Abstract
Fstl1 is a TGF‐β superfamily binding protein which involved in many pathological processes. The function of Fstl1 has been widely elucidated, but its structural characterization has not been explored. Here we solved the high‐resolution crystal structure of FK domain of murine Fstl1, analyzed its unique characteristics, and investigated its contribution to the function of full‐length Fstl1. We found that Fstl1‐FK forms a stable dimer in both solution and crystal, which suggest that this protein may function as a dimer during its interaction with TGF‐β, a molecule known to form dimer during activation process. We also found this FK domain is indispensable for the proper function of Fstl1 during the transduction of TGF‐β signaling. These observations provide important insights into the understanding of Fstl1 and may facilitate the exploration of this molecule in clinical study.
Keywords: crystal structure, FK domain, Fstl1, lung fibrosis, TGF‐β signaling
Short abstract
PDB Code: 6JZA
1. INTRODUCTION
Follistatin‐like 1 (Fstl1) is a secreted, 308 amino‐acid matricellular protein belonging to a group of matrix‐related factors that mediate cell–matrix interactions but do not serve primarily structural roles.1, 2 Expression of Fstl1 is generally associated with tissue undergoing remodeling, either during normal developmental process or as a response to injury.3, 4 During embryogenesis, the expression of Fstl1 is temporarily and spatially regulated and loss‐of‐function experiments have unveiled an important role for Fstl1 in dorsoventral axis establishment, lung,5 skeletal,6 and ureter developments.7 Fstl1 has been implicated in several human diseases. Findings on Fstl1 in cardiovascular disease,7, 8 cancer,9, 10, 11 arthritis,12, 13, 14, 15, 16 lung fibrosis,17 and obesity18, 19 emphasize its potential as both biomarkers and targets for the management of the disease process. Although the functions and mechanisms of Fstl1 have not been completely understood, a growing body of literatures have identified the critical roles of Fstl1 in the regulation of cell survival,20, 21 proliferation,22, 23 differentiation,20, 22 migration,23, 24 as well as inflammation,16 and immune modulation.25 Fstl1 is first discovered as a TGF‐β1‐induced protein,26 and has also been shown to function largely through regulating TGF‐β/BMP signaling,5, 17 although, in various disease models, different signaling pathways, like activation of AKT, AMPK, or ERK–MAPK, are linked to Fstl1.1
The sequence of Fstl1 is highly conserved throughout vertebrate evolution.27 Comparison of the human and mouse protein sequences shows a very high degree of similarity (94.4%), although the signal peptide is species‐variable.28 The mouse protein consists of an N‐terminal region homologous to follistatin (FS) and a domain containing two EF‐hand calcium‐binding sites (EC domain), followed by a C‐terminal domain with homology to the von Willebrand factor type C‐like (VWFC) domain. The FS domain is also termed as FK domain because it can be subdivided into a follistatin/osteonectin‐like EGF (FOLN) domain and Kazal domain, both of which could exist individually in various proteins. Based on the presence of a follistatin domain and an extracellular calcium domain, Fstl1 is a member of the secreted protein acidic rich in cysteines (SPARC) family. However, Fstl1 is a unique member with the lack of calcium‐binding properties of its EC domain, disputing whether Fstl1 share any functional similarities to the other members of the SPARC protein family.
We have previously reported that Fstl1 has broad profibrotic activities in pulmonary fibrosis, showing more functional similarity to SPARC but not follistatin.29 To further investigate Fstl1, we utilized a structural biology method to explore the biochemical characteristics about the functional domain engaged in pulmonary fibrosis. We expressed and purified full‐length murine Fstl1, followed by crystallization screening. Diffractable crystals were obtained after a long time incubation, but the solved structure revealed that only N‐terminal FK domain was present in the crystals. The structure of FK domain (Fstl1‐FK) solved here shows a stable dimer packed head‐to‐tail anti‐parallel, and the tight contact between FOLN and Kazal domain indicates that these two parts might function as a compact unit for full‐length Fstl1. For this reason, we named these two parts as a whole FK domain and studied its function in TGF‐β responsiveness in vitro and in bleomycin model in vivo.
2. RESULTS
2.1. Crystal structure of Fstl1‐FK domain
The full‐length Fstl1 without leading peptide was expressed in Drosophila S2 cells in secreted form, and purified protein was used for crystallization. The crystals appeared in crystallization drops were used for data collection and structure determination.30 Unfortunately, after solving the structure, we found that only N‐terminal FOLN‐Kazal region were present in the crystal structure (Figure 1a), so we analyzed this structure and studied the function of this domain subsequently.
Figure 1.
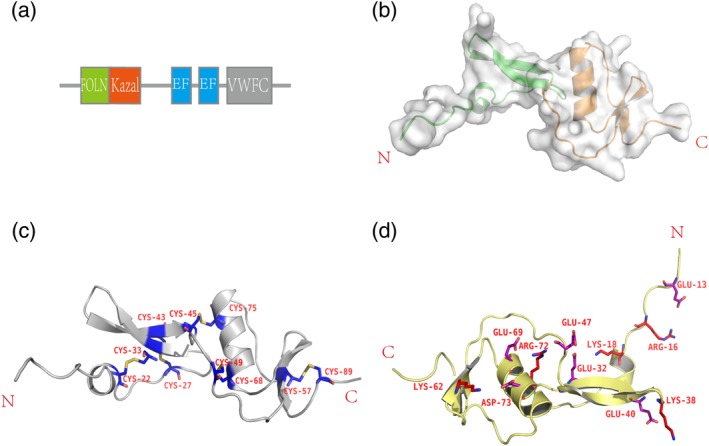
The overall structure of Fstl1‐FK domain. (a) Diagrammatic representation of Fstl1. Fstl1 contains FOLN and Kazal domain at N‐terminus, with two‐EF‐hand domain in the middle and VWFC domain at C‐terminus. (b) Overall structure of Fstl1‐FK domain. Fstl1‐FK domain consists of FOLN domain (green) and Kazal domain (orange). (c) Disulfide bonds. Disulfide bonds stabilize the overall structure of Fstl1‐FK domain. Five pairs of highly conserved disulfide bonds are highlighted by yellow sticks, and cysteines are labeled in dark blue color. (d) Salt bridges on the molecular surface. Glutamic acid and aspartic acid are colored in purple, arginine and lysine are colored in red. The numbers in the labels correspond to the amino acid sequence
Despite several crystal structures of FOLN‐Kazal region (FK domain) from Follistatin family have been solved, the building of initial model of Fstl1‐FK for our crystal structure by molecular replacement failed due to low sequence similarity. Fstl1‐FK contains 10 cysteine residues constituting 5 pairs of disulfide bonds. By taking advantage of anomalous signal of sulfur, the structure was solved by single wavelength anomalous diffraction (SAD) method. The overall structure of Fstl1‐FK is similar to that of follistatin, but the FOLN and Kazal domains pack more tightly to each other than the latter, indicating a less flexibility between two domains (Figure 1b). The five pairs of disulfide bonds, which are highly conserved within Follistatin family, are critical for the stability of whole folding (Figure 1c). The hydrogen bonds and salt bridges formed on the molecular surface also contribute to the stability of the overall structure (Figure 1d).
