Abstract
Patients with heart failure (HF) and preserved (HFpEF) or borderline preserved ejection fraction (HFbEF) outnumber patients with HF and reduced ejection fraction (HFrEF), but limited data exist on outcomes in community-based populations of these patients. We examined clinical outcomes in a diverse population of adults with HFrEF, HFbEF, and HFpEF. All adults with diagnosed HF from 2005 to 2012 in Kaiser Permanente Northern California were categorized by left ventricular systolic function as HFpEF (EF≥ 50%), HFbEF (EF 41–49%), or HFrEF (EF≤ 40%). Demographics, clinical characteristics, and therapies were obtained from electronic records. Outcomes included death, HF hospitalization, and HF-related emergency department (ED) visit. In 28,914 eligible HF patients, there were 52% HFpEF, 16% HFbEF, and 32% HFrEF, with mean age 72.8 years and 45% women. During median follow-up of 3.5 years, crude rates (per 100 person-years) of death, HF hospitalization, and HF-related ED visit were 14.5 (95% CI 14.3 to 14.7), 15.8 (15.5 to 16.0), and 38.2 (37.8 to 38.5), respectively. Compared with HFrEF patients, adjusted hazard ratios of death, HF hospitalization, and HF-related ED visit for HFpEF patients were 0.82 (0.79 to 0.85), 0.72 (0.68 to 0.75), and 0.94 (0.90 to 0.99), respectively, and for HFbEF patients were 0.84 (0.79 to 0.88), 0.79 (0.73 to 0.84), and 0.90 (0.84 to 0.96), respectively. In conclusion, within a large community-based HF cohort, adjusted rates of death, HF hospitalization, and HF-related ED visits were similar in HFpEF and HFbEF patients, but higher in HFrEF patients. Regardless of systolic function, however, long-term mortality and morbidity in all HF patients remain high, reinforcing the need for novel strategies to improve long-term outcomes.
In 2013, the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) categorized heart failure (HF) patients into 3 groups based on left ventricular ejection fraction (EF): HF with reduced EF (≤40%) HF and preserved ejection fraction (HFrEF), HF with borderline preserved EF (41% to 49%) HF and borderline preserved ejection fraction (HFbEF), and HF with preserved EF (≥50%) (HFpEF).1 Due to an aging population, improved treatment of coronary artery disease, and parallel rises in cardiovascular risk factors, the epidemiology of HF has shifted and more than half of incident HF cases and adults hospitalized for HF now have HFpEF or HFbEF.2–5 Studies comparing hospitalization and mortality in HFpEF, HFbEF, and HFrEF patients have shown variable results, but suggest similar or lower event rates in HFpEF and HFbEF patients.6–11 These studies, however, have had modest sample sizes with limited racial, ethnic, and geographic diversity, or were limited to elderly and hospitalized Medicare beneficiaries. Emergency department (ED) utilization independent of hospitalization, as well as long-term mortality and hospitalization in outpatients diagnosed with HF has not been well-studied. To address these limitations, we evaluated the characteristics and outcomes, including ED utilization, of a large, diverse, community-based population of adults with HFrEF, HFbEF, and HFpEF identified from both hospital and outpatient care settings.
Methods
The source population included members from Kaiser Permanente Northern California (KPNC), a large integrated healthcare delivery system providing comprehensive inpatient, ED, and ambulatory care to >4.1 million members in northern and central California. The KPNC membership has broad sociodemographic diversity and is highly representative of the local and statewide population.12 The KPNC Virtual Data Warehouse (VDW) served as the primary data source for subject identification and characterization. The VDW is comprised of datasets populated with linked demographic, administrative, ambulatory pharmacy, outpatient laboratory test results, and health care utilization data for KPNC members.13 The KPNC institutional review board approved the study, and a waiver of consent was obtained due to the nature of the study.
We first identified all adults aged ≥21 years with diagnosed HF based on either having been hospitalized with a primary discharge diagnosis of HF and/or having ≥ 3 ambulatory visits coded for HF with at least 1 visit being with a cardiologist from January 1, 2005 to December 31, 2012. We used the following International Classification of Diseases, 9th Edition (ICD-9) codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9. Previous studies have shown a positive predictive value of >95% for admissions with a primary discharge diagnosis of HF based on these codes when compared against chart review and Framingham clinical criteria.14 For the outpatient HF definition, we required ≥3 ambulatory visits with associated HF diagnoses, with ≥1 of the visits being to a cardiologist to enhance specificity for having HF. Index date was defined as the date when a patient first met the criteria for HF during the study period.
We ascertained information on quantitative and/or qualitative assessments of left ventricular systolic function from the results of echocardiograms, radionuclide scintigraphy, other nuclear imaging modalities, and left ventriculography test results available from site-specific databases complemented by manual chart review. We classified patients into categories of preserved, borderline, and reduced EF. We defined preserved EF as either a reported EF ≥50% and/or based on a physician’s qualitative assessment of preserved or normal systolic function. Borderline preserved EF was defined as a reported EF in 41% to 49% and/or physician’s qualitative assessment of mildly reduced systolic function, and reduced EF was defined either by a reported EF ≤40% and/or based on a physician’s qualitative assessment of moderate, moderate to severe, or severe systolic dysfunction.
