Abstract
Background
Data standardization is essential in electronic health records (EHRs) for both clinical practice and retrospective research. However, it is still not easy to standardize EHR data because of nonidentical duplicates, typographical errors, or inconsistencies. To overcome this drawback, standardization efforts have been undertaken for collecting data in a standardized format as well as for curating the stored data in EHRs. To perform clinical big data research, the stored data in EHR should be standardized, starting from laboratory results, given their importance. However, most of the previous efforts have been based on labor-intensive manual methods.
Objective
We aimed to develop an automatic standardization method for eliminating the noises of categorical laboratory data, grouping, and mapping of cleaned data using standard terminology.
Methods
We developed a method called standardization algorithm for laboratory test–categorical result (SALT-C) that can process categorical laboratory data, such as pos +, 250 4+ (urinalysis results), and reddish (urinalysis color results). SALT-C consists of five steps. First, it applies data cleaning rules to categorical laboratory data. Second, it categorizes the cleaned data into 5 predefined groups (urine color, urine dipstick, blood type, presence-finding, and pathogenesis tests). Third, all data in each group are vectorized. Fourth, similarity is calculated between the vectors of data and those of each value in the predefined value sets. Finally, the value closest to the data is assigned.
Results
The performance of SALT-C was validated using 59,213,696 data points (167,938 unique values) generated over 23 years from a tertiary hospital. Apart from the data whose original meaning could not be interpreted correctly (eg, ** and _^), SALT-C mapped unique raw data to the correct reference value for each group with accuracy of 97.6% (123/126; urine color tests), 97.5% (198/203; (urine dipstick tests), 95% (53/56; blood type tests), 99.68% (162,291/162,805; presence-finding tests), and 99.61% (4643/4661; pathogenesis tests).
Conclusions
The proposed SALT-C successfully standardized the categorical laboratory test results with high reliability. SALT-C can be beneficial for clinical big data research by reducing laborious manual standardization efforts.
Keywords: standardization, electronic health records, data quality, data science
Introduction
Background
As the volume of digitized medical data generated from real-world clinical settings explosively increases owing to the wide adoption of electronic health records (EHRs), there are mounting expectations that such data offer an opportunity to find high-quality medical evidence and improve health-related decision making and patient outcomes [1-6]. EHR data collected during clinical care can support knowledge discovery that allows critical insights into clinical effectiveness, medical product safety surveillance in real-world settings, clinical quality, and patient safety interventions [1,7-12]. In recent years, interest is growing in conducting multi-institutional studies for earning strength in analysis using EHR data, such as the Observational Health Data Sciences and Informatics [13], National Patient-Centered Clinical Research Network [14], and Electronic Medical Records and Genomics network [15], by standardizing EHR data from multiple institutions [16-21].
Indeed, significant promising values are expected from using EHR. However, a substantial number of studies have mentioned that clinical data in EHR may not be of sufficient quality for research [22-27]. Compared with well-organized research cohorts or repositories, EHR systems are typically designed for hospital operations and patient care [28]. For example, a system may use local terminology that allows unmanaged synonyms and abbreviations. Thus, data of the same concept can be stored under different notations across different systems. Therefore, if these duplicate notations are not merged into a single concept, it can distort the results of a study. In addition, if local data are not mapped to standard terminologies, such as the systematized nomenclature of medicine (SNOMED) and logical observation identifiers names and codes (LOINC), performing multicenter research would require extensive labor.
Several EHR data standardization guidelines and tools for laboratory test name have been published [29-32], but there have been relatively few studies on data cleaning methodology for categorical laboratory data [33,34]. The label of laboratory results tends to be managed well for insurance claims, whereas laboratory results data, especially categorical results, are not well harmonized even in a single institution. Categorical laboratory results are usually written as free texts; different notations are used by departments or doctors, leading to significant data noise. Thus, harmonizing data becomes more challenging because it requires not only intensive labor but also clinical knowledge.
Objectives
To resolve this drawback, there is a growing demand for data processing guidance and mapping tools for categorical laboratory data. In this study, we proposed a new automatic standardization algorithm for categorical laboratory results data, called standardization algorithm for laboratory test—categorical results (SALT-C). This algorithm was designed to help data curators by minimizing human intervention.
Methods
Overview
The original laboratory data used in this study are extracted from the clinical data warehouse (CDW) of Samsung Medical Center in Korea. The CDW contains deidentified clinical data of over 3,700,000 patients, including inpatient, outpatient, and emergency room patients, since 1994. The target dataset consists of 59,574,124 categorical laboratory results from 817 laboratory tests. This study focused on categorical data generated by machines; observation data, such as from health examination and allergy tests, were excluded even if sorted in categorical values.
Defining the Categorical Laboratory Results Value Sets and Mapping Terminology
Before developing SALT-C, 5 value sets were predefined as a reference. The value sets were defined as follows. First, we analyzed the distribution of laboratory tests with their results. Second, from the most frequent laboratory tests, we defined the value set of each laboratory test by consulting physicians and referring to SNOMED value sets. Finally, we identified 5 common value sets by combing the value sets with similar values (Table 1). The value sets of the 5 categories were mapped into SNOMED identifiers, as SNOMED is the most popular international standard for clinical terminology. The mapping results are shown in Multimedia Appendix 1.
Table 1.
