Abstract
The sensory hair cells of the inner ear undergo apoptosis after acoustic trauma or aminoglycoside antibiotic treatment, causing permanent auditory and vestibular deficits in humans. Previous studies have demonstrated a role for caspase activation in hair cell death and ototoxic injury that can be reduced by concurrent treatment with caspase inhibitors in vitro. In this study, we examined the protective effects of caspase inhibition on hair cell death in vivo after systemic injections of aminoglycosides. In one series of experiments, chickens were implanted with osmotic pumps that administrated the pan-caspase inhibitor z-Val-Ala-Asp(Ome)-fluoromethylketone (zVAD) into inner ear fluids. One day after the surgery, the animals received a 5 d course of treatment with streptomycin, a vestibulotoxic aminoglycoside. Direct infusion of zVAD into the vestibule significantly increased hair cell survival after streptomycin treatment. A second series of experiments determined whether rescued hair cells could function as sensory receptors. Animals treated with streptomycin displayed vestibular system impairment as measured by a greatly reduced vestibulo-ocular response (VOR). In contrast, animals that received concurrent systemic administration of zVAD with streptomycin had both significantly greater hair cell survival and significantly increased VOR responses, as compared with animals treated with streptomycin alone. These findings suggest that inhibiting the activation of caspases promotes the survival of hair cells and protects against vestibular function deficits after aminoglycoside treatment.
Keywords: auditory, hair cell, vestibular, apoptosis, caspase inhibitors, vestibular ocular reflex
Introduction
Hair cells are mechanoreceptors in the inner ear that detect sound, head position, and head movement. Aminoglycoside antibiotics are toxic to hair cells, and patients treated with large doses of aminoglycosides often incur permanent vestibular and auditory deficits (Hinshaw and Feldman, 1945). Morphological evidence from many vertebrate species suggests that the loss of hair cells in response to aminoglycoside treatment occurs via apoptosis (Jørgensen, 1981, 1991; Forge, 1985; Li et al., 1995; Forge and Li, 2000; Matsui et al., 2002a). Neuronal apoptosis is an evolutionarily conserved form of cell death that occurs through an orderly series of cellular events (Raff, 1998) and results in the activation of caspases, which trigger a proteolytic cascade that leads to the degradation of the cytoplasmic and nuclear proteins of the cell (for review, see Salvesen and Dixit, 1997). Cell-permeable peptides [e.g., the tripeptide z-Val-Ala-Asp(Ome)fluoromethylketone (zVAD)] can inhibit caspase activation in various neurons (for review, see Salvesen and Dixit, 1997). Although numerous studies have examined the role of caspase inhibitors in preventing neuronal death in vitro, few studies have investigated whether the rescued cells remain functional in vivo. Intracerebroventricular administration of the zVAD, however, promoted neuronal survival in transgenic mouse models of neurodegeneration (Ona et al., 1999; Li et al., 2000b), and behavioral tests implied that the saved neurons were functional.
Previous studies have demonstrated that caspase inhibitors prevent apoptosis in auditory and vestibular hair cells in vitro (Liu et al., 1998; Forge and Li, 2000; Cunningham et al., 2002; Matsui et al., 2002a; Cheng et al., 2003). In the present study, we first determined the effects of caspase inhibition on hair cell death in vivo by examining hair cell densities in vestibular organs after systemic injections of aminoglycosides and simultaneous infusion of caspase inhibitors into the inner ear. Additional experiments quantified hair cell function after rescue with caspase inhibitors. In all vertebrates, head motion produces hair cell responses and compensatory eye movements that function to stabilize visual gaze (Dickman and Angelaki, 1999; Dickman et al., 2000). Aminoglycoside treatment in chickens results in reduced vestibulo-ocular reflex (VOR) (Carey et al., 1996) and vestibulocolic reflex (VCR) (Goode et al., 1999, 2001) responses to rotational motion. To determine whether rescued semicircular and otolith vestibular hair cells were functional after combined zVAD and streptomycin treatment, we measured three-dimensional eye-movement responses for the horizontal VOR (HVOR) and the off-vertical axis reflex (OVAR), respectively. Treatment with zVAD provided significant protection from ototoxic insult to both the vestibular receptors and VOR.
Preliminary reports of portions of these data have been published previously (Matsui et al., 2002b).
Materials and Methods
Two series of experiments were performed. In the first experimental series, the general caspase inhibitor zVAD was delivered into the inner ear labyrinth. Chicks also received concurrent streptomycin treatment, and hair cell survival was examined. In the second experimental series, protection of vestibular function was examined by measuring the VOR after systemic administration of zVAD and streptomycin.
Animals
White Leghorn chickens (Gallus domesticus) were obtained from Charles River SPAFAS (North Franklin, CT) or Ideal Poultry Breeding Farms (Cameron, TX) and housed at the animal care facilities at either the Beth Israel Deaconess Hospital (series 1) or the Central Institute for the Deaf (series 2). Chicks were received at 3–14 d of age and observed for 7–18 d to ensure that they were healthy. Surgeries were performed at 21 d of age. All experimental protocols were approved by the Institutional Animal Care and Use Committees at the Central Institute for the Deaf, Washington University in St. Louis, and Children's Hospital Boston and conform to The Society for Neuroscience and the National Institutes of Health animal use guidelines.
Experiment series 1: efficacy of zVAD delivery in vivo
Osmotic pump implantation. Chicks were anesthetized using inhaled isoflurane that was delivered via endotracheal intubation as described by Roberson et al. (2000b). Surgical sites on the head and back were cleaned with a depilatory agent followed by a biocidal wipe. The post-auricular area was injected with 0.1 cc of bupivacaine hydrochloride (AstraZeneca) for postoperative anesthetic. Enrofloxacin (Baytril; Bayer) was given sublingually for antibiotic prophylaxis. A thermal incision was made behind the left ear revealing the mastoid process. A portion of the mastoid bone was removed to expose the anterior semicircular canal and the surface of the anterior ampulla. A stainless steel cannula from a brain infusion kit (outer diameter 0.36 mm; DURECT Corporation, Cupertino, CA) was modified so that the infusion cannula was inserted into a flexible plastic tube until the cannula tip projected out ∼1 mm (Roberson et al., 2000a). The cannula was placed in a fistula created in the vestibule postero-superior to the anterior ampulla and secured with dental acrylic.
An osmotic pump (model 2002, 0.5 μl/hr; DURECT Corporation) was filled with 200 μl of either 50 or 100 μm zVAD (Enzyme Systems Products, Livermore, CA) or 0.01% DMSO dissolved in saline (carrier). We chose to use zVAD in our studies because previous evidence suggested that the drug had a long half-life (Ona et al., 1999; Li et al., 2000b) and that zVAD promoted vestibular hair cell survival in vitro (Matsui et al., 2002a). The pump was placed in a subcutaneous pocket created on the animal's back and connected to the cannula via a plastic catheter tunneled under the skin. Care was taken to ensure that there were no air bubbles in the tubing or pump. The incisions over the back and ear were closed with nylon suture.
Streptomycin treatment. Animals received daily intramuscular injections of either streptomycin (1200 mg/kg; Sigma, St. Louis, MO) or saline for 3 or 5 d, beginning 1 d after the osmotic pump surgery. Streptomycin sulfate was chosen because it preferentially causes lesions within the striolar region of the chick utricle both in vivo (Weisleder and Rubel, 1992, 1993) and in vitro (Matsui et al., 2000) and not in the auditory organ, the basilar papilla. Age-matched chicks received saline injections and served as controls. One day after the last drug treatment, the animals were killed, and the utricles were removed from the pump-implanted ear and placed into 4% paraformaldehyde for 20 min followed by buffer rinse and storage in PBS for later calretinin immunohistochemistry processing (see below).
