Abstract
Recent advances in BOLD fMRI scan techniques have substantially improved spatial and temporal resolution, currently reaching to sub-millimeter and sub-second levels respectively. Unfortunately, there remain physiological barriers that prevent achieving this resolution in practice. BOLD contrast relies on the hemodynamic response to neuronal activity, whose associated cerebral blood oxygenation (CBO) changes may spread over several millimeters and last several seconds. Recent reports have suggested that significant improvements may be possible with cerebral blood volume (CBV)-weighted fMRI, which highlights the CBV changes rather than the BOLD changes associated with the hemodynamic response. Nevertheless, quantitative comparisons between CBV and BOLD are sparse, in particular regarding their temporal characteristics in human brain.
To address this, we studied a cohort of subjects that received injection of ferumoxytol, an intravascular iron-oxide based contrast agent that introduces strong CBV contrast. An event-related visual stimulus paradigm was used to compare the impulse response (IR) for CBV and BOLD contrast, obtained with and without ferumoxytol, respectively. Experiments performed at 7 T (n=5) at 1.2–1.5 mm spatial and 1 s temporal resolution showed that the onset time and time-to-peak of the CBV IR averaged 0.8 and 3.5 s respectively, both 0.6 s shorter than the BOLD IR. While significant, these improvements are relatively small and not expected to lead to practical advantages for the extraction of temporal information about neural activity.
Nonlinearities in the observed IR were also compared and found to be similar between the CBV and BOLD, indicating that these are likely not caused by a “ceiling” effect in the CBO response, but rather support a previously proposed model of vascular compliance, in which changes in vascular tone elicited by a preceding stimulus affect the IR.
Introduction
Continued technological advancements have greatly increased the temporal and spatial resolution of acquisition methods for functional magnetic resonance imaging (fMRI). Modern techniques that combine high magnetic field strength (Ugurbil 2012), array detectors (Keil 2013), and parallel imaging (Larkman 2007), allow data to be acquired at sub-millimeter spatial and sub-second temporal resolutions, several-fold better than what was possible in the original Blood Oxygen Level Dependent (BOLD) fMRI studies from the early 1990s (Bandettini 1992, Kwong 1992, Ogawa 1992). These improved resolutions potentially allow fMRI experiments to capture a much larger portion of the rich spatiotemporal structure of neural activity, including functional differences between cortical columns and layers and processes at the sub-second scale of human cognition.
Despite these technological advances, the physiological processes underlying BOLD contrast ultimately limit the fMRI resolution in practice. In fact, the BOLD contrast that most fMRI studies depend on generally blurs the neuronal activity over several millimeters and several seconds (Shmuel 2007). This blurring is a result of the cascade of cerebral blood volume (CBV), cerebral blood flow (CBF) and cerebral blood oxygenation (CBO) changes that follow neuronal activity, which jointly contribute to BOLD contrast (Buxton 1998). Impulse response (IR) measurements suggest that the BOLD temporal resolution achievable in practice may be limited to about 4 s (de Zwart 2005), while the spatial resolution may be well above 1 mm, depending on the contribution of venous vessels downstream from the site of activity (Shmuel 2007, Turner 2002).
Several studies have suggested that some of the hemodynamic blurring in fMRI can be avoided by sensitizing the experiment to CBF or CBV contrast. CBV-weighting (CBVw) may be achieved with intravascular contrast agents that alter the T1 or T2* MRI relaxation time (Belliveau 1991, Mandeville 2004), or by introducing T1 weighting with spin inversion pulses as is done in the Vascular Space Occupancy (VASO) technique (Lu 2003). Tissue or blood-selective inversion pulses can also be used to generate CBF-weighting (CBFw) (Williams 1992). Both CBVw and CBFw fMRI would reduce the contribution of the downstream venous vasculature compared to BOLD fMRI, resulting in reduced spatial and temporal blurring. Indeed, CBVw and CBFw fMRI studies on cats have confirmed this notion and demonstrated improved spatial discrimination between cortical columns (Kim 2002). Recent work has extended this finding to humans, showing improved detection of activity differences between cortical laminae with VASO-based CBV contrast (Huber 2017).
Despite these advantages in spatial resolution of CBVw and CBFw fMRI, less clear are the potential advantages in temporal resolution. CBFw and VASO-based CBVw MRI generally have coarse temporal sampling inherent to the spin inversion preparation and are therefore not conducive to high temporal resolution fMRI. CBVw fMRI based on intravenous injection of iron oxide, on the other hand, is (minimally) invasive and has found only very limited application in humans. Previously reported fMRI IR measurements on animals are somewhat ambivalent, as they have indicated both increases and decreases in temporal resolution (Leite 2002, Silva 2007). These ambiguities may relate to methodological differences between the IR measurement approaches, as well as species-related differences in physiology. Nevertheless, there are indications that the width of the CBV IR in rat may be as short as 1–1.4 s (Hirano 2011, Silva 2007), well below the ~4 s BOLD fMRI resolution in humans, and distinctly shorter than the approximately 2 s BOLD IR width found in equivalent BOLD experiments in rat. Such a narrow IR would substantially improve fMRI temporal resolution and motivate the development of noninvasive CBVw or CBFw methods with high temporal sampling rate. Such methods may help narrow the gap between temporal resolution of fMRI and that of the underlying neuronal signaling, and in some cases improve studying the temporal order in which brain regions engage in support of behavioral tasks (Yu 2014).
To further investigate these potential increases in fMRI temporal resolution, our study set out to measure the CBV IR in humans injected with iron oxide for a clinical research protocol. Using a dedicated event-related visual paradigm at 7 T, we determined IR timing and compared it to the BOLD IR timing measured on the same subjects in absence of the contrast agent. These experiments furthermore yielded information about temporal nonlinearities of the CBV and BOLD IR, which we investigated to clarify the mechanism of previously observed nonlinearities in comparable BOLD experiments.
Materials and Methods
Volunteers and Ferumoxytol Dosage
BOLD and CBVw fMRI experiments were performed with T2*-weighted MRI in the presence and absence of ferumoxytol. Ferumoxytol is an ultra-small super-paramagnetic iron oxide (USPIO) consisting of particles ranging 17 – 31 nm in diameter and a blood half-life in excess of 10 hours (10–14 h (Li 2005)). Intravenous injection was performed with a typical adult clinical dose of 510 mg (6.8 ± 1.6 mg/kg), two of which in a one-week period are prescribed for treatment of iron-deficiency anemia, in compliance with a protocol approved by the institutional review board and following informed consent. Both BOLD and CBVw experiments were performed on the same healthy volunteers (n=5, age 37.7 ± 9.5 y). BOLD fMRI was performed prior to ferumoxytol injection, or at least 3 weeks after injection, when the ferumoxytol had cleared the blood.