Five common value sets.
| Category | Value set |
| Urine color tests | Clear, cloudy, orange, purple, brown, green, blue, red, black, yellow, dark yellow, pink, turbid, milky white, amber, straw, colorless, bloody |
| Urine dipstick tests | Negative, normal, trace, +, ++, +++, ++++ |
| Blood type tests | Rh+, Rh−, weak D, partial D, variant D, A, B, AB, O, cis-AB |
| Presence-finding tests | Positive, negative, weakly positive |
| Pathogenesis tests | Reactive, nonreactive, weakly reactive |
Developing the Automatic Standardizing Algorithm
The overall procedure of SALT-C is described in Figure 1. Using the 5 common value sets developed in the previous step, we designed SALT-C to assign each laboratory test into one of the 5 value set groups (laboratory test categorizer), then assign the actual value to one of the standardized categorical items in the corresponding value set (laboratory data categorizer). Multimedia Appendix 2 demonstrates the entire process in detail. The following subsections will describe each method. SALT-C was written in Python. The source code of SALT-C can be downloaded using the GitHub link [35].
Figure 1.
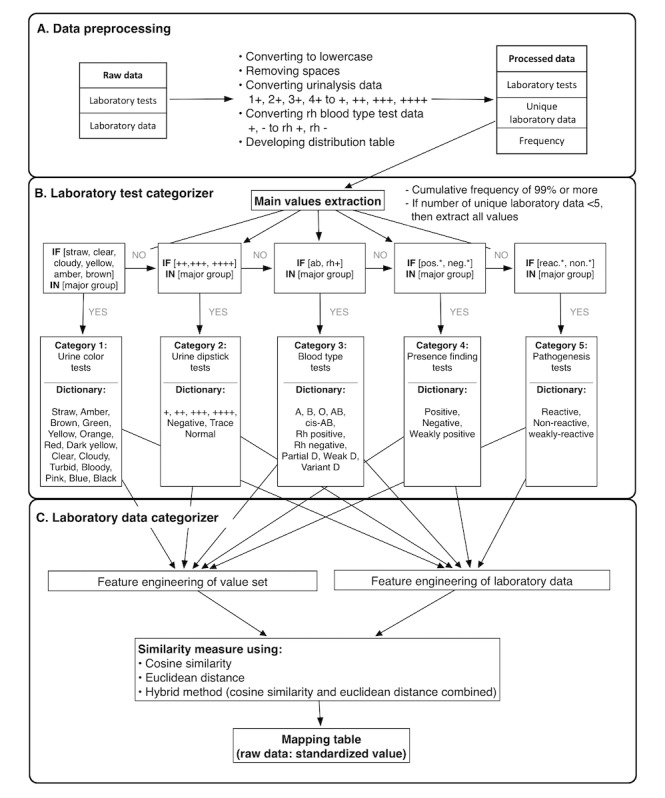
Process of the proposed standardization algorithm for laboratory tests—categorical results (SALT-C). neg: negative; pos: positive.
Data Extraction and Preprocessing
First, SALT-C extracts categorical laboratory data from a database or a comma-separated values format. Second, it preprocesses the extracted data with several methods: (1) applying the general data cleaning rules (ie, uppercase to lowercase and removing spaces from both sides), (2) correcting the abbreviation of - to rh – and + to rh + in Rh blood type laboratory data to distinguish it from the other - data of other laboratory tests, (3) formatting the urinalysis data. For example, results of urinalysis 4+ need to be converted into ++++, which has SNOMED concept identifier 260350009.
Extraction of the Main Values From Each Laboratory Test
SALT-C creates a distribution table for each laboratory test to extract the representative values. The distribution table is implemented in the following order: classify the data for each laboratory test, calculate the frequency of the data, and organize them in descending order. After the creation of the distribution table, the main values of each test are extracted. Only the data with a cumulative frequency of 99.5% or more are extracted as main values.
In performing the experiments by changing the cumulative frequency, 99.5% seemed the most reasonable threshold, empirically. If there are less than 5 values in a laboratory test, then all the values are extracted as main values because the categorizer may not work properly if too few values are extracted as main values.
Laboratory Test Categorizer
Once the main values are extracted in the previous step, they are used to categorize the laboratory tests into 5 groups according to a rule-based categorizer, as in Figure 1. If one or more main values of a laboratory test are included in one of the predefined value sets, as in Table 1, the laboratory test is categorized into the corresponding category. The laboratory test categorizer proceeds in a specific order of test categories (urine color, urine dipstick, blood type, presence finding, and pathogenesis) until the laboratory test is assigned to a single category; most laboratory tests have + and - data as their main values and can be misclassified if they are not ordered.
We included the following when designing the laboratory test categorizer, to prevent laboratory tests from being assigned to incorrect categories: (1) correction of - and + data to rh− and rh+ when they related to blood type tests, (2) classification of tests that have ++, +++, and ++++ as main values in advance so that + data would not affect the subsequent classification, and (3) classification of blood type–related tests as a subsequent step; the remaining tests are classified into the presence-finding or pathogenesis category.
Character-Level Vectorization
In SALT-C, we choose the character-level vectorization to represent laboratory data. By vectorizing, only a limited number of alphabets of laboratory data are used, instead of laboratory test names. The scheme consists of alphabets (a-z) and special characters (-, _, and +). All data are represented as vectors with the number of characters corresponding to the scheme features. This process is described in Figure 2, with examples of the feature representation of urine dipstick tests category data.
Figure 2.
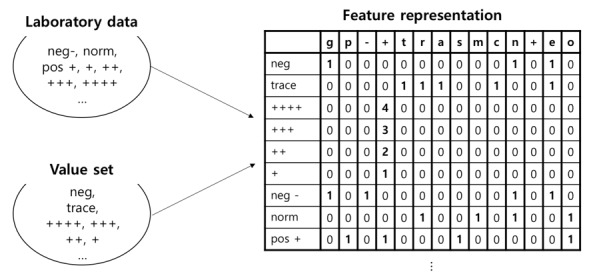
Character level vectorization. neg: negative; norm: normal; pos: positive.
Data Cleaning Using Similarity Measure
After all of the words are vectorized, a similarity score is calculated between a laboratory data point and each of the values in the standardized value set, and then the most similar value is selected. As a method of measuring similarity, we used and compared cosine similarity measure, Euclidean distance, and a hybrid method. The hybrid method was used to select the most similar value calculated by Euclidean distance when there are 2 or more same cosine similarity values.
