Abstract
Objectives
Nitric oxide is an endogenous substance that preserves the myocardial function in patients with heart failure. Asymmetric dimethylarginine (ADMA) is a competitive inhibitor of endogenous nitric oxide synthase. We sought to explore the association between the left ventricular (LV) function as assessed with two-dimensional echocardiography and the serum level of ADMA in nondiabetic patients without significant coronary artery disease.
Patients and methods
Eighty-seven consecutive patients with normal LV ejection fractions were included in this cross-sectional study. The ADMA serum level was measured, and the longitudinal deformation indices of the LV myocardium were evaluated using two-dimensional speckle-tracking echocardiography (2DSTE).
Results
The systolic strain, the systolic strain rate, and the early and late diastolic strain rates as evaluated with 2DSTE were not statistically significantly different between the patients with normal ADMA serum levels and those with increased ADMA serum levels. The two study groups were also not significantly different in terms of the systolic and diastolic myocardial velocities obtained with tissue Doppler.
Conclusion
Our findings showed no statistically significant correlations between the serum ADMA level and the 2DSTE-derived indices of the longitudinal deformation of the LV myocardium in our nondiabetic patients without significant coronary artery stenosis and with normal LV ejection fractions.
Keywords: asymmetric dimethylarginine, left ventricle, two-dimensional speckle-tracking echocardiography
Introduction
Nitric oxide (NO) is an endogenous substance that preserves the myocardial function in a background of heart failure 1. NO is produced by nitric oxide synthase (NOS), an enzyme found in cardiomyocytes and the endothelium 2. Through enzymatic pathways, not only does NO lead to the production of phosphokinase G, which is involved in cardiac contraction 3, but also it ultimately contributes toward the preservation of cyclic guanosine monophosphate by stabilizing phosphodiesterase 5, and thus protects the cardiomyocyte function against deterioration 4,5. Animal studies have shown that an increase in the production of NOS contributes toward the attenuation of myocardial infarction injury and the ensuing heart failure 6.
Asymmetric dimethylarginine (ADMA) is produced from the proteolysis of the proteins that contain methylated arginine 7. A competitive inhibitor of endogenous NOS, ADMA results in a reduction in NO production 8. The ADMA serum level is elevated in patients with heart failure, and a chronic accumulation of ADMA in cardiomyocytes can set in motion cardiomyocyte dysfunction 3,9. Research on healthy volunteers has shown that an infusion of ADMA results in a diminished cardiac output 10. It has also been shown previously that a chronic infusion of ADMA contributes toward an increase in the levels of vascular angiotensin-converting enzymes and oxidative stress 11. A clinical study reported an association between ADMA and adverse cardiovascular events in patients with heart failure 12,13. In light of such evidence, it can be postulated that the serum ADMA level may be associated with a decreased left ventricular (LV) function.
Two-dimensional speckle-tracking echocardiography (2DSTE) is a widely used method for the LV function assessment in some conditions and is capable of detecting subtle myocardial dysfunction by evaluating myocardial deformation 14.
The main aim of the present study was to assess the association between the LV function as determined with the 2DSTE-derived indices of longitudinal deformation and the serum level of ADMA in nondiabetic patients without significant coronary artery disease.
Patients and methods
Study population
Eighty-seven consecutive patients who were admitted to our hospital for selective coronary angiography between January 2017 and March 2017 were included in the present study. The inclusion criteria were sinus rhythm and a left ventricular ejection fraction (LVEF) of more than 50%. The exclusion criteria included the presence of significant coronary artery disease (>50% stenosis), diabetes, cardiomyopathy, any-degree valvular stenosis, more-than-mild valvular regurgitation, and LV hypertrophy (LV mass index in men>115 g/m2 and in women>95 g/m2), history of cancer, inflammatory diseases, cardiac surgery, pacemaker implantation, coronary angioplasty, heart failure, and poor echocardiography window. Venous sampling for cell blood count and biochemistry analysis was performed before coronary angiography after 12 h of fasting. The day after coronary angiography, venous samples were obtained for the evaluation of the ADMA serum level. The samples were stored at −70°C and subsequently analyzed in a single session using a commercial kit (DLD; Diagnostika GMBH, Hamburg, Germany) by the enzyme linked-immunoassay method. The patients were divided into patients with normal ADMA serum levels and those with high ADMA serum levels according to a previously introduced cut-off point (>0.9 µmol/l) 15. The laboratory staff was not informed about the echocardiography data. The study proposal was approved by our institutional review board, and informed written consent was obtained from all the participants.