Experiment series 2: physiological testing of vestibular function
Head stud implantation. For three-dimensional eye-movement recordings, a head stud was implanted in each bird so that the horizontal semi-circular canals were parallel to the earth vertical axis (EVA) rotation plane. Animals were anesthetized with isoflurane, and an incision was made along the midline of the skull. A specially designed head stud (square aluminum tubing) was secured to the skull using stainless steel screws and dental acrylic mixed with ampicillin (5%) powder. The skin was approximated to the acrylic cap and secured with cyanoacrylate glue (Vetbond Tissue Adhesive). After surgery, a single dose of buprenorphin hydrochloride (Buprenex; 10 mg/kg) was administered for postoperative analgesia, and enrofloxacin was given as a prophylactic antibiotic. Animals were then returned to the animal facility to recover.
Eye coil implantation. Three-dimensional eye movements were recorded from the left eye using a three-field coil system (CNC Engineering, Seattle, WA) and a dual-search coil (Robinson, 1963). The three-field coil system was secured to a servo-controlled three-axis rotator system that was mounted on a linear sled (Neurokinetics, Pittsburgh, PA). The dual-search coil consisted of two miniature 80-turn watch-maker coils that were glued orthogonally to each other (Hess and Dieringer, 1991), with cyanoacrylate glue applied to the base of the coil to form a “contact lens.” Proparacaine hydrochloride (0.5% Bupivicaine) was topically applied to the cornea of the eye, and the dual-search coil was placed on the corneal surface. A small drop of tissue glue was administered to the contact lens before placement on the eye to eliminate slippage (Hess, 1990; Hess and Dieringer, 1991; Dickman and Angelaki, 1999).
After testing, the animal was removed from the rotator, proparacaine hydrochloride was again applied to the cornea, and the search coil was removed with a saline rinse. The cornea was then treated with a neomycin/bacitracin ophthalmic ointment. Occasionally during the experimental procedure the coil became displaced from the cornea. This was immediately apparent in the oscilloscope traces because saccades and post-saccadic oscillations were absent. When this occurred, the experimental session was stopped, and the data were discarded.
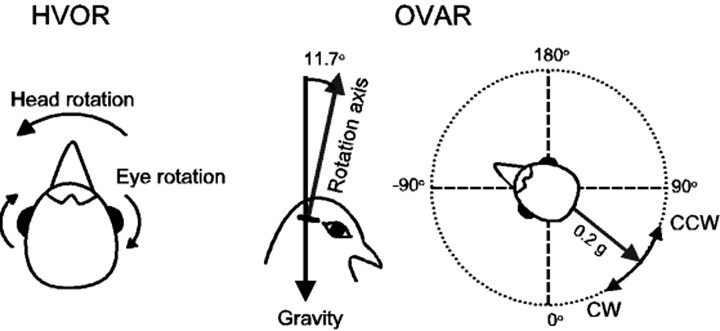
Experimental and stimulation procedure. All experiments were performed with awake animals placed in the dark to eliminate visual cues. Animals were restrained by wrapping an elastic bandage around the wings and body, placed into a foam padded body holder, and attached to the motion delivery system using the implanted head stud. The head was centered in the magnetic field with the major plane of the horizontal semicircular canals oriented parallel to the EVA rotation plane. All stimulus waveforms were produced with a microcomputer and a peripheral interface (1401 plus; Cambridge Electronic Design, Cambridge, UK) using custom script files written for simultaneous stimulus delivery and data acquisition. Eye-position signals were amplified, filtered, and recorded on-line (digitized rate of 833 Hz). Spontaneous eye movements were recorded with animals stationary while objects of interest moved through the visual field. These eye movements were analyzed to produce a primary position vector that served as a calibration point for subsequent evoked responses. Sinusoidal EVA rotations were delivered to elicit HVOR responses. Frequencies ranged between 0.01 and 2 Hz and were delivered at a constant peak velocity of 20°/sec, except for the 2 Hz stimulus, which was mechanically limited to 10°/sec. OVAR stimulation consisted of constant velocity rotation, in both the clockwise (CW) and counterclockwise (CCW) directions, about an axis that was tilted 11.5° away from earth vertical. OVAR rotations ranged from 0.0278 to 0.333 Hz. The OVAR stimulus produced a resultant linear acceleration vector of 0.2 × g, which was sinusoidally modulated relative to the otolith organ receptor as the animal was rotated 360°. All animals had normative HVOR and OVAR responses recorded at posthatch day 28, before other experimental manipulations, so that each animal could serve as its own control. Each animal was then assigned to one of four treatment groups (see below). After 5 d of drug treatment, each animal was again tested for HVOR and OVAR responses on posthatch day 33. Thus, all animals were age matched to eliminate developmental response differences.
Systemic treatment with zVAD and streptomycin. Animals that were being tested for vestibulo-ocular responses received systemic daily injections for 5 d of (1) zVAD (1.5 mg/kg) and streptomycin (1200 mg/kg), (2) streptomycin alone, (3) zVAD alone, or (4) saline, beginning 1 d after the normative eye movements were recorded. We chose to inject the animals with the zVAD as opposed to implanting the animals with osmotic pumps for two reasons. First, it was of interest to determine whether systemic administration of zVAD resulted in a protective effect. Second, we wanted to reduce the possibility of vestibular dysfunction caused by violating the vestibule with a surgical cannula. One day after the last drug treatment (posthatch day 33), HVOR and OVAR responses were obtained. The animals were then anesthetized with 20 mg/kg sodium pentobarbital (Nembutal) and 20 mg/kg ketamine hydrochloride (Ketaset) and placed in a stereotaxic frame. A midline incision was made over the occipital bone, and the horizontal and posterior canals were exposed. The canals were fenestrated and the left ear was perfused with an aldehyde solution composed of 3% glutaraldehyde, 2% paraformaldehyde, and 1% acrolein in 0.1 m sodium cacodylate buffer so that the tissue could be processed for scanning electron microscopy. The right ear was perfused with 4% paraformaldehyde in 0.1 m phosphate buffer for 20–30 min so that the tissue could be processed for calretinin immunohistochemistry. The semicircular canal ampullas and otolith maculae were dissected free and placed in buffer. The otoconia were removed from the utricle and saccule using a stream of buffer from a syringe. The left endorgans were stored in fixative overnight at 4°C, whereas the right endorgans were stored in PBS at 4°C.
Tissue processing
Calretinin labeling. To quantitatively assess the extent of hair cell survival, hair cells were identified using an antibody for calretinin (see Fig. 1 A) (Rogers, 1989). All immunohistochemistry occurred at room temperature with thorough PBS washes between them, unless noted otherwise. Fixed specimens were incubated in 90% methanol with 0.03% H2O2 for 20 min, followed by incubation in a blocking solution consisting of PBS, 2% normal horse serum (NHS), 1% BSA, and 0.2% Triton X-100 for 2 hr. The tissue was then placed immediately into a rabbit anti-calretinin primary antibody (1:2000, in PBS/2% NHS; Chemicon, Temecula, CA) and incubated overnight at 4°C. The next day, inner ear sensory organs were incubated in biotinylated goat anti-rabbit IgG antibody (1:150, in PBS/0.1% NHS; Vector Laboratories, Burlingame, CA) for 2 hr, followed by avidin–biotin–horseradish peroxidase complex (Vector Laboratories) for 90 min. Specimens were then reacted with DAB (Vector Laboratories) for 5 min and mounted on microscope slides in glycerol/PBS (9:1).
