Abstract
Background
Acute myocardial infarction (AMI) is a common disease with high morbidity and mortality around the world. The aim of this research was to determine the differentially expressed genes (DEGs), which may serve as potential therapeutic targets or new biomarkers in AMI.
Methods
From the Gene Expression Omnibus (GEO) database, three gene expression profiles (GSE775, GSE19322, and GSE97494) were downloaded. To identify the DEGs, integrated bioinformatics analysis and robust rank aggregation (RRA) method were applied. These DEGs were performed through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses by using Clusterprofiler package. In order to explore the correlation between these DEGs, the interaction network of protein-protein internet (PPI) was constructed using the STRING database. Utilizing the MCODE plug-in of Cytoscape, the module analysis was performed. Utilizing the cytoHubba plug-in, the hub genes were screened out.
Results
57 DEGs in total were identified, including 2 down- and 55 upregulated genes. These DEGs were mainly enriched in cytokine-cytokine receptor interaction, chemokine signaling pathway, TNF signaling pathway, and so on. The module analysis filtered out 18 key genes, including Cxcl5, Arg1, Cxcl1, Spp1, Selp, Ptx3, Tnfaip6, Mmp8, Serpine1, Ptgs2, Il6, Il1r2, Il1b, Ccl3, Ccr1, Hmox1, Cxcl2, and Ccl2. Ccr1 was the most fundamental gene in PPI network. 4 hub genes in total were identified, including Cxcl1, Cxcl2, Cxcl5, and Mmp8.
Conclusion
This study may provide credible molecular biomarkers in terms of screening, diagnosis, and prognosis for AMI. Meanwhile, it also serves as a basis for exploring new therapeutic target for AMI.
1. Introduction
Acute myocardial infarction (AMI), which represents the main public health issue around the world, is a common cardiac emergency with substantial morbidity and mortality. In the last two or three decades, although a downtrend of AMI has been observed because of the economic development and advances in medical science, its morbidity is still very high at about 44.57 in 100,000 people in China in 2013 [1]. Besides, the death rate of AMI was estimated to increase by 5.6 times from 1987 to 2014 [2]. Therefore, it is growing important for AMI to develop an early diagnosis and proper treatment strategy to prevent the occurrence of sudden mortality.
Fortunately, with the development of gene chip technique, more and more gene expression spectra were tested by gene chip technique in cardiovascular clinic and study. Microarray analysis was widely used in peripheral blood of patients with myocardial infarction [3] and the myocardium of mice [4]. Through microarray analysis, the potential genes associated with AMI will be obtained. For example, through the microarray analysis of GSE48060, Yuan Gao et al. [3] found that the MAX, BCL3, NCOA7, CCL5, and GTF3C2 might play a key role in AMI development, which provided valuable reference for future research. Many studies have found that early growth response factor 1 (EGR1) induces myocardial injury after AMI. Using bioinformatics analysis, Pan et al. [5] found that miR-146a can regulate the expression of EGR1. It offers help as treatment for AMI. Under many stringent states, including ischemia reperfusion, Heat shock proteins (Hsps) are produced. Novo G et al. [6] expounded the clinical significance and pathogenetic role of Hsp60 and HO-1 in AMI using bioinformatics analysis. Heart failure (HF) is a common complication after AMI. Qian C et al. [7] found that the DEGs, including FOS, THBS1, CXCL8, and ITGA2B from the microarray data of GSE59867, may play a vital role in the occurrence and development of HF after AMI. In recent years, integrated bioinformatics analysis method is heavily used in cancer. For example, utilizing integrated bioinformatics analysis method, Guangwei et al. [8] reported the novel therapeutic targets for colorectal neoplasms. However, integrated bioinformatics analysis method is rarely employed in cardiovascular disease.
In this research, three gene expression datasets, including GSE775, GSE19322, and GSE97494, were downloaded from the GEO database. These datasets were screened to identify the DEGs in each dataset. Next, using the RRA approach [9], a total of 57 DEGs, including 2 down- and 55 upregulated genes, were identified. Using Clusterprofiler [10], GO and KEGG analyses were performed, respectively. It was obviously shown that these DEGs were enriched in AMI-related functions and pathways. Then the PPI network was established by using the STRING database. The module analysis filtered out 18 key genes, including Cxcl5, Arg1, Cxcl1, Spp1, Selp, Ptx3, Tnfaip6, Mmp8, Serpine1, Ptgs2, Il6, Il1r2, Il1b, Ccl3, Ccr1, Hmox1, Cxcl2, and Ccl2. Ccr1 was the most fundamental gene in PPI network. 4 hub genes in total were identified, including Cxcl1, Cxcl2, Cxcl5, and Mmp8. Our result may provide a novel pathway for diagnosis and treatment of the AMI in the future.
2. Methods
2.1. Affymetrix Macroarray Data
Utilizing the keywords “myocardial infarction,” we screened the GEO database. Three GEO datasets were found, including GSE775 contributed by Schinke et al., GSE19322 contributed by Hunt et al., and GSE97494 contributed by Chikata et al. These gene expression profiles of AMI were downloaded based on GPL81 platform of Affymetrix Murine Genome U74A Version 2 Array, GPL339 platform of Affymetrix Mouse Expression 430A Array, and GPL6246 platform of Affymetrix Mouse Gene 1.0 ST Array, respectively. There were 18 samples that were from the region between the LAD artery and the apex of the mice, 9 mice within 24 hours after AMI and 9 sham-operated mice within 24 hours. Detailed information about the datasets is listed in Table 1. Through the R software package, the download files were handled.
Table 1.
Details for GEO AMI data.
| Reference | Sample | GEO | Platform | AMI | sham-operation |
|---|---|---|---|---|---|
| Schinke et al. (2003) | LV myocardium | GSE775 | GPL81 | 3 | 3 |
| Hunt et al. (2010) | LV myocardium | GSE19322 | GPL339 | 4 | 4 |
| Chikata et al. (2017) | LV myocardium | GSE97494 | GPL6246 | 2 | 2 |
Abbreviation: GEO, Gene Expression Omnibus. AMI, acute myocardial infarction. LV, left ventricular.
