Abstract
Abstinence from chronic use of addictive drugs triggers an aversive withdrawal syndrome that compels relapse and deters abstinence. Many features of this syndrome are common across multiple drugs, involving both affective and physical symptoms. Some of the network signaling underlying withdrawal symptoms overlaps with activity that is associated with aversive mood states, including anxiety and depression. Given these shared features, it is not surprising that a particular circuit, the dorsal diencephalic conduction system, and the medial habenula and interpeduncular nucleus (MHb-IPN) in particular, have been identified as critical to the emergence of aversive states that arise both as a result, and independently, of drug addiction. As the features of this circuit continue to be characterized, the MHb-IPN axis is emerging as a viable target for therapeutics to aid in the treatment of addiction to multiple drugs of abuse as well as mood-associated disorders.
Keywords: opioid, alcohol, psychomotor stimulants, medial habenula, interpeduncular nucleus, epithalamus, reward, withdrawal, depression, anxiety, stress
Introduction
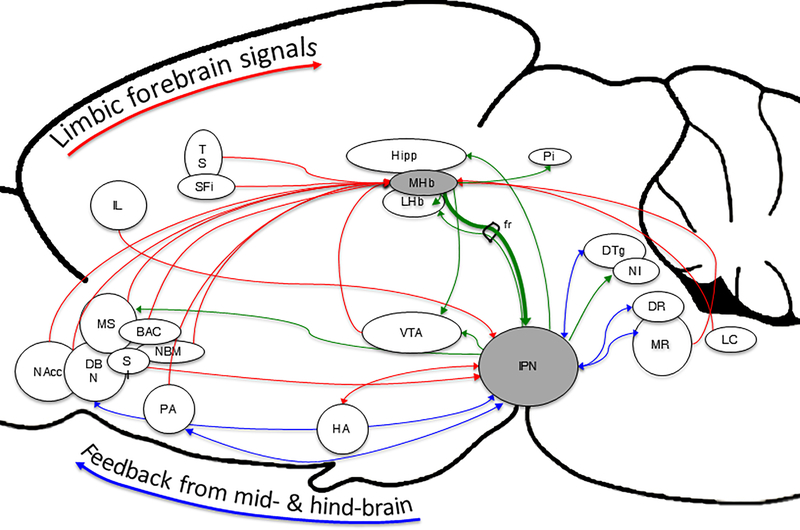
Drug addiction is a substantial public health and societal burden, causing over $700 billion in annual costs associated with crime, lost work productivity, and healthcare (National Center for Chronic Disease et al. 2014, Sacks et al. 2015, Center 2011). Although they have unique pharmacological effects, all addictive drugs share actions on the dopaminergic system that contribute to their rewarding effects (Di Chiara & Imperato 1988). The withdrawal syndrome that follows cessation of chronic use has also been identified as having common substrates. Anxiety and mood disorders, the first and second most common psychiatric disorders in the United States (Kessler et al. 2005), may also reflect alterations in overlapping circuits. Interestingly, research characterizing the mechanisms underlying addiction, withdrawal, and aversive moods states has implicated a common pathway. The medial habenula (MHb) and interpeduncular nucleus (IPN) are two components of the dorsal diencephalic conduction system (DDC), a highly evolutionarily conserved pathway which, along with the medial forebrain bundle, conveys signals from the limbic forebrain to the midbrain and hindbrain (Bianco & Wilson 2009, Okamoto et al. 2012). In higher vertebrates, the DDC has evolved to be a significant pathway by which the forebrain regulates midbrain motivation and reward circuitry (Sutherland 1982). The epithalamic MHb receives inputs from a variety of structures, including the triangular septal nucleus, septofimbral nucleus, ventral tegmental area, and raphe nuclei (Herkenham & Nauta 1977, Lecourtier & Kelly 2007, Phillipson & Pycock 1982). Studies also indicate that the MHb receives inputs from the nucleus accumbens (Sutherland 1982), locus coeruleus and superior cervical ganglion (Gottesfeld 1983), diagonal band nucleus and medial septum (Qin & Luo 2009), as well as the median raphe nucleus (Sutherland 1982, Conrad et al. 1974). Some studies suggest that the MHb may project to the pineal body (Ronnekleiv & Moller 1979, Guglielmotti & Cristino 2006), and it may also send sparse efferents to the VTA (Cuello et al. 1978), as well as extend boutons en passant to the LHb (Kim & Chang 2005). However, the MHb sends its most dense efferent projections to the mesencephalic IPN through the core of the fasciculus retroflexus (FR) (Herkenham & Nauta 1979), which, in turn, sends efferents to a wide variety of mid- and hindbrain structures implicated in regulating affective states. Those structures include the dorsal tegmental nucleus (Shibata & Suzuki 1984), hippocampus (Shibata & Suzuki 1984, Baisden et al. 1979, Wyss et al. 1979), lateral hypothalamus (Massopust & Thompson 1962, Morley 1986, Kemali & Guglielmotti 1982, Smith et al. 1980), ventral tegmental area (Hayakawa et al. 1981, Smaha & Kaelber 1973), septum, preoptic area, and nucleus of the diagonal band (Smaha & Kaelber 1973, Morley 1986). Additionally, there are data indicating projections from the IPN to the dorsal and median raphe nuclei (Groenewegen et al. 1986, Behzadi et al. 1990), as well as the lateral habenula (Massopust & Thompson 1962, Morley 1986). Though the major source of innervation in the IPN arrives from the MHb, there is evidence of afferents arriving from structures including the horizontal limbs of the diagonal band nucleus (Contestabile & Flumerfelt 1981), substantia innominata (Vertes & Fass 1988), infralimbic region of the medial prefrontal cortex (Takagishi & Chiba 1991), preoptic nucleus (Shibata et al. 1986), hypothalamic nuclei (Contestabile & Flumerfelt 1981, Hamill & Jacobowitz 1984), supramammillary nucleus (Contestabile & Flumerfelt 1981, Hamill & Jacobowitz 1984), raphe nuclei (Conrad et al. 1974), nucleus incertus (Hamill & Jacobowitz 1984) and dorsal tegmental nucleus (Hamill & Jacobowitz 1984). While some of these anatomical studies were conducted recently, some were conducted many years ago, so corroborative reproducibility experiments are likely warranted, given the availability of more targeted tracer techniques. A summary of these afferent and efferent projections for the MHb and IPN is available in figure 1 and tables 1 and 2.
Figure 1.
Afferent and efferent connections of the MHb-IPN pathway. The medial habenula-interpeduncular nucleus pathway unites forebrain limbic with midbrain & hindbrain motivation & reward signaling. The medial habenula receives afferent inputs from a wide variety of forebrain limbic structures, and the interpeduncular nucleus sends efferent projections to a variety of midbrain & hindbrain structures implicated in the neurophysiology underlying addiction and a variety of mood-related psychiatric conditions. Red lines indicate afferent projections to the MHb or IPN, and green lines identify efferent
Table 1.
MHb Afferent & Efferent Connections
| Afferent | Efferent | ||
|---|---|---|---|
| Structure | Reference | Structure | Reference |
| Triangular Septum | Herkenham & Nauta, 1977 | Interpeduncular Nucleus | Morley, 1986; Herkenham & Nauta, 1979 |
| Medial Septal Nucleus | Qin & Luo, 2009 | Pineal Body | Ronnekleiv & Moller, 1979; Guglielmotti & Cristino, 2006 |
| Diagnoal Band Nucleus | Qin & Luo, 2009 | Lateral Habenula | Kim & Chang, 2005 |
| Septofimbral Nucleus | Herkenham & Nauta, 1977 | VTA | Cuello, et al., 1978 |
| VTA | Herkenham & Nauta, 1977 | ||
| Interfascicular Nucleus of the VTA | Phillipson & Pycock, 1982 | ||
| Locus Coeruleus | Gottesfeld, 1983 | ||
| Superior Cervical Ganglion | Gottesfeld, 1983 | ||
| Preoptic Area | Groenewegen, 1986; Herkenham & Nauta, 1977 | ||
| Median Raphe Nucleus | Conrad, et al., 1974; Sutherland, 1982 | ||
| Nucleus Basalis of Meynert | Herkenham & Nauta, 1977 | ||
| Nucleus Accumbens | Sutherland, 1982 | ||
Table 2.
