal of Cardiology. 2015;187:246–251. doi: 10.1016/j.ijcard.2015.03.261. [DOI] [PubMed] [Google Scholar]
- 47.Cullen L. A., Mills N. L. Point: The use of sex-specific cutpoints for high-sensitivity cardiac troponin assays. Clinical Chemistry. 2016;63(1):261–263. doi: 10.1373/clinchem.2016.254672. [DOI] [PubMed] [Google Scholar]
- 48.Apple F. S., Quist H. E., Doyle P. J., Otto A. P., Murakami M. M. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clinical Chemistry. 2003;49(8):1331–1336. doi: 10.1373/49.8.1331. [DOI] [PubMed] [Google Scholar]
- 49.Apple F. S., Panteghini M., Ravkilde J., Mair J., Wu A. H. B., Tate J., et al. Quality specifications for B-type natriuretic peptide assays. Clinical Chemistry. 2005;51(3):486–493. doi: 10.1373/clinchem.2004.044594. [DOI] [PubMed] [Google Scholar]
- 50.Redfield M. M., Rodeheffer R. J., Jacobsen S. J., Mahoney D. W., Bailey K. R., Burnett J. C., Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. Journal of the American College of Cardiology. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 51.Wang T. J., Larson M. G., Levy D., et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. American Journal of Cardiology. 2002;90(3):254–258. doi: 10.1016/S0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 52.Wang T. J., Larson M. G., Levy D., et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. The New England Journal of Medicine. 2004;350(7):655–663. doi: 10.1056/nejmoa031994. [DOI] [PubMed] [Google Scholar]
- 53.Luchner A., Behrens G., Stritzke J., et al. Long-term pattern of brain natriuretic peptide and N-terminal pro brain natriuretic peptide and its determinants in the general population: contribution of age, gender, and cardiac and extra-cardiac factors. European Journal of Heart Failure. 2013;15(8):859–867. doi: 10.1093/eurjhf/hft048. [DOI] [PubMed] [Google Scholar]
- 54.Hallengren E., Almgren P., Engström G., et al. Fasting levels of high-sensitivity growth hormone predict cardiovascular morbidity and mortality. Journal of the American College of Cardiology. 2014;64(14):1452–1460. doi: 10.1016/j.jacc.2014.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho J. E., Liu C., Lyass A., et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. Journal of the American College of Cardiology. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniels L. B., Clopton P., Laughlin G. A., Maisel A. S., Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. American Heart Journal. 2014;167(5):674–682.e1. doi: 10.1016/j.ahj.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dieplinger B., Egger M., Poelz W., Gabriel C., Haltmayer M., Mueller T. Soluble ST2 is not independently associated with androgen and estrogen status in healthy males and females. Clinical Chemistry and Laboratory Medicine. 2011;49(9):1515–1518. doi: 10.1515/CCLM.2011.239. [DOI] [PubMed] [Google Scholar]
- 58.Coglianese E. E., Larson M. G., Vasan R. S., et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the framingham heart study. Clinical Chemistry. 2012;58(12):1673–1681. doi: 10.1373/clinchem.2012.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melander O., Belting M., Manjer J., et al. Validation of plasma proneurotensin as a novel biomarker for the prediction of incident breast cancer. Cancer Epidemiology Biomarkers & Prevention. 2014;23(8):1672–1676. doi: 10.1158/1055-9965.EPI-13-1200. [DOI] [PubMed] [Google Scholar]
- 60.Melander O., Maisel A. S., Almgren P., et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. Journal of the American Medical Association. 2012;308(14):p. 1469. doi: 10.1001/jama.2012.12998. [DOI] [PubMed] [Google Scholar]
- 61.Apple F. S., Ler R., Murakami M. M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clinical Chemistry. 2012;58(11):1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 62.Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A. S., Katus H. A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical Chemistry. 2010;56(2):254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 63.Koerbin G., Tate J., Potter J. M., Cavanaugh J., Glasgow N., Hickman P. E. Characterisation of a highly sensitive troponin I assay and its application to a cardio-healthy population. Clinical Chemistry and Laboratory Medicine. 2012;50(5):871–878. doi: 10.1515/cclm-2011-0540. [DOI] [PubMed] [Google Scholar]
