Abstract
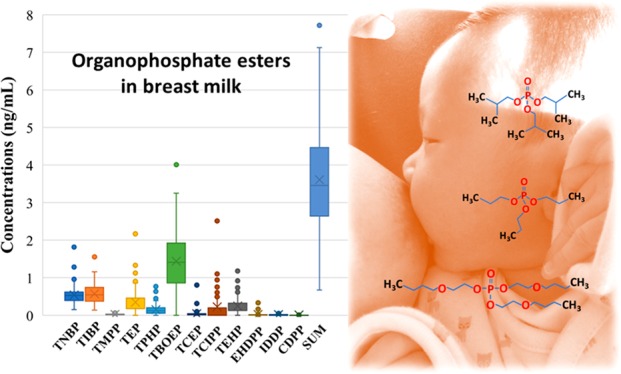
Organophosphate esters (OPEs) are used in consumer products as flame retardants and plasticizers. Little is known, however, about the occurrence and profiles of OPEs in human milk. In this study, 14 OPEs were measured in 100 breast milk samples collected from the United States during the period of 2009–2012, using high-performance liquid chromatography and tandem mass spectrometry. The sum concentrations of 14 OPEs in human milk ranged from 0.670 to 7.83 ng/mL, with a mean value of 3.61 ng/mL. The highest mean concentration was found for tris-2-butoxyethyl phosphate (TBOEP, 1.44 ± 0.789 ng/mL), followed by tri-iso-butyl phosphate (TIBP, 0.569 ± 0.272 ng/mL) and tri-n-butyl phosphate (TNBP, 0.539 ± 0.265 ng/mL), which were the dominant OPEs found in breast milk at detection frequencies of >80%. No significant differences were observed between various maternal/infant characteristics and OPE concentrations (p > 0.05), except for TBOEP, for which the median concentrations in Hispanic mothers (0.765 ng/mL) were 2 times lower than those in non-Hispanic mothers (1.48 ng/mL) (p < 0.05). On the basis of the recommended daily milk ingestion rate, the average and the highest daily intakes of total OPEs were calculated to be in the range of 300–542 and 504–911 ng (kg of body weight)−1 day–1, respectively. The estimated daily intakes of OPEs did not exceed the current reference doses. Our study establishes baseline data for OPE exposure in breast-fed American children.
Introduction
It is challenging to assess the health risks of infant exposure to environmental chemicals present in breast milk, which is a complex and dynamic mixture of endogenous (i.e., fats, water, proteins, carbohydrates, vitamins, and antibodies) and exogenous (i.e., xenobiotics from foods, pharmaceuticals, and the environment) substances delivered to infants through breastfeeding.1,2 Newborns have immature metabolic and immune systems and are prone to exposure to high burdens of exogenous chemicals relative to their body size.3 Breast milk is a major source of exposure to xenobiotics in newborns. For this reason, breast milk has been recognized as one of the important matrices for monitoring organic chemicals, such as polychlorinated biphenyls (PCBs), dioxin/furans, perfluoroalkyl substances (PFASs), polybrominated diphenyl ethers (PBDEs), and bisphenol A (BPA), by the United Nations Environment Program.4−10 Whereas studies have provided evidence of the presence of legacy contaminants in human milk, little is known about the occurrence of organophosphorus flame retardants and plasticizers in human milk from the United States. A few studies from Europe and Asia have reported the presence of organophosphorus flame retardants and plasticizers in breast milk.11−14
Organophosphate esters (OPEs) are widely and increasingly used in consumer products. There are concerns, however, with regard to human exposure to and potential health effects associated with these chemicals. Laboratory animal studies have shown that certain OPEs are neurotoxic, carcinogenic, and reproductive toxicants as well as endocrine disruptors.15−18 Tris(2-ethylhexyl) phosphate (TEHP), tris-2-butoxyethyl phosphate (TBOEP), and tri-n-butyl phosphate (TNBP) were reported to elicit pregnane X receptor agonist activity.19 Triphenyl phosphate (TPHP), TNBP, TBOEP, and tris(2-chloroethyl) phosphate (TCEP) were developmental neurotoxicants.20 Tris-2-chloroisopropyl phosphate (TCIPP) and TCEP were found to affect genes involved in immune responses and steroid hormone biosynthesis and affect xenobiotic metabolic pathways.21 Tris(1,3-dichloroisopropyl) phosphate (TDCIPP) is listed as a known carcinogen by the Consumer Product Safety Commission.22 TCEP has been reported to exert adverse effects on key biologic receptors and genes of vertebrate animals.23 Aryl-OPEs were reported as developmental toxicants,24 and TPHP was reported to disturb homeostasis of sex steroid hormones in human adrenal cells (H295R)16 and to be associated with increased serum thyroxine levels, especially in women.17
Environmental agencies are assessing the risks of OPEs to promulgate regulatory decisions.25 Nevertheless, production and use of OPEs, which are high-production volume chemicals,26 have increased sharply worldwide, from 296000 tons in 2004 to 500000 tons in 2011. OPEs are present ubiquitously in various environmental matrices, including water, air, house dust, and foodstuffs.27−32 The exposure of humans, especially infants and children, to OPEs is a great concern. Accurate determination of exposure levels and profiles of OPEs in infants is necessary to determine potential health risks.33
In this study, 100 human milk samples collected from the United States were analyzed for 14 OPEs. The demographic information was used to determine if they were predictive of or associated with OPE concentrations in human milk. The daily intake of OPEs through the ingestion of breast milk was estimated to assess potential health risks in breast-fed infants.