Figure 1.
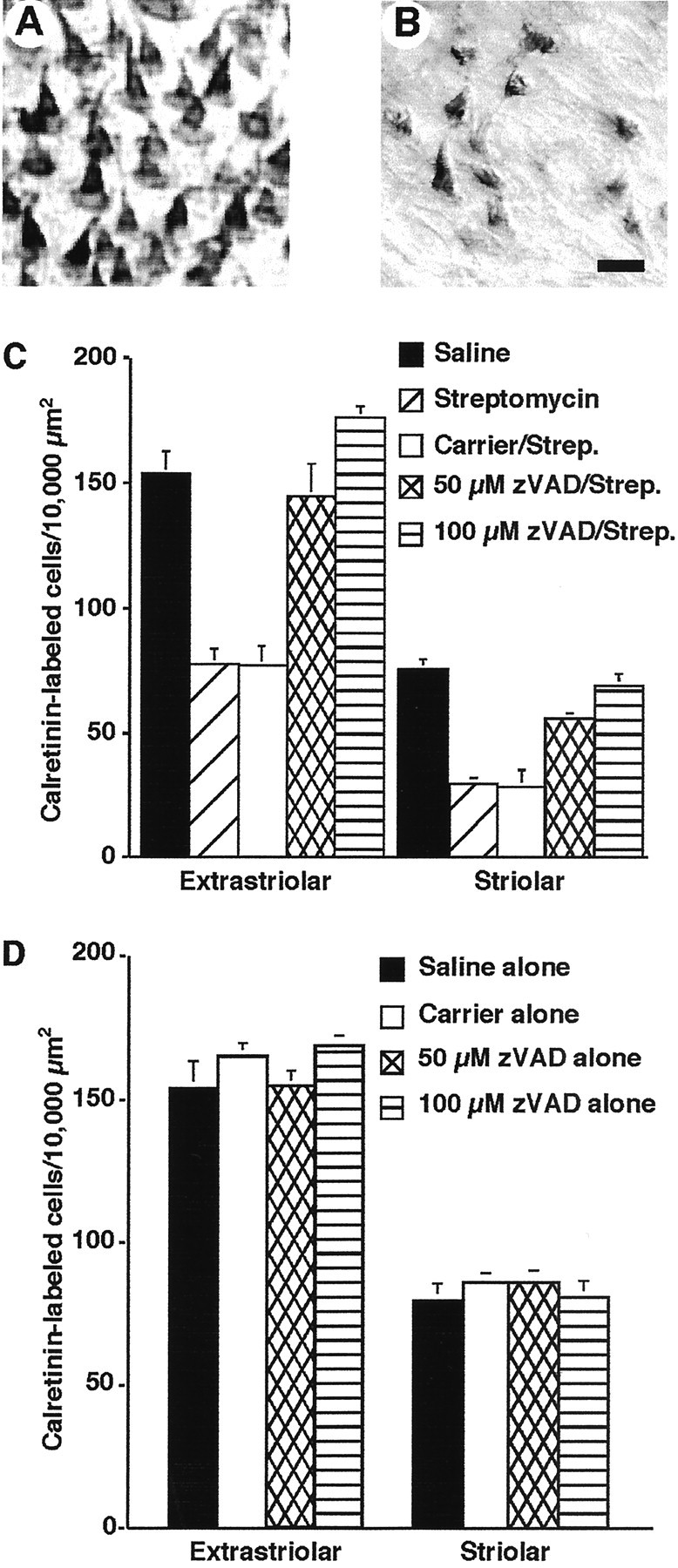
Direct infusion of zVAD into the vestibule promotes hair cell survival after concurrent treatment with streptomycin sulfate. A, Photomicrograph of hair cells stained for calretinin immunohistochemistry after treatment with 100 μm zVAD and 5 d of streptomycin (1200 mg/kg). B, Photomicrograph of calretinin-labeled hair cells after treatment with the carrier and streptomycin. C, Mean hair cell counts (±SEM) for the striolar and extrastriolar regions of the utricle after treatment with saline, streptomycin alone, carrier plus streptomycin, or 50 and 100 μm zVAD and streptomycin treatment. D, Mean hair cell counts for control, saline, carrier alone, 50 μm zVAD alone, or 100 μm zVAD alone treated animals. Calretinin-labeled cells were quantified in 10,000 μm 2 regions of both the extrastriolar (6 regions per organ) and striolar (4 regions per organ) areas. n = 6–8 organs.
Scanning electron microscopy. To assess hair cell survival in treated and control animals, left ear endorgans from VOR animals were processed for scanning electron microscopy. Endorgans were dehydrated in serial acetone washes (70, 90, 95, 100, 100%), followed by two washes in tetramethylsilane (Electron Microscopy Services, Fort Washington, PA), and placed in a 37°C oven to sublimate. The tissue was then mounted on aluminum studs, sputter-coated with palladium, and subsequently viewed on a Hitachi 2600 scanning electron microscope at 20–25 kV.
Data analysis
Counts of calretinin-labeled cells. Whole-mount preparations were examined using video microscopy and a Zeiss Axiovert 135 microscope. Cell counts were made directly from a video monitor using a 100 × 100 μm calibrated template. Two types of hair cells, designated type I and type II, are present in the vestibular organs and can be differentiated on the basis of morphology and innervation (Wersäll, 1956; Jørgensen and Christensen, 1989). Type I hair cells are located in the central region of the horizontal canal and the striolar region of the utricular and saccular maculas. Type II hair cells are found throughout the crista and macula (Jørgensen and Christensen, 1989; Si et al., 2003). Antibodies recognizing calretinin selectively label both types of hair cells in the chick vestibular system (Rogers, 1989; Matsui et al., 2002a). In the utricle, calretinin-labeled cells were counted from six regions in the extrastriolar region and in four striolar regions. In the saccule, calretinin-labeled cells were counted from three regions in both the extrastriola and striola. In the horizontal canal, calretinin-labeled cells were counted from two different central regions. All counting was performed by an investigator blinded to the treatment group. Care was taken to avoid the lateral limits of the sensory epithelium because these regions occasionally contained areas of epithelial damage resulting from the surgical dissection. The regions were averaged to obtain an estimate of the number of surviving hair cells per 10,000 μm 2 for each specimen.
Counts of stereocilia bundles. We used previously published methods to quantify stereocilia bundles in scanning electron micrographs of the avian vestibular organs (Dye et al., 1999). Three horizontal canals and utricles from each treatment group were studied. Within each organ, three regions of the sensory epithelium were photographed at a magnification of 1250×. The numbers of stereocilia bundles in one striolar and two extrastriolar regions were quantified from each utricle. The striolar region was identified in the normal animals by the reversal of the stereocilia bundle morphological polarization. The reversal line was placed into the center of the field for quantification. Stereocilia bundles within a specified 7500 μm 2 rectangular window were counted. For immature hair cells, the identification of a kinocilium was required so that the cells could be counted. The hair cell counts for each region within each treatment group were then normalized to 10,000 μm 2 and averaged.