Stimulus Paradigm
Both BOLD and CBVw IR were measured with the same event-related paradigm, based on a pseudo-random (m-sequence) distribution of 800 ms long visual stimuli (Kellman 2003). A binary m-sequence, otherwise known as a maximum length sequence (MLS), has a flat frequency response, and is an efficient means to estimate impulse responses, which can simply be derived via circular cross-correlation when the m-sequence is used as the stimulus paradigm and signal steady state is assured. It is not only used in neuroscience (Reid 1995), but also in various other fields, such as acoustics (Schroeder 1979) and telecommunication (Zaccarin 1993). This approach allowed the estimation of first-order (linear) IR kernels to isolated stimuli, as well as interaction terms representing nonlinear aspects of the response to closely spaced stimuli. The latter phenomenon was analyzed to investigate the source of previously observed nonlinear effects in BOLD contrast (de Zwart 2009).
A minimum gap of 200 ms was maintained between stimuli to avoid neuronal nonlinearities (de Zwart 2009). Paradigm duration for both the BOLD and CBVw measurements was 600 s. Block-design paradigms using the same visual stimuli were based on alternating 30 s duration stimulation and rest periods. These scans with a total duration of 300 s were employed for the selection of a region-of-interest (ROI).
Visual stimuli were presented using an in-house designed paradigm in Presentation software (Neuro Behavioral Systems, Inc., Berkeley, CA, USA). The stimulus consisted of a radial checkerboard that contrast-reversed every 66.7 ms (4 frames @ 60 Hz display refresh rate), or at 7.5 Hz, projected on a screen in the MRI scanner bore with an approximate visual angle of 19.2º × 14.5º (horizontal × vertical angle for the 4:3 ratio images). During stimulus ‘off’ periods, a uniform gray field was displayed with the same mean luminance level as the checkerboard. To minimize saccades, the subjects were instructed to fixate on a dot in the center of the display that alternated between red and pink at random intervals ranging from 4 to 16 s. To ensure and evaluate compliance, volunteers were instructed to press a button (fORP 932, Current Designs Inc, Philadelphia, PA, USA) in response to color changes. Button presses were recorded by the Presentation software together with timing information about the presented image frames and a scanner volume trigger at the beginning of each fMRI volume, which was used to synchronize stimulus presentation to the MR scanner clock.
Magnetic Resonance Imaging
Experiments were performed at 7 T (Magnetom 7T, Siemens, Erlangen, Germany). Gradient-echo echo-planar imaging (EPI) images were acquired with three-fold in-plane accelerated SENSE (Pruessmann 1999) parallel imaging using the following parameters for all but one volunteer: 1.5 × 1.5 × 1.2 mm3 nominal resolution (144 × 108 matrix size at 216 × 162 mm2 field-of-view); 28 slices with 0.3 mm inter-slice gap; 16.2 ms echo time (TE); 1 s repetition time (TR); 60° nominal flip angle. One (the first) volunteer was scanned at a higher resolution, 1.2 × 1.2 × 1.2 mm3 with 18 slices (180 × 132 matrix size at 216 × 158 mm2 field-of-view), which required a longer TE of 24.5 ms. All other scan parameters were identical. Both BOLD and CBVw fMRI scans were performed at the same TE, which was a compromise between the TEs optimal for each individual contrast but simplified correction of CBVw data for residual BOLD effects (see Appendix A), and for this reason shorter than the 30–50 ms range typically used for BOLD fMRI at 7 T.
The scan plane was placed parallel to the calcarine fissure, using a sagittal MP-RAGE anatomical scan for reference. Screen shots of the slice prescription were saved to aid in reproducing this prescription in the second scan session. To quantify the effect of ferumoxytol on T2* relaxation, multi-gradient-echo (mGRE) anatomical scans were also performed at the same resolution, and in the same slices, as the fMRI scans. Other scan parameters were: 11 echoes; 3.0 – 10.7 ms TE (0.77 ms echo spacing); 25° flip angle; 500 ms TR (54 s acquisition time). For the volunteer with the higher-resolution fMRI, the mGRE scan was acquired with: 6 echoes; 5.0 – 10.0 ms TE (1.0 ms echo spacing); 35° flip angle; 500 ms TR (66 s total acquisition time). In one subject, the mGRE anatomical scan for the scan session without contrast agent was omitted.
Data Analysis
Following image reconstruction, the volumes of each fMRI time-series were aligned to the 10th volume in the BOLD-weighted block paradigm. The block paradigm data for both BOLD and CBVw experiments were subsequently analyzed using a general linear model that accounted for a hemodynamic response function (HRF) convolved paradigm and 2 polynomial trends, as described previously (de Zwart 2009). As in that previous publication, a truncated Gaussian function with a standard deviation (σ) of 3.5 s and a delay from stimulus to the top of the response of 3.5 s was used. A threshold for exceeding voxel-wise t-score was used to select voxels that were significantly activated in both the BOLD and CBVw fMRI scans. This threshold, dependent on the number of voxels in the individual brain masks, was a t-score of 5.22 ± 0.05 (mean ± standard deviation over volunteers), accounting for multiple comparisons.
The event-related fMRI data was analyzed by lag-dependent correlation as described earlier (Kellman 2003). This allowed extraction of both linear and nonlinear kernels of the IR (Kellman 2003). Prior to this analysis, the 600 s run was split in two 300 s segments, from which linear trends (e.g. for drift correction) were removed by comparing the mean signal in 11 bins before and after each analyzed 255-bin segment. The first 40 bins of the 300 s segment, during which stimuli were presented based on the tail-end of the paradigm to assure steady state of the signal, were discarded (see (de Zwart 2009) for more details). During correlation analysis of each 255-bin segment, 10-fold temporal sinc-interpolation was performed yielding a nominal 0.1 s resolution. Correction for slice-timing was subsequently performed, again using sinc-interpolation.
