Abstract
Background
Fumaria species (Fumariacea) has traditionally been used in wound healing in Iranian folk medicine. However, with the discovery of newer agents, its use has faded off into total obscurity. This study explored the wound healing potential of a gel containing 10% Fumaria vaillantii Loisel through topical application of total extract in a model of excisional as well as incisional wound healing in albino Wistar rats.
Methods
Rats were anesthetized, and excisional skin wound was established using a sterilized surgical scissors. The animals were then treated with 10% F.vaillantii topical gel formulation along with the gel base. The treatments were administered once a day after the injury for 21 days. For topical treatment, the hydrogel was formulated and evaluated for chemical and physical characteristics. Histopathological analysis with hematoxylin and eosin (H&E) was used for microscopic examination of the skin tissues on 21-day-old sections of excision wound. To verify collagen formation, hydroxyproline determination was performed 21 days post wound healing. Breaking strength was determined in a 10-day-old incision wound by the uniaxial tensile test.
Results
Topical administration of F.vaillantii gel formulation significantly enhanced skin wound closure on the 6th post-wounding day compared to both gel base and the negative control, indicating an accelerated wound healing process, while a significant difference was observed on 10th and 14th post –wound days in F.vaillantii treatment compared to the negative control groups. Gel formulation prepared with a 10% F. vaillantii extract exhibited a response in terms of wound epithelialization, angiogenesis and number of hair follicles at wound area better than the gel base on the 21st post-wound day. Application of gel base produced further advantages by increasing hydroxyproline content and collagen fiber thickness. Our results on incision wound model were supported by histopathological data indicating the role of gel base in the enhancement of breaking strength.
Conclusion
Traditional use of Fumaria species in the skin diseases was justified in this study by revealing the increase in wound healing activity after hydrogel containing F. vaillantii total extract administration.
Graphical abstract
Electronic supplementary material
The online version of this article (10.1186/s12906-019-2645-y) contains supplementary material, which is available to authorized users.
Keywords: Wound healing, Fumaria vaillantii, Topical hydrogel, Excision, Incision
Background
Wounds, which happen due to physical and chemical injuries or microbial infections, are inevitable events in life. The aim of wound healing is to bring back the structure and function of the injured tissues to the nearly pre-wound conditions. In other words, healing is an intricate body’s natural process to regenerate the integrity of damaged tissue [1–3], thus the collaborative efforts of many different tissues and cell lineages are vital for healing. Several stages are involved in a wound healing process including inflammatory, proliferation and finally remodeling phases [2, 4]. Research on drugs capable of managing each step would be of great interest in modern biomedical sciences [1]. Interestingly, up to 80% of the world’s population use herbal medicines in the treatment of various skin disorders, and known drugs obtained from plant sources have proved to enhance the healing of different wounds [1, 5]. In Iranian folklore, medicinal plants are used to heal skin wounds [6]; however, the potential of many traditional herbal extracts in wound healing still remains unexplored.
Fumaria genus belongs to Fumariaceae family and comprises 60 species of herbaceous flowering plants with a worldwide distribution, particularly in Asia [7]. Fumaria vaillantii Loisel. is one the seven species grown in different parts of Iran with the local name of “Shatareh” [8]. Aerial parts of F.vaillantii as an infusion have widely used by the traditional healers in the treatment of psoriasis, jaundice and fever [8]. Interestingly, the ancient Iranians used Fumaria spp. as a topical formulation, traditionally named “Zemad “or “Marham” to heal skin disorders [9, 10]. Most notably, native people of Jandagh, located in the central part of Iran, empirically used Fumaria vaillantii L. for the healing of skin wounds [6]. A vast majority of pharmacological properties including antioxidant, anti-inflammatory, anti-fungal and anti-bacterial have also been reported for this plant, which was attributed to the presence of diverse secondary metabolites [11–15]. Among a large number of compounds present in this species, alkaloids and flavonoids are two important classes [16–18]. With inflammation as an important stage involved in wound healing, Fumaria vaillantii possessing anti-inflammatory as well as antioxidant properties, due to the presence of metabolites, seems to be effective in the primary stages of wound repair [11, 19]. To the best of our knowledge, only a single study has been performed on the wound healing potential of F. indica extract [19] . To further our knowledge, the present study was undertaken to assay the wound healing property of F.vaillantii gel formulation in both an excision and an incision wound models in rats.
Recently, wound dressing has taken a large attraction due to creating a moist environment to support faster wound healing [20]. Amongst the existing dressings, hydrogels are reported to be optimal for use in all steps of wound healing as a drug delivery system [21]. In the present work, we aimed to formulate a hydrogel for topical administration of F.vaillantii extract, which is suitable for dermatological application including wound healing. To the best of our knowledge, this is a first report on the therapeutic potential of hydrogel formulation composed of F.vaillantti extract as a wound healing promoter in an in vivo model.