HVOR data analysis The three-dimensional eye-position signals and primary position search coil calibration values were used to express the eye movement responses as rotation vectors (Ehorz, Ever, and Etor) in head coordinates (Haustein, 1989; Van Optstal, 1993). Positive eye movements were defined as leftward (horizontal), downward (vertical), and upper pole rotation to the right (clockwise) relative to the standard head reference frame. The eye position vectors were then desaccaded (including post-saccadic oscillations) using a semi-automated program (MATLAB) that allows user validation and manual adjustment of the rejection window. The desaccaded eye position vectors were differentiated to produce eye velocity vectors (dE/dt). From the position vectors (E) and velocity vectors (dE/dt), an angular eye velocity Ω vector with components Ωhor, Ωver, and Ωtor, in head-fixed coordinates was calculated (Hepp, 1990) as Ω = 2(dE/dt + (E × dE/dt))/(1 + E 2).
Once the angular velocity components were derived, each component was averaged over multiple stimulus cycles (3–30, depending on frequency), and the averaged traces were fit with a sine curve using a least-squares algorithm (MATLAB). The fitted mean sine curves were used to calculate gain and phase values for horizontal, vertical, and torsional angular eye velocity components. Gain was expressed as the ratio of peak angular eye velocity to peak rotation velocity. Phase was expressed as the difference (degrees) between peak angular eye velocity and peak rotation velocity.
OVAR data analysis. For the OVAR data, the desacadded mean eye position values for the horizontal, vertical, and torsional eye-movement components were determined from the least-squares fit to the averaged responses obtained from multiple stimulus cycles (4–10, dependent on frequency) using the equation E(t) = Eo + Ep cos(ωt + φ). The mean eye position was described as the sum of a DC offset position (Eo) and a modulation term [Ep cos(ωt + φ)]. For OVAR stimulation, the modulation term was elicited as a function of head position relative to gravity (changing linear acceleration). Each component of slow-phase eye velocity was fit to the equation Ω(t) = Ωv+Ωp cos(ωt + φ′) using the least-squares method. The slow-phase eye velocity was calculated as the sum of the steady-state “bias velocity” of nystagmus (Ωv-ovar) (visually negligible: <1%) and the modulation slow-phase velocity [Ωp cos(ωt + φ′)]. The modulation sensitivities for OVAR slow-phase velocity were calculated as the ratio of peak eye velocity (Ωp in degrees per second) to peak linear acceleration (g). The modulation phase values were expressed as the difference (degrees) between peak eye velocity and head linear acceleration. Estimates of the sensitivity and phase values for OVAR responses were obtained from rotations in both the CW and CCW directions. Because the sensitivity, phase, and spatial orientation values for the eye-movement components observed during OVAR depend on the underlying spatial and temporal properties of the otolith-ocular responses, a modified procedure similar to that used to characterize neural response vectors during OVAR was used (Angelaki, 1992; Angelaki and Hess, 1996). A detailed description of the procedure has been published previously (Angelaki and Hess, 1996; Dickman and Angelaki, 1999). The component response vector sensitivity was defined as the average of the calculated sensitivities to CW and CCW response vectors ((CW + CCW)/2). The temporal phase was computed as the angle (degrees) between each of the maximal response vectors and the direction of the component spatial orientation vector ((CW - CCW)/2).
Statistical analysis
Statistical analyses for hair cell counts were performed using either an unpaired two-sample t test assuming unequal variances (Excel; Microsoft Corporation) or a one-way ANOVA (SigmaStat; SPPS Science Corporation). Post hoc comparisons, when appropriate, used the Tukey–Kramer or Scheffe's test. VOR responses were compared using a multivariable repeated-measures ANOVA (Statistica; StatSoft).
Results
Ototoxic agents can be administered as a series of systemic injections that produce rapid hair cell loss in the chick vestibular system (Weisleder and Rubel, 1992, 1993). To develop an optimum dose for these experiments, 2- to 3-week-old chickens received daily intramuscular injections of 1200 mg/kg streptomycin sulfate for 3 or 5 d. After 3 d of injections, streptomycin-treated chicks had a noticeable head tremor. The frequency of the tremor appeared to increase with each successive day, indicating that the vestibular system was being severely affected by the aminoglycoside regimen. After either 3 or 5 d of streptomycin treatment, the utricles were processed for calretinin immunohistochemistry, to label surviving hair cells (Rogers, 1989; Matsui et al., 2002a). Calretinin labeling was particularly evident in the stereocilia bundles (Fig. 1A). If the stereocilia bundle was missing, the apical portion of the remaining hair cell was labeled (data not shown). Utricles examined after 3 d of streptomycin treatment displayed a 40% reduction in hair cell density (Table 1). The density reductions were similar in both the striola (t(10) = 2.2; p < 0.001) and extrastriolar regions (t(9) = 2.2; p < 0.001) of the utricular maculas. Five days of streptomycin treatment resulted in a 50% reduction in hair cell density in the extrastriolar region (t(16) = 2.1; p < 0.001) and a 60% reduction in the striolar region (t(13) = 2.2; p < 0.001).
Table 1.
Time course for hair cell death after 3 or 5 d streptomycin injections in vivo
|
|
Extrastriolar |
Striolar |
||||
|---|---|---|---|---|---|---|
|
|
Saline
|
Streptomycin (1200 mg/kg)
|
Saline
|
Streptomycin (1200 mg/kg)
|
||
| 3 d injections | 154.8 ± 8.5 | 97.0 ± 3.4 | 80.7 ± 5.1 | 35.3 ± 2.2 | ||
| (63.0 ± 2.2%) | (43.7 ± 2.7%) | |||||
| 5 d injections | 153.3 ± 8.7 | 77.2 ± 6.0 | 75.0 ± 3.8 | 29.2 ± 2.3 | ||
|
|
|
(50.3 ± 3.9%)
|
|
(38.9 ± 3.1%)
|
||
Chickens received daily injections of streptomycin or saline, and utricles were processed for calretinin immunohistochemistry. Calretinin-labeled cells were quantified in 10,000 μm2 regions of both the extrastriolar (six regions per organ) and striolar (four regions per organ) areas. Mean values (±SEM) from 9-12 organs are shown. The percentage of surviving hair cells (relative to controls) is presented below the hair cell densities.
zVAD infusion promotes hair cell survival after aminoglycoside treatment (experiment series 1)
Hair cell survival after ototoxic insult can be enhanced by treatment with the general caspase inhibitors Boc-Asp(Ome)fluoromethylketone (BAF) or zVAD in vitro (Forge and Li, 2000; Cunningham et al., 2002; Matsui et al., 2002a; Cheng et al., 2003). To determine whether zVAD promoted hair cell survival in vivo after streptomycin treatment, chickens were implanted with osmotic pumps filled with either zVAD or carrier and received daily injections of either streptomycin or saline for 3–5 d. After the last day of treatment, the utricles were removed from the ears with the implanted osmotic pump (ipsilateral), fixed, and processed for immunohistochemistry. After 3 d of streptomycin treatment, animals that had received zVAD (either 50 or 100 μm) concurrently had significantly more hair cells in both striolar and extrastriolar regions than control animals (Table 2). The mean cell counts for the zVAD/streptomycin-treated utricles were not significantly different from saline-injected controls (p > 0.5).
Table 2.
