Abstract
Cardiovascular disease (CVD) is a well-recognized complication of diabetes. Although the association of type 2 diabetes with CVD has been well described, the mechanisms, risk stratification and screening strategies of CVD in type 1 diabetes (T1D) are less understood. This review aims to evaluate recent literature and guidelines regarding CVD in T1D. At the cellular level, the early stage of CVD is characterized by endothelial dysfunction. Recent studies have shown that endothelial function is unaffected in younger T1D patients but there is a significant degree of endothelial dysfunction in the older T1D population compared with healthy age-matched controls, highlighting the importance of the endothelial dysfunction in T1D as a major age-dependent cardiovascular risk factor. T1D risk assessment tools have been developed similar to those seen in type 2 diabetes. Foremost among these are the Danish Steno Type 1 risk engine, the Swedish T1D risk score, the Scottish T1D risk score and the QRISK risk calculator. The latter risk prediction tool is used for all patients but contains T1D as an independent risk variable and has the advantage of being derived from, and validated in, a large and diverse population. The latest version (QRISK3) is likely to be recommended for routine use in T1D patients in upcoming guidelines by the National Institute of Clinical Excellence. Mortality in adults with T1D is increasingly due to CVD. This is driven by hyperglycaemia-mediated oxidative stress and vascular inflammation, resulting in atherosclerosis and cardiac autonomic neuropathy. Coronary artery disease is the most significant contributor to CVD and in T1D, has a propensity towards a more silent and severe form. Routine screening of coronary artery disease does not alter outcomes and is therefore not recommended; however, risk prediction tools are being developed to aid identification of high-risk individuals for aggressive risk factor modification strategies.
Keywords: cardiovascular disease, risk assessment, screening, type 1 diabetes
Introduction
Cardiovascular disease (CVD) is a term that encompasses a number of conditions affecting the heart and major blood vessels. This review will focus on the effects of type 1 diabetes (T1D) on coronary artery disease (CAD); however, there is significant overlap between the disease process of CAD, cerebrovascular disease and peripheral vascular disease (PVD).
The prevalence of T1D is increasing worldwide and CVD is a major cause of mortality in T1D. Although acute diabetic complications such as ketosis and hypoglycaemia are more likely to cause death in younger T1D patients, CVD begins to predominate as patients become older 1. With life expectancy in T1D ever-increasing, there is no longer a significant disparity between mortality from CVD in T1D and type 2 diabetes (T2D). A WHO multinational cohort study found that CVD accounted for 44% of deaths in T1D compared with 52% in T2D 2. This public health issue affects both sexes, with a recent Danish study of 4821 T1D patients demonstrating that CVD was the main cause of death in 31 and 30% of men and women, respectively 3.
Although there have been numerous studies describing the relationship between CVD and T2D, there are far fewer for T1D. This is despite evidence that the relative risk (RR) of CVD in patients with T1D is ∼10 times that of the general population 4. This review will analyse the literature surrounding the mechanisms, risk assessment and screening of CVD in T1D.
Mechanisms
Vascular dysfunction is the hallmark of CVD and its pathophysiology in diabetes can be broadly categorized as endothelial dysfunction and chronic vascular inflammation which result in atherosclerosis and subsequent vascular obstruction. In the macrovasculature, this leads to CAD, PVD and cerebrovascular disease, whereas in the microvasculature such as the vasa nervorum, cardiac autonomic nerve conduction is disrupted leading to an autonomic neuropathy that contributes significantly to cardiovascular morbidity and mortality.
Endothelial dysfunction
The vascular endothelium is a complex metabolically active organ which plays a crucial role in vascular homoeostasis. Endothelial dysfunction is the deleterious change in autoregulatory endothelial physiology, resulting in impaired paracrine signalling between the vascular endothelium and smooth muscle cells. These changes are thought to be the precursor to the development of atherosclerosis.
Numerous studies have shown that patients with T1D are more likely to have endothelial dysfunction. This is thought to be due to a direct effect of hyperglycaemia on the endothelium. The postulated mechanism is discussed later in this review. In vitro, arteries harvested from normal rats, which were subsequently exposed to exogenous hyperglycaemia, exhibit attenuated endothelial-dependent relaxation 5. In vivo, studies have also supported this notion by demonstrating that hyperglycaemiamediated by oral glucose loading, diminishes endothelial function in diabetic and nondiabetic patients 6.