IPN Afferent & Efferent Connections
While both are fairly small anatomical structures, the MHb and IPN host the synthesis and release of a wide variety of neurotransmitters. Studies have identified acetylcholine (McCormick & Prince 1987), substance P (SP) (Burgunder & Young 1989, De Biasi et al. 2016, Jackson et al. 2015), glutamate and GABA (Qin & Luo 2009), norepinephrine (Gottesfeld 1983), serotonin (Kinsey et al. 2001), ATP (Edwards et al. 1992, Sperlagh et al. 1998), interleukin-18 (Sugama et al. 2002), and a host of neuropeptides in the MHb-IPN pathway (McLaughlin et al. 2015, Kopp et al. 2002). Additionally, the MHb-IPN circuit has been implicated in mechanisms that mediate some of the acute and aversive features of withdrawal from multiple drugs, including alcohol, opiates, nicotine, and other stimulants. This review will focus on studies that specifically implicate the DDC, and the MHb-IPN pathway in particular, in the neurophysiology associated with both addiction and mood-related psychiatric conditions, with an eye towards the possibility of identifying druggable targets within the pathway that may yield therapeutic benefits for the treatment of both sets of conditions.
Alcohol
The pharmacological activity of ethanol encompasses a broad range of targets in the central nervous system, and studies have indicated that the MHb-IPN circuit represents a significant component of its affective and behavioral effects. Local cerebral glucose utilization rates in alcohol-preferring rats are significantly elevated in both divisions of the habenular complex relative to non-preferring rats (Smith et al. 2001). Data from our lab have identified an interaction between ethanol and nicotine withdrawal, as well as a role played by nAChRs in the MHb or IPN in ethanol withdrawal. Specifically, intraperitoneal injection of a non-selective nAChR antagonist, mecamylamine, is capable of precipitating a withdrawal syndrome in mice chronically treated with ethanol. Withdrawal symptoms are also exhibited when mecamylamine is infused into the MHb or IPN, but not the ventral tegmental area (VTA) or hippocampus (Perez et al. 2015), indicating that nAChR blockade in the MHb-IPN circuit is sufficient to precipitate the ethanol withdrawal syndrome. Another recent finding has identified changes in signaling molecules associated with apoptosis, inflammation, neurodegeneration, and senescence in the habenula, among other structures, following chronic treatment with ethanol (Roux et al. 2015). Receptor knock-out studies have shown that neuropeptide Y (NPY) acts as an important regulator of alcohol intake, with knock-out mice exhibiting increased alcohol ingestion relative to wild-type mice (Thiele et al. 2002). More recently, it was shown that alcohol-preferring rats exhibit an absence of NPY mRNA in the MHb while non-preferring rats do have a signal of mRNA for the neuropeptide (Hwang et al. 2004). Taken together, the literature suggests that activity in the MHb-IPN circuit likely modulates the effects and ingestive behavior of alcohol, as well as its withdrawal symptoms. Variations between individuals in the signaling within this circuit may underlie predispositions to pathological intake patterns.
Opioids
Drug overdose represents the leading cause of accidental death in the United States (Medicine 2016), and addiction to opioids, including both illicit substances, such as heroin, and prescription analgesics, such as oxycodone and fentanyl, drive this epidemic (Prevention 2015). Interestingly, overdose fatalities, sales, and substance use disorder treatment admissions have increased in parallel from 1999 to 2008 (Paulozzi 2014), indicating a significant need for improved treatments for substance abuse disorder and opioid addiction in particular.
One of the densest regions of opioid receptor expression is the DDC, and the MHb, FR, and IPN are particularly rich with expression (Gackenheimer et al. 2005, Gardon et al. 2014, Zhu et al. 1998, Sim-Selley et al. 1999). Additionally, there is a plethora of neurotransmitters that the MHb-IPN circuit synthesizes or is sensitive to, including acetylcholine, neurokinins, interleukin-18 (IL-18) (Viswanath et al. 2013, Sugama et al. 2002), and purines (Pankratov et al. 2009, Pankratov et al. 2006, Kanjhan et al. 1999) that may be modulated by the activities of opioids. Given the broad efferent targets of the IPN that are known to regulate affect and substance use, including the raphe nuclei, nucleus incertus, lateral septum, lateral dorsal tegmentum (LDTg), and hypothalamus (Sutherland 1982, Ryan et al. 2011, Bianco & Wilson 2009, Morley 1986, Gardon et al. 2014), the MHb-IPN circuit is likely an anatomical node, centrally involved in the signaling underlying both acute effects of, and withdrawal from, opioids. Evidence over the past several decades has corroborated such a role. For example, lesions of the MHb have been observed to induce hyperalgesia and increase the analgesic efficacy of morphine (Meszaros et al. 1985), and morphine is capable of inducing analgesia when infused directly into the habenular complex (Cohen & Melzack 1985). When evaluating the effects of intracranial self-stimulation of the VTA on opioid release, a unique reduction of endogenous opioid binding was observed in the MHb (Stein 1993). Altered acetylcholinesterase (AChE) activity is observed in the MHb following chronic morphine administration, and precipitation of withdrawal with the opioid receptor antagonist, naloxone, results in altered AChE activity in the IPN (Neugebauer et al. 2013). Additionally, chronic morphine administration induced a trend towards increased nAChR expression in the MHb (Neugebauer et al. 2013). Acute administration of 18-methoxycoronaridine (18-MC), an α3β4 nAChR antagonist, reduced signs of naltrexone-precipitated withdrawal from morphine (Rho & Glick 1998), an effect that appears to be mediated by activity in the MHb and IPN (Panchal et al. 2005, Taraschenko et al. 2007). 18-MC was also observed to reduce morphine self-administration upon intracranial infusion into the MHb or IPN (Glick et al. 2006). In the MHb, RSK2, a component of the ribosomal S6 kinase 90kDa family, which act as substrates of extracellular-regulated kinases 1 & 2 to regulate cytosolic and nuclear targets, has been identified as critical to morphine-induced analgesia (Darcq et al. 2012). Finally, the LDTg, an efferent target of the IPN, has been shown to exhibit significant increases in vesicular ACh transporter markers following chronic morphine administration (Bajic et al. 2015, Gardon et al. 2014). Altogether, these data implicate the DDC, and MHb-IPN circuit in particular, in the signaling underlying some of the acute effects of opioids, as well as aspects of their addictive properties. Furthermore, adaptations in cholinergic components may represent a significant facet of these changes.