2.2. Screening for DEGs
In order to find out DEGs of each GEO dataset, utilizing the R software and annotation package, the platform and series matrix file(s) were converted. These DEGs in AMI and sham operation group samples were analyzed by utilizing the limma package [11] in R. Log2(fold change) (log2FC) > 1 and a corrected p value < 0.05 were used as the cut-off criteria of DEGs samples.
2.3. Integration of Microarray Data
Through limma packet analysis, we obtained the list of DEGs of the three microarray datasets. The list of down- and upregulated genes in the microarray data was saved. Subsequently, using the RRA approach, the comparison of multiple ranked gene lists was performed.
2.4. GO and KEGG Pathway Enrichment Analyses
Biological functions of the DEGs obtained from the integration of microarray data were explored with GO analysis using Clusterprofiler which is an R package utilized to compare the biological themes among gene clusters. Similarly, in order to identify the enrichment signaling pathways of DEGs, KEGG pathway analysis was performed by utilizing the Clusterprofiler package. A corrected p < 0.05 was the cut-off criterion.
2.5. PPI Network Integration, Modules Analysis, and Selection of Hub Genes
In order to identify the interaction between PPI, the PPI network was built using the STRING (version 11) online database. The highest confidence of the argument of interactions was set at >0.4. To draw an interaction of DEGs, the Cytoscape (version 3.6.1) software was used to visualize and analyse the PPI network. In order to find modules of the whole network, the Molecular Complex Detection (MCODE) plug-in of the Cytoscape software was applied. The hub genes were identified by using the plug-in cytoHubba [12] of the Cytoscape software, including Density of Maximum Neighborhood Component (DMNC) and Maximal Clique Centrality (MCC).
3. Results
3.1. Identification of DEGs in GSE775, GSE19322, and GSE97494
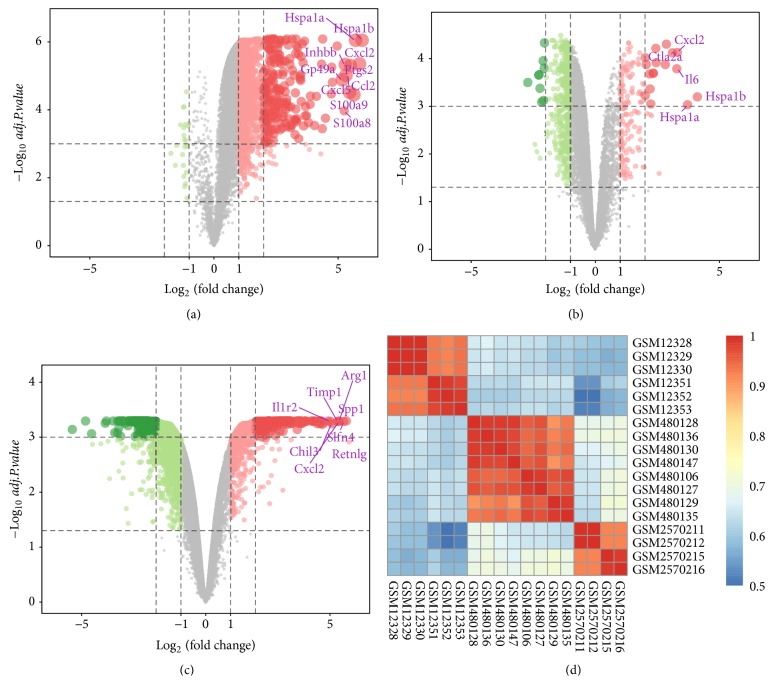
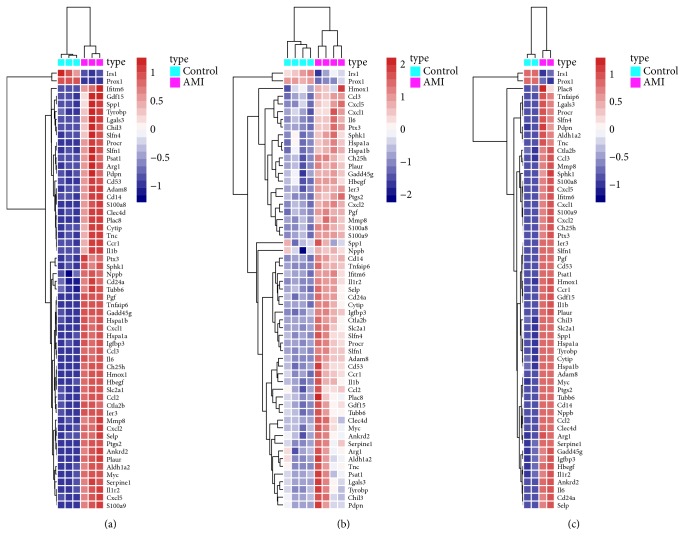
Three expression microarray datasets, including GSE775, GSE19322, and GSE97494, were used to perform background correction and quartile data normalization by the limma package. Meanwhile, using the limma package (log2FC >1, corrected p <0.05), the GSE775 dataset was screened and 2149 DEGs were obtained, including 23 down- and 2126 upregulated genes. Using the same methodology, 597 DEGs were obtained from the GSE19322 dataset, including 446 down- and 151 upregulated genes, and 4534 DEGs were confirmed from the GSE97494 dataset, including 3879 down- and 655 upregulated genes. Many DEGs in two sets of sample data of each microarray, three microarrays in total, are shown in Figures 1(a)–1(c), also known as the volcano plots of DEGs. In order to evaluate the biological repeatability, we drew an association diagram, which indicated that the biological repeatability of the sample was well, as shown in Figure 1(d). Three cluster heatmaps of the 57 DEGs in each microarray are shown in Figures 2(a)–2(c).
Figure 1.
Volcano plot of gene expression profile data in AMI samples and sham-operation ones and correlation coefficient analysis diagram. Notes: (a) volcano plot of GSE775, (b) volcano plot of GSE19322, and (c) volcano plot of GSE97494. The red, green, and gray points represent upregulated genes, downregulated genes, and nondifferentially expressed genes, respectively. They are screened on the basis of log2FC >1.0 and a corrected p <0.05. (d) Correlation coefficient analysis diagram. Each column and row, respectively, represents one sample. Red represents strong correlation between samples and grey represents weak correlation between samples. Different shades of colors indicate the different correlation degree. Abbreviation: AMI, acute myocardial infarction; DEGs, differentially expressed genes; FC, fold change.
