Abstract
Gut symbionts can augment resistance to pathogens by stimulating host immune responses, competing for space and nutrients, or producing antimicrobial metabolites. Gut microbiota of social bees, which pollinate many crops and wildflowers, protects hosts against diverse infections and might counteract pathogen-related bee declines. Bumble bee gut microbiota, and specifically abundance of Lactobacillus “Firm-5” bacteria, can enhance resistance to the trypanosomatid parasite Crithidia bombi. However, the mechanism underlying this effect remains unknown. We hypothesized that the Firm-5 bacterium Lactobacillus bombicola, which produces lactic acid, inhibits C. bombi via pH-mediated effects.
Consistent with our hypothesis, Lactobacillus bombicola spent medium inhibited C. bombi growth via reduction in pH that was both necessary and sufficient for inhibition. Inhibition of all parasite strains occurred within the pH range documented in honey bees, though sensitivity to acidity varied among strains. Spent medium was slightly more potent than HCl, D-, and L-lactic acids for a given pH, suggesting that other metabolites also contribute to inhibition. Results implicate symbiont-mediated reduction in gut pH as a key determinant of trypanosomatid infection in bees. Future investigation into in vivo effects of gut microbiota on pH and infection intensity would test the relevance of these findings for bees threatened by trypanosomatids.
Keywords: microbiome, pH-dependent interactions, cell-free supernatant, pollinator decline, lactic acid bacteria, flagellates, competition, parasite strain variation, Firmicutes, Trypanosomatidae
INTRODUCTION
Both animals and plants associate with symbiotic bacterial communities that provide functional benefits to their hosts (Mueller and Sachs, 2015). The gut microbiota comprise some of the largest and most well-studied communities of host-associated symbionts (Kamada et al., 2013). The bacterial communities that colonize the gut and skin epithelia interact directly with potential pathogens in the environment, and can influence infection by stimulation of the host immune system, competition for space and nutrients, and production of inhibitory substances such as organic acids and bacteriocins (Dillon and Dillon, 2004; Round and Mazmanian, 2009; Daskin et al., 2014; Raymann and Moran, 2018). Interactions between the gut microbiota and pathogens of bees is an emerging area of research with both fundamental and applied importance (Gaggìa et al., 2018; Raymann and Moran, 2018). Elucidation of the antipathogenic potential of the bee microbiota may ultimately help to preserve the pollination services provided by both wild and managed bees, which improve yields of over two-thirds of common agricultural crops (Klein et al., 2007) and contribute to the >$150B per year in economic value supplied by animal pollination (Gallai et al., 2009).
The gut microbiota of corbiculate (“pollen basket”) bees, including honey and bumble bees, comprises a common core of five bacterial clades: Snodgrassella (Betaproteobacteria), Gilliamella (Gammaproteobacteria), Bifidobacterium, and Lactobacillus clades “Firmicutes-4” and “Firmicutes-5” (Kwong et al., 2017b). In addition to these core symbionts, bees may be infected by a variety of bacterial, fungal, protozoal, and viral pathogens (Evans and Spivak, 2010), many of which are shared between wild and managed bees (Graystock et al., 2016), can elevate mortality (Fürst et al., 2014), and have been implicated in declines of bee populations on multiple continents (Cameron et al., 2011; Schmid-Hempel et al., 2014; Goulson et al., 2015). Both core and non-core microbiota have been found to stimulate immunity and enhance bee resistance to pathogens (Evans and Lopez, 2004; Engel et al., 2016; Kwong et al., 2017a; Raymann and Moran, 2018). For example, depletion or perturbation of the gut microbiota increased the severity of bacterial, fungal, and protozoal infections in honey bees (Schwarz et al., 2016; Kwong et al., 2017a; Raymann and Moran, 2018), whereas supplementation with core and hive-associated bacteria improved survival of infected larvae and adults (Forsgren et al., 2010; Vásquez et al., 2012; Kwong et al., 2017a). Several studies have shown direct inhibitory effects of gut and hive-associated symbionts against common bee pathogens (Evans and Armstrong, 2006; Sabaté et al., 2009; Praet et al., 2018), which suggests a parsimonious explanation for the effects of symbionts on infection.
The gut microbiota of bumble bees (Bombus spp.) has been repeatedly associated with resistance to infection with the trypanosomatid gut parasite Crithidia bombi (Koch and Schmid-Hempel, 2012; Koch et al., 2012; Cariveau et al., 2014; Mockler et al., 2018). This parasite has a variety of negative effects on bees. Symptoms include reduced rates of foraging, pollen collection, and learning for worker bees (Shykoff and Schmid-Hempel, 1991; Gegear et al., 2005, 2006); and reduced winter survival and spring nest-founding success for queen bees (Brown et al., 2003). Infection with C. bombi can also exacerbate susceptibility to co-occurring stressors such as starvation, pesticides (Fauser-Misslin et al., 2014), and nectar alkaloids (Palmer-Young et al., 2017b). Trypanosomatid infections appear to be common in corbiculate bees, afflicting over half of individuals in some honey and bumble bee populations (Schmid-Hempel and Ebert, 2003; Cornman et al., 2012), and have been correlated with honey bee colony collapses (Cornman et al., 2012; Ravoet et al., 2013) and native bumble bee declines (Schmid-Hempel et al., 2014). Both the presence and composition of the bumble bee microbiota may improve resistance to C. bombi. For example, germ-free rearing conditions and treatment with antibiotics both resulted in higher infection intensity in B. terrestris (Koch and Schmid-Hempel, 2011, 2012). In contrast, absolute and relative abundance of select core gut bacteria were correlated with resistance to infection in both field surveys and fecal transplant experiments (Koch and Schmid-Hempel, 2011, 2012; Cariveau et al., 2014; Mockler et al., 2018). Just 3 taxa—Snodgrassella, Gilliamella, and Lactobacillus Firm-5—generally account for over 80% of the total gut bacteria in bumble bees (Koch and Schmid-Hempel, 2012; Billiet et al., 2017; Mockler et al., 2018). In both B. terrestris and B. impatiens, inoculation with microbiota rich in Lactobacillus Firm-5 resulted in resistance to C. bombi infection (Koch and Schmid-Hempel, 2012; Mockler et al., 2018). However, no study has examined the mechanisms by which these microbes influence parasite growth.
Lactobacillus Firm-5 is a group of lactic acid-producing bacteria (Praet et al., 2015). One way that they might increase host resistance to parasites is via modification of gut chemistry. Lactic acid fermentation results in production of organic acids that lower pH and inhibit growth of organisms that cause spoilage and infection (Adams and Hall, 1988; Lindgren and Dobrogosz, 1990; Glass et al., 1992; Russell and Diez-Gonzalez, 1998). Indeed, lactic acid bacteria have a long history of use in food preservation in both human and insect societies (Salminen and Wright, 2004; Anderson et al., 2014). In the host intestine, lactic acid bacteria can inhibit enteric pathogens, such as Salmonella and E. coli (Gorbach, 1990). This inhibition may reflect stimulation of the host immune system (Presser et al., 1997; Cox et al., 2010), including that of insects (Evans and Lopez, 2004; Evans and Armstrong, 2006). However, Lactobacillus-mediated inhibition of pathogens is most simply explained by the direct antimicrobial activity of Lactobacillus metabolites. These metabolites, which include lactic acid and bacteriocins (Lindgren and Dobrogosz, 1990), may reduce the suitability of the gut environment for pathogens.
