Abstract
Objectives
The aim of this study was to investigate the outcomes of left atrial appendage occlusion (LAAO) in high bleeding risk patients suffering atrial fibrillation (AF) and to analyze the different antithrombotic therapies following the intervention.
Background. Methods
This monocentric study included 68 patients with nonvalvular AF with an absolute contraindication to OAT or at high bleeding risk. Follow-up was done with a clinical visit at 3-6-12 months.
Results
Successful LAAO was achieved in 67/68 patients. At discharge, 32/68 patients were on dual antiplatelet therapy (APT), 34/68 were without any antithrombotic therapy or with a single antiplatelet drug, and 2/68 were on anticoagulant therapy. At three-month follow-up visit, 73.6% of the patients did not receive dual APT, of whom 14.7% had no thrombotic therapy and 58.9% were on single antiplatelet therapy. During a follow-up of 1.4 ± 0.9 years, 3/62 patients had late adverse effects (2 device-related thrombus without clinical consequences and 1 extracranial bleeding). The device-related thrombosis was not related to the antithrombotic therapy.
Conclusions
LAAO is feasible and safe and prevents stroke in patients with AF with contraindication to oral anticoagulant therapy. After LAAO, single antiplatelet therapy seems to be a safe alternative to dual antiplatelet therapy, especially in patients at high bleeding risk. No benefit has been observed with dual APT.
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its incidence and prevalence are constantly increasing [1]. The prevalence of AF in the general population is about 2% and increases with age, with a lifetime risk of about 15% [2]. Its prevalence has been projected to increase in the US to 12.1 million cases in 2030 [3].
AF is an independent risk factor for ischemic stroke and thromboembolic events, which significantly increase mortality and morbidity and can cause serious disabilities. Annual stroke rate in AF patients is about 5%, and at least 20% of all ischemic strokes are associated with AF [4]. Despite some risk factors have been identified [5–8], the natural history of its development is largely unpredictable. Oral anticoagulant therapy (OAT) is the cornerstone of management of AF patients at increased stroke risk [4]. CHA2DS2-VASc [9] is the thromboembolic risk assessment score recommended by the European Society of Cardiology [4], the American Heart Association [10], and the American College of Cardiology [10]. OAT is recommended in male patients with CHA2DS2-VASc ≥2 and in female patients with CHA2DS2-VASc ≥3; OAT should be considered in male patients with CHA2DS2-VASc =1 and in female patients with CHA2DS2-VASc =2. Two classes of antithrombotic drugs, Vitamin K Antagonists (VKA) and Nonvitamin K antagonist Oral Anticoagulants (NOACs), are recommended for the prevention of ischemic stroke in AF.
NOACs have proved equally effective with a lower risk of cerebral haemorrhage [11–15].
The decision to prescribe antithrombotic drugs must necessarily involve an assessment of the risk of stroke against the risk of major bleeding. The HAS-BLED [16] is the most widely used haemorrhagic risk score. A HAS-BLED score ≥3 identifies patients with high risk of haemorrhage, but this is not a criterion for exclusion from OAT [4]. Many other conditions confer an increased risk per se without affecting bleeding scores [17–22].
Left atrial appendage (LAA) occlusion offers an alternative mechanical approach [23, 24] to reduce cardioembolic risk in AF patients [25]. The rationale for LAA occlusion is based on the strong evidence that more than 90% of thrombi during nonvalvular AF originate in the LAA [26].
Currently, European guidelines recommended LAA occlusion in patients with AF and contraindications for long term anticoagulation (class IIb indication, level of evidence B) [4]. Clinical data are derived from real life registries due to the obvious difficulties to randomize patients with a contraindication to anticoagulation.
Compared to OAC therapy, LAA closure reduced the risk of life-threatening bleeding events, such as haemorrhagic stroke [27]. In the recently published EWOLUTION trial, LAA closure appeared safe and effective, obtaining an ischemic stroke rate as low as 1.1% [28]. Similarly, LAA closure with the ACP, now replaced by Amulet device, showed a favourable outcome for the prevention of AF-related thromboembolism [29].
After LAA occlusion, one of the most important problem is thrombus formation on the device surface.
