Abstract
Glucagon-like peptide 1 (GLP-1)-based therapies reduce hyperglycaemia in type 2 diabetes. Diabetes cardiovascular comorbidity remains prevalent, although current treatments are effective at reducing hyperglycaemia. GLP-1 exerts specific actions on the cardiovascular system in both healthy individuals and patients with cardiovascular pathology, and GLP-1 therapies have improved the cardiovascular profile of diabetic patients. GLP-1 exerts its action by binding to its receptor (GLP-1 receptor) at the cell surface. Mechanistically, it is not clear how GLP-1 therapies exert beneficial effects on the cardiovascular system. It is difficult to arrive at any conclusions on the ability of GLP-1 receptor agonism to reduce cardiovascular disease from animal/human studies because of varying experimental designs. This review highlights recent findings from long-term human GLP-1 therapy studies, and summarizes postulated mechanisms as to how GLP-1 receptor agonism may alleviate cardiovascular disease.
Keywords: cardiovascular disease, cardiovascular outcome trials, cardiovascular system, glucagon-like peptide 1, glucagon-like peptide 1 receptor, major adverse cardiac event, type 2 diabetes
Type 2 diabetes (T2D) is a chronic complex multifactorial disease with an incompletely understood aetiology and pathogenesis 1,2. The majority of T2D patients are overweight (60–90% in western countries), implying that diets involving excessive nutrient consumption cause disease pathogenesis 3. However, this does not explain how individuals with a BMI of less than or equal to 25 develop T2D and the majority of overweight individuals remain disease free 2,4. Interestingly, ∼50% of T2D patients are not overweight in Japan 5. It is also noteworthy that overweight patients have been reported to have a lower mortality rate because of cardiovascular disease (CVD) than normal-weight patients, termed ‘the obesity paradox’ 6. The obesity paradox implies that the diabetic phenotype promotes CVD independent of patient BMI. Despite current treatments being effective at reducing hyperglycaemia, diabetes cardiovascular (CV) comorbidity remains prevalent, and therefore novel therapies are desirable: ∼75% of diabetic patients die from CVD 7. Evidence suggests that glucagon-like peptide 1 (GLP-1) exerts specific actions on the cardiovascular system (CVS) in both healthy individuals and patients with CV pathology, and GLP-1 therapies have improved the CV profile of diabetic patients 8,9.
The best-characterized function of GLP-1 is its promotion of the incretin effect 9. The incretin effect is reduced in T2D – incretin hormones account for less than 20% of the insulin release after glucose ingestion in T2D patients compared with 70% in nondiabetic individuals 10. The current consensus is that GLP-1 levels are normal in T2D, but its action is reduced 11. GLP-1 has a very short half-life (∼1.5 min) because of its rapid proteolytic degradation in the plasma by dipeptidyl peptidase IV (DPP-IV) enzymes 11. The DPP-IV resistant GLP-1 analogues are effective at reducing hyperglycaemia in T2D patients as they prolong the GLP-1 response because of their extended half-lives 8. However, GLP-1 also has extrapancreatic functions (Fig. 1) 9. Importantly, GLP-1 therapies induce weight loss, which is associated with reducing CVD in diabetic and nondiabetic patients 8,9. GLP-1-based therapies also appear to exert other specific actions in diabetes 9.
Fig. 1.
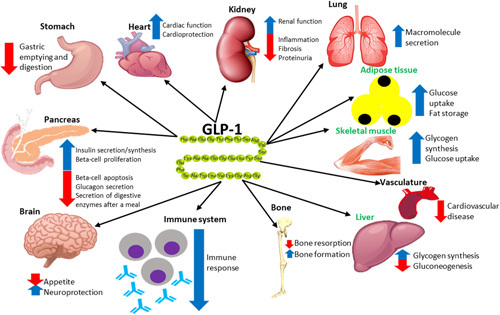
Summary of the effects that glucagon-like peptide 1 (GLP-1) has on various organs. Organs highlighted in green do not express GLP-1 receptor, but GLP-1 has mediated direct insulin-like effects during experimental settings. This figure is adapted from de Graaf and colleagues 8,12–16.
