Abstract
Background:
Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor. Metformin may potentiate mTOR inhibition by sirolimus while mitigating its adverse effects. We conducted a pilot study to test this hypothesis.
Methods:
Patients with advanced solid tumor were treated with sirolimus for 7 days followed by randomization to either sirolimus with metformin (Arm A) or sirolimus (Arm B) until day 21. From day 22 onwards, all patients received sirolimus and metformin. The primary aim was to compare the change in phospho-p70S6K (pp70S6K) in peripheral blood mononuclear cells (PBMC) from day 8 to day 22 using a two-sample t test. Secondary aims were objective response rate, toxicity and other serum pharmacodynamic biomarkers (e.g., fasting glucose, triglycerides, insulin, C-peptide, IGF-1, IGF-1R, IGF-BP, leptin).
Results:
24 patients were enrolled, with 18 evaluable for the primary endpoint. The was no significant difference in mean change in pp70S6K in arm A versus arm B (−0.12 vs. −0.16; P =0.64). Similarly, there were no significant differences in other serum pharmacodynamic biomarkers. There were no partial responses. There were no dose-limiting or unexpected toxicities.
Conclusions:
Adding metformin to sirolimus, although well tolerated, was not associated with significant changes in pp70S6K in PBMC or other serum pharmacodynamic biomarkers.
Impact:
Combining metformin with sirolimus did not improve mTOR inhibition.
Keywords: Metformin, sirolimus, pharmacodynamics, solid tumors, p70S6K, mTOR
Introduction
The mammalian target of rapamycin (mTOR) is downstream of PI3K signaling and plays a central role in cell growth, metabolism and autophagy [1]. Upregulation of this pathway has been observed in numerous cancers including breast, lung, liver, glioblastoma, lymphoma and kidney [2]. Sirolimus, also known as rapamycin, is an mTOR complex 1 (mTORC1) inhibitor. Sirolimus was approved by the U.S. Food and Drug Administration (FDA) for prevention of orthotopic organ transplant rejection in 1999 under the brand name Rapamune [3]. There is no FDA approved oncology indication for sirolimus. Everolimus, temsirolimus and sirolimus all exhibit similar mTORC1 inhibition [4], but there has been little commercial interest in the development of sirolimus for the treatment of cancer.
The use of mTORC1 inhibitors is limited by potential toxicities (such as fatigue, pneumonitis, hyperglycemia, hyperlipidemia, and lymphopenia), and lack of durable responses. The latter is believed to be secondary to feedback upregulation of AKT causing pathway escape [2, 5]. To address this, combined mTORC1 and mTORC2 inhibitors, dual PI3K and mTOR inhibitors, and combinations of mTOR inhibitors with MEK or RAF inhibitors are currently under investigation in clinical trials [6]. These strategies can be expensive with uncertain benefit. Thus, combining mTORC1 inhibitors with another drug that could potentiate their efficacy, mitigate their adverse effects and is inexpensive could be very useful. Metformin, an oral hypoglycemic drug, has established mechanistic properties that might fulfill this role.
Metformin is a generic drug of biguanide class with an excellent safety profile that makes it an attractive anti-cancer agent. In epidemiological studies, metformin use is associated with reduced incidence and mortality of colorectal, breast, lung, prostate, ovarian and pancreatic cancer [7–13]. There is a growing body of evidence that metformin exerts anti-cancer activity by both cell autonomous and systemic effects [14]. The systemic effects are mainly through its anti-hyperglycemic action of suppressing hepatic glucose output (inhibition of gluconeogenesis and glycogenolysis), increasing peripheral tissue (skeletal muscle and adipocytes) insulin sensitivity and decreasing intestinal absorption of glucose [15].
The cell autonomous effects of metformin include inhibition of mitochondrial oxidative phosphorylation through the LKB1-AMPK pathway [16–18] (Figure 1). AMPK is a central regulator of cellular energy metabolism. The activation of AMPK leads to (a) suppression of mTOR and subsequent downstream effectors including p70S6K and 4E-BP1, resulting in inhibition of protein synthesis, cell growth and proliferation [19]; (b) inhibition of 3-hydroxy-3-methyl-glutaryl-CoA (HMG CoA) reductase resulting in inhibition of lipid and sterol biosynthesis; and (c) systemic inhibition of growth promoting factors including glucose, insulin, insulin-like growth factor 1 (IGF-1), insulin-like growth factor 1 receptor (IGF-1R), insulin-like growth factor binding protein (IGF-BP) and leptin, whereas metformin causes an increase in adiponectin that exerts anti-cancer effects.