Materials and Methods
Sample Collection
Breast milk samples were obtained from the archives and sample repository of the Vanguard phase of the U.S. National Children’s Study (NCS). In brief, a total of 100 breast milk samples were collected from the United States in Tennessee (n < 10), Wisconsin (n = 21), South Dakota (n = 29), Maine (n < 10), New York (n = 12), Pennsylvania (n = 17), North Carolina (n < 10), and California (n = 11) between 2009 and 2012. Further details of the samples were reported previously.8 Mothers were 19–40 years of age (mean of 29.8 years) and in good health, with no documented occupational exposure to OPEs. Demographic information about mothers and children was obtained from the NCS archives and is given in Table S1. The Institutional Review Board of the New York State Department of Health reviewed and approved the analysis of samples. The NCS obtained informed consent from all participants. The samples were selected randomly from the NCS repository, stored in cryogenic vials (Nalgene, Thermo Fisher Scientific, Roskilde, Denmark), and kept on dry ice during transportation to Wadsworth Center. The breast milk samples were stored at −20 °C until further analysis.
Analytical Methods
Fourteen triester OPEs were analyzed: trimethyl phosphate (TMP), triethyl phosphate (TEP), tripropyl phosphate (TPP), TNBP, tri-iso-butyl phosphate (TIBP), TEHP, TBOEP, TCEP, TCIPP, TPHP, trimethylphenyl phosphate (TMPP), cresyl diphenyl phosphate (CDPP), isodecyl diphenyl phosphate (IDDP), and 2-ethylhexyl diphenyl phosphate (EHDPP). The method for the extraction of OPEs in breast milk samples was similar to that described previously.27 Briefly, 0.5 mL of breast milk was liquid–liquid extracted with 5 mL of 0.5% formic acid in acetonitrile and then purified by dispersive solid phase extraction (d-SPE) sorbents. Detailed information about the extraction and instrumental analysis by high-performance liquid chromatography and tandem mass spectrometry (HPLC–MS/MS) is provided in Table S2.
Quality Assurance/Quality Control
To reduce the background levels of OPEs, all experimental steps were performed in a clean fume hood. Polypropylene (PP) tubes and centrifuge tube filters were precleaned with HPLC-grade n-hexane, methanol, and acetonitrile, in that order, prior to use. MgSO4 was weighed in clean glass tubes and baked in a muffle furnace at 450 °C for 6 h. Four procedural blanks were analyzed in parallel with every batch of 20 samples to check for background levels of contamination. Twelve compounds, except TPP and TMP, were found in procedural blanks at concentrations that ranged from 0.001 to 0.18 ng/mL. Matrix spikes were prepared by fortifying two different concentrations of target analytes (1 and 5 ng of each) into cow milk purchased from a local supermarket (cow milk was used as a surrogate for breast milk due to its similar composition)4 and passed through the entire analytical procedure. The matrix spike recoveries ranged from 51% to 112% for individual compounds. Recoveries of the eight labeled internal standards (TMP-d9, TEP-d15, TPP-d21, TNBP-d27, TEHP-d51, TCEP-d12, TCIPP-d18, and TPHP-d15), which were spiked into all samples prior to extraction, ranged from 42% to 70%, except for that of TEHP-d51, which was 10%. A relatively low recovery of TEHP-d51 was due to the matrix effect, which was accounted for by the isotope dilution method of quantification. A 10-point calibration curve was constructed with standard solutions of the target analytes in a concentration range of 0.05–50 ng/mL, with a regression coefficient of >0.99. Duplicate injections of samples and midpoint calibration standards were performed after every 20 samples to ensure precision and accuracy of each analytical run. The limit of quantification (LOQ) was calculated according to Armbruster34 when the target compounds, which ranged from 0.005 to 0.390 ng/mL, were present in blanks; the LOQ was set at 10 times the signal-to-noise ratio of the instrument when the compounds were not present in blanks, which were 0.023 and 0.166 ng/mL for TPP and TMP, respectively. Further details of the method and QA/QC data are listed in Table S3.
Data Analysis
The mean concentrations of target analytes found in procedural blanks were subtracted from the sample values. Concentrations below the LOQ were assigned one-half the value of the LOQ, and nondetects (NDs) were set to zero for basic statistical analysis. Percentiles were calculated when >80% of the samples had concentrations above the LOQ. Because the data were not normally distributed, nonparametric statistical tests were applied to assess the statistical significance. Spearman’s correlation analysis was used to examine compound-specific associations. The Mann–Whitney U test and Kruskal–Wallis H test were used to compare differences in individual OPE concentrations with demographic variables: maternal age, parity, education, prepregnancy body mass index (BMI), family income, ethnicity, race, residential geographic area, infant gender, prematurity, birth weight, and birth season. Statistical significance was set at p < 0.05 level. All statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago, IL).