Echocardiography
All the patients underwent transthoracic echocardiography the day after coronary angiography by the same cardiologist, who was highly experienced in echocardiography. The left lateral decubitus position was selected for the patients during echocardiography while one-lead ECG monitoring was performed using a 2–4 MHz probe in a commercial setting (Samsung Medison, Seoul, South Korea). The cardiologist was blinded to the laboratory data. The LV septal and posterior wall thicknesses, the LV mass by the M-mode method, the biplane LV end-diastolic and end-systolic volumes by the modified Simpson method, the left atrial anterior–posterior diameter, the mitral flow wave peak velocity (E and A waves), the deceleration time of the E wave obtained with pulsed wave, and the septal and lateral mitral annuli wave peak velocities (s′, e′, and a′) obtained with pulsed-wave tissue Doppler were measured according to the recommendations of the American Society of Echocardiography 16,17. The averaged s′, e′, and a′ were reported. The averaged e′ was used for the calculation of the E/e′ ratio. For 2DSTE, three consecutive cardiac cycles from the apical window – consisting of two-chamber, three-chamber, and four-chamber views – were acquired at a frame rate of 40–80 frames/s in expiration and stored in the setting. The endocardial borders of the LV in all the cited views were traced at end-systole from one side to another side of the mitral annulus before the epicardial border was traced automatically with software. The corrections required for the traced borders were made in this stage, and their accuracy was confirmed subsequently. In the next stage, it was checked whether these traced borders followed the endocardial and epicardial motions and all the stages were repeated if there was an inaccuracy. With software, each LV wall was divided into three sections automatically and the longitudinal strain and strain rate curves for each section were depicted. The strain curve included one negative peak, and the strain rate curve featured one negative [systolic strain rate (SRS)] and one early [early diastolic strain rate (SRE)] systolic peaks, and another late diastolic positive peak (SRA) (Fig. 1). After several attempts, the sections where the obtained signals were not interpretable were excluded. The peaks for each section were measured, and the global value of each of these indices of longitudinal deformation was calculated by averaging the accepted sections. A total of 1524 (97.3%) sections were finally accepted for analysis.
Fig. 1.
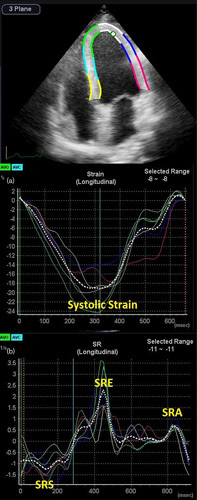
Two-dimensional speckle-tracking echocardiography in the apical four-chamber view. (a) Strain curve and (b) strain rate curve. SRA, late diastolic longitudinal strain rate; SRE, early diastolic longitudinal strain rate; SRS, systolic longitudinal strain rate.
Statistical analysis
The continuous data were presented as means and standard deviations if they were normally distributed; otherwise, they were presented as medians and interquartile ranges. The categorical data were described as frequencies and percentages. The continuous data were compared using the Student t-test if they were normally distributed; otherwise, they were compared using the Mann–Whitney U-test. The categorical data were compared using the χ2-test. The correlations between the ADMA serum level and the LVEF and the 2DSTE-derived indices of the longitudinal deformation of the LV myocardium were evaluated using the Pearson test. Multiple variable linear regression models were applied to explore the correlation between the ADMA serum level and the 2DSTE-derived indices of the LV longitudinal myocardial deformation adjusted for potential confounders – including the BMI, the systolic and diastolic blood pressures, and the LV end-diastolic volume index. The interobserver and intraobserver variabilities were evaluated using a coefficient variation. The statistical analyses were carried out using IBM SPSS statistics for Windows (version 23.0) (IBM Corp., Armonk, New York, USA). A P value of equal to or less than 0.05 was considered statistically significant.
Results
The demographic, clinical, and laboratory data of the patients with normal and increased ADMA levels are presented in Table 1.
Table 1.