Methods
Plant material and extraction procedure
F.vaillantii plant was collected from North of Iran in August 2014. A voucher specimen (No. 6563 TEH) was deposited at the Herbarium of Faculty of Pharmacy at Tehran University of Medical Sciences and authenticated by Dr. Gholamreza Amin. Following separating the aerial parts of the plant, they were dried in the dark for 3 days. Total extract was prepared by thoroughly mixing 320 g of dried powder with ethanol: water (80:20) three times at room temperature for 72 h via maceration procedure. The extracts were evaporated to dryness and then kept at 4 °C.
Gel formulation
Weighed quantities of different thickening agents including HPMC (Hydroxypropyl methylcellulose) 4000 cP, HPMC 15 cP and Carbomer 940, which were kind donations of Hakim Pharmaceutical Company, were separately added to distilled water and allowed to soak for 24 h. The hydroalcoholic extract of Fumaria vaillantii (10%) was solubilized in propylene glycol (Sepidaj, Iran). The latter solution was transferred to the aqueous dispersion of each thickening agents, individually at different concentrations. The mixtures were then stirred gradually to find the best formulation. Triethanolamine was added to neutralize carbomer solution.
Evaluation of the gel
pH measurement
In order to determine the pH of the gel, the glass electrode was completely dipped into a solution containing 10% of the hydrogel in deionized water.
Rheology of the gel
Viscosity was determined at 25 °C using a Brookfield digital viscometer-RVDV-III (Brookfield, Massachusetts, USA) and spindle no. 52 at different rpm.
Centrifugation test
Hydrogels were centrifuged at 6000 rpm for 30 min and then examined for phase separation.
Animals
In our study, male albino Wistar rats weighing 220–250 g were purchased from the National Animal Center (Pasteur Institute of Karaj) and maintained in a 12/12-h light–dark cycle, with food and water supplied ad libitum. Animals were treated in accordance with the guidelines approved by the animal ethics committee of Pasteur Institute of Iran (IR.PII.REC.1397.027, 2019-01-09). Animals were divided into three groups (n = 5). Group I: Received administration of 10% F.vaillantii hydrogel formulation. Group II: Received topical application of gel base. Group III: Served as the negative control. The animals were euthanized with intra-peritoneal injection of sodium pentobarbital at 60 mg/kg on the 10th and 21st days after wounding.
Wound healing activity assessment
Excision and incision models were used to investigate the wound healing activity of gel formulation of F.vaillantii total extract.
Excision wound model
Rats were anesthetized using ketamine + xylazine and the hair on the back was clipped with electric clippers. Cutaneous square wounds of 225 mm2 width with 2 mm depth were inflicted on the depilated ethanol-sterilized dorsal thoracic region of rats with the help of sterilized surgical scissors under a semi-aseptic condition. The F.vaillantii hydrogel formulation along with the gel base was topically applied once a day for 21 days. Wound photos were taken and the wound area was measured in mm2 by putting a transparent sheet over it. Upon tracing the wound margin by a permanent marker, the wound area was recorded using graph paper and Image J software on 2nd, 4th, 6th, 8th, 10th, 12th, 14th 16th,18th and 21st post wound days to monitor the percentage of wound closure. The percentage of wound contraction was calculated using the formula [22]: (Initial wound size – specific day wound size)/ Initial wound size × 100.
The healed wound along with the surrounding skin obtained on day 21 were excised and then dissected into two equal parts to be further examined by histopathological analysis and hydroxyproline level determination method.
Histopathological evaluation
The healing tissues obtained from all the three groups of animals in our excision wound model were processed for histopathological analysis. Following fixation of the tissue samples in 10% formalin, samples were dehydrated using graded alcohol series. Afterwards, skin samples were cleared in xylene and then embedded in paraffin wax. Serial sections of 5 μm were prepared and stained with hematoxylin and eosin (H&E) for routine histopathological evaluation. All slides were investigated in a blinded manner by a pathologist.
Hydroxyproline determination
Hydroxyproline contents were spectrophotometrically measured by Woessner’s method [23]. In brief, after the weighed tissue samples were hydrolyzed in 6 N HCl for 18 h at 115 °C, the residue was evaporated to dryness and remixed with a known volume of water. Following incubation of one ml of the sample with 0.5 ml of 0.05 M chloramine T solution (Sigma-Aldrich, USA) for 20 min at room temperature, 1 ml of Erlich’s solution was added and further incubated for 15 min at 60 °C. Absorbance was measured at 550 nm using a spectrophotometer (Cecil Company, UK). Hydroxyproline level was calculated from a linear standard curve and presented as μg/100 mg of dry content.
Incision wound model
Rats were anesthetized with ketamine + xylazine and the dorsal fur of the animals was shaved whit an electric clipper to make the incision wound. Incision of 3 cm was made at least 2 cm lateral to the vertebral column and parallel to it by a sharp scalpel with sufficient care. Afterwards, the incision was closed with surgical sutures at intervals of 1 cm. The hydrogel containing 10% of Fumaria vaillantii L. total extract and the gel base were topically applied once a day, starting from the initial day for 10 days. Sutures were removed on the 8th day and breaking strength of the healed wound and normal skin were measured using uniaxial tensile test (Model Z 2.5, Zwick GmbH & Co, Ulm-Einsingen, Germany) [24] on the 10th day.