Figure 2.
Hierarchical clustering heatmap of DEGs, which was screened on the basis of log2FC >1.0 and a corrected p <0.05. Notes: (a) GSE775 data, (b) GSE19322 data, and (c) GSE97494 data. Red represents that the expression of genes is relatively upregulated. Blue represents that the expression of genes is relatively downregulated. Gray represents the expression of genes without significant changes. Abbreviation: DEGs, differentially expressed genes; FC, fold change.
3.2. Identification of DEGs in AMI Utilizing Integrated Bioinformatics Analysis
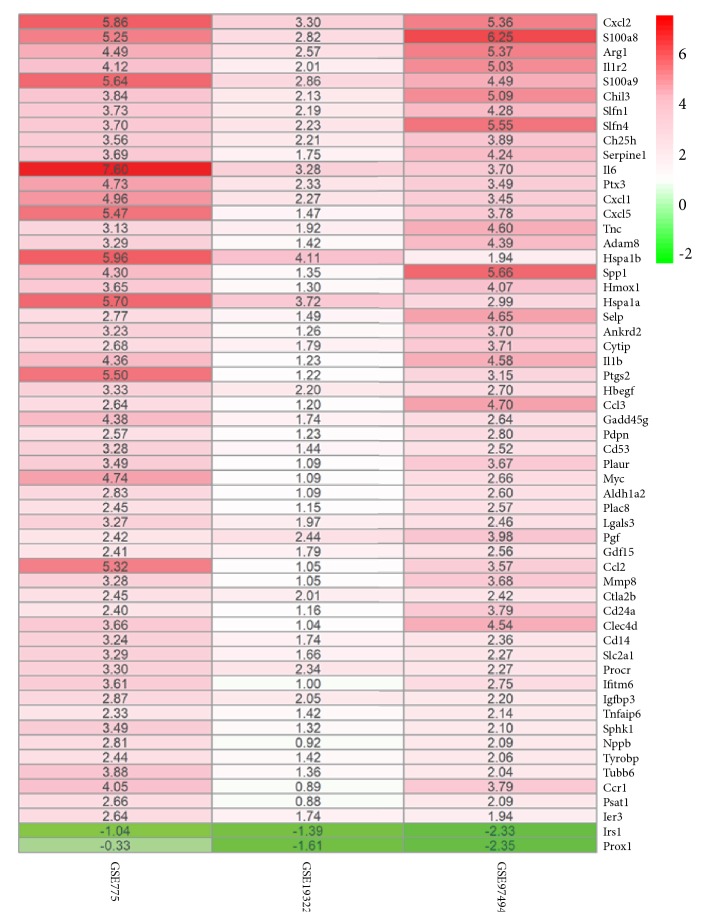
Using the RRA method according to Log2FC >1 and a corrected p <0.05, the list of DEGs of the three microarray datasets were analyzed. A total of 57 DEGs were determined by rank analysis, including 2 down- and 55 upregulated genes, as shown in Table 2. The heatmap of the 57 DEGs was drawn by heatmap package, which is shown in Figure 3.
Table 2.
Screening DEGs in AMI by integrated microarray.
| DEGs | Gene names |
|---|---|
| Upregulated | Cxcl2,S100a8,Arg1,Il1r2,S100a9, Chil3,Slfn1,Slfn4,Ch25h,Serpine1,Il6,Ptx3,Cxcl1, Cxcl5,Tnc,Adam8,Hspa1b,Spp1,Hmox1,Hspa1a, Selp,Ankrd2,Cytip,Il1b,Ptgs2,Hbegf,Ccl3, Gadd45g,Pdpn,Cd53,Plaur,Myc,Aldh1a2,Plac8, Lgals3,Pgf,Gdf15,Ccl2,Mmp8,Ctla2b,Cd24a, Clec4d,Cd14,Slc2a1,Procr,Ifitm6,Igfbp3, Tnfaip6,Sphk1,Nppb,Tyrobp,Tubb6,Ccr1, Psat1,Ier3 |
| Downregulated | Irs1,Prox1 |
Abbreviation: DEGs, differentially expressed genes.
Figure 3.
The heatmap of differentially expressed genes. Notes: each column and row represents one dataset and one gene, respectively. Red and green represent logFC >0 and logFC <0, respectively. The logFC values are shown in each rectangle. The gradual color ranged from green to red represents the changing process from downregulation to upregulation. Abbreviation: FC, fold change.
3.3. GO Analysis of DEGs
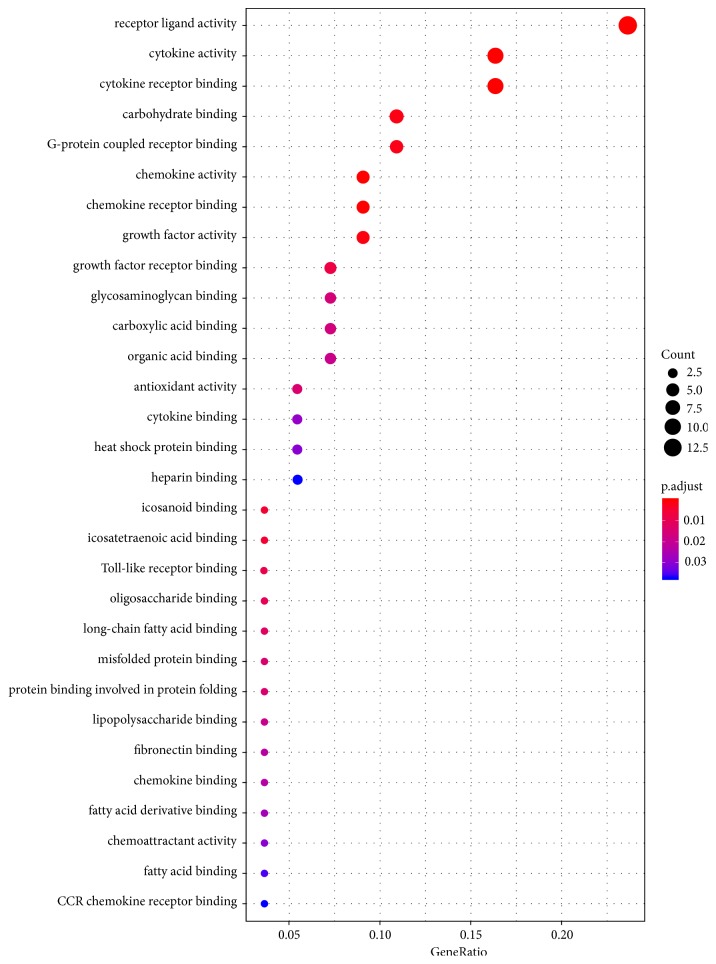
Using Clusterprofiler package, biological annotation of the DEGs obtained by RRA approach was performed. The down- and upregulated genes with p value <0.05 were obtained from GO functional enrichment. From GO functional enrichment analysis, we identified that these DEGs were mainly enriched in the following functional categories, including receptor ligand activity, cytokine activity, cytokine receptor binding, G-protein coupled receptor binding, carbohydrate binding, chemokine activity, and chemokine receptor binding. GO analyses are shown in Figure 4. Meaningful results of the GO analysis of DEGs in AMI are listed in Table 3.