In a study of 30 T1D patients with a mean age of 46 years compared with 25 nondiabetic controls with a mean age of 57 years, flow-mediated dilatation (a measure of endothelial function) was found to be reduced in the T1D groups 7. Such differences in endothelial function are not detectable in a study of younger patients with T1D with a mean age of 21 years 8. Aging is characteristically associated with abnormalities of the vascular smooth muscle and endothelium that promote endothelial dysfunction. Thus, although younger T1D patients appear to be unaffected by endothelial dysfunction, older T1D patients appear to be more at risk than their nondiabetic counterparts.
Vascular inflammation and atherosclerosis
Chronic vascular inflammation is now recognized to be the basis of diabetic vasculopathy and atherosclerosis. The latter is no longer considered to be simply a disorder of lipid metabolism. Vascular inflammation precedes the discovery of lipid laden plaque and this results in cytokine-mediated monocyte recruitment, which accumulate in the subendothelial layer of the intima where they differentiate into macrophages. Inflammatory cytokines upregulate the expression of macrophage scavenger receptors which facilitate the phagocytosis of low-density lipoprotein (LDL) 9. Inflammatory cytokines also downregulate expression of cytoplasmic cholesterol transporters resulting in lipid accumulation within the macrophages, giving them a histological foamy appearance. When these foam cells accrue, a fatty streak develops and is covered by a smooth muscle cap where it may remain in this state until a plaque rupture event occurs. As vascular inflammation is so pivotal in atherosclerosis, there has been significant work to determine how T1D patients develop chronic vascular inflammation.
A unifying theory suggests that hyperglycaemia itself causes both endothelial dysfunction and vascular inflammation through a number of distinct metabolic processes involving oxidative stress.
Role of oxidative stress
In endothelial cells, glucose can pass freely through the plasma membrane in an insulin-independent manner. Intracellular hyperglycaemia promotes a number of pathways, including mitochondrial production of the reactive oxygen species superoxide (O2−). In the presence of endothelial nitric oxide, O2− reacts to form peroxynitrite (ONOO−) which causes oxidative DNA damage, signalling the production of the nuclear enzyme poly(ADP-ribose) polymerase (PARP), a key DNA repair enzyme. PARP requires nicotinamide adenine dinucleotide (NAD+) as a substrate for generating NAD ribose monomers. Hence, activation of PARP causes an intracellular ‘energy crisis’, which rapidly depletes NAD+ and subsequently slows the rate of glycolysis, electron transport and ATP formation. Endothelial dysfunction is precipitated by the resulting disruption of ATP-sensitive endothelial ion channels which play a key role in regulating the resting membrane potential and thus intracellular calcium (Ca2+) levels, by decreasing Ca2+ influx through voltage-gated Ca2+ ion channels. Reduced intracellular Ca2+ inhibits the Ca2+-dependent endothelial nitric oxide synthase, which diminishes the production of nitric oxide, a potent vasodilator which otherwise diffuses into adjacent vascular smooth muscle cells and generates cyclic GMP by activating guanylate cyclase. Cyclic GMP promotes intracellular Ca2+ sequestration in vascular smooth muscle cells resulting in vasorelaxation.
Hyperglycaemia not only promotes endothelial dysfunction but can also lead to vascular inflammation by encouraging the production of proinflammatory cytokines. Intracellular glucose is converted to the plasma membrane-associated secondary messenger diacylglycerol that activates protein kinase C. Subsequently, the post-transcriptional factor, NF-κB, is stimulated to promote the expression of proinflammatory genes.
Chronic hyperglycaemia also activates the polyol pathway, resulting in sorbitol and fructose accumulation which leads to the formation of advanced glycosylation end products. The nonenzymatic modification of plasma proteins causes a number of complications including: (1) alteration of enzymatic activity, (2) interference of receptor recognition, (3) generation of reactive species and proinflammatory cytokines. The resulting advanced glycosylation end product collagen cross-linking leads to increased vascular stiffness, systolic hypertension and reduced myocardial compliance 10.
Cardiac autonomic neuropathy
Cardiac autonomic neuropathy (CAN) is a microvascular complication that results in impaired cardiac autonomic function. It is a powerful predictor of cardiovascular mortality due to the increased risk of arrhythmias, silent myocardial infarctions (MIs) and sudden cardiorespiratory arrest. A meta-analysis of 15 studies of CAN, with follow-up mortality data, showed that the pooled estimated relative mortality risk was 3.45 [95% confidence interval (CI): 2.66–4.67, P<0.001] 11.
