Abstract
Background:
Perfluorooctanoate (PFOA) has been used extensively in the manufacture of both commercial and household products. PFOA serum concentrations have been associated with adverse health effects, including lower body mass in children and infants.
Objective:
To determine if there is an association between PFOA and body mass, insulin and lipid profile in exposed young girls.
Methods:
We conducted a cross-sectional study of PFAS environmental biomarkers and insulin resistance in 6 to 8 year-old girls from Greater Cincinnati (n=353). In 2004–2006, blood samples were obtained to measure polyfluoroalkyl substances (PFAS), fasting insulin, glucose and lipids. Clinical exams included anthropometric measurements and pubertal maturation staging. Linear regression and mediation analyses, specifically structural equation modeling (SEM), were used to determine the strength and direction of the relationships between PFAS, pubertal maturation status, body mass index (BMI), cholesterol and insulin resistance.
Results:
The median PFOA (7.7ng/ml) was twice the National Health and Nutrition Examination Survey (2005–2006). Only PFOA, a PFAS sub-species, showed statistically significant relationships with the outcomes. In regression models, PFOA was associated with decreased BMI and waist-to-height ratio (p=0.0008; p=0.0343), HDL-cholesterol (p=0.0046) and had a borderline inverse association with the HOMA Index of insulin resistance (p=0.0864). In SEM, PFOA retained an inverse relationship with BMI (p<0.0001) but the relationships with HOMA and HDL-cholesterol were no longer statistically significant. Pubertal initiation (Tanner breast or pubic stage 2 or greater) and BMI were associated with increased HOMA Index (p<0.0001).
Conclusions:
These findings suggest PFOA exposure in young girls affects both BMI and ultimately insulin resistance. In mediation analysis with puberty in the model, the direct effects of PFOA on insulin resistance and were reduced.
1. INTRODUCTION
Recent studies have linked exposures to environmental toxicants with both pre-and post-puberty adiposity. Environmental chemicals can alter gene expression affecting cell signaling pathways that regulate energy homeostasis and possibly perturb insulin resistance and cholesterol fractions.
Environmental chemicals such as per- and polyfluoroalkyl substances (PFAS) are commonly used in manufacturing and are widely detected in humans biospecimens due to the prolonged half-life of 3–4 years (Li et al., 2017). PFAS have entered the environment as a result of the extensive use of fluorochemicals in industrial and consumer products. The Environmental Working Group estimates that 110 million Americans could have PFAS contaminated drinking water (Andrews, 2018). One of the most investigated derivatives of perflouoroalkyl compounds is perfluorooctanoic acid (PFOA). Although concentrations of PFOA and other PFAS in human serum have decreased over the past five to ten years as a result of changes in manufacturing processes, potential health effects of PFAS exposure remain relevant because of persistent past exposure. In the 2005–2006 NHANES cycle, the reported median PFOA serum concentration in children 12–19 years was 3.8 ng/mL (CDC, 2019). Levels of PFOA in young girls in the Cincinnati cohort of the Breast Cancer and the Environment Research Program (n=353) were much higher during that period, with a median value of 7.3 ng/mL and a 99.7% detection level. PFOA values for these girls ranged from less than the limit of detection (LOD) to 55.9 ng/mL (Pinney et al., 2014). Only one participant had a value < LOD. The girls in this study were primarily exposed through the release of PFAS in drinking water from an industrial site in West Virginia (Pinney et al., 2014).
PFOA exposure during critical developmental periods has been reported to result in altered health outcomes, including changes in birthweight and weight during childhood, and lipid profile changes, depending on the timing of the PFOA exposure. In an analysis using NHANES cross-sectional data from children aged 12–19 years, investigators found that only perfluorohexane sulfonic acid (PFHxS), but not any other PFAS, had an inverse association with insulin resistance, assessed by the homeostatic model assessment (HOMA), and was directly related to total cholesterol in both girls and boys, and to low density lipoprotein (LDL) cholesterol in boys. PFOA was noted to have a direct relationship with high density lipoprotein (HDL) cholesterol in girls (Nelson et al., 2010). Multiple other cross-sectional studies have examined the relationship between maternal or childhood exposure to PFAS, weight at birth or body mass index (BMI) during childhood, and glucose and lipid homeostasis, most reporting a decrease in insulin resistance but disparate findings regarding body weight and blood lipids (Domazet et al., 2016; Fleisch et al., 2017; Lin et al., 2009; Liu et al., 2018). However, other studies have reported that PFAS exposure during childhood leads to increased BMI, adiposity, and cholesterol levels in adults, indicating that the effects from PFAS may be dependent on the specific age during the window of exposure and the age at the time the outcome is assessed (Barry et al., 2014; Betts, 2014; Fitz-Simon et al., 2013; Steenland et al., 2009).
Elevated serum insulin concentrations in the setting of normal or elevated glucose levels is reflective of a state of insulin resistance, which clinically is often associated with obesity but can also be diagnosed in a normal weight individual. Pubertal development is itself associated with a state of insulin resistance (Kelsey and Zeitler, 2016). Early pubertal development (Tanner stage 2) is associated with higher serum insulin levels (Biro et al., 2010b) corresponding to increased insulin resistance, perhaps reflecting effects from higher growth hormone levels during the accelerated pubertal growth spurt that occurs with early puberty. Female children with higher BMI enter puberty at younger ages (Biro et al., 2013; Biro, 2003) although the mechanisms by which this occurs are not well understood (Jasik and Lustig, 2008). Given the previously described childhood PFAS exposure studies and mixed findings of health effects, we sought to use structural equation modeling (SEM) to describe the relationship between serum PFAS concentrations in school age children, body weight, serum insulin, glucose and lipids (cholesterol and triglycerides) in the puberty study cohort. SEM allowed us to describe the direction of the relationships, incorporate modifying factors such as pubertal maturation, and to test whether PFOA had a direct or indirect relationship on the outcomes, specifically outcomes of BMI and insulin resistance.