Direct application of zVAD promotes hair cell survival in vivo after 3 d of streptomycin treatment
|
|
|
Streptomycin (1200 mg/kg) |
||||||
|---|---|---|---|---|---|---|---|---|
|
|
Saline Alone
|
Alone
|
Carrier
|
50 μm zVAD
|
100 μm zVAD
|
|||
| Extrastriolar | 154.8 ± 8.5 | 108.5 ± 5.4 | 79.7 ± 4.7 | 146.1 ± 12.6 | 147.4 ± 12.6 | |||
| (70.1 ± 3.5%) | (51.5 ± 3.0%) | (94.4 ± 8.1%) | (95.2 ± 8.1%) | |||||
| Striolar | 80.7 ± 5.1 | 36.6 ± 10.4 | 25.4 ± 2.2 | 51.8 ± 9.1 | 59.6 ± 7.5 | |||
|
|
|
(45.4 ± 12.9%)
|
(31.5 ± 2.7%)
|
(64.2 ± 11.3%)
|
(74.0 ± 9.3%)
|
|||
Chickens were implanted with an osmotic pump containing zVAD or carrier and received daily injections of streptomycin or saline for 3 d. One day after the last drug treatment, the animals were killed, and the utricles were removed from the ear containing the pump and processed for calretinin immunohistochemistry. Calretinin+ cells were quantified in 10,000 μm2 regions of both the extrastriolar (six regions per organ) and striolar (four regions per organ) areas. Mean values (±SEM) from four to eight organs are shown. The percentage of surviving hair cells (relative to controls) is presented below the hair cell densities.
After 5 d of streptomycin treatment, more hair cells were present in zVAD-treated animals (Fig. 1A,C) when compared with either streptomycin alone or carrier/streptomycin-treated animals (Fig. 1B,C). Treatment with 100 μm zVAD resulted in nearly full protection, because there was no significant difference in hair cell density of either sensory region between the saline-treated animals and 100 μm zVAD/streptomycin-treated animals (p > 0.5). These results suggest that caspase inhibitors promote hair cell survival during concurrent systemic treatment with streptomycin.
To determine whether zVAD had any direct effects on hair cells, three additional chickens were implanted with osmotic pumps filled with zVAD, and three other animals were implanted with pumps filled with carrier and then allowed to survive for 6 d. The animals were then killed, and the utricles were removed and processed for calretinin immunohistochemistry. Hair cells appeared healthy and morphologically normal in all experimental and control conditions (data not shown). There was no significant difference in hair cell density between any of the experimental conditions and age-matched nontreated animals (Fig. 1D) (p > 0.5).
Systemic injection of zVAD promotes hair cell survival after streptomycin treatment (experiment series 2)
The previous experiments demonstrated that infusion of zVAD directly into the inner ear reduced the ototoxic effects of streptomycin. It was also of interest to determine whether systemic delivery of zVAD would promote hair cell survival. Animals received daily systemic injections of (1) zVAD (1.5 mg/kg) and streptomycin (1200 mg/kg), (2) streptomycin alone, (3) zVAD alone, or (4) saline for a total of 5 d. To characterize the pattern of hair cell loss or protection, the utricular and saccular maculas, along with the horizontal crista ampularis, were examined for hair cell density. As shown in Figures 2, 3, 4, little hair cell death (e.g., fused stereocilia bundles, hair cell “blebbing,” or extruded hair cells) was observed in organs of control animals injected with saline alone of the horizontal canal (Fig. 2A), the utricle (Fig. 3A), or the saccule (Fig. 4A). In contrast, extensive hair cell loss was apparent in the horizontal canal (Fig. 2C), the utricle (Fig. 3C), and the saccule (Fig. 4C) of animals treated for 5 d with streptomycin. Widespread fusion of stereocilia bundles was observed in the crista ampullaris of the horizontal canal (data not shown). In contrast, very little hair cell pathology was observed in either the utricle or saccular maculas. Instead, stereocilia bundles were absent from the striolar region of both sensory organs. After 5 d of concurrent treatment of zVAD and streptomycin, more hair cells were present in all vestibular organs examined (Figs. 2E,3E,4E), as compared with streptomycin-alone organs (Fig. 5). Also, little hair cell degeneration was observed in stereocilia bundles in any of the organs after concurrent treatment of zVAD and streptomycin.
Figure 2.
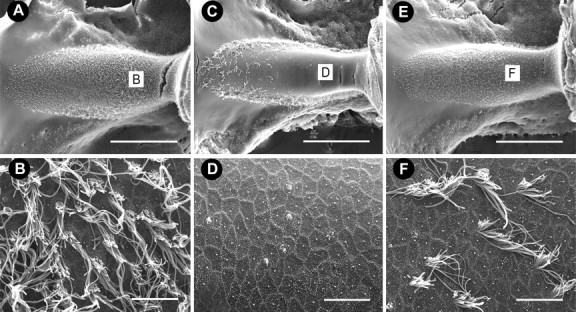
Scanning electron micrographs of the horizontal crista ampullaris. A, Low magnification of the crista from an untreated animal with numerous hair cells throughout the sensory epithelium. B, High magnification of the central apical region. C, Low-magnification image from an animal treated with 5 d of streptomycin (1200 mg/kg) showed that surviving hair cells were present only at the edge of the epithelium. Hair cells with mature stereocilia are absent from the central apical region of the crista (C, D). E, Low-magnification image from an animal treated with 5 d of zVAD (1.5 mg/kg) and streptomycin. F, Central apical region of horizontal crista ampullaris shows only moderate hair cell loss. Scale bars: low magnification, 500 μm; high magnification, 10 μm.
Figure 3.
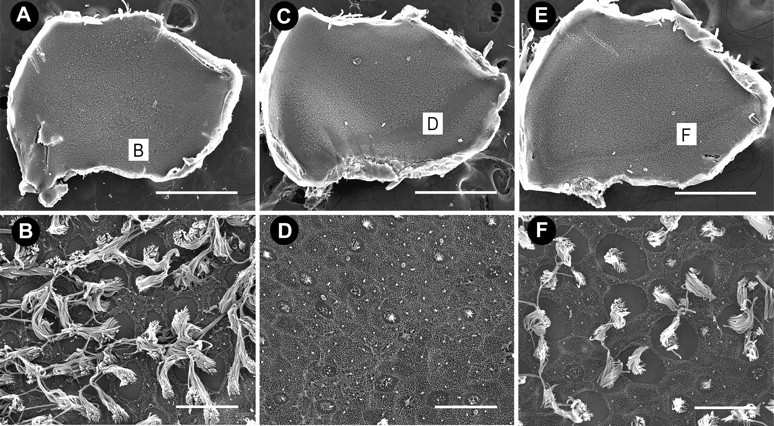
Scanning electron micrographs of utricles. A, Low-magnification image of a control utricle with densely populated hair cells. B, High-magnification image of the striolar region of the same utricle as shown in A. C, After 5 d of streptomycin treatment, hair cell damage is predominately localized to the striolar region with extensive stereociliary loss evident along the entire length of the striola (D). E, After 5 d of zVAD and streptomycin treatment, some hair cell loss is evident in the striola, but most hair cells are still present. Scale bars: low magnification, 500 μm; high magnification, 10 μm.
Figure 4.
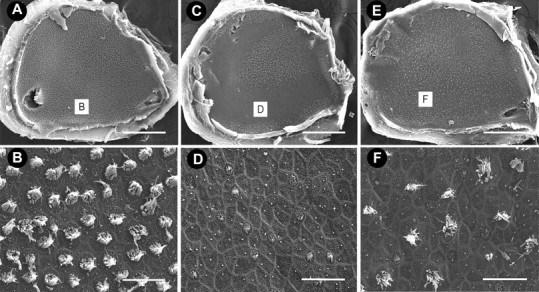
Scanning electron micrographs of saccules. A, Low-magnification image of a control saccule, densely populated by hair cells. B, High-magnification image of the striolar region of the same saccule as shown in A. C, After 5 d of streptomycin treatment, hair cell damage is predominately localized to the striolar region with extensive stereociliary loss evident along the entire length of the striola (D). E, After 5 d of zVAD and streptomycin treatment, hair cell loss is evident in the striolar region (F), but many hair cells are still present when compared with animals treated with streptomycin alone. Scale bars: low magnification, 500 μm; high magnification, 10 μm.