Current manufacturer's instructions recommend the continuation of dual antiplatelet therapy (APT) for at least 3 months after the procedure. However, this therapeutic strategy might be a problem, considering their intrinsic high risk of bleeding.
In this study, we aimed to assess the feasibility and the safety of LAA closure and to compare the different strategies for postimplant antithrombotic therapy in a high bleeding risk population.
2. Methods
Sixty-eight patients underwent LAA occlusion between February 2014 and October 2017. All the procedures were performed at the regional referral Center of Ospedale San Francesco, Nuoro, Italy, by two operators (GC and PM). Eight patients were treated with ACP™ and AMPLATZER™ Amulet™ device (St. Jude Medical, St. Paul, MN, USA) and 60 patients with WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA). Anthropometric and clinical data and indication to LAA closure were collected. CHA2DS2-VASc and HAS-BLED scores were calculated in each patient. Transesophageal echocardiography (TEE) was performed the day before the procedure to rule out LAA thrombus and to get accurate device sizing. Each procedure was performed under general anesthesia by femoral vein approach and transseptal puncture under fluoroscopic and TEE guidance. Five thousand units of heparin were administered intravenously. The activated clotting time was kept above 250 seconds during the procedure. Transthoracic echocardiography or TEE was performed at day 1 after implantation to confirm appropriate device implantation and to exclude residual device-related leak or thrombosis.
Technical success was defined as the successful implantation of device. Procedural success was defined as technical success without major procedure-related complications.
Postimplant antithrombotic was precribed and tailored according to the risk of bleeding of each patient. In particular, the choice of therapy was based on the history of previous major bleeding and global bleeding risk.
Events were labelled as “early” if they occurred within 7 days of the procedure or before discharge and “late” when they occurred after 7 days. Peripheral embolism, death, haemorrhagic stroke, and persistent need for OAT were also recorded.
Primary indication for LAA closure was considered in patients with two or more indications.
Patients signed a written informed consent before undergoing the procedure.
Patients were followed clinically at 1, 3, 6, and 12 months. TEE was performed at 3 and 12 months.
2.1. Statistical Analysis
Continuous traits were reported as mean and standard deviation. Categorical traits were reported as absolute frequency and percentages. The expected incidence of thromboembolic or bleeding events were calculated as the mean of each individual annual risk according to the patient's CHA2DS2-VASc and HAS-BLED scores. Thromboembolism reduction was calculated as follows: (expected % − observed % event rate)/expected % event rate. All analyses were conducted using Stata 12.0 statistical package.
3. Results
3.1. Patients
Mean age was 73.6 ± 8.7 years and 19.1% were female. The sample had a very high annual stroke risk (4.4%) and a very high estimated bleeding risk (5.8%), as shown in Table 1. Major comorbidities were ischemic heart disease (33.8%) and end-stage chronic kidney disease (33.8%).
Table 1.
CHAD2DS2-VASc and HAS-BLED scores.
| Value | CHAD2DS2-VASc | HAS-BLED |
|---|---|---|
| 0 | 1 (1.5) | N/A |
| 1 | N/A | 1 (1.5%) |
| 2 | 15 (22.1%) | 17 (25.0%) |
| 3 | 14 (20.6%) | 26 (38.2%) |
| 4 | 17 (25.0%) | 15 (22.1%) |
| 5 | 16 (23.5%) | 9 (13.2%) |
| 6 | 3 (4.4%) | N/A |
| 7 | 2 (2.9%) | N/A |
|
| ||
| Mean | 3.7 ± 1.4 | 3.2 ± 1.0 |
| Predicted annual risk | 4.4% | 5.8% |
Indications for LAA closure were a history of intracranial haemorrhage (32.4%), history of ischemic stroke during anticoagulant therapy (4.4%), high risk of bleeding (23.4%), end-stage chronic kidney disease (19.1%), and chronic liver disease and labile INR (10.3%). However, two or more indications frequently coexisted, thereby highlighting the extreme frailty of these patients (Figure 1).
Figure 1.