In the bee gut, Lactobacillus Firm-5 has been shown to have a disproportionately large effect on gut metabolomics. In honey bees, mono-inoculation with Firm-5 accounted for over 80% of the changes seen in bees inoculated with a full complement of gut microbes (Kešnerová et al., 2017). Firm-5 isolates also showed in vitro inhibitory activity against the pathogens Paenibacillus larvae and Melisococcus plutonius (Praet et al., 2018). The high relative abundance of the Firm-5 clade in bumble bees (often >30% total bacteria (Koch and Schmid-Hempel, 2012; Billiet et al., 2017)), combined with its consistent association with resistance to trypanosomatid infection, suggests that Lactobacillus Firm-5 plays a major role in bumble bee resistance to trypanosomatid parasites.
We hypothesized that Lactobacillus Firm-5 enhances resistance to trypanosomatid parasites primarily by modifying the pH of the enteric environment. To test this hypothesis, we measured the inhibitory effects of spent medium from Lactobacillus bombicola, a member of the Firmicutes-5 clade that is ubiquitous in the gut microbiota of corbiculate bees, including Bombus spp. (Kwong et al., 2017b; Billiet et al., 2017; Praet et al., 2018), on in vitro growth of several strains of C. bombi. We predicted that spent medium from L. bombicola would inhibit C. bombi growth, that the acidity of spent medium would be both necessary and sufficient to account for parasite inhibition, and that C. bombi strains would vary in sensitivity to spent medium.
MATERIALS AND METHODS
Overview of experiments.
Three experiments were conducted to evaluate effects of spent medium from L. bombicola cultures on growth of C. bombi. Spent medium was generated by growth of L. bombicola in MRS broth for 3 d, followed by sterile filtration to remove live cells. For C. bombi growth assays, the MRS-based spent medium (or MRS broth control) was diluted 1:1 in fresh, Crithidia-specific “FPFB” medium (Salathé et al., 2012). (1) The Neutralization Experiment tested whether spent medium would inhibit growth, and whether acidity of the spent medium was necessary or sufficient for inhibition. (2) The Acidification Experiment tested for variation in pH-dependent growth inhibition due to various sources of acidity. (3) The Strain Variation Experiment tested for variation in sensitivity to spent medium among different parasite strains.
Cell Cultures.
Lactobacillus bombicola strain 70–3, isolated from Bombus lapidarius collected near Ghent Belgium (isolate “28288T” (Praet et al., 2015)), was obtained from the DSMZ and grown in 2 mL screw-cap tubes in MRS broth (Research Products International, Mt. Prospect, IL) with 0.05% cysteine at 27 °C. Crithidia bombi cell cultures were isolated from bumble bee intestines by flow cytometry-based single cell sorting as described previously (Salathé et al., 2012). Cultures originated from wild infected bumble bees. Strains VT1 (Vermont, USA, 2013, courtesy Rebecca Irwin) and IL13.2 (Illinois, USA, 2013, courtesy Ben Sadd) originated from B. impatiens workers. Strains S08.1 (Switzerland, 2008, courtesy Ben Sadd) originated from B. terrestris. These same cell lines have been used to assess effects of phytochemicals on parasite growth (Palmer-Young et al., 2017a). Briefly, cells from fecal samples were sorted into 96-well plates containing “FPFB” culture medium with 10% heat-inactivated fetal bovine serum and incubated at 27 °C. Cultures with successful growth and absence of visible contamination were transferred to vented, 25 cm2 tissue culture flasks, grown to high density, and cryopreserved at −80 °C until several weeks before the experiments began (Salathé et al., 2012). Culture identity was confirmed as C. bombi based on glyceraldehyde 3-phosphate dehydrogenase and cytochrome b gene sequences. Cultures were inspected weekly to verify absence of contamination.
The Neutralization and Acidification Experiments were performed with a line of strain “VT1” that had been in continuous culture for 2 months at the start of the experiments presented here, with transfers to fresh medium every 3–4 d. This line is referred to as “VT1*” in the Multi-Strain Experiment, to differentiate it from the more recently thawed line of the same strain. All other strains in the Multi-Strain Experiment were thawed 20 d prior to the assay and transferred to fresh medium every 3–7 d, depending on growth.
Generation of spent medium.
To generate spent medium, L. bombicola aliquots were transferred to 8 mL fresh medium and grown in screw-cap 14 mL conical tubes at 27 °C for 3 d. The resulting spent medium (net OD630 nm = 0.500–0.700) was sterile-filtered through a 0.22 μm membrane and stored at −20 °C until use in experiments (not more than 2 weeks). Fresh MRS medium, incubated under identical conditions, was used as a control.
Neutralization Experiment.
To evaluate the inhibitory effects of spent medium, neutralized spent medium, and acidified fresh medium, growth of C. bombi was compared across four MRS-based treatments: Spent medium (“Spent”, initial pH 4.8), spent medium neutralized to pH 6.2 with 1 M NaOH (“Neutralized spent”), fresh MRS medium (“Fresh”, pH 6.2), and fresh MRS medium acidified to pH 4.8 with 1 M HCl (“Acidified fresh”). The C. bombi culture was diluted to an OD of 0.020 in fresh FPFB medium (pH 5.88). The resulting cell suspension (100 μL) was added to wells of a 96-well plate containing an equal volume of the MRS-based treatment medium, resulting in a final net OD630 nm of 0.010. Growth was measured twice daily by optical density (630 nm) over the ensuing 48 h on an EL-800 plate reader spectrophotometer (Biotek, Winooski, VT). Net optical density at each time point was computed by subtracting OD of wells containing the corresponding MRS-based treatment medium and FPFB medium without C. bombi; this controlled for any differences in optical density that occurred independent of C. bombi growth. The experiment included 18 replicate wells per treatment.
Acidification Experiment.
To compare variation in growth inhibition across different sources of acidity, growth of C. bombi was compared in dilutions of spent medium (initial pH 4.65), and in fresh medium acidified with D-lactic acid (pH 4.82), L-lactic acid (pH 4.77), or HCl (pH 4.73). Each base medium was diluted with fresh MRS medium to 0, 20, 40, 60, 80, or 100% of initial concentration. The final pH of each treatment was measured with a pH meter (‘Orion Star’, Thermo, Waltham, MA) after combination with an equal volume of FPFB medium. Growth of C. bombi (initial OD 0.010) was evaluated with a 96-well plate assay as in the Neutralization Experiment above. The experiment included 12 replicate wells per concentration of each acidification treatment. To verify the relative potency of spent vs. acidified medium against different parasite strains, the experiment was repeated with two C. bombi strains (IL13.2 and VT1*) tested in parallel against two of the four acidification treatments (spent medium and HCl-acidified medium); results are shown in Supplementary Figure 2.
Strain Variation Experiment.