Results and Discussion
OPE Concentrations and Profiles in Breast Milk
Mean concentrations and detection frequencies of OPEs in breast milk are summarized in Table 1. Twelve target OPEs, except TPP and TMP, were found in breast milk, with detection frequencies that ranged from 12% to 97%, corroborating the previous findings of the widespread occurrence of OPEs or their metabolites in human urine, breast milk, placenta, plasma, hair, and nails.12,13,35−37 TBOEP, TNBP, TIBP, TMPP, and TPHP were quantified in 55–97% of the samples, whereas other compounds were found only in 12–37% of the samples. The highest mean concentration was found for TBOEP (1.44 ± 0.789 ng/mL), followed by TIBP (0.569 ± 0.272 ng/mL), TNBP (0.539 ± 0.265 ng/mL), TEP (0.350 ± 0.333 ng/mL), TEHP (0.245 ± 0.244 ng/mL), TCIPP (0.221 ± 0.326 ng/mL), and TPHP (0.149 ± 0.146 ng/mL), in that decreasing order. The sum concentrations of 12 OPEs (∑OPEs) ranged from 0.670 to 7.83 ng/mL, with a mean value of 3.61 ± 1.40 ng/mL, which was comparable to that reported in pooled human milk samples from Sweden (median of 3.37 ng/g, converted from the average lipid content)11 and was >2 times higher than that reported in cow milk (median of 1.55 ng/g) collected from the United States.27 A significant correlation (0.341 < r < 0.762; p < 0.01) was found among the concentrations of TNBP, TIBP, TMPP, and TBOEP in breast milk samples (Table S4), suggesting a common source for these four compounds.
Table 1. Distribution of Concentrations of Organophosphate Flame Retardants (nanograms per milliliter) in Breast Milk (n = 100) from the United States.
| procedural blank | LOQa | % DFb (>LOQ) | AMc,f | SDd,f | 25th | 50th | 75th | 95th | range | |
|---|---|---|---|---|---|---|---|---|---|---|
| TNBP | 0.184 | 0.301 | 88 | 0.539 (0.488–0.591) | 0.265 | 0.390 | 0.525 | 0.620 | 1.01 | <LOQ to 1.82 |
| TIBP | 0.176 | 0.275 | 88 | 0.569 (0.515–0.625) | 0.272 | 0.373 | 0.545 | 0.735 | 1.09 | <LOQ to 1.55 |
| TMPP | 0.002 | 0.010 | 85 | 0.021 (0.019–0.024) | 0.012 | 0.010 | 0.020 | 0.030 | 0.050 | NDe to 0.060 |
| TEP | 0.154 | 0.354 | 32 | 0.350 (0.287–0.418) | 0.333 | g | g | g | g | ND to 2.17 |
| TPHP | 0.032 | 0.109 | 55 | 0.149 (0.119–0.178) | 0.146 | g | g | g | g | ND to 0.760 |
| TPP | ND | 0.023 | 0 | ND | g | g | g | g | g | ND |
| TBOEP | 0.012 | 0.114 | 97 | 1.44 (1.29–1.58) | 0.789 | 0.855 | 1.41 | 1.92 | 2.95 | ND to 4.01 |
| TCEP | 0.002 | 0.026 | 37 | 0.036 (0.022–0.055) | 0.086 | g | g | g | g | ND to 0.800 |
| TCIPP | 0.128 | 0.390 | 20 | 0.221 (0.162–0.289) | 0.326 | g | g | g | g | ND to 2.51 |
| TEHP | 0.058 | 0.256 | 35 | 0.245 (0.197–0.291) | 0.244 | g | g | g | g | ND to 1.23 |
| EHDPP | 0.002 | 0.010 | 25 | 0.022 (0.012–0.034) | 0.058 | g | g | g | g | ND to 0.340 |
| IDDP | 0.005 | 0.023 | 19 | 0.013 (0.011–0.016) | 0.014 | g | g | g | g | ND to 0.070 |
| CDPP | 0.001 | 0.005 | 12 | 0.005 (0.002–0.008) | 0.016 | g | g | g | g | ND to 0.080 |
| TMP | ND | 0.166 | 0 | ND | g | g | g | g | g | ND |
| ∑OPEsf | 3.61 (3.35–3.88) | 1.40 | 2.64 | 3.46 | 4.47 | 6.07 | 0.670–7.83 |
LOQ, limit of quantification.
DF, detection frequency.
AM, arithmetic mean (99% confidence intervals).
SD, standard deviation.
ND, not detected.
Values below LOQ were estimated using LOQ/2 and nondetects were set to zero for statistical analysis.
Percentiles are not given because of their low DF (<80%).