GLP-1 induces its effects by acting as an agonist to the glucagon-like peptide 1 receptor (GLP-1R). The effects induced by GLP-1 vary in different tissues as GLP-1R is coupled to a range of intracellular signalling pathways in different tissues, each of which promotes the desired physiological response elicited by receptor activation 12,17. GLP-1 also indirectly affects organs through the insulin secretion that it promotes – ∼28% of the postprandial insulin released into circulation is because of the action of this hormone 13,18. GLP-1R knockout and knockdown studies in mice have shown that the ability of GLP-1 to act as an incretin hormone is dependent on the presence of its receptor in islet β-cells 19,20. GLP-1R is a class B G-protein-coupled receptor, consisting of a large hydrophilic N-terminal extracellular domain, seven hydrophobic transmembrane α-helices (TM1-7) joined by three hydrophilic extracellular loops (ECL1-3) and three intracellular loops (ICL1-3), and an intracellular C-terminal domain. The GLP-1R C-terminal domain interacts with heterotrimeric G-proteins that consist of α, β and γ subunits that activate downstream signalling pathways upon agonist (GLP-1) binding (Fig. 2) 17,21–24.
Fig. 2.
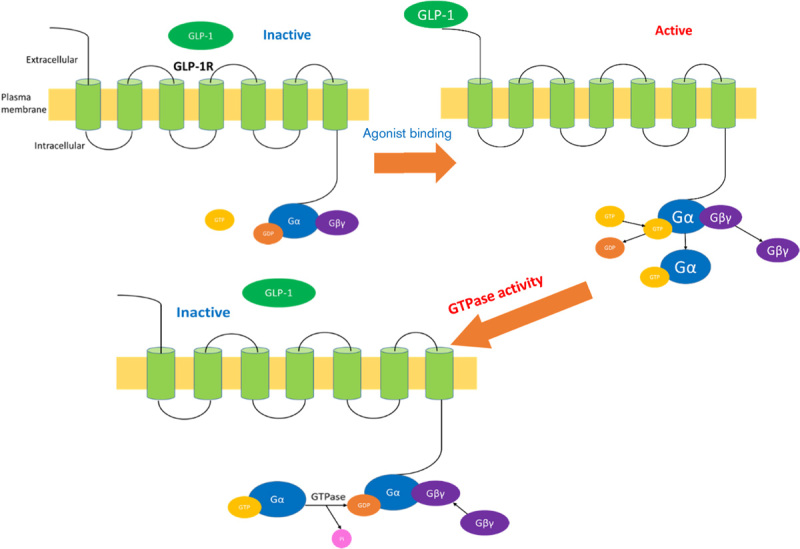
The canonical model of glucagon-like peptide 1 (GLP-1) receptor activation. Upon GLP-1 binding, the Gα subunit is activated by exchanging GDP for GTP, and then the G-protein subunits dissociate. Both the active Gα and the Gβγ subunits activate downstream effectors to propagate G-protein-coupled receptor signalling. The intrinsic GTPase activity converts the Gα subunit bound GTP to GDP, and the G-protein subunits then reassociate, ready for the arrival of a new agonist. This figure is adapted from Thompson and colleagues 11,17.
There are currently five licenced GLP-1-based therapies, one of which has two modes of delivery (exenatide as twice-daily Byetta or once-weekly Bydureon) 25, and semaglutide is being considered by regulatory authorities 26. These have differing levels of efficacy on glycaemic control and weight loss, perhaps because of differing GLP-1R activation of these drugs – for example, because of different penetration of the central nervous system 27. Both animal and human studies generally suggest that GLP-1 therapies exert beneficial CV actions 9.