Manual Validation
We performed manual validation by adjudicating a total of 167,936 laboratory unique values that SALT-C predicted as labels. We examined the accuracy of the predicted labels calculated by the similarity measure. Three medical providers were recruited to manually verify data. Two of them examined the total data set and another person was involved to determine the final adjudication in the case of a discrepancy. The mean of the similarity scores for correct, incorrect, and unclassified data were identified.
Results
Dataset Descriptive Statistics
Distribution of Laboratory Tests
A total of 817 categorical laboratory tests and 59,574,124 test results were selected from the source database. The most frequent laboratory test was urinalysis (43,559,493, 73.12%), followed by hepatitis B blood (5,219,770, 8.76%), ABO/Rh blood type (3,261,992, 5.85%), hepatitis C blood (1,653,741, 2.77%), rapid plasma reagin (1,044,173, 1.75%), venereal disease research laboratory (551,980, 0.93%), Treponema pallidum latex agglutination (527,454, 0.89%), HIV (464,507, 0.73%), and hepatitis B blood test (1,653,741, 2.77%). Other tests had a rate of less than 0.5%. Additional results are described in Multimedia Appendix 3.
Distribution of Laboratory Data
Frequency distribution tables for laboratory data were created for the 817 laboratory tests. Representative distribution tables for each of the 5 categories are described in Figures 3-7 as histogram charts.
Figure 3.
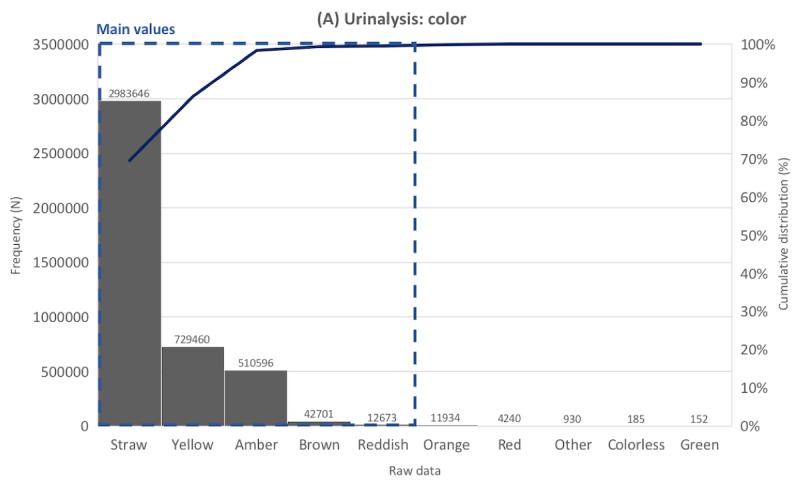
Distribution of laboratory tests data. Example laboratory test in the urine color tests category.
Figure 7.
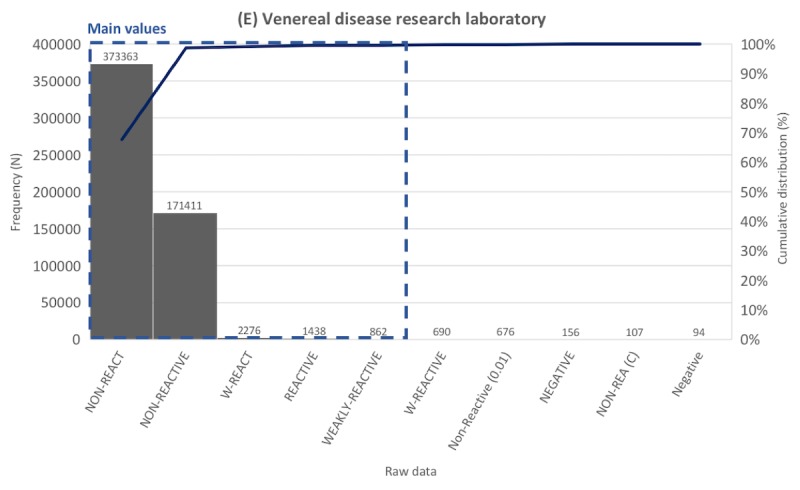
Distribution of laboratory tests data. Example laboratory test in the pathogenesis tests category.
In the color test of urinalysis (Figure 3), there were 4,296,997 data points, of which 132 values were unique before preprocessing. The most common value was Straw, accounting for 69.43%, followed by Yellow (16.97%), and Amber (11.88%). Other data comprised less than 1%. Straw, Yellow, Amber, and Brown were extracted as main values according to the criterion that only data with a cumulative frequency of 99.5% or less are extracted as main values. The main values had various synonyms or typos and abbreviations. For example, the number of different notations that should be corrected as Straw was 151, for example, Starw, ]traw, Strow, Strwa, traw, ]Straw, and steaw.
As for the blood detection test in urinalysis (Figure 4), there were 4,296,700 data points, of which 235 values were unique before preprocessing. Various synonyms of the main values were identified, including typos and abbreviations. For example, trace had 29 such notation variations: 10 tr, 25 tr, tr -, 5 tr, tr, 10 trace, and 10 trt. The most common value was neg -, accounting for 52.32%, followed by 10 tr (13.73%), 25 + (11.73%), 250 ++++ (6.89%), 50 ++ (6.60%), and 150 +++ (4.16%). Other data comprised less than 1%. Items neg -, 10 tr, 25 +, 250 ++++, and 50 ++ were extracted as main values.
Figure 4.
