Abstract
Corneal decellularization represents a promising alternative source of human donor with global shortage. Multiple methods have been developed for the preparation of decellularized porcine corneal stroma. However, most strategies relied on long-time treatment to facilitate the entry of detergents or nucleases, which may cause irreversible ultrastructural damage. Here, we developed a rapid decellularization method for porcine corneal stroma through the combined mild detergent sodium N-lauroyl glutamate (SLG) and supernuclease. Compared with traditional methods, the novel decellularization method allowed the efficient removal of xenoantigen DNA within 3 h, while retaining the ultrastructure, transparency, and mechanical properties of porcine corneas. When transplanted in rabbit model for 1 month, the decellularized porcine corneal grafts presented favorable transparency and biocompatibility without immune rejection. Therefore, the combined use of detergent SLG and supernuclease may serve as a promising method for the clinical use of decellularized porcine cornea.
Keywords: Porcine cornea, decellularization, sodium N-lauroyl glutamate, supernuclease, xenotransplantation
Introduction
Cornea is the outermost transparent tissue covering the eye, which makes it vulnerable to trauma or infection, thereby causing irreversible transparency loss and even blindness. Corneal blindness affects millions of patients worldwide and has risen as a global health challenge.1 As the most common transplant, corneal transplantation is apparently the first choice for the treatment of corneal blindness.2 Corneal stroma accounts for 90% of the corneal thickness, and several equivalents, such as the polymethacrylates synthetic keratoprosthesis, collagen biosynthetic implants, and cell-based replacements, have been developed for the substitution of diseased stroma.3 However, these substitutes have been reported to induce serious complications, suture intolerance, or unsatisfactory recovery of visual acuity.4,5 Therefore, decellularized xenogenic cornea represents a promising alternative source of human donor.
Decellularization aims to eliminate the immune barrier of xenografts by removing xenogenic cells and antigens, meanwhile saving the native extracellular matrix (ECM) as tissue scaffold.6,7 By now, multiple acellular ECM has been approved for clinical applications by replacing the damaged tissue or organs when homeotransplantation is not available.8–11 For cornea, multiple methods have been developed for the preparation of decellularized porcine corneas (DPCs)12–22; however, one common shortcoming is about the long elution time for the removal of xenogenic components, thereafter causing corneal over-swelling and declining, or even losing corneal transparency. Consistently, unsatisfactory corneal transparency and vision restoration have been observed in the clinical trials after the transplantation of traditional DPCs.23
Enzymes such as nuclease, trypsin, and dispase have been commonly used for decellularization, on account of their function in cleaving nucleic acid sequences and therefore aiding in the removal of nucleotides after cell lysis in tissues.24–26 However, the decellularization efficiency by using enzymes alone is not satisfactory for clinical needs, as it is difficult to remove cellular residues and themselves from the heterologous tissue, which may impede recellularization or evoke adverse immune responses,27–30 and the long-standing processing time could disrupt ECM ultrastructure, resulting in the loss of needed ECM constituents, such as collagen, laminin, fibronectin, elastin, and glycosaminoglycan (GAG).31 As a genetically engineered endonuclease, benzonase has been used for decellularizing dermis and corneas.32,33 Benzonase can quickly infiltrate into corneal stroma, efficiently degrade all forms of DNA and RNA without proteolytic activity, and also easily be removed by repeated washing.34 Supernuclease is a homologous nuclease of benzonase. Currently, there are no reports on the application of supernuclease in the field of decellularization, but it is a potential reagent to process a functional decellularization effect as benzonase does.
Detergents like sodium dodecyl sulfate (SDS) and Triton X-100 were commonly used in traditional decellularization methods. SDS can effectively remove nuclear remnants and cytoplasmic proteins from dense tissues such as dermis, kidney, and lung, but usually accompanies with ultrastructural damage and biological components loss, such as GAG, growth factors, and collagen.35–37 Triton X-100 can effectively remove cell residues from thicker tissues such as valve conduits, although less effective than SDS, but tissue damage is still unavoidable.38,39 With commendable properties, like mild, strong decontamination, and good compatibility, amino acid-based surfactants such as sodium N-lauroyl glutamate (SLG) and sodium N-lauroyl sarcosinate (SLS) have been widely used in daily supplies like skin care products, shower gels, and detergents.40,41 They may also have potential effects like solubilizing cell membranes, dissociating DNA from proteins, and therefore effectively removing cellular residues from tissue.42,43 Thereby, as a substitute for traditional methods, the combined use of supernuclease and amino acid surfactant might be a potential solution for optimizing the traditional decellularization methods.
In this present study, we aimed to develop a better decellularization method by using SLG and supernuclease, in order to overcome the structural and functional impairment of DPCs processed by traditional decellularization methods. For the recommendation of an optimum method, we then tested the key properties of DPCs decellularized by our and another two traditional methods, and examined the postoperative symptoms of rabbit corneas after the implementation of lamellar keratoplasty (LKP) with DPCs.
Materials and methods
Animals
Fresh porcine eyes (4- to 6-month-old Duroc swine, Yingzhou farm, Guangzhou, China) were obtained within 2 h post-mortem and washed with phosphate-buffered saline (PBS) containing 1 mg/mL tobramycin. Following the mechanical removal of epithelium, the corneas were excised and cut into lamellas with thickness of 500 μm (for in vitro evaluation) or 300 μm (for animal experiments) and diameter of 10 mm using a microkeratome (Evolution3E, Moria S.A., France). Male New Zealand white rabbits aged 10 weeks and weighing 2–2.5 kg were used as animal recipients. All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of DPC
In order to compare the decellularization effects of detergents, the porcine corneal stroma was separately treated with 0.5% SLG (Sigma-Aldrich, St. Louis, MO, USA), SLS (Sigma-Aldrich), SDS (Solarbio, Beijing, China), or Triton X-100 (Solarbio), supplemented with 200 U/mL supernuclease (Sino Biological, Beijing, China) in PBS for 2 h at room temperature under shaking condition (100 r/min).
In order to compare the characterization of DPCs, the native porcine corneal stroma was treated with one of three decellularization methods as described below. Method A was the decellularization method we presented in this study by using SLG and supernuclease. Methods B and C were two traditional decellularization methods reported by previous studies.14,15 A summary of the decellularization methods is shown in Table 1.