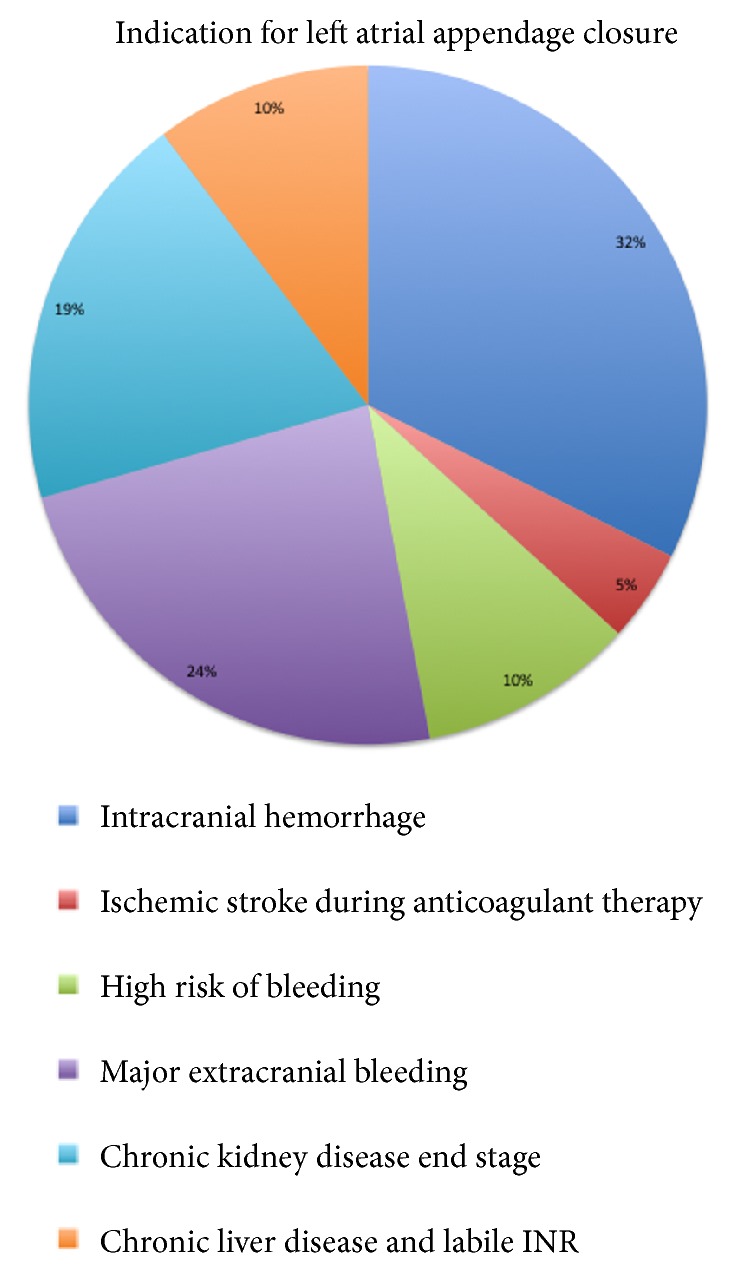
Indication for left atrial appendage closure. Main indications for LAA occlusion were a history of intracranial haemorrhage, high risk of bleeding, end-stage chronic kidney disease and chronic liver disease. All the patients had a contraindication to oral anticoagulant therapy.
LAA morphology, established with a TEE and angiography combination, was as follows: 14 subjects (20.6%) having cactus type, 20 (29.4%) windsock, 17 (25.0%) chicken wing, and 17 (25.0%) cauliflower.
Successful LAA closure was achieved in 67/68 patients.
3.2. Procedure and Periprocedural Events
Two patients experienced major periprocedural complications. The first one had a periprocedural embolic stroke which improved after mechanical thrombectomy. Notably, any thrombotic formation had been shown by intraprocedural TEE. No neurological deficit was reported at the follow-up neurologic visit. The other patient developed massive intracranial haemorrhage as a consequence of dual APT. This patient was affected by cerebral amyloid angiopathy and experienced the event the day after the procedure, approximately 24 hours after the first administration of the antiplatelet therapies.