Confirmation of quercetin in total extract by HPLC
Quercetin content was evaluated using Waters LC 600 model chromatograph (Waters, Massachussets, USA) coupled with UV detector. Separation was performed by isocratic elution at a flow rate of 1 ml/min on Acclaim™ 120 C18 analytical column (150 mm × 4.6 mm; 5 μm). Mobile phase was a mixture of 2% acetic acid in water and acetonitrile in a 2:1 ratio and the sample injection volume was 20 μl. Stock solution of total extract of F.vaillantii was prepared (10 mg/ 10 ml) in ethanol and then passed through a 0.45 μm membrane filter. A 370 nm wavelength was applied for analysis. Peak areas were integrated automatically by Waters Software.
Statistical analysis
The data are expressed as mean ± SEM of at least triplicate determinations, and comparisons were based on ANOVA followed by Tukey’s post test using a GraphPad Prism 6.0 Software. A value of p < 0.05 was considered as statistically significant.
Results
Formulation and physical evaluation of the hydrogels
Among different gelling agents used, only HPMC 4000 cP (2.5%) formed a viscous mixture and the others could not increase viscosity of the formulation. Visual examination showed that the prepared F.vaillantii hydrogel was stable after being subjected to centrifugation.
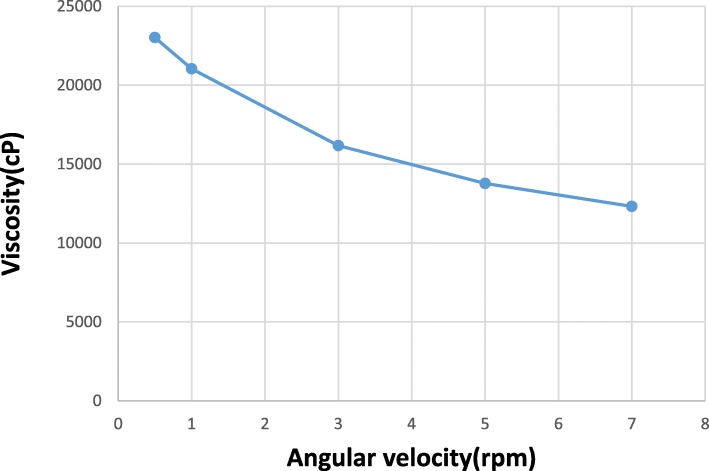
Rheology is an important parameter as it affects the spreadability of the topical formulations on the skin surface. Rheological behavior of the hydrogel formulation containing HPMC 4000 cP with neutral pH of 6.4 exhibited a desirable non-Newtonian shear thinning pseudo plastic type of flow, i.e. viscosity decreases at increasing angular velocity Fig. 1.
Fig. 1.
Rheological behavior of hydrogel formulation of F.vaillantii total extract using HPMC 4000 cP (2.5%)
The stability studies indicated no color fading for F.vaillantii prepared hydrogel 6 months after development. The pH of the hydrogel formulation was found to be within the range of 5.8–6.4, which lies in the normal pH range of the skin [25]. The rheology of the hydrogel was found to be the same after 6 months of storage at room temperature.
Wound healing activity
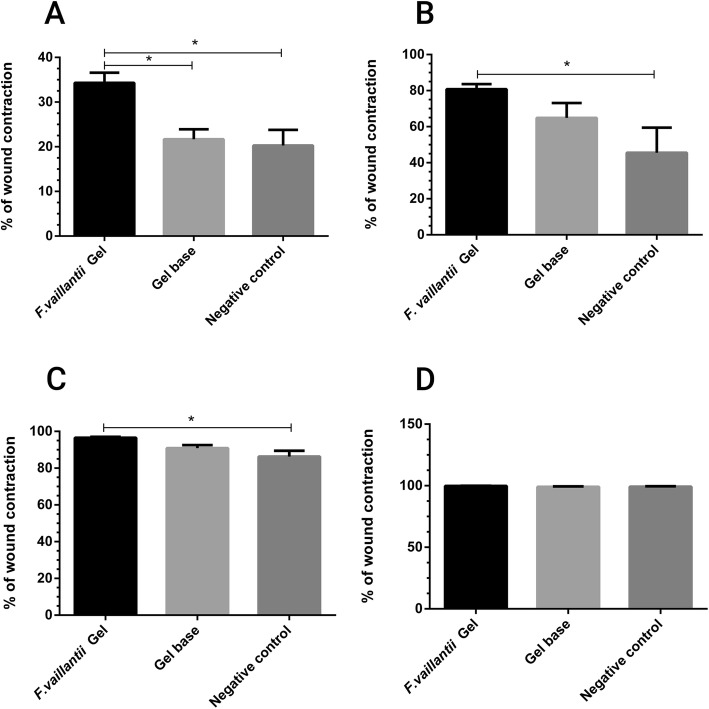
Wound contraction indicates the rate of increase of healed area. In other words, the faster the wound closure, the more effective would be the medication [26]. To study the rate of wound contraction and subsequent epithelialization, an excision wound model was established. The mean wound area was calculated in the control group as well as the treated groups as indices of wound closure within 21 days. Application of F. vaillantii gel formulation exhibited a considerable potential in wound healing activity. The macroscopic alterations of the wound area within 21 days are displayed in Fig. 2. On the day 6, F.vaillantii -treated group showed the highest percentage of wound contraction (35%) compared to both negative group (20%) and the group treated with gel base (20%) (Fig. 3). Furthermore, a significant difference (p < 0.5) was observed between F. vaiilantii gel-treated animals and the control on the days 10 and 14, and the wound was found to be almost healed on day 21 in the treated groups with no scar. Interestingly, the percentage closure of wound area was considerable on the 14 and 21 post-wounding days in animals treated with gel base. Noteworthy is that the wound closure rate was much slower in the treated rats on days 14 and 21 when compared with the control rats (Fig. 3).