Table 1.
Detailed procedures of the decellularization methods.
| Step | Method A | Method B | Method C |
|---|---|---|---|
| 1 | Native porcine corneal stroma (500 μm deep) | Native porcine corneal stroma (500 μm deep) | Native porcine corneal stroma (500 μm deep) |
| 2 | Agitate in 0.5% SLG and 200 U/mL supernuclease for 2 h at room temperature | Soak in ultrapure water for 12 h | Shake in 0.5% SDS for 24 h at 4°C |
| 3 | Rinse 6 times in PBS (10 min each time) at 100 r/min | Agitate in 2M NaCl for 30 min | Rinse 8 times in PBS for 16 h |
| 4 | Rinse in 0.9% NaCl for 10 min at 100 r/min | Agitate in ultrapure water for 30 min | Wash 3 times in PBS supplemented with 200 U/mL penicillin and 200 U/mL streptomycin for 3 h |
| 5 | Dehydrate in glycerol | Repeat above two processes for 3 times | Dehydrate in glycerol |
| 6 | Co60 irradiation (8 kGy) | Rinse in 0.2% Triton X-100 for 6 h | Co60 irradiation (8 kGy) |
| 7 | Wash in PBS | ||
| 8 | Dehydrate in glycerol | ||
| 9 | Co60 irradiation (8 kGy) | ||
| Total time | About 3 h | >21 h | >43 h |
SLG: sodium N-lauroyl glutamate; SDS: sodium dodecyl sulfate; PBS: phosphate-buffered saline.
Evaluations of corneal transparency, thickness, moisture content, and light transmittance
The transparency of DPCs were imaged using a black letter “A” on white paper. The thickness of corneas were tested before and after decellularization using a thickness gauge (547-301, Mitutoyo, Japan), and the thickening rate was calculated using the following equation: Thickening rate = (TS – TI) / TI × 100%, where TI and TS showed the corneal thickness before and after decellularization, respectively. To evaluate the moisture content, the swollen and dry weights of the DPCs were measured, the moisture content was calculated using the equation: Moisture content = (WS – WD) / WS × 100%, where WS was swollen weight of DPCs and WD was the dry weight after the dehydration in air. To evaluate the light transmittance, DPCs were fixed on specimen chamber, and the light transmittance was measured every 100 nm wavelength ranging from 300 to 800 nm wavelengths by using a UV-visible recording spectrophotometer (SpectraMax M2, Molecular Devices, MD). All characteristics were tested with the native porcine corneal stroma as normal controls.
Histological and immunostaining
The corneas were embedded in paraffin or O.C.T. compound. Paraffin corneal sections (4 μm) were stained with hematoxylin and eosin (H&E), and viewed under a light microscope (Olympus, Japan). Cryostat sections (7 μm) were used for the immunofluorescence staining and hematoxylin staining. For immunofluorescence staining, the primary antibodies was rabbit polyclonal antibody against porcine collagen I (1:100, Abcam), and the secondary antibodies were FITC donkey anti-rabbit IgG (1:1000, Invitrogen, Carlsbad, CA, USA). The 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) was used to label nuclei, and the corneal sections were viewed under an epifluorescence microscope (Nikon, Tokyo, Japan). Each condition was evaluated with the native porcine corneal stroma as control.
Ultrastructural analysis
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were performed to evaluate the corneal ultrastructure after decellularization. For the preparation of TEM examination, corneas were fixed in 2.5% glutaraldehyde in 0.1 M PBS for 4 h, and postfixed with 1% osmium tetroxide in 0.1 M PBS for 2 h, followed by the dehydration in a graded series of ethanol, embedding in Epon812 resin, and sections (60 nm) making. Then, processed sections were observed and photographed under the TEM (JEM-1200EX, JEOL, Japan). After that, the fiber diameter, number, and spacing were analyzed by Nano Measurer 1.2 software. For SEM, tissues were fixed with 2.5% glutaraldehyde in 4°C for 4 h, dehydrated in a graded series of ethanol, critical-point dried for 4 h, gold-coated with platinum (IB-3, Eiko, Japan), and then observed and photographed under the SEM (JSM-840, JEOL, Japan).
Quantitative analysis
The DNA was extracted and quantified according to the manufacturer’s instructions of GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) and DNA quantification kit (Invitrogen). Collagen content was tested according to the manufacturer’s instructions of sirius red collagen detection kit (Chondrex, Seattle, WA, USA), and GAG content was tested according to the manufacturer’s instructions of GAG enzyme-linked immunosorbent assay (ELISA) detection kit (Heng Kang Tiansheng, Beijing, China). All above-mentioned tests were performed both in the DPCs and native porcine corneal stroma.
Mechanical testing
The biomechanical properties of DPCs were examined by a static material testing machine (HZ1007E, HenZhun, CN) with a 10 kg load cell. Corneal stroma samples (dimension: 3 mm × 7 mm, n = 5) were clamped with a 3 mm clip distance and stretched at a constant cross-head speed at 2 mm/min. Deformation was recorded when the force was greater than 0.098 N. The break point was determined once the force declined by more than 10%. The measurements were analyzed with TM2101v5.5 Data Analysis Software, and the native porcine corneal stroma was also tested as normal controls.
Cell culture
Simian virus 40–immortalized human corneal epithelial cells (HCECs) were provided by professor Choun-Ki Joo from School of Medicine, Catholic University of Korea (Seoul, Korea). To evaluate the cytotoxicity of DPC, HCECs were seeded into a 24-well plate with a density of 2 × 103 cells per well and incubated in normal culture medium, the Dulbecco’s modified Eagle’s medium/F12 medium (DMEM/F12, Invitrogen) containing 10% fetal bovine serum (FBS, Invitrogen) for 6 days. For conditioned culture medium, one DPC was dipped in 10 mL culture medium for 48 h at 37°C and 5% CO2 culture environment. Cells were counted every 24 h using TC20™ Automated Coulter Counter (BIO-RAD). To evaluate the cytocompatibility of DPC, each DPC was placed into a 6-cm culture dish. HCECs were suspended in culture medium (DMEM/F12 containing 10% FBS) and then seeded evenly into these DPC-loaded dishes at a density of 1 × 104 cells per dish. Then, the DPCs were collected for SEM after 3 days of culture with conditions of 37°C and 5% CO2.