Clinical and laboratory data of the patients with normal serum asymmetric dimethylarginine levels and those with high serum asymmetric dimethylarginine levels
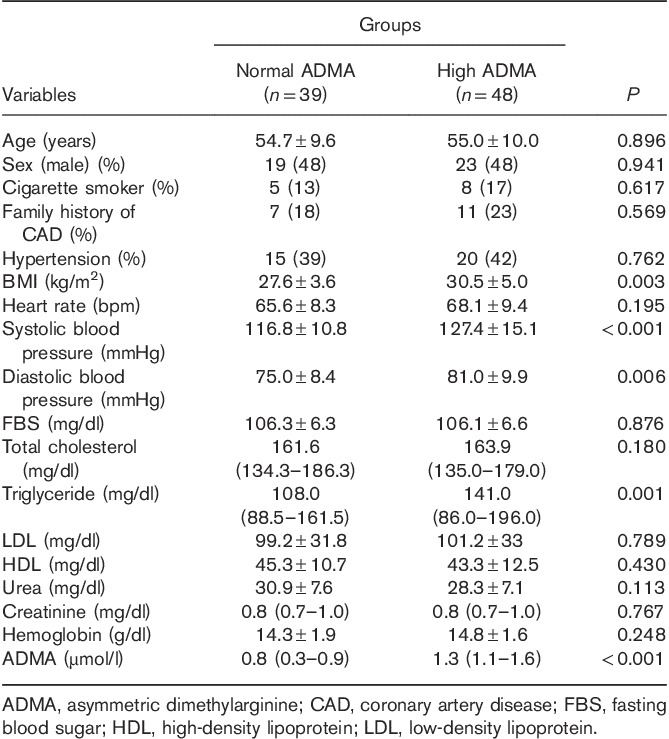
The systolic and diastolic blood pressures were higher in the patients with increased ADMA levels, but their mean was within the normal range in both groups. The BMI was higher in the patients with elevated ADMA levels than in their counterparts with normal ADMA levels. The echocardiographic parameters are shown in Table 2.
Table 2.
Echocardiography data of the patients with normal serum asymmetric dimethylarginine levels and those with high serum asymmetric dimethylarginine levels
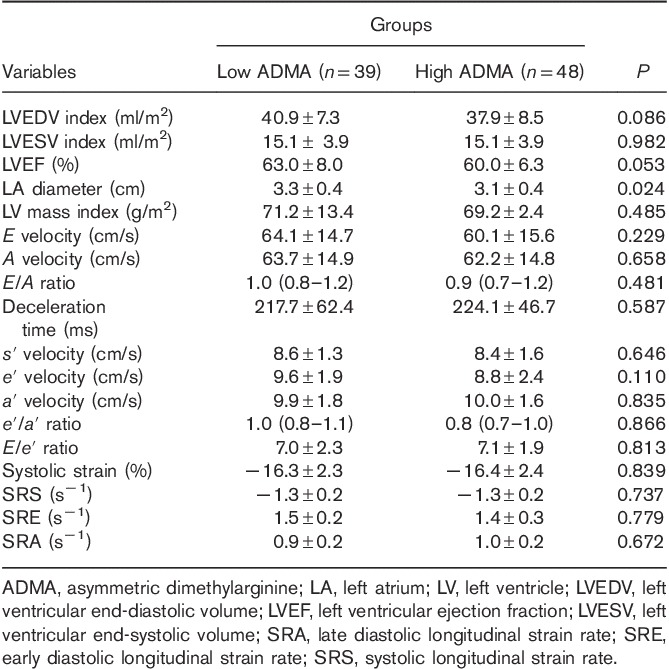
With the exception of the left atrial diameter, there were no statistically significant differences in the standard echocardiography parameters and the tissue Doppler echocardiography markers between the two groups. The two study groups were also not statistically significantly different vis-à-vis the 2DSTE-derived indices of the longitudinal deformation of the LV myocardium.
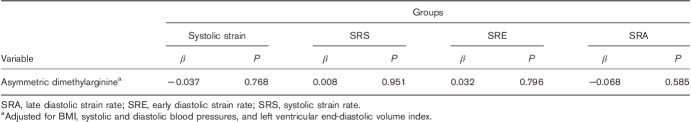
The mean ADMA serum level in the study population was 1.0 µmol/l (0.8–1.3 µmol/l). The ADMA level was correlated with the systolic strain (r=0.009 and P=0.931), the SRS (r=−0.025 and P=0.821), the SRE (r=−0.002 and P=0.987), the SRA (r=0.026 and P=0.813), and the LVEF (r=−0.225 and P=0.035). The multiple variable linear regression models showed that the correlation between the ADMA serum level and the 2DSTE-derived indices of the LV longitudinal myocardial deformation adjusted for potential confounders was not statistically significant (Table 3).