Fig. 2.
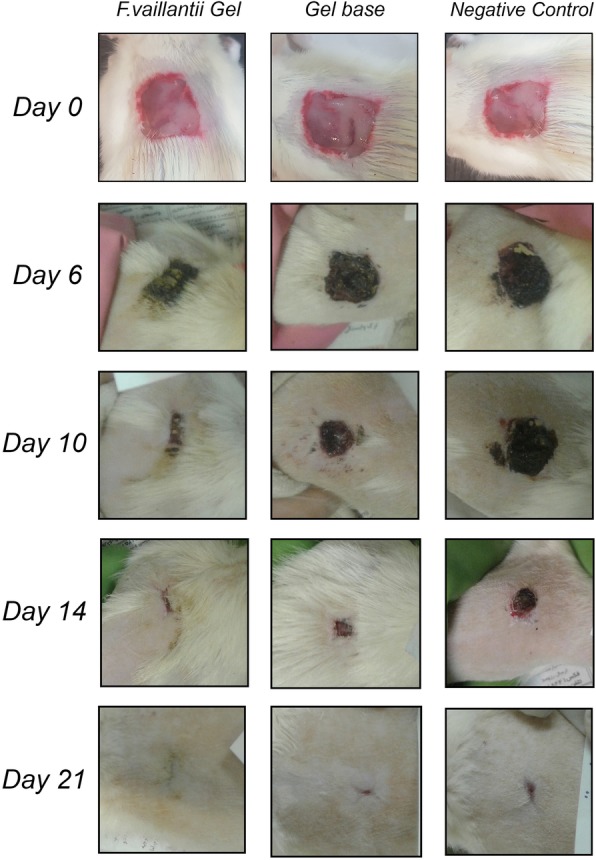
Photographs of the macroscopic observations of excision wound on days 6, 10, 14, 21. The rats were subjected to topical administration of F.vaillantii gel extract and gel base
Fig. 3.
Percentage of wound contraction in the excision wound model upon administration of F. vaillantii gel formulation on different days; a) Day 6, b) Day 10, c) Day 14 and d) Day 21. Values are expressed as mean ± SEM of 5 animals in each group. *p < 0.05 versus negative control (one-way ANOVA, followed by Tukey’s test)
To assess the breaking strength, the incision wound study was performed on the day 10 regenerated tissues. The results of the different parameters including maximum mechanical strength (fmax, N); stress (N/mm2); absorbed energy (area up to f max; N × mm); deformation (mm); stiffness (N/mm) of the uniaxial tensile test are reported in Table 1. According to this table,topical application of either F.vaillantii formulated gel or gel base led to an increase in tensile strength compared to the negative control group after 10 days, although none of the tensile strength in the treated groups differed significantly from that of the control group. It is worthy to note that the groups receiving F.vaillantii hydrogel or gel base exerted more or less the same effect on the breaking strength of healing tissue. Indeed, very little development of breaking strength was observed following inclusion of the F.vaillantii extract. The mean of breaking strength in the healthy skin was much higher than that of the other groups.
Table 1.