To compare susceptibility to spent medium across different C. bombi strains and degrees of acclimation to the culture environment, inhibitory concentration of spent medium was compared across four cell lines: the “VT1*” line of strain VT1 that had been used for the above experiments, and by this time had been in continuous culture for 3 months; and strains VT1, IL13.2, and S08.1 that had been thawed 20 d prior to the experiment. Six dilutions of spent medium (0–100%) were prepared in fresh MRS medium; final pH of each treatment was measured after combination with an equal volume of FPFB medium. Growth of each line of C. bombi (initial OD 0.010) was evaluated with a 96-well plate assay as in the Neutralization Experiment above. The experiment included 12 replicate wells per cell line and spent medium concentration.
Statistical analysis.
Statistical analysis was performed in R v3.4.3 for Windows (R Core Team, 2014). For the Acidification and Strain Variation Experiments, dose-response curves to relate growth rate to pH were estimated with the drm function in R package drc (Ritz et al., 2015). Under the conditions of the experiment (initial OD = 0.010, incubation temperature 32 °C), Crithidia bombi growth was found to be exponential over the first 24 h of incubation (Supplementary Figure 1). Therefore, the rate of growth (log2(OD/OD0)/t) over the first incubation interval (0–21 h) was used as the response variable for the dose-response models. A log-logistic function, with the lower asymptote fixed at 0, was fitted to the growth measurements for each acidification treatment (Acidification Experiment) or cell line (Strain Variation Experiment), using final pH of the treatment medium at the start of the experiment (i.e., after combination of the MRS-based treatment with an equal volume FPFB medium) as the predictor variable. The 95% confidence intervals for EC50 pH (i.e., the pH that inhibited growth by 50% relative to the unacidified control treatment), and 95% CI’s for ratios of EC50 pH among acidification treatments and strains, were estimated using the delta method (drc function EDcomp (Ritz et al., 2015)). Acidification treatments and strains were considered significantly different when the 95% confidence interval for the ratio of their EC50 pH values did not include 1.
RESULTS
Neutralization Experiment: acidity-dependent inhibition of C. bombi by spent medium.
We found that L. bombicola spent medium completely inhibited growth of C. bombi cell cultures (Figure 1). However, neutralized spent medium had no inhibitory effect, indicating that acidity of the spent medium was necessary for inhibition (Figure 1). Moreover, acidification of fresh (Lactobacillus-specific) MRS medium to pH 4.8 with L-lactic acid led to a level of growth inhibition that was comparable to that caused by pH 4.8 spent medium (Figure 1). This demonstrated that spent medium from L. bombicola inhibited C. bombi growth, and that changes in pH were necessary and qualitatively sufficient to account for this inhibition.
Figure 1. Spent medium from Lactobacillus bombicola inhibited growth of Crithidia bombi (Strain VT1); acidity of the spent medium was necessary and sufficient for inhibition.
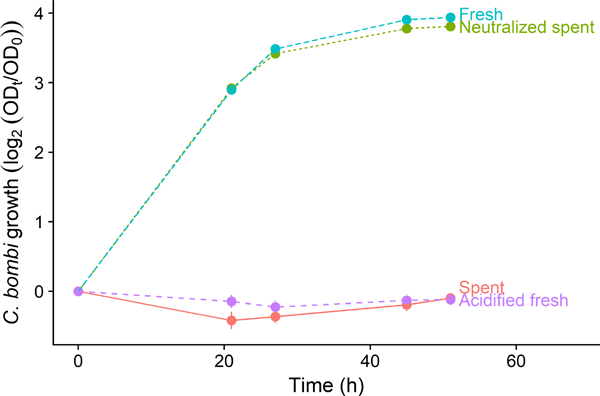
Spent medium (“Spent”, red solid line) completely inhibited parasite growth. However, spent medium neutralized to pH 6.2 with NaOH (“Neutralized spent”, green dotted line) resulted in no inhibition relative to the fresh medium control (“Fresh”, light green dashed line), demonstrating that acidity was necessary for inhibition. Fresh medium acidified to pH 4.8 with lactic acid (“Acidified fresh”, blue dashed line) showed that acidity was sufficient for complete growth inhibition. Final pH of both spent medium and acidified fresh medium was 5.0 after combination with equal volume of fresh Crithidia medium (pH 5.9). Y-axis represents approximate number of parasite cell divisions, as measured by optical density (630 nm). Points and error bars show means and 95% confidence intervals (n = 36 wells per treatment).
Acidification Experiment: pH-dependent inhibition of C. bombi with different sources of acidity.
Having established pH-dependent growth inhibition, we conducted a follow-up experiment to quantify C. bombi growth rates across a range of pH values, and to compare the relative inhibitory effects of L. bombicola spent medium with that of three other sources of acidity: HCl, L-lactic acid (the form produced by animal cells (Ewaschuk et al., 2002)), and D-lactic acid (the form produced by L. bombicola (Praet et al., 2015)). We used log-logistic models to estimate and compare the EC50 pH (i.e., the pH that inhibited growth by 50% relative to the unacidified control treatment) for each source of acidity. All four sources of acidity resulted in qualitatively similar inhibition of C. bombi (Figure 2A), with considerable inhibition achieved within the pH range previously measured in honey bee guts (vertical lines in Figure 2A, from (Zheng et al., 2017); no data are available on pH of bumble bee guts). However, the EC50 pH varied somewhat across sources of acidity, with a hierarchy of pH-dependent inhibitory potency in the order HCl < L-lactic acid < D-lactic acid < spent medium (Figure 2B, see Supplementary Information Table S1 for table of EC50 values and Supplementary Data S1 for model parameters, confidence intervals on EC50 ratios, and raw data). An additional experiment with a second C. bombi strain (‘IL13.2’) confirmed the greater potency of spent medium relative to HCl-acidified medium across multiple parasite genotypes (Supplementary Figure 2).
Figure 2. Different sources of acidity varied in pH-dependent inhibitory potency against C. bombi (Strain VT1).
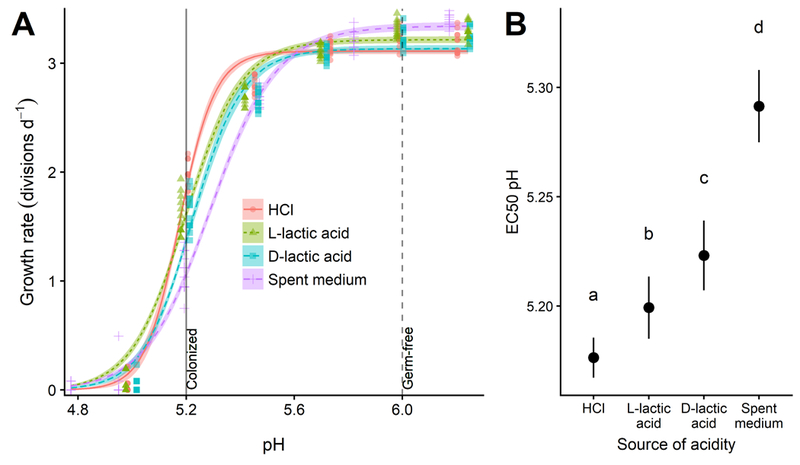
(A) Dose-response curves relating pH to growth rate. X-axis shows final pH of treatment medium after combination of L. bombicola spent medium or acidified MRS medium with an equal volume of Crithidia-specific FPFB medium. Y-axis represents growth rate over first 21 h of incubation, measured as number of doublings per day by optical density (OD 630 nm). Lines and shaded bands represent model predictions and standard errors for each source of acidity. Points show raw data for each replicate well (n = 12). Vertical lines correspond to pH values measured in ileum and rectum of microbe-colonized and germ-free honey bees (Zheng et al., 2017), and are shown as an estimate of gut pH range in bumble bees. (B) 50% inhibitory concentrations (EC50, i.e., the concentration that inhibits growth by 50%) for different sources of acidity. Point estimates and 95% confidence intervals are derived from model fits shown in panel (A). Higher EC50 pH estimates correspond to higher inhibitory potency for a given level of acidity. Different lower-case letters indicate statistically significant (P < 0.05) differences in pairwise comparisons of EC50 pH by source of acidity.