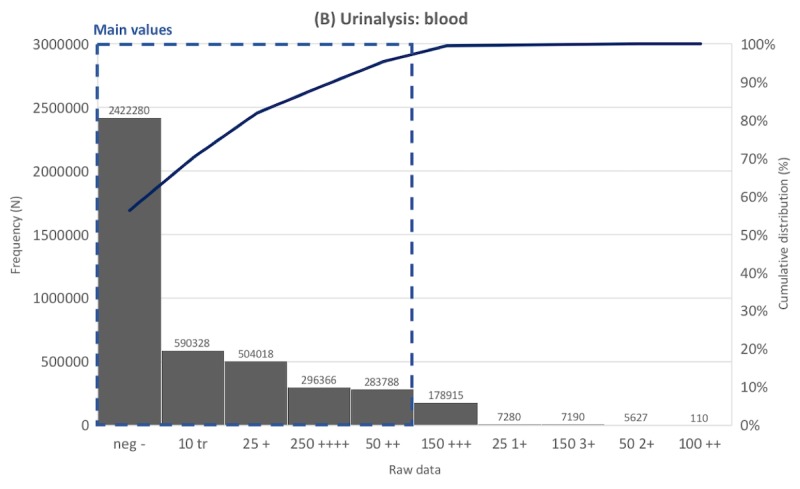
Distribution of laboratory tests data. Example laboratory test in the urine dipstick tests category.
In ABO blood type laboratory tests (Figure 5), there were 1,630,995 data points, of which 53 values were unique before preprocessing. The most common value was A, accounting for 34.17%, followed by O (27.42%), B (27.08%), and AB (11.15%). Other data consisting of blood group variant (ie, A1, A2, A3, Ax, Am, Ael, and Aend) comprised less than 1%. A, O, B, and AB were extracted as main values.
Figure 5.
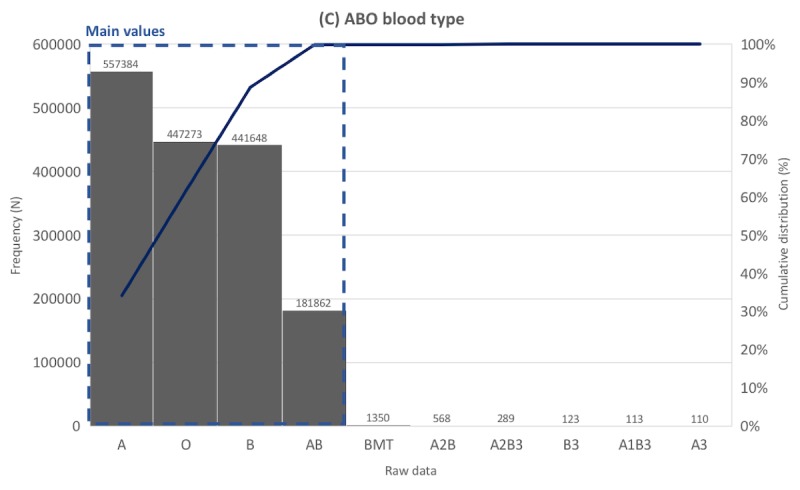
Distribution of laboratory tests data. Example laboratory test in the blood type tests category.
As the representative case of the presence-finding tests category, the antihepatitis B surface antibody laboratory test (Figure 6) had 1,190,631 data points, of which 56,134 were unique values before preprocessing. The most common value was NEG (2.00), accounting for 11.66%, followed by POS (>1000) (11.09%), NEGATIVE (10.14%), and NEG (0.01) (1.81%). Other data comprised less than 1%. NEG (2.00), POS (>1000), NEGATIVE, and NEG (0.01) were extracted as main values. Laboratory tests belonging to this category usually had data composed of numbers and letters; thus, the number of unique values was far higher compared with other categories.
Figure 6.
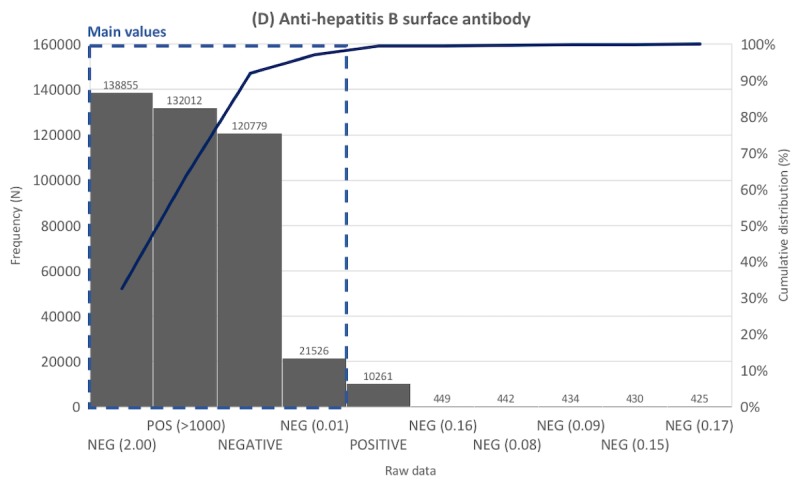
Distribution of laboratory tests data. Example laboratory test in the presence finding tests category.
As the representative case of the pathogenesis tests category, the venereal disease research laboratory test had 551,980 data points, of which 130 were unique values before preprocessing (Figure 7). The most common value was NON-REACT, accounting for 67.64%, followed by NON-REACTIVE (31.05%). Other data comprised less than 1%. NON-REACT, NON-REACTIVE, W-REACT, REACTIVE, and WEAKLY-REACTIVE were extracted as main values.
Categorization Results and 5 Common Value Sets
Overall, 5 categories and common value sets were created, and 480 laboratory tests were categorized into their corresponding group by the categorizer (Table 2). A total of 337 laboratory tests could not be classified. However, most of these uncategorized tests are not commonly used as these codes have been extinguished or temporarily issued for system testing. In addition, they only account for 0.61% of the raw data.