Nicotine and Psychomotor Stimulants
The role of the lateral division of the habenular complex (LHb) in regulating dopaminergic activity in the VTA via the rostromedial tegmental nucleus has been established, and represents an important mechanism by which aversion and addiction are modulated (Barrot et al. 2012, Sanchez-Catalan et al. 2016, Jean-Richard Dit Bressel & McNally 2014, Quina et al. 2015). Both the LHb and MHb send dense efferent projections through the fasciculus retroflexus, and anatomical studies suggest that projections emerging from the MHb course through the core and those from the LHb through the sheath of this dense fiber bundle (Bianco & Wilson 2009). Degeneration of dopaminergic fibers in the caudate following chronic exposure to psychomotor stimulants like amphetamines, was observed decades ago (Ellison et al. 1978). In addition to dopaminergic fibers, similar degeneration has been observed in axons populating the sheath of the FR following chronic exposure to cathinone, cocaine, amphetamine, methamphetamine, and MDMA (Ellison 2002, Carlson et al. 2000). In rats treated with cocaine, increased expression of Fos-protein, a marker of neuronal activation, was observed in the MHb associated with cue-induced reinstatement (James et al. 2011). Additionally, similar to its interference with opioid-derived reward, 18-MC administration results in reduced methamphetamine self-administration in rats (Glick et al. 2000). Once again, direct infusions into the MHb and/or IPN induced similar reductions of self-administration, with what appears to be greater efficacy when infused into the IPN, suggesting that activity in the MHb-IPN circuit likely mediates this effect (Glick et al. 2008). Finally, methamphetamine and cocaine have been shown to increase extracellular concentrations of ACh in the IPN, with cocaine inducing a dose-dependent biphasic effect (Hussain et al. 2008).
Probably the best-characterized activity of a drug in the MHb-IPN pathway is that of nicotine, perhaps attributable to the considerable density of a variety of nAChRs that populate the structure (Mugnaini et al. 2002). Studies have suggested that up 90–100% of MHb neurons express nAChRs, with the majority containing the α3, α4, α5, β2, and/or β4 subunits (Viswanath et al. 2013, Sheffield et al. 2000). Some data suggest that approximately 20% of nAChRs in the MHb expressed by neurons that project to the IPN contain the α5 subunit (Picciotto & Kenny 2013, Grady et al. 2009). In the IPN, high levels of α2 subunit-containing nAChRs can be found (De Biasi & Salas 2008, Grady et al. 2009), and the distributions of nAChRs composed of specific subunit combinations can help distinguish subnuclei within both the MHb and IPN (Shih et al. 2014).
As many reviews of the effects of nicotine in this circuit have been written over the years (McLaughlin et al. 2015, De Biasi et al. 2014, De Biasi & Dani 2011, Dani & De Biasi 2001, Jackson et al. 2015), this section will focus on recent advances in characterizing the effects of chronic use and withdrawal from nicotine in the MHb-IPN pathway. For example, it was recently shown that, during withdrawal from nicotine, the spontaneous action potential frequencies in MHb cholinergic neurons are doubled after mice are administered nicotine relative to mice in withdrawal treated with saline (Gorlich et al. 2013). Further, these studies demonstrated that the pacemaking activities of MHb cholinergic neurons are determined by the activities of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, and pharmacological inhibition of these HCN channels resulted in the manifestation of nicotine withdrawal-associated behaviors, including both somatic and anxiety-associated symptoms. A study from our lab showed that nicotine enhances the intrinsic excitability of MHb neurons by activating α5- containing nAChRs, which results in the facilitation of neurokinin release onto NK1 and NK3 receptors (Dao et al. 2014). Notably, pharmacological blockade of NK1 & NK3 receptors in the MHb of mice chronically treated with nicotine resulted in the precipitation of somatic symptoms of nicotine withdrawal. Further implicating the α5-containing nAChRs in the MHb in the physiology of nicotine addiction and withdrawal, mice lacking expression of the subunit self-administer doses of nicotine at levels that are aversive to wild-type mice, and virus-mediated re-expression of the subunit in the MHb rescues self-administration to levels resembling those consumed by wild type mice (Fowler et al. 2011a). Additionally, mice lacking the α5 nAChR subunit exhibit reduced IPN activation following exposure to nicotine relative to wild type mice, suggesting a significant role played by this subunit in the MHb-IPN pathway in determining the range of nicotine doses capable of facilitating activity in brain reward circuitry (Fowler et al. 2011b, Fowler et al. 2013). Mice lacking the α2 nAChR subunit exhibit elevations of both glutamate and GABA in the IPN, suggesting that α2-containing nAChRs may participate in the signaling underlying the effects of nicotine (Lotfipour et al. 2013). Studies have also demonstrated a significant role played by IPN signaling in somatic symptoms of nicotine withdrawal by the IPN, with GABAergic neuronal activation enhanced by increased glutamate release, perhaps from the MHb (Zhao-Shea et al. 2013). This group also showed that pharmacological inhibition of NMDA receptor activation reduced symptoms of withdrawal, suggesting a glutamatergic signal from the MHb playing a significant role in the nicotine withdrawal syndrome. Following chronic nicotine exposure, mecamylamine infusion into the MHb or IPN has been observed to induce anxiety-associated behaviors and symptoms of nicotine withdrawal (Zhao-Shea et al. 2015, Salas et al. 2009). Given that mecamylamine infusion into nicotine-naïve mice does not result in significant changes in anxiety-associated behavior, the MHb-IPN axis represents a node of neuroplastic adaptations resulting from chronic nicotine exposure that underlie the affective and somatic symptoms of nicotine withdrawal upon cessation.
Accordingly, while not necessarily a direct pharmacological target of most psychomotor stimulants apart from nicotine, the MHb-IPN circuit likely represents a system that modulates the acute effects of psychomotor stimulants, and contributes to signaling underlying withdrawal and relapse. A summary of the signaling associated with drug action in this pathway is available in table 3.
Table 3.
Roles in the actions of drugs
| MHb | IPN | ||||
|---|---|---|---|---|---|
| Technique | Finding | Reference | Technique | Finding | Reference |
| Local cerebral glucose utilization | Higher in MHb of EtOH-preferring rats | Smith, D. G, et al., 2001 | Naloxone-precipitated opioid withdrawal | Altered AChE activity in the IPN | Neugebauer, N. M., et al., 2013 |
| IP nAChR antagonist injection | Precipitates EtOH withdrawal syndrome | Perez, E., et al., 2015 | 18-MC IPN infusion | Reduced morphine self-administration | Glick, S. D., et al. 2006 |
| NPY Receptor knock-out | Knock-out mice ingest more EtOH | Roux, A. et al., 2015 | 18-MC IPN infusion | Reduced methamphetamine self-administration | Glick, S. D., et al. 2000 |
| In situ hybridization | EtOH-preferring mice lack NPY mRNA in MHb | Thiele, T.E., et al., 2002; Hwang, B.H., et al., 2004 | Methamphetamine & cocaine administration | Increased extracellular [ACh] | Hussain, R. J., et al., 2008 |
| MHb lesion | Hyperalgesia, increase analgesic efficacy of morphine | Meszaros, J., et al., 1985 | |||
| Intracranial infusion to MHb | Analgesia | Cohen, S. R., et al., 1985 | |||
| VTA ICSS | Reduced endogenous opioid binding in MHb | Stein, E. A., et al., 1993 | |||
| Chronic morphine administration | Altered AChE activity in the MHb | Neugebauer, N. M., et al., 2013 | |||
| Chronic morphine administration | Trend to increased MHb nAChR expression | Neugebauer, N. M., et al., 2013 | |||
| 18-MC MHb infusion | Reduced morphine self-administration | Glick, S. D., et al. 2006 | |||
| RSK2 knock-out in MHb | Reduced morphine analgesia | Darcq, E., et al., 2012 | |||
| Cocaine cue-induced reinstatement | Increased Fos-protein in MHb | James, M. H. et al., 2011 | |||
| 18-MC MHb infusion | Reduced methamphetamine self-administration | Glick, S. D., et al. 2000 | |||
Depression-like behavior
In addition to playing a role in the acute effects of multiple drugs and the manifestation of their withdrawal, the MHb-IPN circuit has been implicated in mood-associated conditions. For example, one group worked to identify brain regions exhibiting activation during uncontrollable stress that then correlated with a subsequent manifestation of helplessness behaviors (Mirrione et al. 2014). Using positron emission tomography (PET) with rats, the habenula and lateral septum emerged as central in a network of brain regions, corroborating a role played by these structures in vulnerability to uncontrollable stress (Mirrione et al. 2014). An evaluation of the effects of chronic mild stress in rats on somatostatin (SST) receptor expression and SST release revealed that this signaling system significantly changes in the MHb (Faron-Gorecka et al. 2016). In fact, SST2 receptor expression in the MHb, and peripheral SST concentration in plasma, were identified as particularly sensitive biochemical indicators of whether an animal would be stress responsive or non-responsive. An effort to characterize changes in the expression of microRNA (miRNA) species in the MHb and LHb of rats that underwent learned helplessness identified six miRNAs that were significantly altered (Svenningsen et al. 2016). The miRNA species identified are associated with MAPK, neutrophin, and ErbB signaling pathways.