A potential confound in the CBVw scan is the presence of BOLD effects (Mandeville 2004). To account for these, their magnitude was first estimated on a voxel-by-voxel basis from the BOLD-weighted scans, after which they were removed as detailed in Appendix A. Removal was based on the assumption that the blood deoxyhemoglobin and ferumoxytol concentration induced changes have additive effects on tissue R2* (=1/T2*) relaxivity. The resulting contrast was then referred to as CBV contrast (as opposed to the “CBVw” contrast prior to removal). The assumption of independent R2* contributions for deoxyhemoglobin and ferumoxytol is likely not quite accurate. Any intravenous contribution to the BOLD signal will become invisible in the presence of a paramagnetic contrast agent. Also, since CBV in turn is also a contributor to the BOLD signal, minor residual BOLD effects may remain after correction. Therefore, for completeness we present results for both CBV and CBVw data below.
To illustrate the effect of the contrast agent on the fMRI signal, R2* maps were calculated from the mGRE data by performing least-squares fitting of a mono-exponential decay curve to each voxel time course as a function of echo time. This yielded a decay rate (R2*) and amplitude for each voxel.
Impulse Response Fitting
From the IR data, characteristic timing parameters were calculated using a fitting procedure based on the following gamma-variate function:
| (1) |
Here, a1 is the onset time (OT), and a2 determines the full-width at half-maximum (FWHM). The time to peak (TTP) then follows from the OT and FWHM. The FWHM and TTP were numerically computed from the fitted response curve using a 1 ms temporal resolution. Since earlier work (Silva 2007) has shown that particularly the CBV response displays a return-to-baseline that does not correspond to the model described by Eq. 1, only the initial part of the measured IR for each voxel was fitted, namely the IR from the moment of stimulus onset to 1 s beyond an estimate of TTP. This estimate of TTP was first determined for each voxel by taking the time at which IR response amplitude was maximum (minimum for CBV data) during the first 20 s post stimulus onset. Results from a fit of Eq. 1 to the mean response over all voxels for each data type (BOLD, CBVw, or CBV) were used as an initial guess for voxel-wise fitting. Voxels for which the fit failed to converge, or where the fit’s R2 value was below 0.5 for one or more data types, were ignored. Histograms for OT, TTP, and FWHM were computed for the remaining voxels using 0.1 s bin size.
Effect of Gaussian Blurring
Due to potential spatial heterogeneity in IR timing associated with the dependence of the hemodynamic response on local vascular geometry, fMRI spatial and temporal resolutions may to some extent be interdependent. For example, one would expect that in such heterogeneous regions, a larger voxel size would blur the IR and lead to larger FWHM. To investigate this, together with possible effect on SNR, m-sequence data analysis and subsequent voxel-wise IR fitting were repeated on copies of the time series images that were subjected to in-plane Gaussian blurring with 2, 3, 4 and 5 mm kernels. Voxels within the same, block-paradigm derived functional ROI were analyzed.
Results
R2* Changes Following Ferumoxytol Administration
Analysis of the anatomical mGRE data showed that the presence of paramagnetic ferumoxytol leads to notable shortening of the T2* relaxation time. Figure 1 compares the calculated R2* map for an example slice for the baseline mGRE scan (left) to the same slice in the corresponding mGRE scan from the scan session after ferumoxytol injection. For the 8594 voxels in the functional ROIs (see Block Paradigm Results, below), analyzed for 3 out of the 5 volunteers, R2* was found to be 39.7 ± 24.6 s−1 (mean ± standard deviation) at baseline, versus 78.3 ± 47.7 s−1 in the presence of ferumoxytol. For the remaining 2 volunteers, one was found not task-compliant and was excluded (see fMRI Task Compliance, below), for the other the mGRE scan prior to ferumoxytol administration was mistakenly not acquired (R2* in the 3121 functional-ROI voxels for that volunteer averaged 110.1 s−1 in the presence of ferumoxytol). Summarized, averaged over 3 volunteers, ferumoxytol roughly doubled the R2* relaxation rate in the voxels of interest.
Figure 1:

R2* relaxation rate maps for one slice for one volunteer for both the baseline-BOLD scan and the CBVw scan in the presence of ferumoxytol. Data show the approximately two-fold increase in R2*, or twofold reduction in T2*, that the presence of ferumoxytol engenders.
fMRI Task Compliance
Inspection of the button-box response data for the various fMRI runs indicated that four out of five volunteers performed both event-related and block-design tasks with high accuracy, resulting in a button press within 1 s following a dot color change 92.0 % of the time (± 1.4 % standard error over volunteers). The remaining volunteer, however, only showed 28.8 % response accuracy, as well as barely any visual activation in the block paradigm task at baseline, both suggestive of low task compliance, presumably due to drowsiness. This volunteer was excluded from further analysis.
Block Paradigm Results
All four task-compliant volunteers showed robust activation in both BOLD and CBVw scans with grossly similar spatial distribution. Figure 2 shows an example of t-score maps for one volunteer as well as subject-averaged signal BOLD and CBVw signal time-courses for all 11715 voxels (2929 ± 730 per volunteer) active in both scans. As expected, the CBVw signal decreased with stimulation, consistent with increased T2* relaxation with activation-induced blood volume increases. Average activation induced signal changes were 4.1 ± 0.2 % and −5.6 ± 1.1 % (mean ± standard error over volunteers) for BOLD and CBVw data respectively.
Figure 2:

(a) Example block paradigm activation t-score maps for 4 slices from the volunteer for which higher-resolution data were acquired (1.2 × 1.2 × 1.2 mm3 resolution, 24.5 ms TE), superimposed on the first volume of the EPI time-series data. The top row shows BOLD fMRI response, the bottom row CBVw fMRI response. (b) Average response time-courses for BOLD and CBVw block paradigm experiments in all 11715 voxels from 4 volunteers that were significantly activated in both BOLD and CBVw block paradigm scans. The negative contrast in CBVw fMRI, a signal decrease when CBV increases in the presence of paramagnetic ferumoxytol contrast agent, is clearly evident. Conventional BOLD shows positive contrast, a signal increase with activation, during the same task. Stimulus-on periods are identified using yellow boxes on the time axis.