Biocompatibility analysis
The biocompatibility was evaluated by rabbit corneal micropocket implantation and anterior chamber implantation. For rabbit corneal micropocket implantation, the corneal stroma decellularized by SLG and supernuclease (300 µm, 3 mm diameter) was implanted into the rabbit stromal pockets. For rabbit anterior chamber implantation, the corneal stroma decellularized by SLG and supernuclease (300 µm, 3 × 3 mm2 size) was implanted into the rabbit anterior chambers. After the operation, ofloxacin eye drops (Santen, Osaka, Japan) were topically applied twice a day. Slit lamp observation were used to assess the transparency changes and the anterior chamber response of DPCs at 10 days postoperation.
LKP in rabbits
Ten corneal stromata decellularized by SLG and supernuclease (300 µm, 7.5 mm diameter) were implanted into the right corneas of 10 rabbits by LKP. Briefly, for LKP, a 200 µm deep, 7.5-mm diameter circular incision was made under general anesthesia by using a Hessbarg–Barron vacuum trephine. A lamellar dissection was then dissected using a fine operating knife along a natural uniform stratum in the corneal stroma to remove the host epithelium and anterior stroma. The grafts were fixed into the recipient bed by 16 interrupted stitches with 10-0 nylon sutures. After LKP, tobramycin–dexamethasone eye ointment was used 3 times daily for 7 days, followed by tobradex and ofloxacin eye drops. Clinical examinations were followed up, including the assessment of epithelial integrity (by fluorescein staining), corneal optical clarity (by slit lamp observation), neovascularization, and graft degradation. Optical coherence tomography (OCT) was used to assess the grafts’ attachment and the interlayer effusion. The infiltration of keratocytes into the implants was assessed by using confocal microscopy. At 2 and 4 weeks postoperation, three rabbits were euthanized for each time point, and the corneas were harvest and prepared for H&E staining; while the remaining four rabbits were used for long-term observation. The contralateral unoperated corneas were served as controls.
Statistical analysis
Values were shown as means ± SD. The SPSS 17.0 software was used for statistical analysis, and the differences between two groups were compared with the Student’s t test. Statistical significance was defined as p < 0.05.
Results
Comparison of detergents for decellularization
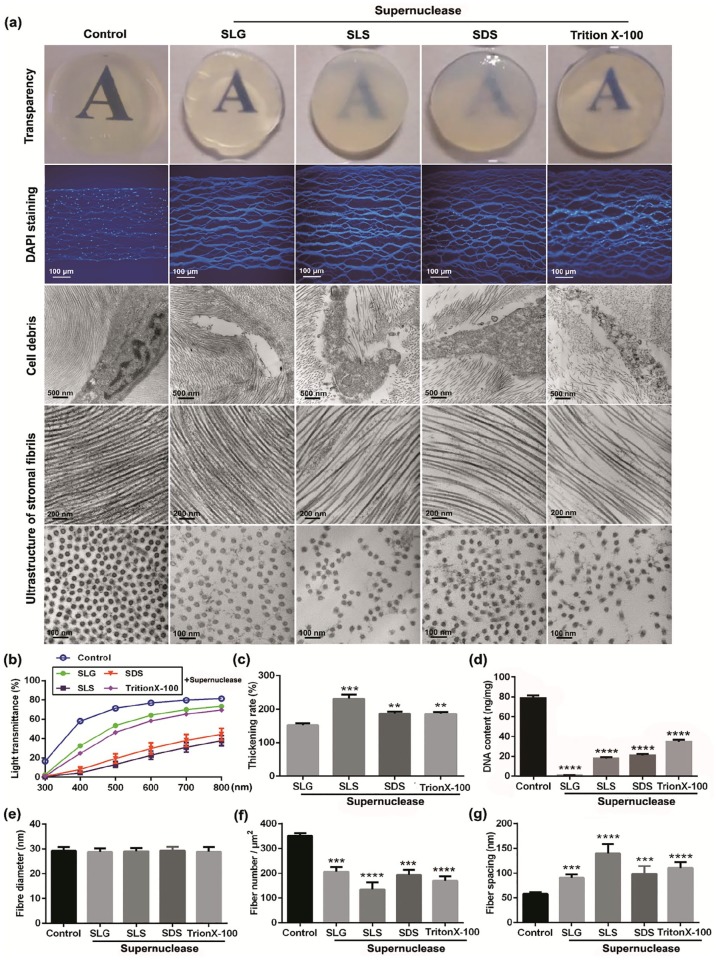
For the purpose of finding a better detergent for corneal decellularization, fresh porcine corneal stroma with the thickness of 500 µm was separately treated with 0.5% SLG, SLS, SDS, or Triton X-100, combined with 200 U/mL supernuclease in PBS for 2 h. Results showed that corneas were almost transparent after the combined treatment with SLG and supernuclease, while the transparency was declined in varying degrees after the treatment with other three detergents compared with native corneal stroma. The remaining nuclei were examined by DAPI staining, which indicated that only SLG plus supernuclease treated corneas showed no obvious residual nuclei. TEM was used to examine the ultrastructural changes of corneal stromal fibrils and nucleus, results showed that the alignment of corneal stromal fibrils was regular and the cellular nuclei were almost entirely removed after the treatment with SLG and supernuclease, while the integrity and regular alignment of stromal fibrils were slightly disordered in corneas treated with other presented detergents, accompanied with destroyed nuclei structure and residual cracked nuclei (Figure 1(a)). Compared with the characteristics of native porcine corneal stroma, the declines of light transmittance and the increases of corneal thickness vary differently in corneas treated, respectively, with above-mentioned detergents (Figure 1(b) and (c)). SLG plus supernuclease treatment had better efficiency in DNA removal (from 78.90 ± 1.48 to 1.02 ± 0.07 ng/mg) than other three treatments (17.83 ± 0.82 ng/mg of SLS + supernuclease, 20.78 ± 0.89 ng/mg of SDS + supernuclease, 34.57 ± 1.25 ng/mg of Triton X-100 + supernuclease), with more than 98% of total DNA being removed (Figure 1(d)), although the fiber numbers of unit area were decreased and the fiber spacings were increased in different degrees in all kinds of DPCs compared with native porcine corneal stroma (Figure 1(e)–(g)). These results suggest that the combined use of SLG and supernuclease has better capacity on the removal of xenogenic cells and antigens, and the preservation of the transparency and the ultrastructure of corneal stroma.