Though the MHb is a relatively small structure, it can be segregated into subnuclei based upon localization of neurotransmitter and transporter protein expression in specific regions. The MHb is glutamatergic, and is often sub-divided into a dorsal sub-region, which expresses SP, and a ventral sub-region, which expresses ACh (Kobayashi et al. 2013). Some studies suggest that it can be further divided based on transporter protein and receptor densities (Aizawa et al. 2012). Moreover, efferent projections from the MHb target the IPN topographically, with efferents from the dorsal MHb targeting the lateral IPN, those from medial regions of the MHb terminating in the ventral IPN, and those from the lateral MHb terminating in the dorsal IPN (Bianco & Wilson 2009). Given the neurochemical differences in these topographic projections, it is not surprising that studies suggest these anatomical distinctions correspond to different functional roles (Ichijo & Toyama 2015). As altered physical activity and anhedonia are diagnostic indicators of major depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorders (Association 2013), the MHb has been evaluated as a regulator of analogous behaviors like wheel running activity (WRA) in rodents (Hsu & Wang 2014). Working with a genetic ablation model of the dorsal subnucleus of the MHb, reduced WRA and sucrose preference was observed, both of which are indicative of depressive-like behaviors. Interestingly, despite no known direct synaptic connection with dopaminergic populations, mice exhibited a significant preference for optogenetic intracranial self-stimulation of the dorsal MHb (Hsu & Wang 2014). Conversely, inhibition of the terminals arriving in the IPN from the dorsal MHb yielded place aversion. This corroborates studies that characterized metabolic differences in brain regions of rats bred for susceptibility to helplessness, which exhibited significantly increased markers of metabolic activity in both the lateral and medial habenula (Shumake et al. 2003). Using the same metabolic profiling method in Holtzman rats, a strain that exhibits susceptibility to stress-evoked helplessness, Padilla and colleagues characterized the effects of a two-week treatment with fluoxetine following a learned helplessness paradigm (Padilla et al. 2011). The group identified a protective effect of fluoxetine against depression-like behaviors in the forced swim test relative to rats treated with vehicle. This protective effect correlated with changes in regional metabolic activity in a variety of brain networks, including the habenular complex, IPN, and dorsal raphe nucleus (Padilla et al. 2011). In particular, fluoxetine treatment was associated with comparatively stronger positive correlations with prefrontal regions, including the dorsal, orbital, and prelimbic cortices. The IPN was positively correlated with the lateral orbital cortex in rats treated with fluoxetine, and this correlation was not present in vehicle-treated rats. Finally, metabolic markers in the dorsal raphe become somewhat positively correlated with the habenula in fluoxetine-treated rats, while strongly negatively correlated among the vehicle-treated group.
Due to challenges derived from the small size and sub-cortical location of the MHb-IPN circuit, the majority of studies characterizing its function have been performed in animal models. However, a few studies with humans have corroborated a role played by the circuit in depression. Accumulating literature over the past decade indicates that ketamine may represent a novel pharmacological treatment strategy for major depressive disorder (Han et al. 2016, Burger et al. 2016, Zarate et al. 2006, Murrough et al. 2013). When evaluating changes in cerebral glucose metabolism using PET, 20 unmedicated patients with treatment-resistant major depressive disorder were scanned before and after a ketamine infusion. The habenular complex, among several other brain regions, exhibited reduced metabolism in association with a rapid antidepressant effect of ketamine (Carlson et al. 2013). Finally, a post-mortem study of sections from the brains of patients diagnosed with various mood disorders identified significant reductions in the volumes, cell numbers, and mean cell areas in the MHb of depressive patients (Ranft et al. 2010).
Anxiety, fear, and stress
While anxiety and fear have been associated for quite some time with anatomical structures including the amygdala, hippocampus, hypothalamus, and periaqueductal gray, recent research has implicated the DDC in these behaviors as well (Okamoto & Aizawa 2013). A genetic lesion study indicates that selective elimination of MHb afferents arriving from two forebrain structures, the triangular septum and bed nucleus of the anterior commissure, results in disrupted anxiety- and fear-associated behaviors (Yamaguchi et al. 2013). In particular, lesions of the projections from the triangular septum, which terminate in the ventral subnucleus of the MHb, disrupted anxiety-associated behavior. Conversely, lesions of projections arriving from the bed nucleus of the anterior commissure, which terminate in the dorsal subnucleus of the MHb, disrupted fear-associated behavior. Following both acute and chronic restraint stress in rats, significant elevations of the pro-inflammatory cytokine, IL-18, have been observed in the dorsal MHb (Sugama et al. 2002). Mast cells are another immune system-associated signaling component that appears to be sensitive to environmental stressors and aversive mood states (Georgin-Lavialle et al. 2016, Nautiyal et al. 2008, Silver & Curley 2013, Frenzel & Hermine 2013). Mice treated with a 3-week behavioral subordination paradigm, either via exposure to an aggressor or placement in a clean cage, exhibited increased numbers of mast cells in the habenula, thalamus, and hypothalamus (Cirulli et al. 1998).
When characterizing fear-associated behavior in zebrafish, inhibition or lesion of the dorsal habenula (analogous to MHb in mammals) resulted in elevated freezing behaviors in response to a conditioned fear stimulus (Agetsuma et al. 2010, Lee et al. 2010). Both control and habenula-disrupted fish froze upon first exposure to an electric shock, but as subsequent shocks were administered, control fish exhibited reduced freezing behavior while those with disrupted habenular function exhibited no such behavioral adaptation. Another study identified behavioral signatures of elevated baseline anxiety in zebrafish following dorsal habenula lesions, including responses to novel environments and alarm substance secretion in response to overhead shadows (Mathuru & Jesuthasan 2013). This has led to the suggestion that reciprocal connectivity between the IPN, raphe nuclei, and dorsal tegmental region may be critical to behavioral responses to stressors. Furthermore, activity in the habenular complex may be an upstream determining factor in selection of behavioral strategies to cope with stressors (Okamoto et al. 2012, Jesuthasan 2012). A unique characteristic of the habenular complex is its asymmetry, with the left habenula larger than its right counterpart (Ahumada-Galleguillos et al. 2016, Hetu et al. 2016). When this asymmetry is lost in zebrafish, behavioral assays indicated elevated manifestations of anxiety, as well as elevated cortisol levels in response to stressors (Facchin et al. 2015).
While the size of the habenular complex renders current neuroimaging technology incapable of distinguishing the LHb from the MHb in human studies, some studies indicate a role played by the habenular complex in the pathophysiology of bipolar disorder (BD). In a study using high-resolution magnetic resonance imaging (MRI), it was found that patients diagnosed with BD who had either never been medicated, or had been un-medicated for at least two months, exhibited smaller habenular volumes than healthy controls (Savitz et al. 2011).