Figure 4.
GO enrichment analyses of DEGs in AMI. Notes: X-axis indicates the percentage that each functional group gene, respectively, accounts for the total genes. Y-axis represents different functional groups (also named as different GO terms). The size of the dot indicates the number of genes in different functional groups, and the color of the dot reflects the different p-value range. The bigger the gene count, the bigger the dot size is. The gradual color ranged from blue to red represents the changing process of p-value from big to small value. Abbreviations: AMI, acute myocardial infarction; DEGs, differentially expressed genes; GO, gene ontology.
Table 3.
GO analysis of genes associated with AMI.
| Term | Description | Count | P-value |
|---|---|---|---|
| GO:0048018 | receptor ligand activity | 13 | 8.99E-11 |
| GO:0005125 | cytokine activity | 9 | 2.63E-09 |
| GO:0008009 | chemokine activity | 5 | 5.77E-08 |
| GO:0005126 | cytokine receptor binding | 9 | 7.10E-08 |
| GO:0042379 | chemokine receptor binding | 5 | 5.14E-07 |
| GO:0045236 | CXCR chemokine receptor binding | 3 | 4.59E-06 |
| GO:0008083 | growth factor activity | 5 | 2.57E-05 |
| GO:0030246 | carbohydrate binding | 6 | 4.20E-05 |
| GO:0001664 | G-protein coupled receptor binding | 6 | 6.03E-05 |
| GO:0050542 | icosanoid binding | 2 | 0.000248517 |
| GO:0050543 | icosatetraenoic acid binding | 2 | 0.000248517 |
| GO:0070851 | growth factor receptor binding | 4 | 0.000399461 |
| GO:0035325 | Toll-like receptor binding | 2 | 0.000428787 |
| GO:0036041 | long-chain fatty acid binding | 2 | 0.000743064 |
| GO:0070492 | oligosaccharide binding | 2 | 0.000743064 |
| GO:0016209 | antioxidant activity | 3 | 0.000961581 |
| GO:0005539 | glycosaminoglycan binding | 4 | 0.001232506 |
| GO:0019956 | chemokine binding | 2 | 0.00149192 |
| GO:0051787 | misfolded protein binding | 2 | 0.00149192 |
| GO:0001530 | lipopolysaccharide binding | 2 | 0.001619175 |
| GO:0031406 | carboxylic acid binding | 4 | 0.001701432 |
| GO:0043177 | organic acid binding | 4 | 0.001850987 |
| GO:0019955 | cytokine binding | 3 | 0.002141304 |
| GO:0001968 | fibronectin binding | 2 | 0.002329934 |
| GO:1901567 | fatty acid derivative binding | 2 | 0.002815131 |
| GO:0044183 | protein binding involved in protein folding | 2 | 0.003914772 |
| GO:0031072 | heat shock protein binding | 3 | 0.004127547 |
| GO:0005504 | fatty acid binding | 2 | 0.004741625 |
| GO:0008201 | heparin binding | 3 | 0.005037273 |
| GO:0048020 | CCR chemokine receptor binding | 2 | 0.005409964 |
3.4. KEGG Pathway Analysis of DEGs
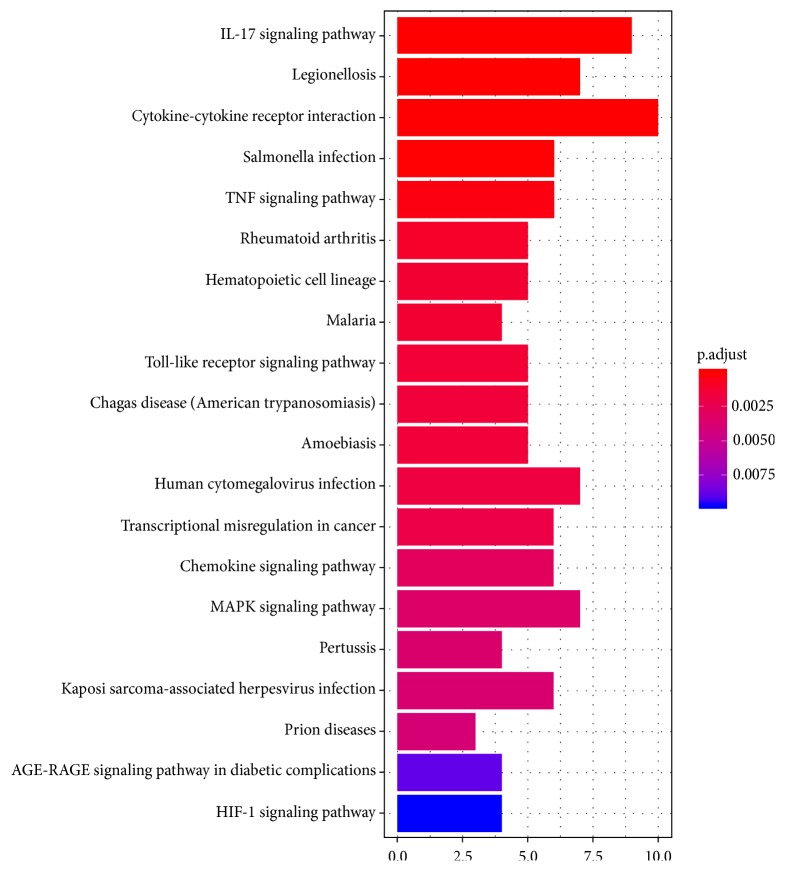
Top 20 KEGG pathway analyses of DEGs are shown in Table 4 and Figure 5. Table 4 shows that these DEGs were primarily enriched in the cytokine-cytokine receptor interaction, Chemokine signaling pathway, TNF signaling pathway, and so on.