Figure 1.
Characteristics of corneas after the treatment with different decellularization detergents: (a) General images of corneal transparency, immunofluorescence images stained with DAPI, and TEM micrographs of stromal fibrils and nucleus (n = 4 per group). (b) Light transmittance at different wavelengths of visible light spectrum (n = 4 per group). Graphical representation of (c) corneal thickening rate, (d) DNA content, (e) fiber diameter, (f) fiber number, and (g) fiber spacing of DPCs relative to the native corneal stroma (n = 4 per group).
**p < 0.01; ***p < 0.001; ****p < 0.0001.
Corneal characteristics after decellularization
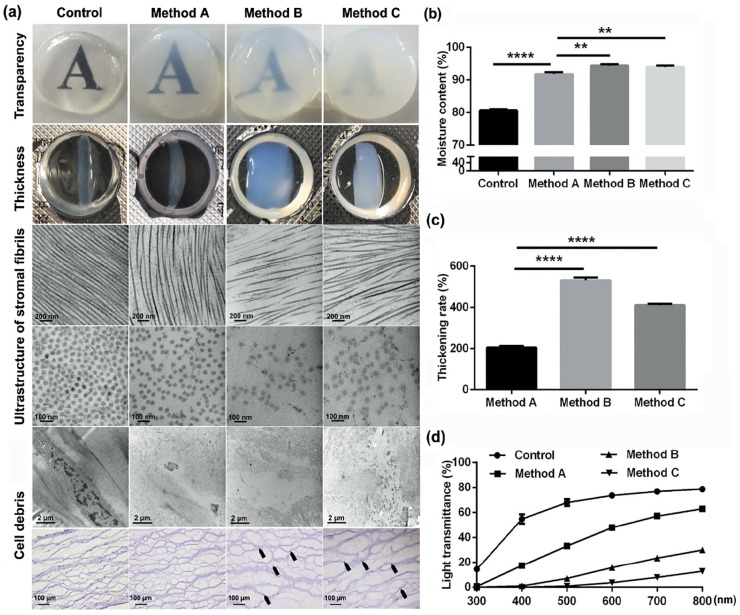
To investigate the characteristics of DPCs in different groups, corneal macroscopic transparency, thickness, stromal fibril ultrastructure, and light transmittance have been tested. Macroscopic transparency of porcine corneal stroma decellularized by three presented methods was declined compared to control corneal stroma, and swelling thickness of DPCs by performing method B and C increased more severely than by performing method A (Figure 2(a)). TEM results showed that the alignment of the stromal fibrils had more serious disorder and more cracked nuclei were left in corneal stroma treated with method B or C, than in corneal stroma treated with method A (Figure 2(a)). Detection of corneal moisture content showed that all DPCs had higher level of moisture content compared to control corneal stroma, while method A–treated DPCs (91.80 ± 0.37%) had apparently lower level of moisture content than corneal stroma treated with other two methods (method B: 94.42 ± 0.25%; method C: 94.04 ± 0.23%; p < 0.01; Figure 2(b)). Besides, the thickening rate of method A–treated corneal stroma had more than doubled (204.90 ± 3.95%), which is still lower than the thickening rate of corneal stroma treated with method B (531.90 ± 7.93%) or method C (410.30 ± 3.69%) (Figure 2(c)). Moreover, although the light transmittances of all DPCs were lower than that of the native porcine corneal stroma, method A–treated corneal stroma had better light transmittances than that of corneal stroma treated with method B or C (Figure 2(d)).
Figure 2.
Corneal characteristics after decellularization: (a) General images of corneal transparency, thickness. TEM micrographs and hematoxylin staining of stromal fibrils and nucleus (n = 5 per group). Graphical representation of (b) moisture content and (c) thickening rate (n = 5 per group). (d) Light transmittance of DPCs and native corneal stroma at different wavelengths (n = 5 per group).
**p < 0.01; ****p < 0.0001.
Characteristics of DPCs after rehydration
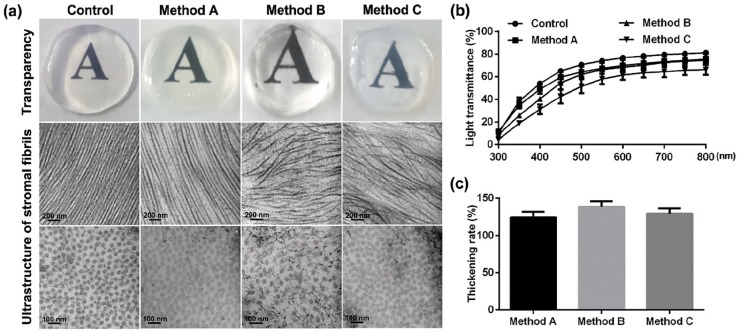
After decellularization, DPCs were submerged in glycerol for dehydration and followed by rehydration in normal saline for 90 s. All rehydrated DPCs showed good transparency, but only the corneal stroma decellularized with SLG and supernuclease maintained the integrity and regular alignment of stromal fibrils, but significant damages of stromal fibrils were observed in another two groups (Figure 3(a)). In addition, there were only slightly lower capacity of light transmittances of DPCs than that of native porcine corneal stroma (Figure 3(b)), while no significant changes of the thickening rate appeared in these three groups of DPCs after rehydration (Figure 3(c)).
Figure 3.
Characteristics of DPCs after rehydration: (a) Representative images of corneal transparency, and TEM micrographs of stromal fibrils and nucleus (n = 5 per group). (b) Light transmittance at different wavelengths of visible light spectrum (n = 5 per group). (c) Graphical representation of the thickening rate of DPCs and native corneal stroma after the rehydration with normal saline for 90 s.