Studies have also implicated the IPN in regulating anxiety and fear. Early studies of the IPN identified a role for the structure in the retention of avoidance conditioning. Rats were trained to perform a jumping response following a visual stimulus to avoid a shock. After a learning period, a group of trained rats were treated with electrolytic lesions of the IPN. Following recovery from the procedure, rats re-learned the task and retention of the response was compared to controls. Rats with IPN lesions exhibited comparatively inferior retention of the response, though exhibited other signatures of a fear reaction, implying a role played by IPN signaling in specific components of fear learning (Thompson 1960). Following chronic nicotine administration, increased corticotropin releasing factor (CRF) synthesis is observed in dopaminergic neurons in the VTA, which appear to send efferents to the ventral IPN (Zhao-Shea et al. 2015). This is accompanied by an increase in CRF1 receptor expression in a particular subnucleus of the ventral IPN, and withdrawal induces release of CRF by the VTA onto ventral IPN neurons. Blockade of CRF1 receptor binding in the IPN was shown to reduce anxiety-associated behavior generated during withdrawal from nicotine. Table 4 presents a compilation of studies characterizing the role of this pathway in mood-associated conditions.
Table 4.
Roles in Mood Disorders
| MHb | IPN | ||||
|---|---|---|---|---|---|
| Technique | Finding | Reference | Technique | Finding | Reference |
| PET in stress-treated rats | Hb identified as central in network of brain regions | Mirrione, M. M., et al., 2014 | Inhibition of afferent terminals in IPN | Place aversion | Hsu, Y. W., et al., 2014 |
| Chronic mild stress in rats | Altered SST release and SST2 receptor expression in MHb distinguish stress-responsive from non-responsive | Faron-Gorecka, A. et al., 2016 | Stress-evoked helplessness treated with fluoxetine in rats | Fluoxetine was protective, correlated with altered metabolic activity in IPN | Padilla, E., et al., 2011 |
| Learned helplessness in rats | Altered miRNA associated with MAPK, neutrophin, and ErbB in MHb & LHb | Svenningsen, K., et al., 2016 | Electrolytic lesion of IPN | Inferior retention of conditioned avoidance response | Thompson, R., 1960 |
| Genetic ablation of dorsal MHb | Reduced wheel running activity | Hsu, Y. W., et al., 2014 | Chronic nicotine administration | Increased CRF release to ventral IPN | Zhao-Shea, R., et al., 2015 |
| Optogenetic self-stimulation of dorsal MHb | Preference for stimulation | Hsu, Y. W., et al., 2014 | Chronic nicotine administration | Increased CRF1 receptor expression in ventral IPN | Zhao-Shea, R., et al., 2015 |
| Rats bred for susceptibility to helplessness | Increased markers of metabolic activity in MHb & LHb | Shumake, J. et al., 2003 | Blockade of CRF1 receptor in IPN | Reduced anxiety-associated behaviors during nicotine withdrawal | Zhao-Shea, R., et al., 2015 |
| Stress-evoked helplessness treated with fluoxetine in rats | Fluoxetine was protective, correlated with altered metabolic activity in Hb | Padilla, E., et al., 2011 | |||
| PET in depressed patients treated with ketamine | Reduced metabolism in the Hb | Carlson, P. J., et al., 2013 | |||
| Post-mortem study of brain sections of patients with depression | Reduced volume, cell number, and mean cell areas in MHb | Ranft, K., et al., 2010 | |||
| Elimination of MHb afferents from TS & BAC | Disrupted anxiety-associated behaviors | Yamaguchi, T., et al., 2013 | |||
| Acute & chronic restraint stress | Elevated IL-18 in dorsal MHb | Sugama, S., et al., 2002 | |||
| Subordination paradigm | Elevated mast cell numbers in the Hb | Cirulli, F., et al., 1998 | |||
| Inhibition or lesion of dMHb in zebrafish | Elevated freezing behavior, elevated anxiety, elevated alarm substance secretion | Agetsuma, M., et al., 2010; Lee, A., et al., 2010; Mathuru, A.S., et al., 2013 | |||
| MRI of un-medicated patients with bipolar disorder | Reduced Hb volumes | Savitz, J.B., et al., 2011 | |||
Conclusion
The dorsal diencephalic conduction system is emerging as an important node in the pathophysiology of addiction to multiple drugs, including alcohol, opioids, and nicotine. In addition to this involvement in substance abuse and addiction, the DDC also appears to regulate affect and mood-associated psychiatric conditions. Given the high comorbidity of substance use disorders and psychiatric illnesses, it makes sense that both conditions engage some overlapping pathways. For example, approximately one-third of individuals diagnosed with major depressive disorder also have a substance use disorder (excluding nicotine dependency), with almost half of all patients with depression having a family history of substance use disorders (Davis et al. 2008). Individuals with both disorders tend to experience earlier onsets of depression, greater persistence of the disorders, and exhibit more suicide attempts. When considering another addictive drug, nicotine, individuals diagnosed with mental illnesses are approximately twice as likely to smoke tobacco (Lasser et al. 2000), and studies have shown that 81.8% of individuals diagnosed with bipolar disorder, and 76.8% diagnosed with generalized anxiety disorder have smoked daily for at least a month (Lasser et al. 2000). Anxiety disorders have been implicated in alcohol, opioid, and stimulant abuse (Vorspan et al. 2015). Among adolescents, the majority of substance use disorders occur among youth with prior psychiatric disorders, with alcohol abuse observed in 17.3% and drug abuse observed in 20% of those diagnosed with prior anxiety disorders (Conway et al. 2016). A relationship between anxiety, stress, depression, and other psychiatric conditions and increased nicotine consumption was established many years ago (Lasser et al. 2000, Feldner et al. 2007, Morissette et al. 2007, Patton et al. 1998, Zvolensky et al. 2005, Kassel et al. 2003, Cougle et al. 2010), and a recent study has indicated that this dynamic has not changed, despite declining overall usage over the past 6 decades (Prochaska et al. 2016). This relationship even translates to a unique puff volume among those with psychiatric conditions, with smokers who have a history of panic attacks exhibiting increased puff volumes relative to those without histories of panic attacks (Farris et al. 2016). Accordingly, mounting data indicates involvement of the DDC, and the MHb-IPN pathway in particular, in both addiction to multiple drugs of abuse and mood-associated conditions, and the MHb-IPN circuit may represent a junction at which signaling underlying both sets of conditions occurs. Given the presence of nAChRs composed of unique subunit compositions in the MHb-IPN pathway (Antolin-Fontes et al. 2015), this circuit may yield strategic targets for pharmacological therapeutics to improve treatment outcomes of both sets of conditions.
Acknowledgements
This effort for this work was supported by grants from the National Institutes of Health DA0941, P50CA179546 (MDB), NS21229, DA09411(JAD), and T32DA028874 (IAM) and.
Abbreviations
- 18-MC
18-methoxycoronaridine
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ATP
adenosine triphosphate
- BAC
bed nucleus of the anterior commissure
- BD
bipolar disorder
- CRF1
Corticotropin-releasing factor
- DDC
dorsal diencephalic conduction system
- DTg
dorsal tegmentum
- FR
fasciculus retroflexus
- HA
hypothalamic nuclei
- HCN
hyperpolarization-activated cyclic nucleotide-gated channels
- Hipp
hippocampus
- IL-18
interleukin-18
- IL
infralimbic region of the medial prefrontal cortex
- IPN
Interpeduncular nucleus
- LC
locus coeruleus
- LDTg
lateral dorsal tegmentum
- LHb
lateral habenula
- MDMA
3,4-methylenedioxymethamphetamine
- MHb
medial habenula
- miRNA
microRNA
- MRI
magnetic resonance imaging
- MS
medial septum
- NAChRs
nicotinic acetylcholine receptors
- NBM
Nucleus Basalis of Meynert
- NI
nucleus incertus
- NK1
neurokinin-1
- NK3
neurokinin-3
- NMDA
N-methyl-D-aspartate receptor
- NPY
neuropeptide Y
- PA
Preoptic area
- PET
positron emission tomography
- Pi
pineal body
- RN
raphe nuclei
- Sfi
septofimbrial nucleus
- SI
Substantia innominate
- SP
substance P
- SST
somatostatin
- TS
triangular septum
- VTA
ventral tegmental area
- WRA
wheel running activity
Footnotes
Conflict of interests
All authors declare that they do not have any conflicts of interest to report.