Table 2.
Laboratory test categorization.
| Category | Classified laboratory tests | |
| Number | Representative laboratory tests | |
| Urine color tests | 2 | Urinalysis: color, turbidity |
| Urine dipstick tests | 14 | Urinalysis: glucose, protein, ketones, hemoglobin, urobilinogen, bilirubin, leukocyte esterase |
| Blood type tests | 3 | Rh type, ABO group |
| Presence-finding tests | 453 | Hepatitis C virus antibody, Anti-HIV antibody, hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B e-antigen, barbiturate screen, opiate screen, toxoplasma antibody, rubella antibody |
| Pathogenesis tests | 8 | Rapid plasma reagin, venereal disease research laboratory (VDRL), Treponema pallidum latex agglutination, VDRL (cerebrospinal fluid), Treponema pallidum |
As shown in Table 2, 2 laboratory tests were categorized into the urine color tests category: one was the test for urine color and the other was the test for urine turbidity. The urine dipstick tests category included 2 sets of urinalysis tests, each consisting of 7 tests (glucose, protein, ketones, hemoglobin, urobilinogen, bilirubin, and leukocyte esterase) for checking the level of presence in urine. The blood type tests consisted of 2 tests related to blood type and 1 Rh type test. Most of the tests that have positive and negative data were categorized into the presence-finding tests. The pathogenesis tests category included 8 laboratory tests that were mostly related to sexually transmitted disease screening.
Manual Validation of Similarity Measure Results
We examined 3 similarity measures, namely, cosine similarity, Euclidean distance, and hybrid method. For the mapping results of values, the hybrid method showed a 97.82% accuracy compared with cosine similarity (93.20%) and Euclidean distance (97.64%). For the mapping results of data, the hybrid method, with 99.99% accuracy, was also the most accurate compared with cosine similarity (93.78%) and Euclidean distance (99.96%), as shown in Table 3. Therefore, when using SALT-C with the hybrid method as a similarity measure, nearly all of the raw data were mapped to the target value. As for the unique laboratory values, the algorithm predicted labels with the following accuracy values: 97.62% (urine color tests), 97.54 (urine dipstick tests), 94.64% (blood type tests), 99.68% (presence-finding tests), and 99.61% (pathogenesis tests). Approximately 0.002% of the raw data that did not contain enough information for terminology mapping or were severely distorted were excluded from the analysis interpretation.
Table 3.
Manual validation in unlabeled data.
| Category | Cosine similarity | Euclidean distance | Hybrid method | ||||
| Value | Data | Value | Data | Value | Data | ||
| Urine color, n (%) | |||||||
| Correct | 123 (97.6) | 8,592,841 (>99.99) | 122 (96.8) | 8,592,835 (0.49) | 123 (97.6) | 8,592,841 (>99.99) | |
| Incorrect | 3 (2.4) | 140 (<0.01) | 4 (3.2) | 146 (<0.01) | 3 (2.4) | 140 (<0.01) | |
| Urine dipstick, n (%) | |||||||
| Correct | 162 (79.8) | 28,747,699 (93.96) | 198 (97.5) | 30,594,572 (>99.99) | 198 (97.5) | 30,594,572 (>99.99) | |
| Incorrect | 41 (20.2) | 1,846,897 (6.04) | 5 (2.5) | 24 (<0.01) | 5 (2.5) | 24 (<0.01) | |
| Blood type, n (%) | |||||||
| Correct | 50 (89) | 3,261,963 (>99.99) | 53 (95) | 3,261,994 (>99.99) | 53 (95) | 3,261,994 (>99.99) | |
| Incorrect | 6 (11) | 44 (<0.01) | 3 (5) | 13 (<0.01) | 3 (5) | 13 (<0.01) | |
| Presence finding, n (%) | |||||||
| Correct | 162,291 (99.68) | 14,788,631 (99.97) | 162,296 (99.69) | 14,788,663 (99.97) | 162,291 (99.68) | 14,788,631 (99.97) | |
| Incorrect | 514 (0.32) | 4021 (0.03) | 509 (0.31) | 3989 (0.03) | 514 (0.32) | 4021 (0.03) | |
| Pathogenesis, n (%) | |||||||
| Correct | 4643 (99.61) | 1,944,729 (99.98) | 4638 (99.51) | 1,941,960 (99.84) | 4643 (99.61) | 1,944,729 (99.98) | |
| Incorrect | 18 (0.39) | 283 (0.01) | 23 (0.49) | 3052 (0.16) | 18 (0.39) | 283 (0.01) | |
Discussion
Principal Findings
The primary goal for this study was to find the way to efficiently map raw data to international standard terms. The first thing we did was to find standard value sets or code lists related to categorical laboratory test results. There are some value sets publicly available at SNOMED, LOINC, and Value Set Authority Center, but these were scattered, requiring an integrated dictionary to identify the spectrum of categorical laboratory data. Without an integrated reference dictionary, it is hard for researchers to convert their data into standard codes systemically, given that these data contain many synonyms, typos, and abbreviations. Such a situation has impeded easy organization and aggregation into standard terminology, as medical providers’ help is needed.
In this study, we identified 5 common value sets for categorical laboratory results by analyzing the distribution of laboratory tests with their results, by consulting with medical doctors, and by referring to laboratory tests’ SNOMED child codes. We found that 99.39% of the categorical test values fell into these value sets. As most of the categorical laboratory results were urinalysis data and data related to positive, negative, reactive, and nonreactive findings, and given that many researchers struggle with urinalysis data processing, we designed the value sets to handle as much urinalysis data as possible. The value sets developed in this study can be used for EHR interoperability, such as using Fast Health Interoperable Resources and Clinical Document Architecture. We continue to expand the values of value sets by applying SALT-C to several EHR databases internationally; furthermore, we are registering categorical laboratory value sets at Value Set Authority Center.