Follow-up occurred through December 31, 2013, with subjects censored if they either disenrolled from the health plan or reached the end of study follow-up. Primary outcomes were HF hospitalization, HF-related ED visit, and death from any cause. To account for potential competing risks, a secondary composite outcome made of each of the primary outcomes was obtained. Hospitalizations and ED visits were identified from the VDW, and encounters for HF were based on a primary diagnosis for HF using the same inclusion criteria ICD-9 codes. Deaths were identified from hospital and billing claims databases, administrative health plan databases, state death certificate registries, and Social Security Administration files as available at each site. These approaches have yielded >97% vital status information in previous studies.14, 15
We ascertained information on coexisting illnesses based on diagnoses or procedures using relevant ICD-9 codes, laboratory results, or filled outpatient prescriptions from health plan hospitalization discharge, ambulatory visit, laboratory, and pharmacy databases, as well as regional diabetes mellitus and cancer registries.16,17 We collected baseline and follow-up data on diagnoses of acute myocardial infarction; unstable angina; coronary revascularization; stroke or transient ischemic attack; atrial fibrillation or flutter; ventricular fibrillation or tachycardia; mitral or aortic valvular heart disease; peripheral arterial disease; rheumatic heart disease; receipt of a pacemaker, cardiac resynchronization therapy, or an implantable cardioverter defibrillator; dyslipidemia; hypertension; diabetes mellitus; hospitalized bleed; dementia; depression; chronic lung disease; chronic liver disease; and systemic cancer based on previously described ICD-9 codes and Current Procedure Terminology procedure codes.15
We ascertained available ambulatory results for systolic and diastolic blood pressure, serum LDL and HDL cholesterol, estimated glomerular filtration rate using the CKDEPI estimating equation, urinary protein dipstick measurements, and blood hemoglobin level on or before the index date and during follow-up. We also captured longitudinal receipt of prescribed statins, ACE inhibitors or angiotensin receptor blockers, β blockers and diuretics based on dispensings found in ambulatory pharmacy databases using previously described methods.18
Analyses were conducted using SAS statistical software, version 9.3 (Cary, North Carolina). We compared baseline characteristics across EF groups using ANOVA or nonpara-metric tests for continuous variables and chi-square tests for categorical variables. Given the large sample size, we compared standardized mean differences using a D value >0.10, focusing only on differences in baseline characteristics that were clinically meaningful. We calculated event rates (per 100 person-years) with 95% confidence intervals and compared Kaplan-Meier survival curves for each outcome across study groups using log-rank tests. We then used multivariable extended Cox regression models with time-varying covariates to examine the independent association in systolic function group and adverse events. Models were adjusted for age, gender, and any other variables at entry (Table 1) that differed across groups with a D ≥ 0.10, with application of a robust sandwich estimator to account for clustering of multiple observations within the same subject.
Table 1.
Baseline characteristics of 28,914 adults with heart failure identified from 2005 to 2012, overall and stratified by left ventricular systolic function
| Characteristic | Overall N = 28,914 | HFpEFN= 14,883 | HFbEF N = 4,657 | HFrEF N = 9,374 |
|---|---|---|---|---|
| Mean age ± SD (years) | 72.8 ± 12.7 | 74.7 ± 11.9 | 72.1 ± 12.4 | 70.3 ± 13.4 |
| Age categories (years) | ||||
| <45 | 776 (2.7 %) | 243 (1.6 %) | 115 (2.5 %) | 418(4.5 %) |