Chronic GLP-1R agonism in rodents was reported to reduce blood pressure (BP) and prevent hypertension 28–31. However, acute GLP-1 infusion in rodents increases heart rate and BP 9,32. Human short-term clinical trials have reported conflicting findings as acute GLP-1 therapy has had no effect on BP and heart rate, or increased both 9. GLP-1 analogue-based and DPP-IV inhibition-based therapies reduced plasma lipid levels in healthy and diabetic rodents, and the same therapies also had similar effects on T2D patients 9,33,34. However, one study found that exenatide treatment for 24 weeks had no effect on lipid profiles 35. Rodent in-vitro studies have reported vasorelaxant actions of GLP-1, and different studies postulated different mechanisms as to how this was achieved 9. In-vivo rodent studies have reported that GLP-1 induces vasodilation of certain blood vessels and promotes vasoconstriction of others, as well as improving endothelial function 9,36,37. Similarly, improved blood flow and endothelial function has been reported in diabetic individuals in response to acute GLP-1 therapies 9,38,39. Interestingly, exendin-4 treatment did not affect short-term triglyceride exposure-induced endothelial dysfunction in rat femoral artery, which suggests that the reported in-vivo beneficial actions of GLP-1 therapies on the endothelium likely occur by extracardiovascular GLP-1 signalling 40. Rodent and human studies have provided evidence that GLP-1 therapies have antiatherosclerotic actions and angiogenic effects 9,41,42. GLP-1 therapies have been reported to reduce levels of proinflammatory molecules (associated with atherosclerotic development) in patients 9, and one study reported that anti-inflammatory benefits persisted for 12 weeks in obese T2D patients after a single exenatide injection 43. Finally, GLP-1 treatments during rodent and human in-vitro/in-vivo studies have been reported to exert beneficial effects on the myocardium such as protection against diabetic cardiomyopathy 9.
Long-term human studies have shown that chronic DPP-IV inhibition did not significantly confer any CV benefits 44,45. In contrast, chronic GLP-1-based treatments showed multiple CV benefits in diabetic patients 9. Four cardiovascular outcome trials have been reported for GLP-1 analogues: ELIXA 46, LEADER 47, SUSTAIN 6 26 and EXSCEL 48 trials have tested lixisenatide, liraglutide, semaglutide and exenatide, respectively. ELIXA showed no advantage over placebo in terms of influencing the primary outcome of a four-point major adverse cardiac event, which included CV mortality, nonfatal myocardial infarction, nonfatal stroke and hospital admission for unstable angina 46. The other cardiovascular outcome trials all used a three-point major adverse cardiac event primary end-point (excluding unstable angina); superiority was found for liraglutide and semaglutide (albeit not a prespecified analysis for semaglutide), but not for exenatide. All-cause mortality benefit was shown for liraglutide and exenatide, but not for semaglutide, although the latter agent was tested in a smaller study. The ELIXA population all had a CV event within 180 days of the study start, and it is reasonable to assume that the stability of their coronary lesions would have made any drug effect difficult to determine. Conversely, the EXSCEL study included 27% of patients at a much lower CV risk, which may have resulted in the marginal lack of statistical superiority. Examination of components of the primary end-point showed heterogeneity between the two trials that reported reduced CVD with GLP-1 analogues, with the LEADER superiority being driven by a significant reduction in CV mortality, whereas SUSTAIN 6 superiority was largely because of a reduction in nonfatal stroke. In both of these studies, the mechanisms by which these drugs reduced CV outcomes are elusive. A moderator analysis of the LEADER study suggests that the reductions in glycated haemoglobin, weight and systolic BP were insufficient to account for all of the benefits. Recently, an analysis of severe hypoglycaemia has also been shown not to influence the outcome. The slow separation of the event curves in the Kaplan–Meier plots from both LEADER and SUSTAIN 6 suggests an impact on the atherosclerotic process, which is in contrast to that observed with the sodium-glucose cotransporter 2 inhibitors 49.