Impulse Response Estimate for an Isolated 800-ms Duration Stimulus
Calculated IRs for BOLD, CBVw, and CBV scans are summarized in Figure 3. Shown are estimated responses to an isolated (800 ms duration) stimulus. It is seen that on average, the peak (negative) CBVw IR amplitude is approximately 1.5 times the (positive) BOLD peak IR amplitude when expressed as a percentage of the baseline signal level. The integral of the response over the first 15 seconds after stimulus onset for the (BOLD-corrected) CBV response is 2.71 ± 0.62 (mean ± SE over volunteers) times larger than for BOLD. For the (uncorrected) CBVw response this ratio is 1.78 ± 0.64, substantially smaller. At the same time, it should be realized that these larger CBV(w) responses do not directly translate into improved contrast-to-noise ratio (CNR): due to the reduced baseline T2* (and thus signal level) in the presence of ferumoxytol, the functional ROI averaged CNRs are 0.80 ± 0.24 and 1.26 ± 0.21 for CBVw and CBV respectively, relative to that for BOLD. The latter value is in reasonable agreement with a CBV/BOLD CNR ratio estimate of 1.41 for 7 T at TE = 16.2 ms based on a previously reported model (Mandeville 2004). Note that this model assumes an optimal dose of USPIO, a condition possibly not met with the 510-mg dose used here.
Figure 3:
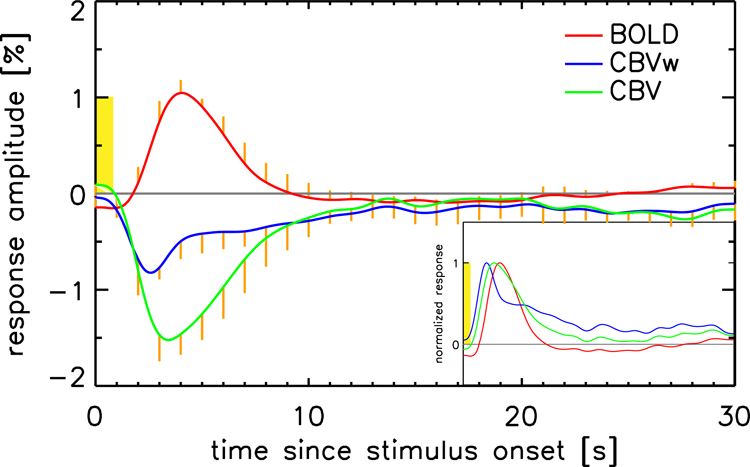
The measured impulse response functions to an isolated, 800 ms duration visual stimulus (7.5 Hz contrast-inverting checkerboard, stimulus timing shown in yellow) are shown for both the BOLD (red) and CBVw (blue) fMRI experiments. The mean response over voxels per volunteer was computed, these mean responses were then averaged over the 4 volunteers. The response in the presence of ferumoxytol can be corrected for the BOLD response that still contributes to the activation-induced signal change in those data by subtracting a scaled version of the corresponding BOLD response for each voxel (see text and Appendix A for details). This BOLD-corrected CBV curve is shown in green. The insert shows normalized and rectified versions of these curves to illustrate the earlier onset and slower return to baseline of the CBVw and CBV impulse responses. Standard error over volunteers is shown in orange either above or below each trace (‘one-sided’) to minimize clutter.
Other than polarity and amplitude differences, the BOLD, CBVw, and CBV IRs also show substantial shape differences. To illustrate this further, we inverted both CBV-related IRs and normalized all IRs to their maximum amplitudes; see insert in Figure 3. Particularly strong differences are seen between BOLD and CBVw responses with the latter showing a biphasic character similar to earlier observations (Silva 2007): a fast initial response followed by a long tail. These differences are reduced in the BOLD-corrected CBV data, which also no longer show a prominent biphasic response. CBV data continues to show an earlier OT and TTP than the BOLD IR however.
Figure 4 shows the result of convoluting the mean BOLD and CBVw IR functions measured in the m-sequence experiments with a 30-second duration box car (broken lines). Data show a good correspondence with the average responses observed in the block-paradigm BOLD and CBVw experiments (solid lines).
Figure 4:
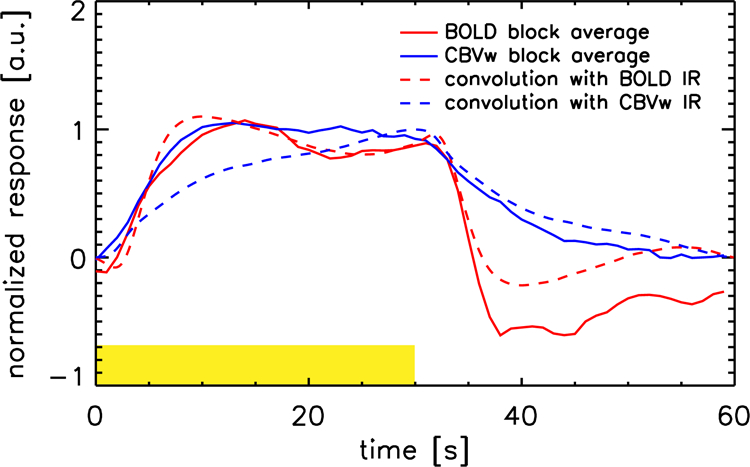
Plot of the mean responses to the block-paradigm visual stimuli, averaged over volunteers and stimulus blocks 1–4 (solid lines). The 5th block was not included since the experiment ended before signal returned to baseline. The CBVw response was inverted. Broken lines show the result of convoluting a 30-second duration box car with the first 30 seconds post stimulus onset of BOLD and CBVw IR (see Figure 3). The yellow box indicates the time that stimuli were presented.
Impulse Response Timing
For the average IRs over volunteers, the fitted OTs were 1.2 s for BOLD, 0.6 s for CBV and 0.7 s for CBVw. The TTP was 4.1 s for BOLD, 3.5 s for CBV, and 2.6 s for CBVw. Fit results for FWHM were 4.0 s for BOLD, 4.0 s for CBV and 2.6 s for CBVw, but these values are less informative since most of the downslope was not taken into account when fitting the gamma-variate model. For CBV(w), that model does not properly describe IR return to baseline, as can be seen in Figure S3 in Supplementary Material. This would require a dual gamma variate fit, see Figure S4. FWHM determined directly from the measured IR per volunteer was respectively 3.9 ± 0.6 s or BOLD, 4.7 ± 0.8 for CBV and 4.4 ± 1.3 s for CBVw (mean ± standard error over volunteers).
It should be realized that the IR width calculated from ROI- or volunteer-averaged signal might in part represent heterogeneity in response timing across vessels, possibly grossly over-estimating the average of voxel-specific IR widths. This heterogeneity may differ between CBV and BOLD data, affecting interpretation of IR differences.