Strain Variation Experiment: sensitivity to spent medium differs across C. bombi strains and rate of growth in culture (Figure 3).
Figure 3. Crithidia bombi pH sensitivity varied according to strain identity and rate of growth in culture.
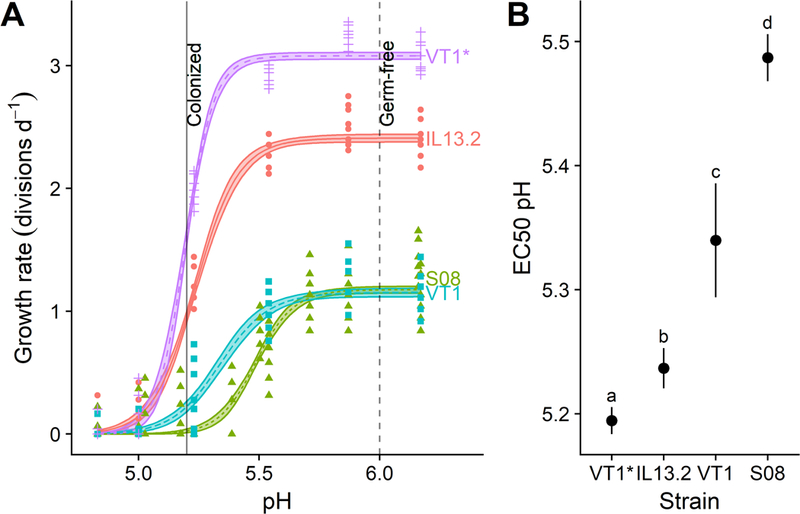
(A) Dose-response curves relating pH to growth rate for different C. bombi strains. X-axis shows final pH of treatment medium after combination of L. bombicola spent MRS medium with an equal volume of Crithidia-specific FPFB medium. Y-axis represents growth rate over first 20 h of incubation, measured as number of doublings per day by optical density (OD 630 nm). Lines and shaded bands represent model predictions and standard errors for each strain. Points show raw data for each replicate well (n = 12). Vertical lines correspond to pH values measured in ileum and rectum of microbe-colonized and germ-free honey bees (Zheng et al., 2017), and are shown as an estimate of gut pH range in bumble bees. Strains “VT1*” and “VT1” are the same strain, but “VT1*” had been grown in continuous culture for 3 months prior to the experiment, whereas “VT1” and all other strains had been thawed from cryopreserved stock 3 weeks prior. (B) 50% inhibitory concentrations (EC50) for each strain. Point estimates and 95% confidence intervals are derived from model fits shown in panel (A). Higher EC50 pH estimates correspond to higher sensitivity to acidity. Different lower-case letters indicate statistically significant (P < 0.05) differences in pairwise comparisons of EC50 pH by source of acidity. For all strains, inhibition occurred within the pH range measured in honey bee guts (data from (Zheng et al., 2017)).
We tested the dose-dependent effects of spent medium across three C. bombi strains, including two lines of strain VT1—the line that had been used for the above experiments and kept in continuous culture over the preceding 3 months (“VT1*), and a second line that had been thawed 20 d prior to the experiment (“VT1”). Values for EC50 pH showed statistically and biologically meaningful variation across cell lines (Figure 3). The line most thoroughly acclimated to the culturing conditions, VT1*, exhibited both the fastest growth (Figure 3A) and the lowest sensitivity to spent medium, as indicated by its low EC50 pH value (Figure 3B); strain IL13.2 exhibited the second-fastest growth and the second-lowest sensitivity to spent medium. Strains VT1 and S08.1 had similar growth rates in the absence of spent medium (Figure 3A), but had significantly different EC50 pH values (Figure 3B; see Supplementary Information Table S1 for table of EC50 values and Supplementary Data S1 for model parameters, confidence intervals on EC50 ratios, and raw data). All C. bombi strains suffered growth inhibition within the pH range (5.2–6.0) documented in the honey bee hindgut (Zheng et al., 2017) (Figure 3A).
DISCUSSION
Our results show that production of acids by the core bumble bee hindgut symbiont, L. bombicola, is both necessary and sufficient to inhibit growth of the widespread trypanosomatid parasite C. bombi. The growth-inhibitory effects of L. bombicola-acidified spent medium were qualitatively similar across C. bombi isolates and occurred within a pH range that is physiologically realistic for the bee hindgut where C. bombi establishes. Because Lactobacilli and other acid-producing bacteria are dominant members of the bumble bee gut microbiota, our results suggest that the inhibitory effects of bumble bee gut microbiota on C. bombi infection intensity can be largely attributed to microbial acidification of the gut. Our findings provide a mechanistic basis to understand how microbiota may affect trypanosomatid infection in corbiculate bees that share a core microbiome (Kwong et al., 2017b) and can be infected with identical and related parasites, including trypanosomatids (McMahon et al., 2015; Schwarz et al., 2015; Tripodi et al., 2018).
Inhibitory activity of L. bombicola spent medium is driven by production of acids.
The effects of L. bombicola spent medium on C. bombi growth could be explained by the acidity of the spent medium. Environmental pH is a recognized driver of interactions in microbial communities, where species vary in how they alter the pH of their surroundings and in the pH range at which they can grow (Morton et al., 2017; Ratzke and Gore, 2018). In honey bees, microbial colonization with core symbionts resulted in acidification of the hindgut lumen (Zheng et al., 2017). In our cell culture experiments, a reduction in pH that corresponds to the difference between guts of germ-free (pH ~6.0) and normal, hive-reared honey bees (pH ~5.2 (Zheng et al., 2017)) profoundly inhibited growth of C. bombi. Our findings indicate that symbiont-mediated gut acidification could act as an important filter that prevents trypanosomatid establishment and defends bees against infection.
Spent medium was more strongly inhibitory than expected based on pH alone.
Whereas all four tested acids inhibited C. bombi in a dose-dependent fashion, the EC50 pH varied across sources of acidity (Figure 2). Both enantiomers of lactic acid were more inhibitory than HCl for a given pH. This finding is consistent with prior work on bacteria, which showed growth inhibition at relatively high pH when lactic acid, rather than HCl, is used as the acidulant (Adams and Hall, 1988; Glass et al., 1992). For example, the inhibitory pH of E. coli was 0.1 pH units higher with lactic acid, rather than HCl, as the acidulant (Glass et al., 1992). This increased activity reflects the fact that lactic acid and other weak acids are undissociated at low pH, which allows them to penetrate membranes of target cells with relative ease. Once inside the relatively alkaline cytoplasm of the cell, the acid dissociates, and disrupts the proton motive force necessary for energy production and homeostasis (Russell and Diez-Gonzalez, 1998).