References
- Agetsuma M, Aizawa H, Aoki T et al. (2010) The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nature neuroscience, 13, 1354–1356. [DOI] [PubMed] [Google Scholar]
- Ahumada-Galleguillos P, Lemus CG, Diaz E, Osorio-Reich M, Hartel S and Concha ML (2016) Directional asymmetry in the volume of the human habenula. Brain structure & function. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Kobayashi M, Tanaka S, Fukai T and Okamoto H (2012) Molecular characterization of the subnuclei in rat habenula. The Journal of comparative neurology, 520, 4051–4066. [DOI] [PubMed] [Google Scholar]
- Antolin-Fontes B, Ables JL, Görlich A and Ibañez-Tallon I (2015) The Habenulo-Interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology, 96, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP (2013) Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Baisden RH, Hoover DB and Cowie RJ (1979) Retrograde demonstration of hippocampal afferents from the interpeduncular and reuniens nuclei. Neuroscience letters, 13, 105–109. [DOI] [PubMed] [Google Scholar]
- Bajic D, Soiza-Reilly M, Spalding AL, Berde CB and Commons KG (2015) Endogenous cholinergic neurotransmission contributes to behavioral sensitization to morphine. PloS one, 10, e0117601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S and Jhou TC (2012) Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32, 14094–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi G, Kalen P, Parvopassu F and Wiklund L (1990) Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective D-[3H]aspartate labelling of possible excitatory amino acid inputs. Neuroscience, 37, 77–100. [DOI] [PubMed] [Google Scholar]
- Bianco IH and Wilson SW (2009) The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 364, 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Capobianco M, Lovern R, Boche B, Ross E, Darracq MA and McLay R (2016) A Double-Blinded, Randomized, Placebo-Controlled Sub-Dissociative Dose Ketamine Pilot Study in the Treatment of Acute Depression and Suicidality in a Military Emergency Department Setting. Military medicine, 181, 1195–1199. [DOI] [PubMed] [Google Scholar]
- Burgunder JM and Young WS 3rd (1989) Neurokinin B and substance P genes are co-expressed in a subset of neurons in the rat habenula. Neuropeptides, 13, 165–169. [DOI] [PubMed] [Google Scholar]
- Carlson J, Armstrong B, Switzer RC 3rd and Ellison G (2000) Selective neurotoxic effects of nicotine on axons in fasciculus retroflexus further support evidence that this a weak link in brain across multiple drugs of abuse. Neuropharmacology, 39, 2792–2798. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC et al. (2013) Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biological psychiatry, 73, 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center, U. S. D. o. J. N. D. I. (2011) National Drug Threat Assessment. (Justice D. o. ed.).
- Cirulli F, Pistillo L, de Acetis L, Alleva E and Aloe L (1998) Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain, behavior, and immunity, 12, 123–133. [DOI] [PubMed] [Google Scholar]
- Cohen SR and Melzack R (1985) Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain research, 359, 131–139. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Leonard CM and Pfaff DW (1974) Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. The Journal of comparative neurology, 156, 179–205. [DOI] [PubMed] [Google Scholar]
- Contestabile A and Flumerfelt BA (1981) Afferent connections of the interpeduncular nucleus and the topographic organization of the habenulo-interpeduncular pathway: an HRP study in the rat. The Journal of comparative neurology, 196, 253–270. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen J, Husky MM, He JP and Merikangas KR (2016) Association of Lifetime Mental Disorders and Subsequent Alcohol and Illicit Drug Use: Results From the National Comorbidity Survey-Adolescent Supplement. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 280–288. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Zvolensky MJ, Fitch KE and Sachs-Ericsson N (2010) The role of comorbidity in explaining the associations between anxiety disorders and smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 12, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Emson PC, Paxinos G and Jessell T (1978) Substance P containing and cholinergic projections from the habenula. Brain research, 149, 413–429. [DOI] [PubMed] [Google Scholar]
- Dani JA and De Biasi M (2001) Cellular mechanisms of nicotine addiction. Pharmacology, biochemistry, and behavior, 70, 439–446. [DOI] [PubMed] [Google Scholar]
- Dao DQ, Perez EE, Teng Y, Dani JA and De Biasi M (2014) Nicotine enhances excitability of medial habenular neurons via facilitation of neurokinin signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34, 4273–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Befort K, Koebel P, Pannetier S, Mahoney MK, Gaveriaux-Ruff C, Hanauer A and Kieffer BL (2012) RSK2 signaling in medial habenula contributes to acute morphine analgesia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 37, 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM and Frazier E (2008) Major depression and comorbid substance use disorders. Current opinion in psychiatry, 21, 14–18. [DOI] [PubMed] [Google Scholar]
- De Biasi M and Dani JA (2011) Reward, addiction, withdrawal to nicotine. Annual review of neuroscience, 34, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, McLaughlin I and Klima ML (2016) Chapter 18 - Nicotine and Neurokinin Signaling A2 - Preedy, Victor R In: Neuropathology of Drug Addictions and Substance Misuse, pp. 189–200. Academic Press, San Diego. [Google Scholar]
- De Biasi M, McLaughlin I, Perez EE, Crooks PA, Dwoskin LP, Bardo MT, Pentel PR and Hatsukami D (2014) Scientific overview: 2013 BBC plenary symposium on tobacco addiction. Drug and alcohol dependence, 141, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M and Salas R (2008) Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Experimental biology and medicine (Maywood, N.J.), 233, 917–929. [DOI] [PubMed] [Google Scholar]
- Di Chiara G and Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America, 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ and Colquhoun D (1992) ATP receptor-mediated synaptic currents in the central nervous system. Nature, 359, 144–147. [DOI] [PubMed] [Google Scholar]
- Ellison G (2002) Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 12, 287–297. [DOI] [PubMed] [Google Scholar]
- Ellison G, Eison MS, Huberman HS and Daniel F (1978) Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science (New York, N.Y.), 201, 276–278. [DOI] [PubMed] [Google Scholar]
- Facchin L, Duboue ER and Halpern ME (2015) Disruption of Epithalamic Left-Right Asymmetry Increases Anxiety in Zebrafish. The Journal of neuroscience : the official journal of the Society for Neuroscience, 35, 15847–15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faron-Gorecka A, Kusmider M, Kolasa M et al. (2016) Chronic mild stress alters the somatostatin receptors in the rat brain. Psychopharmacology, 233, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Brown LA, Goodwin RD and Zvolensky MJ (2016) Panic attack history and smoking topography. Drug and alcohol dependence, 171, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA and Zvolensky MJ (2007) Smoking, traumatic event exposure, and post-traumatic stress: a critical review of the empirical literature. Clinical psychology review, 27, 14–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ and Kenny PJ (2011a) Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature, 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ and Kenny PJ (2011b) Habenular α5* nicotinic receptor signaling controls nicotine intake. Nature, 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Tuesta L and Kenny PJ (2013) Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology, 10.1007/s00213-00013-03235-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel L and Hermine O (2013) Mast cells and inflammation. Joint, bone, spine : revue du rhumatisme, 80, 141–145. [DOI] [PubMed] [Google Scholar]