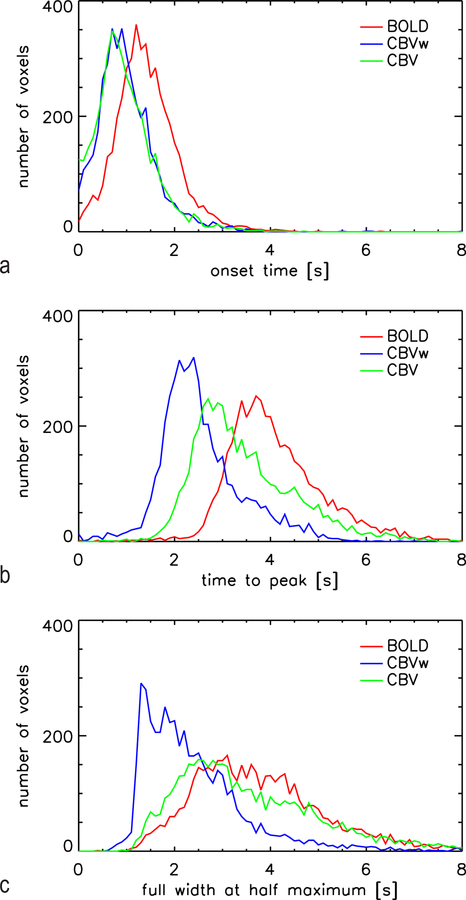
To address the issue of response heterogeneity, we also performed voxel-by-voxel fitting with the gamma-variate function (Equation 1). Results are summarized in OT, TTP, and FWHM histograms (Figure 5), as well as in Table 1. These histograms extend further to the left (shorter/faster) for CBV data, indicating that in a substantial fraction of the voxels, the CBV IRs have earlier OT and TTP, and smaller FWHM. For CBVw, 70.5% of the voxels had both earlier OT and TTP than for BOLD, as did 64.8% of the CBV voxels. Results are only shown for the 4691 voxels in which fits for all three IR converged. The CBVw fit was most likely to fail, yielding a good fit in only 5833 of the 11715 functional ROI voxels, compared to a successful fit for 10013 voxels for BOLD and 9146 for CBV. The low CNR of CBVw IR appeared to be the main culprit, judged from the fact that fit convergence markedly improved for Gaussian-blurred data, e.g. the CBVw fit was successful for 8833 out of 11715 voxels for the 5 mm blurred data. Improved fitting reliability was the most notable effect of spatially blurring the data, as there was no notable shift in any of the histogram distributions (results not shown). The latter indicates that already at a 1.2–1.5 mm nominal spatial resolution, there are no discernable differences in intra-voxel vascular composition (e.g. arteriolar-dominated versus vein-dominated voxels). This is further evidenced by data shown in Figure S1 in the supplementary material, which shows voxel-wise scatter plots of various parameter pairs. These plots show no distinct pools of voxels, as would be expected for notable variations in intra-voxel vascular composition. Mean values (± standard error over 4691 voxels) for the fitted OT, TTP and FWHM on a voxel-by-voxel basis are shown in Table 1.
Figure 5:
Histograms for voxel-wise fitted onset time, time-to-peak and full-width at half-maximum for the 4783 voxels in which the fit for all three IR types converged. These data indicate that a pool of voxels shows clearly faster response onset in CBV(w) fMRI than BOLD fMRI. Time to peak is also faster for CBV fMRI albeit correcting for residual BOLD diminished this difference. Uncorrected CBVw data show smaller response width, however correction for residual BOLD largely increases response width to the level of BOLD data.
Table 1:
Results of voxel-wise fitting of the measured impulse response function using a gamma-variate function. Results shown are mean and standard error for the 4691 functional ROI voxels in which fits for all 3 data types were successful.
| OT (± SE) [s] | TTP (± SE) [s] | FWHM (± SE) [s] | |
|---|---|---|---|
| BOLD | 1.35 ± 0.01 | 4.14 ± 0.01 | 3.84 ± 0.02 |
| CBVw | 0.72 ± 0.02 | 2.58 ± 0.01 | 2.46 ± 0.02 |
| CBV | 0.78 ± 0.01 | 3.48 ± 0.02 | 3.71 ± 0.03 |
It is well established that the onset of the BOLD response is delayed relative to stimulus onset, and there is evidence to suggest that the CBV response onset precedes that of BOLD (Buxton 1998, Silva 2007). This is attributed to the notion that blood volume and flow changes are the initial hemodynamic changes that lead to, and are required for, the blood oxygenation changes that dominate during much of the BOLD response. We therefore hypothesized that onset time would be less sensitive to residual BOLD contribution in the CBVw fMRI experiment, or possible over-compensation for residual BOLD by the proposed correction. This is confirmed by the data presented in Figure 5, which shows only minimal effect of BOLD correction on OT distribution. In contrast, IR TTP and FWHM distributions more closely resemble those of BOLD for the uncorrected (CBVw) data than for the corrected CBV data.
Nonlinearities in the Presence of Closely Spaced Stimuli
The response to an 800 ms stimulus in isolation, as shown in Figure 3, is not the direct result of correlation analysis of the m-sequence data, which yields the average response to all events in the paradigm (half of which are preceded by another, identical stimulus the previous second). These measured responses are affected by any interaction between consecutive closely-spaced stimuli in the paradigm. These interaction terms are separated during m-sequence analysis, see (de Zwart 2009), allowing their effect to be quantified and compensated for (Figure 6). Analysis of these nonlinear interaction in both BOLD and CBV data shows similar effects in both cases (Figure 6). For both contrasts, interactions were dispersive in character, meaning they led to lower and broader responses. Peak amplitudes of the interactions were 35.8% ± 2.3% and 36.7% ± 5.8% of the maximum IR for BOLD and CBV over the first 10 seconds after stimulus onset, respectively. These results suggest that a dispersive hemodynamic nonlinearity, rather than a blood oxygenation ceiling effect, is the most likely explanation for the observed interaction between closely spaced stimuli.
Figure 6:

Solid lines show the 10-fold sinc-interpolated measured average impulse response to stimuli in the m-sequence experiment for both conventional BOLD (red) and CBVw signal (blue). These measured responses are affected by any interaction between consecutive closely-spaced stimuli in the paradigm. These interaction terms are separated during m-sequence analysis, see (de Zwart 2009). Time-courses for the interaction terms between two directly adjacent ‘on’ stimuli (200 ms inter stimulus interval) are shown using long strokes. These interaction terms can be used to correct the measured IR, yielding the IR to an isolated 800 ms visual stimulus (short strokes). The later correspond to the curves shown in Figure 3. The timing of the 800 ms stimulus is shown in yellow. Standard error over volunteers is shown in green (one-sided) and is omitted for the average response traces since those data are shown in Figure 3.