| 45–54 | 1986 (6.9 %) | 768 (5.2 %) | 351 (7.5 %) | 867 (9.2 %) |
| 55–64 | 4620 (16.0 %) | 1999 (13.4 %) | 804 (17.3 %) | 1817(19.4%) |
| 65–74 | 7360 (25.5 %) | 3705 (24.9 %) | 1255 (26.9 %) | 2400 (25.6 %) |
| 75–84 | 9414 (32.6 %) | 5266 (35.4 %) | 1446 (31.1 %) | 2702 (28.8 %) |
| ≥85 | 4758 (16.5 %) | 2902 (19.5 %) | 686 (14.7 %) | 1170(12.5 %) |
| Woman | 13,001 (45.0 %) | 8250 (55.4 %) | 1774 (38.1 %) | 2977 (31.8%) |
| Race | ||||
| White | 22,098 (76.4 %) | 11,642 (78.2%) | 3577 (76.8 %) | 6879 (73.4 %) |
| Black | 3183 (11.0%) | 1424 (9.6 %) | 534(11.5 %) | 1225 (13.1 %) |
| Asian / Pacific Islander | 2804 (9.7 %) | 1429 (9.6 %) | 423 (9.1 %) | 952 (10.2 %) |
| Other | 829 (2.9 %) | 388 (2.6 %) | 123 (2.6 %) | 318(3.4%) |
| Hispanic | 3484 (12.0 %) | 1772(11.9 %)* | 559(12.0%)* | 1153 (12.3 %) |
| Acute myocardial Infraction | 4097 (14.2 %) | 1683 (11.3 %) | 792 (17.0 %)* | 1622 (17.3 %) |
| Coronary bypass | 1781 (6.2 %) | 880 (5.9 %)* | 361 (7.8 %) | 540 (5.8 %) |
| Percutaneous coronary intervention | 3316(11.5 %) | 1481 (10.0 %) | 663 (14.2 %) | 1172(12.5 %) |
| Ischemic stroke or transient ischemic attack | 1786 (6.2 %) | 988 (6.6 %) | 277 (5.9 %)* | 521 (5.6 %) |
| Atrial fibrillation or flutter | 9211 (31.9%) | 5405 (36.3 %) | 1423 (30.6 %) | 2383 (25.4 %) |
| Ventricular tachycardia or fibrillation | 591 (2.0%) | 153 (1.0%) | 102 (2.2 %) | 336 (3.6 %) |
| Mitral and/or aortic valvular disease | 6149 (21.3 %) | 3572 (24.0 %) | 964 (20.7 %) | 1613 (17.2%) |
| Peripheral arterial disease | 1903 (6.6 %) | 999 (6.7 %) | 343 (7.4 %) | 561 (6.0 %) |
| Rheumatic heart disease | 434(1.5 %) | 269 (1.8 %) | 56(1.2%)* | 109(1.2%) |
| Cardiac resynchronization therapy | 320(1.1 %) | 94 (0.6 %) | 67(1.4%)* | 159(1.7%) |
| Implantable cardioverter defibrillator | 717 (2.5 %) | 114 (0.8%) | 112(2.4%) | 491 (5.2%) |
| Pacemaker | 1815 (6.3 %) | 874 (5.9 %) | 319(6.8%)* | 622 (6.6 %) |
| Dyslipidemia | 22,734 (78.6 %) | 11,674 (78.4%) | 3815(81.9%) | 7245 (77.3 %) |
| Hypertension | 23,173 (80.1 %) | 12,768 (85.8 %) | 3754 (80.6 %) | 6651 (71.0%) |
| Diabetes mellitus | 12,027 (41.6 %) | 6398 (43.0 %) | 2049 (44.0 %) | 3580 (38.2 %) |
| Hospitalized bleed | 1568 (5.4 %) | 910(6.1 %) | 255 (5.5 %) | 403 (4.3 %) |
| Diagnosed dementia | 1269 (4.4 %) | 700 (4.7 %) | 201 (4.3 %)* | 368 (3.9 %) |
| Diagnosed depression | 4943 (17.1 %) | 2839 (19.1 %) | 778 (16.7 %) | 1326(14.1 %) |
| Chronic lung disease | 10,597 (36.7 %) | 5974 (40.1 %) | 1638 (35.2 %) | 2985 (31.8%) |
| Chronic liver disease | 809 (2.8 %) | 469 (3.2 %) | 111 (2.4 %)* | 229 (2.4 %) |
| Systemic cancer | 2637 (9.1 %) | 1460 (9.8 %) | 410(8.8%)* | 767 (8.2 %) |
| Systolic blood pressure (mm Hg) | ||||
| ≥180 | 648 (2.2 %) | 424 (2.8 %) | 99 (2.1 %) | 125 (1.3 %) |
| 160–179 | 1716(5.9%) | 1078 (7.2 %) | 262 (5.6 %) | 376 (4.0 %) |
| 140–159 | 4954 (17.1 %) | 2833 (19.0 %) | 817(17.5%) | 1304 (13.9 %) |
| 130–139 | 5795 (20.0 %) | 3174(21.3 %) | 900 (19.3 %) | 1721 (18.4%) |
| 121–129 | 4888 (16.9 %) | 2501 (16.8 %) | 805 (17.3 %) | 1582(16.9%) |
| ≤120 | 10,692 (37.0 %) | 4812(32.3 %) | 1735 (37.3 %) | 4145 (44.2 %) |
| Missing | 221 (0.8 %) | 61 (0.4 %) | 39 (0.8 %) | 121 (1.3 %) |
| Diastolic blood pressure (mm Hg) | ||||
| ≥110 | 201 (0.7 %) | 78 (0.5 %) | 31 (0.7 %) | 92 (1.0 %) |
| 100–109 | 474(1.6%) | 195 (1.3 %) | 83 (1.8 %) | 196 (2.1 %) |
| 90–99 | 1426 (4.9 %) | 662 (4.4 %) | 230 (4.9 %) | 534 (5.7 %) |
| 85–89 | 1472 (5.1 %) | 700 (4.7 %) | 236 (5.1 %) | 536 (5.7 %) |
| 81–84 | 1898 (6.6 %) | 968 (6.5 %) | 310(6.7 %) | 620 (6.6 %) |
| ≤80 | 23,222 (80.3 %) | 12,219(82.1 %) | 3728 (80.1 %) | 7275 (77.6 %) |
| Baseline estimated glomerular filtration rate (ml/min/1.73 m2) | ||||
| ≥90 | 2538 (8.8 %) | 1106(7.4%) | 404 (8.7 %) | 1028(11.0%) |
| 60–89 | 10,218 (35.3 %) | 5095 (34.2 %) | 1676 (36.0 %) | 3447 (36.8 %) |