- Gackenheimer SL, Suter TM, Pintar JE, Quimby SJ, Wheeler WJ, Mitch CH, Gehlert DR and Statnick MA (2005) Localization of opioid receptor antagonist [3H]-LY255582 binding sites in mouse brain: comparison with the distribution of mu, delta and kappa binding sites. Neuropeptides, 39, 559–567. [DOI] [PubMed] [Google Scholar]
- Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D and Kieffer BL (2014) Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience, 277, 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Moura DS, Salvador A et al. (2016) Mast cells’ involvement in inflammation pathways linked to depression: evidence in mastocytosis. Molecular psychiatry, 21, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM and Dickinson HA (2000) 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport, 11, 2013–2015. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM and Maisonneuve IM (2006) 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. European journal of pharmacology, 537, 94–98. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM and Maisonneuve IM (2008) Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. European journal of pharmacology, 599, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD and Ibanez-Tallon I (2013) Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proceedings of the National Academy of Sciences of the United States of America, 110, 17077–17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z (1983) Origin and distribution of noradrenergic innervation in the habenula: a neurochemical study. Brain research, 275, 299–304. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F and Gotti C (2009) Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29, 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW and Nauta WJ (1986) Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. The Journal of comparative neurology, 249, 65–102. [DOI] [PubMed] [Google Scholar]
- Guglielmotti V and Cristino L (2006) The interplay between the pineal complex and the habenular nuclei in lower vertebrates in the context of the evolution of cerebral asymmetry. Brain research bulletin, 69, 475–488. [DOI] [PubMed] [Google Scholar]
- Hamill GS and Jacobowitz DM (1984) A study of afferent projections to the rat interpeduncular nucleus. Brain research bulletin, 13, 527–539. [DOI] [PubMed] [Google Scholar]
- Han Y, Chen J, Zou D et al. (2016) Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatric disease and treatment, 12, 2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Seki M and Zyo K (1981) Studies on the efferent projections of the interpeduncular complex in cats. Okajimas folia anatomica Japonica, 58, 1–15. [DOI] [PubMed] [Google Scholar]
- Herkenham M and Nauta WJ (1977) Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. The Journal of comparative neurology, 173, 123–146. [DOI] [PubMed] [Google Scholar]
- Herkenham M and Nauta WJ (1979) Efferent connections of the habenular nuclei in the rat. The Journal of comparative neurology, 187, 19–47. [DOI] [PubMed] [Google Scholar]
- Hetu S, Luo Y, Saez I, D’Ardenne K, Lohrenz T and Montague PR (2016) Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Human brain mapping, 37, 2602–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YW and Wang SD (2014) Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. 34, 11366–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain RJ, Taraschenko OD and Glick SD (2008) Effects of nicotine, methamphetamine and cocaine on extracellular levels of acetylcholine in the interpeduncular nucleus of rats. Neuroscience letters, 440, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Suzuki R, Lumeng L, Li TK and McBride WJ (2004) Innate differences in neuropeptide Y (NPY) mRNA expression in discrete brain regions between alcohol-preferring (P) and -nonpreferring (NP) rats: a significantly low level of NPY mRNA in dentate gyrus of the hippocampus and absence of NPY mRNA in the medial habenular nucleus of P rats. Neuropeptides, 38, 359–368. [DOI] [PubMed] [Google Scholar]
- Ichijo H and Toyama T (2015) Axons from the medial habenular nucleus are topographically sorted in the fasciculus retroflexus. Anatomical science international, 90, 229–234. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M and Damaj MI (2015) New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology, 96, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW and Dayas CV (2011) Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience, 199, 235–242. [DOI] [PubMed] [Google Scholar]
- Jean-Richard Dit Bressel P and McNally GP (2014) The role of the lateral habenula in punishment. PloS one, 9, e111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuthasan S (2012) Fear, anxiety, and control in the zebrafish. Developmental neurobiology, 72, 395–403. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L and Ryan AF (1999) Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. The Journal of comparative neurology, 407, 11–32. [PubMed] [Google Scholar]
- Kassel JD, Stroud LR and Paronis CA (2003) Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological bulletin, 129, 270–304. [DOI] [PubMed] [Google Scholar]
- Kemali M and Guglielmotti V (1982) The connections of the frog interpeduncular nucleus (ITP) demonstrated by horseradish peroxidase (HRP). Experimental brain research, 45, 349–356. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR and Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U and Chang SY (2005) Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. The Journal of comparative neurology, 483, 236–250. [DOI] [PubMed] [Google Scholar]
- Kinsey AM, Wainwright A, Heavens R, Sirinathsinghji DJ and Oliver KR (2001) Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mRNAs in the rat brain. Brain research. Molecular brain research, 88, 194–198. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Sano Y, Vannoni E et al. (2013) Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Frontiers in behavioral neuroscience, 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH and Hokfelt T (2002) Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience, 111, 443–532. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D and Bor DH (2000) Smoking and mental illness: A population-based prevalence study. Jama, 284, 2606–2610. [DOI] [PubMed] [Google Scholar]
- Lecourtier L and Kelly PH (2007) A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews, 31, 658–672. [DOI] [PubMed] [Google Scholar]
- Lee A, Mathuru AS, Teh C, Kibat C, Korzh V, Penney TB and Jesuthasan S (2010) The habenula prevents helpless behavior in larval zebrafish. Current biology : CB, 20, 2211–2216. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Byun JS, Leach P, Fowler CD, Murphy NP, Kenny PJ, Gould TJ and Boulter J (2013) Targeted Deletion of the Mouse α2 Nicotinic Acetylcholine Receptor Subunit Gene (Chrna2) Potentiates Nicotine-Modulated Behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 10.1523/JNEUROSCI.4731-1512.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massopust LC Jr. and Thompson R (1962) A new interpedunculodiencephalic pathway in rats and cats. The Journal of comparative neurology, 118, 97–105. [DOI] [PubMed] [Google Scholar]
- Mathuru AS and Jesuthasan S (2013) The medial habenula as a regulator of anxiety in adult zebrafish. Frontiers in neural circuits, 7, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA and Prince DA (1987) Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience, 7, 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA and De Biasi M (2015) Nicotine withdrawal. Current topics in behavioral neurosciences, 24, 99–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine A. S. o. A. (2016) Opioid Addiction 2016 Facts & Figures.