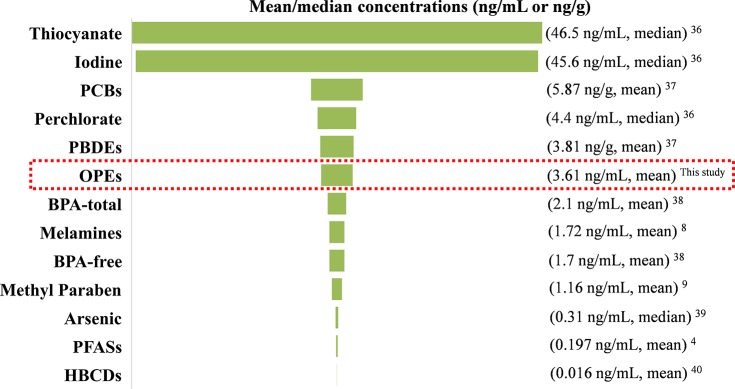
A wide range of environmental chemicals have been reported to occur in human milk from the United States previously, and the reported mean/median concentrations of each of those chemicals are summarized in Figure 1. The mean concentrations of OPEs (3.61 ng/mL) in breast milk were lower than those of thiocyanate (median of 46.5 ng/mL),38 iodine (median of 45.6 ng/mL),38 PCBs (mean of 5.87 ng/g, converted from the average lipid content),39 perchlorate (median of 4.4 ng/mL),38 and PBDEs (mean of 3.81 ng/g, converted from the average lipid content)39 but were ∼2 times higher than the sum concentrations of melamine and its derivatives (mean of 1.72 ng/mL),8 BPA (mean of 2.1 ng/mL),40 and methyl paraben (mean of 1.16 ng/mL).9 Moreover, the mean concentrations of OPEs in breast milk were 1–2 orders of magnitude higher than those found for arsenic,41 PFASs,4 and hexabromocyclododecanes (HBCDs)42 (mean/median, range of 0.31–0.016 ng/mL). These results further support the significance of OPFR exposure in humans.
Figure 1.
Comparison of mean/median concentrations of organophosphate esters (OPEs) measured in this study with those of other chemicals reported previously in human milk from the United States. Abbreviations: PCBs, polychlorinated biphenyls; PBDEs, polybrominated diphenyl ethers; BPA, bisphenol A; PFASs, perfluoroalkyl substances; HBCDs, hexabromocyclododecanes.
To the best of our knowledge, OPE concentrations in breast milk were previously reported from Sweden,11 Australia,43 Spain,13 Japan, the Philippines, and Vietnam.12 The same OPE analogues determined in earlier studies with those of ours were included for comparison (Figure S2). Among the 12 OPEs reported in our study, TBOEP was the predominant compound (40%), which was followed in abundance by TIBP (16%) and TNBP (15%). The composition of OPEs, however, varied widely in breast milk samples from various countries. For example, TMP accounted for 26% of the total OPE concentrations in breast milk from Spain (n = 20), followed by TBOEP (20%), TCIPP (17%), and TPHP (17%). In contrast, TMP was not detected in our samples. TCIPP was predominant (55%) in human milk from Sweden (n = 6), whereas TCIPP accounted for only 6% of the total OPE concentrations, with a low detection frequency in our study. TCEP (65%) and TPHP (29%) were the major OPEs found in human breast milk from the Philippines (n = 41), and TNBP accounted for 65% and 68% of the total OPE concentrations in human milk from Japan (n = 20) and Vietnam (n = 26), respectively. The concentrations of nine OPEs analyzed in breast milk from Australia (n = 3) were below the method limits of detection. Differences in the composition of OPEs in human breast milk samples from various countries may be related to the usage patterns of OPEs in these countries.36 Nevertheless, our comparison of OPE profiles in breast milk across various countries is tempered by the differences in the number of OPE analogues measured in each of the studies and the sample size. The average annual production volume of TBOEP in the United States was between 454 and 4540 tons in 2012 (Table S5).44 The widespread use of TBOEP in the United States is reflected in its occurrence in freshwater fish from Lake Ontario and herring gull eggs.45−47
Demographic Characteristics and OPE Concentrations in Breast Milk
We examined the differences in the concentrations of four major OPEs, namely, TNBP, TIBP, TMPP, and TBOEP, with various demographic features (Table S6). No significant differences were observed between various maternal/infant characteristics and OPE concentrations (p > 0.05), except for TBOEP, for which the median concentration in Hispanic mothers (0.765 ng/mL) was 2-fold lower than that in non-Hispanic mothers (1.48 ng/mL) (Mann–Whitney U test; p = 0.041). Several studies have reported significant associations between urinary total or individual OPE metabolites and demographic characteristics, such as age, gender, smoking status, and BMI.36,43,48−52 In addition, studies have shown that body burdens of persistent organic contaminants decline in women with a history of breastfeeding.4,53 The concentrations of OPEs in breast milk were significantly lower in multiparas than in primiparas in Vietnam, whereas an opposite trend existed for Japanese mothers.12 We did not find significant associations between OPE concentrations in breast milk and maternal characteristics (age or BMI) or temporal factors (season), nor did we find significant differences in OPE concentrations between parity, which may be attributed to the short half-life of OPEs in human bodies and the fact that the concentrations in breast milk reflect daily exposures.8 It is worth noting that small sample sizes analyzed in studies are subject to certain biases.