- 64.Schwarzenberger J. C., Sun L. S., Pesce M. A., et al. Sex-based differences in serum cardiac troponin I, a specific marker for myocardial injury, after cardiac surgery. Critical Care Medicine. 2003;31(3):689–693. doi: 10.1097/01.CCM.0000055442.84685.4D. [DOI] [PubMed] [Google Scholar]
- 65.Salton C. J., Chuang M. L., O’Donnell C. J., et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. Journal of the American College of Cardiology. 2002;39(6):1055–1060. doi: 10.1016/S0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 66.Fernández-Jiménez R., López-Romero P., Suárez-Barrientos A., et al. Troponin release overestimates infarct size in presence of left ventricular hypertrophy. Journal of the American College of Cardiology. 2012;60(7):640–641. doi: 10.1016/j.jacc.2012.02.067. [DOI] [PubMed] [Google Scholar]
- 67.Piro M., Della Bona R., Abbate A., Biasucci L. M., Crea F. Sex-related differences in myocardial remodeling. Journal of the American College of Cardiology. 2010;55(11):1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 68.White H. D. Pathobiology of Troponin Elevations: Do editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. Journal of the American College of Cardiology. 2011;57(24):2406–2408. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 69.Ostadal B., Netuka I., Maly J., Besik J., Ostadalova I. Gender differences in cardiac ischemic injury and protection—experimental aspects. Experimental Biology and Medicine. 2009;234(9):1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- 70.Ostadal B., Ostadal P. Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. British Journal of Pharmacology. 2014;171(3):541–554. doi: 10.1111/bph.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raparelli V., Elharram M., Shimony A., Eisenberg M. J., Cheema A. N., Pilote L. Myocardial infarction with no obstructive coronary artery disease: Angiographic and clinical insights in patients with premature presentation. Canadian Journal of Cardiology. 2018;34(4):468–476. doi: 10.1016/j.cjca.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Mendelsohn M. E., Karas R. H. The protective effects of estrogen on the cardiovascular system. The New England Journal of Medicine. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 73.Sribhen K., Piyophirapong S., Wannasilp N. Cardiac troponin T concentrations in healthy adolescents. Clinica Chimica Acta. 2010;411:1542–1543. doi: 10.1016/j.cca.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 74.Cangemi R., Romiti G. F., Campolongo G., et al. Gender related differences in treatment and response to statins in primary and secondary cardiovascular prevention: The never-ending debate. Pharmacological Research. 2017;117:148–155. doi: 10.1016/j.phrs.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 75.Saenger A., Beyrau R., Braun S., et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clinica Chimica Acta. 2011;412:748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 76.Franzini M., Lorenzoni V., Masotti S., et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: A multicenter study in Italy. Clinica Chimica Acta. 2015;438:376–381. doi: 10.1016/j.cca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Mingels A., Jacobs L., Michielsen E., Swaanenburg J., Wodzig W., van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clinical Chemistry. 2008;55(1):101–108. doi: 10.1373/clinchem.2008.106427. [DOI] [PubMed] [Google Scholar]
- 78.Kimenai D. M., Henry R. M., van der Kallen C. J., et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart. 2016;102(8):610–616. doi: 10.1136/heartjnl-2015-308917. [DOI] [PubMed] [Google Scholar]