- Meszaros J, Gajewska S and Tarchalska-Krynska B (1985) Habenulo-interpeduncular lesions: the effects on pain sensitivity, morphine analgesia and open-field behavior in rats. Polish journal of pharmacology and pharmacy, 37, 469–477. [PubMed] [Google Scholar]
- Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W and Henn FA (2014) Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Frontiers in human neuroscience, 8, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW and Zimering RT (2007) Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychological bulletin, 133, 245–272. [DOI] [PubMed] [Google Scholar]
- Morley BJ (1986) The interpeduncular nucleus. International review of neurobiology, 28, 157–182. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Merlo Pich E, Chiamulera C and Bunnemann B (2002) Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. The European journal of neuroscience, 16, 1633–1646. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC et al. (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. The American journal of psychiatry, 170, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Chronic Disease, P., Health Promotion Office on, S. and Health (2014) Reports of the Surgeon General In: The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US), Atlanta (GA). [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW and Silver R (2008) Brain mast cells link the immune system to anxiety-like behavior. Proceedings of the National Academy of Sciences of the United States of America, 105, 18053–18057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS and Picciotto MR (2013) Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacology, biochemistry, and behavior, 109, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Agetsuma M and Aizawa H (2012) Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Developmental neurobiology, 72, 386–394. [DOI] [PubMed] [Google Scholar]
- Okamoto H and Aizawa H (2013) Fear and anxiety regulation by conserved affective circuits. Neuron, 78, 411–413. [DOI] [PubMed] [Google Scholar]
- Padilla E, Shumake J, Barrett DW, Sheridan EC and Gonzalez-Lima F (2011) Mesolimbic effects of the antidepressant fluoxetine in Holtzman rats, a genetic strain with increased vulnerability to stress. Brain research, 1387, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal V, Taraschenko OD, Maisonneuve IM and Glick SD (2005) Attenuation of morphine withdrawal signs by intracerebral administration of 18-methoxycoronaridine. European journal of pharmacology, 525, 98–104. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA and Verkhratsky A (2009) P2X receptors and synaptic plasticity. Neuroscience, 158, 137–148. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A and North RA (2006) Vesicular release of ATP at central synapses. Pflugers Archiv : European journal of physiology, 452, 589–597. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M and Bowes G (1998) Depression, anxiety, and smoking initiation: a prospective study over 3 years. American journal of public health, 88, 1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi J, Mack Rudd (2014) Opioid Painkiller Prescribing, Where You Live Makes a Difference. (C. f. D. C. a. P. National Center for Injury Prevention and Control; ed.). Atlanta, GA. [Google Scholar]
- Perez E, Quijano-Carde N and De Biasi M (2015) Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 40, 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT and Pycock CJ (1982) Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Experimental brain research, 45, 89–94. [DOI] [PubMed] [Google Scholar]
- Picciotto MR and Kenny PJ (2013) Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harbor perspectives in medicine, 3, a012112–a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention C. f. D. C. a. (2015) Number and Age-Adjusted Rates of Drug-poisoning Deaths Involving Opioid Analgesics and Heroin: United States, 2000–2014. (N. C. f. H. Statistics; ed.). [Google Scholar]
- Prochaska JJ, Das S and Young-Wolff KC (2016) Smoking, Mental Illness, and Public Health. Annual review of public health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C and Luo M (2009) Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience, 161, 827–837. [DOI] [PubMed] [Google Scholar]
- Quina LA, Tempest L, Ng L, Harris JA, Ferguson S, Jhou TC and Turner EE (2015) Efferent pathways of the mouse lateral habenula. The Journal of comparative neurology, 523, 32–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B and Bernstein HG (2010) Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychological medicine, 40, 557–567. [DOI] [PubMed] [Google Scholar]
- Rho B and Glick SD (1998) Effects of 18-methoxycoronaridine on acute signs of morphine withdrawal in rats. Neuroreport, 9, 1283–1285. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK and Moller M (1979) Brain-pineal nervous connections in the rat: an ultrastructure study following habenular lesion. Experimental brain research, 37, 551–562. [DOI] [PubMed] [Google Scholar]
- Roux A, Muller L, Jackson SN et al. (2015) Chronic ethanol consumption profoundly alters regional brain ceramide and sphingomyelin content in rodents. ACS chemical neuroscience, 6, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Ma S, Olucha-Bordonau FE and Gundlach AL (2011) Nucleus incertus--an emerging modulatory role in arousal, stress and memory. Neuroscience and biobehavioral reviews, 35, 1326–1341. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE and Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. American journal of preventive medicine, 49, e73–79. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J and De Biasi M (2009) Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29, 3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M and Barrot M (2016) Response of the Tail of the Ventral Tegmental Area to Aversive Stimuli. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Nugent AC, Bogers W et al. (2011) Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biological psychiatry, 69, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW and Lester RA (2000) Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology, 39, 2591–2603. [DOI] [PubMed] [Google Scholar]
- Shibata H and Suzuki T (1984) Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: a retrograde horseradish peroxidase study. Brain research, 296, 345–349. [DOI] [PubMed] [Google Scholar]
- Shibata H, Suzuki T and Matsushita M (1986) Afferent projections to the interpeduncular nucleus in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. The Journal of comparative neurology, 248, 272–284. [DOI] [PubMed] [Google Scholar]
- Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA and Drenan RM (2014) Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34, 9789–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Edwards E and Gonzalez-Lima F (2003) Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain research, 963, 274–281. [DOI] [PubMed] [Google Scholar]
- Silver R and Curley JP (2013) Mast cells on the mind: new insights and opportunities. Trends in neurosciences, 36, 513–521. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Daunais JB, Porrino LJ and Childers SR (1999) Mu and kappa1 opioid-stimulated [35S]guanylyl-5’-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience, 94, 651–662. [DOI] [PubMed] [Google Scholar]
- Smaha LA and Kaelber WW (1973) Efferent fiber projections of the habenula and the interpeduncular nucleus. An experimental study in the opossum and cat. Experimental brain research, 16, 291–308. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK and Murphy JM (2001) Alcohol-naive alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol-nonpreferring (NP) and Wistar rats. Alcoholism, clinical and experimental research, 25, 1309–1316. [PubMed] [Google Scholar]
- Smith OA, Astley CA, DeVito JL, Stein JM and Walsh KE (1980) Functional analysis of hypothalamic control of the cardiovascular responses accompanying emotional behavior. Federation proceedings, 39, 2487–2494. [PubMed] [Google Scholar]
- Sperlagh B, Magloczky Z, Vizi ES and Freund TF (1998) The triangular septal nucleus as the major source of ATP release in the rat habenula: a combined neurochemical and morphological study. Neuroscience, 86, 1195–1207. [DOI] [PubMed] [Google Scholar]
- Stein EA (1993) Ventral tegmental self-stimulation selectively induces opioid peptide release in rat CNS. Synapse (New York, N.Y.), 13, 63–73. [DOI] [PubMed] [Google Scholar]
- Sugama S, Cho BP, Baker H, Joh TH, Lucero J and Conti B (2002) Neurons of the superior nucleus of the medial habenula and ependymal cells express IL-18 in rat CNS. Brain research, 958, 1–9. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ (1982) The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience and biobehavioral reviews, 6, 1–13. [DOI] [PubMed] [Google Scholar]
- Svenningsen K, Veno MT, Henningsen K, Mallien AS, Jensen L, Christensen T, Kjems J, Vollmayr B and Wiborg O (2016) MicroRNA Profiling in the Medial and Lateral Habenula of Rats Exposed to the Learned Helplessness Paradigm: Candidate Biomarkers for Susceptibility and Resilience to Inescapable Shock. PloS one, 11, e0160318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M and Chiba T (1991) Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain research, 566, 26–39. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM and Glick SD (2007) 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse (New York, N.Y.), 61, 547–560. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT and Pedrazzini T (2002) Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience, 22, Rc208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R (1960) Interpeduncular nucleus and avoidance conditioning in the rat. Science (New York, N.Y.), 132, 1551–1553. [DOI] [PubMed] [Google Scholar]
- Vertes RP and Fass B (1988) Projections between the interpeduncular nucleus and basal forebrain in the rat as demonstrated by the anterograde and retrograde transport of WGA-HRP. Experimental brain research, 73, 23–31. [DOI] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, Molfese DL and Salas R (2013) The medial habenula: still neglected. Frontiers in human neuroscience, 7, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorspan F, Mehtelli W, Dupuy G, Bloch V and Lepine JP (2015) Anxiety and substance use disorders: co-occurrence and clinical issues. Current psychiatry reports, 17, 4. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW and Cowan WM (1979) A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience, 4, 463–476. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T and Nakanishi S (2013) Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron, 78, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS and Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry, 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L et al. (2015) Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nature communications, 6, 6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD and Tapper AR (2013) Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Current biology : CB, 23, 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS and Pintar JE (1998) Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience, 18, 2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW and McLeish AC (2005) Smoking and panic attacks, panic disorder, and agoraphobia: a review of the empirical literature. Clinical psychology review, 25, 761–789. [DOI] [PubMed] [Google Scholar]