Table 4.
Top 20 KEGG pathway enrichment analyses of DEGs associated with AMI.
| pathway | ID | Gene count | p-value | adjust p-value | Genes |
|---|---|---|---|---|---|
| IL-17 signaling pathway | mmu04657 | 9 | 2.32E-10 | 3.30E-08 | Cxcl2,S100a8,S100a9,Il6,Cxcl1,Cxcl5,Il1b,Ptgs2,Ccl2 |
| Legionellosis | mmu05134 | 7 | 6.85E-09 | 4.87E-07 | Cxcl2,Il6,Cxcl1,Hspa1b,Hspa1a,Il1b,Cd14 |
| Cytokine-cytokine receptor interaction | mmu04060 | 10 | 6.80E-07 | 3.22E-05 | Cxcl2,Il1r2,Il6,Cxcl1,Cxcl5,Il1b,Ccl3,Gdf15,Ccl2,Ccr1 |
| Salmonella infection | mmu05132 | 6 | 1.38E-06 | 4.88E-05 | Cxcl2,Il6,Cxcl1,Il1b,Ccl3,Cd14 |
| TNF signaling pathway | mmu04668 | 6 | 1.03E-05 | 0.000292216 | Cxcl2,Il6,Cxcl1,Il1b,Ptgs2,Ccl2 |
| Rheumatoid arthritis | mmu05323 | 5 | 3.97E-05 | 0.00093865 | Il6,Cxcl5,Il1b,Ccl3,Ccl2 |
| Hematopoietic cell lineage | mmu04640 | 5 | 7.17E-05 | 0.001297709 | Il1r2,Il6,Il1b,Cd24a,Cd14 |
| Malaria | mmu05144 | 4 | 7.31E-05 | 0.001297709 | Il6,Selp,Il1b,Ccl2 |
| Toll-like receptor signaling pathway | mmu04620 | 5 | 8.73E-05 | 0.001377696 | Il6,Spp1,Il1b,Ccl3,Cd14 |
| Chagas disease (American trypanosomiasis) | mmu05142 | 5 | 0.000105431 | 0.001497126 | Serpine1,Il6,Il1b,Ccl3,Ccl2 |
| Amoebiasis | mmu05146 | 5 | 0.000120813 | 0.001559588 | Arg1,Il1r2,Il6,Il1b,Cd14 |
| Human cytomegalovirus infection | mmu05163 | 7 | 0.000153378 | 0.00181497 | Il6,Il1b,Ptgs2,Ccl3,Myc,Ccl2,Ccr1 |
| Transcriptional misregulation in cancer | mmu05202 | 6 | 0.000179856 | 0.001964578 | Il1r2,Il6,Gadd45g,Myc,Cd14,Igfbp3 |
| Chemokine signaling pathway | mmu04062 | 6 | 0.000291266 | 0.002954267 | Cxcl2,Cxcl1,Cxcl5,Ccl3,Ccl2,Ccr1 |
| MAPK signaling pathway | mmu04010 | 7 | 0.000358914 | 0.003397722 | Hspa1b,Hspa1a,Il1b,Gadd45g,Myc,Pgf,Cd14 |
| Pertussis | mmu05133 | 4 | 0.000404457 | 0.003589552 | Il6,Cxcl5,Il1b,Cd14 |
| Kaposi sarcoma-associated herpesvirus infection | mmu05167 | 6 | 0.000451092 | 0.003767942 | Cxcl2,Il6,Cxcl1,Ptgs2,Myc,Ccr1 |
| Prion diseases | mmu05020 | 3 | 0.00050479 | 0.003982228 | Il6,Hspa1a,Il1b |
| AGE-RAGE signaling pathway in diabetic complications | mmu04933 | 4 | 0.001183968 | 0.008848603 | Serpine1,Il6,Il1b,Ccl2 |
| HIF-1 signaling pathway | mmu04066 | 4 | 0.001367781 | 0.009711245 | Serpine1,Il6,Hmox1,Slc2a1 |
Figure 5.
Top 20 KEGG pathway enrichment analyses of DEGs in AMI. Notes: X-axis indicates gene count; Y-axis represents different pathways. The column color reflects p-value: red represents the smallest value; blue represents the biggest value. The gradual color ranged from blue to red represents the changing process of p-value from big to small value. Abbreviations: AMI, acute myocardial infarction; DEGs, differentially expressed genes; KEGG, Kyoto Encyclopedia of Genes and Genomes.
3.5. Establishing the PPI Network, Conducting Modules Analysis, and Selection of Hub Genes
In order to ulteriorly explore the biological characteristics of these DEGs, a PPI network was created using the STRING database. There were 56 nodes and 240 edges in this network, including 2 down- and 54 upregulated genes (see the supplementary document (available here)), as shown in Figure 6(a). Subsequently, a vital module was confirmed from the whole network, a total of 18 nodes and 117 edges in this module, as shown in Figure 6(b). 18 key genes in total were identified, including Cxcl5, Arg1, Cxcl1, Spp1, Selp, Ptx3, Tnfaip6, Mmp8, Serpine1, Ptgs2, Il6, Il1r2, Il1b, Ccl3, Ccr1, Hmox1, Cxcl2, and Ccl2. Ccr1 was the most key gene in PPI network. These genes in the module were mainly enriched in the cytokine-cytokine receptor interaction, TNF signaling pathway, Toll-like receptor signaling pathway, and chemokine signaling pathway, as shown in Table 4. Utilizing the cytoHubba plug-in, Cxcl1, Cxcl2, Cxcl5, and Mmp8 hub genes were screened out, as shown in Figure 6(c).