2. MATERIALS AND METHODS
2.1. Study Design and Participants
Over 1,200 girls were recruited for the puberty study of Breast Cancer and Environment Research Program (BCERP). Between 2004 and 2006, young girls ages 6 to 8 were recruited at three sites to evaluate the effect of environmental chemical and metal exposure on the age of pubertal initiation (Biro et al., 2010a).
The current study was limited to the participants of the Cincinnati cohort of the BCERP. Of the 379 girls, approximately 85% were recruited from public and parochial schools and the remaining 15% (with a first or second degree family member with breast cancer) were recruited through the Breast Cancer Registry of Greater Cincinnati. The sampling frame for recruitment at the Cincinnati site consisted of elementary schools whose administration had agreed to participate. Parents of age-eligible girls were given information about that study, and then, if interested, contacted the study coordinator who screened them for their daughter’s eligibility. Most frequently voiced parental reasons for not participating included concerns about the breast palpitation, pubic area observation and phlebotomy included in the protocol. Exclusion criteria included a history of growth disorders or diabetes at the time of presentation.
Clinical study visits were conducted on a semi-annual basis with a study visit window of ± four weeks. The Institutional Review Boards from the University of Cincinnati, Cincinnati Children’s Hospital Medical Center and the Center for Disease Control and Prevention approved the study. Written informed consent was obtained from the participant’s parents or guardians and assent from the girl. This cross-sectional analysis used data from the first clinical visit including anthropometric measurements, clinical pubertal maturation assessments and serum concentrations of PFAS for study participants (N=353). The analysis of insulin resistance was restricted to the 311 girls on whom we also had fasting glucose and fasting insulin measurements.
2.2. Serum PFAS concentrations
Using the CDC’s standardized sample collection and processing protocol, serum from the initial study visit was assayed for PFAS concentrations. All materials used in collecting blood and processing the serum were pre-screened for the presence of PFAS by CDC. Serum was stored in pre-screened polypropylene cryovials and placed in −80°C freezer s until shipment to the CDC for PFAS measurement. Methods for assessing PFAS biomarker concentrations in serum have previously been defined (Kato et al., 2011; Kuklenyik et al., 2005). Further details on assay of this serum have been previously published (Pinney et al., 2014).
2.3. Insulin, Glucose and Insulin Sensitivity
Fasting insulin (I0, measured in μg/mL) and glucose (G0, measured in mg/dL) concentrations were measured in plasma by the Cincinnati Children’s Hospital Medical Center (CCHMC) laboratory. Glucose concentration was measured using the hexokinase technique, on a Hitachi analyzer. Intra-assay variability is 1.0%, inter-assay variability 1.7%. Insulin was measured by chemiluminescence; intra-assay variability is 1.5–2.3%, inter-assay variability 2.6–5.9%.
Insulin Sensitivity (SI) was assessed with the Insulin Sensitivity Index (ISI0) and Quantitative Insulin Sensitivity Index (QUICKI). Insulin Resistance was calculated with Homeostatic Model Assessment (HOMA-IR). The Insulin Sensitivity Index was defined by [104/(I0 *G0)] (Hanson et al., 2000). QUICKI was defined by [1/(log (I0) + log (G0))] (Katz et al., 2017) and HOMA-IR was defined by [(G0*I0)/405] (Matthews et al., 1985). For girls in early stages of puberty, fasting insulin and glucose are thought to be most accurate for calculating IS (Adam et al., 2011). QUICKI, calculated from 1/[log(plasma insulin level) × (plasma glucose level)], has been correlated to euglycemic clamp measurements in children and adolescents (r = .91) (Gungor et al., 2004), and in obese children and adolescents (r = .89) (Conwell et al., 2004).
2.4. Cholesterol and Triglyceride
Fasting triglycerides, total cholesterol and HDL also were measured in plasma at the CCHMC laboratory on a Hitachi analyzer using enzymatic colorimetric procedures for measurement of cholesterol and triglyceride, and the modified Laboratories of Lipid Research Clinics method for measurement of HDL cholesterol (Hainline et al., 1986). The LDL cholesterol concentration was calculated using Friedewald equation, unless the triglycerides were elevated and direct quantification of LDL-C was performed. Intra-assay variability was 1.5% and inter-assay was 0.8% for total cholesterol, and 1.5% and 1.8% respectively for triglycerides.
2.5. Pubertal Maturation
Trained clinicians used both observation and breast palpation to determine pubertal maturation stage at each study visit. Thelarche was defined by the onset of breast development, and pubarche as the first appearance of pubic hair (both sexual maturation rating stage 2, based on Marshall and Tanner criteria) (Marshall and Tanner, 1969). The methods for determining maturation have been defined elsewhere (Biro et al., 2010a). For the structural equation analysis, we considered the ‘puberty’ modifier variable to be present with reaching sexual maturation rating Stage 2 or greater for either thelarche and/or pubarche.