2.2. Dimerization of Fstl1‐FK
From the crystal structure, a dimer formed through two‐fold symmetric axis could be identified (Figure 2a), which covers an area of more than 1,400 å2, indicating a stable dimer may form when Fstl1 plays functions under physiological conditions. The dimer is stabilized by two interactions, one from the packing of β‐sheets in FOLN domains, the other from interaction between N‐terminal peptide and the Kazal domain of the accompanying molecule (Figure 2b). To further address the stability of the dimer observed in crystal structure, the FK domain was expressed in E. coli and purified to homogeneity. From the gel filtration chromatography, Fstl1‐FK exists as both dimer and monomer in solution (Figure 2c), and analysis by analytical ultracentrifugation (AUC) further confirmed the existence of the dimer (Figure 2d). Co‐immunoprecipitate of GFP‐tagged and HA‐tagged Fstl1‐FK also agrees with the existence of Fstl1 dimer in living cells (Figure 2e). Consistent with this observation, we have previously reported that purified full‐length Fstl1 also forms dimer in solution.30 The FK domain from Follistatin can also be eluted as both monomer and dimer in gel filtration column, indicating these protein might play their roles in a dimer form.
Figure 2.
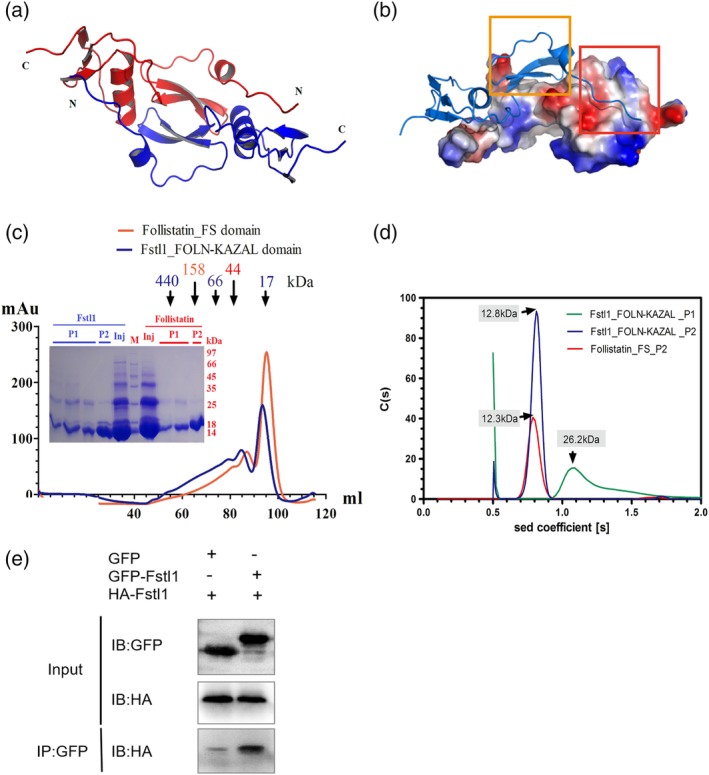
Dimerization of Fstl1‐FK domain. (a) Ribbon representation of the dimer formed in the crystals. (b) The dimer is stabilized by two interactions: One from β‐sheets in FOLN domain (orange rectangle), the other from N‐terminal peptide and Kazal domain (red rectangle). (c) The results of size exclusion chromatography of Follistatin‐FS domain and Fstl1‐FK domain. Samples were loaded on Hiload 16/60 Superdex 200 column for purification. Orange line represents Follistatin‐FS domain, blue line represents Fstl1‐FK domain. Eluted fractions were also detected by SDS‐PAGE. M represents molecular mass standards. P1 and P2 means Peak 1 and Peak 2 on size exclusion column; Inj (injection) means the sample before loaded onto column. (d) Analytical ultracentrifuge analysis of Fstl1‐FK domain and Follistatin‐FS domain. Sedimentation coefficient distribution of the fractions from size exclusion column was shown. Peak 1 (green) and Peak 2 (blue) of Fstl1‐FK domain were detected as dimer and monomer respectively. For Follistatin‐FS domain, only Peak 2 was measured (red), since Peak 1 precipitates easily during AUC experiment. (e) Co‐immunoprecipitate of GFP‐Fstl1‐FK and HA‐Fstl1‐FK. Two proteins were co‐expressed in 293T cells and pulled‐down by GFP‐trap beads, then detected by anti‐HA antibody
2.3. The comparison of Fstl1‐FK with FOLN/Kazal domains from other proteins
After searching for the structure homologues of Fstl1‐FK using the DALI server, we found that BM‐40/SPARC (PDB code: 1BMO), Follistatin‐Related protein 3 (PDB code: 2KCX), Follistatin (PDB code: 1LR9) and Human Complement Factor I (PDB code: 2XRC) are the best hits with high Z‐scores. By aligning Fstl1 with the other four proteins based on the structures (Figure 3a), we identified several common characteristics owned by these proteins. Firstly, the position of five pairs of disulfide bonds in these proteins is invariant, which may be indispensable for the stability of the overall structure. Secondly, compared to the FOLN domain, the Kazal domain is more conserved. In addition, the length of the loop between FOLN and Kazal domain is variable, implying that the relative flexibility between the FOLN and Kazal domain is diverse amongst these proteins. To understand the unique structural property of Fstl1, we analyzed the structure differences between Fstl1 and the other four proteins.