Stress (N/mm2), maximum mechanical strength (fmax,N), absorbed energy (area up to fmax(N × mm)), deformation (mm), stiffness(N/mm) 10 days post wound healing a
| Parameters | Stress (N/mm2) | fmax (N) | Area up to fmax(N × mm) | Deformation (mm) | Stiffness(N/mm) |
|---|---|---|---|---|---|
| Groups | |||||
| F.vaillantii Gel | 0.46 ± 0.06 | 19.08 ± 2.40 | 240.8 ± 30.63 | 22.49 ± 2.58 | 1.38 ± 0.15 |
| Gel base | 0.47 ± 0.05 | 19.99 ± 2.10 | 259.2 ± 53.09 | 23.34 ± 2.46 | 1.36 ± 0.10 |
| Negative control | 0.41 ± 0.08 | 18.03 ± 4.20 | 164.5 ± 68.26 | 14.52 ± 3.65 | 1.29 ± 0.14 |
| Normal skin | 0.77 ± 0.02* | 34.52 ± 3.83* | 600.3 ± 35.91**** | 30.49 ± 1.95** | 1.49 ± 0.15 |
aValues are mean ± SEM of 5 animals in each group. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0001 were considered significant compared to negative control group
Histopathological study
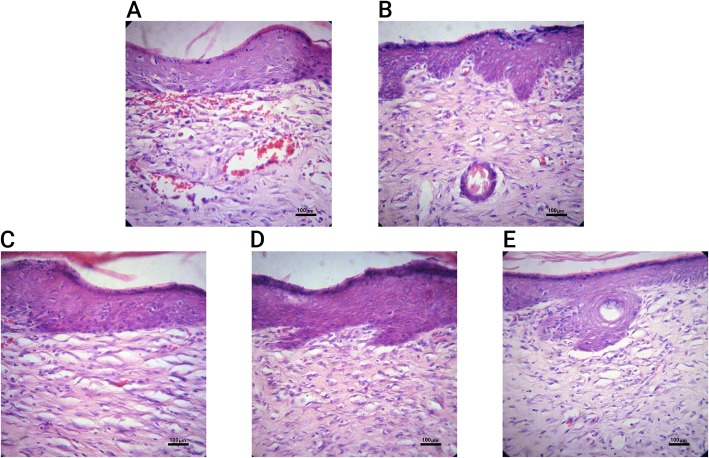
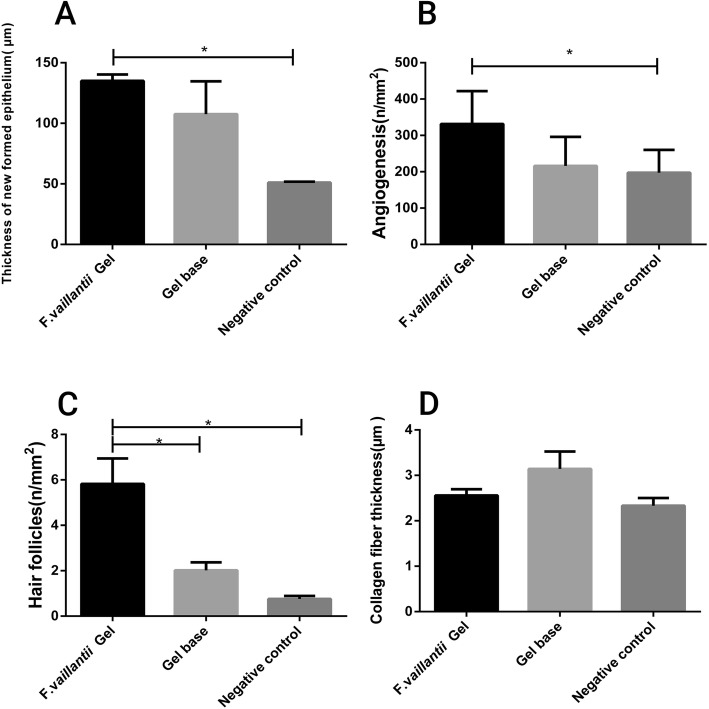
In our experiment on the untreated group (negative control), the thickness of the newly formed epithelium was significantly (p < 0.05) lower than that of the group submitted to the topical administration of F.vaillantii hydrogel (Fig. 4a and d). Following 21 days of surgery, collagen fibers, fibroblasts along with mature hair follicles were clearly established as shown in Fig. 4e. However, collagen fibrils were not well-organized and presented as sparse and irregular shapes in the negative control group (Fig. 4a). Inversely, in both gel base and F.vaillantii hydrogel groups, the dermis layer was regular with hair follicles and angiogenesis more than the control group (Figs. 4b, c, d and 5a, b, c). Nevertheless, the amount of collagen was almost the same in all experimental groups with a minute increase in the gel base treated group (Fig. 5d).
Fig. 4.
Microscopic photographs of histopathological evaluation of wound healing in the negative control, gel base and F.vaillantii gel extract administered. Skin sections display H& E stained in epidermis and dermis. The original magnification was 400×. a) Negative control: 21-old-wound tissue; b) Gel base group: 21-old-wound tissue treated with gel base; c),d),e) 21-old-wound tissue treated with F.vaillantii gel extract
Fig. 5.
Comparison of a) epithelium thickness; b) angiogenesis (n/mm 2); c) number of hair follicles and d) collagen fiber thickness values of F.vaillantii gel extract, gel base and negative control on excision wound model. Values are mean ± SEM of 5 animals in each group. *p < 0.05 was considered significant
Hydroxyproline content
Collagen, a component of growing cells, is synthesized in the healing tissues. Thus, to further confirm the synthesized collagen in the wound areas, hydroxyproline content was determined (Fig. 6). Our results exhibited no significant difference in collagen deposition between F.vaillantii hydrogel-treated and the negative control groups, although the group receiving no treatment possessed the lowest content of hydroxyproline. Interestingly, gel base administration resulted in an insignificant increase in the amount of collagen.
Fig. 6.