CAN in T1D is thought to occur due to a combination microvascular disease of the vasa nervorum and neuronal oxidative stress, leading to diminished autonomic nerve conduction 12. Sympathetic and parasympathetic involvement results in a sympathovagal imbalance. The subclinical phase manifests as decreased heart rate variability, whereas the early clinical phase of CAN is characterized by a resting tachycardia. In the advanced stage, there is loss of postural heart rate reflexes, hypertension, exercise intolerance, QT prolongation with arrhythmia, left ventricular dysfunction, reduced sympathetically-mediated coronary vasodilation and silent myocardial ischaemia.
Noninvasive assessment of CAN involves cardiovascular autonomic reflex tests. Parasympathetic dysfunction can be assessed by measuring the heart rate variability seen in deep breathing and postural changes. Sympathetic dysfunction can be tested by measuring the blood pressure (BP) response to a postural change and to isometric exercise such as sustained handgrip.
In a study of 195 diabetic patients, the prevalence of CAN was 62% in patients with T1D, compared with 39% with T2D. CAN was diagnosed (according to the CAN subcommittee of the Toronto Consensus Panel) as the presence of at least two abnormal cardiovascular autonomic reflex tests. The most significant predictors for the presence of CAN were age, diabetes duration, haemoglobin A1c (HbA1c), peripheral neuropathy and carotid intimal medial thickening on ultrasonography 13.
Diabetic autoimmune myocarditis
A recent study suggests that the post-MI period in diabetic patients may differ between T1D and T2D. The study used newly developed assays to investigate levels of cardiac autoantibodies post-MI in T1D, T2D and control patients. Following a mean duration of 4.5 years, cardiac autoantibody titres were found to be elevated in 15 (83%) of 18 T1D patients, whereas only 3 (15%) of 20 T2D patients and 3 (4%) of 78 healthy control participants. It has therefore been postulated that T1D patients are not only more likely to experience CVD, but are at risk of experiencing a post-MI inflammatory autoimmune myocarditis. The resulting MRI-detectable scarring and oedema may reduce left ventricular systolic function with corresponding prognostic implications 14. This provides a potentially exciting line of future investigation and management.
Risk assessment
By the time they are diagnosed, half of all patients with T2D will show signs of complications 15. T1D, in contrast, is diagnosed at a much earlier age, providing greater scope for risk factor modification in preventing recognized cardiovascular complications. This is particularly important as the life expectancy of patients with T1D is ever-increasing and diabetes is itself a major risk factor for CVD, the onset of which occurs 10–15 years earlier than in nondiabetic controls 16.
Risk prediction helps to identify patients with a high likelihood of future CVD events. In these patients, primary prevention measures prevention measures should be considered to modify the risk profile.
Risk prediction
Although there are specific scoring tools to estimate the 10-year risk of CVD in T2D patients such as the UK Prospective Diabetes Study risk engine 17, there have been a lack of corresponding validated risk prediction tools specific to T1D. The 2013 European Society of Cardiology (ESC) guidelines specifically recommend that risk assessment tools developed for the general population should not be used to assess cardiovascular risk in the diabetic population 18. Consequently, new risk prediction models specific to T1D patients have been developed.
A recent Danish study described a prediction model for estimating the 5- and 10-year cumulative risk of a CVD event (defined as CAD, CVD, heart failure or PVD) in T1D. Following observation of 4306 T1D patients over a 12-year period, the model was derived from a Poisson regression analysis of data collected on relevant risk factors. The final model includes 10 risk factors: age, sex, diabetic duration, systolic BP, LDL cholesterol, HbA1c, albuminuria, glomerular filtration rate, smoking and exercise. External validation of this model was performed using a different population of 2119 patients with T1D. The 5-year CVD event was found to be highly accurate, with a concordance statistic of 0.826 (95% CI: 0.769–0.839) 19. Fewer than 19% of the validation cohort were followed up for 10 years. Therefore, the validation was only performed for 5-year CVD risk prediction. Another limitation of this risk engine is that the model was based on patients of predominately Danish ancestry (>90%). It is not clear if this model will be as accurate in other countries with more diverse ethnicities.