Figure 3.
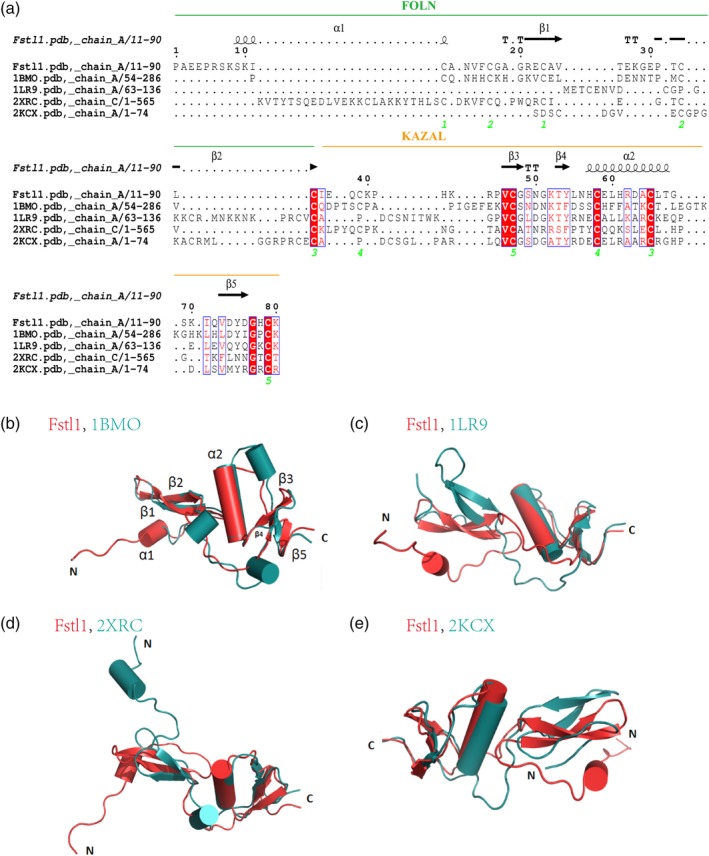
Structural comparison between Fstl1‐FK domain and other proteins. (a) The amino acid sequence alignment of Fstl1‐FK with the other four proteins which have similar structures. The secondary structure elements are shown above the alignment. Conserved residues are highlighted in red. The green number below the alignment represents the conserved cysteines. (b) Superimposition of Fstl1‐FK domain (red) with BM‐40 FS domain (blue, PDB code 1BMO). (c) Superimposition of Fstl1‐FK domain (red) with Follistatin FS domain (blue, PDB code 1LR9). (d) Superimposition of Fstl1‐FK domain (red) with human complement factor I (blue, PDB code 2XRC). (e) Superimposition of Fstl1‐FK domain (red) with Fstl3 FS domain (blue, PDB code 2KCX)
BM‐40 has the best similarity with Fstl1 in a R.M.S.D. of 1.7 å. Compared to BM‐40, Fstl1 has an extra short helix α1 on its N‐terminus. BM‐40 has two inserted short α‐helices within the loop between sheets β2 and β3, resulting in an enlarged surface (Figure 3b). In addition, an extra α‐helix is inserted between helix α2 and sheet β5 in BM‐40, leading to a distinguishable protruding surface.
The R.M.S.D. between Fstl1 and Follistatin (Figure 3c) or Fstl3 (Figure 3e) are 5.4 å and 4.1 å, respectively. Their Kazal domains are similar, and the main structural differences are located on N‐terminal FOLN region. The unique α1‐helix of Fstl1 is not available in the other two structures. Furthermore, the relative position of FOLN to the Kazal domain is significantly different amongst these structures.
The R.M.S.D. between Fstl1 and Human complement factor I is 9.2 å. The N‐terminal helix α1 region preceding β1‐sheet protrudes in opposite directions (Figure 3d). The packing pattern of FOLN and Kazal domain is similar. There is a short helix α1 in the loop linking FOLN and Kazal domain in Human complement factor I. In addition, the anti‐parallel sheet β1‐β2 in Fstl1 is longer than that in Human complement factor I.
2.4. The possible binding specificity of FK domains
Follistatin family interacts with TGF‐β proteins to regulate the latter triggered signaling in the cells, a process that is accompanied with large conformational changes for Follistatin family proteins before and after interactions occur. Several complex structures of these interactions have been reported previously, but not including Fstl1. By comparing the structure of free Fstl1 with homologues in these complexes, we could provide a basis for exploring the structural changes of Fstl1 during its interaction with other proteins.
BM‐40 binds specifically with collagen to form a complex (PDB code: 2V53). Overall, Fstl1 has the similarity with BM‐40 in BM‐40‐collagen complex in a R.M.S.D. of 2.2 å. The structure of BM‐40 changed significantly following binding with collagen. Compared to the free BM‐40, the FOLN domain of BM‐40 in the complex moves closer towards collagen, and the loop following Kazal‐Like domain acquires a large conformational change (Figure 4a). Based on this observation, we speculate that, upon encountering the interacting partners, the possible conformational change of Fstl1 may also include its loop region between FK and EGF domains. Further superimposition displays that the N‐terminus of Fstl1 is unique; this region has more charged residues, which forms a special region with low electrostatic potential (Figure 4b). We can also observe the extremely different electrostatic potential in the loops between sheet β2 and β3 for these two proteins. In this region, Fstl1 is more basic, in contrary to the acidic property in BM‐40 (Figure 4b). In addition, we noticed that there is an extra α‐helix in the loop region between helix α2 and sheet β5 in BM‐40, which includes several basic residues contributing to a local high electrostatic potential (Figure 4b). Overall, the different electrostatic potential on the surface between Fstl1 and BM‐40 may dictate their different binding partners.