We also found very slightly but significantly higher potency of D-lactic acid relative to L-lactic acid (Figure 2). L-lactic acid is the enantiomer more often produced by trypanosomatids, including by Leishmania species that are closely related to C. bombi (Bringaud et al., 2006), and L-lactic acid dehydrogenase has been found in the genome of honey bee-infective trypanosomatids (Runckel et al., 2014). It is possible that C. bombi can use oxidative phosphorylation (Bringaud et al., 2006) to metabolize this enantiomer, and thereby reduce its toxicity, or that they have greater resistance to the enantiomer that they produce through their own metabolism. For example, L. bulgaricus produced D-lactic acid, and were less inhibited by this self-generated enantiomer than by L-lactic acid (Benthin and Villadsen, 1995).
Prior measurements of the pH range and sources of acidity in honey bee gut both indicate that pH-mediated inhibition of trypanosomatids is achievable for corbiculate bees. The pH of a symbiont-colonized honey bee hindgut (~5.2 (Zheng et al., 2017)) was similar to the EC50 pH for HCl (5.18) and lower than the EC50 pH due to spent medium (5.29). In vitro, L. bombicola produced exclusively D-lactic acid (Praet et al., 2015), which was the most inhibitory (i.e., highest EC50 pH) of the pure acids tested here (Fig 3). Moreover, the specific acids that are found in the gut environment are generally more inhibitory for a given pH than those tested here. In the honey bee hindgut, the most abundant acids were acetic acid and succinic acid (Zheng et al., 2017). Both acetic acid (pKa = 4.95) and succinic acid (pKa = 4.2 for first deprotonation and 5.6 for second deprotonation) have relatively high pKa’s relative to lactic acid (pKa = 3.86) and HCl (pKa = −7). These high pKa values mean that at low pH, the acids are found in their more potent, undissociated form. As a result, these high-pKa acids generally have antimicrobial effects that are even stronger than those of lactic acid for a given pH (Adams and Hall, 1988). Indeed, lactic and acetic acids can have synergistic effects against growth of E. coli (Adams and Hall, 1988), with lactic acid producing a low pH that increases the fraction of undissociated acetic acid (Adams and Hall, 1988).
Spent medium was slightly more inhibitory than all pure acids for a given pH (Figure 3). Because neutralization completely removed the inhibitory activity of spent medium, it appears that some aspect of the spent medium—whether the existence of some metabolite or the relative lack of nutrients—is only inhibitory at low pH. In other words, something about the spent medium is potentiated by an acidic environment. For example, low pH could facilitate solubility or penetration of non-lactic acid components, such as bacteriocins; this type of synergy was seen in other studies of Lactobacillus spp. (Fayol-Messaoudi et al., 2005; Keersmaecker et al., 2006). Within the bee gut, synergistic effects could also occur between organic acids and toxins produced by other members of the microbiota (Praet et al., 2015; Steele et al., 2017).
Strains varied in sensitivity to spent medium.
We found that sensitivity to spent medium varied by C. bombi strain and rate of growth in culture. Strain VT1 that had been in continuous culture for 3 months and had the fastest growth rate was the least sensitive to spent medium, followed by the next-fastest strain IL13.2, the recently thawed line of Strain VT1, and strain S08.1. The comparison between the recently thawed VT1 and S08.1 strains—which had similar maximal growth rates, but different levels of sensitivity to spent medium—indicates that pH sensitivity can have a genotypic basis and is not purely driven by the overall growth rate in culture. Strains of C. bombi have been shown to be both genetically and phenotypically diverse, and to vary in growth rate (Imhoof and Schmid-Hempel, 1998), infectivity (Barribeau et al., 2014), and responses to host diet composition (Sadd, 2011), phytochemicals (Palmer-Young et al., 2016), and microbiota (Koch and Schmid-Hempel, 2012). Our study documents variation in pH sensitivity within an ecologically relevant range of pH that is likely representative of the environment in the bumble bee gut.
Our results showed some growth inhibition of all strains—and complete inhibition of some strains—within the pH range measured in the gut of honey bees (Zheng et al., 2017). The pH found in a germ-free gut (5.8–6.0) was favorable for growth, consistent with the high C. bombi infection intensities found in germ-free and antibiotic-treated bees (Koch and Schmid-Hempel, 2011), whereas pH of a symbiont-colonized gut (<5.2) would be expected to inhibit growth of all strains. Thus, pH sensitivity may constrain ability of strains to colonize certain host genotypes (Barribeau et al., 2014) or enterotypes (Koch and Schmid-Hempel, 2012; Li et al., 2015) characterized by low gut pH. For example, inoculation with a microbiota high in Lactobacillus Firm-5 resulted in lower overall infection intensity and favored infection with a single parasite strain that was less successful in bees inoculated with microbiota low in Firm-5 (Koch and Schmid-Hempel, 2012). We hypothesize that low-pH conditions favor strains that are more tolerant, whereas high-pH conditions favor strains that are strong competitors. Further experiments are needed to investigate the extent to which parasite populations are selected for pH tolerance, and possible trade-offs between growth rate, infectivity, or tolerance to environmental stressors and insect immune factors. Environment-dependent selection for these other traits could maintain variation in pH tolerance within parasite populations.
Gut microbiota-driven changes in pH may explain patterns of trypanosomatid infection in bees.
The pH-dependent inhibition demonstrated here is consistent with past surveys and experiments that showed negative correlations between abundance of acid-producing gut symbionts and C. bombi infection intensity, and with associations between microbiota composition and relative infectivity of different parasite strains. The Bombus gut microbiota is dominated by three taxa—Lactobacillus Firm-5, Gilliamella, and Snodgrassella, that made up over 80% of total gut bacteria (Koch and Schmid-Hempel, 2012; Billiet et al., 2017). In fecal transplant experiments of B. terrestris and B. impatiens, relative abundances of Lactobacillus Firm-5 and Gilliamella were negatively correlated with C. bombi infection intensity (Koch and Schmid-Hempel, 2011, 2012; Koch et al., 2012; Mockler et al., 2018). Both Lactobacillus and Gilliamella ferment sugars to produce acids (Engel et al., 2012; Kešnerová et al., 2017).
In contrast, Snodgrassella abundance was not correlated with resistance to C. bombi in either B. terrestris or B. impatiens (Koch and Schmid-Hempel, 2012; Mockler et al., 2018), and pre-inoculation with S. alvi reduced resistance to trypanosomatid infection in A. mellifera (Schwarz et al., 2016). Whereas Lactobacillus and Gilliamella spp. produce acids from sugars, Snodgrassella consumes organic acids (Kwong and Moran, 2013; Kešnerová et al., 2017). This metabolic activity could elevate gut pH to levels that are more hospitable to trypanosomatids. On the other hand, Snodgrassella abundance was negatively correlated with infection prevalence in a field survey (Cariveau et al., 2014). This correlation might reflect the association of Snodgrassella with acid-producing Gilliamella (Kešnerová et al., 2017), rather than inhibitory effects of Snodgrassella per se. Snodgrassella and Gilliamella form a biofilm that lines the ileum (Engel et al., 2012) and could competitively inhibit trypanosomatid attachment to the gut wall (Gorbunov, 1996; Schwarz et al., 2015). Further study would be needed to determine the relative contributions of gut acidification versus biofilm formation to microbiota-induced inhibition of parasites in bee guts. It would be intriguing to investigate how Snodgrassella abundance alters gut pH, and whether the effects of this symbiont on gut pH are outweighed by its direct competition with trypanosomatids for space along the gut wall, or by its contribution to anoxic gut environments (Zheng et al., 2017) that can be toxic to insect gut trypanosomatids (Bringaud et al., 2006). Because Apis and Bombus share a core gut microbiota (Martinson et al., 2011; Kwong and Moran, 2016), we hypothesize that trypanosomatid parasites of honey bees (Schwarz et al., 2015) and bumble bees interact with similar communities of gut symbionts, and that the same pH-altering symbiont taxa could govern parasite establishment in both host genera.