2.6. Anthropometry
Height, weight, hip, and waist measurements were obtained by trained clinicians. Each study participant was wearing only a study-supplied t-shirt and no shoes. Waist and hip circumferences were measured to the nearest 0.1 cm using a fiberglass measuring tape when arms were relaxed at the participant’s side and the feet were together. The waist was measured at its narrowest point and the hip its widest point. Height was measured in cm using a stadiometer and weight was measured in kg by a digital scale. The reported measures are an average of two measurements or three if the two measurements differed by more than 0.5 cm or 0.2 kg. Waist-to-height ratio was calculated as waist circumference divided by height. Waist-to-hip ratio was calculated as waist circumference divided by hip circumference. Body mass index (weight/height2) was reported as a Z score (distance between a participant’s ratio and the mean) and percentile, using these values obtained from the Centers for Disease Control’s 2000 growth curve reference table (CDC, 2010), which is both age and sex specific but not race specific.
A Tanita TBF-300A machine was used to measure total fat mass and fat mass percent. Total lean body mass was calculated from total body mass, based on an assumed hydration fraction of the lean tissue. Fat mass was reported by the Tanita as the difference between lean body mass and total body weight (Prentice, 2007). Fat mass percentage was defined as the proportion of fat mass to the total body weight. The RJL systems bioelectrical impedance analyzer was used to measure bioelectrical resistance.
2.7. Covariates
Additional covariates selected a priori include race/ethnicity and age in months. The parent or guardian identified the race/ethnicity of their daughter as Black, non-Hispanic white, Asian, or Hispanic. For the purposes of this report, race/ethnicity was classified as black (including black Hispanic), nonblack Hispanic, non-Hispanic white, or non-Hispanic Asian. Participants could belong to only one race/ethnicity category. Race/ethnicity for this study was categorized as Black versus all other since the numbers in other categories (Asian, nonblack Hispanic) were very small. Participant age in months was modeled as a continuous variable.
2.8. Statistical Analyses
Following the usual practice, PFAS values below the limit of detection (LOD) were converted to the value of LOD/√2 (Wolff et al., 2005). All data preparation and regression analyses were conducted using SAS (version 9.2, SAS Institute, Cary, NC). All structural equation analyses were conducted using Mplus (version 5.2, Muthѐn & Muthѐn, Los Angeles, CA). The model assumptions for regression and SEM were first verified. An alpha level of less than or equal to 0.05 was used for determining statistical significance.
We performed regression modeling on the health outcomes for each of these six serum PFAS concentrations: 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH), perfluorodecanoate (PFDeA), perfluoronanoate (PFNA), PFOA, perfluorooctane sulfonate (PFOS), and perfluorohexane sulfonate (PFHxS). The health outcomes included I0, BMIz, ISI0, QUICKI, HOMA-IR, waist-to-height. The models incorporated not only serum PFAS concentration levels as the independent variable but also information on race and age in months. Race and age were forced to remain in the regression models regardless of their statistical significance.
In order to investigate the correct direction of the relationship between PFOA and the outcomes, SEM were constructed. SEM is a statistical method utilizing the maximum likelihood estimator in which multiple related regression relationships may be modeled. Variables in SEM may be both independent and dependent depending on their relationship with one another. Mediators, variables which are both independent and dependent, are able to “mediate” the effects of the covariates on the dependent variables (Succop et al., 1998). In this multilevel modeling, the assumption of independence is assumed at the lowest level of the hierarchy (Muthén and Muthén, 2010). Structural equation model building began initially by investigating the direction of relationship between PFOA and the health outcomes as well as other univariate models to determine the causal pathways. A strategy of backward elimination was then employed using all possible endogenous pathways and covariates that might affect the casual chain. Insignificant effects were removed. Next, single forward model building was performed to allow insignificant variables the ability to reenter the model. Over fifty models were run to determine the direction of the relationships and determine the best fit of the models. For models without Monte Carlo simulation, models with insignificant Chi Square values, Root Mean Square (RSMEA) values less than 0.06 and Comparative Fit Indexes (CFI) greater than 0.9 were considered. Ultimately Monte Carlo integration was used to stabilize the models. Only the Akaike (AIC) was used to measure model fit in SEM with a lower AIC equating to a better fit.
3. RESULTS
3.1. Demographic Characteristics
The mean age of the girls included in this study was 7.08 years with a range of 6.00 to 8.83 years. A majority of the girls were non-Hispanic White, with small proportions of Hispanic (<4%) and Asian (<1%), all combined in one category for analyses (66.3%). About one-third of the girls were Black (33.7%). Tanner breast staging was obtained on all 353 of the girls at entry into the study and 15.01% had reached thelarche at the time of the first study visit. Pubic hair staging was obtained on 351 of the girls with 8.78% having reached pubarche. Only eight girls had achieved both thelarche and pubarche. Additional descriptive statistics are shown in Table 1.
Table 1-.
Study Population Demograhic Characteristics (n=353)
| Standard Deviation |
||||
|---|---|---|---|---|
| N | (%) | Mean | ||
| Age (years) | 7.08 | 0.62 | ||
| Race/Ethnicity | ||||
| Black | 119 | (33.7%) | ||
| All Other | 234 | (66.3%) | ||
| Tanner Breast Stage (Thelarche) | ||||
| 0 or 1 | 300 | (84.99%) | ||
| ≥2 | 53 | (15.01%) | ||
| Tanner Pubic Stage (Pubarche) | ||||
| 0 or 1 | 320 | (90.65%) | ||
| ≥2 | 31 | (8.78%) | ||
| missing | 2 | (<1%) | ||
| Thelarche and/or Pubarche | ||||
| Neither | 277 | (78.47%) | ||
| Either or both | 76 | (21.53%) | ||
| Weight (kg) | 25.95 | 6.07 | ||
| Height (cm) | 123.50 | 7.08 | ||
| Waist Circumfrence (cm) | 55.57 | 5.92 | ||
| BMI% | 59.68 | 30.38 | ||
| BMIz score | 0.38 | 1.07 | ||
| Waist-to-height ratio | 0.44 | 0.04 |
Note: BMI=body mass index (kg/meters2)
3.2. PFAS Concentrations, Insulin and Glucose
Of eight PFASs measured, six had detectable serum concentrations in over 70% of the girls and are included in these analyses (Table S-1). Three PFASs (Me-PFOSA-AcOH, PFHxS, and PFOA) had a study median serum concentration that was about twice the NHANES 2005–2006 median (Table S-1). Table S-2 reports the mean values for fasting glucose (mean=84.83 mg/dL) and insulin (mean=12.44 μg/mL). Seventeen percent (n=53) of the girls had fasting insulin concentrations that were above the upper bound of the clinical reference interval of 15.5 μg/mL(LabCorp, 2001). The median BMI percentile of these girls was 88.65% and of these girls, 21 had BMIs less than the 85th percentile (not in Table 2). Of those 21 girls, the median BMI percentile was 50.08% and the average BMIz was 0.11.