Daily Intake of OPEs via Breast Milk
An infant’s daily consumption of breast milk can vary, depending on the infant’s age and solid food intake.4 The U.S. Environmental Protection Agency (EPA) estimated an average daily breast milk consumption rate for infants by age.54 Estimated daily intakes (EDIs) of OPEs were calculated on the basis of the measured concentrations and the consumption rates of breast milk by infants between the ages of 0 and 12 months.
The respective mean and the highest EDIs of ∑OPEs were 542 and 911 ng [kg of body weight (bw)]−1 day–1 for infants less than 1 month of age, 505 and 850 ng (kg of bw)−1 day–1 for infants from 1 to 3 months of age, 397 and 668 ng (kg of bw)−1 day–1 for infants from >3 to 6 months of age, and 300 and 504 ng (kg of bw)−1 day–1 for infants from >6 to 12 months of age, respectively (Table 2). The EDI decreased with an increase in age, which can be explained by increasing body weight and decreasing milk ingestion level with age.
Table 2. Estimated Daily Intakes (EDI; nanograms per kilogram of body weight per day) of Organophosphorus Esters (OPEs) through Breastfeeding in U.S. Infants.
| from
birth to <1 month [150 mL (kg of bw)−1 day–1]c |
from
1 to 3 months of age [140 mL (kg of bw)−1 day–1]c |
from
>3 to 6 months of age [110 mL (kg of bw)−1 day–1]c |
from
>6 to 12 months of age [83 mL (kg of bw)−1 day–1]c |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RfDa [ng (kg of bw)−1 day–1] | mean | max | mean | max | mean | max | mean | max | |
| TNBP | 2.40 × 103 | 80.9 | 273 | 75.5 | 255 | 59.3 | 200 | 44.7 | 151 |
| TIBP | b | 85.4 | 233 | 79.7 | 217 | 62.6 | 171 | 47.2 | 129 |
| TMPP | 1.30 × 103 | 3.15 | 9.00 | 2.94 | 8.40 | 2.31 | 6.60 | 1.74 | 4.98 |
| TEP | 1.25 × 105 | 52.5 | 326 | 49.0 | 304 | 38.5 | 239 | 29.1 | 180 |
| TPHP | 7.00 × 103 | 22.4 | 114 | 20.9 | 106 | 16.4 | 83.6 | 12.4 | 63.1 |
| TPP | b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBOEP | 1.50 × 103 | 216 | 602 | 202 | 561 | 158 | 441 | 120 | 333 |
| TCEP | 2.20 × 103 | 5.40 | 120 | 5.04 | 112 | 3.96 | 88.0 | 2.99 | 66.4 |
| TCIPP | 3.60 × 103 | 33.2 | 377 | 30.9 | 351 | 24.3 | 276 | 18.3 | 208 |
| TEHP | 3.50 × 104 | 36.8 | 185 | 34.3 | 172 | 27.0 | 135 | 20.3 | 102 |
| EHDPP | 6.00 × 102 | 3.30 | 51.0 | 3.08 | 47.6 | 2.42 | 37.4 | 1.83 | 28.2 |
| IDDP | b | 1.95 | 10.5 | 1.82 | 9.80 | 1.43 | 7.70 | 1.08 | 5.81 |
| CDPP | b | 0.750 | 12.0 | 0.700 | 11.2 | 0.550 | 8.80 | 0.420 | 6.64 |
| TMP | b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ∑OPEs | b | 542 | 911 | 505 | 850 | 397 | 668 | 300 | 504 |
The reference doses (RfD) reported by the U.S. EPA have been used to describe noncarcinogenic risks of environmental toxicants.55 The EDIs were compared with the corresponding RfD values reported for OPEs.55,56 The average and highest EDIs of TNBP and TBOEP were 1–2 orders of magnitude lower than the RfDs; the average and highest EDIs of TPHP, TCEP, TCIPP, EHDPP, TEHP, and TMPP were 2–3 orders of magnitude lower than the RfDs. The average and highest hazard quotient (HQ; ratio of EDI to RfD) values of the target OPEs were up to 5 orders of magnitude below 1 (HQ ranged from 7.01 × 10–5 to 4.01 × 10–1 < 1). These results are similar to those reported previously for six pooled Swedish breast milk samples and 87 Asian breast milk samples.11,12 These results should be interpreted with caution, however, due to the lack of a consensus RfD value for OPEs. Moreover, the concentrations of OPEs are expected to fluctuate in breast milk throughout the feeding period due to the rapid metabolism and elimination of OPEs.1 Nevertheless, breastfeeding has been widely recommended due to a wide range of health benefits for nursing infants, including protection against infection, an improvement in cognitive development, enhancement of the immune system, and provision of balanced and adequate nutrients. Our results establish baseline data on infant exposure to OPEs through breast milk.