Another risk prediction model for T1D is the Swedish T1D risk score 20. In this observational study, 3661 T1D patients between the ages of 30 and 65 years were assessed for 5 years between 2002 and 2007 to determine risk factors for CVD. The eight variables used to elaborate a risk equation for 5-year CVD risk were: diabetic duration, age of onset, log ratio total cholesterol : high density lipoprotein (HDL) cholesterol, log HbA1c, log systolic BP, smoking, macroalbuminaemia and previous CVD. The model produced a concordance statistic of 0.83, with a sensitivity and specificity of 72 and 77%, respectively. This model was validated on a separate cohort of 4484 patients giving a concordance statistic of 0.8 and a sensitivity and specificity of 62 and 77%, respectively. Similar to the Danish Steno risk engine, this model requires validation in different populations.
Although many risk prediction models are limited by small sample sizes, a risk prediction model has recently been devised using the Scottish National dataset, containing data on 26 680 adult T1D patients collected between 2004 and 2014. CVD event rates determined the most important risk factors to be: age, sex, HbA1c, estimated glomerular filtration rate, HDL, diabetes duration, smoking status, antihypertensive treatment and statin therapy. This risk prediction model could be used to calculate individual risk estimates for patients; however, it has not yet been independently validated.
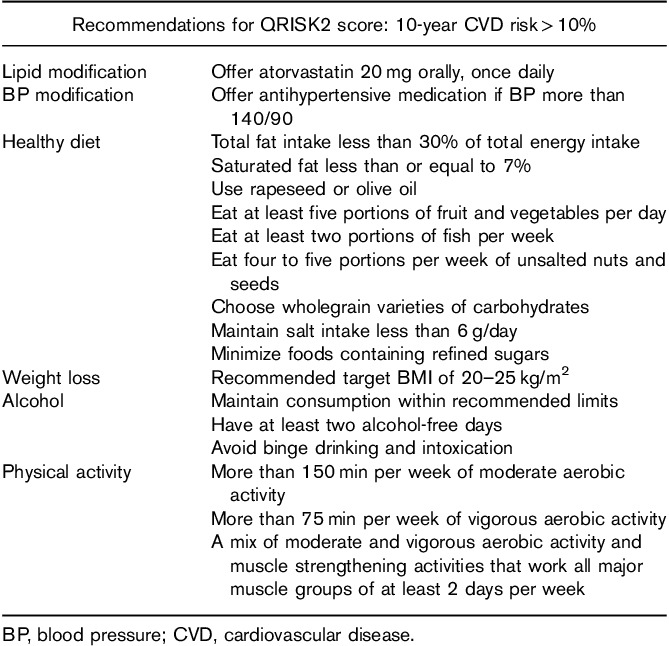
The most widely used CVD scoring system in the UK is the QRISK predictive algorithm, which estimates the 10-year risk of developing CVD. The second iteration of the algorithm (QRISK2) was updated in 2008 to include additional risk factors such as T2D. Since then, it has been updated annually and now includes T1D as a separate independent variable. The latest version (QRISK3) has recently been validated by the original developers of the QRISK score using Cox proportional hazards models in a large cohort study of over 7.8 million primary care patients 21. CVD was defined as angina, MIs, transient ischaemic attacks and strokes; it is important to note that PVD was not included as an end point. The mean predicted risks and observed risks matched closely. Given such a large sample size, the study is likely to be representative of the population. The latest National Institute of Clinical Excellence (NICE) guidelines advocate the use of QRISK2 for assessing CVD risk in all nondiabetic and T2D patients between the ages of 40–84 years. A score of greater than 10% over 10 years is considered high risk, with primary measures recommended (Table 1). However, when NICE published these guidelines in 2014, the QRISK2 score did not include T1D as a separate variable. Therefore, NICE recommend the use of any scoring tools in assessing CVD risk in T1D 22. Consequently, NICE recommends a personalized CVD risk assessment of patients with T1D by assessing each patient’s individual risk factor profile. A recent NICE surveillance report suggests that the next iteration of revised guidelines is likely to endorse the use of QRISK3 in T1D risk assessment 23. Our understanding of the modifiable risk factors in the context of T1D is outlined below.
Table 1.
National Institute of Clinical Excellence recommendations for QRISK score: 10-year cardiovascular disease risk more than 10% 22
Hypertension
Systemic hypertension is more common among patients with T1D than age-matched and sex-matched controls (43 vs. 15%, P<0.001) 24.