| 45–59 | 6499 (22.5 %) | 3430 (23.0 %) | 1037 (22.3 %) | 2032 (21.7 %) |
| 30–44 | 5082 (17.6 %) | 2826 (19.0 %) | 796(17.1 %) | 1460 (15.6 %) |
| 15–29 | 2402 (8.3 %) | 1414 (9.5 %) | 349 (7.5 %) | 639 (6.8 %) |
| <15 | 383 (1.3 %) | 231 (1.6%) | 65 (1.4 %) | 87 (0.9 %) |
| Dialysis | 779 (2.7 %) | 430 (2.9 %) | 161 (3.5 %) | 188 (2.0 %) |
| Missing | 1013 (3.5 %) | 351 (2.4 %) | 169 (3.6 %) | 493 (5.3 %) |
| Baseline Dipstick Proteinuria | ||||
| Negative, trace or missing | 23,132 (80.0%) | 11,652 (78.3 %) | 3697 (79.4 %) | 7783 (83.0 %) |
| 1+ | 2611 (9.0%) | 1457 (9.8 %) | 410(8.8%) | 744 (7.9 %) |
| 2+ | 1794 (6.2 %) | 973 (6.5 %) | 286 (6.1 %) | 535 (5.7 %) |
| 3+ | 1377 (4.8 %) | 801 (5.4 %) | 264 (5.7 %) | 312(3.3 %) |
| Baseline hemoglobin (g/dL) | ||||
| ≥13.0 | 14,157 (49.0%) | 6621 (44.5 %) | 2332(50.1 %) | 5204 (55.5 %) |
| 12.0–12.9 | 5276(18.2%) | 2919(19.6%) | 857(18.4%) | 1500 (16.0 %) |
| 11.0–11.9 | 4091 (14.1 %) | 2436 (16.4 %) | 612 (13.1 %) | 1043 (11.1 %) |
| 10.0–10.9 | 2467 (8.5 %) | 1513 (10.2%) | 399 (8.6 %) | 555 (5.9 %) |
| 9.0–9.9 | 1134 (3.9%) | 695 (4.7 %) | 158 (3.4 %) | 281 (3.0%) |
| <9.0 | 539(1.9%) | 322 (2.2 %) | 89(1.9%) | 128(1.4%) |
| Missing | 1250 (4.3 %) | 377 (2.5 %) | 210 (4.5 %) | 663 (7.1 %) |
| Missing | 221 (0.8 %) | 61 (0.4 %) | 39 (0.8 %) | 121 (1.3 %) |
| HDL cholesterol (g/dL) | ||||
| ≥60 | 4696 (16.2 %) | 2737 (18.4 %) | 669 (14.4 %) | 1290 (13.8 %) |
| 50–59 | 5197(18.0%) | 2818 (18.9 %) | 779 (16.7 %) | 1600(17.1 %) |
| 40–49 | 8305 (28.7 %) | 4306 (28.9 %) | 1358 (29.2 %) | 2641 (28.2 %) |
| 35–39 | 4060 (14.0 %) | 1991 (13.4 %) | 707 (15.2 %) | 1362 (14.5 %) |
| <35 | 4475 (15.5 %) | 2090 (14.0 %) | 819(17.6%) | 1566 (16.7 %) |
| Missing | 2181 (7.5 %) | 941 (6.3 %) | 325 (7.0 %) | 915 (9.8 %) |
| LDL cholesterol (g/dL) | ||||
| ≥200 | 285 (1.0%) | 122 (0.8 %) | 59 (1.3 %) | 104(1.1 %) |
| 160–199 | 1033 (3.6 %) | 512(3.4%) | 157 (3.4 %) | 364 (3.9 %) |
| 130–159 | 2732 (9.4 %) | 1345 (9.0 %) | 455 (9.8 %) | 932 (9.9 %) |
| 100–129 | 6297 (21.8 %) | 3325 (22.3 %) | 975 (20.9 %) | 1997 (21.3 %) |
| 70–99 | 10,555 (36.5 %) | 5537 (37.2 %) | 1696 (36.4 %) | 3322 (35.4 %) |
| <70 | 5678 (19.6 %) | 3037 (20.4 %) | 968 (20.8 %) | 1673 (17.8 %) |
| Missing | 2334 (8.1 %) | 1005 (6.8 %) | 347 (7.5 %) | 982 (10.5 %) |
| Baseline medication use | ||||
| ACE inhibitor/angiotensin II receptor blocker | 18,312(63.3 %) | 9192 (61.8%) | 3082 (66.2 %) | 6038 (64.4 %) |
| Aldosterone receptor blocker | 2032 (7.0 %) | 676 (4.5 %) | 327 (7.0 %) | 1029(11.0%) |
| Beta blocker | 19,334 (66.9 %) | 10,331 (69.4%) | 3235 (69.5 %) | 5768 (61.5 %) |
| Calcium channel blocker | 9055 (31.3 %) | 5859 (39.4 %) | 1401 (30.1 %) | 1795 (19.1 %) |
| Digoxin | 4507 (15.6 %) | 2078 (14.0 %) | 692 (14.9 %) | 1737(18.5%) |
| Hydralazine | 2529 (8.7 %) | 1466 (9.9 %) | 387 (8.3 %) | 676 (7.2 %) |
| Diuretic | 18,958 (65.6 %) | 10,287 (69.1 %) | 2971 (63.8%) | 5700 (60.8 %) |
| Nitrates | 6980 (24.1 %) | 3376 (22.7 %) | 1292 (27.7 %) | 2312(24.7%) |
| Statin | 17,428 (60.3 %) | 8989 (60.4 %) | 2950 (63.3 %) | 5489 (58.6 %) |
| Other lipid-lowering drug | 1569 (5.4 %) | 812(5.5%)* | 295 (6.3 %) | 462 (4.9 %) |
| Antiplatelet agent | 3406(11.8%) | 1602 (10.8 %) | 680 (14.6 %) | 1124(12.0%) |
| Anticoagulant | 7178 (24.8 %) | 3982 (26.8 %) | 1128 (24.2%) | 2068 (22.1 %) |
| Non-steroidal anti-inflammatory drug | 3232(11.2%) | 1754(11.8 %) | 502 (10.8 %)* | 976 (10.4 %) |
All comparisons in preserved EF versus reduced EF and borderline EF versus reduced EF groups were statistically significant using a p value <0.05 except where noted by an asterisk. ACE = angiotensin converting enzyme; HDL = high density lipoprotein; HFbEF = heart failure with borderline ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LDL = low density lipoprotein; SD = standard deviation.