The laboratory data categorizer (Figure 1) measures the distance metrics between the standard item (eg, negative) and the laboratory test categorical values using a vector space model. We used the following method to increase computational efficiency and accuracy: (1) we only used the alphabets included in laboratory data, instead of alphabetical lists, as features and (2) we excluded duplicated characters in the standard term as much as possible. For example, negative and positive data were converted to neg – and posi to reduce similarity. We also attempted other string-matching methods, such as K-means clustering and Levenshtein distance; however, these 2 methods performed poorly. We demonstrated that the combination of cosine similarity and Euclidean distance method could give the best accuracy for laboratory test data, exceeding the performance of other measures. This hybrid model was complementary: the cosine similarity method selects the standard term with the most similar vector direction, and if the most similar vector direction is more than one, then the model adopts the closest value using the Euclidean distance method. For example, +++ 6 data have the same cosine similarity scores for +, ++, +++, and ++++, respectively, but Euclidean distance indicates +++ is the closest value. Usually, the cosine similarity is more accurate than Euclidean distance because it is less sensitive to the length or character order of terms; in some cases, the cosine similarity can be more accurate when combined with the Euclidean distance method. If there is a predefined code list table, it is more accurate to find the closest standard term by measuring the distance between the standard values and the data to be corrected; otherwise, the K-means method can be an alternative.
Limitation
Our study has a number of limitations to be considered. First, we validated SALT-C through one institution; thus, it may not be generalizable to other institutions’ data. However, our manual validation of 167,936 data points proved the high performance of SALT-C. When it comes to applying this algorithm to other institutions, the framework suggested in this study can be used to process categorical laboratory data, and the accuracy of the algorithm can be increased by adding more values to the value sets. Second, we only targeted the diagnostic test results from devices, whereas data from observational health examinations, such as past history, family history, and manual allergy test results, were excluded. In the case of processing allergy test results, it is much more efficient to treat it as a regular expression method, so we did not include it in the algorithm. We believe that observational health examination data should be managed using a different table (ie, excluded from the laboratory test result table); the terms and structure of reporting these data are not well standardized, and as such, we were unable to include them in this study. Third, meaningless data or data that do not correspond to any values in the value sets were assigned standard values randomly. In this case, we suggest 2 solutions: (1) if the similarity scores measured by cosine similarity or Euclidean distance between the actual data and each of the standard values in the value set are the same, then these data need manual mapping; (2) as these data do not take up much of the total dataset, the rate of manual mapping will decrease by selecting the dataset corresponding to 95% of the cumulative frequency from the beginning. Fourth, we grouped blood group A variants such as A1, A2, A3, Ax, Am, Ael, and Aend into A, blood group B variants such as B1, B2, B3, Bx, Bm, Bel, and Bend into B, and cis-AB into AB. However, it is more accurate to categorize blood group variants into subgroup [36,37]. We recommend modifying SALT-C algorithm depends on purpose of research regarding blood type.
Future work
For the short-to-medium term, we plan to validate SALT-C algorithm with multiple institutions and add more values sets that covers more laboratory tests. In addition, as a series of SALT algorithm, we aim to develop standardization algorithm for laboratory test—allergy (SALT-A) that handles allergy data and standardization algorithm for laboratory test—blood culture (SALT-BC) that deals with semistructuralized blood culture results.
Conclusions
We developed SALT-C, an algorithm that supports mapping of categorical laboratory data to the SNOMED-clinical terms (CT), and applied it to a large, long-period EHR system database. Previous studies on laboratory data processing have focused on the automatic mapping of laboratory test names or the standardization of numeric laboratory data [30-32,38]; however, we focused on categorical values of laboratory tests. Although SNOMED CT or LOINC standardize categorical laboratory test results, there is no widely accepted process of assigning standard codes to unstructured data fields.
There is an increasing need to aggregate and standardize EHR data to aid discovery of high-quality medical evidence and improve health-related decision making and patient outcomes. However, guidelines and automated methods for systemically converting disparate categorical laboratory data to standard terminology have been left to future work. The value sets and automated method suggested in this study may improve data interoperability and could be used for implementing standardized clinical data warehouse while reducing the manual effort of converting data. We plan to validate SALT-C through applying it at multisite institutions as well as expanding the value sets.
Acknowledgments
This study was supported by Samsung Medical Center grant #SMX1162111 and funded by the Ministry of Trade, Industry and Energy (grant number 20001234). This study was supported by Institute for Information and Communications Technology Promotion grant funded by the Korea government (Ministry of Science and ICT; 2018-0-00861, Intelligent SW Technology Development for Medical Data Analysis).
Abbreviations
- CDW
clinical data warehouse
- CT
clinical terms
- EHR
electronic health record
- LOINC
logical observation identifiers names and codes
- SALT-C
standardization algorithm for laboratory test—categorical results
- SNOMED
systematized nomenclature of medicine
Appendix
Mapping table of value sets.
The flow of SALT-C algorithm.