Content determination and histological staining
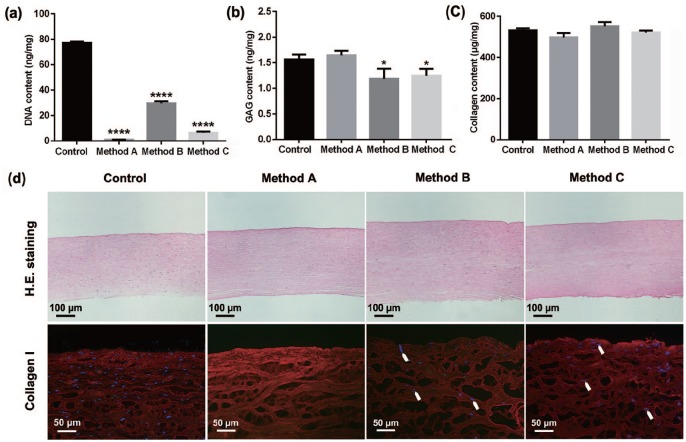
Quantitative analysis revealed that the decellularization treatments significantly reduced the total DNA of porcine corneal stroma, from 76.96 ± 0.60 ng/mg of native control to 1.02 ± 0.03 ng/mg of method A, 29.42 ± 0.95 ng/mg of method B, or 6.43 ± 0.50 ng/mg of method C, which indicated a better efficiency of DNA removal by performing method A, with more than 98% of total DNA removed in DPCs under the combined treatment of SLG and supernuclease (Figure 4(a)). Furthermore, except the slight reduction of GAG content in DPCs treated by methods B and C, the porcine GAG and collagen contents had no remarkable changes in all DPCs compared to control corneal stroma (Figure 4(b) and (c)), which were further confirmed by the results of H&E and collagen I staining (Figure 4(d)).
Figure 4.
Content determination of porcine components in porcine corneal stroma with or without decellularization. Quantification of remaining (a) DNA content, (b) GAG content, and (c) collagen content in native and decellularized porcine corneal stroma (n = 4 per group). (d) Representative images of H&E staining and immunofluorescence staining (collagen I) of native and decellularized porcine corneal stroma (n = 4 per group, arrows showed residual nuclei).
*p < 0.05; ****p < 0.0001.
Mechanical testing
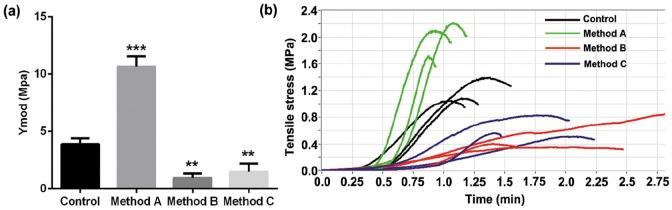
Tensile testing experiment was used to examine the mechanical properties of corneal stroma, and Young’s modulus (Ymod) was used to represent the elasticity of corneal stroma in the case of being stretched. Interestingly, corneal stroma treated with SLG plus supernuclease had a much higher value of Ymod (10.63 ± 0.54 MPa) when compared with native porcine corneal stroma (3.89 ± 0.30 MPa), which was in sharp contrast to corneal stroma treated with another two traditional decellularization methods showing significant declines on Ymod value (method B: 0.95 ± 0.22 MPa, method C: 1.50 ± 0.41 MPa) (Figure 5(a)). The dynamic variation of tensile strength showed that, although the corneal stroma treated with SLG and supernuclease only maintained a short time before rupture under the action of external forces, a greater tensile strength showed in DPCs processed with method A than in native and another two traditional DPCs (Figure 5(b)).
Figure 5.
Mechanical properties of porcine corneas with or without decellularization: (a) Young’s modulus (Ymod) and (b) tensile stress (n = 6 per group).
**p < 0.01; ***p < 0.001.
Cytotoxicity, cytocompatibility, and biocompatibility of porcine corneas decellularized with SLG and supernuclease
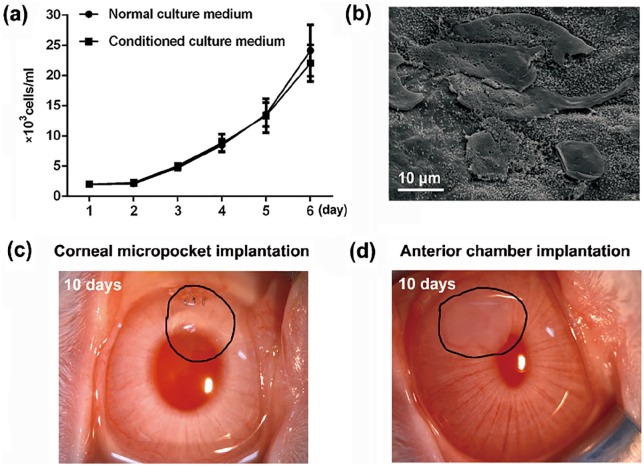
To determine the cytotoxicity of corneal stroma decellularized with SLG and supernuclease, proliferation ability of HCECs was detected when cultured in the conditioned culture medium after the incubation of DPCs. During 6 days’ cultivation, no significant differences was found between the experimental and control groups (Figure 6(a)). The SEM results showed that HCECs could be well adhered to and grown on the surface of DPCs, without significant changes of cell morphology at 3 days after cultivation, indicating the good cytocompatibility of DPCs processed by SLG and supernuclease (Figure 6(b)). For the test of biocompatibility, corneal stroma decellularized by SLG and supernuclease was implanted into the rabbit stromal pockets and anterior chambers. The DPCs presented good transparency and no obvious anterior chamber response at 10 days after transplantation (Figure 6(c) and (d)).
Figure 6.
Cytotoxicity, cytocompatibility, and biocompatibility examinations of porcine corneal stroma decellularized with SLG and supernuclease: (a) The proliferation curve of HECEs during 6 days’ cultivation in normal or conditioned culture medium (n = 3 per group). (b) SEM micrographs of HCECs at 3 days after the cultivation on the surface of a DPC (n = 3). (c) Rabbit corneal micropocket implantation results and (d) rabbit anterior chamber implantation results at 10 days postoperation (n = 3 per group).