Results
In 28,914 eligible adults with HF identified during the study period, 51.5% had HFpEF, 16.1% had HFbEF, and 32.4% had HFrEF. More than half (55.4%) of the cohort was identified using outpatient diagnostic HF criteria. Demographic and clinical characteristics were similar in systolic function groups, but HFpEF patients were notably older with a higher proportion of women. They were also more likely to have a history of hypertension, atrial arrhythmia, stroke, hospitalized bleed, proteinuria, or valvular heart disease, and less likely to have a history of myocardial infarction, percutaneous coronary intervention, or implantable cardioverter defibrillator relative to HFrEF patients (Table 1). The clinical and demographic profile of HFbEF patients was largely intermediate from HFpEF to HFrEF patients, although HFbEF patients were more likely to have had a history of coronary bypass (Table 1). The cohort mean age was 72.8 years with 45% women and notable racial and ethnic diversity (Table 1).
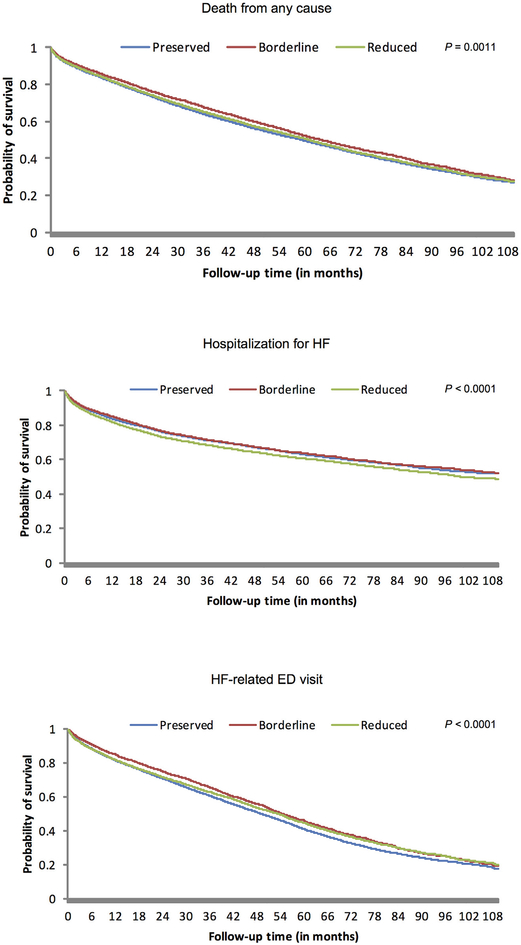
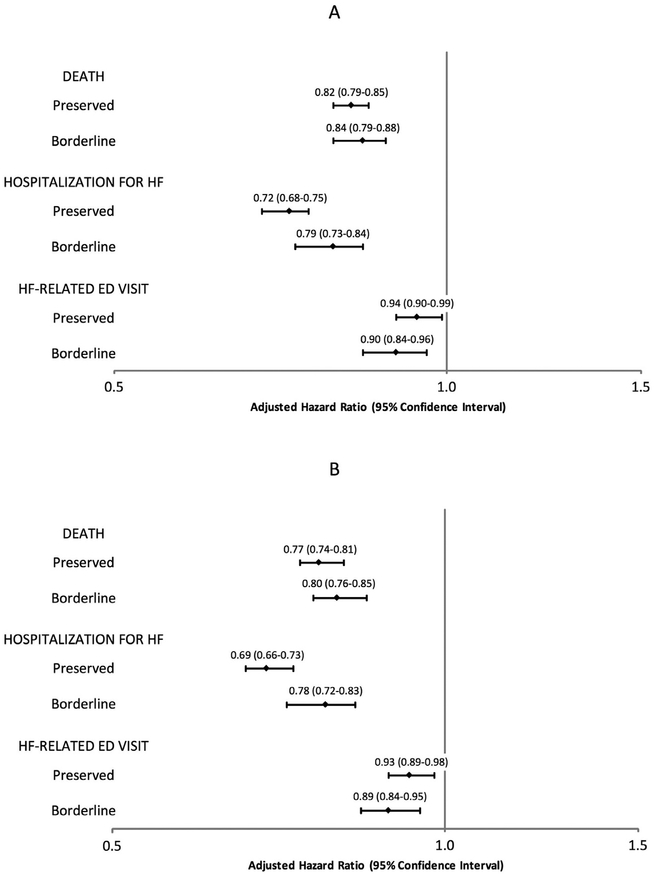
Median follow-up was 3.5 (interquartile range 1.4 to 6.3) person-years and the overall crude rate of death from any cause was 14.5 per 100 person-years (95% CI 14.3 to 14.7). Crude death rates were highest in HFpEF patients and lowest in HFbEF patients (Table 2), with HFpEF patients experiencing the lowest survival probability over time (Figure 1). After adjustment for the wide range of potential confounding factors and patient characteristics previously described, we observed significantly lower adjusted rates of death in HFpEF and HFbEF patients relative to HFrEF patients (Figure 2). Adjustment for longitudinal use of HF-specific and cardioprotective medications, including ACE inhibitors, angiotensin II receptor blockers, β blockers, diuretics, aldosterone antagonists, and statins did not substantially affect these findings (Figure 2).