This is the first study of the occurrence of OPEs in human milk from the United States. OPE concentrations in the breast milk of U.S. mothers were similar to those reported for PBDEs but 2-fold higher than the concentrations of melamine measured in the same samples. Our OPE concentrations in breast milk were similar to those reported previously for Swedish mothers and were >2-fold higher than those reported in cow milk from the U.S. markets. The composition of OPEs varied widely in breast milk from various countries, in which TBOEP, TNBP, and TIBP were the predominant compounds found in U.S. breast milk. The concentrations of TBOEP in Hispanic mothers were 2-fold lower than those in non-Hispanic mothers in the United States. The EDIs of OPEs through breast milk did not exceed the current RfDs. In addition to triester OPEs, the metabolites of OPEs should be investigated in further studies to assess the risks of OPEs in breast-fed infants.
Acknowledgments
This research was prepared using National Children’s Study Research Materials obtained from the NCS Vanguard Data and Sample Archive and Access System and does not necessarily reflect the opinions or views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health (NIH). The analysis portion of the research reported herein was supported in part by the National Institute of Environmental Health Sciences of the NIH via Grant U2CES026542-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Authors thank all of the study participants for providing samples.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.estlett.9b00394.
Demographic characteristics of study participants (Table S1), chemical properties and MS/MS parameters of target OPE compounds (Table S2), information about instrument performance (Table S3), correlation analysis (Table S4), production volumes of OPEs (Table S5), and statistical comparison of select OPE compounds with demographic features (Table S6) and profiles of OPEs in milk samples from different countries (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lehmann G. M.; LaKind J. S.; Davis M. H.; Hines E. P.; Marchitti S. A.; Alcala C.; Lorber M. Environmental chemicals in breast milk and formula: Exposure and risk assessment implications. Environ. Health Perspect. 2018, 126, 096001. 10.1289/EHP1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajewska-Szmyt M.; Sinkiewicz-Darol E.; Gadzala-Kopciuch R. The impact of environmental pollution on the quality of mother’s milk. Environ. Sci. Pollut. Res. 2019, 26, 7405–7427. 10.1007/s11356-019-04141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias P.; Rodriguez-Dozal S.; Baltazar-Reyes M. C.; Gold-Bouchot G.; Zapata-Perez O.; Loreto-Gomez C.; Riojas-Rodriguez H. Persistent organic pollutants in serum and breast milk of fertile-aged women. Rev. Int. Contam. Ambiental 2019, 35, 281–293. 10.20937/RICA.2019.35.02.02. [DOI] [Google Scholar]
- Tao L.; Kannan K.; Wong C. M.; Arcaro K. F.; Butenhoff J. L. Perfluorinated compounds in human milk from Massachusetts, USA. Environ. Sci. Technol. 2008, 42, 3096–3101. 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- Weldon R. H.; Barr D. B.; Trujillo C.; Bradman A.; Holland N.; Eskenazi B. A pilot study of pesticides and PCBs in the breast milk of women residing in urban and agricultural communities of California. J. Environ. Monit. 2011, 13, 3136–3144. 10.1039/c1em10469a. [DOI] [PubMed] [Google Scholar]
- Daniels J. L.; Pan I. J.; Jones R.; Anderson S.; Patterson D. G.; Needham L. L.; Sjodin A. Individual characteristics associated with PBDE levels in US human milk samples. Environ. Health Perspect. 2010, 118, 155–160. 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind J. S.; Berlin C. M.; Sjodin A.; Turner W.; Wang R. Y.; Needham L. L.; Paul I. M.; Stokes J. L.; Naiman D. Q.; Patterson D. G. Do Human Milk Concentrations of Persistent Organic Chemicals Really Decline During Lactation? Chemical Concentrations During Lactation and Milk/Serum Partitioning. Environ. Health Perspect. 2009, 117, 1625–1631. 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Kannan K. Occurrence of melamine and its derivatives in breast milk from the United States and its implications for exposure in infants. Environ. Sci. Technol. 2019, 53, 7859. 10.1021/acs.est.9b02040. [DOI] [PubMed] [Google Scholar]
- Hines E. P.; Mendola P.; von Ehrenstein O. S.; Ye X. Y.; Calafat A. M.; Fenton S. E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg J.; Hanberg A.; Berglund M.; Skerfving S.; Remberger M.; Calafat A. M.; Filipsson A. F.; Jansson B.; Johansson N.; Appelgren M.; Hakansson H.; et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008, 116, 334–339. 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist A. M.; Olofsson U.; Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010, 12, 943–951. 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- Kim J. W.; Isobe T.; Muto M.; Tue N. M.; Katsura K.; Malarvannan G.; Sudaryanto A.; Chang K. H.; Prudente M.; Viet P. H.; et al. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 2014, 116, 91–97. 10.1016/j.chemosphere.2014.02.033. [DOI] [PubMed] [Google Scholar]