Table 2-.
Linear Regression of the Effects of PFOA on health outcomes
| BMIz | Waist to Height | Waist to Hip Ratio | Resistance | Log (Tanita Fat | Tanita Fat Free | Tanita Fat Mass % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | β | p value | β | p value | β | p value | β | p value | |
| n= | 352 | 353 | 353 | 351 | 329 | 329 | 344 | |||||||
age in months and race (African American vs. other) forced to be included in the model
3.3. Regression modeling examining the association between PFASs and body composition, insulin sensitivity and resistance, and triglyceride and cholesterol.
PFAS concentration measurements did not follow a normal distribution and therefore underwent natural log-transformations. Log-transformed values of I0, ISI0, Fat Mass and HOMA were also used in the analyses to normalize the distributions. All other measurements displayed normal distributions.
Regression analyses were conducted to estimate the effect of the PFAS on the outcomes of BMIz, waist-to-height ratio, insulin, glucose and IS and insulin resistance variables. Covariates forced to be included in all models included race and age in months. Serum concentration levels of PFOA, Me-PFOSA-AcOH, PFDeA, and PFOS were found to have statistically significant inverse effects on BMIz and waist-to-height ratio (Table 2 and S-3). As the serum concentration increased, BMIz of the girls decreased and waist-to-height ratio decreased. In addition, race was directly associated with BMIz for all of the PFAS except PFOA.
Only PFOA had a borderline statistical association with the girls’ fasting insulin levels (Table 3 and S-3). Both Me-PFOSA-AcOH and PFOA had borderline direct effect on insulin sensitivity (both ISI0 and QUICKI) (p=0.10) and borderline inverse effect on insulin resistance (HOMA-IR) (p=0.09) (Tables 3 and S-3).
Table 3-.
Linear Regression of the Effects of PFOA (ng/mL) on insulin sensitivity
| Glucose mg/dl | Log (Insulin) | 10^4 / (Insulin | 1/(log (Glucose) + | (Glucose*lnsulin)/ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | β | p value | β | p value | |
| log (PFOA) | −1.1125 | 0.2074 | −0.0895 | 0.0935 | 0.1025 | 0.0864 | 0.0021 | 0.1052 | −0.1025 | 0.0864 |
| Age* | 0.0806 | 0.2039 | 0.0159 | <.0001 | −0.0167 | 0.0001 | −0.0004 | 0.0001 | 0.0167 | 0.0001 |
| Race (AA)* | −0.7829 | 0.4636 | 0.1351 | 0.0368 | −0.1230 | 0.0894 | −0.0024 | 0.1161 | 0.1230 | 0.0894 |
| n= | 311 | 309 | 309 | 309 | 309 | |||||
age in months and race (African American vs. other) forced to be included in the model
Because no significant associations between the other PFAS species and metabolic biomarkers of the outcomes were detected, only the relationship between PFOA serum concentration and insulin resistance was examined in additional linear regression analyses. BMIz was added to the linear regression model as an independent variable (Table S-4). When BMIz was included in the model along with the interaction of PFOA and BMIz, the effects of PFOA on insulin, ISI, QUICKI and HOMAIR dissipated (not included in table S4). This is most likely due to the collinearity between BMI and insulin concentrations.
To further understand the relationship between PFOA and BMI, we conducted regression analyses to investigate the relationship between PFOA and fat mass, fat mass percent (proportion of fat to total body weight) and body impedance. The results using this model showed a statistically significant inverse relationship between the PFOA and fat mass and fat mass percent indicating that as the serum PFOA concentration increased, the fat mass percent decreased (p=0.0009) (Table 2). There was no association of PFOA with body impedance.
PFOA serum concentration was directly associated with serum HDL concentration (p=0.0046), but no association was seen with total serum cholesterol, triglyceride concentration, nor the calculated value of non-HDL cholesterol. There was a direct relationship between African American race and HDL, but an inverse relationship with triglyceride concentration (Table S-5).