Hypertension has been shown to have a linear relationship with the risk of CAD and stroke. This is due to the deleterious effects of mechanical stress and sheer-related injury on the vasculature that increase endothelial dysfunction and promote plaque rupture, respectively. Chronic hypertension also increases left ventricular afterload resulting in hypertrophy. The resulting reduction in ventricular compliance causes diastolic heart failure.
Although there are no large randomized clinical trials comparing the outcomes of antihypertensive therapy in T1D patients, there is a general consensus that BP should be strictly controlled. There is a general consensus that BP in T1D should be strictly controlled. This largely comes from extrapolation of trials involving T2D, which showed that stricter BP control reduced macrovascular complications. However, the recommended target BP differs in various guidelines. The American Diabetes Association 2014 guidelines recommend a target BP of less than 140/80 mmHg, but a position statement in 2017 has suggested that patients with T1D and T2D should have a BP target of less than 140/90 mmHg. Furthermore, these guidelines recommend lifestyle modification for patients with a BP of more than 120/80 mmHg and initiating pharmacological therapy if the BP exceeds 140/90 mmHg. An angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) were recommended as first-line treatments. If the initial BP is at least 160/100 mmHg, initial pharmacological therapy with two antihypertensive agents is recommended 25.
NICE guidelines instead recommend a target BP of less than 135/85 mmHg (unless there is coexisting albuminuria or two or more features of metabolic syndrome, where the target is less than 130/80 mmHg) 21.
ESC guidelines from 2013 recommend a BP target of less than 140/85 in all diabetic patients. As these patients often have nocturnal hypertension, administration of antihypertensive agents should be considered at bedtime (ideally after evaluation of a 24-h ambulatory BP profile). ACE inhibitors or ARBs are recommended as first-line treatments 18.
The National Heart, Lung and Blood Institute and the American Diabetes Association (ADA) recommend BP assessment in each clinic visit and aiming for a target of less than 130/80 mmHg. Lifestyle modifications alone are recommended for patients with a BP greater than 120/80 mmHg while those greater than 140/80 mmHg are treated with ACE inhibitors or ARBs.
Hyperlipidaemia
Hyperlipidaemia is an important modifiable risk factor for the onset of CVD. Lipid metabolism is affected in T1D as insulin deficiency reduces the activity of lipoprotein lipase, resulting in elevated triglyceride levels and lower HDL-C. Triglyceride concentration is a particularly strong CVD predictor in diabetes 26. Patients who have well-controlled diabetes have serum lipids similar to the general population; however, it has been reported that even when absolute lipid levels are normal, the apolipoproteins of T1D patients are potentially more atherogenic as they are cholesteryl ester-enriched 27.
Although there is no trial data on the efficacy of statins in the younger population with T1D, a meta-analysis of 14 randomized trials investigated the effect of cholesterol lowering in 1466 T1D patients with a mean age of 55 years. After a mean follow-up of 4.3 years, there was a lower CVD rate ratio (RR) in the T1D group receiving cholesterol reduction therapy versus the control group (RR=0.79; 95% CI: 0.62–1.01) 28.
NICE guidelines recommend considering statin therapy in all adults with T1D, while statins should be routinely offered to any adult meeting any of the following criteria: (1) aged older than 40 years, (2) diabetic duration more than 10 years, (3) established nephropathy, (4) have other CVD risk factors. The recommended initial starting drug and dose is atorvastatin 20 mg. Patients with established CVD are recommended to be treated with atorvastatin 80 mg without the need for a formal risk assessment. NICE do not recommend routinely offering fibrates, nicotinic acid, bile acid sequestrants or omega-3 compounds in patients with T1D.
National Heart, Lung and Blood Institute and ADA guidelines recommend diet control and fasting lipid levels every 2 years in patients with low-risk lipid profiles, aiming for a LDL less than 2.6 mmol/l and non-HDL-C of less than 3.4 mmol/l. Patients with high-risk lipid profiles were recommended to be commenced on statin therapy with a target LDL less than 2.6 mmol/l for primary prevention and LDL less than 1.8 mmol/l for secondary prevention.