Figure 1:
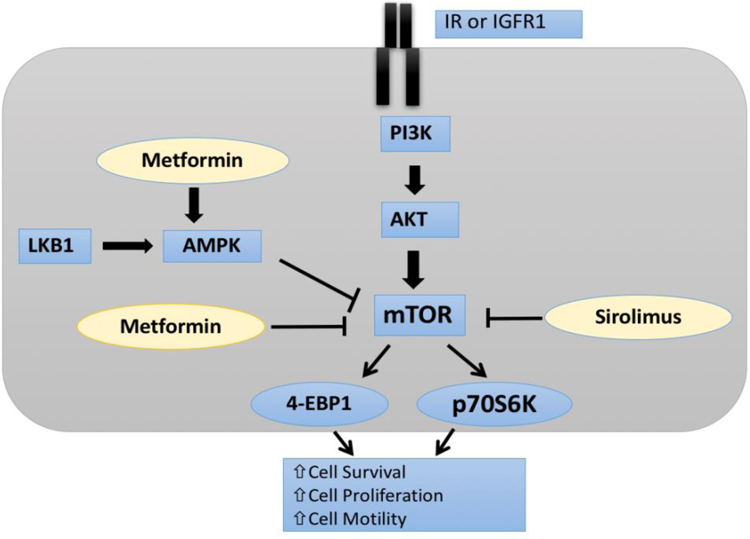
Potential mechanisms of action of sirolimus and metformin. Sirolimus directly inhibits mTOR whereas metformin mainly leads to mTOR inhibition through activation of AMPK, although metformin can also directly inhibit mTOR. mTOR inhibition ultimately leads to decrease in p70S6K.
Given the role of mTOR signaling and potential synergistic activity of combining sirolimus and metformin in patients with advanced solid tumors, we conducted an open-label, randomized study to test the hypotheses that (a) the combination of metformin plus sirolimus would result in greater reduction of phospho-p70S6K (pp70S6K) than sirolimus alone in peripheral blood mononuclear cells (PBMC); (b) the combination of metformin plus sirolimus would result in decreased levels of serum biomarkers including fasting insulin, C-peptide, glucose, triglycerides, IGF-1, IGF-BP1, IGF-BP3 and leptin, but an increase in adiponectin in peripheral blood; and (c) sirolimus-induced toxicity, especially hyperglycemia and hypertriglyceridemia, would be mitigated by combining sirolimus with metformin.
Materials and Methods
Eligibility criteria
Patients were eligible if they were aged 18 years or older with a histologically or cytologically confirmed solid tumor that was metastatic or unresectable and for which standard curative or palliative measures did not exist or were no longer effective. Patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1 and adequate hematologic function (absolute neutrophil count [ANC] > 1500/μl, hemoglobin > 9g/dL and platelets > 100,000/μl), renal function (creatinine < 1.4 mg/dl for females or < 1.5 mg/dl for males) and acceptable hepatic disease markers (total bilirubin < 1.5x upper limit of normal [ULN], SGOT and SGPT < 2.5x ULN for patients without liver metastases or SGOT and SGPT < 5x ULN for patients with liver metastases).
Patients were excluded for prior treatment with an mTOR inhibitor (including sirolimus) resulting in grade 3 or greater toxicities and if they were already on treatment with metformin, unless metformin could be held for 4 weeks prior to the start of the study. Additional exclusion criteria included fasting glucose > 200 mg/dL, fasting triglycerides > 300 mg/dL, concurrent use of any proton pump inhibitors (which decrease the absorption of metformin) [14, 20], history of lactic acidosis or metabolic acidosis (blood pH < 7.35), and uncontrolled diabetes (defined as a hemoglobin A1c > 8%).
The study protocol was approved by the University of Chicago Institutional Review Board and all patients provided written informed consent.
Study design and treatments
We conducted an open-label, randomized study (randomization was performed using the method of permuted blocks) in which all eligible patients were started on sirolimus 3 mg daily alone for the first 7 days (lead-in period); cycle 1, days 1–8 (see Figure 2). Sirolimus in the tablet formulation (1 mg tablets) was taken at approximately the same time every day, and was taken consistently either with or without food. Before starting sirolimus, blood was collected for baseline measurement of pharmacodynamic biomarkers in PBMC. The blood was collected again for pharmacodynamic biomarkers on cycle 1, day 8 to assess the effect of sirolimus alone on these biomarkers. In our previous study, the effect of sirolimus on pp70S6K was observed within 48 hours of the first dose [21]. On day 8, patients were randomized 1:1 to metformin extended-release (XL) (500 mg daily for a week and then increased to 1000 mg daily on day 15 as tolerated) in addition to sirolimus (Arm A) or continued on sirolimus alone (Arm B) for two weeks (until day 22). Metformin XL was taken with the evening meal and was swallowed whole and never crushed or chewed. A repeat biomarker evaluation was done on cycle 1, day 22 to assess the effect of adding metformin XL on these pharmacodynamic biomarkers. From day 22 onwards, all patients received the combination of sirolimus and metformin XL.