Figure 6.
Establishment of PPI network, modules analysis, and Venn diagram. (a) Whole PPI network. Circles and lines represent genes and the interaction of proteins between genes, respectively. The red represents the upregulated genes. The green represents the downregulated genes. (b) PPI network of module. Circles and lines represent genes and the interaction of proteins between genes, respectively. The red represents the upregulated genes. (c) Venn diagram of mutual hub genes based on two methods. Abbreviation: PPI, protein-protein interaction.
4. Discussion
AMI is one of the common kinds of coronary heart disease with high morbidity and mortality all over the world. In recent years, the number of patients with AMI is increasing annually. Controlling the number of patients with AMI and exploring the molecular mechanism of AMI are urgent to be solved.
In the study, using integrated bioinformatics and RRA analysis method, a total of 57 DEGs, including 2 down- and 55 upregulated genes, were identified from the GSE775, GSE19322, and GSE97494 database. From GO functional enrichment analysis, we identified that these DEGs were mainly enriched in the following functional categories, including receptor ligand activity, cytokine activity, cytokine receptor binding, G-protein coupled receptor binding, carbohydrate binding, chemokine activity, and chemokine receptor binding. Through KEGG pathway enrichment analysis, we found that the DEGs were chiefly enriched in the pathway of cytokine-cytokine receptor interaction, MAPK signaling pathway, TNF signaling pathway, Toll-like receptor signaling pathway, and chemokine signaling pathway. Utilising the STRING database, the PPI network was constructed. The module analysis filtered out 18 key genes, including Cxcl5, Arg1, Cxcl1, Spp1, Selp, Ptx3, Tnfaip6, Mmp8, Serpine1, Ptgs2, Il6, Il1r2, Il1b, Ccl3, Ccr1, Hmox1, Cxcl2, and Ccl2. Ccr1 was the most fundamental gene in PPI network. Four hub genes in total were filtered out, including Cxcl1, Cxcl2, Cxcl5, and Mmp8. Most of these genes in AMI have been reported, which indicated that the results of integrated bioinformatics analysis were reliable.
Chemokine (C-C motif) receptor 1 (Ccr1), the highest score, was identified from the module. Ccr1 is inflammation-associated gene, which may be a novel biomarker for the diagnosis and prognosis of AMI [13]. It exerts an important role in controlling inflammation [14]. Significantly, during the pathogenesis of AMI, inflammation of the coronary artery is the key process [15, 16]. We found that Ccr1 mainly enriched in cytokine-cytokine receptor interaction and chemokine signaling pathway from the KEGG pathway analysis, which may be a direction of future research for diagnosis and treatment of AMI. In mice, chemokine (C-X-C motif) ligand 2 (Cxcl2) plays a kind of the potent neutrophil chemoattractants [17]. Using pharmacologic inhibition of circulating Cxcl2, researchers found neutrophil recruitment reduced at the site of myocardial infarction and injury within the infarcted myocardium alleviated [17]. Expression of Cxcl2 and Cxcl5 in AMI was elevated, which aggravated acute inflammation after myocardial injury and promoted cardiac rupture [18, 19]. From the KEGG pathway analysis, we found that Cxcl2 mainly enriched in cytokine-cytokine receptor interaction, TNF signaling pathway, and chemokine signaling pathway. Thus, Cxcl2 may play a key role in regulating cardiac remodeling following myocardial infarction (MI). Chemokine (C-C motif) ligand 3 (Ccl3) is also an important circulating chemokine. Tineke et al. [20] showed that CCL3 is highly upregulated in patients with AMI. Vandervelde et al. clearly showed that the Ccl3 mRNA expression was upregulated in ischemic myocardium [21]. These evidences indicated that Ccl3 is closely associated with myocardial ischemia. Our study found that Ccl3 was primarily enriched in cytokine-cytokine receptor interaction, Toll-like receptor signaling pathway, and chemokine signaling pathway. In experimental models of AMI, the innate immune response was induced through activation of Toll-like receptor (TLR)2 and TLR4 on circulating blood cells, which increases infarct size and influences ventricular remodeling [22, 23]. In the model of myocardial infarction, pharmacological inhibition of TLR2 or TRL4 can decrease monocyte inflow into the infarcted region, decrease the infarct area, and enhance myocardial remodeling [24–26]. From the above evidence, we identified the importance of cytokine-cytokine receptor interaction and chemokine signaling pathway in the occurrence and development of AMI.
Prostaglandin-endoperoxide synthase 2 (PTGS2, also named as COX-2), which can increase the neoplastic process by promoting proliferation, suppressing apoptosis, and angiogenesis, is an enzyme during conversion of arachidonic acid to prostaglandins [27]. PTGS2 has high expression in every kind of tumor, which was usually induced by cancer promoters, oncogenes, and cytokines [28]. PTGS2 gene associated with the decreasing risk of stroke and MI has been demonstrated [29]. Therefore, it plays a crucial role in treatment of MI. From KEGG pathway analysis, we found that PTGS2 was enriched in TNF signaling pathway. It has been reported that TNF signaling pathway was associated with cardiac remodeling following MI [30]. So we speculate that PTGS2 exerts an important role on regulating cardiac remodeling following MI through TNF signaling pathway. We look forward to the result being confirmed by future experiments.
Among these genes, a novel gene Tnfaip6 (tumor necrosis factor-stimulated gene-6) was obviously differentially expressed in AMI. Interestingly, this gene was mainly reported in inflammatory bowel disease [31]. According to integrated bioinformatics analysis, we speculated that Tnfaip6 may play an important role in AMI, which could be a novel target for the treatment of AMI. Thus, further studies are needed in order to verify it.