Do gut microbiota shape resistance to opportunistic infection via alteration of gut pH?
In the context of prior experiments, our results suggest a pH-mediated role of the bee microbiota in defense against opportunistic infection. Multiple studies have correlated lack of core gut bacteria, or abundance of non-core bacteria, with bee infection. In North America and Europe, high diversity of non-core bacterial species correlated with higher prevalence of the pathogens Crithidia and Nosema (Koch et al., 2012; Cariveau et al., 2014). Similarly, in a survey of Bombus spp. in China, bees could be grouped into two microbial enterotypes (Li et al., 2015). One enterotype was dominated by core symbionts Snodgrassella and Gilliamella, while the other enterotype comprised non-core Hafnia, Enterobacteriaceae, and Serratia, The latter two are thought to be opportunistic pathogens (Kwong et al., 2017a; Raymann and Moran, 2018). Finally, higher susceptibility to pathogens was found in germ-free honey and bumble bees (Koch and Schmid-Hempel, 2011; Kwong et al., 2017a; Mockler et al., 2018; Raymann and Moran, 2018).
Acidification of the bee gut lumen by symbionts (Zheng et al., 2017) could explain how a strong core microbiota resists invasion by pathogens. This hypothesis is consistent with the dominant role of pH in determination of bacterial communities in soil (Morton et al., 2017; Ratzke and Gore, 2018), and correlations between low gastric acidity and opportunistic infection in human subjects (Stark and Nylund, 2016). However, other explanations for the effects of core gut microbiota on parasites—such as physical competition for space and resources, enhancement of the immune response, and improvement of host nutritional status (Raymann and Moran, 2018)—also deserve consideration. It is possible that different microbial assemblages are optimal for different functions. For example, a microbiome that produces abundant acids might be optimal for direct defense against parasites, whereas other community compositions might be provide more benefits to immune system regulation, or to nutritional sufficiency that improves resistance or tolerance to infection (Scrimshaw et al., 1959; Brown et al., 2003). Further research is needed to investigate variation in gut pH within and across species, and possible causative relationships between microbiome composition and bee health under different environmental circumstances.
Conclusion.
Our results build on prior associations between microbiome and infection intensity to provide mechanistic insights into how the bee gut microbiota are likely to influence trypanosomatid infection in bumble bees and possibly other corbiculate bee species. Our findings of pH-dependent parasite inhibition in vitro suggest that gut pH could be a critical determinant of trypanosomatid growth in vivo, a hypothesis that could be tested in both manipulative and observational studies that relate gut pH to trypanosomatid establishment. The role of gut pH in resistance to other enteric pathogens, and the selective forces acting on symbionts and parasites to create and tolerate different levels of acidity, warrants further study as part of continued investigations into the functional significance of the bee microbiome.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ben Sadd for providing strains IL13.2 and S08.1, Rebecca Irwin for providing the bees from which Strain VT1 was established, Guang Xu and Ben Sadd for sharing DNA sequences, and two anonymous reviewers for comments that improved the manuscript.
FINANCIAL SUPPORT
This project was funded by a National Science Foundation Postdoctoral Research Fellowship to EPY (NSF-DBI-1708945); USDA NIFA Hatch funds (CA-R-ENT-5109-H), NIH (5R01GM122060–02), and NSF MSB-ECA (1638728) to QSM; and an NSF-CAREER grant (IOS 1651888) to TRR. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL STANDARDS
Not applicable.
DATA AVAILABILITY
All data are supplied in the Supplementary Information, Data S1.
REFERENCES
- Adams MR and Hall CJ (1988). Growth inhibition of food-borne pathogens by lactic and acetic acids and their mixtures. International Journal of Food Science & Technology 23, 287–292. doi: 10.1111/j.1365-2621.1988.tb00581.x. [DOI] [Google Scholar]
- Anderson KE, Carroll MJ, Sheehan T, Mott BM, Maes P and Corby-Harris V (2014). Hive-stored pollen of honey bees: many lines of evidence are consistent with pollen preservation, not nutrient conversion. Molecular Ecology 23, 5904–5917. doi: 10.1111/mec.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barribeau SM, Sadd BM, du Plessis L and Schmid-Hempel P (2014). Gene expression differences underlying genotype-by-genotype specificity in a host–parasite system. Proceedings of the National Academy of Sciences 111, 3496–3501. doi: 10.1073/pnas.1318628111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthin S and Villadsen J (1995). Different inhibition of Lactobacillus delbrueckii subsp. bulgaricus by D- and L-lactic acid: effects on lag phase, growth rate and cell yield. Journal of Applied Bacteriology 78, 647–654. doi: 10.1111/j.1365-2672.1995.tb03111.x. [DOI] [Google Scholar]
- Billiet A, Meeus I, Van Nieuwerburgh F, Deforce D, Wäckers F and Smagghe G (2017). Colony contact contributes to the diversity of gut bacteria in bumblebees (Bombus terrestris). Insect Science 24, 270–277. doi: 10.1111/1744-7917.12284. [DOI] [PubMed] [Google Scholar]
- Bringaud F, Rivière L and Coustou V (2006). Energy metabolism of trypanosomatids: Adaptation to available carbon sources. Molecular and Biochemical Parasitology 149, 1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Brown MJF, Schmid-Hempel R and Schmid-Hempel P (2003). Strong context-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology 72, 994–1002. [Google Scholar]
- Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF and Griswold TL (2011). Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences 108, 662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariveau DP, Elijah Powell J, Koch H, Winfree R and Moran NA (2014). Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus). The ISME Journal 8, 2369–2379. doi: 10.1038/ismej.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman RS, Tarpy DR, Chen Y, Jeffreys L, Lopez D, Pettis JS, vanEngelsdorp D and Evans JD (2012). Pathogen Webs in Collapsing Honey Bee Colonies. PLOS ONE 7, e43562. doi: 10.1371/journal.pone.0043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AJ, Pyne DB, Saunders PU and Fricker PA (2010). Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. British Journal of Sports Medicine 44, 222–6. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- Daskin JH, Bell SC, Schwarzkopf L and Alford RA (2014). Cool Temperatures Reduce Antifungal Activity of Symbiotic Bacteria of Threatened Amphibians – Implications for Disease Management and Patterns of Decline. PLOS ONE 9, e100378. doi: 10.1371/journal.pone.0100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RJ and Dillon VM (2004). The gut bacteria of insects: nonpathogenic Interactions. Annual Review of Entomology 49, 71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Engel P, Martinson VG and Moran NA (2012). Functional diversity within the simple gut microbiota of the honey bee. Proceedings of the National Academy of Sciences 109, 11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Kwong WK, McFrederick Q, Anderson KE, Barribeau SM, Chandler JA, Cornman RS, Dainat J, Miranda J. R. de, Doublet V, Emery O, Evans JD, Farinelli L, Flenniken ML, Granberg F, Grasis JA, Gauthier L, Hayer J, Koch H, Kocher S, Martinson VG, Moran N, Munoz-Torres M, Newton I, Paxton RJ, Powell E, Sadd BM, Schmid-Hempel P, Schmid-Hempel R, Song SJ, Schwarz RS, vanEngelsdorp D and Dainat B (2016). The bee microbiome: impact on bee health and model for evolution and ecology of host-microbe interactions. mBio 7, e02164–15. doi: 10.1128/mBio.02164-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD and Armstrong T-N (2006). Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecology 6, 4. doi: 10.1186/1472-6785-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD and Lopez DL (2004). Bacterial Probiotics Induce an Immune Response in the Honey Bee (Hymenoptera: Apidae). Journal of Economic Entomology 97, 752–756. doi: 10.1093/jee/97.3.752. [DOI] [PubMed] [Google Scholar]