Table 3.
Adjusted association between the asymmetric dimethylarginine level group and the two-dimensional speckle-tracking echocardiography-derived indices
The interobserver variability was 8.7% for the systolic strain, 9.1% for the SRS, 8.8% for the SRE, and 9.2% for the SRA and the intraobserver variability was 6.5% for the systolic strain, 6.8% for the SRS, 7.5% for the SRE, and 8.0% for the SRA.
Discussion
In the current study, we evaluated the longitudinal deformation of the LV myocardium in the context of elevated and normal ADMA serum levels in patients without significant coronary artery stenosis and diabetes. We found that the 2DSTE-derived markers of the longitudinal deformation of the LV myocardium were not statistically significantly different between the patients with elevated ADMA serum levels and those with normal ADMA serum levels. The LVEF was, however, correlated with the ADMA serum level. Our study is the first study of its kind to evaluate longitudinal myocardial deformation in patients with a normal LVEF and an increased ADMA serum level.
Von Haehling et al. 9 reported an elevation in the serum level of ADMA in their patients with chronic heart failure in comparison with their normal participants. Our study population had no significant systolic dysfunction.
Poreba et al. 18 showed that the ADMA serum level was elevated in their hypertensive patients with diastolic dysfunction compared with their hypertensive patients without diastolic dysfunction. In their study, the definition of the diastolic dysfunction was the same as that for heart failure with a normal EF. The fact that our study population had no history of heart failure and that the E/e′ in all the study participants was less than 14 illustrates the difference in the study populations between the two studies.
Tang et al. 19 reported that, in their patients with systolic LV dysfunction (LVEF<35%), the serum level of ADMA was correlated with the Doppler-derived indices of the diastolic function such as the mitral E/A ratio, the deceleration time, and the E/e′ ratio. In our study, only patients with an LVEF of greater than 50% were included.
Zhao et al. 20 found an association between an elevated ADAM serum level and a lower LVEF in their patients on peritoneal dialysis. Bartnicki et al. 21, in a study on patients with chronic kidney disease, reported that the patients with higher ADMA serum levels had lower LVEFs than those with lower serum levels of ADMA. In our study, none of the patients had chronic kidney disease.
Our results indicated a correlation between the LVEF and the ADMA serum level, which is in agreement with previous studies 20,21. Nonetheless, in the interpretation of this finding, the following points should be taken into account. First, the calculation of the LVEF using the Simpson biplane method is a semiquantitative method that is operator dependent. In other words, it rests on geometrical assumptions and requires a precise visualization of the apex 22,23. Second, biplane methods fail to visualize the third dimension. Third, the circumferential strain (not evaluated in the present study) makes a more pronounced contribution toward the LVEF than does the longitudinal strain 24, although some investigators consider the LVEF and strain as two different entities of ‘muscular pump’ and ‘hemodynamic compression pump’, respectively 25. Fourth, despite the absence of a correlation in our patients with a normal systolic function between the ADMA serum level and the longitudinal deformation of the LV myocardium, there may be such a correlation in patients suffering from heart failure with a preserved or reduced EF. Fifth, myocardial changes in patients with increased ADMA serum levels may prove too subtle for the detection zone of 2DSTE, necessitating the use of more advanced technologies such as cardiac MRI. Last but not the least, although two subject groups of our study were different regarding to the ADMA serum level, but arginine/ADMA ratio may have been the same in both groups.
Study limitations
The present cross-sectional single-center investigation with a low sample size should be considered a pilot study. We had no access to three-dimensional echocardiography or cardiac MRI for the evaluation of our patients, and nor was it possible for us to measure the serum arginine level and the serum arginine/ADMA ratio. Other salient weaknesses of our study are that we measured the deformation indices only in one direction and that the results are generalizable only to similar patients.
Conclusion
According to our findings, there were no statistically significant differences between the patients with normal ADMA levels and those with elevated serum ADMA levels in the 2DSTE-derived indices of the longitudinal deformation of the LV myocardium such as the systolic strain, the SRS, the SRE, and the SRA in our nondiabetic patients without significant coronary artery stenosis and with normal LVEFs.