Figure 5.
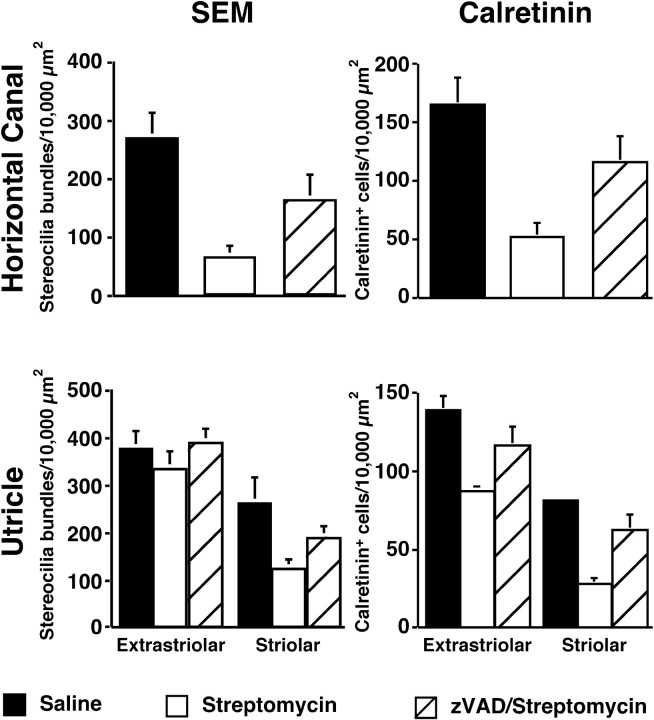
Systemic injections of zVAD promote hair cell survival. Chickens were given daily simultaneous injections of (1) zVAD (1.5 mg/kg) and streptomycin (1200 mg/kg), (2) streptomycin alone, or (3) saline alone for 5 d. Hair cells (either stereocilia bundles or calretinin + cells) were quantified in 10,000 μm2 regions from the central region of the horizontal canal and both the extrastriolar and striolar areas of the utricle. For stereocilia bundle densities, means (±SD) represent three sampled regions per organ in the horizontal canal. In the utricle, two regions per organ of the extrastriolar region and one region per organ in the striolar region were sampled. For calretinin densities, means (±SD) represent two regions sampled in each horizontal canal. In the utricle, six regions per organ in the extrastriolar region and four regions per organ in the striolar region were sampled. More hair cells were present in both sensory organs after 5 d of treatment of zVAD/streptomycin, when compared with animals receiving streptomycin alone. Significantly fewer hair cells were present in all sampled regions of zVAD/streptomycin-treated animals when compared with animals receiving saline alone (p < 0.01). n = 3–5 organs.
Type I hair cells are found predominantly in the central area of the horizontal canal and the striolar region of the utricle and saccule (Jørgensen, 1989; Si et al., 2003; Zakir et al., 2003). Few stereociliary bundles remained in either the apical region of the horizontal canal (Figs. 2D, 5) or the striolar region of the utricle (Figs. 3D, 5) and saccule (Fig. 4D) in animals that received streptomycin alone, suggesting that streptomycin targeted mainly type I hair cells. In contrast, more stereociliary bundles remained in the central region of the horizontal canal (Figs. 2F, 5) and the striolar region of the utricle (Figs. 3F, 5) and saccule (Fig. 4F) in animals that received concurrent treatment with zVAD and streptomycin. Interestingly, the proportional loss of hair cells was greater for the horizontal canal (Fig. 5), followed by the saccule (data not shown), followed by the least effect in the utricle (Fig. 5).
Scanning electron microscopy provides only a “surface” view of the sensory epithelia, and there may be surviving hair cells present in the sensory epithelia despite the absence of stereocilia bundles (Baird et al., 2000; Gale et al., 2002). Therefore, organs from opposite sides of the same animals were immunolabeled for calretinin, which identifies hair cells in the avian ear (Rogers, 1989; Matsui et al., 2002a). Significantly more hair cells were present in the central region of the horizontal canal (Fig. 5) (t(10) = 2.2; p < 0.001) and both the striolar (t(6) = 2.4; p < 0.05) and extrastriolar (t(5) = 2.5; p < 0.05) regions of the utricle (Fig. 5) of zVAD/streptomycin-treated animals when compared with animals that received streptomycin alone. Hair cell densities were also obtained from the saccules of control animals (extrastriolar = 199.7 ± 25.1; striolar = 138 ± 16.4). Significant hair cell protection was observed in both the extrastriolar region (153.9 ± 45.8; t(18) = 2.2; p < 0.001) and the striolar region (67.1 ± 20.1; t(14) = 2.2, p < 0.001) of saccules from zVAD/streptomycin-treated animals when compared with animals that had received streptomycin alone (extrastriolar = 82.8 ± 27.6; striolar = 32.9 ± 8.0).
Unlike direct infusion of zVAD into the vestibule, concurrent systemic treatment of zVAD with streptomycin did not provide full protection (Fig. 5). Treatment with zVAD increased hair cell survival in the central region of the horizontal canal, when compared with streptomycin alone (difference between streptomycin and zVAD/streptomycin treatment as a percentage of controls). Additionally, treatment with zVAD increased hair cell survival in the utricle by 21% in the extrastriolar region and 43% in the striolar region. Finally, treatment with zVAD increased hair cell survival in the saccule by 36% in the extrastriolar region and 25% in the striolar region. As expected, there was no difference in hair cell densities of any of the organs from animals treated with only zVAD when compared with control organs (data not shown).