- Evans JD and Spivak M (2010). Socialized medicine: Individual and communal disease barriers in honey bees. Journal of Invertebrate Pathology 103, Supplement, S62–S72. doi: 10.1016/j.jip.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Zello GA, Naylor JM and Brocks DR (2002). Metabolic acidosis: separation methods and biological relevance of organic acids and lactic acid enantiomers. Journal of Chromatography B 781, 39–56. doi: 10.1016/S1570-0232(02)00500-7. [DOI] [PubMed] [Google Scholar]
- Fauser-Misslin A, Sadd BM, Neumann P and Sandrock C (2014). Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. Journal of Applied Ecology 51, 450–459. doi: 10.1111/1365-2664.12188. [DOI] [Google Scholar]
- Fayol-Messaoudi D, Berger CN, Coconnier-Polter M-H, Moal VL-L and Servin AL (2005). pH-, Lactic Acid-, and Non-Lactic Acid-Dependent Activities of Probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Applied and Environmental Microbiology 71, 6008–6013. doi: 10.1128/AEM.71.10.6008-6013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E, Olofsson TC, Váasquez A and Fries I (2010). Novel lactic acid bacteria inhibiting Paenibacillus larvae. Apidologie 41, 99–108. doi: 10.1051/apido/2009065. [DOI] [Google Scholar]
- Fürst MA, McMahon DP, Osborne JL, Paxton RJ and Brown MJF (2014). Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggìa F, Baffoni L and Alberoni D (2018). Probiotics for Honeybees’ Health In Probiotics and Prebiotics in Animal Health and Food Safety, pp. 219–245. Springer, Cham: doi: 10.1007/978-3-319-71950-4_9. [DOI] [Google Scholar]
- Gallai N, Salles J-M, Settele J and Vaissière BE (2009). Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics 68, 810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- Gegear RJ, Otterstatter MC and Thomson JD (2005). Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Animal Behaviour 70, 209–215. doi: 10.1016/j.anbehav.2004.09.025. [DOI] [Google Scholar]
- Gegear RJ, Otterstatter MC and Thomson JD (2006). Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proceedings. Biological sciences / The Royal Society 273, 1073–8. doi: 10.1098/rspb.2005.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass KA, Loeffelholz JM, Ford JP and Doyle MP (1992). Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Applied and Environmental Microbiology 58, 2513–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach SL (1990). Lactic Acid Bacteria and Human Health. Annals of Medicine 22, 37–41. doi: 10.3109/07853899009147239. [DOI] [PubMed] [Google Scholar]
- Gorbunov PS (1996). Peculiarities of life cycle in flagellate Crithidia bombi (protozoa, trypanosomatidae). Zoologicheskii Zhurnal 75, 808–810. [Google Scholar]
- Goulson D, Nicholls E, Botías C and Rotheray EL (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957. [DOI] [PubMed] [Google Scholar]
- Graystock P, Blane EJ, McFrederick QS, Goulson D and Hughes WOH (2016). Do managed bees drive parasite spread and emergence in wild bees? International Journal for Parasitology: Parasites and Wildlife 5, 64–75. doi: 10.1016/j.ijppaw.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoof B and Schmid-Hempel P (1998). Single-clone and mixed-clone infections versus host environment in Crithidia bombi infecting bumblebees. Parasitology 117, 331–336. [DOI] [PubMed] [Google Scholar]
- Kamada N, Chen GY, Inohara N and Núñez G (2013). Control of pathogens and pathobionts by the gut microbiota. Nature Immunology 14, 685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keersmaecker D, C.j S, Verhoeven TLA, Desair J, Marchal K, Vanderleyden J and Nagy I (2006). Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiology Letters 259, 89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Kešnerová L, Mars RAT, Ellegaard KM, Troilo M, Sauer U and Engel P (2017). Disentangling metabolic functions of bacteria in the honey bee gut. PLOS Biology 15, e2003467. doi: 10.1371/journal.pbio.2003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham S, Kremen C and Tscharntke T (2007). Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B: Biological Sciences 274, 303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H and Schmid-Hempel P (2011). Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proceedings of the National Academy of Sciences of the United States of America 108, 19288–19292. doi: 10.1073/pnas.1110474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H and Schmid-Hempel P (2012). Gut microbiota instead of host genotype drive the specificity in the interaction of a natural host-parasite system. Ecology Letters 15, 1095–1103. doi: 10.1111/j.1461-0248.2012.01831.x. [DOI] [PubMed] [Google Scholar]
- Koch H, Cisarovsky G and Schmid-Hempel P (2012). Ecological effects on gut bacterial communities in wild bumblebee colonies. The Journal of animal ecology 81, 1202–10. doi: 10.1111/j.1365-2656.2012.02004.x. [DOI] [PubMed] [Google Scholar]
- Kwong WK and Moran NA (2013). Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. International Journal of Systematic and Evolutionary Microbiology 63, 2008–2018. doi: 10.1099/ijs.0.044875-0. [DOI] [PubMed] [Google Scholar]
- Kwong WK and Moran NA (2016). Gut microbial communities of social bees. Nature Reviews Microbiology 14, 374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK, Mancenido AL and Moran NA (2017a). Immune system stimulation by the native gut microbiota of honey bees. Open Science 4, 170003. doi: 10.1098/rsos.170003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK, Medina LA, Koch H, Sing K-W, Soh EJY, Ascher JS, Jaffé R and Moran NA (2017b). Dynamic microbiome evolution in social bees. Science Advances 3, e1600513. doi: 10.1126/sciadv.1600513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Powell JE, Guo J, Evans JD, Wu J, Williams P, Lin Q, Moran NA and Zhang Z (2015). Two gut community enterotypes recur in diverse bumblebee species. Current Biology 25, R652–R653. doi: 10.1016/j.cub.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Lindgren SE and Dobrogosz WJ (1990). Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiology Letters 87, 149–164. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- Martinson VG, Danforth BN, Minckley RL, Rueppell O, Tingek S and Moran NA (2011). A simple and distinctive microbiota associated with honey bees and bumble bees. Molecular Ecology 20, 619–628. doi: 10.1111/j.1365-294X.2010.04959.x. [DOI] [PubMed] [Google Scholar]