Figure 4.
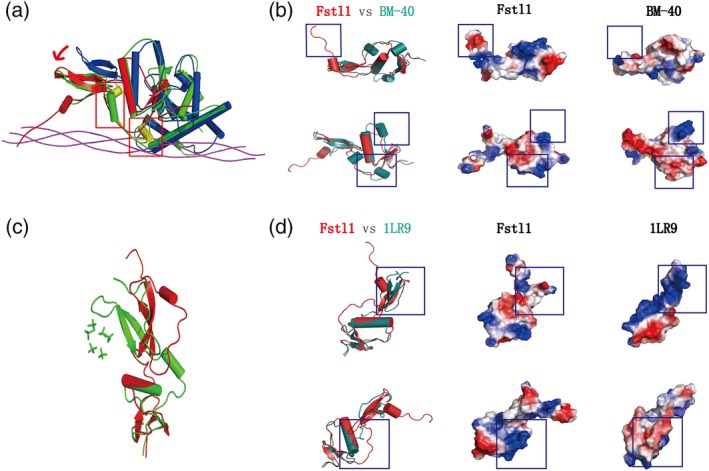
The possible binding specificity of FK domains. (a) Superimposition of Fstl1‐FK domain (red) with free BM‐40 (green, PDB code 1BMO) and BM‐40‐collagen complex (PDB code 2V53, blue for BM‐40 and magenta for collagen). The loop region linking N‐terminal β‐sheet and kazal region was colored by yellow. Red arrow indicated the region with dramatic conformational shift. (b) The differences of electrostatic potential distribution between Fstl1‐FK domain (red) and BM40 (green). The different regions were labeled in the blue rectangles. (c) Superimposition of Fstl1‐FK domain (red) with Follistatin‐heparin analogs (green, PDB code 1LR7). (d) The differences of electrostatic potential distribution between Fstl1‐FK domain (red) and Follistatin (green, PDB code 1LR9). The different regions were labeled in the blue rectangles
Secondly, we compared the structure of Fstl1 with FS1 domain of Follistatin in complex with heparin analogs (PDB codes: 1LR7, 1LR8, 1LR9) (Figure 4c). We found that the binding region of heparin analogs in Follistatin is a basic‐residue‐enriched region, which forms a long strip on the surface of Follistatin to enclose heparin analogs. However, we did not find the corresponding region on the surface of Fstl1 (Figure 4d), indicating an unlikely interaction between Fstl1 with heparin analogs. Moreover, the loop region between FOLN and Kazal is significantly different in two proteins, with distinctive electrostatic potential, indicating the potential differences of the physiological functions for two proteins.
2.5. FK domain is essential for the proper function of Fstl1 in vitro
Fstl1 has been shown to enhance bleomycin‐induced lung fibrosis through regulating TGF‐β1 responsiveness.17 To identify Fstl1 functional domain, we constructed full‐length Fstl1 (pc‐Fstl1), FK domain deletion mutant (pc‐Fstl1‐△FK) and FK domain alone (pc‐Fstl1‐FK) (Figure 5a). We firstly tested if FK domain is essential for Fstl1 to promote TGF‐β1‐induced expression mesenchymal marker (N‐cadherin) in A549 cells. The expression of N‐cadherin was detected by immunoblotting. As shown in Figure 5b, overexpression of full‐length Fstl1 or Fstl1‐FK, but not Fstl1‐△FK, enhanced TGF‐β1 induced N‐cadherin expression in A549 cells.
Figure 5.
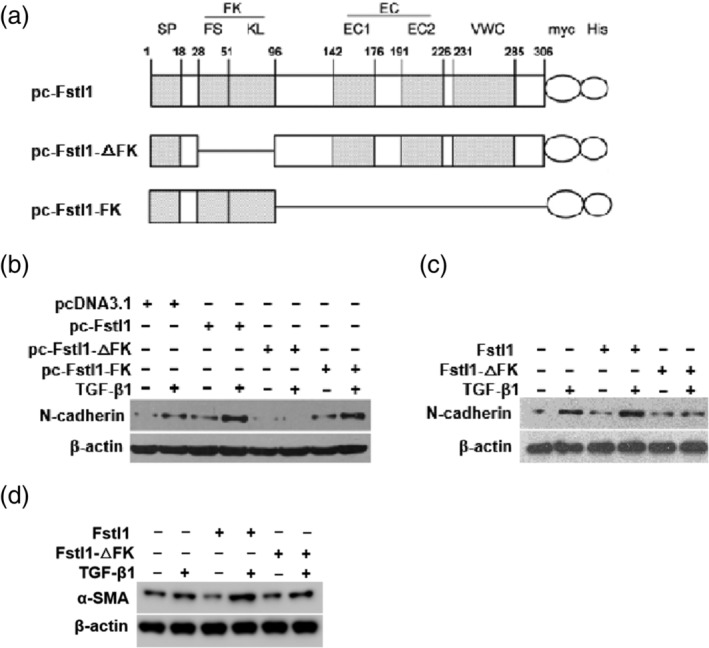
FK domain is essential for Fstl1 in promoting TGF‐β1 signaling in lung epithelial cell and mouse embryonic fibroblasts (MEF). (a) Schematic representation of myc and His‐tagged full‐length Fstl1 (1–306 aa), FK domain deletion mutant and only FK domain mutant. (b) A549 cells were transfected with empty vector or Fstl1, Fstl1‐△FK and Fstl1‐FK plasmid for 48 hr, then treated with TGF‐β1 (5 ng/mL) for 48 hr. Cell lysates were immunoblotted with N‐cadherin and β‐actin (loading control throughout) antibodies. Shown were representative blots from three independent experiments. (c, d), A549 cells (c) or MEF cells (d) were treated with TGF‐β1 (5 ng/mL) and Fstl1 (100 ng/mL) or Fstl1‐△FK mutant protein (100 ng/mL) for 48 hr or 24 hr. Immunoblotting was performed to detect the protein levels of N‐cadhein, α‐SMA and β‐Actin. Shown were representative blots from three independent experiments
To further confirm the importance of FK domain for Fstl1 function, we generated and purified biologically active recombinant Fstl1 and Fstl1‐△FK protein by Drosophila S2 expression system. Then we treated A549 cells (Figure 5c) with TGF‐β1 and Fstl1 or Fstl1‐△FK mutant protein. As expected, administration of Fstl1‐△FK mutant protein failed to promote TGF‐β1‐induced N‐cadherin as that of full‐length Fstl1 in A549 cells. Similar observation was also confirmed in another in vitro model of TGF‐β1‐induced fibroblast activation (upregulation of α‐SMA expression) in MEF cells (Figure 5d). These data indicate that FK domain is essential for Fstl1 to regulate TGF‐β1 responsiveness in vitro.