Smoking
Smoking is a well-recognized major risk factor for all types of CVD by causing chronic vascular inflammation and endothelial dysfunction. The adverse effects of smoking are particularly notable in PVD and CAD. A comparison of international registries has demonstrated a varying prevalence of smoking in T1D patients in different countries. The prevalence of smoking was found to be 10% in the USA T1D Exchange Registry and 24% in the Prospective Diabetes Follow-up Registry in Germany. This likely reflects differences in antismoking public health policy. The majority of T1D smokers were under the age of 50 and had a male preponderance across all ages (12 vs. 9% T1D Exchange Registry; 28 vs. 20% Prospective Diabetes Follow-up Registry). Smokers were more likely to have an elevated HbA1c compared with non-smokers [8.5 vs. 7.9% (70 vs. 62 mmol/mol); P<0.001] and an adverse lipid profile with elevated triglycerides (1.62 vs. 1.35 mmol/l; P<0.0001) after adjusting for age, sex, diabetic duration, ethnicity and lipid lowering medication 29.
Glycaemic control
Long-term glycaemic control is an important predictor of CVD events. Even after adjusting for age and other traditional risk factors, HbA1c correlates closely with risk of CVD. The 10-year DCCT trial demonstrated that glycaemic control was the most significant modifiable univariate risk factor for CVD 30. Interestingly, the extent of atherosclerotic plaques determined by intravascular ultrasound has been shown to correlate closely with mean HbA1c collected prospectively over 18 years after adjustment for total cholesterol and age (P<0.05) 31. Glycaemic control has also shown to be an extremely important modifiable predictor of the development of CAN 32. NICE guidelines recommend measuring the HbA1c every 3–6 months with a target level of less than or equal to 48 mmol/mol (6.5%) to minimize the risk of long-term vascular complications 22. The ADA recommends monitoring the HbA1c every 3 months, with a target level of less than or equal to 7.0%.
Guidelines for addressing modifiable risk factors have been proposed by NICE (Table 1). Here, they suggest addressing risk factors if the 10-year QRISK2 score for CVD is greater than 10% with the aim of reducing risk to less than 10%.
Screening
Early aggressive CAD and silent presentation have made CVD the leading cause of death among patients with diabetes. compared with T2D, CVD in T1D is more likely to present insidiously and have a more significant index disease severity 4. Consequently, screening of CAD in asymptomatic T1D patients has been the subject of intense interest.
Effective screening relies on the screening population having a premorbid phase which is easily detectable by widely available investigations with a high degree of accuracy. CAD screening is problematic due to the difficulty in identifying and quantifying atherosclerotic plaque. A resting ECG is often normal despite the presence of severe CAD if there is no ischaemia at rest. Exercise ECGs have similarly been shown to be an unreliable predictor of CAD 33 due to a low sensitivity (70%) and specificity (75%) 34. Other investigations, such as stress echocardiography and myocardial perfusion imaging are not widely available, highly interpreter dependent and usually involve the injection of stressor and radionucleotide compounds, respectively. Invasive catheter coronary angiography is also unsuitable for population screening due to the significant resources and personnel required. Furthermore, the inherent procedural risks outweigh any potential benefits.
Computed tomography scanners are now widespread and offer a non-invasive detailed assessment of the coronary anatomy. In all symptomatic patients, NICE now recommend computed tomography coronary angiography (CTCA) as the first-line investigation 35. Consequently, recent studies have investigated whether CTCA screening of asymptomatic T1D patients is beneficial.
In the Factor-64 trial 36, 900 asymptomatic T1D and T2D patients with no prior CAD history and a disease duration of at least 3 years, were randomly assigned to CTCA screening or standard guidelines-based (non-screening) diabetic care. Patients found to have severe CAD were recommended to undergo invasive coronary angiography, while those with moderate disease recommended to have functional stress imaging assessment using adenosine or regadenoson. At a mean follow-up of 4 years, the primary outcome event rates (mortality, nonfatal MI or hospitalization for unstable angina) were not significantly different between CTCA (6.2%) and control groups (7.6%). This highlights that the identification and treatment of asymptomatic CAD does not significantly change the clinical outcome.
These findings correlate with data relating to non-diabetic patients in whom screening of CAD in asymptomatic individuals is not recommended 37. Consequently, ESC guidelines now rate the screening of a silent MI in an asymptomatic high-risk diabetic patient as a class IIb indication with level of evidence rating of ‘C’ (indicating that the benefits are likely to outweigh the risks but there is low quality of evidence to support the implementation). Nevertheless, it is worth noting that a negative CTCA has been demonstrated to be an excellent predictor of good prognosis in asymptomatic diabetic patients. In a prospective screening study of 525 patients between 2005 and 2013, the event rate (all-cause mortality, nonfatal MI or late revascularization) with a normal CTCA was found to be just 3% 38.