Distribution of categorical laboratory tests.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Lopez MH, Holve E, Sarkar IN, Segal C. Building the informatics infrastructure for comparative effectiveness research (CER): a review of the literature. Med Care. 2012 Jul;(50 Suppl):S38–48. doi: 10.1097/MLR.0b013e318259becd. [DOI] [PubMed] [Google Scholar]
- 2.Reiz AN, de la Hoz MA, García MS. Big data analysis and machine learning in intensive care units. Med Intensiva. 2018 Dec 24; doi: 10.1016/j.medin.2018.10.007. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Safran C, Bloomrosen M, Hammond WE, Labkoff S, Markel-Fox S, Tang PC, Detmer DE, Expert Panel Toward a national framework for the secondary use of health data: an American Medical Informatics Association white paper. J Am Med Inform Assoc. 2007;14(1):1–9. doi: 10.1197/jamia.M2273. http://europepmc.org/abstract/MED/17077452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner MG, Embi PJ. Toward reuse of clinical data for research and quality improvement: the end of the beginning? Ann Intern Med. 2009 Sep 1;151(5):359–60. doi: 10.7326/0003-4819-151-5-200909010-00141. [DOI] [PubMed] [Google Scholar]
- 5.Sanson-Fisher RW, Bonevski B, Green LW, D'Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007 Aug;33(2):155–61. doi: 10.1016/j.amepre.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Embi PJ, Payne PR. Evidence generating medicine: redefining the research-practice relationship to complete the evidence cycle. Med Care. 2013 Aug;51(8 Suppl 3):S87–91. doi: 10.1097/MLR.0b013e31829b1d66. [DOI] [PubMed] [Google Scholar]
- 7.Banda JM, Evans L, Vanguri RS, Tatonetti NP, Ryan PB, Shah NH. A curated and standardized adverse drug event resource to accelerate drug safety research. Sci Data. 2016 May 10;3:160026. doi: 10.1038/sdata.2016.26. http://europepmc.org/abstract/MED/27193236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banda JM, Halpern Y, Sontag D, Shah NH. Electronic phenotyping with APHRODITE and the observational health sciences and informatics (OHDSI) data network. AMIA Jt Summits Transl Sci Proc. 2017;2017:48–57. doi: 10.1038/clpt.2013.47. http://europepmc.org/abstract/MED/28815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bie S, Coloma PM, Ferrajolo C, Verhamme KM, Trifirò G, Schuemie MJ, Straus SM, Gini R, Herings R, Mazzaglia G, Picelli G, Ghirardi A, Pedersen L, Stricker BH, van der Lei J, Sturkenboom MC, EU-ADR Consortium The role of electronic healthcare record databases in paediatric drug safety surveillance: a retrospective cohort study. Br J Clin Pharmacol. 2015 Aug;80(2):304–14. doi: 10.1111/bcp.12610. doi: 10.1111/bcp.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holve E, Segal C, Lopez MH. Opportunities and challenges for comparative effectiveness research (CER) with electronic clinical data: a perspective from the EDM forum. Med Care. 2012 Jul;(50 Suppl):S11–8. doi: 10.1097/MLR.0b013e318258530f. [DOI] [PubMed] [Google Scholar]
- 11.Pacurariu AC, Straus SM, Trifirò G, Schuemie MJ, Gini R, Herings R, Mazzaglia G, Picelli G, Scotti L, Pedersen L, Arlett P, van der Lei J, Sturkenboom MC, Coloma PM. Useful interplay between spontaneous ADR reports and electronic healthcare records in signal detection. Drug Saf. 2015 Dec;38(12):1201–10. doi: 10.1007/s40264-015-0341-5. http://europepmc.org/abstract/MED/26370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh S, Platt R, Steiner JF, Brown JS. Comparative-effectiveness research in distributed health data networks. Clin Pharmacol Ther. 2011 Dec;90(6):883–7. doi: 10.1038/clpt.2011.236. [DOI] [PubMed] [Google Scholar]
- 13.Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, Suchard MA, Park RW, Wong IC, Rijnbeek PR, van der Lei J, Pratt N, Norén GN, Li YC, Stang PE, Madigan D, Ryan PB. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–8. http://europepmc.org/abstract/MED/26262116. [PMC free article] [PubMed] [Google Scholar]
- 14.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578–82. doi: 10.1136/amiajnl-2014-002747. http://europepmc.org/abstract/MED/24821743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman O, Kuivaniemi H, Tromp G, Faucett WA, Li R, Manolio TA, Sanderson SC, Kannry J, Zinberg R, Basford MA, Brilliant M, Carey DJ, Chisholm RL, Chute CG, Connolly JJ, Crosslin D, Denny JC, Gallego CJ, Haines JL, Hakonarson H, Harley J, Jarvik GP, Kohane I, Kullo IJ, Larson EB, McCarty C, Ritchie MD, Roden DM, Smith ME, Böttinger EP, Williams MS, eMERGE Network The electronic medical records and genomics (eMERGE) network: past, present, and future. Genet Med. 2013 Oct;15(10):761–71. doi: 10.1038/gim.2013.72. http://europepmc.org/abstract/MED/23743551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland MR, Tatonetti NP, Hripcsak G. Development and validation of a classification approach for extracting severity automatically from electronic health records. J Biomed Semantics. 2015;6:14. doi: 10.1186/s13326-015-0010-8. https://jbiomedsem.biomedcentral.com/articles/10.1186/s13326-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010 Jun;48(6 Suppl):S45–51. doi: 10.1097/MLR.0b013e3181d9919f. [DOI] [PubMed] [Google Scholar]
- 18.Cafri G, Banerjee S, Sedrakyan A, Paxton L, Furnes O, Graves S, Marinac-Dabic D. Meta-analysis of survival curve data using distributed health data networks: application to hip arthroplasty studies of the International Consortium of Orthopaedic Registries. Res Synth Methods. 2015 Dec;6(4):347–56. doi: 10.1002/jrsm.1159. [DOI] [PubMed] [Google Scholar]