DPC-dependent LKP in rabbit
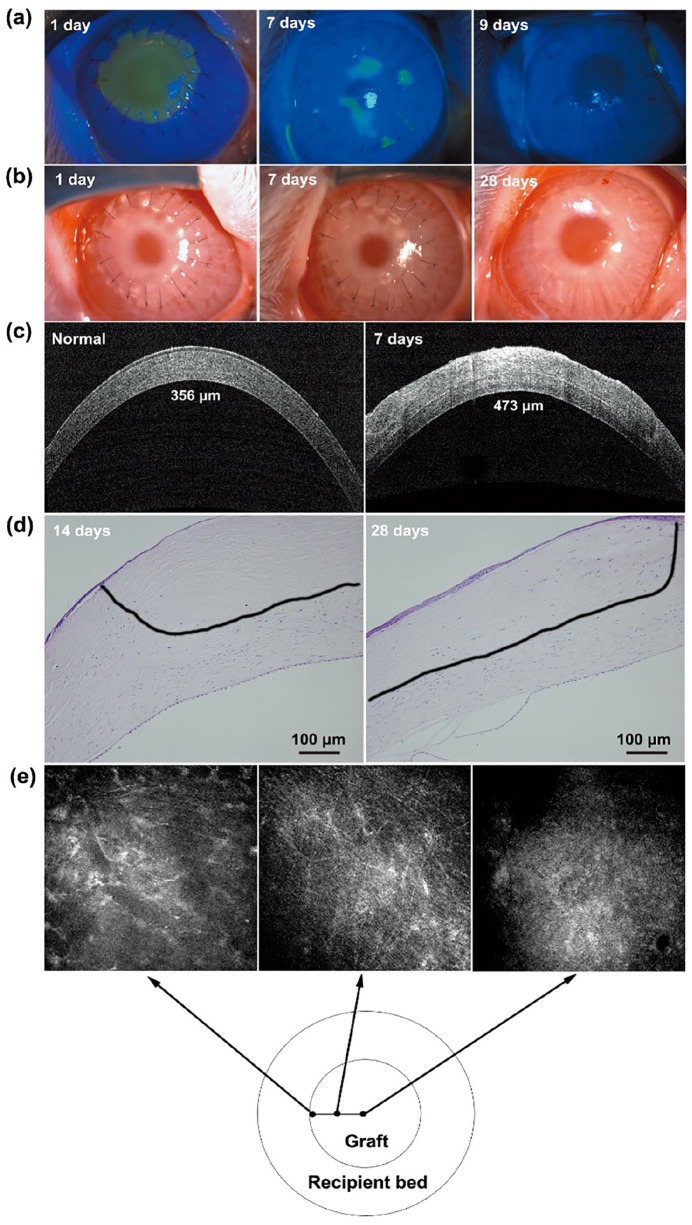
After the decellularization with SLG and supernuclease, DPCs (300 mm thickness) were implanted into rabbit corneas as lamellar grafts. The DPCs presented favorable re-epithelialization in 7–9 days after transplantation (Figure 7(a)), accompanied by an adequate transparency and good biocompatibility without degradation and neovascularization at 28 days after transplantation (Figure 7(b)). Well-reconstructive corneas were demonstrated by OCT test, with an average central corneal thickness of the rabbit eyes at 460 ± 15 μm and no obvious interlayer exudation at 7 days after transplantation (Figure 7(c)). In addition, representative images of H&E staining indicated that the host corneal stromal cells had migrated into the grafts (black line) at 28 days after operation (Figure 7(d)), and this migration was also verified by confocal microscopy at 4 months after operation (Figure 7(e)).
Figure 7.
Lamellar transplantation with porcine corneal stroma decellularized with SLG and supernuclease in rabbits: (a) Corneal re-epithelialization results detected by fluorescence staining at 1, 7, and 9 days after transplantation. (b) General appearance of eyes examined by slit lamp at 1, 7, and 28 days after transplantation. (c) OCT photographs of normal and decellularized corneal stroma at 7 days after transplantation. (d) H&E staining of the DPCs at 14 and 28 days after transplantation. (e) Confocal microscopy photographs of DPCs at 4 months after transplantation.
Discussion
Corneal blindness affects millions of patients worldwide and the first therapeutic option is the implementation of keratoplasty.1,2 To compensate the shortage of human donors, corneal decellularization has become a promising technique for the substitution of corneal scaffolds. However, in order to facilitate the entry of decellularization regents into the xenografts, the over-swelling of porcine corneas is inevitable, thereby causing the decline and even loss of corneal transparency and visual acuity.13–15,23 Shortening the treatment time had been identified as a useful way to preserve the ultrastructure and characteristics of stromal fibrils during corneal decellularization through suppressing the over-swelling of porcine corneal stroma. Here we provide a modified method by using the mild detergent SLG and supernuclease for the decellularization of porcine corneal stroma. Results indicated that our decellularization method presented a higher removal efficiency (within 3 h) of the xenogenic porcine antigens, and protected the DPCs with a normal-like ultrastructural integrity and transparency. Moreover, the better structural and functional advances were presented in the DPCs treated with SLG and supernuclease compared with porcine corneal stroma decellularized by traditional decellularization methods. Corneal features are shown in Table 2.
Table 2.
Corneal features after decellularization by new and traditional methods.
| New method |
Traditional methods |
|||
|---|---|---|---|---|
| Method A | Method B | Method C | ||
| Corneal transparency | Declined slightly | Declined seriously | ||
| Alignment of the stromal fibrils | Regular | Irregular | ||
| Residual nuclei | No obvious | Obvious | ||
| Thickening rate (%) | 204.90 ± 3.95 | 531.90 ± 7.93 | 410.30 ± 3.69 | |
| p < 0.0001 (compared with method A) | ||||
| Moisture content (%) | 91.80 ± 0.37 | 94.42 ± 0.25 | 94.04 ± 0.23 | |
| p < 0.01 (compared with method A) | ||||
| DNA clearance rate | About 98.67% | About 61.77% | About 91.65% | |
| GAG content (ng/mg) | No significant changes (1.65 ± 0.05, p > 0.05) | Significant decreased | ||
| (1.19 ± 0.11, p < 0.05) | (1.25 ± 0.08, p < 0.05) | |||
| Mechanical properties | Young’s modulus | Significant increase | Significant decline | |
| Tensile stress | Great in a short time | Weak in a long time | ||
GAG: glycosaminoglycan.