Figure 2:
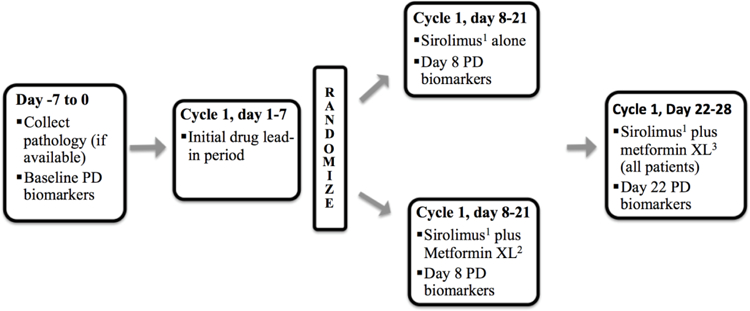
Study design showing screening phase (day −7 to 0), initial lead-in phase (day 1–7), randomized phase (day 8–21) and post-randomization phase (day 22–28). The pharmacodynamic (PD) biomarkers were collected at baseline (day −7 to 0), day 8 and day 22.
1 Sirolimus 3 mg daily, oral tablet
2 Metformin XL started at 500 mg daily for day 8–14 and increased to 1000 mg daily on day 15 if there is no metformin related toxicity ≥ grade 2. If a patient had a grade 2 toxicity due to metformin they were maintained on metformin XL 500 mg daily for the remainder of the study. If the patient develops toxicity ≥ grade 3 they were taken off study.
3 Sirolimus 3 mg daily and metformin XL 1000 mg daily; metformin XL started at 500 mg daily for those not previously receiving it and titrated up to 1000 mg daily after one week (as above).
Patients were continued on combination therapy until disease progression or unacceptable toxicity. In case of an unacceptable toxicity defined as grade ≥ 3 per the National Cancer Institute (NCI) Common Toxicity Criteria, version 4.0, one or both drugs were held (depending upon attribution) until the toxicity resolved to grade ≤1 and the patient was restarted on drug(s) reduced by one dose level (sirolimus 2 mg daily; metformin XL 500 mg daily). Patients who experienced toxicity ≥ grade 3 at the lowest dose were taken off study (i.e., no further dose reduction was allowed).
The primary objective of the study was to compare the change in the pharmacodynamic biomarker pp70S6K in PBMC with the combination of metformin XL and sirolimus to that with sirolimus alone.
Assessments
Evaluations during the study period included complete medical history, physical examinations and laboratory tests including hematologic and metabolic profiles. Toxicities were assessed using the NCI Common Toxicity Criteria, version 4.0. Patients with measurable disease were assessed for objective tumor response and duration of response every 8 weeks according to RECIST (version 1.1) criteria [22].
Biomarker Assessment/Correlatives:
We hypothesized that the metformin would increase the efficacy of sirolimus by inhibition of mTOR signaling. As described above, mTOR inhibition by metformin is at least partially through the LKB1-AMPK pathway, as compared to sirolimus, which causes mTOR inhibition through FKBP12. Additionally, activation of AMPK causes phosphorylation of ULK1, which induces autophagy [23]. Based on these hypotheses, we assessed the putative target markers. Blood samples (about 6 tablespoons of blood) were collected at baseline (cycle 1 day 1), cycle 1 day 8 and cycle 1 day 22 for the research related blood tests as listed.
Quest Diagnostics performed testing of serum fasting glucose, fasting triglycerides, insulin, insulin C-peptide, IGF-1, IGF-BP1, IGF-BP3, leptin and adiponectin. Enzyme-linked immunosorbent assays (ELISA) were used for measuring insulin, insulin C-peptide, IGF-1, IGF-BP1, IGF-BP3, leptin and adiponectin. The time points and assays for pp70S6K are described below.
Peripheral blood CD3+ T cell pp70S6K
It has been reported that the phosphorylation of p70S6 kinase (p70S6K) at threonine 389 is most closely correlated with mTOR activity in vivo [24] and our group has reported correlation between the sirolimus dose and phosphorylation of p70S6K [21]. In the present study, we measured p70S6K phosphorylation at Thr389 in PBMCs as a biomarker of sirolimus and metformin effect in vivo.