The presence of CAD on computed tomography has also been shown to increase the likelihood of certain microvascular complications, suggesting a common pathogenesis. In a recent Canadian study, 69 long-standing T1D patients underwent coronary artery calcification scoring by CTCA and the incidence of microvascular complications assessed. Patients with high coronary calcium scores (≥300 Agatston Units of coronary calcium) were found to have an increased incidence of microvascular complications such as large-nerve fibre neuropathy and retinopathy when compared with age-matched and sex-matched comparator group of 73 patients without diabetes who underwent the same investigations 39.
Conclusion
CVD is the principal cause of death in T1D adults with disease duration over 20 years. Diabetic vasculopathy is driven by hyperglycaemia-mediated endothelial dysfunction and vascular inflammation, which lead to the development of atherosclerosis and autonomic dysfunction.
Among the subtypes of CVD, CAD predominates and the prognosis in T1D is more unfavourable than in T2D due to an increased likelihood of involvement of all three coronary territories with severe and often silent disease. This is due to a more adverse risk profile (greater diabetic duration and increased likelihood of hypertension) together with an increased propensity of developing cardiac autonomic neuropathy.
Routine screening of asymptomatic patients is currently not recommended, as this does not alter clinical outcomes and the first line UK CAD screening test (CTCA) involves radiation exposure. Current guidelines do not differentiate the screening or treatment of diabetic patients from their nondiabetic counterparts, despite the added risk conferred by the diabetic status.
In the future, T1D risk prediction tools may offer a valuable insight on those who are found to be particularly high risk. Once identified, these patients may warrant further clinical work up in specialist Cardiology clinics to precisely determine the best clinical course. New prediction models based on T1D populations, have recently been developed using Danish (Steno type 1 risk engine), Swedish and Scottish registries. Although potentially valuable in aiding risk stratification, these risk engines are limited by relatively small sample sizes, limited population diversity and a lack of external validation, respectively. The NICE QRISK calculator overcomes these challenges; however, until recently, has not been recommended in the cardiovascular assessment of the T1D patients. Updated guidelines are likely to recommend use of the new QRISK3 calculator in T1D.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Morimoto A, Onda Y, Nishimura R, Sano H, Utsunomiya K, Tajima N, et al. Cause-specific mortality trends in a nationwide population-based cohort of childhood-onset type 1 diabetes in Japan during 35 years of follow-up: the DERI Mortality Study. Diabetologia 2013; 56:2171–2175. [DOI] [PubMed] [Google Scholar]
- 2.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001; 44 (Suppl 2):14–21. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen ME, Almdal TP, Carstensen B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia 2013; 56:2401–2404. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute–National Institute of Diabetes and Digestive and Kidney Diseases Working Group on cardiovascular complications of type 1 diabetes mellitus. Circulation 2005; 111:3489–3493. [DOI] [PubMed] [Google Scholar]
- 5.Bohlen HG, Lash JM. Topical hyperglycemia rapidly suppresses EDRF-mediated vasodilation of normal rat arterioles. Am J Physiol 1993; 265:219–225. [DOI] [PubMed] [Google Scholar]
- 6.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999; 34:146–154. [DOI] [PubMed] [Google Scholar]
- 7.Besic H, Jeraj L, Spirkoska A, Jezovnik MK, Poredoš P. Deterioration of endothelial function of micro- and macrocirculation in patients with diabetes type 1 and 2. Int Angiol 2017; 36:354–361. [DOI] [PubMed] [Google Scholar]
- 8.Heier M, Espeland CN, Brunborg C, Seljeflot I, Margeirsdottir HD, Hanssen KF, et al. Preserved endothelial function in young adults with type 1 diabetes. PLoS ONE 2018; 13:0206523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl) 2017; 95:1153–1165. [DOI] [PubMed] [Google Scholar]
- 10.Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens 2001; 14:475–486. [DOI] [PubMed] [Google Scholar]
- 11.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003; 26:1895–1901. [DOI] [PubMed] [Google Scholar]