- McMahon DP, Fürst MA, Caspar J, Theodorou P, Brown MJF and Paxton RJ (2015). A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. Journal of Animal Ecology 84, 615–624. doi: 10.1111/1365-2656.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler BK, Kwong WK, Moran NA and Koch H (2018). Microbiome Structure Influences Infection by the Parasite Crithidia bombi in Bumble Bees. Applied and Environmental Microbiology 84, e02335–17. doi: 10.1128/AEM.02335-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JT, Sanders J, Quinn RA, McDonald D, Gonzalez A, Vázquez-Baeza Y, Navas-Molina JA, Song SJ, Metcalf JL, Hyde ER, Lladser M, Dorrestein PC and Knight R (2017). Balance Trees Reveal Microbial Niche Differentiation. mSystems 2, e00162–16. doi: 10.1128/mSystems.00162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller UG and Sachs JL (2015). Engineering Microbiomes to Improve Plant and Animal Health. Trends in Microbiology 23, 606–617. doi: 10.1016/j.tim.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Palmer-Young EC, Sadd BM, Stevenson PC, Irwin RE and Adler LS (2016). Bumble bee parasite strains vary in resistance to phytochemicals. Scientific Reports 6, 37087. doi: 10.1038/srep37087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young EC, Sadd BM, Irwin RE and Adler LS (2017a). Synergistic effects of floral phytochemicals against a bumble bee parasite. Ecology and Evolution 7, 1836–1849. doi: 10.1002/ece3.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young EC, Hogeboom A, Kaye AJ, Donnelly D, Andicoechea J, Connon SJ, Weston I, Skyrm K, Irwin RE and Adler LS (2017b). Context-dependent medicinal effects of anabasine and infection-dependent toxicity in bumble bees. PLOS ONE 12, e0183729. doi: 10.1371/journal.pone.0183729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praet J, Meeus I, Cnockaert M, Houf K, Smagghe G and Vandamme P (2015). Novel lactic acid bacteria isolated from the bumble bee gut: Convivina intestini gen. nov., sp. nov., Lactobacillus bombicola sp. nov., and Weissella bombi sp. nov. Antonie van Leeuwenhoek 107, 1337–1349. doi: 10.1007/s10482-015-0429-z. [DOI] [PubMed] [Google Scholar]
- Praet J, Parmentier A, Schmid‐Hempel R, Meeus I, Smagghe G and Vandamme P (2018). Large-scale cultivation of the bumblebee gut microbiota reveals an underestimated bacterial species diversity capable of pathogen inhibition. Environmental Microbiology 20, 214–227. doi: 10.1111/1462-2920.13973. [DOI] [PubMed] [Google Scholar]
- Presser KA, Ratkowsky DA and Ross T (1997). Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Applied and Environmental Microbiology 63, 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ratzke C and Gore J (2018). Modifying and reacting to the environmental pH can drive bacterial interactions. PLOS Biology 16, e2004248. doi: 10.1371/journal.pbio.2004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravoet J, Maharramov J, Meeus I, De Smet L, Wenseleers T, Smagghe G and de Graaf DC (2013). Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLOS ONE 8, e72443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann K and Moran NA (2018). The role of the gut microbiome in health and disease of adult honey bee workers. Current Opinion in Insect Science 26, 97–104. doi: 10.1016/j.cois.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz C, Baty F, Streibig JC and Gerhard D (2015). Dose-response analysis using R. PLOS ONE 10, e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL and Mazmanian SK (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology 9, 313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runckel C, DeRisi J and Flenniken ML (2014). A draft genome of the honey bee trypanosomatid parasite Crithidia mellificae. PLOS ONE 9, e95057. doi: 10.1371/journal.pone.0095057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JB and Diez-Gonzalez F (1998). The effects of fermentation acids on bacterial growth. Advances in Microbial Physiology 39, 205–234. [DOI] [PubMed] [Google Scholar]
- Sabaté DC, Carrillo L and Carina Audisio M (2009). Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Research in Microbiology 160, 193–199. doi: 10.1016/j.resmic.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Sadd BM (2011). Food-environment mediates the outcome of specific interactions between a bumblebee and its trypanosome parasite. Evolution 65, 3001, 2995. [DOI] [PubMed] [Google Scholar]
- Salathé R, Tognazzo M, Schmid-Hempel R and Schmid-Hempel P (2012). Probing mixed-genotype infections I: Extraction and cloning of infections from hosts of the trypanosomatid Crithidia bombi. PLOS ONE 7, e49046. doi: 10.1371/journal.pone.0049046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S and von Wright, A. (2004). Lactic Acid Bacteria: Microbiological and Functional Aspects, Third Edition CRC Press. [Google Scholar]
- Schmid-Hempel P and Ebert D (2003). On the evolutionary ecology of specific immune defence. Trends in Ecology & Evolution 18, 27–32. [Google Scholar]
- Schmid-Hempel R, Eckhardt M, Goulson D, Heinzmann D, Lange C, Plischuk S, Escudero LR, Salathé R, Scriven JJ and Schmid-Hempel P (2014). The invasion of southern South America by imported bumblebees and associated parasites. Journal of Animal Ecology 83, 823–837. doi: 10.1111/1365-2656.12185. [DOI] [PubMed] [Google Scholar]
- Schwarz RS, Bauchan GR, Murphy CA, Ravoet J, de Graaf DC and Evans JD (2015). Characterization of Two Species of Trypanosomatidae from the Honey Bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. Journal of Eukaryotic Microbiology 62, 567–583. doi: 10.1111/jeu.12209. [DOI] [PubMed] [Google Scholar]
- Schwarz RS, Moran NA and Evans JD (2016). Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proceedings of the National Academy of Sciences 113, 9345–9350. doi: 10.1073/pnas.1606631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw NS, Taylor CE and Gordon JE (1959). Interactions of Nutrition and Infection. American Journal of Medical Sciences 237, 367–403. [PubMed] [Google Scholar]
- Shykoff JA and Schmid-Hempel P (1991). Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 22, 117–125. doi: 10.1051/apido:19910204. [DOI] [Google Scholar]
- Stark CM and Nylund CM (2016). Side Effects and Complications of Proton Pump Inhibitors: A Pediatric Perspective. The Journal of Pediatrics 168, 16–22. doi: 10.1016/j.jpeds.2015.08.064. [DOI] [PubMed] [Google Scholar]
- Steele MI, Kwong WK, Whiteley M and Moran NA (2017). Diversification of Type VI Secretion System Toxins Reveals Ancient Antagonism among Bee Gut Microbes. mBio 8, e01630–17. doi: 10.1128/mBio.01630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi AD, Szalanski AL and Strange JP (2018). Novel multiplex PCR reveals multiple trypanosomatid species infecting North American bumble bees (Hymenoptera: Apidae: Bombus). Journal of Invertebrate Pathology 153, 147–155. doi: 10.1016/j.jip.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Vásquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L and Olofsson TC (2012). Symbionts as Major Modulators of Insect Health: Lactic Acid Bacteria and Honeybees. PLOS ONE 7, e33188. doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Powell JE, Steele MI, Dietrich C and Moran NA (2017). Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proceedings of the National Academy of Sciences 201701819. doi: 10.1073/pnas.1701819114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