The blood sample was drawn at baseline (cycle 1 day 1), day 8 and day 22 of cycle 1. PBMCs were isolated by Ficoll density gradient centrifugation, followed by total CD3+ T cell isolation using the Human T cell Enrichment Kit (Miltenyi. Inc, Bergisch Gladbach, Germany). The T cells were cryopreserved until further analysis. One the day of analysis, the cryopreserved CD3+ T cells were thawed, washed, and counted. Half of the T cells were cultured with media as control, while the other half were cultured with PMA + Ionomycin at concentrations of 50ng/ml and 500ng/ml, respectively, for 3 hours at 37°C in an incubator with 5% CO2. After the stimulation, T cells were centrifuged, washed once with cold PBS, and lysed in RIPA buffer for 30 minutes on ice. The lysates were centrifuged and the supernatant was collected and mixed with Western blot sample buffer. The samples were then denatured at 95°C in a water bath for 5 minutes and loaded onto a 10% SDS-PAGE gel (Bio-rad Laboratories, Hercules, California). Proteins were then transferred onto an immubilon-FL membrane (Fisher Scientific, IPFL00010, Hampton, New Hampshire). The membrane was blocked in Odyssey blocking buffer (Li-cor Inc., Lincoln, Nebraska) for one hour at room temperature (RT) with agitation. After blocking, the membrane was incubated with rabbit anti-human phospho-p70 S6 kinase antibody (Cell Signaling Technology, Danvers, Massachusetts) overnight at 4°C. The membrane was washed three times with TBST, then incubated with IRDye 680LT donkey anti-rabbit IgG (Li-cor Inc.) for one hour at RT. After three washes with TBST, the membrane was re-blocked with mouse anti-human beta-actin antibody (Cell Signaling Technology) for one hour at RT, washed three times with TBST, and incubated with IRDye 800CW goat anti-mouse IgG (Li-cor Inc.) for one hour at RT. After three washes and re-blocking, the membrane was incubated with rabbit anti-human phospho-Erk1,2 antibody (Cell Signaling Technology) for one hour at RT. The membrane was washed three times with TBST, incubated with IRDye 680LT donkey anti-rabbit IgG (Li-cor Inc.) for one hour at RT. After three washes with TBST, the image of the membrane was captured using Odyssey Fc Imager analyzed using Image Studio. The antibody against beta-actin was used as a loading control. pErk was also used as a control as treatment with PMA and ionomycin leads to increased phosphorylation of Erk (pErk), and phosphorylation of Erk is not affected by the mTOR-S6K pathway.
Statistical Considerations
pp70S6K (the primary endpoint) and other biomarkers (plasma insulin, insulin C-peptide, glucose, IGF-1, IGF-BP1, IGF-BP3, leptin and adiponectin) were measured at baseline, on day 8 (end of the sirolimus lead-in period), and on day 22 (14 days post randomization). Within-patient changes were calculated on a log scale (equivalent to log ratio) and compared between the two treatment arms using a two-sample t-test. Using the data from Cohen et al [21], we assumed 0.55 as the standard deviation (SD) of the ratio for day 22 compared to day 8. Further, we estimated that we would require n=64 patients (32 per treatment arm) to provide 80% statistical power assuming no change in the patients randomized to continue sirolimus alone, versus a 50% decrease in those randomized to sirolimus plus metformin (log ratio of −0.693). This calculation assumed a SD on the log scale of approximately 0.55/0.50 = 1.10 (via the delta method) and was based on a one-sided Student’s t-test at the alpha=0.05 significance level.
Results
Patient Demographics
A total of 24 patients were enrolled, at which time an unplanned interim analysis was conducted due to a lower than anticipated accrual rate. There were 11 patients in the sirolimus plus metformin group (arm A) and 13 patients in the sirolimus alone group (arm B). The patient characteristics are summarized in Table 1.
Table 1:
Patient Characteristics, number (%)
| Variable | Sirolimus plus Metformin N = 11 | Sirolimus N = 13 |
|---|---|---|
| Age in years; Median (range) | 58 (26–66) | 56 (18–80) |
| Gender | ||
| Male | 4 (36.4) | 5 (38.5) |
| Female | 7 (63.6) | 8 (61.5) |
| Histology | ||
| Lung (Adenoid Cystic, adeno CA, carcinoid) | 1 (9.1) | 2 (15.4) |
| Breast (Ductal or Mucinous CA) | 0 | 3 (23.1) |
| Colon adeno CA | 2 (18.2) | 0 |
| Endometrial adeno CA | 4 (36.4) | 0 |
| Gastric or GEJ adeno CA | 0 | 2 (15.4) |
| Neuroendocrine tumor | 2 (18.2) | 1 (7.7) |
| Others | 2 (18.2) | 5 (38.5) |
| Prior Lines of Therapy (range 0–15) | ||
| 0 | 2 (18.2) | 6 (46.2) |
| 1 | 3 (27.3) | 1 (7.7) |
| 2 | 3 (27.3) | 3 (23.1) |
| 3 | 3 (27.3) | 2 (15.4) |
| ≥ 4 | 0 | 1 (7.7) |
| Performance Status (baseline) | ||
| 0 | 8 (72.7) | 7 (53.8) |
| 1 | 3 (27.3) | 6 (46.2) |
Phospho-p70S6K Analysis
A total of 18 patients were evaluable for the primary endpoint (8 in arm A; 10 in arm B). Six patients failed to pass quality control due to a low number of CD3+ T cells. The mean change in pp70S6K from day 8 to day 22, expressed as the log of the ratio of pp70S6K on D22 relative to D8, was −0.12 (SD=0.13) in arm A versus −0.16 (SD=0.29) in arm B (Figures 3 and 4). There was no significant difference between the two arms (P= 0.64; Table 2). Analysis of within group changes showed that sirolimus (alone) decreased mean pp70S6K significantly on day 8 compared to day 1 (from 0.13 to 0.03, p=0.01); however, there was no change in mean pp70S6K from day 8 to day 22 (from 0.03 to 0.03, p=0.74; Table 3). Adding metformin to sirolimus also did not significantly decrease the mean pp70S6K on day 22 compared to day 8 (from 0.03 to 0.02, p=0.11; Table 3).