3.3. Pharmacological Therapy and Follow-Up
At discharge, only 32 patients (47.1%) were on dual APT. About 50% of the sample was discharged without any antithrombotic therapy (7.3%) or with a single antiplatelet drug (42.7%), as described in Table 2. Two patients were discharged with an anticoagulant therapy: one for a concomitant procedure of AF ablation and one for a recently diagnosed deep vein thrombosis. Both patients were treated with DOACs for three months. At three months follow-up visit, 73.6% of the patients did not receive dual APT, of whom 14.7% had no thrombotic therapy and 58.9% were on single antiplatelet therapy. At 12 months of follow-up, only 8 patients were receiving dual APT; the main indication for dual APT persistence was generally represented by the presence of a coronary stent.
Table 2.
Antithrombotic therapy before and after left atrial appendage closure.
| Pretreatment | Posttreatment, discharge | Posttreatment, 3 months | Posttreatment, 6/12 months | |
|---|---|---|---|---|
| n | 68 | 68 | 68 | 62 |
| No antithrombotic therapy | 16 (23.5%) | 5 (7.3%) | 10 (14.7%) | 20 (32.3%) |
| Single anti-platelet therapy | 12 (17.7%) | 29 (42.7%) | 40 (58.9%) | 34 (54.8%) |
| Dual anti-platelet therapy | 23 (33.8%) | 32 (47.1%) | 16 (23.5%) | 8 (12.9%) |
| Anticoagulant therapy | 17 (25.0%) | 2 (2.9%) | 2 (2.9%) | 0 (0.0%) |
Six patients had incomplete follow-up: 5 had the LAA closure less than one year ago and 1 patient was lost. During the follow-up (mean time was 1.4 ± 0.9 years), three patients out of 62 had late adverse effects. Two of them had device-related thrombus without clinical consequences and one had extracranial bleeding. Two device-related thromboses were detected accidentally at TEE in patients implanted with WATCHMAN device. This finding was apparently not related to antithrombotic therapy. Indeed, one patient was receiving dual APT (ticagrelor twice a day and aspirin) and one was receiving a DOAC at the appropriate dose. The first one was treated adding enoxaparin to dual APT; the other was treated replacing DOAC with enoxaparin. In both cases thrombus was dissolved after another month of therapy with no clinical consequences. The patient who developed extracranial bleeding was affected by haemophilia and was discharged without any antithrombotic therapy. Three patients died during follow-up, but none of the deaths were related to the procedure or device. Indeed, two patients died of cancer and one of hepatic failure.
3.4. Prevention of Thromboembolic and Haemorrhagic Events
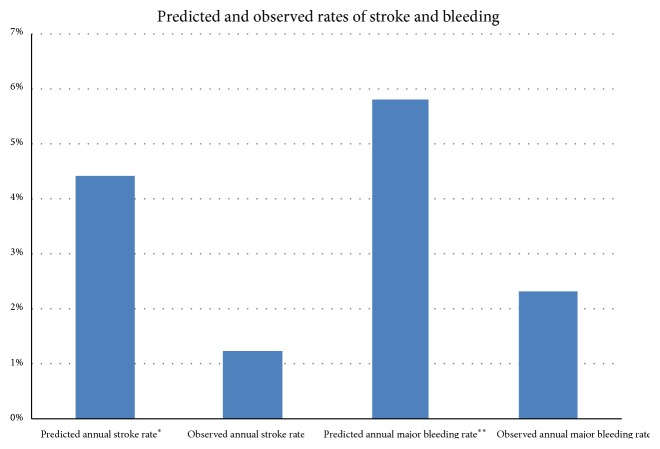
Considering the expected major bleeding rate of 6%, as calculated by HAS-BLED score, and the observed annual major bleeding rate of 2%, we obtained a 60% reduction in bleeding events (Figure 2). The expected annual risk of thromboembolism of the sample, according to the CHA2DS2-VASc score, was 4.4%. We recorded only one thromboembolic cerebral event in periprocedural setting and any cerebral event during the follow-up.
Figure 2.
Predicted and observed rates of stroke and major bleeding. Effectiveness and safety of LAAO in reducing thromboembolic events and haemorrhagic complications. ∗Calculated from CHAD2DS2-VASc score. ∗∗Calculated from HAS-BLED score.