Discussion
General Remarks
Availability of human subjects receiving injections with a strong intravascular T2* contrast agent allowed us to study temporal aspects of the CBV response to brief visual stimulation and compare this with the BOLD response in the same subjects with and without USPIO contrast agent. After correction for BOLD contamination in the CBVw data, we found that the CBV IR occurred earlier than the BOLD response, consistent with previous work on animals (Hirano 2011, Kennerley 2010, Silva 2007). Nevertheless, CBV IR width varied across the visual cortex but generally was similar to that of the BOLD IR. This is possibly the result of an inadequate correction for residual BOLD, as uncorrected CBVw data showed a distinctly narrower response. In addition, similar nonlinearities due to interactions between closely spaced stimuli were observed between the two contrasts, consistent with the notion that with BOLD, dispersive nonlinearities originate from delayed vascular compliance (Behzadi 2005, de Zwart 2009), as opposed to a blood oxygenation ceiling effect.
Impulse Response Timing Differences Between BOLD and CBV Contrast
In the human visual experiments described here, the onset-time difference between CBV and BOLD response averaged about half a second. This difference is substantial and consistent with the notion that the two contrasts could emphasize different parts of the vasculature, with CBV providing a weighting towards the arterial side of the vascular bed, as was suggested in previous work, which showed that CBV changes to brief stimuli are concentrated to the arterial side of the vasculature (Hillman 2007). Another explanation for this observation is that capillary and venous transit time delays the blood oxygenation increase signal in BOLD, but that this transit time plays a reduced role in the onset of the venous CBV increase. The slow component in the observed CBVw response could also reflect a venous contribution, albeit the spatial resolution of our data does not allow us to verify that hypothesis. This finding of earlier onset suggests CBV contrast may have advantages in discerning the sequence of neural events in fMRI experiments. One potential application is the distinction between laminar activity, for which onset-time information may be beneficial (Yu 2014). However, practical application of this phenomenon and use of onset-time information without contrast-agent injection may require development of new, rapid CBV- and CBF-weighted techniques, as current technology generally has poor (multiple second) temporal resolution.
Whereas the CBV IR started earlier than BOLD, differences in FWHM of BOLD and CBV IR were less striking. In some portion of pixels, CBV FWHM was smaller than that of BOLD, but the overall histogram distributions looked rather similar. One possibility is that the venous side of the vasculature, with possibly a more sluggish hemodynamic response, may also contribute to CBV contrast as it is susceptible to passive dilation in response to upstream pressure changes. To test this hypothesis, fit results for CBV were plotted on a voxel basis as a function of BOLD results for that parameter (supplementary material, Figure S1). This did not reveal distinct classes of voxels with different CBV IR characteristics. Depending on whether a voxel’s signal was dominated by either venous or arterial effects, one would expect the latter to have an earlier (and possibly narrower) IR, particularly in CBV IR. As the FWHM of the IR is indicative of the ability to distinguish neural events that are closely spaced in time, CBV techniques may be of limited advantage in this regard.
It should be noted that the 3 T monkey experiments by Leite at al. (Leite 2002) did not report a distinct OT difference between BOLD and CBVw fMRI, however notably longer stimuli (minimum duration 4 s) were used in those experiments. For proper comparison, one would need to convolve our IR kernels with the full (4 s) stimulus length. The slow return to baseline of our CBVw IR, and lack of a post-stimulus undershoot, both evident in Figure 3, then leads to a delay in both TTP and return to baseline of the response to longer stimuli. This is illustrated by Figure 4, in which the mean response in the block paradigm data (30 s on/off) for BOLD and CBVw is compared to the result of convolving a 30 s duration stimulus with the mean BOLD and CBVw IR derived from the m-sequence experiments. These data demonstrate the slower rise and return to baseline of CBVw when compared to BOLD, responsible for the measured longer time constants for CBV versus BOLD in the presence of long stimuli (Leite 2002, Qiu 2012).
Work in rodents by Hirano and coworkers (Hirano 2011), on the other hand, using sub-second stimuli, did find a significantly shorter IR OT for CBV compared to BOLD, albeit only on the order of 100 ms. The authors attribute the small size of this difference (much smaller than their estimate for the arteriole-venule transit time of about 0.5 s), to an arterial contribution to the BOLD signal. It is also possible that the larger difference in onset time in human is due to differences in vasculature, especially the increased prevalence of large veins. Because the capillary-to-venous transit time is longer for large diameter veins (because of their increased down-stream distance from the site of arteriolar and capillary dilation with activation), they tend to delay and widen the BOLD response. Blood volume effects, on the other hand, may be more immediate, as they are not limited by transit of a blood oxygenation change through the vasculature.
It should be noted that the biphasic behavior of CBVw IR, with slow return to baseline, is less apparent in CBV IR, following the above described removal of residual BOLD (e.g. see normalized curves in Figure 3). This slow component in CBVw could therefore in large part reflect the effect of uncorrected residual BOLD in the CBVw experiment, instead of a true characteristic of the CBV response. The observation that a similar effect is observed in block paradigm data from USPIO-based CBVw-fMRI (Qiu 2012), but generally not found in VASO data (Huber 2015, Huber 2014), would be supportive of that hypothesis. An impromptu reanalysis of the rat data from the Silva paper (Silva 2007), including a correction for residual BOLD, did however show some residual slow component signal (see Supplementary Figure S5). The relatively small number of voxels in our analysis of those data makes this finding somewhat inconclusive however, requiring this issue to be further investigated.
Work by Huber et al. has demonstrated that the averaging of CBV data in units of % signal change, as was done in this work, could lead to a reduced relative contribution of larger pial arteries (Huber 2015). Conversion to units of ml would amplify this large-vessel signal, which seems undesirable for accurate spatial localization of CBVw-fMRI.
Finally, it should be pointed out that the HRF chosen for block paradigm analysis to some extent affects which voxels are included in the functional ROI, a source of potential bias. However, very similar IR functions were found using an HRF with twice increased TTP and width, showing that this effect was minimal (data not shown).