- Beser M. I.; Pardo O.; Beltran J.; Yusa V. Determination of 21 perfluoroalkyl substances and organophosphorus compounds in breast milk by liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Anal. Chim. Acta 2019, 1049, 123–132. 10.1016/j.aca.2018.10.033. [DOI] [PubMed] [Google Scholar]
- He C.; Toms L. M. L.; Thai P.; Van den Eede N.; Wang X. Y.; Li Y.; Baduel C.; Harden F. A.; Heffernan A. L.; Hobson P.; et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ. Int. 2018, 111, 124–130. 10.1016/j.envint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Xu Q. L.; Wu D.; Dang Y.; Yu L. Q.; Liu C. S.; Wang J. H. Reproduction impairment and endocrine disruption in adult zebrafish (Danio rerio) after waterborne exposure to TBOEP. Aquat. Toxicol. 2017, 182, 163–171. 10.1016/j.aquatox.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ji K.; Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 2012, 114, 173–181. 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Preston E. V.; McClean M. D.; Henn B. C.; Stapleton H. M.; Braverman L. E.; Pearce E. N.; Makey C. M.; Webster T. F. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int. 2017, 101, 158–164. 10.1016/j.envint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Kojima H.; Takeuchi S.; Itoh T.; Iida M.; Kobayashi S.; Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 2013, 314, 76–83. 10.1016/j.tox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Sun L.; Tan H.; Peng T.; Wang S.; Xu W.; Qian H.; Jin Y.; Fu Z. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2016, 35, 2931–2940. 10.1002/etc.3477. [DOI] [PubMed] [Google Scholar]
- Krivoshiev B. V.; Beemster G. T. S.; Sprangers K.; Blust R.; Husson S. J. A toxicogenomics approach to screen chlorinated flame retardants tris(2-chloroethyl) phosphate and tris(2-chloroisopropyl) phosphate for potential health effects. J. Appl. Toxicol. 2018, 38, 459–470. 10.1002/jat.3553. [DOI] [PubMed] [Google Scholar]
- Babich M. A.CPSC Staff Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam. 2006; pp 1–75. https://www.cpsc.gov/s3fs-public/pdfs/ufurn2.pdf.
- Wu Y.; Su G. Y.; Tang S.; Liu W.; Ma Z. Y.; Zheng X. M.; Liu H. L.; Yu H. X. The combination of in silico and in vivo approaches for the investigation of disrupting effects of tris (2-chloroethyl) phosphate (TCEP) toward core receptors of zebrafish. Chemosphere 2017, 168, 122–130. 10.1016/j.chemosphere.2016.10.038. [DOI] [PubMed] [Google Scholar]
- Du Z. K.; Wang G. W.; Gao S. X.; Wang Z. Y. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat. Toxicol. 2015, 161, 25–32. 10.1016/j.aquatox.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Rodil R.; Quintana J. B.; Reemtsma T. Liquid chromatography-tandem mass spectrometry determination of nonionic organophosphorus flame retardants and plasticizers in wastewater samples. Anal. Chem. 2005, 77, 3083–3089. 10.1021/ac048247s. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Fang J. Z.; Ren L.; Fan R. F.; Zhang J. Q.; Liu G. H.; Zhou L.; Chen D. Y.; Yu Y. X.; Lu S. Y. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ. Pollut. 2018, 235, 358–364. 10.1016/j.envpol.2017.12.092. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Kannan K. Concentrations and dietary exposure to organophosphate esters in foodstuffs from Albany, New York, United States. J. Agric. Food Chem. 2018, 66, 13525–13532. 10.1021/acs.jafc.8b06114. [DOI] [PubMed] [Google Scholar]
- Poma G.; Sales C.; Bruyland B.; Christia C.; Goscinny S.; Van Loco J.; Covaci A. Occurrence of organophosphorus flame retardants and plasticizers (PFRs) in Belgian foodstuffs and estimation of the dietary exposure of the adult population. Environ. Sci. Technol. 2018, 52, 2331–2338. 10.1021/acs.est.7b06395. [DOI] [PubMed] [Google Scholar]
- Poma G.; Glynn A.; Malarvannan G.; Covaci A.; Darnerud P. O. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem. Toxicol. 2017, 100, 1–7. 10.1016/j.fct.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Meeker J. D.; Stapleton H. M. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 2010, 118, 318–323. 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. P.; Xiong L. L.; Li D. K.; Chen C. J.; Cao Q. Monitoring and exposure assessment of organophosphorus flame retardants in source and drinking water, Nanjing, China. Environ. Monit. Assess. 2019, 10.1007/s10661-019-7239-0. [DOI] [PubMed] [Google Scholar]
- Cao Z. G.; Zhao L. C.; Zhang Y. C.; Ren M. H.; Zhang Y. J.; Liu X. T.; Jie J. Y.; Wang Z. Y.; Li C. H.; Shen M. H.; et al. Influence of air pollution on inhalation and dermal exposure of human to organophosphate flame retardants: A case study during a prolonged haze episode. Environ. Sci. Technol. 2019, 53, 3880–3887. 10.1021/acs.est.8b07053. [DOI] [PubMed] [Google Scholar]
- Lehmann G. M.; Verner M. A.; Luukinen B.; Henning C.; Assimon S. A.; LaKind J. S.; McLanahan E. D.; Phillips L. J.; Davis M. H.; Powers C. M.; et al. Improving the risk assessment of lipophilic persistent environmental chemicals in breast milk. Crit. Rev. Toxicol. 2014, 44, 600–617. 10.3109/10408444.2014.926306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D. A.; Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [PMC free article] [PubMed] [Google Scholar]