4. Discussion
We described a single-center cohort of patients who underwent percutaneous LAA occlusion.
Safety of the procedure was confirmed by the low frequency of early complications. Indeed, we achieved a successful device implantation in 67 out of 68 patients (98.5%), which is higher in comparison to the 91% in the PROTECT AF study and to the 95% in the CAP Registry [30], but comparable to the 98.5% of EWOLUTION registry [31].
Percutaneous LAA closure is a relatively safe technique with complications mostly related to the operator's experience [32]. In our Center, the procedures were performed only by two skilled operators, thus explaining the high frequency of successful device implantation.
The current instructions for use, provided by manufacturers and updated after publication of follow-up results of EWOLUTION trial [28], suggest at least three months dual APT after device implantation. The goal of antiplatelet treatment following implantation of intracardiac device is to prevent thrombus formation on its surface before its complete endothelialization [23, 25]. In our study, 2 patients had device-related thrombus (3.2%). This result is similar to those obtained in other studies and confirmed in a recent large meta-analysis, which showed an overall incidence of 3.9% with a low rate of neurological complications [33]. As reported by other trials and registries [28, 33, 34], the device-related thrombosis in our study was apparently not related to the antithrombotic therapy. Indeed, one patient was receiving dual APT for acute coronary syndrome and the other one was receiving OAT for deep venous thrombosis.
About 50% of the population study was discharged without any antithrombotic therapy or with a single APT and this proportion grew to 73.6% at three-month follow-up visit. Nevertheless, our rate of device-related thrombosis is similar to those obtained in other studies with higher prescription rates of dual APT. For example, in EWOLUTION trial [28] the average time to dual APT discontinuation was 6 months and only 13% of patients were discharged on single antiplatelet therapy or with any antithrombotic drug. Similar observations have been made in studies on antithrombotic therapy after transcatheter aortic valve implantation and a recent large meta-analysis confirmed the lack of benefit from dual APT compared to single APT in these patients [35]. This and other recent observations [36] suggest that dual APT following devices implantation may be an overtreatment. A possible explanation is that the mechanism leading to device thrombosis in the left atrium is different from those that cause stent thrombosis in coronary arteries. Indeed, while coronary stent thrombosis is linked to a high shear stress with consequent platelet activation, the left atrium thrombosis is associated with a very low shear stress with the formation of a fibrin-rich clot [37]. Thus, it is possible that dual APT, that is extremely effective in preventing coronary stent thrombosis, could have only a small impact in preventing atrial device thrombus formation, causing rather an increase in bleeding.
This exploratory hypothesis requires large confirmatory trials that compare dual APT, single APT, and OAT following the LAA occlusion.
The very low rate of antithrombotic drugs prescription in our study was due to patient selection. Indeed, population study was at very high bleeding risk. Mean HAS-BLED score was 3.2 ± 1.0 and the proportion of patients with a HAS-BLED score ≥3 was 73.5% (comparing the EWOLUTION trial [31], mean HAS-BLED score was 2.3 ± 1.2 and proportion of patients with a HAS-BLED score ≥3 was 40%). Another relevant result of our study is the 60% reduction in the observed bleeding rate. Confirming previous data, our findings showed that LAA occlusion is associated with a significant reduction in bleeding [29, 36], mainly driven by the discontinuation of antithrombotic drug.
4.1. Limitations
We acknowledged some limitations of this study. First, the lack of randomization does not allow a control group treatment and the comparison of events (bleeding and ischemic stroke) has been based on estimated scores. In addition, the relative short period of follow-up might increase the possibility of underestimating event rates, although it is plausible that the first month after the procedure represents the most critical period after LAA closure.
5. Conclusions
LAA occlusion is feasible and safe and prevents stroke in patients with AF with contraindication to OAT. Although it is possible that after LAA occlusion single antiplatelet therapy is effective as dual antiplatelet therapy, specific studies on the topic are needed.
Abbreviations
- LAA:
Left atrial appendage
- LAAO:
Left atrial appendage occlusion
- AF:
Atrial fibrillation
- OAT:
Oral anticoagulant therapy
- VKA:
Vitamin K antagonists
- NOACs:
Nonvitamin k antagonist Oral Anticoagulants
- ACP:
AMPLATZER™ Cardiac Plug
- TEE:
Transesophageal echocardiography.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Pierluigi Merella and Gavino Casu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects.