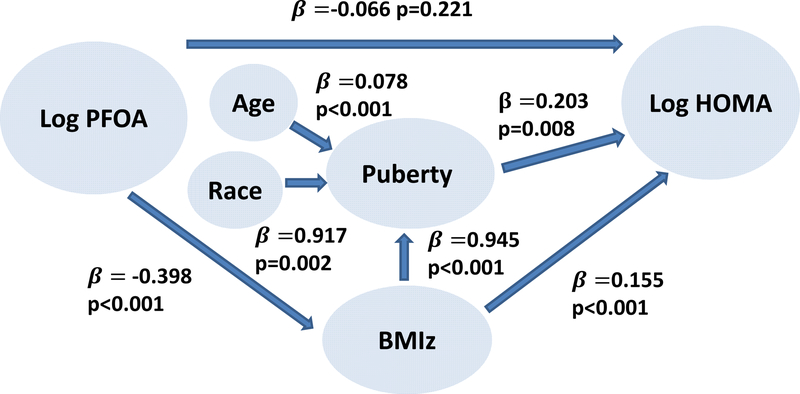
3.4. Structural Equation models (SEM) examining PFOA as a predictor of adiposity and insulin resistance
The final SEM indicated that serum PFOA concentration in girls aged 6–8 years was inversely related to their BMIz, which then had a direct effect on HOMA (Figure 1). Any borderline effect of PFOA on HOMA dissipated when pubertal maturation and BMIz were added to the model. Furthermore, pubertal maturation acted as an effect modifier of the relationship between BMIz and HOMA in that BMIz was a significant determinant of early pubertal maturation, and pubertal maturation was a significant determinant of increased HOMA. As previously described, race (African American) and increased BMI are determinants of earlier pubertal maturation (Biro et al., 2013). Although there was a significant effect of PFOA on HDL in the regression models, when HDL was added to the final SEM, HDL had no significant effect on HOMA and was ultimately not included.
Figure 1.
Structural equation model of the complex relationships between PFOA, BMI and insulin resistance.
4. DISCUSSION
To our knowledge, this is the one of the first studies to investigate the combined relationships between PFAS, serum insulin and glucose, serum lipids, anthropometric measures and early stage pubertal maturation in girls from 6 to 8 years, using structural equations to demonstrate the direction of the associations and allow for mediators, both of which lend strength to this study. Linear regression is not able to show the direction of the relationship between various measures, but can only be used to determine if a relationship exists in a pre-determined direction. SEM also allowed us to postulate richer relationships between the variables as not all variables in SEM need to have direct relationships on the ultimate outcome.
An earlier publication (Hillman et al., 2013) using much of the same cohort but without any inclusion of environmental exposure, reported similar results regarding the relationships among BMIz, waist-to-height ratio, breast maturation and insulin sensitivity. However, they used a statistical approach (SOBEL’s test for significance) which limited testing for the presence of indirect effects to using regression models and presumes an a priori distinction between the dependent variable and the independent variable (Sobel, 2013). The authors assumed that the effect on insulin sensitivity by pubertal maturation was mediated via BMIz. SEM used in the present analysis did not predetermine the delineation between the dependent and independent variables since the variable could be independent in one model and dependent in another model. The current approach with SEM provided evidence that the predominant effect of PFOA was on BMIz, and BMIz’s effect on insulin resistance was mediated by pubertal maturation.
Previous studies of this cohort (Herrick et al., 2017; Pinney et al., 2014) suggest that the primary exposure route for greater Cincinnati girls was through drinking water and that the source was an industrial facility upriver from Cincinnati, in West Virginia. Since production at this facility has been curtailed since 2002 and ceased in 2011, it is likely that the serum concentrations of these greater Cincinnati girls were higher around the time of their birth and infancy (most between 1998 and 2000) than they were at the time of this study. Serum concentrations measured in a Cincinnati area adult cohort in the 1990s were high (adjusted geometric mean 16.4 ng/mL) and our PBPK modeling suggested a 3.8 year half-life (Herrick et al., 2017).
Studies in humans have shown that low-dose intrauterine exposure to PFOA disrupts normal fetal growth and development. Most studies have reported an inverse association between maternal serum PFOA concentrations and birth outcomes such as birth weight, head circumference, gestational age and length and ponderal index (Apelberg et al., 2007; Fei et al., 2007; Lee et al., 2013; Starling et al., 2017). Additionally, prenatal concentrations of PFOA in mothers in Project Viva (median 5.6 ng/mL) were associated with an increase in BMI and fat mass in mid-childhood (mean 7.7 years) in their daughters, but not with HOMA-IR (Fleisch et al., 2017; Mora et al., 2017), reflecting a well-described developmental in utero programming pattern in which an environmental exposure results in decreased birthweight followed by increased weight and adiposity later in life (Simmons, 2005; Symonds, 2007; Hoffman et al., 2017). However, other studies of PFAS exposure in childhood and adolescence reported an inverse association with weight and insulin resistance in childhood. Among a sub-cohort of children in Project Viva, with serum PFOA concentrations (geometric mean 4.2 ng/mL) somewhat lower than that of girls in our study, there was an inverse relationship between serum PFOA and HOMA-IR which was more pronounced in prepubertal female children, median age 7.6 years. (Fleisch et al., 2017). In a study of young children in the New York City area, PFHxS was associated with decreased insulin resistance (Koshy et al., 2017). We report similar relationships with linear regression analyses in our cohort, and our findings from the SEM analyses add information about the direction of the relationship
Findings in studies of PFAS exposure and effects in adolescent health have had mixed results. In the European Youth Heart Study cohort, childhood PFOA exposure (median 9.0 ng/mL) was associated with elevated BMI at age 15 years, and also increased skinfold thickness and waist circumference at age 21 years. In multi-variable models, childhood PFOA exposure (at 9 years) was inversely related to HOMA-IR at age 15 and 21 years (Domazet et al., 2016). In NHANES 1999–2000 and 2003–2004, adolescents (mean age 15.5 years), PFNA serum concentration was associated with hyperglycemia but also lower HDL cholesterol (Lin et al., 2009).