Table 2.
Crude rates of events per 100 person-years, by category of left ventricular systolic function
| Left ventricular systolic function group | Death from any cause | Hospitalization for heart failure | Heart failure-related emergency department visit |
|---|---|---|---|
| Preserved | 14.8(14.5–15.1) | 14.8(14.5–15.1) | 40.5 (39.9–41.0) |
| Borderline | 13.6 (13.1–14.1) | 15.0 (14.5–15.6) | 36.0 (35.2–36.8) |
| Reduced | 14.4 (14.1–14.8) | 17.7 (17.3–18.1) | 35.7 (35.1–36.3) |
| Overall | 14.5 (14.3–14.7) | 15.8(15.5–16.0) | 38.2 (37.8–38.5) |
Crude event rates reported per 100 person-years with 95% CI in parentheses.
Figure 1. Kaplan-Meier curves for primary outcomes by category of left ventricular systolic function.
Kaplan-Meier Curves for each left ventricular systolic function group are shown for the primary outcomes (from top to bottom): death from any cause, hospitalization for HF, and HF-related ED visit. Differences in the probabilities for all outcomes in left ventricular systolic function groups were statistically significant. ED = emergency department; HF = heart failure.
Figure 2. Multivariable association of left ventricular systolic function group with clinical outcomes, with and without adjustment for long-term medication use.
(A) Multivariable association of outcomes not adjusting for long-term medication use. (B) Multivariable association of outcomes adjusting for long-term medication use. Adjusted hazard ratios are reported relative to patients with HF and reduced ejection fraction. 95% confidence intervals are shown in parentheses. ED = emergency department; HF = heart failure.
The overall rate of hospitalization for HF was 15.8 per 100 person-years (95% CI 15.5 to 16.0). Crude rates of hospitalization for HF were significantly lower in HFpEF and HFbEF patients when compared with HFrEF patients (Table 2). Crude rates of HF hospitalization were highest in HFrEF patients throughout (Figure 1). After adjustment for potential confounders, HFpEF, and HFbEF patients experienced significantly lower rates of hospitalization for HF than patients with HFrEF (Figure 2). Further adjustment for longitudinal medication use did not materially affect the results for HFpEF and HFbEF patients (Figure 2).
Overall, the rate of HF-related ED visits was 38.2 per 100 person-years (95% CI 37.8 to 38.5). Compared with HFrEF patients, crude rates of HF-related ED visits were similar for HFbEF patients, but significantly higher in HFpEF patients (Table 2). The probability of a HF-related ED visit was similar from HFpEF to HFrEF patients until 2 years of follow-up, after which HFpEF patients demonstrated a persistently higher risk of experiencing a HF-related ED visit (Figure 1). After adjustment for potential confounding variables, the rates of HF-related ED visits were only slightly lower in those with HFpEF and HFbEF when compared with patients with HFrEF (Figure 2). As seen with other outcomes, adjustment for longitudinal receipt of HF-specific and cardioprotective medications did not materially affect the results (Figure 2).
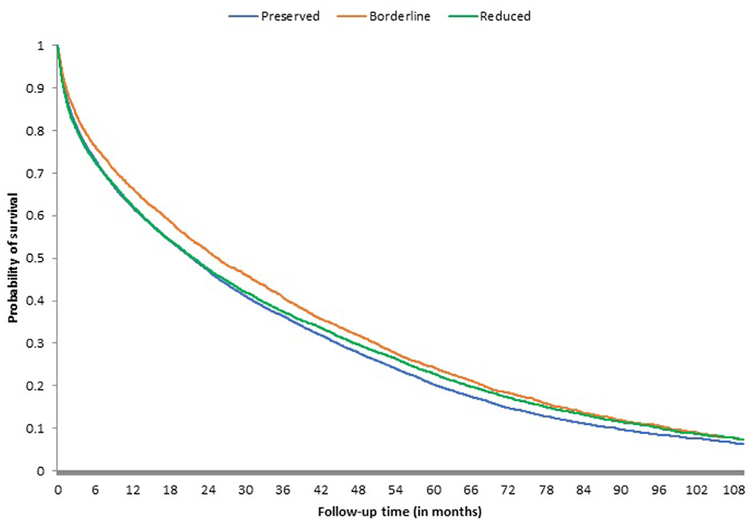
Kaplan-Meier curves of the composite outcome of death, HF hospitalization, or HF-related ED visits suggested no significant competing risks and mirrored the individual primary outcomes across systolic function groups, with HFpEF and HFrEF patients experiencing early and persistently lower rates of event-free survival when compared with HFbEF patients (Figure 3).