- 79.Koerbin G., Tate J. R., Hickman P. E. Analytical characteristics of the Roche highly sensitive troponin T assay and its application to a cardio-healthy population. Annals of Clinical Biochemistry. 2010;47(6):524–528. doi: 10.1258/acb.2010.010033. [DOI] [PubMed] [Google Scholar]
- 80.Mueller T., Egger M., Leitner I., Gabriel C., Haltmayer M., Dieplinger B. Reference values of galectin-3 and cardiac troponins derived from a single cohort of healthy blood donors. Clinica Chimica Acta. 2016;456:19–23. doi: 10.1016/j.cca.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Ungerer J. P., Tate J. R., Pretorius C. J. Discordance with 3 cardiac troponin I and T assays: Implications for the 99th percentile cutoff. Clinical Chemistry. 2016;62(8):1106–1114. doi: 10.1373/clinchem.2016.255281. [DOI] [PubMed] [Google Scholar]
- 82.Peacock W. F., Baumann B. M., Bruton D., et al. Efficacy of high-sensitivity troponin T in identifying very-low-risk patients with possible acute coronary syndrome. JAMA Cardiology. 2018;3(2):104–112. doi: 10.1001/jamacardio.2017.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koerbin G., Abhayaratna W. P., Potter J. M., et al. Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clinical Biochemistry. 2013;46:1636–1643. doi: 10.1016/j.clinbiochem.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Apple F. S., Sandoval Y., Jaffe A. S., Ordonez-Llanos J. Cardiac troponin assays: Guide to understanding analytical characteristics and their impact on clinical care. Clinical Chemistry. 2016;63(1):73–81. doi: 10.1373/clinchem.2016.255109. [DOI] [PubMed] [Google Scholar]
- 85.Aw T., Phua S., Tan S. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clinica Chimica Acta. 2013;422:26–28. doi: 10.1016/j.cca.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 86.Omland T., de Lemos J. A., Holmen O. L., et al. Impact of sex on the prognostic value of high-sensitivity cardiac troponin i in the general population: The hunt study. Clinical Chemistry. 2015;61(4):646–656. doi: 10.1373/clinchem.2014.234369. [DOI] [PubMed] [Google Scholar]
- 87.Zeller T., Ojeda F., Brunner F. J., et al. High-sensitivity cardiac troponin I in the general population – defining reference populations for the determination of the 99th percentile in the Gutenberg Health Study. Clinical Chemistry and Laboratory Medicine (CCLM) 2015;53(5):699–706. doi: 10.1515/cclm-2014-0619. [DOI] [PubMed] [Google Scholar]
- 88.Ji M., Moon H., Hur M., Yun Y. Determination of high-sensitivity cardiac troponin I 99th percentile upper reference limits in a healthy Korean population. Clinical Biochemistry. 2016;49:756–761. doi: 10.1016/j.clinbiochem.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 89.Li S., Zuo Y., Huang W. Establishment of a reference interval for high-sensitivity cardiac troponin I in healthy adults from the Sichuan area. Medicine. 2017;96(14):p. e6252. doi: 10.1097/MD.0000000000006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bossard M., Thériault S., Aeschbacher S., et al. Factors independently associated with cardiac troponin I levels in young and healthy adults from the general population. Clinical Research in Cardiology. 2017;106(2):96–104. doi: 10.1007/s00392-016-1026-5. [DOI] [PubMed] [Google Scholar]
- 91.Rubini Giménez M., Twerenbold R., Boeddinghaus J., et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin t in suspected myocardial infarction. JAMA Cardiology. 2016;1(8):p. 912. doi: 10.1001/jamacardio.2016.2882. [DOI] [PubMed] [Google Scholar]
- 92.Mueller-Hennessen M., Lindahl B., Giannitsis E., et al. Diagnostic and prognostic implications using age- and gender-specific cut-offs for high-sensitivity cardiac troponin T — Sub-analysis from the TRAPID-AMI study. International Journal of Cardiology. 2016;209:26–33. doi: 10.1016/j.ijcard.2016.01.213. [DOI] [PubMed] [Google Scholar]
- 93.Eggers K. M., Jernberg T., Lindahl B. Prognostic importance of sex-specific cardiac troponin t 99th percentiles in suspected acute coronary syndrome. American Journal of Medicine. 2016;129(8):880.e1–880.e12. doi: 10.1016/j.amjmed.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 94.McRae A., Graham M., Abedin T., et al. Sex-specific, high-sensitivity cardiac troponin T cut-off concentrations for ruling out acute myocardial infarction with a single measurement. CJEM. 2018:1–8. doi: 10.1017/cem.2018.435. [DOI] [PubMed] [Google Scholar]