Nonlinearities in the Presence of Closely Spaced Stimuli
In this work, IR functions were measured using an efficient pseudo-random event-related fMRI stimulus paradigm based on the m-sequence, which was set up to minimize neuronal inter-stimulus interactions by assuring a minimal stimulus separation of 200 ms. This allowed measurement of the mean IR function to the brief (800 ms duration) stimuli used, as well as assessment of residual inter-stimulus interactions, comparison of which can be informative of their origin. The interaction-effects in BOLD IR found here are in line with what was found in earlier work investigating vascular nonlinearities using the same stimulus paradigm in visual experiments in humans at 3 T (de Zwart 2009). The effect is a broadening of IR when a stimulus is directly preceded by another. For example, the BOLD data shown in Figures 3 and 6 in this work can be compared to data from Figures 4 and 3 in (de Zwart 2009), respectively.
The temporal signature of the interaction effect of closely spaced stimuli found in CBVw fMRI is on the same time scale as found in BOLD fMRI, and both interaction effects are similar in magnitude (~36% of the main response when comparing the peak amplitude). The presence of nonlinearities in CBV fMRI was previously described by Lu et al. (Lu 2005), where nonlinear effects as a function of stimulus duration were described. Lu et al. were not clear about the source of the nonlinear effects in their work, but suggested they result from the coupling between the fMRI signal and underlying neuronal activity. Another contemporaneous study (Behzadi 2005) focused on nonlinearities in the CBF response to neuronal stimuli. They proposed an arteriolar compliance model that describes the evoked CBF response using a second-order nonlinear feedback model, showing that the baseline vascular state, which can be altered by a preceding neuronal event, affects the CBF IR (and as a result, the CBV response, since CBF is the input that drives changes in CBV in this model). Importantly, the fact that similar interaction effects are found in CBVw fMRI, which predominantly reports the CBV response to activation, confirms the notion put forward by Behzadi et al. (Behzadi 2005) that these observations are predominantly hemodynamic effects. This as opposed to, for example, ceiling effects in the level of blood oxygenation.
Sensitivity of CBVw fMRI
Although the percentage signal change was larger in CBVw fMRI than in BOLD fMRI, the sensitivity was found to be lower (mean t-score for BOLD in the functional ROI was 10.1, versus 8.9 for CBVw), a result of the lower CNR when compared to BOLD fMRI (0.80 ± 0.24 CBVw/BOLD CNR ratio), possibly exacerbated by increased motion sensitivity due to increased spatial contrast in the images acquired in the presence of ferumoxytol. It is well established that the potential CNR-benefit of CBVw-based fMRI wanes with increasing field strength, since the BOLD contrast increases with field strength, whereas USPIO-based CBVw fMRI contrast is proportional to the percentage change in CBV, which is independent of field strength (Mandeville 2012, Mandeville 2004). At the clinical dose used here, possibly exacerbated by wash-out during the 1–3-hour delay post injection before CBVw fMRI was performed, there appears to be no tangible sensitivity gain for CBVw fMRI over BOLD fMRI at 7 T. However, optimizing the USPIO dose and scan parameters independently for BOLD- and CBVw-fMRI will affect the CNR advantage of USPIO-based CBVw fMRI (Zhao 2006).
Comparison with Non-Invasive CBV-based fMRI
MRI techniques that do not require the injection of a contrast agent but are predominantly reflective of CBV changes, allowing for CBV-sensitive fMRI experiments, have also been proposed (Lu 2003, Stefanovic 2005). Of these, VASO (Lu 2003), in which blood signal is selectively suppressed using an inversion pulse, is the most widely used. It has been shown that temporal response onset of VASO is earlier than for BOLD (Wu 2008), consistent with the data shown here. In that work, performed at 1.5 T, for commonly activated pixels, onset time was 7.2 ± 2.4 s for BOLD, 6.2 ± 2.1 s for VASO with tissue suppression and 4.6 ± 2.7 for conventional VASO, where onset time in these block paradigm experiments was defined as the time-to-half-maximum in these block-paradigm experiments. Data shown by Huber (Figure 4 in (Huber 2014)) are suggestive of an earlier onset for VASO than BOLD, but a value for onset time was not reported, though it was estimated to be around 250 ms (private communication with author). The longer contrast-preparation and image-repetition times required in VASO experiments make this value more difficult to measure accurately. Note that CBV changes may in principle occur at both venous and arterial sides of the vasculature, and that their relative contribution in VASO- and USPIO-based fMRI may differ. Furthermore, an in-depth investigation into the origins of VASO signal has shown that it is difficult to make it exclusively depend on CBV effects, but that CBF and extravascular BOLD contrast generally contribute as well (Donahue 2006).
Conclusion
In human brain, the IR of CBV-weighted fMRI has an earlier onset and later offset than the BOLD IR, consistent with findings in rodents. The approximately half a second later BOLD IR onset is attributed to the stronger dependence of the BOLD contrast mechanism on the capillary transit time. The earlier onset of CBV IR may help distinguish between timing differences of neuronal processes that are spatially distinct if short stimuli are used. However, the similar IR widths in CBV and BOLD IRs suggest that these contrasts may have similar discriminative ability for temporally resolving spatially overlapping neuronal processes that are closely spaced in time. Interestingly, temporal nonlinearities observed in the CBV IR showed amplitude and temporal characteristics similar to those observed for BOLD. The latter finding is in agreement with an earlier proposed model of delayed vascular compliance to explain dispersive nonlinearities of temporal interaction.
Supplementary Material
Acknowledgments
The authors thank Drs. Afonso Silva and Alan Koretsky (Laboratory of Functional and Molecular Imaging, NINDS, National Institutes of Health, Bethesda, MD, USA) for discussions. This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Appendix A –. Correcting CBVw fMRI for BOLD contribution
In a gradient echo experiment, the MR signal S decays as a function of echo time t with relaxation constant R2*:
| (A1) |
In a conventional BOLD fMRI experiment, the decreasing concentration of paramagnetic deoxygenated blood during activation engenders an R2* decrease, thus increasing the MR signal at a given echo time from its value at rest (Sr) to an increased value during activation (Sa):
| (A2) |
Expressed as a signal change relative to baseline, this yields:
| (A3) |
Using a Taylor expansion, for small changes in R2*, e−x can be approximated as:
| (A4) |
thus:
| (A5) |
The effect of changing CBV in a given voxel can be expressed similarly. Increases in CBV increases the amount of paramagnetic iron in a voxel, causing an R2* decrease (and thus a signal decrease) during activation. The BOLD- and CBV-induced R2* changes are cumulative in the USPIO experiment (SF(t)):
| (A6) |
Note that the signs of and are not the same during activation, so the two effects counteract in practice.