Conflicts of Interest
Dr. Gavino Casu is a proctor for Boston Scientific and received fees as a consultant. Dr. Patrizio Mazzone is a proctor for Boston Scientific and St. Jude Medical. The other authors declared no conflicts of interest. The authors declared having no financial support for this paper.
Authors' Contributions
All the authors contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
References
- 1.Miyasaka Y., Barnes M. E., Gersh B. J., et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(11):119–125. doi: 10.1161/CIRCULATIONAHA.106.178309. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J., van der Kuip D. A. M., Hofman A., et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. European Heart Journal. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 3.Colilla S., Crow A., Petkun W., Singer D. E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. The American Journal of Cardiology. 2013;112(8):1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European heart journal. 2016;37(6):2893–2962. doi: 10.1016/j.crvasa.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Delitala A. P. Subclinical Hyperthyroidism and the Cardiovascular Disease. Hormone and Metabolic Research. 2017;49(10):723–731. doi: 10.1055/s-0043-117893. [DOI] [PubMed] [Google Scholar]
- 6.Chao T.-F., Lip G. Y. H., Liu C.-J., et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. Journal of the American College of Cardiology. 2018;71(2):122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 7.Shang W., Li L., Huang S., et al. Chronic kidney disease and the risk of new-onset atrial fibrillation: a meta-analysis of prospective cohort studies. PLoS One. 2016;11(5):p. e0155581. doi: 10.1371/journal.pone.0155581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller J. D., Aronis K. N., Chrispin J., et al. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. Journal of the American College of Cardiology. 2015;66(25):2899–2906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Lip G. Y., Nieuwlaat R., Pisters R., Lane D. A., Crijns H. J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 10.January C. T., Wann L. S., Alpert J. S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 11.Connolly S. J., Ezekowitz M. D., Yusuf S., et al. Dabigatran versus warfarin with atrial fibrillation. The New England Journal of Medicine. 2009;361:1139–1151. doi: 10.1517/14656560903530691. [DOI] [PubMed] [Google Scholar]
- 12.Patel M. R., Mahaffey K. W., Garg J., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. The New England Journal of Medicine. 2011;365(24):883–891. doi: 10.1056/NEJMc1112233. [DOI] [PubMed] [Google Scholar]
- 13.Granger C. B., Alexander J. H., McMurray J. J., et al. Apixaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2011;365:981–992. doi: 10.1517/13543784.2012.696611. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano R. P., Ruff C. T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. The New England Journal of Medicine. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 15.Ruff C. T., Giugliano R. P., Braunwald E., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. The Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 16.Lip G. Y. H., Frison L., Halperin J. L., Lane D. A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score. Journal of the American College of Cardiology. 2011;57(2):173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Merella P., Lorenzoni G., Marziliano N., et al. Nonvalvular atrial fibrillation in high-hemorrhagic-risk patients: state of the art of percutaneous left atrial appendage occlusion. Journal of Cardiovascular Medicine. 2018;20(1):1–9. doi: 10.2459/JCM.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 18.Weis N., Cowan S., Hallager S., et al. Vertical transmission of hepatitis B virus during pregnancy and delivery in Denmark. Scandinavian Journal of Gastroenterology. 2017;52(2):178–184. doi: 10.1080/00365521.2016.1244704. [DOI] [PubMed] [Google Scholar]
- 19.DeSimone C. V., Graff-Radford J., El-Harasis M. A., Rabinstein A. A., Asirvatham S. J., Holmes D. R. Cerebral amyloid angiopathy: diagnosis, clinical implications, and management strategies in atrial fibrillation. Journal of the American College of Cardiology. 2017;70(9):1173–1182. doi: 10.1016/j.jacc.2017.07.724. [DOI] [PubMed] [Google Scholar]