Wei Gong et al. [32] have found that trimetazidine can prevent cardiac rupture in mice with AMI through inhibiting the expression of Mmp2 and Mmp9, which indicates that the MMP family may be associated with cardiac remodeling after AMI. Matrix metalloproteinase-8 (Mmp8), a member of the MMP family, has gained growing attention in recent years. Previous research had only identified that types I, II, and III collagens are the substrates of Mmp8. However, in recent years, an increasing number of other proteins were detected as the substrates of Mmp8, including chemokine (C-X-C motif) ligand 5 (CXCL5) [33], macrophage inflammatory protein-1 [34], chemokine (C-X-C motif) ligand 11 (CXCL11) [35], and angiotensin-1 [36]. Research indicated that Mmp8 can regulate the function and behavior of multiple cell types, including stem/progenitor cells [37], endothelial cells [38], smooth muscle cells [39], and neutrophils [34]. Study showed that gingival crevicular fluid Mmp8 concentrations significantly increase in patients with AMI [40]. Bioinformatics analysis indicates that Mmp8 may be associated with prognosis of AMI. Nevertheless, little is known about the relation between Mmp8 and cardiac remodeling. Therefore, more experiments were needed to verify it in the future.
It is noticeable that there have been papers researching the differentially expressed genes in AMI. However, the results of those papers were somewhat different from ours. The following reasons may account for this phenomenon: (1) some studies [3, 13], which have been reported, are peripheral blood microarray analysis of patients with AMI. Nevertheless, our study is microarray analysis of LV myocardium of mouse with AMI. Because the sample origin and the timing of specimen collection are different [4], which leads to somewhat different results, (2) different batches of microarray analysis, to some extent, also have somewhat different results; (3) compared with other studies of AMI, our study provides an integrated bioinformatics analysis of DEGs of AMI by means of statistical methods. We may provide credible results. Of course, it is important that the results are validated in follow-up experiments.
5. Conclusion
In conclusion, our study provides an integrated bioinformatics analysis of DEGs of AMI. This research provides numerous genes associated with AMI. This study may provide credible molecular biomarkers in terms of screening, diagnosis, and prognosis for AMI. Meanwhile, it also serves as a basis for exploring new therapeutic target for AMI. Compared with other studies of AMI, innovation point and merit of our current study was that the RRA method was utilized for the first time in exploring DEGs in AMI study. This study also has certain limitations. In this study, 18 microarrays were only screened, which is not enough. The limited sample size may easily lead to false positive results. Therefore, to verify the current findings, it is necessary to perform more experiments.
Acknowledgments
This study was funded by the Project of Young and Middle-aged Talent Cultivation of Fujian Provincial Health System, China (Grant No. 2013-ZQN-JC-30).
Data Availability
The data used to support the findings of this study are included within the supplementary information file.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
A PPI network: there were 56 nodes and 240 edges in this network, including 2 down- and 54 upregulated genes (see the supplementary document).
References
- 1.Wang D. Z., Shen C. F., Zhang Y., et al. Fifteen-year trend in incidence of acute myocardial infarction in Tianjin of China. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;45(2):154–159. doi: 10.3760/cma.j.issn.0253-3758.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Chang J., Liu X., Sun Y. Mortality due to acute myocardial infarction in China from 1987 to 2014: Secular trends and age-period-cohort effects. International Journal of Cardiology. 2017;227:229–238. doi: 10.1016/j.ijcard.2016.11.130. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y., Qi G., Guo L., Sun Y. Bioinformatics analyses of differentially expressed genes associated with acute myocardial infarction. Cardiovascular Therapeutics. 2016;34(2):67–75. doi: 10.1111/1755-5922.12171. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T., Zhao L., Cao X., et al. Bioinformatics analysis of time series gene expression in left ventricle (LV) with acute myocardial infarction (AMI) Gene. 2014;543(2):259–267. doi: 10.1016/j.gene.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Pan J., Alimujiang M., Chen Q., Shi H., Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction−induced myocardial damage via downregulation of early growth response factor 1. Journal of Cellular Biochemistry. 2019;120(3):4433–4443. doi: 10.1002/jcb.27731. [DOI] [PubMed] [Google Scholar]
- 6.Novo G., Cappello F., Rizzo M., et al. Hsp60 and heme oxygenase-1 (Hsp32) in acute myocardial infarction. Translational Research. 2011;157(5):285–292. doi: 10.1016/j.trsl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Qian C., Chang D., Li H., Wang Y. Identification of potentially critical genes in the development of heart failure after ST‐segment elevation myocardial infarction (STEMI) Journal of Cellular Biochemistry. 2018;120(5):7771–7777. doi: 10.1002/jcb.28051. [DOI] [PubMed] [Google Scholar]
- 8.Sun G., Li Y., Peng Y., et al. Identification of differentially expressed genes and biological characteristics of colorectal cancer by integrated bioinformatics analysis. Journal of Cellular Physiology. 2019 doi: 10.1002/jcp.28163. [DOI] [PubMed] [Google Scholar]
- 9.Kolde R., Laur S., Adler P., Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics. 2012;28(4):573–580. doi: 10.1093/bioinformatics/btr709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G., Wang L.-G., Han Y., He Q.-Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A Journal of Integrative Biology. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie M. E., Phipson B., Wu D., et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):p. e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin C.-H., Chen S.-H., Wu H.-H., Ho C.-W., Ko M.-T., Lin C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Systems Biology. 2014;8(4, article no. S11) doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J., Gao C., Wang R., Xiao C., Yang M. Genes associated with inflammation and the cell cycle may serve as biomarkers for the diagnosis and prognosis of acute myocardial infarction in a Chinese population. Molecular Medicine Reports. 2018;18(2):1311–1322. doi: 10.3892/mmr.2018.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liehn E. A., Merx M. W., Postea O., et al. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. Journal of Cellular and Molecular Medicine. 2008;12(2):496–506. doi: 10.1111/j.1582-4934.2007.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aukrust P., Halvorsen B., Yndestad A., et al. Chemokines and cardiovascular risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(11):1909–1919. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 16.Tereshchenko I. P., Petrkova J., Voevoda M. I., et al. CCL5/RANTES gene polymorphisms in slavonic patients with myocardial infarction. Mediators of Inflammation. 2011;2011:6. doi: 10.1155/2011/525691.525691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montecucco F., Lenglet S., Braunersreuther V., et al. Single administration of the CXC chemokine-binding protein evasin-3 during ischemia prevents myocardial reperfusion injury in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(7):1371–1377. doi: 10.1161/ATVBAHA.110.206011. [DOI] [PubMed] [Google Scholar]