HVOR responses
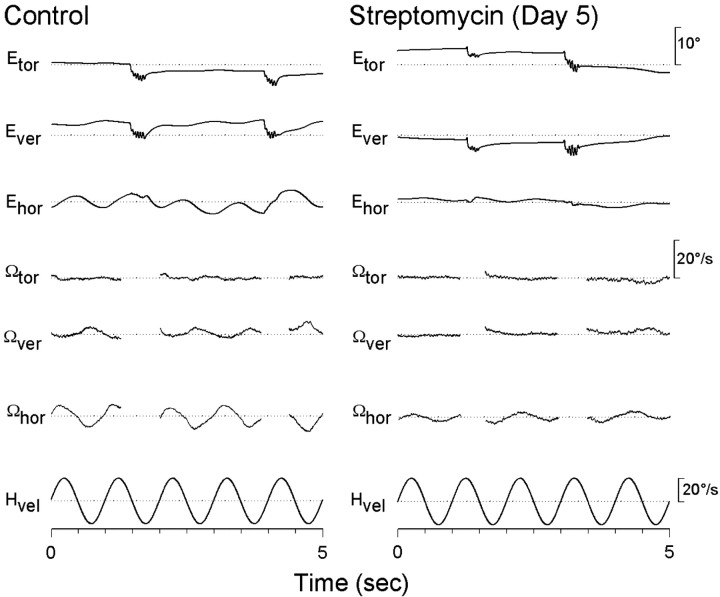
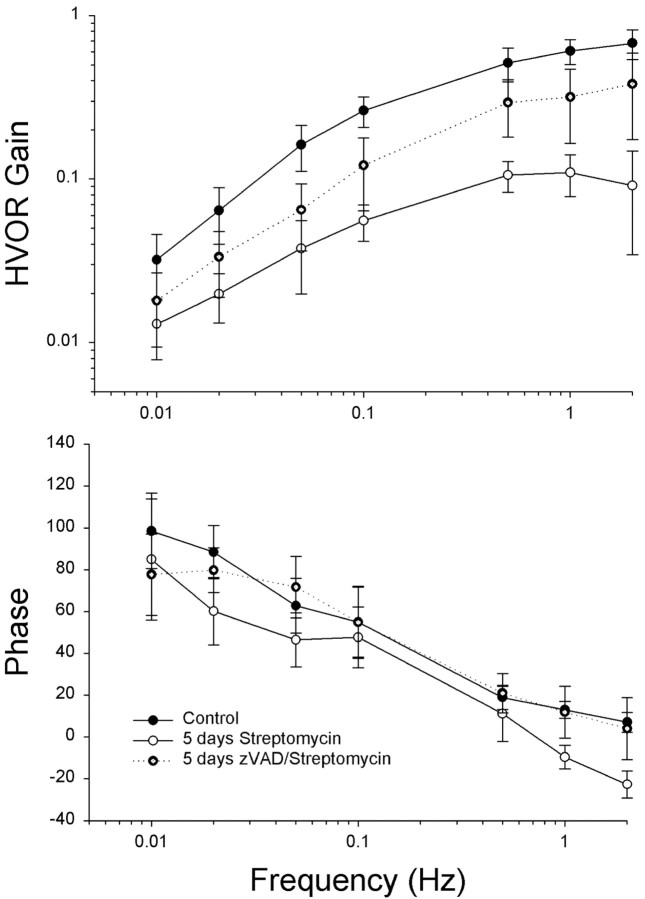
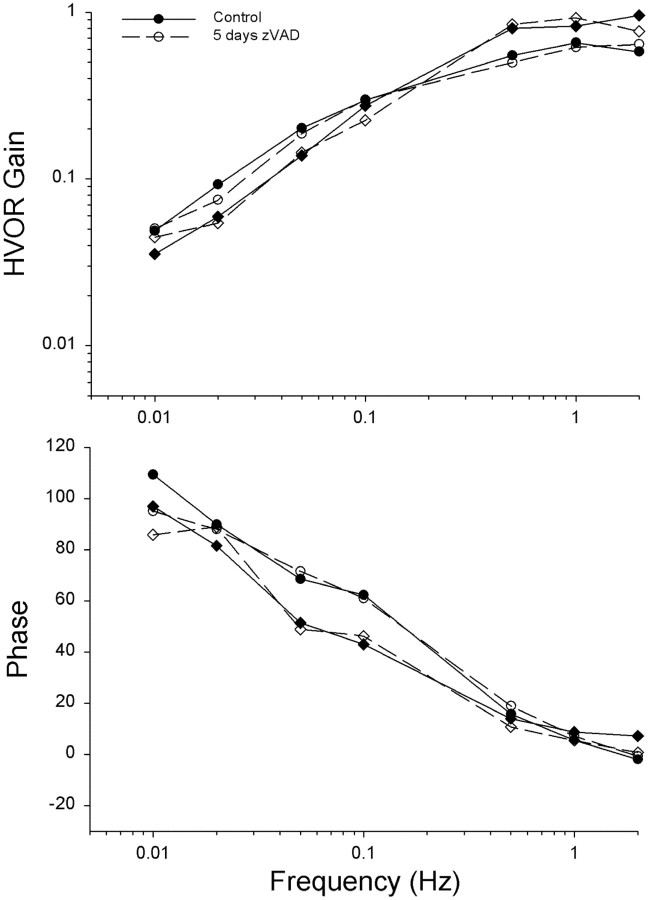
The previous experiments demonstrated that infusion of zVAD directly into the inner ear or delivered systemically reduced the ototoxic effects of streptomycin. It was also of interest to determine whether the surviving hair cells were functional. In all vertebrates, head motion produces hair cell responses and compensatory eye movements that function to stabilize visual gaze (Dickman and Angelaki, 1999; Dickman et al., 2000). To quantify compensatory eye movements resulting from stimulation of the horizontal canal, animals were rotated around the EVA (Fig. 6). Normative eye movements were recorded from 4-week-old animals using eye-search coils, so that each animal would serve as its own control. As shown in Figure 7, HVOR responses consisted primarily of a compensatory horizontal component with no or small vertical and torsional components. The slow phase gain (horizontal eye velocity/head velocity) and phase of the elicited horizontal component during EVA rotation was dependent on stimulus frequency (Fig. 8). At the lowest frequency tested (0.01 Hz), the gain of the HVOR response was extremely small (0.03 ± 0.01), but the gain increased as stimulus frequency increased from 0.26 ± 0.05 at 0.1 Hz to 0.68 ± 0.14 at 2 Hz. At the higher stimulus frequencies tested (0.5–2 Hz), the slow phase gain and phase values remained relatively constant. In general, the HVOR displayed high-pass filter characteristics similar to those described in other bird studies (Carey et al., 1996; Dickman et al., 2000).
Figure 6.
Rotation stimulation paradigms for horizontal vestibulo-ocular reflex (HVOR) and off-vertical axis rotation (OVAR). To stimulate hair cells in the horizontal canal, the chick was rotated around the EVA at frequencies ranging from 0.01 to 2 Hz with a constant peak velocity of 20°/sec, except for the 2 Hz stimulus, which was mechanically limited to 10°/sec. Compensatory eye movements were measured. To stimulate the utricle, OVAR was used to deliver low- to mid-frequency (0.0.0278–0.333 Hz) linear acceleration stimulation with the rotation axis tilted 11.7° (0.2 gm) relative to EVA. Both clockwise (CW) and counterclockwise (CCW) OVAR rotations were delivered.
Figure 7.
Three-dimensional eye-movement recordings from a normal chicken and a streptomycin-treated chicken rotated relative to EVA. HVOR responses are 1 Hz sinusoidal EVA rotations (20°/sec peak velocity). The top three traces represent torsional (Etor), vertical (Ever), and horizontal (Ehor) eye-position records with both slow and fast phase movements. The bottom three traces indicate torsional (Ωtor), vertical (Ωver), and horizontal (Ωhor) slow-phase eye velocity with the fast phases and post-saccadic eye oscillations removed. Upward deflection in the top three traces indicates counterclockwise, downward, and leftward eye movements, respectively. Dotted lines show zero eye velocity. The bottom trace represents head velocity (upward deflection equals leftward).
Figure 8.
Mean frequency response functions for the HVOR in streptomycin- and zVAD/streptomycin-treated animals. Mean (±SD) horizontal slow-phase eye velocity gain and phase values are plotted for control (filled circles), streptomycin only (open circles; n = 5 animals), and zVAD/streptomycin (star circles; n = 5 animals) treatment conditions. Each animal served as its own control. Gain values are presented as eye velocity/head velocity and phase values as degrees lead relative to head velocity.