Mechanistically, it is not clear how GLP-1-based therapies exert beneficial effects on the CVS, but studies have suggested several pathways (Fig. 3). According to the postulated mechanisms, it appears that GLP-1 therapies mediate these effects directly and/or by promoting the incretin effect 2. By promoting the incretin effect, GLP-1-based therapies alleviate the potential of the diabetic phenotype to promote CVD by reducing hyperglycaemia and hyperlipidaemia, as well as by improving blood flow systemically because of increasing vascular nitric oxide production 8,9. These therapies also appear to promote the effects by direct mechanisms as well 9. In addition, studies have found that GLP-1 therapies improve angiogenesis, reduce atherosclerosis progression, increase cardiac function and improve the prognosis of cardiac ischaemia by the incretin effect and extrapancreatic GLP-1R activity 8,9. Chronic GLP-1 therapies have also been reported to prevent hypertension, and evidence suggests that this was achieved by CV GLP-1R activity 9.
Fig. 3.
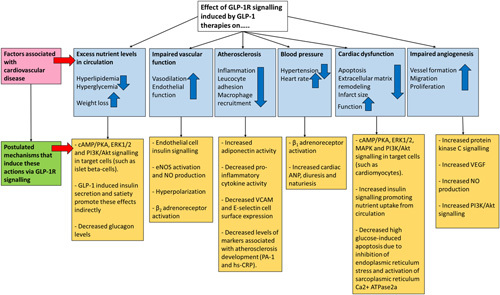
Reported benefits of glucagon-like peptide 1 receptor (GLP-1R) agonism on cardiovascular disease. A summary of the reported benefits of glucagon-like peptide 1 (GLP-1)-based therapies on reducing cardiovascular disease burden in patients and in experimental settings, and the postulated mechanisms as to how this was achieved. This figure is adapted from Tate and colleagues 9,11. ANP, atrial natriuretic peptide; eNOS, endothelial nitric oxide synthase; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
Currently, several studies are testing the chronic effects of other GLP-1 analogues 9. It is difficult to arrive at any conclusions on the conflicting findings on the ability of GLP-1-based therapies to reduce CVD from animal and human studies: animal species and trial design differed between animal studies, and trial design and cohorts varied between human studies. The notion that the ‘inactive’ forms of GLP-1 may have direct effects on the CVS warrants investigation 50. The effect of allosteric GLP-1R agonists (discussed in the study by Thompson and Kanamarlapudi 11) on the CVS is another area of future research. A better understanding of T2D aetiology/pathogenesis and how the disease phenotype promotes CVD, as well as further elucidation of GLP-1 activity/targets could provide better insight into the therapeutic potential of GLP-1R agonism to reduce T2D-associated CVD.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Affourtit C. Mitochondrial involvement in skeletal muscle insulin resistance: a case of imbalanced bioenergetics. Biochim Biophy Acta 2016; 1857:1678–1693. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103:137–149. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am 2010; 39:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Obesity and overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. [Accessed 15 October 2017].