Acknowledgements
This study was done by financial support of Tehran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carnicer R, Crabtree MJ, Sivakumaran V, Casadei B, Kass DA. Nitric oxide synthases in heart failure. Antioxid Redox Signal 2013; 18:1078–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YH. Nitric oxide signalling and neuronal nitric oxide synthase in the heart under stress. F1000Res 2017; 6:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Hou L, Xu D, Chen A, Yang L, Zhuang Y, et al. Effect of asymmetric dimethylarginine (ADMA) on heart failure development. Nitric Oxide 2016; 54:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Zhang P, Xu Z, Yue W, Zhuang Y, Chen Y, Lu Z. S-nitrosylation of PDE5 increases its ubiquitin-proteasomal degradation. Free Radic Biol Med 2015; 86:343–351. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, et al. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation 2010; 121:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awada HK, Hwang MP, Wang Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: aspects to consider and proteins to deliver. Biomaterials 2016; 82:94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tain YL, Hsu CN. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins (Basel) 2017; 9:E92.28272322 [Google Scholar]
- 8.Shin S, Thapa SK, Fung HL. Cellular interactions between L-arginine and asymmetric dimethylarginine: Transport and metabolism. PLoS ONE 2017; 12:e0178710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Haehling S, Bode-Böger SM, Martens-Lobenhoffer J, Rauchhaus M, Schefold JC, Genth-Zotz S, et al. Elevated levels of asymmetric dimethylarginine in chronic heart failure: a pathophysiologic link between oxygen radical load and impaired vasodilator capacity and the therapeutic effect of allopurinol. Clin Pharmacol Ther 2010; 88:506–512. [DOI] [PubMed] [Google Scholar]
- 10.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 2003; 23:1455–1459. [DOI] [PubMed] [Google Scholar]
- 11.Veresh Z, Debreczeni B, Hamar J, Kaminski PM, Wolin MS, Koller A. Asymmetric dimethylarginine reduces nitric oxide donor-mediated dilation of arterioles by activating the vascular renin-angiotensin system and reactive oxygen species. J Vasc Res 2012; 49:363–372. [DOI] [PubMed] [Google Scholar]
- 12.Dückelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol 2007; 27:2037–2042. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CP, Lin SJ, Chung MY, Lu TM. Asymmetric dimethylarginine predicts clinical outcomes in ischemic chronic heart failure. Atherosclerosis 2012; 225:504–510. [DOI] [PubMed] [Google Scholar]
- 14.Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail 2017; 19:307–313. [DOI] [PubMed] [Google Scholar]
- 15.Kusnierova P, Vsiansky F, Pleva L, Plevova P, Safarcik K, Svagera Z. Reference intervals of plasma matrix metalloproteinases 2, 3, and 9 and serum asymmetric dimethylarginine levels. Scand J Clin Lab Invest 2015; 75:508–513. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28:1–39. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, III, Dokainish H, Edvardsen T. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2016; 29:277–314. [DOI] [PubMed] [Google Scholar]
- 18.Poręba R, Gać P, Poręba M, Derkacz A, Chachaj A, Mazur G, Szuba A. Left ventricular diastolic dysfunction and plasma asymmetric dimethylarginine concentration in persons with essential hypertension. Arch Med Sci 2015; 11:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J 2008; 29:2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JR, Zhang DY, Sun DZ. Correlation research on ADMA plasma levels and left ventricular function of peritoneal dialysis patients. Int J Clin Exp Med 2014; 7:4455–4460. [PMC free article] [PubMed] [Google Scholar]
- 21.Bartnicki P, Kowalczyk M, Franczyk-Skóra B, Baj Z, Rysz J. Evaluation of endothelial (dys)function, left ventricular structure and function in patients with chronic kidney disease. Curr Vasc Pharmacol 2016; 14:360–367. [DOI] [PubMed] [Google Scholar]
- 22.Feigenbaum H, Mastouri R, Sawada S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J 2012; 76:1550–1555. [DOI] [PubMed] [Google Scholar]
- 23.Sitia S, Tomasoni L, Turiel M. Speckle tracking echocardiography: a new approach to myocardial function. World J Cardiol 2010; 2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, et al. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 2017; 70:942–954. [DOI] [PubMed] [Google Scholar]
- 25.De Keulenaer GW, Brutsaert DL. Systolic and diastolic heart failure are overlapping phenotypes within the heart failure spectrum. Circulation 2011; 123:1996–2004. [DOI] [PubMed] [Google Scholar]