- 18.Mylonas K. J., Turner N. A., Bageghni S. A., et al. 11β-HSD1 suppresses cardiac fibroblast CXCL2, CXCL5 and neutrophil recruitment to the heart post MI. Journal of Endocrinology. 2017;233(3):315–327. doi: 10.1530/JOE-16-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usui S., Chikata A., Takatori O., et al. Endogenous muscle atrophy F-box is involved in the development of cardiac rupture after myocardial infarction. Journal of Molecular and Cellular Cardiology. 2019;126:1–12. doi: 10.1016/j.yjmcc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 20.de Jager S. C., Kraaijeveld A. O., Grauss R. W., et al. CCL3 (MIP-1α) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. Journal of Molecular and Cellular Cardiology. 2008;45(3):446–452. doi: 10.1016/j.yjmcc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Vandervelde S., van Luyn M. J., Rozenbaum M. H., Petersen A. H., Tio R. A., Harmsen M. C. Stem cell-related cardiac gene expression early after murine myocardial infarction. Cardiovascular Research. 2007;73(4):783–793. doi: 10.1016/j.cardiores.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Arslan F., de Kleijn D. P., Pasterkamp G. Innate immune signaling in cardiac ischemia. Nature Reviews Cardiology. 2011;8(5):292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 23.Timmers L., Pasterkamp G., de Hoog V. C., Arslan F., Appelman Y., de Kleijn D. P. V. The innate immune response in reperfused myocardium. Cardiovascular Research. 2012;94(2):276–283. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 24.Chong A. J., Shimamoto A., Hampton C. R. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. The Journal of Thoracic and Cardiovascular Surgery. 2004;128(2):170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Timmers L., Sluijter J. P. G., van Keulen J. K., et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circulation Research. 2008;102(2):257–264. doi: 10.1161/circresaha.107.158220. [DOI] [PubMed] [Google Scholar]
- 26.Shimamoto A., Chong A. J., Yada M., et al. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(1):I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 27.Gray R. T., Cantwell M. M., Coleman H. G., et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clinical and Translational Gastroenterology. 2017;8(4):p. e91. doi: 10.1038/ctg.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J., Shim M. COX-2 inhibitor NS-398 suppresses doxorubicin-induced p53 accumulation through inhibition of ROS-mediated Jnk activation. Molecular Carcinogenesis. 2016;55(12):2156–2167. doi: 10.1002/mc.22458. [DOI] [PubMed] [Google Scholar]
- 29.Ross S., Eikelboom J., Anand S. S., et al. Association of cyclooxygenase-2 genetic variant with cardiovascular disease. European Heart Journal. 2014;35(33):2242–2248. doi: 10.1093/eurheartj/ehu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J., Moon M., Yan L., et al. Cellular FLICE-inhibitory protein protects against cardiac remodelling after myocardial infarction. Basic Research in Cardiology. 2012;107(1):p. 239. doi: 10.1007/s00395-011-0239-z. [DOI] [PubMed] [Google Scholar]
- 31.Yu Q., Zhang S., Wang H., et al. TNFAIP6 is a potential biomarker of disease activity in inflammatory bowel disease. Biomarkers in Medicine. 2016;10(5):473–483. doi: 10.2217/bmm.16.9. [DOI] [PubMed] [Google Scholar]
- 32.Gong W., Ma Y., Li A., Shi H., Nie S. Trimetazidine suppresses oxidative stress, inhibits MMP-2 and MMP-9 expression, and prevents cardiac rupture in mice with myocardial infarction. Cardiovascular Therapeutics. 2018;36(5):p. e12460. doi: 10.1111/1755-5922.12460. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Steen P. E., Wuyts A., Husson S. J., Proost P., Van Damme J., Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. European Journal of Biochemistry. 2003;270(18):3739–3749. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 34.Quintero P. A., Knolle M. D., Cala L. F., Zhuang Y., Owen C. A. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1α to reduce acute lung inflammation and injury in mice. The Journal of Immunology. 2010;184(3):1575–1588. doi: 10.4049/jimmunol.0900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox J. H., Dean R. A., Roberts C. R., Overall C. M. Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. The Journal of Biological Chemistry. 2008;283(28):19389–19399. doi: 10.1074/jbc.M800266200. [DOI] [PubMed] [Google Scholar]
- 36.Van Lint P., Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine & Growth Factor Reviews. 2006;17(4):217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Q., Zhang F., Lin L., et al. Functional role of matrix metalloproteinase-8 in stem/progenitor cell migration and their recruitment into atherosclerotic lesions. Circulation Research. 2013;112(1):35–47. doi: 10.1161/circresaha.112.274019. [DOI] [PubMed] [Google Scholar]
- 38.Fang C., Wen G., Zhang L., et al. An important role of matrix metalloproteinase-8 in angiogenesis in vitro and in vivo. Cardiovascular Research. 2013;99(1):146–155. doi: 10.1093/cvr/cvt060. [DOI] [PubMed] [Google Scholar]
- 39.Xiao Q., Zhang F., Grassia G., et al. Matrix metalloproteinase-8 promotes vascular smooth muscle cell proliferation and neointima formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(1):90–98. doi: 10.1161/atvbaha.113.301418. [DOI] [PubMed] [Google Scholar]
- 40.Ehlers V., Willershausen I., Kraft J., Münzel T., Willershausen B. Gingival crevicular fluid MMP-8-concentrations in patients after acute myocardial infarction. Head & Face Medicine. 2011;7(1) doi: 10.1186/1746-160X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A PPI network: there were 56 nodes and 240 edges in this network, including 2 down- and 54 upregulated genes (see the supplementary document).
Data Availability Statement
The data used to support the findings of this study are included within the supplementary information file.