2.6. FK domain is essential for the proper function of Fstl1 in vivo
To further investigate whether FK mutant (Fstl1‐△FK) protein blocks Fstl1 activities in bleomycin‐model of lung fibrosis, we intravenously administrated PBS or Fstl1‐△FK protein in mice at indicated time points after intratracheal bleomycin injection (Figure 6a). We have previously shown the induction of Fstl1 after bleomycin injury. Here, the administration of FK deletion mutant protein effectively dampened bleomycin‐induced lung fibrosis phenotype, as determined by H&E staining (Figure 6b). Collagen accumulation in lung tissue treated with FK mutant was significantly reduced, as determined by Masson's trichrome staining (Figure 6b) and hydroxyproline content (Figure 6c). The attenuated fibrosis by FK mutant was further supported by the reduced accumulation of myofibroblasts, as determined by α‐SMA expression in mice lung tissues (Figure 6d). These results indicate that FK‐deletion mutant protein of Fstl1 attenuates bleomycin‐induced lung fibrosis, which possibly provide a novel therapeutic strategy for patients with progressive pulmonary fibrosis.
Figure 6.
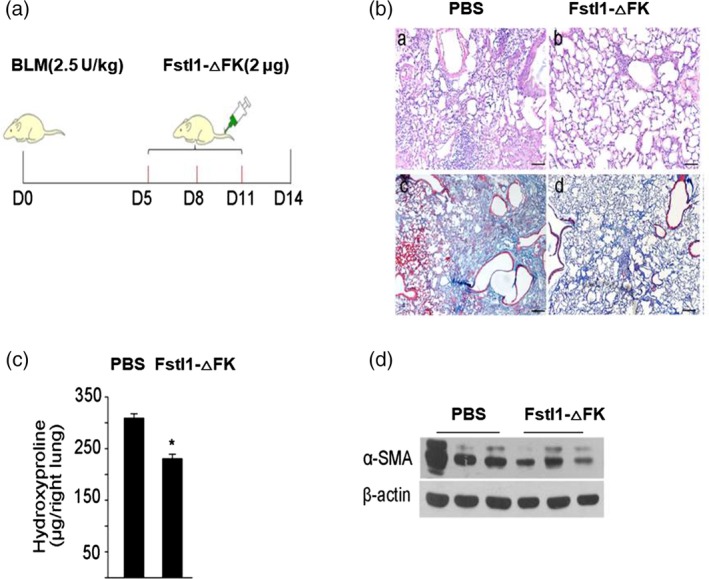
Fstl1 deletion mutant protein attenuates lung fibrosis after bleomycin challenge in vivo. (a) C57BL/6J mice were subjected to intratracheal injection of 2.5 U/kg bleomycin treatment, then intravenous administration of PBS or Fstl1‐△FK protein (2 μg/mouse) at 5, 8, 11 day. (b) H&E (a, b) and masson trichrome staining of collagen (c, d) on lung sections from PBS or Fstl1‐△FK protein injection mice after bleomycin treatment. Representative images of the staining are shown (n = 4 per group). (c) Hydroxyproline contents in lung tissues from PBS or Fstl1‐△FK protein injection mice after bleomycin treatment were measured (n = 4 per group; *, p < .05). (d) Mouse lung tissues were harvested for western blot analysis. Immunoblotting was performed to detect the protein levels of α‐SMA and β‐actin (n = 4 per group), three repetitive experiments were shown for each group
3. DISCUSSION
Fstl1 is a TGF‐β superfamily binding protein and has been identified as a bone morphogenetic protein 4 (BMP4) antagonist controlling embryonic development.1 Fstl1 is also involved in many pathological processes, including carcinogenesis and metastasis, inflammatory and autoimmune diseases, cardiovascular diseases, and organ fibrosis.16, 31, 32, 33, 34 Despite extensive studies for Fstl1 have been performed, no structural information is available for this important molecule. In our report, we solved the high‐resolution crystal structure of FK domain of Fstl1, analyzed its unique characteristics, and investigated its contribution to the function of full‐length Fstl1. We found that, Fstl1‐FK forms a stable dimer in both solution and crystal, which suggest that this protein may function as a dimer during its interaction with TGF‐β, a molecule which has been known to function in a dimer form. These observations, despite still preliminary, provide important information for our understanding of Fstl1.
Fstl1 consists of FK domain, EC domain and VWC domain, belongs to Fst‐SPARC family. Its sequence has low homology with other members of Fst‐SPARC family, including follistatin and BM‐40/SPARC/osteonectin. The FK domain of Fstl1 does not bind to activin like follistatin,35 nor does its EC domain bind to collagen like SPARC.28 So far, few studies have been reported on Fstl1 functional domain. The EC domain of Fstl1 was demonstrated to be nonfunctional.36 However, Li et al. showed that the EC domain was critical for Fstl1 suppress sensory afferent transmission.37 Recently, Fstl1 has been chosen as a promising target for treatment of lung fibrosis.17 In our study, we found that FK domain of Fstl1 is more similar to corresponding domain of BM‐40, rather than follistatin (Figure 4b), and is indispensable for the proper function of Fstl1 during the activation of TGF‐β signaling and regulating lung fibrosis in vitro and in vivo. Here our structural and functional data provided the elucidation of the function of this molecule, and contributed to the progress of our manipulation of this molecule for clinical study.