- 19.Gini R, Schuemie M, Brown J, Ryan P, Vacchi E, Coppola M, Cazzola W, Coloma P, Berni R, Diallo G, Oliveira JL, Avillach P, Trifirò G, Rijnbeek P, Bellentani M, van der Lei J, Klazinga N, Sturkenboom M. Data extraction and management in networks of observational health care databases for scientific research: a comparison of EU-ADR, OMOP, mini-sentinel and matrice strategies. EGEMS (Wash DC) 2016;4(1):1189. doi: 10.13063/2327-9214.1189. http://europepmc.org/abstract/MED/27014709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si Y, Weng C. An OMOP CDM-based relational database of clinical research eligibility criteria. Stud Health Technol Inform. 2017;245:950–4. doi: 10.1093/jamia/ocx019. http://europepmc.org/abstract/MED/29295240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waitman LR, Aaronson LS, Nadkarni PM, Connolly DW, Campbell JR. The greater plains collaborative: a PCORnet clinical research data network. J Am Med Inform Assoc. 2014;21(4):637–41. doi: 10.1136/amiajnl-2014-002756. http://europepmc.org/abstract/MED/24778202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liaw ST, Rahimi A, Ray P, Taggart J, Dennis S, de Lusignan S, Jalaludin B, Yeo A, Talaei-Khoei A. Towards an ontology for data quality in integrated chronic disease management: a realist review of the literature. Int J Med Inform. 2013 Jan;82(1):10–24. doi: 10.1016/j.ijmedinf.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Kahn MG, Raebel MA, Glanz JM, Riedlinger K, Steiner JF. A pragmatic framework for single-site and multisite data quality assessment in electronic health record-based clinical research. Med Care. 2012 Jul;(50 Suppl):S21–9. doi: 10.1097/MLR.0b013e318257dd67. http://europepmc.org/abstract/MED/22692254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan KS, Fowles JB, Weiner JP. Review: electronic health records and the reliability and validity of quality measures: a review of the literature. Med Care Res Rev. 2010 Oct;67(5):503–27. doi: 10.1177/1077558709359007. [DOI] [PubMed] [Google Scholar]
- 25.Callahan TJ, Bauck AE, Bertoch D, Brown J, Khare R, Ryan PB, Staab J, Zozus MN, Kahn MG. A comparison of data quality assessment checks in six data sharing networks. EGEMS (Wash DC) 2017 Jun 12;5(1):8. doi: 10.5334/egems.223. http://europepmc.org/abstract/MED/29881733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnum JF. The misinformation era: the fall of the medical record. Ann Intern Med. 1989 Mar 15;110(6):482–4. doi: 10.7326/0003-4819-110-6-482. [DOI] [PubMed] [Google Scholar]
- 27.Botsis T, Hartvigsen G, Chen F, Weng C. Secondary use of EHR: data quality issues and informatics opportunities. Summit Transl Bioinform. 2010 Mar 1;2010:1–5. http://europepmc.org/abstract/MED/21347133. [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn MG, Callahan TJ, Barnard J, Bauck AE, Brown J, Davidson BN, Estiri H, Goerg C, Holve E, Johnson SG, Liaw S, Hamilton-Lopez M, Meeker D, Ong TC, Ryan P, Shang N, Weiskopf NG, Weng C, Zozus MN, Schilling L. A harmonized data quality assessment terminology and framework for the secondary use of electronic health record data. EGEMS (Wash DC) 2016;4(1):1244. doi: 10.13063/2327-9214.1244. http://europepmc.org/abstract/MED/27713905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JY, Sun Y. A system for automated lexical mapping. J Am Med Inform Assoc. 2006;13(3):334–43. doi: 10.1197/jamia.M1823. http://europepmc.org/abstract/MED/16501186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parr SK, Shotwell MS, Jeffery AD, Lasko TA, Matheny ME. Automated mapping of laboratory tests to LOINC codes using noisy labels in a national electronic health record system database. J Am Med Inform Assoc. 2018 Oct 1;25(10):1292–300. doi: 10.1093/jamia/ocy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan AN, Griffith SP, Moore C, Russell D, Rosario Jr AC, Bertolli J. Standardizing laboratory data by mapping to LOINC. J Am Med Inform Assoc. 2006;13(3):353–5. doi: 10.1197/jamia.M1935. http://europepmc.org/abstract/MED/16501183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidahussein M, Vreeman DJ. A corpus-based approach for automated LOINC mapping. J Am Med Inform Assoc. 2014;21(1):64–72. doi: 10.1136/amiajnl-2012-001159. http://europepmc.org/abstract/MED/23676247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo H, Kim K, Cha K, Lee JY, Mun H, Cho SJ, Chung JI, Pyo JH, Lee K, Kang M. Application of efficient data cleaning using text clustering for semistructured medical reports to large-scale stool examination reports: methodology study. J Med Internet Res. 2019 Jan 8;21(1):e10013. doi: 10.2196/10013. https://www.jmir.org/2019/1/e10013/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinis T. Data analysis: approximation aids handling of big data. Nature. 2014 Nov 13;515(7526):198. doi: 10.1038/515198d. [DOI] [PubMed] [Google Scholar]
- 35.GitHub Inc. [2019-08-12]. rpmina/SALT_C https://github.com/rpmina/SALT_C.
- 36.Cho D, Kim SH, Jeon MJ, Choi KL, Kee SJ, Shin MG, Shin J, Suh S, Yazer MH, Ryang D. The serological and genetic basis of the cis-AB blood group in Korea. Vox Sang. 2004 Jul;87(1):41–3. doi: 10.1111/j.1423-0410.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 37.Westman JS, Olsson ML. ABO and other carbohydrate blood group systems. In: Mark F, Anne E, Steven S, Connie W, editors. Technical Manual. Bethesda, Maryland: aaBB Press; 2017. pp. 265–94. [Google Scholar]
- 38.Yoon D, Schuemie MJ, Kim JH, Kim DK, Park MY, Ahn EK, Jung E, Park DK, Cho SY, Shin D, Hwang Y, Park RW. A normalization method for combination of laboratory test results from different electronic healthcare databases in a distributed research network. Pharmacoepidemiol Drug Saf. 2016 Mar;25(3):307–16. doi: 10.1002/pds.3893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapping table of value sets.
The flow of SALT-C algorithm.
Distribution of categorical laboratory tests.