Figure 3:
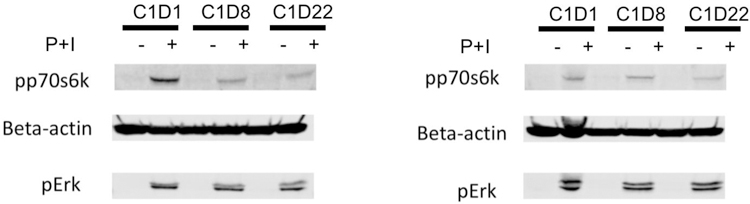
Western blots showing pp70S6K, pERK, and Beta-actin expression in a patient treated with sirolimus alone left panel (A) and a patient treated with sirolimus plus metformin group in the right panel (B). Reduced level of pp70S6K can be seen in both left (A) and right (B) panel.
Abbreviations: P, phorbol 12-myristate 13-acetate (PMA); I, ionomycin; C, cycle; D, day
Figure 4:
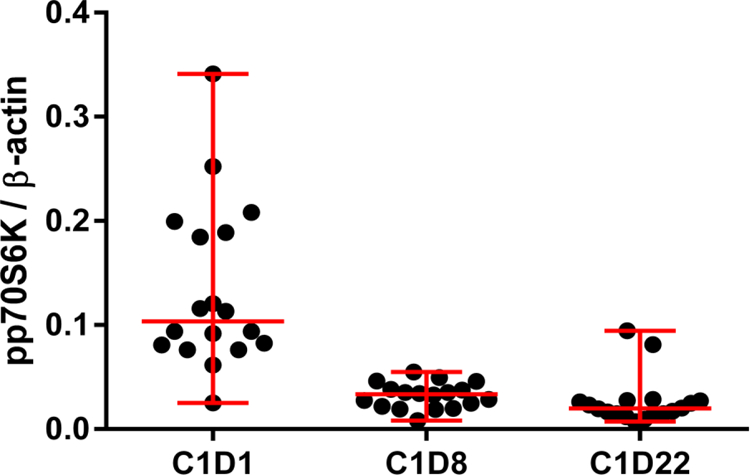
Phospho-p70S6K levels detected by Western blotting in each patient (shown as solid black circle) from sirolimus plus metformin and sirolimus group on cycle (C) 1, day (D) 1, C1D8 and C1D22. Marked decrease in the average level of pp70S6K can be seen from C1D1 to C1D8 which was not as dramatic from C1D8 to C1D22.
Table 2:
Pharmacodynamic Biomarkers
| Biomarkers Mean (SD) | Sirolimus plus Metformin N= 8 (44.4%) | Sirolimus N=10 (55.6%) | Log ratio P-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 22 | Day 1 | Day 8 | Day 22 | D22/D8 | D22/D1 | |
| PP70S6k | 0.13 (0.05) | 0.03 (0.01) | 0.02 (0.006) | 0.13 (0.09) | 0.03 (0.01) | 0.03 (0.03) | 0.64 | 0.47 |
| Fasting glucose (mg/dl) | 100.7 (11.9) | 94 (13) | 91.6 (8.1) | 99.4 (17.7) | 97.5 (16) | 104.8 (29.8) | 0.11 | 0.05 |
| Fasting Triglyceride (mg/dl) | 112.3 (49.2) | 136.3 (58.8) | 128.3 (39.8) | 112.9 (31.8) | 116.9 (37.7) | 162.9 (82.1) | 0.17 | 0.46 |
| Insulin (uIU/mL) | 9.2 (8.1) | 7.7 (4.0) | 6.8 (5) | 7.8 (3.2) | 6.9 (3.9) | 7.7 (4) | 0.25 | 0.75 |
| C-peptide (ng/mL) | 2.9 (1.4) | 2.1 (1.1) | 2.0 (1.1) | 2.4 (1.3) | 2.1 (1.4) | 2.2 (1.3) | 0.31 | 0.15 |
| IGF-1 (ng/mL) | 154.8 (69.5) | 147.5 (69.2) | 142.6 (69) | 150.5 (45.7) | 143.2 (40) | 134.7 (41) | 0.48 | 0.57 |
| IGF-BP1 (ng/mL) | 17.2 (11.6) | 20 (16.1) | 20.2 (11.5) | 16.2 (13) | 16 (13.4) | 20.6 (15) | 0.71 | 0.55 |
| IGF-BP3 (ng/mL) | 4.1 (0.7) | 4.4 (0.7) | 4.4 (0.9) | 4.1 (1.3) | 4 (1.1) | 4.2 (1) | 0.66 | 0.88 |
| Leptin v(ng/mL) | 9.9 (8.9) | 9.2 (9.9) | 7.1 (6.9) | 6.9 (2.7) | 6.2 (2.8) | 5.3 (2.9) | 0.41 | 0.29 |
| Adiponectin (mcg/mL) | 9.6 (6.7) | 10.9 (6.4) | 11.3 (7.8) | 11.3 (4.6) | 13.2 (5.2) | 14.1 (6) | 0.33 | 0.53 |
Table 3:
Within Group Changes: PP70S6K
| PP70S6K | Day 1 Mean (SD) | Day 8 Mean (SD) | Day 22 Mean (SD) | P-Value D22/D8 | P-Value D22/D1 | P-Value D8/D1 |
|---|---|---|---|---|---|---|
| Sirolimus plus Metformin | 0.13 (0.05) | 0.03 (0.01) | 0.02 (0.006) | 0.11 | 0.0005 | 0.0007 |
| Sirolimus | 0.13 (0.09) | 0.03 (0.01) | 0.03 (0.03) | 0.74 | 0.009 | 0.01 |
Other Biomarkers
Of the 21 patients evaluable for serum pharmacodynamic biomarkers, there were no significant differences by adding metformin XL to sirolimus in fasting glucose (P=0.11), fasting triglycerides (P=0.17), insulin (P=0.25), insulin C-peptide (P=0.31), IGF-1 (P=0.48), IGF-BP1 (P=0.71), IGF-BP3 (P=0.66), leptin (P=0.41) and adiponectin (P=0.33) [Table 2]. There were also no statistically significant differences between the two treatment groups in biomarker changes from day 1 to day 22 with the exception of fasting glucose (p=0.05) [Table 2].
Response Evaluation
A total of 17 patients were evaluable for response by RECIST 1.1 criteria based on being on therapy until the time of the first on-treatment CT at 8 weeks. The best response was stable disease in 9 patients; 4 patients in the sirolimus plus metformin XL arm and 5 patients in the sirolimus alone arm. The duration of stable disease ranged from 2.3 months to 16.7 months. The longest duration of stable disease (16.7 months) was observed in a patient with lung neuroendocrine tumor on sirolimus alone.
Adverse Events
The combination therapy with sirolimus and metformin XL was generally well tolerated and there were no significant unexpected toxicities. Only one patient was not increased to metformin XL 1000 mg daily (continued on 500 mg daily) due to grade 1 diarrhea and grade 1 fatigue. None of the patients required dose reduction of metformin XL or sirolimus due to adverse effects.