Residual heterogenic cellular components in xenografts, like DNA fragments, are considered as the major inflammatory inducers for recipients after xenotransplantation,44 thereby the removal of cell debris is very important to avoid a severe immunological response.45 Our decellularization method removed more than 98% of the major xenogeneic DNA, and there was barely cracked nuclei left showed by electron microscopy examination, which largely lowered the risk of postoperative immunological incompatibilities. Besides, growth factors and cytokines in ECM are vital components for the maintenance of cell migration, proliferation, and phenotype,46,47 as well as for the support of the host remodeling process postoperation,31,48 of which, GAG content serves as an useful indicator for the evaluation of the physiological components loss for porcine corneas after decellularization. Previous research found that, after the transplantation of corneal grafts with low GAG contents, the surrounding stromal cells can hardly infiltrate into the implants.13 Our study showed that GAG content in DPCs treated with SLG and supernuclease maintained a conventional level as in native corneal stroma, indicating the high efficacy of our decellularization method in saving biological components of porcine corneal stroma. Besides, the high proliferation ability of HCECs, the good transparency of transplanted grafts, and no inflammatory response induced by DPCs in anterior chamber indicated the barely cytotoxicity and good biocompatibility of porcine corneal stroma decellularized by our developed method. In addition, a good recovery condition was observed about the reconstructed corneas in rabbits after DPC-dependent LKP, showing a favorable re-epithelialization ability of the grafts within 9 days and a clear initiation of keratocytes infiltration into the transplanted grafts within 2 weeks.
A uniform diameter, arranged between 25 and 35 nm,49 and well-aligned lateral order of corneal collagen fibrils are requirements for the accomplishment of corneal transparency. In this present study, TEM results indicated that stroma treated with SLG and supernuclease demonstrated a normal-like ultrastructure resemble native corneas, with fibril diameter ranging from 27.26 to 30.26 nm. However, although the transparency and light transmittance of the corneal stroma decellularized by traditional methods was significantly enhanced, and no significant alteration of collagen content had appeared after glycerol treatment, TEM analysis confirmed a significant disruption of collagen architecture in those traditional DPCs. Therefore, the application effects of glycerol on transparency improvement might disguise the internal structural damages of porcine cornea stroma caused by the over-swelling during decellularization process, but a foreseeable postoperative complication is about the transparency decline of the reconstructed corneas because of the removal of glycerol. Furthermore, good postoperative outcomes were showed in rabbits after performing the LKP with porcine corneal stroma decellularized with SLG and supernuclease, showing an eligible thickness of the reconstructed rabbit corneas, and no significant corneal edema appeared.
In addition, a high mechanical strength is essential for the survival of decellularized corneal grafts, as they need to withstand the stitch tear during the surgery, as well as the membrane stresses caused by intraocular pressure and external forces.50 GAG plays a hydrophilic role in ECM, which is linked to polypeptide core to connect two collagen fibrils and provide the intermolecular force.51,52 After the decellularization of porcine corneas with traditional methods, the reduction of GAG content and the irregular arrangement of fibers in corneal stroma may result in the decrease of mechanical strength in DPCs. However, with the application of SLG and supernuclease, the stromal structure of DPCs was well preserved, meanwhile, tensile strength and Ymod of DPCs increased on account of the collagen crosslinking induced by low dosage of gamma irradiation,53 which may result in the increase of mechanical strength.
Based on the superior characteristics of the presented DPCs, the combined use of detergent SLG and supernuclease may serve as a promising method for the clinical use of DPCs, and provide a reference method for decellularization in other fields.
Conclusion
The combined use of SLG and supernuclease is a recommendable method for the production of DPCs, which demonstrated a high removal efficiency of xenogenic cells and antigens in porcine corneas, together with the better protective function on ultrastructural integrity and biological characteristics of corneal matrix fibers compared with traditional decellularization methods.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by Taishan Scholar Program (20150215, 20161059), Key Project of National Natural Science Foundation of China (81530027), and the Innovation Project of Shandong Academy of Medical Sciences.
ORCID iD: Qingjun Zhou  https://orcid.org/0000-0002-7770-9275
https://orcid.org/0000-0002-7770-9275
References
- 1. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001; 79(3): 214–221. [PMC free article] [PubMed] [Google Scholar]
- 2. Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation. Lancet 2012; 379: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 3. Brunette I, Roberts CJ, Vidal F, et al. Alternatives to eye bank native tissue for corneal stromal replacement. Prog Retin Eye Res 2017; 59: 97–130. [DOI] [PubMed] [Google Scholar]
- 4. Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr Opin Ophthalmol 2017; 28: 390–396. [DOI] [PubMed] [Google Scholar]
- 5. Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med 2010; 2(46): 46ra61. [DOI] [PubMed] [Google Scholar]
- 6. Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 2011; 13: 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong ML, Wong JL, Vapniarsky N, et al. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 2016; 92: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerson CJ, Elkins RC, Goldstein S, et al. Structural integrity of collagen and elastin in SynerGraft(R) decellularized-cryopreserved human heart valves. Cryobiology 2012; 64(1): 33–42. [DOI] [PubMed] [Google Scholar]
- 9. Cosentino M, Kanashiro A, Vives A, et al. Surgical treatment of Peyronie’s disease with small intestinal submucosa graft patch. Int J Impot Res 2016; 28(3): 106–109. [DOI] [PubMed] [Google Scholar]
- 10. Loo YL, Haider S. The use of porcine acellular dermal matrix in single-stage, implant-based immediate breast reconstruction: a 2-center retrospective outcome study. Plast Reconstr Surg Glob Open 2018; 6(8): e1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Ambra L, Berti S, Feleppa C, et al. Use of bovine pericardium graft for abdominal wall reconstruction in contaminated fields. World J Gastrointest Surg 2012; 4(7): 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Z, Zhou Y, Li N, et al. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 2009; 30(21): 3513–3522. [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto Y, Funamoto S, Sasaki S, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010; 31(14): 3941–3948. [DOI] [PubMed] [Google Scholar]