- Liu L. Y.; Salamova A.; He K.; Hites R. A. Analysis of polybrominated diphenyl ethers and emerging halogenated and organophosphate flame retardants in human hair and nails. J. Chromatogr. A 2015, 1406, 251–257. 10.1016/j.chroma.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Gong X.; Lin W.; Liu Y.; Wang Y.; Wu M.; Kannan K.; Ma J. Metabolites of organophosphate ester flame retardants in urine from Shanghai, China. Environ. Res. 2018, 164, 507–515. 10.1016/j.envres.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Ding J. J.; Xu Z. M.; Huang W.; Feng L. M.; Yang F. X. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci. Total Environ. 2016, 554, 211–217. 10.1016/j.scitotenv.2016.02.171. [DOI] [PubMed] [Google Scholar]
- Leung A. M.; Braverman L. E.; He X.; Schuller K. E.; Roussilhes A.; Jahreis K. A.; Pearce E. N. Environmental perchlorate and thiocyanate exposures and infant serum thyroid function. Thyroid 2012, 22, 938–943. 10.1089/thy.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J.; Holden A.; Sharp M.; Tanner M.; Williams-Derry C.; Hooper K. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere 2007, 67, S307–S317. 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Mendonca K.; Hauser R.; Calafat A. M.; Arbuckle T. E.; Duty S. M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health 2014, 87, 13–20. 10.1007/s00420-012-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan C. C.; Cottingham K. L.; Jackson B. P.; Farzan S. F.; Gandolfi A. J.; Punshon T.; Folt C. L.; Karagas M. R. Estimated exposure to arsenic in breastfed and formula-fed infants in a United States cohort. Environ. Health Perspect. 2015, 123, 500–506. 10.1289/ehp.1408789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan C. C.; Abdallah M. A.-E.; Wu N.; Heiger-Bernays W.; McClean M. D.; Harrad S.; Webster T. F. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environ. Sci. Technol. 2012, 46, 12146–12153. 10.1021/es302638d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Toms L. L.; Thai P.; Van den Eede N.; Wang X.; Li Y.; Baduel C.; Harden F. A.; Heffernan A. L.; Hobson P.; et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ. Int. 2018, 111, 124–130. 10.1016/j.envint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Chemical Data Access Tool (CDAT). Chemical Data Reporting (CDR) Results 2016 (data set). https://www.epa.gov/chemical-data-reporting/2016-chemical-data-reporting-results.
- Giraudo M.; Douville M.; Houde M. Chronic toxicity evaluation of the flame retardant tris (2-butoxyethyl) phosphate (TBOEP) using Daphnia magna transcriptomic response. Chemosphere 2015, 132, 159–165. 10.1016/j.chemosphere.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Porter E.; Crump D.; Egloff C.; Chiu S.; Kennedy S. W. Use of an avian hepatocyte assay and the avian toxchip polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ. Toxicol. Chem. 2014, 33, 573–582. 10.1002/etc.2469. [DOI] [PubMed] [Google Scholar]
- Chen D.; Letcher R. J.; Chu S. Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography-tandem quadrupole mass spectrometry. J. Chromatogr. A 2012, 1220, 169–174. 10.1016/j.chroma.2011.11.046. [DOI] [PubMed] [Google Scholar]
- Lu S. Y.; Li Y. X.; Zhang T.; Cai D.; Ruan J. J.; Huang M. Z.; Wang L.; Zhang J. Q.; Qiu R. L. Effect of e-waste recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress. Environ. Sci. Technol. 2017, 51, 2427–2437. 10.1021/acs.est.6b05462. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li W. H.; Martinez-Moral M. P.; Sun H. W.; Kannan K. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environ. Int. 2019, 122, 213–221. 10.1016/j.envint.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.; Lu S.; Huang M.; Zhou M.; Zhou Z.; Zheng H.; Jiang Y.; Bai X.; Zhang T. Urinary metabolites of organophosphate flame retardants in 0–5-year-old children: Potential exposure risk for inpatients and home-stay infants. Environ. Pollut. 2018, 243, 318–325. 10.1016/j.envpol.2018.08.051. [DOI] [PubMed] [Google Scholar]
- Wei B.; Goniewicz M. L.; O’Connor R. J.; Travers M. J.; Hyland A. J. Urinary metabolite levels of flame retardants in electronic cigarette users: A study using the data from NHANES 2013–2014. Int. J. Environ. Res. Public Health 2018, 15, 201. 10.3390/ijerph15020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; English K.; Baduel C.; Thai P.; Jagals P.; Ware R. S.; Li Y.; Wang X.; Sly P. D.; Mueller J. F. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ. Res. 2018, 164, 262–270. 10.1016/j.envres.2018.02.040. [DOI] [PubMed] [Google Scholar]
- Hardell E.; Carlberg M.; Nordstrom M.; van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci. Total Environ. 2010, 408, 4412–4419. 10.1016/j.scitotenv.2010.06.029. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Exposure factors handbook: 2011 edition (final). 2011. https://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=236252.
- U.S. Environmental Protection Agency . Regional screening levels (RSLs)-user’s guide. 2018. https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide.
- van den Eede N.; Dirtu A. C.; Neels H.; Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011, 37, 454–461. 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.