4. EXPERIMENTAL PROCEDURES
4.1. Molecular cloning
The full length of mFstl1 without signal peptide encoding the amino acid between 20 and 306 was constructed into pMT/Bip‐His A (Invitrogen) for Fstl1 protein production. The full length of mFstl1 without signal peptide was constructed into pcDNA3.1/myc‐His (−) A vector for functional assay. Several mutants were constructed respectively. Construct of Fstl1‐ΔFK lacks the Follistatin and Kazal domain. Construct of Fstl1‐FK consists of Follistatin and Kazal domain only. All of the constructs were verified by DNA sequencing.
The FK domain of Fstl1 was inserted into pET30‐His vector (Invitrogen) for prokaryotic expression. The FS domain of Follistatin was also inserted into pET30‐His vector for prokaryotic expression and analytical ultracentrifuge (AUC) analysis.
4.2. Expression, purification, and crystallization of Fstl1
Drosophila S2 cells were seeded into T25 culture flask, and were transfected with a mixture containing 5 μg pMT/Bip‐mFstl1‐His, 0.3 μg selecting vector pCoBLAST, and 8 μL transfection reagent cellfectin (Invitrogen). After 5 hr incubation, transfection buffer was replaced by fresh SFX‐INSECT medium (Hyclone). Two days post transfection, medium with Blasticidin (25 μg/mL) was added for stably cell line selection. The selection medium was renewed every 3 days for about 3 weeks until stable cell lines grow up.
For large scale S2 cells culture, the seed cells were inoculated in to 200 mL SFX‐INSECT medium in a 500 mL spinning flask at a ratio of 1:10. The cells were cultured at 27°C with a constant stirring rate of 120 rpm. When the cell density reached 5 × 106 cells/mL, CuSO4 was added with a final concentration of 0.5 mM to induce protein expression. The supernatant of the culture was harvested 3 days later. Supernatant containing secreted Fstl1 was filtered, concentrated, and transferred into the Ni‐NTA binding buffer (50 mM Tris, pH 8.0, 500 mM NaCl, 10 mM imidazole) by the Amicon Ultra spin filters (Amicon Stirred Cell, Model 8003).
Recombinant Fstl1 with 6 × His tag was captured by Ni‐NTA affinity column. Following a thorough wash, the protein was eluted from Ni‐NTA column with elution buffer (50 mM Tris–HCl pH 8.0, 500 mM NaCl, 250 mM imidazole), then was concentrated and subsequently the 6 × His tag was removed with TEV protease. The protein was further purified by an ion exchange Q‐Sepharose and a HiLoad 16/600 Superdex 200 gel filtration column (GE Healthcare) in a buffer containing 50 mM Tris–HCl, pH 8.0, 500 mM NaCl).
Initial crystallization screening was carried out by the sitting‐drop vapor diffusion method at 293 K with a protein concentration of 10 mg/mL. The small crystals were obtained in Index kit condition No.20 (0.1 M HEPES pH 7.5, 1.4 M Sodium citrate tribasic dihydrate). The crystallization condition was optimized by changing the concentration of protein, the gradient of the precipitant, and the pH of the solution. Finally, crystals that were suitable for data collection were obtained under the condition of 0.1 M HEPES, pH 7.5, and 1.2 M sodium citrate tribasic dihydrate. Unfortunately, despite the full‐length Fstl1 was used for crystallization, the proteins is degraded quickly in the crystallizing solution. The protein fragment containing in the crystals is only approximate 14 KD on molecular weight detected by SDS‐PAGE, and the N‐terminal sequencing revealed that this small fragment is attributed to the FK domain of Fstl1.
4.3. Data collection and structure determination
Diffraction data were collected in an in‐house X‐ray facility at 100 K at a wavelength of 1.5418 å using a Rigaku MM‐007HF X‐ray source equipped with an R‐AXIS HTC image plate detector. A high‐quality native dataset was collected using 1° oscillation per frame for 180°, with a crystal‐to‐detector distance of 170 mm. The data set was indexed, integrated and scaled using the HKL‐2000 package.
Despite several crystal structures of FOLN‐Kazal region (FK domain) from Follistatin family have been solved, the building of initial model of Fstl1‐FK for our crystal structure by molecular replacement failed due to low sequence similarity. Fstl1‐FK contains 10 cysteine residues constituting 5 pairs of disulfide bonds. By taking advantage of anomalous signal of sulfur, the structure was solved by single wavelength anomalous diffraction (SAD) method. Initial sulfur sites were discovered by SHELX, and further phasing was resolved by Phenix.autosol. Initial model was built by Phenix.autobuild. COOT and Phenix.refine was used repeatedly to further rebuild and refine the model. The quality of the final model was validated with PROCHECK. The results of data collection and structure refinement were summarized in Table 1. The coordinates and structure factors were deposited to Protein Data Bank with entry 6JZA.
Table 1.
X‐ray crystallographic data and refinement statistics for Fstl1‐FK
| Data collection | |
| Space group | P6 2 |
| Wavelength (å) | 1.5418 |
| Unit cell dimensions | |
| a (å) | 45.73 |
| b (å) | 45.73 |
| c (å) | 68.97 |
| α, β, γ (°) | 90, 90, 120 |
| Molecules per ASUa | 1 |
| Resolution (å)b | 30–2.3 (2.37–2.30) |
| Completeness (%)b | 99.89 (99.98) |
| Redundancyb | 9.95 (8.14) |
| No. of total reflectionsb | 36,506 (3,785) |
| No. of unique reflectionsb | 3,669 (465) |
| I/σ b | 25.3 (2.5) |
| R sym b , c | 5.2 (35.3) |
| Refinement statistics | |
| Resolution (å) | 2.30 |
| No. of reflections | 3,669 |
| R work/R free (%)d , e | 20.20/23.57 |
| No. of atoms | |
| Protein | 652 |
| Water | 34 |
| B‐factors (å2) | |
| Protein | 49.11 |
| Water | 40.77 |
| R.m.s. deviations | |
| Bond length (å) | 0.010 |
| Bond angle (°) | 1.253 |
| Ramachandran analysis | |
| Favored (%) | 96.20 |
| Allowed (%) | 3.80 |
| Outliers (%) | 0 |
ASU, asymmetric unit.