Discussion
In this open-label, randomized study we found that adding metformin XL to sirolimus, although well tolerated, was not associated with significant changes in pp70S6K or other pharmacodynamic biomarkers. Based on an unplanned interim analysis, the trial was terminated early because it was estimated that the probability of finding a significant difference supporting our hypothesis by the end of the study was extremely low. While our trial did not find a significant difference in pp70S6K levels between treatment arms comparing day 8 and day 22, the within group analysis of pp70S6k showed a decrease in pp70S6K on day 22 compared to day 8 in the sirolimus and metformin group (p=0.11) compared to no change in sirolimus alone group (p=0.74).
The novelty of our study design is that we used a commercially available mTOR inhibitor, sirolimus, and combined it with a commercially available diabetes drug, metformin, to potentially improve the efficacy and reduce the toxicities of mTOR inhibition. Additionally, we used a unique design (randomization after a one-week lead-in) and attempted to measure the efficacy of the drug combination using a peripheral blood biomarker, pp70S6K. The accrual goal of 64 patients is relatively efficient for evaluating a combination strategy with a biomarker endpoint, and we were able to close the study early for futility after only 18 evaluable patients. A similar design could be used for other combination strategies with a biomarker endpoint, perhaps with a pre-planned interim analysis.
One limitation of our study is that pp706SK in PBMC may not be the optimal biomarker to use for mTOR inhibition, as it is not established as a predictive biomarker of response. However, it was selected as the best biomarker to use based on relevant literature and our own institutional experience from a previous trial [21]. Peralba and colleagues studied the pharmacodynamic effects of CCI-779 (temsirolimus), an ester of sirolimus, for clinical use [25]. Using healthy volunteers, they found a 40% inter-individual and 14% intra-individual variability in p70S6K activity in PBMC over one week, suggesting little spontaneous variation over time. Experiments in mice bearing breast cancer cell line MDA-468 and treated with CCI-779 showed that the decrease in p70S6K activity was equivalent in PBMCs and tumor tissue. Also, they reported an association between inhibition of p70S6K activity and CCI-779 treatment starting 24 hours after treatment, irrespective of the dose of CCI-79 (25, 75, or 250 mg) used to treat patients with renal cell cancer. Similarly, Tabernero and colleagues studied tumor samples and skin biopsies for expression of p70S6K, phosphorylated eukaryotic initiation factor 4G (peIF-4G), p4E-BP1 and pAKT in a phase I study of 55 advanced solid tumor patients treated with everolimus [26]. In the cohort with daily dosing (5 mg or 10 mg), they found near complete inhibition of p70S6K on day 22 when compared to baseline (day 0); however, in about 50% of patients, pAKT was increased on day 22 when compared to baseline (day 0). There was good concordance in mTOR pathway inhibition between skin and tumor tissue. Similar results were seen with weekly dosing of everolimus. Additionally, there was no difference between early (24 hours after everolimus administration) and sustained (24 hours before next weekly dose administration) inhibition of p70S6K. Because of the small number of patients achieving clinical benefit, they could not assess p70S6K as a predictive biomarker. Similar findings were reported in another phase I trial with advanced solid tumor patients [27].