Figure 3. Kaplan-Meier curves for the composite outcome of death from any cause, HF hospitalization, or HF-related ED visit.
Kaplan-Meier Curves for each left ventricular systolic function group are shown for the composite outcome of death from any cause, HF hospitalization, or HF-related ED visit. Differences in the probabilities for all outcomes in left ventricular systolic function groups were statistically significant. ED = emergency department; HF = heart failure.
Discussion
Within a large, diverse community-based cohort of patients with HF, we observed high rates of death, HF hospitalization, and ED visits for HF across all categories of left ventricular systolic function. Crude rates of death observed in HFpEF patients in our study were higher than those seen in the I-PRESERVE and CHARM-Preserved clinical trials, but similar to previous large cohorts from the Cardiovascular Health Study and Olmsted County, suggesting that HFpEF patients seen in usual clinical care may represent a higher-risk population than those in clinical trials.19–22 Our findings are consistent with results from the Meta-Analysis Global Group in Chronic Heart Failure who report similar crude death rates in HFpEF and HFrEF patients.6 Studies of hospitalized or fee-for-service Medicare-enrolled HF patients saw higher mortality across all EF groups compared with our results.7,10,11 The adjusted rate of death for HFpEF patients in our study was 18% lower than for HFrEF patients, which is less than the 32% lower adjusted hazard ratio seen in the Meta-Analysis Global Group in Chronic Heart Failure analysis,6 but consistent with earlier cohort studies.7,11,23 Classification of HF patients in these earlier studies, however, was variable with EF distinctions often inconsistent with current ACCF/AHA guidelines and results from patients cared for in previous treatment eras. These studies also could not systematically account for variation in medication and procedure use in patients as we were able to.
A unique strength of our study was the evaluation of long-term mortality and HF hospitalization in HFbEF patients. Previous studies evaluating outcomes in this group have reported intermediate 1-year mortality and hospitalization risk for HFbEF patients when compared with HFrEF and HFpEF patients.8,10,24 Our findings revealed similar adjusted rates of death and HF hospitalization in HFbEF and HFpEF patients, with notably higher rates in HFrEF patients. Rather than an intermediate risk profile, we observed that HFbEF patients have comparable clinical risks to HFpEF patients. Our results also vary from previous studies of HF patients in the Get with the Guidelines-Heart Failure (GWTG-HF) registry that observed no significant differences in adjusted mortality in systolic function groups at 1 or 5 years of follow-up.8,10 The observed mortality rates in HFbEF patients in these studies, however, were much higher than those seen in our cohort and likely due to differences in the studied populations.8,10 The GWTG-HF studies included only patients aged ≥65 years who were enrolled in Medicare fee-for-service health plans and studied after an index HF hospitalization, whereas our cohort consisted of a community-based population of adults aged ≥21 years with HF diagnosed either as an outpatient or through hospitalization.8,10 Excluding younger HF patients and outpatients diagnosed with HF, who are known to have lower rates of in-hospital and 1-year mortality, likely biases the previous GWTG-HF studies toward higher adverse event rates.25,26 By including these patients in the study, our findings are more representative of the entire HFbEF population in the community.
The evaluation of HF-related ED visits independent of hospitalization was also unique to our study. ED visits for HF compose nearly 1% of all ED visits in the United States, carry high economic costs, and are associated with high rates of hospitalization and recidivism.27–29 A previous Kaiser Permanente Southern California and Northwest study found higher ED visit rates in HFpEF patients when compared with HFrEF and HFbEF patients.7 However, this study did not identify HF-related ED visits, included a smaller cohort of patients followed only after an index HF hospitalization, and excluded HFbEF patients. We observed minimal adjusted differences in rates of HF-related ED visits across systolic function groups, but a high burden of ED use. The markedly higher rates of HF-related ED visits relative to HF hospitalization we observed in all systolic function groups suggests a large portion of health care utilization by HF patients may be limited to the ED and is not well captured by previous studies evaluating only HF hospitalization.