In a more recent study of NHANES 2013–2014 data, in adults, PFOA was not associated with HOMAIR, but the toxicokinetics of isomers differed. While increased linear PFOA was associated with increased total cholesterol and serum albumin, increased branched PFOA was significantly associated with HDL-C and negatively associated with glycohemoglobin (HbA1C), an indicator of elevated blood glucose) (Liu et al., 2018). In a study of greater Cincinnati adults, the PFOA found in serum was primarily linear (97%) (Herrick et al., 2017), but we have not yet examined the relationship with serum glucose and insulin in this cohort.
In most prior studies examining the relationship between PFOA exposure and the lipid profile in children and adolescents, cholesterol levels increased with PFOA exposure (Khalil et al., 2018; Koshy et al., 2017; Maisonet et al., 2015; Mora et al., 2017; Zeng et al., 2015). Geiger (Geiger et al., 2014) found total and LDL cholesterol were significantly associated with PFOA (mean 4.2 ng/mL) and PFOS exposure (mean 17.7 ng/mL) in a nationally representative sample of adolescents, but not HDL-C and triglycerides. In Taiwanese children, age 12–15 years, PFOS, PFOA and PFNA were positively associated with total, LDL cholesterol and triglycerides, and HDL cholesterol decreased linearly across quantiles of PFOS and PFOA exposure (Zeng et al., 2015). In a study of prenatal PFOA exposure in the Avon longitudinal cohort, lower tertile maternal serum PFOA, but not middle or upper, was directly associated with total cholesterol at age 7, and with LDL-C at age 15 in a non-monotonic pattern of exposure effect (Maisonet et al., 2015). In the Project Viva cohort, higher PFOA (median 4.3 ng/mL) was associated with higher total cholesterol in girls, and higher HDL cholesterol in all children. Higher PFDeA was associated with a higher total/HDL-C ratio (Mora et al., 2018). Investigators of a cross-sectional study of children of the World Trade Center Health Registry and an age matched non-WTC comparison group, ages 0–8 years, with low PFAS exposure levels (1.81 ng/mL and 1.39 ng/mL median serum concentrations of PFOA respectively), reported positive associations of PFOA with triglycerides, total and LDL cholesterol (Koshy et al., 2017). We tested this hypothesis in a cohort of young girls, and did find evidence of significantly increased HDL-C and decreased insulin resistance both directly and indirectly through BMI in SEM models.
Metabolic homeostasis with PFOA exposure has been demonstrated in animal models, with some evidence that this effect is related to the life cycle age at exposure. Alterations in hepatic sterol metabolism and increases in circulating cholesterol levels in obese rats can lead to a range of effects on glucose and insulin levels depending on the age of assessment (Zoltowska et al., 2001). Hines et al., 2009 showed that low-dose in utero exposure to PFOA in mice resulted in increased total body weight in mid-life, elevated organ weight gain and increased rate of weight gain, and increased serum insulin and leptin (Hines et al., 2009). However, this study also demonstrated a ‘critical window’ effect of PFOA exposure, exemplified by high dose PFOA exposure during the post-weaning period resulting in lower body weight and PFOA exposure during adulthood having no effect on body weight gain. Likewise, rodent model studies have provided evidence of alterations in cholesterol with PFOA exposure. When fed diets containing both cholesterol and fat, both male Balb/c mice and especially male and female C57BL/6 mice developed hypercholesterolemia with altered expression of genes associated with hepatic sterol output (bile acid production) but expression of genes associated with sterol input was reduced only in C57BL/6 mice (Rebholz et al., 2016). Spady et al. (1993) suggested that sterol imbalance also may occur in humans.
Although our findings provide some insight into the mechanism of PFOA exposure on body composition in female children, many questions remain. Waist-to-height ratio is an accurate assessment of central adiposity (Biro et al., 2010b), and in our study, waist-to-height ratio was inversely related to PFOA concentration, suggesting less central adiposity in the girls with increased PFOA exposure. Since the girls with higher PFOA levels had both lower BMIs and lower % fat mass, PFOA may be associated with increased mobilization of stored energy in the form of adipose tissue through lipolysis. Free fatty acids and glycerol are hydrolyzed or released from adipose tissue during lipolysis for the mobilization of stored energy during fasting or exercise. We did not measure free fatty acids, but future studies assaying lipodomics would provide key mechanistic data by ascertaining which lipid species are altered after PFOA exposure during pubertal development.
We conducted a cross-sectional analysis within a longitudinal cohort, due to the fact that we had measurements of glucose and insulin only at the time of the baseline examination. Ideally we would have been able to examine these factors in the cohort longitudinally, with repeat measurements of PFOA and the outcome variables, and would have shown whether these relationships changed as the girls matured. We are in the process of conducting longitudinal analyses examining BMI, waist:height and waist:hip ratios. The present study is limited by examining a single population, thus limiting generalizability. Also, humans are exposed to an array of environmental exposures, and we considered only one class of chemicals in this analysis, implicitly assuming that the levels of all others were randomly across the outcomes. However, the PFOA in this population were twice the median NHANES average providing a unique opportunity to examine a higher exposed population. A additional potential limitation are false positive findings created by multiple testing. We examined twelve outcomes in this set of girls. A Bonferoni adjustment would result in a p-value=0.0041. Of the PFOA linear regression models, this would only change the significance of its relationship with waist to height (p=0.0342) and LDL (p=0.0046)
5. CONCLUSIONS
In this cohort of girls age 6 to 8 years, increasing serum PFOA concentrations were associated with decreased BMIz and fat mass percent. Structural equation modeling showed causal direct effects of the young girls’ PFOA serum concentrations on BMIz, and an indirect effect on pubertal maturation through BMI. Structural equations further showed PFOA levels had both a direct effect on HOMA and an indirecteffect through BMI. In these models, pubertal maturation acts as an effect modifier of the relationship between BMIz and HOMA. A further weight reduction in those children who are underweight because of chronic conditions is an unfavorable outcome. From a population perspective, environmental exposures are most likely to have a deleterious effect on those in the tails of the distribution of the outcome. For BMI, it would be those who are either underweight (if the exposure effect is to decrease weight) or obese (if the exposure effect is to increase weight). The long-term implications of the reduction in childhood BMI with childhood PFOA exposure remain to be seen as others have reported that childhood PFOA exposure is associated with increased BMI in adulthood. Our observed exposure-related decrease in BMI may reflect an effect during only one developmental stage in obesity and possibly adipogenesis. Additional longitudinal studies are needed to further probe biological mechanisms and to determine the potential longer-term effects of prepubertal exposure to PFOA.