Since in the experiments described in this work all scan parameters, including echo time t, are identical in the BOLD fMRI and CBVw fMRI experiments, the BOLD contribution to can be removed on the basis of , measured in the BOLD fMRI experiment, for the same voxel, yielding the CBV-induced signal change,.
| (A7) |
This approach is analogous to what was employed in previous work (Kim 2013, Mandeville 2004).
References
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS and Hyde JS, 1992. Time course EPI of human brain function during task activation. Magn Reson Med 25, 390–7 [DOI] [PubMed] [Google Scholar]
- Behzadi Y and Liu TT, 2005. An arteriolar compliance model of the cerebral blood flow response to neural stimulus. Neuroimage 25, 1100–11 [DOI] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN Jr., McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ and Rosen BR, 1991. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254, 716–9 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC and Frank LR, 1998. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39, 855–64 [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Silva AC, van Gelderen P, Kellman P, Fukunaga M, Chu R, Koretsky AP, Frank JA and Duyn JH, 2005. Temporal dynamics of the BOLD fMRI impulse response. Neuroimage 24, 667–77 [DOI] [PubMed] [Google Scholar]
- de Zwart JA, van Gelderen P, Jansma JM, Fukunaga M, Bianciardi M and Duyn JH, 2009. Hemodynamic nonlinearities affect BOLD fMRI response timing and amplitude. Neuroimage 47, 1649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, Edden RA, Pekar JJ and van Zijl PC, 2006. Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med 56, 1261–73 [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM and Boas DA, 2007. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage 35, 89–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Stefanovic B and Silva AC, 2011. Spatiotemporal evolution of the functional magnetic resonance imaging response to ultrashort stimuli. J Neurosci 31, 1440–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Goense J, Kennerley A, Guidi M, Trampel R, Turner R and Moller HE, 2015. Micro- and macrovascular contributions to layer-dependent blood volume fMRI: A multi-modal, multi-species comparison. ISMRM 23rd annual meeting & exhibition, Toronto, ON, Canada p 2114 [Google Scholar]
- Huber L, Goense J, Kennerley AJ, Ivanov D, Krieger SN, Lepsien J, Trampel R, Turner R and Moller HE, 2014. Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7 T. Neuroimage 97, 349–62 [DOI] [PubMed] [Google Scholar]
- Huber L, Handwerker DA, Jangraw DC, Chen G, Hall A, Stuber C, Gonzalez-Castillo J, Ivanov D, Marrett S, Guidi M, Goense J, Poser BA and Bandettini PA, 2017. High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron 96, 1253–1263 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil B and Wald LL, 2013. Massively parallel MRI detector arrays. J Magn Reson 229, 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellman P, Gelderen P, de Zwart JA and Duyn JH, 2003. Method for functional MRI mapping of nonlinear response. Neuroimage 19, 190–9 [DOI] [PubMed] [Google Scholar]
- Kennerley AJ, Mayhew JE, Redgrave P and Berwick J, 2010. Vascular Origins of BOLD and CBV fMRI Signals: Statistical Mapping and Histological Sections Compared. Open Neuroimag J 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG and Duong TQ, 2002. Mapping cortical columnar structures using fMRI. Physiol Behav 77, 641–4 [DOI] [PubMed] [Google Scholar]
- Kim SG, Harel N, Jin T, Kim T, Lee P and Zhao F, 2013. Cerebral blood volume MRI with intravascular superparamagnetic iron oxide nanoparticles. NMR Biomed 26, 949–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R and et al. , 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89, 5675–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman DJ and Nunes RG, 2007. Parallel magnetic resonance imaging. Phys Med Biol 52, R15–55 [DOI] [PubMed] [Google Scholar]
- Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB and Mandeville JB, 2002. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage 16, 283–94 [DOI] [PubMed] [Google Scholar]
- Li W, Tutton S, Vu AT, Pierchala L, Li BS, Lewis JM, Prasad PV and Edelman RR, 2005. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging 21, 46–52 [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ and Van Zijl PC, 2003. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 50, 263–74 [DOI] [PubMed] [Google Scholar]
- Lu H, Soltysik DA, Ward BD and Hyde JS, 2005. Temporal evolution of the CBV-fMRI signal to rat whisker stimulation of variable duration and intensity: a linearity analysis. Neuroimage 26, 432–40 [DOI] [PubMed] [Google Scholar]
- Mandeville JB, 2012. IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage 62, 1000–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, Chen YC, Choi JK, Kim YR, Belen D, Liu C, Kosofsky BE and Marota JJ, 2004. Exogenous contrast agent improves sensitivity of gradient-echo functional magnetic resonance imaging at 9.4 T. Magn Reson Med 52, 1272–81 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H and Ugurbil K, 1992. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 89, 5951–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB and Boesiger P, 1999. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42, 952–62 [PubMed] [Google Scholar]
- Qiu D, Zaharchuk G, Christen T, Ni WW and Moseley ME, 2012. Contrast-enhanced functional blood volume imaging (CE-fBVI): enhanced sensitivity for brain activation in humans using the ultrasmall superparamagnetic iron oxide agent ferumoxytol. Neuroimage 62, 1726–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC and Alonso JM, 1995. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–4 [DOI] [PubMed] [Google Scholar]
- Schroeder MR, 1979. Integrated-Impulse Method Measuring Sound Decay without Using Impulses. Journal of the Acoustical Society of America 66, 497–500 [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK and Ugurbil K, 2007. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage 35, 539–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP and Duyn JH, 2007. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magn Reson Med 57, 1110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B and Pike GB, 2005. Venous refocusing for volume estimation: VERVE functional magnetic resonance imaging. Magn Reson Med 53, 339–47 [DOI] [PubMed] [Google Scholar]
- Turner R, 2002. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage 16, 1062–7 [DOI] [PubMed] [Google Scholar]
- Ugurbil K, 2012. The road to functional imaging and ultrahigh fields. Neuroimage 62, 726–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS and Koretsky AP, 1992. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 89, 212–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Chuang KH, Wai YY, Wan YL, Chen JH and Liu HL, 2008. Vascular space occupancy-dependent functional MRI by tissue suppression. J Magn Reson Imaging 28, 219–26 [DOI] [PubMed] [Google Scholar]
- Yu X, Qian C, Chen DY, Dodd SJ and Koretsky AP, 2014. Deciphering laminar-specific neural inputs with line-scanning fMRI. Nat Methods 11, 55–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccarin D and Kavehrad M, 1993. An Optical Cdma System Based on Spectral Encoding of Led. Ieee Photonics Technology Letters 5, 479–482 [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K and Kim SG, 2006. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage 30, 1149–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.