- 12.Fisher VL, Tahrani AA. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes Metab Syndr Obes 2017; 10:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moţăţăianu A, Maier S, Bajko Z, Voidazan S, Bălaşa R, Stoian A. Cardiac autonomic neuropathy in type 1 and type 2 diabetes patients. BMC Neurol 2018; 18:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipes MA, Galderisi A. Cardiac autoimmunity as a novel biomarker, mediator, and therapeutic target of heart disease in type 1 diabetes. Curr Diab Rep 2015; 15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.[No authors listed]. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 1991; 34:877–890. [PubMed] [Google Scholar]
- 16.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006; 29:798–804. [DOI] [PubMed] [Google Scholar]
- 17.Stevens RJ, Kothari V, Adler AI, Stratton IM. United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001; 101:671–679. [PubMed] [Google Scholar]
- 18.Task Force Members, Rydén L, Grant PJ, Anker SD, Bern C, Cosentino F, Danchin N, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013; 34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 19.Vistisen D, Andersen GS, Hansen CS, Hulman A, Henriksen JE, Bech-Nielsen H, et al. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno Type 1 Risk Engine. Circulation 2016; 133:1058–1066. [DOI] [PubMed] [Google Scholar]
- 20.Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Gudbjörnsdottir S, Swedish National Diabetes R. A new model for 5-year risk of cardiovascular disease in Type 1 diabetes; from the Swedish National Diabetes Register (NDR). Diabet Med 2011; 28:1213–1220. [DOI] [PubMed] [Google Scholar]
- 21.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ Clin Res 2017; 357:2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Clinical Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification. NICE clinical guideline CG181. 2014. Available at: https://www.nice.org.uk/guidance/cg181 [Accessed 6 December 2018]. [PubMed]
- 23.National Institute of Clinical Excellence (NICE). Surveillance report 2018 – Cardiovascular disease: risk assessment and reduction, including lipid modification. NICE guideline CG181. 2014. Available at: https://www.nice.org.uk/guidance/cg181/resources/surveillance-report-2018-cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-2014-nice-guideline-cg181-pdf-6123288665797 [Accessed 6 December 2018]. [PubMed]
- 24.Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care 2005; 28:301–306. [DOI] [PubMed] [Google Scholar]
- 25.De Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40:1273–1284. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal M, Schinske A, Pop-Busui R. Lipids and lipid management in diabetes. Best Pract Res Clin Endocrinol Metab 2014; 28:325–338. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs MJ, Kleisli T, Pio JR, Malik S, L’Italien GJ, Chen RS, et al. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract 2005; 70:263–269. [DOI] [PubMed] [Google Scholar]
- 28.Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125. [DOI] [PubMed] [Google Scholar]
- 29.Hofer SE, Miller K, Hermann JM, deSalvo DJ, Riedl M, Hirsch IB, et al. International comparison of smoking and metabolic control in patients with type 1 diabetes. Diabetes Care 2016; 39:177–178. [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. Eur Heart J 2013; 353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes 2002; 51:2637–2641. [DOI] [PubMed] [Google Scholar]
- 32.[No authors listed]. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998; 41:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiner D, Ryan T, McCabe C, Kennedy JW, Schloss M, Tristani F, et al. Exercise stress testing. Correlations among history of angina, ST-segment response and prevalence of coronary-artery disease in the Coronary Artery Surgery Study (CASS). N Engl J Med 1979; 301:230–235. [DOI] [PubMed] [Google Scholar]
- 34.Gianrosi R, Detrano R, Mulvihill D, Lehmann K, Dubach P, Colombo A, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation 1989; 80:87–98. [DOI] [PubMed] [Google Scholar]
- 35.National Institute of Clinical Excellence (NICE). Chest pain of recent onset: assessment and diagnosis. NICE clinical guideline CG95. 2016. Available at: https://www.nice.org.uk/guidance/cg95 [Accessed 6 December 2018]. [PubMed]
- 36.Muhlestein JB, Lappé DL, Lima JA, Rosen BD, May HT, Knight S, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014; 312:2234–2243. [DOI] [PubMed] [Google Scholar]
- 37.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 38.Van den Hoogen IJ, de Graaf MA, Roos CJ, Leen AC, Kharagjitsingh AV, Wolterbeek R, et al. Prognostic value of coronary computed tomography angiography in diabetic patients without chest pain syndrome. J Nucl Cardiol 2016; 23:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovshin JA, Bjornstad P, Lovblom LE, Bai JW, Lytvyn Y, Boulet G, et al. Atherosclerosis and microvascular complications: results from the canadian study of longevity in type 1 diabetes. Diabetes Care 2018; 41:2570–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]