Supplementary Material
Highlights.
Per- and polyfluoroalkyl substances (PFAS) have been commonly used in manufacturing and are widely detected in humans due to a half-life of 3–4 years
Previous studies have shown PFAS exposure during some developmental windows resulted in decreased weight and changes in the lipid profile
Median levels of PFOA in this study population of girls, primarily exposed through drinking water, were almost twice the NHANES level.
A strong linear relationship was found between PFOA and BMI, PFOA and HDL cholesterol.
Structural equation modeling revealed that the PFOA exposure effect of decreasing BMI consequently decreased insulin resistance.
ACKNOWLEDGMENTS
The late Dr. Paul Succop assisted with the structural equation statistical analyses.
FUNDING SOURCES
Funding acknowledgments: U01ES012770, U01ES019453, U01ES019457, U01ES026119, U0ES029133, P30ES006096, CSTA-UL1RR026314, T32GM063483,. The funding sources had no input into the study design, the collection, analysis and interpretation of the data, the writing of the report and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
Authors have no conflicts of interest to report.
REFERENCES AND RECOMMENDED READINGS
Papers of particular interest, published within the period of review have been highlighted as:
* Of special interest
** Of outstanding interest
- Adam TC, Hasson RE, Lane CJ, Davis JN, Weigensberg MJ, Spruijt-Metz D, Goran MI, 2011. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 34, 994–9. 10.2337/dc10-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D, 2018. Report: Up to 110 million million Americans could have PFAS-contaminated drinking water [WWW Document]. https://www.ewg.org. URL https://www.ewg.org/research/report-110-million-americans-could-have-pfas-contaminated-drinking-water
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR, 2007. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 115, 1670–6. 10.1289/ehp.10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Darrow LA, Klein M, Winquist A, Steenland K, 2014. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ. Res 132, 62–69. 10.1016/j.envres.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Betts KS, 2014. PFOA and high cholesterol: basis for the finding of a probable link. Environ. Health Perspect. 122, A338 10.1289/ehp.122-A338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM et al. , 2003. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J. Pediatr 643–647. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS, 2010a. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126, e583–90. 10.1542/peds.2009-3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, Deardorff J, Herrick RL, Succop PA, Hiatt RA, Kushi LH, Wolff MS, 2013. Onset of breast development in a longitudinal cohort. Pediatrics 132, 1019–1027. 10.1542/peds.2012-3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Huang B, Morrison JA, Horn PS, Daniels SR, 2010b. Body mass index and waist-to-height changes during teen years in girls are influenced by childhood body mass index. J. Adolesc. Health 46, 245–50. 10.1016/j.jadohealth.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2019. Fourth national report on human exposure to environmental chemicals -updated tables, January 2019 1–520.
- CDC, 2010. CDC Growth Charts. 10.1590/S1516-35982002000600018 [DOI]
- Conwell LS, Trost SG, Brown WJ, Batch JA, 2004. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27, 314–9. 10.2337/DIACARE.27.2.314 [DOI] [PubMed] [Google Scholar]
- *.Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK, 2016Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: The European Youth Heart Study. Diabetes Care 39, 1745–51. 10.2337/dc16-0269 [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin J, Tarone R, 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 115, 1677–1682. 10.1289/ehp.10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, Armstrong B, 2013. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology 24, 569–576. 10.1097/EDE.0b013e31829443ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, Gillman MW, Oken E, Sagiv SK, 2017. Early-Life exposure to perfluoroalkyl substances and childhood metabolic function. Environ. Health Perspect. 125, 481–487. 10.1289/EHP303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A, 2014. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 98, 78–83. 10.1016/j.chemosphere.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Gungor N, Saad R, Janosky J, Arslanian S, 2004. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J. Pediatr 144, 47–55. 10.1016/j.jpeds.2003.09.045 [DOI] [PubMed] [Google Scholar]
- Hainline A, Karon JM, Winn CL, Gill JB, 1986. Accuracy and comparability of long-term measurements of cholesterol. Clin. Chem 32, 611–615. [PubMed] [Google Scholar]
- Hanson RL, Pratley RE, Bogardus C, Narayan KMV, Roumain JML, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC, 2000. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am. J. Epidemiol 151, 190–198. 10.1093/oxfordjournals.aje.a010187 [DOI] [PubMed] [Google Scholar]
- He X, Liu Y, Xu B, Gu L, Tang W, 2018. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci. Total Environ. 625, 566–574. 10.1016/j.scitotenv.2017.12.186 [DOI] [PubMed] [Google Scholar]
- **.Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C, Pinney SM, 2017Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environ. Pollut 228, 50–60. 10.1016/j.envpol.2017.04.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hillman JB, Huang B, Pinney SM, Biro FM, 2013. Early pubertal development and insulin sensitivity among school-aged girls: mediation via adiposity. J. Pediatr. Adolesc. Gynecol 26, 47–50. 10.1016/j.jpag.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE, 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell. Endocrinol 304, 97–105. 10.1016/j.mce.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Reynolds RM, Hardy DB 2017. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr. Rev 75(12):951–970. 10.1093/nutrit/nux053 [DOI] [PubMed] [Google Scholar]