Values in parentheses are for the highest resolution shell.
R sym = ∑|I − <I>|/∑<I>, where I is the observed intensity, and <I> is the average intensity of multiple observations of symmetry related reflections.
R = ∑hkl||F obs| − |F calc||/∑hkl|F obs|.
R free is calculated from 5% of the reflections excluded from refinement.
4.4. Analytical ultracentrifugation (AUC)
Sedimentation velocity (SV) experiment was performed using a Beckman/Coulter XL‐I analytical ultracentrifuge with double‐sector or six‐channel centerpieces and sapphirine windows. An additional protein purification step on a HiLoad 16/60 Superdex 200 gel‐filtration column in 50 mM Tris pH 8.0, 200 mM NaCl and 1 mM TCEP was performed before the experiments. SV experiments were conducted at 42,000 rpm, 4°C using absorbance detection and double‐sector cells loaded with approximately 40 μM Fstl1‐FK. The buffer composition (density and viscosity) and protein partial specific volume (V‐bar) were obtained using the program SEDNTERP (http://www.rasmb.bbri.org/). The data were analyzed using the programs SEDFIT and SEDPHAT.
4.5. Mammalian cell culture, transfection and Western blot
Human pulmonary epithelial cells A549 was obtained from American Type Culture Collection. Cells were cultured in DMEM medium supplemented with 10% FBS (HyClone) and antibiotics in 5% CO2 at 37°C in a humidified atmosphere.
pcDNA3.1/myc‐His(−)A‐Fstl1, pcDNA3.1/myc‐His(−)A‐Fstl1‐ΔFK or pcDNA3.1/myc‐His(−)A‐Fstl1‐FK was transiently transfected to A549 cells. pcDNA3.1/myc‐His(−)A was transfected as negative control. Two days post transfection, the cells were washed twice with PBS, then cultured in DMEM/F12 medium without FBS for 1 day starvation. After starvation treatment, TGF‐β1 (R&D System) was added to the culture medium at the concentration of 5 ng/mL for 2 days. The cells were washed twice with PBS before RIPA buffer was added for cell lysis. Cell lysates were harvested for further analysis. The concentration of proteins was measured by BCA protein assay kit (Thermo Pierce). The amount of N‐cadherin was detected by western blot with a polyclonal antibody (Abcam). β‐actin (Sigma) was used as loading control.
Mouse embryonic fibroblast (MEF) were used for analysis the function of Fstl1 and its mutants. 5 ng/mL TGF‐β1 together with 100 ng/mL recombinant Fstl1 or Fstl1‐ΔFK proteins were added into the culture medium of MEF cells. 2 days later, N‐cadherin or α‐SMA were detected by western blot.
4.6. Mice, Bleomycin administration, and Hydroxyproline assay
C57BL/6J mice were treated with bleomycin as the pulmonary fibrosis model. All mice were housed and cared for in a pathogen‐free facility at Nankai University. All animal experiments were approved by the Animal Care and Use Committee at Nankai University. Mice were anaesthetized with chloral hydrate. Then intratracheally injected with bleomycin (Blenoxane, Nippon Kayaku Co. Ltd.) at the dose of 2.5 U/kg to establish pulmonary fibrosis model. After bleomycin treatment, intravenous administration of PBS or Fstl1‐ΔFK recombinant proteins (2 μg/mouse/time point, n = 4 per group) at Day 5, 8, 11. Mice were sacrificed at Day 14 after bleomycin treatment. Lung tissues were collected for further analyses. Collagen contents were measured by a conventional hydroxyproline method.38
4.7. Histological analysis
Lung tissues cleared of blood were fixed in 10% formalin neutral buffered at 4°C overnight, dehydrating and embedding in paraffin. Sections of lung tissues were placed on slides, then deparaffinized, rehydrated and washed before staining. After staining with hematoxylin and eosin (Sigma) or Masson, slides were dehydrated, cleared, and sealed. The slides were visualized by light microscopy.
4.8. Statistical analysis
Data are presented as mean ± SEM. Differences in measured variables between experimental and control group were assessed by using two‐sided Student's t tests. Results were considered statistically significant at p < .05.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
AUTHOR CONTRIBUTIONS
X.L. and W.N. conceived the project and designed the experiments. X.L., L.L., and Y.C. performed the experiments. X.L. and W.N. analyzed the data. X.L. wrote the paper.
ACKNOWLEDGMENTS
This project is supported by the National Natural Science Foundation of China (31870730), Tianjin Natural Science Foundation (17JCZDJC31900), National Key Research and Development Program of China (2017YFD0200900), and the Fundamental Research Funds for the Central Universities, Nankai University (63191123).
Li X, Li L, Chang Y, Ning W, Liu X. Structural and functional study of FK domain of Fstl1. Protein Science. 2019;28:1819–1829. 10.1002/pro.3696
Funding information Fundamental Research Funds for the Central Universities, Nankai University, Grant/Award Number: 63191123; National Key Research and Development Program of China, Grant/Award Number: 2017YFD0200900; National Natural Science Foundation of China, Grant/Award Number: 31870730; Natural Science Foundation of Tianjin City, Grant/Award Number: 17JCZDJC31900
Contributor Information
Wen Ning, Email: ningwen108@nankai.edu.cn.
Xinqi Liu, Email: liu2008@nankai.edu.cn.