- 14. Pang K, Du L, Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 2010; 31(28): 7257–7265. [DOI] [PubMed] [Google Scholar]
- 15. Luo H, Lu Y, Wu T, et al. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 2013; 34(28): 6748–6759. [DOI] [PubMed] [Google Scholar]
- 16. Huang YH, Tseng FW, Chang WH, et al. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater 2017; 58: 238–243. [DOI] [PubMed] [Google Scholar]
- 17. Guler S, Aslan B, Hosseinian P, et al. Supercritical carbon dioxide-assisted decellularization of aorta and cornea. Tissue Eng Part C Methods 2017; 23(9): 540–547. [DOI] [PubMed] [Google Scholar]
- 18. Yoeruek E, Bayyoud T, Maurus C, et al. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol 2012; 90(3): e206–e210. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez-Andrades M, de la CruzCardona J, Ionescu AM, et al. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest Ophthalmol Vis Sci 2011; 52(1): 215–222. [DOI] [PubMed] [Google Scholar]
- 20. Zhu J, Zhang K, Sun Y, et al. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng Part A 2013; 19(21–22): 2412–2425. [DOI] [PubMed] [Google Scholar]
- 21. Xiao J, Duan H, Liu Z, et al. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials 2011; 32(29): 6962–6971. [DOI] [PubMed] [Google Scholar]
- 22. Huang M, Li N, Wu Z, et al. Using acellular porcine limbal stroma for rabbit limbal stem cell microenvironment reconstruction. Biomaterials 2011; 32(31): 7812–7821. [DOI] [PubMed] [Google Scholar]
- 23. Zhang MC, Liu X, Jin Y, et al. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant 2015; 15(4): 1068–1075. [DOI] [PubMed] [Google Scholar]
- 24. Brown BN, Freund JM, Han L, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods 2011; 17(4): 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price AP, England KA, Matson AM, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 2010; 16(8): 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasertsung I, Kanokpanont S, Bunaprasert T, et al. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater 2008; 85(1): 210–219. [DOI] [PubMed] [Google Scholar]
- 27. Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science 2010; 329(5991): 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elder BD, Kim DH, Athanasiou KA. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 2010; 66(4): 722–772; discussion 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang B, Zhang Y, Zhou L, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods 2010; 16(5): 1201–1211. [DOI] [PubMed] [Google Scholar]
- 30. Funamoto S, Nam K, Kimura T, et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010; 31(13): 3590–3595. [DOI] [PubMed] [Google Scholar]
- 31. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32(12): 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertasi G, Cole W, Samsell B, et al. Biological incorporation of human acellular dermal matrix used in Achilles tendon repair. Cell Tissue Bank 2017; 18(3): 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu J, Li Z, Li J, et al. Application of benzonase in preparation of decellularized lamellar porcine corneal stroma for lamellar keratoplasty. J Biomed Mater Res A. Epub ahead of print 22 July 2019. DOI: 10.1002/jbm.a.36760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wen Y, Xiao F, Wang C, et al. The impact of different methods of DNA extraction on microbial community measures of BALF samples based on metagenomic data. Am J Transl Res 2016; 8(3): 1412–1425. [PMC free article] [PubMed] [Google Scholar]
- 35. Helliwell JA, Thomas DS, Papathanasiou V, et al. Development and characterisation of a low-concentration sodium dodecyl sulphate decellularised porcine dermis. J Tissue Eng 2017; 8: 2041731417724011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elebring E, Kuna VK, Kvarnstrom N, et al. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. J Tissue Eng 2017; 8: 2041731417738145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Da Palma RK, Fratini P, SchiavoMatias GS, et al. Equine lung decellularization: a potential approach for in vitro modeling the role of the extracellular matrix in asthma. J Tissue Eng 2018; 9: 2041731418810164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer SR, Chiu B, Churchill TA, et al. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A 2006; 79(2): 254–262. [DOI] [PubMed] [Google Scholar]
- 39. Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 2004; 127(2): 399–405. [DOI] [PubMed] [Google Scholar]
- 40. Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 1997; 61(1): 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Epand RF, Infante MR, Flanagan TD, et al. Properties of lipoamino acids incorporated into membrane bilayers. Biochim Biophys Acta 1998; 1373(1): 67–75. [DOI] [PubMed] [Google Scholar]
- 42. Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc 2006; 1(4): 1872–1878. [DOI] [PubMed] [Google Scholar]
- 43. Giusti S, Bogetti ME, Bonafina A, et al. An improved method to obtain a soluble nuclear fraction from embryonic brain tissue. Neurochem Res 2009; 34(11): 2022–2029. [DOI] [PubMed] [Google Scholar]
- 44. Hussein KH, Park KM, Kang KS, et al. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater Sci Eng C Mater Biol Appl 2016; 67: 766–778. [DOI] [PubMed] [Google Scholar]
- 45. Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009; 30(8): 1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 2004; 12(3–4): 367–377. [DOI] [PubMed] [Google Scholar]
- 47. Wilson SL, Sidney LE, Dunphy SE, et al. Keeping an eye on decellularized corneas: a review of methods, characterization and applications. J Funct Biomater 2013; 4(3): 114–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015; 84: 25–34. [DOI] [PubMed] [Google Scholar]
- 49. Ghezzi CE, Rnjak-Kovacina J, Kaplan DL. Corneal tissue engineering: recent advances and future perspectives. Tissue Eng Part B Rev 2015; 21(3): 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Long K, Liu Y, Li W, et al. Improving the mechanical properties of collagen-based membranes using silk fibroin for corneal tissue engineering. J Biomed Mater Res A 2015; 103(3): 1159–1168. [DOI] [PubMed] [Google Scholar]
- 51. Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 2014; 802: 31–47. [DOI] [PubMed] [Google Scholar]
- 52. Bi Y, Patra P, Faezipour M. Structure of collagen-glycosaminoglycan matrix and the influence to its integrity and stability. Conf Proc IEEE Eng Med Biol Soc 2014; 2014: 3949–3952. [DOI] [PubMed] [Google Scholar]
- 53. Gouk SS, Lim TM, Teoh SH, et al. Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation, and remodeling. J Biomed Mater Res B Appl Biomater 2008; 84(1): 205–217. [DOI] [PubMed] [Google Scholar]