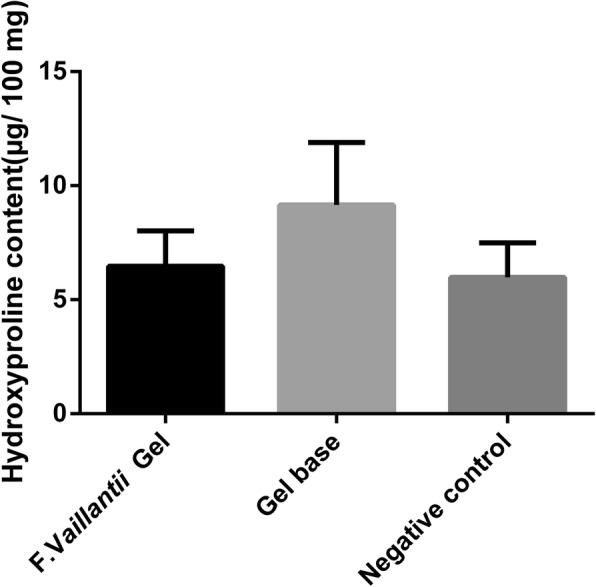
Hydroxyproline content (μg/100 mg skin) in different groups. Values represent mean ± SEM of 5 animals in each group
Quercetin content
HPLC chromatogram further acknowledged the presence of quercetin in the total extract of F.vaillantii (Additional file 1: Figure S1). The retention time (5.5 min) of the peak of quercetin was found in the total extract. The results demonstrated that the total extract of F.vaillantii contained 2.25% quercetin.
Discussion
Breakdown in normal anatomic structure and function of the skin, termed skin wound, which could occur through several causes including physical injuries, may lead to the opening and disruption of the skin [27]. Wound healing is a dynamic process in which dermal and epidermal tissues regenerate as closely as possible to the normal status. A sequence of events occurs to repair the damage following injury. These events have been classified into several stages including inflammatory, proliferative and remodeling [28]. Cytokines are usually released in the inflammatory stage due to the phagocytosis of bacterial pathogens leading to the migration of the cells involved in the proliferative stage. A subsequent chain of events including angiogenesis, collagen deposition, granulation, tissue formation, epithelialization and wound contraction take place at the proliferative stage [29]. In the final stage, collagen remodeling takes place along the tension lines [30]. Non-toxic, novel and cost-benefit therapeutic agents that contribute to increased healing rate, hastened epithelialization, inhibition of bacterial infection and supporting tissue remodeling have gained a great deal of attention among researchers worldwide. Therefore, we herein attempted to make a hydrogel formulation containing 10% F.vaillantii total extract and applied it on an animal model of skin wound to verify the hypothesis that this formulation could demonstrate a distinguished treatment in healing wounds by providing an enhanced tissue repair.
Our results revealed that the topical administration of our hydrogel formulation using HPMC 4000 cP (2.5%) on a rat excision wound model leads to a significant acceleration in wound healing after 6, 10 and 14 days, a finding confirmed by an increased wound contraction compared to the negative control group. This enhanced potential of wound healing may be due to anti-inflammatory, antimicrobial and astringent properties of the plant, which are well documented in the literatures [31]. In this regard, our literature survey identified several phytochemicals including flavonoids, alkaloids, tannins and saponins present in the F.vaillantii total extract [11], which may be responsible for its wound contraction and enhanced rate of epithelialization 21 days post wound healing. This assumption is supported by our previous study indicating a considerable amount of flavonoids in the F.vaillantii total extract [32]. As flavonoids including quercetin are known to possess antioxidant and anti-inflammatory effects [33, 34], and according to the HPLC results performed here, confirming the presence of 2.25% of quercetin in total extract of F.vaillantii, the wound healing activity of F.vaillantii extract may be attributed to this property in the inflammatory phase. Furthermore, the alkaloids present in F.vaillantii are also responsible for its antimicrobial property [7, 35], which in turn leads to a better wound healing in the inflammatory phase.
Hydrogels exhibit several advantages as they provide the required moist environment to the wound area and also act as a suitable carrier for topical administration of substrates. Moreover, they cause a slow release of substances over time. What this information brings out noticeably is that hydrogel formulation can be a suitable candidate to promote wound healing. Thus, we prepared a hydrogel formulation with 10% total extract of F.vaillantii using 2.5% HPMC 4000 cP displaying an optimum consistency and spreadability. Consequently, the proper hydrogel spreading would assist in the uniform administration of the gel to the skin. Additionally, based on our results, our formulated herbal gel contributed to a faster wound healing compared to the negative control group. Surprisingly, collagen fiber thickness and hydroxylproline content appeared to be more or less similar but basically higher in the gel base than F.vaillantii gel formulation -treated groups, which can necessarily be explained by the therapeutic effect of hydrogels alone. In addition, topical administration of F.vaillantii hydrogel was found to significantly increase the number of vessels as well as hair follicles in the gel-treated compared to the negative control wounds. Similar observations have also been reported by Xiao-bo Wu et al. (2012) who concluded that angiogenesis in granulation tissues results in improvement of circulation needed for supplying oxygen and nutrients vital for the healing process [36].