- Jasik CB, Lustig RH, 2008. Adolescent obesity and puberty: the “perfect storm.” Ann. N. Y. Acad. Sci 1135, 265–279. 10.1196/annals.1429.009 [DOI] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 1218, 2133–2137. 10.1016/j.chroma.2010.10.051 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ, 2017. Quantitative insulin sensitivity check index : a simple , in humans accurate method for assessing insulin sensitivity in humans 85, 2402–2410. [DOI] [PubMed] [Google Scholar]
- Kelsey MM, Zeitler PS, 2016. Insulin resistance of puberty. Curr. Diab. Rep 16, 64 10.1007/s11892-016-0751-5 [DOI] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, Hangartner T, Kannan K, 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environ. Res 160, 314–321. 10.1016/j.envres.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M, Honda M, Chu DB, Han X, Shao Y, Kannan K, Urbina EM, Trasande L, 2017. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int 109, 128–135. 10.1016/j.envint.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM, 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal. Chem 77, 6085–6091. 10.1021/ac050671l [DOI] [PubMed] [Google Scholar]
- LabCorp, 2001. Pediatric Reference Ranges [WWW Document]. URLPediatric_Reference_Ranges_End6ocrinology_0981.pdf
- Lee YJ, Kim MK, Bae J, Yang JH, 2013. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 90, 1603–1609. 10.1016/j.chemosphere.2012.08.035 [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Talving P, Jakobsson K 2017. Half-lives of PFOS, PFHxS and PFOS after end exposure to contaminated drinking water. Occupational and Environmental Medicine, 75(1), 46–51. doi: 10.1136/oemed-2017-104651. Epub 2017 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Chen P-C, Lin Y-C, Lin L-Y, 2009. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 32, 702–7. 10.2337/dc08-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-S, Wen L-L, Chu P-L, Lin C-Y, 2018. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ. Pollut 232, 73–79. 10.1016/j.envpol.2017.09.019 [DOI] [PubMed] [Google Scholar]
- Maisonet M, Näyhä S, Lawlor DA, Marcus M, 2015. Prenatal exposures to perfluoroalkyl acids and serum lipids at ages 7 and 15 in females. Environ. Int 82, 49–60. 10.1016/j.envint.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Arch. Dis. Child 44, 291–303. 10.1136/ADC.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, Calafat AM, Ye X, Okene E, Sagiv SK, 2018. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ. Int 111, 1–13. 10.1016/j.envint.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, Ye X, Sagiv SK, 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ. Health Perspect 125, 467–473. 10.1289/EHP246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 2010. MPlus user’s guide: the comprehensive modeling program for applied researchers. Muthén & Muthén. 1–758. [Google Scholar]
- Nelson JW, Hatch EE, Webster TF, 2010* Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect 118, 197–202. 10.1289/ehp.0901165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, Succop P, Sucharew H, Ball KM, Kato K, Kushi LH, Bornschein R, 2014Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ. Pollut 184, 327–334. 10.1016/j.envpol.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, 2007. BIA Technology for assessing body fat an introductory guide for clinical and research applications. Tanita. [Google Scholar]
- Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, Woollett LA, 2016. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol. Reports 3, 46–54. 10.1016/j.toxrep.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R Developmental origins of adult metabolic disease: concepts and controversies. 2005. Trends Endocrinol. Metab 6(8):390–4. 10.1016/j.tem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sobel E, 2013. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Methodol 13, 290–312. 10.2307/270723 [DOI] [Google Scholar]
- Spady D, Woollett L, Dietschy J, 1993. Regulation of plasma ldl-cholesterol levels by dietary cholesterol and fatty acids. Annu. Rev. Nutr 13, 355–81. [DOI] [PubMed] [Google Scholar]
- Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Ye X, Dabelea D, 2017. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the Healthy Start Study. Environ. Health Perspect 125, 067016 10.1289/EHP641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V, 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol 170, 1268–1278. 10.1093/aje/kwp279 [DOI] [PubMed] [Google Scholar]
- Succop P, Bornschein R, Brown K, Tseng CY, 1998. An empirical comparison of lead exposure pathway models. Environ. Health Perspect 106 Suppl 6, 1577–83. 10.1289/ehp.98106s61577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME 2007. Integration of physiological and molecular mechanisms of the developmental origins of adult disease: new concepts and insights. Proc. Nutr. Soc 66(3):422–50. DOI: 10.1017/S002966510700571X [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Lioy PJ, Santella RM, Wang RY, Jones RL, Caldwell KL, Sjödin A, Turner WE, Li W, Georgopoulos P, Berkowitz GS, 2005. Exposures among pregnant women near the World Trade Center site on 11 September 2001. Environ. Health Perspect 113, 739 10.1289/EHP.7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin X.Di, Zhu Y, Li J, Lee YL, Dong GH, 2015. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci. Total Environ 512–513, 364–370. 10.1016/j.scitotenv.2015.01.042 [DOI] [PubMed] [Google Scholar]
- Zoltowska M, Ziv E, Delvin E, Stan S, Bar-On H, Kalman R, Levy E, 2001. Circulating lipoproteins and hepatic sterol metabolism in Psammomys obesus prone to obesity, hyperglycemia and hyperinsulinemia. Atherosclerosis 157, 85–96. 10.1016/S0021-9150(00)00711-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.