- 95.Yang S., Huai W., Qiao R., et al. Age and gender tailored cutoff value of hs-ctnt contributes to rapidly diagnose acute myocardial infarction in chest pain patients. Clinical Laboratory. 2016;62:1451–1459. doi: 10.7754/Clin.Lab.2016.151201. [DOI] [PubMed] [Google Scholar]
- 96.Shah A. S., Anand A., Strachan F. E., et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. The Lancet. 2018;392(10151):919–928. doi: 10.1016/S0140-6736(18)31923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cullen L., Greenslade J. H., Carlton E. W., et al. Sex-specific versus overall cut points for a high sensitivity troponin I assay in predicting 1-year outcomes in emergency patients presenting with chest pain. Heart. 2016;102(2):120–126. doi: 10.1136/heartjnl-2015-308506. [DOI] [PubMed] [Google Scholar]
- 98.Shah A. S., Griffiths M., Lee K. K., et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:p. g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mueller T., Egger M., Peer E., Dieplinger B. 5th generation cardiac troponin I and T assays in clinical routine – A head-to-head comparison with data from the Linz troponin (LITROP) study. Clinica Chimica Acta. 2018;485:195–204. doi: 10.1016/j.cca.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 100.Eggers K. M., Johnston N., James S., Lindahl B., Venge P. Cardiac troponin I levels in patients with non–ST-elevation acute coronary syndrome—The importance of gender. American Heart Journal. 2014;168(3):317–324.e1. doi: 10.1016/j.ahj.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 101.May E. A. B., Bonaca M. P., Jarolim P., et al. Prognostic performance of a high-sensitivity cardiac troponin i assay in patients with Non-ST-Elevation acute coronary syndrome. Clinical Chemistry. 2014;60(1):158–164. doi: 10.1373/clinchem.2013.206441. [DOI] [PubMed] [Google Scholar]
- 102.Moehring B., Mueller M., Rubini Gimenez M., et al. Impact of gender-specific reference values of high-sensitivity troponin T on the prevalence and long-term outcome of acute myocardial infarction. European Heart Journal. 2013;34(suppl 1):P4042–P4042. doi: 10.1093/eurheartj/eht309.P4042. [DOI] [Google Scholar]
- 103.Clerico A., Zaninotto M., Ripoli A., et al. The 99th percentile of reference population for cTnI and cTnT assay: methodology, pathophysiology and clinical implications. Clinical Chemistry and Laboratory Medicine (CCLM) 2017;55(11):1634–1651. doi: 10.1515/cclm-2016-0933. [DOI] [PubMed] [Google Scholar]
- 104.Cediel G., Gonzalez-del-Hoyo M., Carrasquer A., Sanchez R., Boqué C., Bardají A. Outcomes with type 2 myocardial infarction compared with non-ischaemic myocardial injury. Heart. 2017;103(8):616–622. doi: 10.1136/heartjnl-2016-310243. [DOI] [PubMed] [Google Scholar]
- 105.Stein G. Y., Herscovici G., Korenfeld R., et al. Type-II myocardial infarction – patient characteristics, management and outcomes. PLoS ONE. 2014;9(1):p. e84285. doi: 10.1371/journal.pone.0084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pepine C. J., Ferdinand K. C., Shaw L. J., et al. Emergence of nonobstructive coronary artery disease. Journal of the American College of Cardiology. 2015;66(17):1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monneret D., Gellerstedt M., Bonnefont-Rousselot D. Determination of age- and sex-specific 99th percentiles for high-sensitive troponin T from patients: an analytical imprecision- and partitioning-based approach. Clinical Chemistry and Laboratory Medicine (CCLM) 2018;56(5):818–829. doi: 10.1515/cclm-2017-0256. [DOI] [PubMed] [Google Scholar]
- 108.Welsh P., Preiss D., Shah A. S., et al. Comparison between high-sensitivity cardiac troponin t and cardiac troponin i in a large general population cohort. Clinical Chemistry. 2018;64(11):1607–1616. doi: 10.1373/clinchem.2018.292086. [DOI] [PMC free article] [PubMed] [Google Scholar]