- 20.Ronco F., Mazzone P., Hosseinian L., Genovesi S. Recent advances in stroke prevention in patients with atrial fibrillation and end-stage renal disease. Cardiorenal Medicine. 2017;7(3):207–217. doi: 10.1159/000470856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merella P., Casu G., Mazzone P., et al. Atrial fibrillation in severe and end stage renal disease: from oral anticoagulation therapy to percutaneous left atrial appendage occlusion. Giornale Italiano Di Nefrologia: Organo Ufficiale Della Societa Italiana Di Nefrologia. 2019 doi: 10.1177/0394936218823523. [DOI] [PubMed] [Google Scholar]
- 22.Genovesi S., Slaviero G., Porcu L., et al. Implant success and safety of left atrial appendage occlusion in end stage renal disease patients: Peri-procedural outcomes from an Italian dialysis population. International Journal of Cardiology. 2018;262:38–42. doi: 10.1016/j.ijcard.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 23.Casu G., Gulizia M. M., Molon G., et al. ANMCO/AIAC/SICI-GISE/SIC/SICCH consensus document: percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation patients: Indications, patient selection, staff skills, organisation, and training. European Heart Journal Supplements: Journal of the European Society of Cardiology. 2017;19:D333–D353. doi: 10.1093/eurheartj/sux008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casu G., Gulizia M. M., Molon G., et al. ANMCO/AIAC/SICI-GISE/SIC/SICCH Consensus document: Percutaneous left atrial appendage occlusion in patients with nonvalvular atrial fibrillation: Indications, patient selection, competences, organization, and operator training. Giornale Italiano di Cardiologia. 2016;17(7-8):594–613. doi: 10.1714/2330.25054. [DOI] [PubMed] [Google Scholar]
- 25.Meier B., Blaauw Y., Khattab A. A., et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace. 2014;16(10):1397–1416. doi: 10.1093/europace/euu174. [DOI] [PubMed] [Google Scholar]
- 26.Blackshear J. L., Odell J. A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. The Annals of Thoracic Surgery. 1996;61(2):755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 27.Reddy V. Y., Doshi S. K., Sievert H., et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation 2.3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013;127(6):720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 28.Boersma L. V., Ince H., Kische S., et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14(9):1302–1308. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Tzikas A., Shakir S., Gafoor S., et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 30.Reddy V. Y., Holmes D., Doshi S. K., Neuzil P., Kar S. Safety of percutaneous left atrial appendage closure: Results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011;123(4):417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 31.Boersma L. V. A., Schmidt B., Betts T. R., et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. European Heart Journal. 2016;37(31):2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzoni G., Merella P., Pischedda P., Casu G. Percutaneous Management of Left Atrial Appendage Perforation: Keep Calm and Think Fast. Journal of Invasive Cardiology. 2018;30:E126–E127. [PubMed] [Google Scholar]
- 33.Lempereur M., Aminian A., Freixa X., et al. Device-associated thrombus formation after left atrial appendage occlusion: A systematic review of events reported with the Watchman, the Amplatzer Cardiac Plug and the Amulet. Catheterization and Cardiovascular Interventions. 2017;90(1):E111–E121. doi: 10.1002/ccd.26903. [DOI] [PubMed] [Google Scholar]
- 34.Bergmann M. W., Betts T. R., Sievert H., et al. Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: Three-month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroIntervention. 2017;13(7):877–884. doi: 10.4244/EIJ-D-17-00042. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad Y., Demir O., Rajkumar C., et al. Optimal antiplatelet strategy after transcatheter aortic valve implantation: a meta-analysis. Open Heart. 2018;5(1):p. e000748. doi: 10.1136/openhrt-2017-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korsholm K., Nielsen K. M., Jensen J. M., Jensen H. K., Andersen G., Nielsen-Kudsk J. E. Transcatheter left atrial appendage occlusion in patients with atrial fibrillation and a high bleeding risk using aspirin alone for post-implant antithrombotic therapy. EuroIntervention:Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2017;12(17):2075–2082. doi: 10.4244/EIJ-D-16-00726. [DOI] [PubMed] [Google Scholar]
- 37.Reed G. W., Cannon C. P. Triple oral antithrombotic therapy in atrial fibrillation and coronary artery stenting. Clinical Cardiology. 2013;36:585–594. doi: 10.1002/clc.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.