Lastly, Ekshyyan and colleagues studied mTOR pathway inhibition with weekly temsirolimus (25 mg) administration in head and neck squamous cell cancer patients [28]. They found a significant decrease in p70S6K in the tumor tissue and PBMC without significant upregulation of pAKT in the tumor tissue when they compared pre-treatment (day 0) and post-treatment (day 22) samples. In contrast, the significant decrease in p4E-BP1 noted in tumors was not seen in PBMCs. Therefore, the authors concluded that p70S6K will be a better pharmacodynamic biomarker for mTOR inhibitors than p4E-BP1.
Based on the above studies, there is evidence to support using p70S6K in PBMC as a pharmacodynamic biomarker for mTOR inhibitors; however, there is no clear dose-response relationship and there is not enough evidence to conclude whether p70S6K can be predictive of anti-cancer response to mTOR inhibitors. Several studies [25, 27–30] have successfully used Western blot assays to quantitatively measure p70S6K activity. We have validated our assay in a previous study of sirolimus [21] and used the same assay to measure pp70S6K in the current protocol exploring the combination of sirolimus and metformin.
There were several other limitations to our study. First, sirolimus alone effectively suppressed pp70S6K by day 8, and this suppression did not change between day 8 and day 22 (contrary to our hypothesis that feedback upregulation of AKT might cause pathway escape). Given the magnitude of effect of sirolimus alone on the biomarker, it would be difficult to show further changes using the combination of sirolimus and metformin. Second, we were unable to measure some of the other pharmacodynamic biomarkers of the mTOR pathway, such as p4EBP-1 and pAKT. In a study by Zakikhani and colleagues to assess the effect of adding metformin to sirolimus on mTOR pathway inhibition in breast cancer cell lines, they found that both sirolimus and metformin could independently inhibit phospho-mTOR as well as p70S6K; however, the p70S6K suppression was more with sirolimus alone as compared to metformin alone [31]. Adding metformin to sirolimus did not lead to further suppression of p70S6K; however, adding metformin to sirolimus resulted in a decrease of pAKT as opposed to an increase in pAKT with sirolimus alone. Third, the study was designed to compare the changes in pp70S6K between day 8 and day 22; however, these might not be the optimal time points. In fact, the within group analysis of our data showed a significant decrease in pp70S6K from day 1 to day 8 as well as from day 1 to day 22 in both arms, confirming the activity of sirolimus alone as well as sirolimus and metformin. Perhaps a time point later than day 22 would be necessary to see pathway escape causing a rebound in pp706SK with sirolimus alone. Fourth, we evaluated a pharmacodynamic biomarker in circulating CD3+ T cells rather than tumor tissue, and it is possible that these cells do not reflect escape mechanisms occurring in tumors.
Given the low cost and commercial availability of both metformin and sirolimus, this particular combination may be worth further investigation, especially in resource-poor settings and for patients unable to afford more costly cancer therapies. In fact, there are multiple ongoing clinical trials (, , , , , , , , , ) assessing the anti-cancer efficacy of metformin in combination with other drugs[32]. For instance, an ongoing study is evaluating the safety and tolerability of metformin with or without sirolimus as maintenance therapy in patients with pancreatic adenocarcinoma (). Similarly, another study is evaluating the combination of temsirolimus with metformin in advanced solid tumors ().
Conclusion
Despite the potential theoretical benefits of combining sirolimus with metformin, for this trial we conclude that adding metformin to sirolimus did not significantly decrease pp70S6K or favorably alter other PD biomarkers. Based on the interim analysis, we conclude that a larger trial with this design would not likely show benefit.
Acknowledgments
Grant Support: Supported by pilot funding from the University of Chicago Comprehensive Cancer Center and by the Biostatistics and Human Immunologic Monitoring core facilities of the University of Chicago Comprehensive Cancer Center (Support Grant No. P30CA014599).
Abbreviations:
- PBMC
peripheral blood mononuclear cells
- mTOR
mammalian target of rapamycin
- IGF-1
Insulin-like growth factor type 1
- IGF-1R
IGF receptor type 1
- IGF-BP
IGF binding protein
- ORR
objective response rate
- CI
confidence interval
Footnotes
Financial Disclosure/Conflict of interests: Dr. Ratain has provided expert testimony on behalf of generic drug companies regarding the everolimus patents.