- 5.George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: an emerging entity in the era of obesity. World J Diabetes 2015; 6:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo P, Cleland JF, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med 2015; 162:610–618. [DOI] [PubMed] [Google Scholar]
- 7.Ali MK, Narayan KMV, Tandon N. Diabetes & coronary heart disease: current perspectives. Indian J Med Res 2010; 132:584–597. [PMC free article] [PubMed] [Google Scholar]
- 8.De Graaf C, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev 2016; 68:954–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate M, Chong A, Robinson E, Green BD, Grieve DJ. Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. Br J Pharmacol 2015; 172:721–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahrén B. Incretin dysfunction in type 2 diabetes: clinical impact and future perspectives. Diabetes Metab 2013; 39:195–201. [DOI] [PubMed] [Google Scholar]
- 11.Thompson A, Kanamarlapudi V. Type 2 diabetes mellitus and glucagon like peptide-1 receptor signalling. Clin Exp Pharmacol 2013; 3:138. [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87:1409–1439. [DOI] [PubMed] [Google Scholar]
- 13.Donath MY, Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care 2013; 36 (Suppl 2):S145–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab 2013; 15:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 2010; 1:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed J, Kanamarlapudi V. Choi S. Glucagon like peptide-1 (GLP-1). Encyclopedia of signaling molecules. New York, NY: Springer-Verlag Inc.; 2017. 1–9. [Google Scholar]
- 17.Reed J, Kanamarlapudi V. Choi S. GLP-1R. Encyclopedia of signaling molecules. New York, NY: Springer; 2016. 1–12. [Google Scholar]
- 18.Salehi M, Aulinger B, D’Alessio DA. Effect of glycemia on plasma incretins and the incretin effect during oral glucose tolerance test. Diabetes 2012; 61:2728–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest 2012; 122:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Li B, et al. The role of β-cell GLP-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab 2014; 19:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker PLDD. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels 2002; 8:179–188. [PubMed] [Google Scholar]
- 22.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 2000; 289:739–745. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel M, Espinosa R, Michelle MLB, Bell GI. Human glucagon-like peptide-1 receptor gene: localization to chromosome band 6p21 by fluorescence in situ hybridization and linkage of a highly polymorphic simple tandem repeat DNA polymorphism to other markers on chromosome 6. Diabetes 1993; 42:1215–1218. [DOI] [PubMed] [Google Scholar]
- 24.Van Eyll B, Lankat-Buttgereit B, Bode HP, Göke R, Göke B. Signal transduction of the GLP-1-receptor cloned from a human insulinoma. FEBS Lett 1994; 348:7–13. [DOI] [PubMed] [Google Scholar]
- 25.Pisano M. Overview of insulin and non-insulin delivery devices in the treatment of diabetes. Pharmacy and Therapeutics 2014; 39:866–876. [PMC free article] [PubMed] [Google Scholar]
- 26.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz M, Evers A, Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett 2013; 23:4011–4018. [DOI] [PubMed] [Google Scholar]
- 28.Aroor AR, Sowers JR, Jia G, DeMarco VG. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart and Circ Physiol 2014; 307:H477–H492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata K, Kume S, Araki S-i, Sakaguchi M, Chin-Kanasaki M, Isshiki K, et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 2009; 380:44–49. [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013; 19:567–575. [DOI] [PubMed] [Google Scholar]
- 31.Yu MMC, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 2003; 21:1125–1135. [DOI] [PubMed] [Google Scholar]
- 32.Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol 2009; 157:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as Yin and Yang of intestinal lipoprotein production: evidence for predominance of glp-2–stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes 2013; 62:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol 2010; 206:287–296. [DOI] [PubMed] [Google Scholar]
- 35.Moretto TJ, Milton DR, Ridge TD, MacConell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460. [DOI] [PubMed] [Google Scholar]
- 36.Gardiner SMJ, Kemp P, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol 2008; 154:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardiner SMJ, Kemp P, Bennett T, Baker D. Possible involvement of GLP-1 (9–36) in the regional haemodynamic effects of GLP-1 (7–36) in conscious rats. Br J Pharmacol 2010; 161:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013; 36:2346–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004; 287:E1209–E1215. [DOI] [PubMed] [Google Scholar]
- 40.Nathanson D, Erdogdu O, Pernow J, Zhang Q, Nyström T. Endothelial dysfunction induced by triglycerides is not restored by exenatide in rat conduit arteries ex vivo. Regul Pept 2009; 157:8–13. [DOI] [PubMed] [Google Scholar]
- 41.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, et al. Chronic DPP-4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011; 124:2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, et al. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Transl Med 2012; 1:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012; 97:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 45.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 47.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 50.Sharma R, McDonald TS, Eng H, Limberakis C, Stevens BD, Patel S, et al. In vitro metabolism of the glucagon-like peptide-1 (GLP-1)-derived metabolites GLP-1(9-36)amide and GLP-1(28-36)amide in mouse and human hepatocytes. Drug Metab Dispos 2013; 41:2148–2157. [DOI] [PubMed] [Google Scholar]