The establishment of an incision wound model needs to be further worked out in order to determine breaking strength, confirming the wound healing activity of 10% total extract hydrogel of F.vaillantii. In our study, control rats exhibited a wound breaking strength (area up to fmax) of 164.5 ± 68.26 N/mm on the 10th post wound day, whereas gel base and the hydrogel extract-treated groups displayed no significant breaking strength (259.2 ± 53.09 and 240.8 ± 30.63 N /mm), respectively. These data are in agreement with the results of our excision model and highlight the role of the gel base in collagen production, which leads to stabilization of fiber formation and subsequent stable intra- and inter- molecular crosslinks [37, 38].
Conclusion
The present study revealed that the hydrogel formulation containing F.vaillantii total extract (10%) promotes healing of epithelial wounds and enhances wound closure, number of hair follicles as well as angiogenesis at wound sites. This potency may be associated with the individual or synergistic effects of phytochemicals present in the total extract and provide evidence to some ethnomedicinal properties of F.vaillantii. The results of this study strongly encourage one to explore the efficiency of a hydrogel formulation containing a combination of several plant extracts in wound repair.
Additional file
Figure S1. Representative HPLC chromatogram of a) quercetin (5 μg/ml) b) total extract at UV detection λmax = 370 nm. (JPG 4060 kb)
Acknowledgments
The authors are thankful to the Hakim Pharmaceutical Company (Tehran, Iran) for providing some excipients and Mr. Majid Darabi for his contribution in HPLC analysis.
Abbreviation
- F. vaillantii
Fumaria vaillantii
- H&E
Hematoxylin and eosin
- HPLC
High pressure liquid chromatography
- HPMC
Hydroxypropyl methylcellulose
Authors’ contributions
FDR performed all experiments and wrote the manuscript. MA contributed in formulation and analysis. FHAT and SCH helped in animal experiments. GT and FM helped in analysis of incision and histopathology data. MS designed and supervised the study plan. All authors have read and approved the manuscript before submission.
Funding
This work is a part of Pharm.D. thesis, and authors are grateful to the Pasteur Institute of Iran for providing animals and materials for this study. The funding was also used to cover costs of histopathology analysis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Ethics approval and consent to participate
All animals used in the study were handled in accordance with the guidelines approved by the animal ethics committee of Pasteur Institute of Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Davoodi-Roodbordeii, Email: fatemehdavoodi.dr@gmail.com.
Minoo Afshar, Email: minoo_afshar@yahoo.com.
Fatemeh Haji Abas Tabrizi, Email: f.tabrizi65@gmail.com.
Samira Choopani, Email: samirachoopani@yahoo.com.
Giti Torkaman, Email: torkamg@modares.ac.ir.
Fariborz Moayer, Email: fariborz_moayer@yahoo.com.
Mona Salimi, Phone: 98-02164112264, Email: salimimona@pastuer.ac.ir.
References
- 1.Biswas TK, Mukherjee B. Plant medicines of Indian origin for wound healing activity: a review. Int J Low Extrem Wounds. 2003;2(1):25–39. doi: 10.1177/1534734603002001006. [DOI] [PubMed] [Google Scholar]
- 2.Evans P. The healing process at cellular level: a review. Physiotherapy. 1980;66(8):256–259. [PubMed] [Google Scholar]
- 3.Chah K, Eze C, Emuelosi C, Esimone C. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol. 2006;104(1):164–167. doi: 10.1016/j.jep.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 4.Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol. 2002;79(2):249–251. doi: 10.1016/S0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 5.Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol. 2002;83(3):193–199. doi: 10.1016/S0378-8741(02)00195-2. [DOI] [PubMed] [Google Scholar]
- 6.Bahmani M, Asadi-Samani M. A short look to the most important medicinal plants effective on wound healing. J Inj Inflamm. 2016;1(1):e07. [Google Scholar]
- 7.Ivanov I, Vrancheva R, Marchev A, Petkova N, Aneva I, Denev P, Georgiev VG, Pavlov A. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol App Sci. 2014;3(2):296–306. [Google Scholar]
- 8.Amiri MS, Joharchi MR, TaghavizadehYazdi ME. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad. Iran Iran J Pharm Res. 2014;13(1):157–162. [PMC free article] [PubMed] [Google Scholar]
- 9.Jahandideh M, Hajimehdipoor H, Mortazavi SA, Dehpour A, Hassanzadeh G. A wound healing formulation based on Iranian traditional medicine and its HPTLC fingerprint. Iran J Pharm Res. 2016;15:149–157. [PMC free article] [PubMed] [Google Scholar]
- 10.Zargari A. Medicinal plants. Iran: Tehran university press; 1998.