References
- 1.Yap TA, et al. , Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol, 2008. 8(4): p. 393–412. [DOI] [PubMed] [Google Scholar]
- 2.Wander SA, Hennessy BT, and Slingerland JM, Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest, 2011. 121(4): p. 1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napoli KL and Taylor PJ, From beach to bedside: history of the development of sirolimus. Ther Drug Monit, 2001. 23(5): p. 559–86. [DOI] [PubMed] [Google Scholar]
- 4.Dittmer DP, Bhatt AP, and Damania B, Rapalogs in viral cancers. Expert Opin Investig Drugs, 2012. 21(2): p. 135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak M, Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila), 2010. 3(9): p. 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin D, et al. , Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov, 2011. 10(11): p. 868–80. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, et al. , Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer, 2011. 11: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby G, et al. , New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care, 2009. 32(9): p. 1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie CJ, Poole CD, and Gale EA, The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia, 2009. 52(9): p. 1766–77. [DOI] [PubMed] [Google Scholar]
- 10.Garrett CR, et al. , Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer, 2012. 106(8): p. 1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiralerspong S, et al. , Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol, 2009. 27(20): p. 3297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehdev A, et al. , Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer, 2015. 121(7): p. 1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehdev A and O’Neil BH, The Role of Aspirin, Vitamin D, Exercise, Diet, Statins, and Metformin in the Prevention and Treatment of Colorectal Cancer. Curr Treat Options Oncol, 2015. 16(9): p. 43. [DOI] [PubMed] [Google Scholar]
- 14.Pollak MN, Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov, 2012. 2(9): p. 778–90. [DOI] [PubMed] [Google Scholar]
- 15.Bailey CJ and Turner RC, Metformin. N Engl J Med, 1996. 334(9): p. 574–9. [DOI] [PubMed] [Google Scholar]
- 16.Jalving M, et al. , Metformin: taking away the candy for cancer? Eur J Cancer, 2010. 46(13): p. 2369–80. [DOI] [PubMed] [Google Scholar]
- 17.Pierotti MA, et al. , Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene, 2012. [DOI] [PubMed]
- 18.Rattan R, Ali Fehmi R, and Munkarah A, Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol, 2012. 2012: p. 928127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korsse SE, Peppelenbosch MP, and van Veelen W, Targeting LKB1 signaling in cancer. Biochim Biophys Acta, 2012. 1835(2): p. 194–210. [DOI] [PubMed] [Google Scholar]
- 20.Nies AT, et al. , Proton pump inhibitors inhibit metformin uptake by organic cation transporters (OCTs). PLoS One, 2011. 6(7): p. e22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen EE, et al. , Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin Cancer Res, 2012. 18(17): p. 4785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 23.Rubinsztein DC, Codogno P, and Levine B, Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov, 2012. 11(9): p. 709–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris TE and Lawrence JC Jr., TOR signaling. Sci STKE, 2003. 2003(212): p. re15. [DOI] [PubMed] [Google Scholar]
- 25.Peralba JM, et al. , Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research, 2003. 9(8): p. 2887–2892. [PubMed] [Google Scholar]
- 26.Tabernero J, et al. , Dose- and Schedule-Dependent Inhibition of the Mammalian Target of Rapamycin Pathway With Everolimus: A Phase I Tumor Pharmacodynamic Study in Patients With Advanced Solid Tumors. Journal of Clinical Oncology, 2008. 26(10): p. 1603–1610. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell A, et al. , Phase I Pharmacokinetic and Pharmacodynamic Study of the Oral Mammalian Target of Rapamycin Inhibitor Everolimus in Patients With Advanced Solid Tumors. Journal of Clinical Oncology, 2008. 26(10): p. 1588–1595. [DOI] [PubMed] [Google Scholar]
- 28.Ekshyyan O, et al. , Pharmacodynamic evaluation of temsirolimus in patients with newly diagnosed advanced-stage head and neck squamous cell carcinoma. Head & Neck, 2010. 32(12): p. 1619–1628. [DOI] [PubMed] [Google Scholar]
- 29.Boulay A, et al. , Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res, 2004. 64(1): p. 252–61. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann B, p70S6 kinase phosphorylation for pharmacodynamic monitoring. Clinica Chimica Acta, 2012. 413(17–18): p. 1387–1390. [DOI] [PubMed] [Google Scholar]
- 31.Zakikhani M, et al. , Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast cancer research and treatment, 2010. 123(1): p. 271–279. [DOI] [PubMed] [Google Scholar]
- 32.Aljada A and Mousa SA, Metformin and neoplasia: implications and indications. Pharmacol Ther, 2012. 133(1): p. 108–15. [DOI] [PubMed] [Google Scholar]