Our study was strengthened by long-term follow-up of a large, sociodemographically diverse cohort of patients diagnosed with HFpEF, HFbEF, and HFrEF from both inpatient and outpatient settings using current ACCF/AHA criteria. Our ability to use comprehensive electronic medical records with linked inpatient and outpatient pharmacy, laboratory, and health care utilization information, and account for differences in the use of HF-specific and other cardiovascular medications over time is another strength of this study. There are, however, several important limitations. We were unable to gather HF-specific functional status, coronary anatomy, or non-EF echocardiographic parameters on our patients. Our cohort was also composed of insured patients from Northern California whose results may not be representative of all geographic or practice settings. However, the demographic diversity, along with the broad range of illness severity within our cohort, argues for greater generalizability to community-based practice settings than previous studies of patients in clinical trials, receiving care in tertiary care academic medical centers, or identified only through HF hospitalization. Our highly specific outpatient HF diagnostic criteria may have also inadvertently excluded healthier HF patients not seen by a cardiologist. Although high diagnostic specificity could be viewed as a study strength, it also represents a potential limitation. We also could not account for recovery or change in a patient’s EF during the study period. Previous studies report that patients with HF and EF recovery have more benign clinical courses.30 Inclusion of these patients in our HFbEF and HFpEF groups could have contributed to the lower event rates observed. However, patients were categorized based on their EF at the time of diagnosis and we suspect most patients with HF and EF recovery were categorized as HFrEF. This should have lowered event rates in the HFrEF group, and yet despite this we observed higher adjusted event rates in HFrEF patients across all outcomes.
In this large, community-based population of HF patients, adjusted rates of death, HF hospitalization, and ED visits for HF were similar for patients with HFpEF and HFbEF, and modestly higher in those with HFrEF. These findings were not explained by differences in receipt of either HF-specific or other cardioprotective medications. Despite lower rates of long-term mortality seen in our cohort compared with other large registry studies, we demonstrate persistently high rates of adverse events and excess ED utilization in HF patients in the current treatment era regardless of level of systolic function, and reinforce the need for novel strategies to improve long-term outcomes especially in those with HFpEF and HFbEF.
Supplementary Material
Funding:
Supported in part by research grants from the National, Heart, Lung and Blood institute (U19 L091179, RC1 HL099395) and from the National Institute on Aging (U01 AGO34661) of the National Institutes of Health of the United States of America.
Relation with industry: Dr. Alan S. Go has received a research grant through his institution from Novartis.
Footnotes
Disclosure
Harshith R. Avula, Thomas K. Leong, Keane K. Lee, and Sue Hee Sung have no disclosures to report. Alan S. Go has received a research grant through his institution from Novartis as below.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.amjcard.2018.05.036.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. Foundation ACoC, Guidelines AHATFoP. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 2.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011;124:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18:242–252. [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 5.Gurwitz JH, Magid DJ, Smith DH, Goldberg RJ, McManus DD, Allen LA, Saczynski JS, Thorp ML, Hsu G, Sung SH, Go AS. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med 2013;126:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(MAGGIC) M-aGGiCHF. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 7.Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW. Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction. Am J Cardiol 2015;116:1088–1092. [DOI] [PubMed] [Google Scholar]
- 8.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 9.Coles AH, Tisminetzky M, Yarzebski J, Lessard D, Gore JM, Darling CE, Goldberg RJ. Magnitude of and prognostic factors associated with 1-year mortality after hospital discharge for acute decompensated heart failure based on ejection fraction findings. J Am Heart Assoc 2015;4:e002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168:721–730. [DOI] [PubMed] [Google Scholar]
- 11.Coles AH, Fisher K, Darling C, Yarzebski J, McManus DD, Gore JM, Lessard D, Goldberg RJ. Long-term survival for patients with acute decompensated heart failure according to ejection fraction findings. Am J Cardiol 2014;114:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey Internal Division of Research report. Oakland, CA: Kaiser Permanente Northern California Division of Research, 2012:1–15. [Google Scholar]
- 13.Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes 2008;1:138–147. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA 2006;296:2105–2111. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 16.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care 1997;20:1396–1402. [DOI] [PubMed] [Google Scholar]
- 17.Fireman BH, Fehrenbacher L, Gruskin EP, Ray GT. Cost of care for patients in cancer clinical trials. J Natl Cancer Inst 2000;92:136–142. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006;113:2713–2723. [DOI] [PubMed] [Google Scholar]
- 19.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators I-P. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Committees CIa. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–639. [DOI] [PubMed] [Google Scholar]
- 22.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko H, Suzuki S, Yajima J, Oikawa Y, Sagara K, Otsuka T, Matsuno S, Kano H, Uejima T, Koike A, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T. Clinical characteristics and long-term clinical outcomes of Japanese heart failure patients with preserved versus reduced left ventricular ejection fraction: a prospective cohort of Shinken Database 2004–2011. J Cardiol 2013;62:102–109. [DOI] [PubMed] [Google Scholar]
- 24.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol 2013;61:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera R, Pandey A, Ayers CR, Agusala V, Pruitt SL, Halm EA, Drazner MH, Das SR, de Lemos JA, Berry JD. Contemporary Epidemiology of Heart Failure in Fee-For-Service Medicare Beneficiaries Across Healthcare Settings. Circ Heart Fail 2017;10:e004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, McNaughton CD, Heavrin BS, Gheorghiade M, Collins SP. The burden of acute heart failure on U.S. emergency departments. JACC Heart Fail 2014;2:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peacock WF. Using the emergency department clinical decision unit for acute decompensated heart failure. Cardiol Clin 2005;23:569–588. viii. [DOI] [PubMed] [Google Scholar]
- 29.Claret PG, Calder LA, Stiell IG, Yan JW, Clement CM, Borgundvaag B, Forster AJ, Perry JJ, Rowe BH. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. CJEM 2017;20:222–229. [DOI] [PubMed] [Google Scholar]
- 30.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.