REFERENCES
- 1. Sylva M, Moorman AF, van den Hoff MJ. Follistatin‐like 1 in vertebrate development. Birth Defects Res C Embryo Today. 2013;99:61–69. [DOI] [PubMed] [Google Scholar]
- 2. Gagliardi F, Narayanan A, Mortini P. SPARCL1 a novel player in cancer biology. Crit Rev Oncol Hematol. 2017;109:63–68. [DOI] [PubMed] [Google Scholar]
- 3. Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin‐like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. [DOI] [PubMed] [Google Scholar]
- 4. Mattiotti A, Prakash S, Barnett P, van den Hoff MJB. Follistatin‐like 1 in development and human diseases. Cell Mol Life Sci. 2018;75:2339–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geng Y, Dong Y, Yu M, et al. Follistatin‐like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sylva M, Li VS, Buffing AA, et al. The BMP antagonist follistatin‐like 1 is required for skeletal and lung organogenesis. PLoS One. 2011;6:e22616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu J, Qi X, Gong J, et al. Fstl1 antagonizes BMP signaling and regulates ureter development. PLoS One. 2012;7:e32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimano M, Ouchi N, Nakamura K, et al. Cardiac myocyte follistatin‐like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108:E899–E906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mashimo J, Maniwa R, Sugino H, Nose K. Decrease in the expression of a novel TGF β1‐inducible and rasrecision gene, TSC‐36, in human cancer cells. Cancer Lett. 1997;113:213–219. [DOI] [PubMed] [Google Scholar]
- 10. Johnston IM, Spence HJ, Winnie JN, et al. Regulation of a multigenic invasion programme by the transcription factor,AP‐1: Re‐expression of a down‐regulated gene, TSC‐36, inhibits invasion. Oncogene. 2000;19:5348–5358. [DOI] [PubMed] [Google Scholar]
- 11. Sumitomoa K, Kurisakib A, Yamakawab N, et al. Expression of a TGF‐β1 inducible gene, TSC‐36, causes growth inhibition in human lung cancer cell lines. Cancer Lett. 2000;155:37–46. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin‐related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305–1314. [DOI] [PubMed] [Google Scholar]
- 13. Ehara Y, Sakurai D, Tsuchiya N, et al. Follistatin‐related protein gene (FRP) is expressed in the synovial tissues of rheumatoid arthritis, but its polymorphisms are not associated with genetic susceptibility. Clin Exp Rheumatol. 2004;22:707–712. [PubMed] [Google Scholar]
- 14. Miyamae T, Marinov AD, Sowders D, et al. Follistatin‐like protein‐1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–4762. [DOI] [PubMed] [Google Scholar]
- 15. Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin‐like protein 1 promotes arthritis by up‐regulating IFN‐gamma. J Immunol. 2009;182:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong Y, Geng Y, Li L, et al. Blocking follistatin‐like 1 attenuates bleomycin‐induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan N, Sun H, Wang Y, et al. Follistatin‐like 1: A potential mediator of inflammation in obesity. Mediators Inflamm. 2013;2013:752519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Y, Zhou S, Smas CM. Downregulated expression of the secreted glycoprotein follistatin‐like 1 (Fstl1) is a robust hallmark of preadipocyte to adipocyte conversion. Mech Dev. 2010;127:183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Dong Y, Teng X, Cheng M, Shen Z, Chen W. Follistatin‐like 1 attenuates differentiation and survival of erythroid cells through Smad2/3 signaling. Biochem Biophys Res Commun. 2015;466:711–716. [DOI] [PubMed] [Google Scholar]
- 21. Ouchi N, Asaumi Y, Ohashi K, et al. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaly Y, Blair HC, Smith SM, et al. Follistatin‐like protein 1 regulates chondrocyte proliferation and chondrogenic differentiation of mesenchymal stem cells. Ann Rheum Dis. 2015;74:1467–1473. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Mu T, Li T, et al. Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac Cancer. 2017;8:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi DL, Shi GR, Xie J, Du XZ, Yang H. MicroRNA‐27a inhibits cell migration and invasion of fibroblast‐like synoviocytes by targeting follistatin‐like protein 1 in rheumatoid arthritis. Mol Cells. 2016;39:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murakami K, Tanaka M, Usui T, et al. Follistatin‐related protein/follistatin‐like 1 evokes an innate immune response via CD14 and toll‐like receptor 4. FEBS Lett. 2012;586:319–324. [DOI] [PubMed] [Google Scholar]
- 26. Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming‐growth‐factor‐beta 1‐regulated genes, one of which seems to encode a follistatin‐related polypeptide. Eur J Biochem. 1993;217:13–19. [DOI] [PubMed] [Google Scholar]
- 27. Zwijsen A, Blockx H, Van Arnhem W, et al. Characterization of a rat C6 glioma‐secreted follistatin‐related protein (FRP). FEBS Lett. 1994;225:937–946. [DOI] [PubMed] [Google Scholar]
- 28. Hambrock HO, Kaufmann B, Müller S, et al. Structural characterization of TSC‐36/Flik: Analysis of two charge isoforms. J Biol Chem. 2004;279:11727–11735. [DOI] [PubMed] [Google Scholar]
- 29. Trombetta‐eSilva J, Bradshaw AD. The function of SPARC as a mediator of fibrosis. Open Rheumatol J. 2012;6:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L, Li X, Liu X, et al. Expression, characterization, and preliminary X‐ray crystallographic analysis of recombinant murine Follistatin‐like 1 expressed in Drosophila S2 cells. Biosci Trends. 2013;7:93–100. [PubMed] [Google Scholar]
- 31. Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suppressor effect of follistatin‐like 1 in ovarian and endometrial carcinogenesis: A differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. [DOI] [PubMed] [Google Scholar]
- 32. Li D, Wang Y, Xu N, et al. Follistatin‐like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prakash S, Borreguero LJJ, Sylva M, et al. Deletion of Fstl1 (Follistatin‐like 1) from the endocardial/endothelial lineage causes mitral valve disease. Arterioscler Thromb Vasc Biol. 2017;37:e116–e130. [DOI] [PubMed] [Google Scholar]
- 34. Fang Y, Zhang S, Li X, Jiang F, Ye Q, Ning W. Follistatin like‐1 aggravates silica‐induced mouse lung injury. Sci Rep. 2017;7:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawabata D, Tanaka M, Fujii T, et al. Ameliorative effects of follistatin‐related protein/TSC‐36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660–668. [DOI] [PubMed] [Google Scholar]
- 36. Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li KC, Zhang FX, Li CL, et al. Follistatin‐like 1 suppresses sensory afferent transmission by activating Na+,K+‐ATPase. Neuron. 2011;69:974–987. [DOI] [PubMed] [Google Scholar]
- 38. Jiang D, Liang J, Hodge J, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]