- 11.Srivastava S, Choudhary GP. Pharmacognostic and pharmacological study of Fumaria vaillantii Loisel: a review. J Pharmacogn Phytochem. 2014;3(1):194–197. [Google Scholar]
- 12.Rao C, Verma A, Gupta P, Vijayakumar M. Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharma. 2007;57(4):491–498. doi: 10.2478/v10007-007-0039-z. [DOI] [PubMed] [Google Scholar]
- 13.Raza SA, Rashid A, William J, Razzaq A. Evaluation of oxidative stability of sunflower oil at frying temperature in presence of butylated hydroxytoluene and methanolic extracts of medicinally important plants of Pakistan. Int Food Res J. 2014;21(1):331–334. [Google Scholar]
- 14.Jaberian H, Piri K, Nazari J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013;136(1):237–244. doi: 10.1016/j.foodchem.2012.07.084. [DOI] [PubMed] [Google Scholar]
- 15.Moghtader M. In vitro antifungal effects of Fumaria vaillantii Loisel. Essential oil on Aspergillus flavus. J Yeast Fungal Res. 2013;4(2):21–25. [Google Scholar]
- 16.Rajopadhye AA, Upadhye AS. Botanical and phytochemical standardization of Fumaria vaillantii Loisel. Indian J Nat Prod Resour. 2011;2:369–374. [Google Scholar]
- 17.Tripathi YC, Rathore M, Kumar H. On the variation of alkaloidal contents of Fumaria indica at different stages of life span. Anc Sci Life. 1994;13(3–4):271–273. [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta PC, Sharma N, Rao CV. A review on ethnobotany, phytochemistry and pharmacology of Fumaria indica (fumitory) Asian Pac J Trop Biomed. 2012;2(8):665–669. doi: 10.1016/S2221-1691(12)60117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garima P, Rajesh KG, Gupta Shyam S, ChV R. Wound repair and anti-inflammatory potential of Fumaria indica in excision wound-induced rats. Br J Pharm Res. 2014;4(2):257–266. doi: 10.9734/BJPR/2014/5387. [DOI] [Google Scholar]
- 20.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D. Wounds—what should a dressing formulary include. Hosp Pharm. 2002;9:261–266. [Google Scholar]
- 22.Ribeiro Barros Cardoso C, Aparecida Souza M, Amália Vieira Ferro E, Favoreto S, Deolina Oliveira Pena J. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12(2):235–243. doi: 10.1111/j.1067-1927.2004.012216.x. [DOI] [PubMed] [Google Scholar]
- 23.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93(2):440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 24.Asadi MR, Torkaman G, Hedayati M, Mofid M. Role of sensory and motor intensity of electrical stimulation on fibroblastic growth factor-2 expression, inflammation, vascularization, and mechanical strength of full-thickness wounds. J Rehabil Res Dev. 2013;50(4):489–498. doi: 10.1682/JRRD.2012.04.0074. [DOI] [PubMed] [Google Scholar]
- 25.Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–370. doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 26.Prasad V, Dorle AK. Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol. 2006;107(1):38–47. doi: 10.1016/j.jep.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493. doi: 10.1001/archderm.1994.01690040093015. [DOI] [PubMed] [Google Scholar]
- 28.Steidelman W, Digenis A, Tobin G. Impediments to wound healing. Am J Surg. 1998;176(2A Suppl):395–475. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 29.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Esimone CO, Nworu CS, Jackson CL. Cutaneous wound healing activity of a herbal ointment containing the leaf extract of Jatropha curcas L.(Euphorbiaceae) Int J Appl Res Nat Prod. 2008;1(4):1–4. [Google Scholar]
- 31.Bambal V, Wyawahare N, Turaskar A, Deshmukh T. Evaluation of wound healing activity of herbal gel containing the fruit extract of Coccinia indica wight and arn.(cucurbitaceae) Int J Pharm Pharm Sci. 2011;3(4):319–322. [Google Scholar]
- 32.Tabrizi FHA, Irian S, Amanzadeh A, Heidarnejad F, Gudarzi H, Salimi M. Anti-proliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Res Pharm Sci. 2016;11(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- 33.Özbilgin S, Acıkara ÖB, Akkol EK, Süntar I, Keleş H, İşcan GS. In vivo wound-healing activity of Euphorbia characias subsp. wulfenii: Isolation and quantification of quercetin glycosides as bioactive compounds. J Ethnopharmacol. 2018. [DOI] [PubMed]
- 34.Aoudia H, Oomah BD, Zaidi F, Zaidi-Yahiaoui R, Drover JCG, Harrison JE. Phenolics, antioxidant and anti-inflammatory activities of Melia azedarach extracts. Int J Appl Res Nat Prod. 2013;6(2):19–29. [Google Scholar]
- 35.Karou D, Savadogo A, Canini A, Yameogo S, Montesano C, Simpore J, Colizzi V, Traore AS. Antibacterial activity of alkaloids from Sida acuta. Afr J Biotechnol. 2006;5(2):195–200. [Google Scholar]
- 36.Wu X-b, Luo X-q, S-y G, Xu J-H. The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol. 2012;141(3):934–937. doi: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Rahman N, Rahman H, Haris M, Mahmood R. Wound healing potentials of Thevetia peruviana: antioxidants and inflammatory markers criteria. J Tradit Complement Med. 2017;7(4):519–525. doi: 10.1016/j.jtcme.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udupa AL, Kulkarni DR, Udupa SL. Effect of Tridax procumbens extracts on wound healing. Int J Pharmacogn. 1995;33(1):37–40. doi: 10.3109/13880209509088145. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative HPLC chromatogram of a) quercetin (5 μg/ml) b) total extract at UV detection λmax = 370 nm. (JPG 4060 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.