One day after the normative eye recordings, the animals received daily systemic injections of (1) zVAD (1.5 mg/kg) and streptomycin (1200 mg/kg), (2) streptomycin alone, (3) zVAD alone, or (4) saline for a total of 5 d. One day after the last drug treatment, the eye coils were reattached, and eye movements were tested again. Chickens treated with streptomycin alone for 5 d had significantly reduced VOR gains for all stimulus frequencies, as compared with their pretreatment performance (F(1,7) = 19.2; p < 0.005). Mean average gains were <0.11 at all frequencies tested (Fig. 8). The mean HVOR phase values were significantly advanced at all frequencies tested in the control measurements, as compared with streptomycin alone (F(1,7) = 32.0; p < 0.001). A similar loss of gain and change in phase has been reported previously after a 5 d course of streptomycin treatment in comparably aged chickens (Carey et al., 1996). Simultaneous treatment with zVAD and streptomycin, however, resulted in significant protection of HVOR function (F(1,7) = 27.2; p < 0.005). Mean gain values ranged from 0.15 ± 0.06 at 0.1 Hz to 0.47 ± 0.14 at 2 Hz.
The mean gain values were significantly lower after zVAD/streptomycin treatment than during their own control runs, suggesting that zVAD provides incomplete protection (F(1,7) = 27.2; p < 0.001). With the exception of the lowest stimulus frequencies, animals that received streptomycin alone had significantly smaller phase leads with respect to head rotations than did animals that had received concurrent treatment with zVAD and streptomycin (F(1,7) = 24.9; p < 0.005). Animals that were treated simultaneously with zVAD and streptomycin had similar mean phase responses to control animals in the mid-high frequencies (0.05–2 Hz), indicating that the surviving hair cells were indeed functional (Fig. 8B) (p = 0.3).
zVAD treatment does not impair HVOR measurements
As noted, animals that had received concurrent treatment of zVAD and streptomycin had gains that were lower than their pretreatment control performance (Fig. 8). To determine whether zVAD itself had direct effects on vestibular hair cell function, two animals were treated with zVAD alone for 5 d. As shown in Figure 9, zVAD-treated animals had identical gain (Fig. 9A) and phase (Fig. 9B) values, as compared with their pretreatment control performance (p > 0.5).
Figure 9.
Frequency response functions for the HVOR in animals treated with zVAD alone. Slow-phase eye velocity gain and phase values are plotted for pretreatment performance (open symbols) and after 5 d of zVAD treatment (closed symbols; n = 2 animals). Gain values are presented as eye velocity/head velocity and phase values as degrees lead relative to head velocity. Treatment with zVAD had no effect on either the gain or phase.
OVAR responses
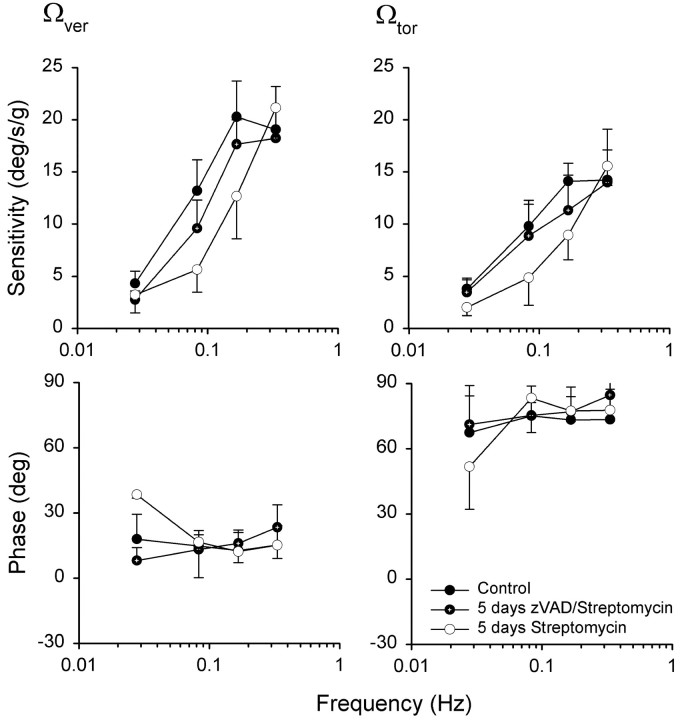
Rotations of the head relative to gravity produce coactivation of both the semicircular canal and otolith receptors of the vestibular system (Fig. 6). The utricle and the saccule are responsible for detecting linear acceleration and head position with respect to gravity. Otolith-ocular responses during OVAR are elicited in lateral-eyed species such as rabbits, rats, and pigeons (Baarsma and Collewijn, 1975; Hess and Dieringer, 1990; Dickman and Angelaki, 1999). Because the rotating gravity stimulus during OVAR is transduced by hair cells, the viability of the otolithic sensory organs can be estimated by the relative size of compensatory eye movements during head rotation (Dickman and Angelaki, 1999).
During off-vertical axis rotations in normal chickens, eye movement steady-state responses consisted primarily of sinusoidal modulations of vertical and torsional components. Similar to other lateral-eyed species, no horizontal eye movement component was observed (Hess and Dieringer, 1990; Dickman and Angelaki, 1999; Maruta et al., 2001). The measurements from zVAD/streptomycin-treated animals were compared with those obtained from animals that were treated only with streptomycin (Fig. 10). Animals that were treated with streptomycin alone had significantly lower vertical (F(1,5) = 9.5; p < 0.05) and torsional (F(1,6) = 5.4; p < 0.06) component gain values at low- to midrange frequencies (0.0278–0.016 Hz), when compared with their pretreatment performance or zVAD/streptomycin-treated animals. Surprisingly, streptomycin-treated animals had normal gains at the highest frequency tested (0.333 Hz; p > 0.05). Simultaneous treatment with zVAD and streptomycin resulted in slightly lower gains across frequencies, but these were not significant from their pretreatment performance (p > 0.5). There was also no phase shift observed in animals treated with streptomycin (Fig. 10) (p > 0.3).
Figure 10.
Mean (±SD) vertical and torsional slow-phase sensitivity and phase values to OVAR stimulation as a function of frequency in streptomycin- and zVAD/streptomycin-treated animals. Mean sensitivity values (degrees per second divided by gravity) were calculated as slow-phase eye velocity/head acceleration for four animals. Phase values (degrees) are shown relative to linear acceleration. Each animal served as its own control.
Discussion
As shown previously, treatment with the aminoglycoside antibiotic streptomycin caused a substantial loss of hair cells and a reduction in vestibular function as measured by the VOR (Carey et al., 1996). Animals that received concurrent administration of streptomycin and the pan-caspase inhibitor zVAD had significantly more hair cells and better vestibular function than did animals treated with streptomycin alone. These results suggest that inhibiting caspase activation during aminoglycoside treatment results in the rescue of viable hair cells and the preservation of vestibular function.
Direct infusion of caspase inhibitors promotes vestibular hair cell survival
Our results demonstrate that direct infusion of zVAD into the labyrinth dramatically reduced the ototoxic effect of streptomycin, as evidenced by no observable loss of utricular hair cells. This result is consistent with other studies in which intracerebroventricular administration of zVAD promoted neuronal survival in mouse neurodegeneration models (Ona et al., 1999; Li et al., 2000b) and reduced the infarct size after transient focal ischemia (Loddick et al., 1996). Most of these studies examined anatomical parameters as opposed to behavioral or physiological measurements. In one study, however, local application of zVAD not only reduced apoptotic neuronal death after traumatic spinal chord injury but also improved behavioral recovery (Li et al., 2000a). Other groups have used osmotic pumps to deliver various drugs to the inner ear, including tetrodotoxin (Brown et al., 1993), growth factors (Miller et al., 1997; Kuntz and Oesterle, 1998; Ruan et al., 1999; Shoji et al., 2000), and free radical scavengers (Song and Schacht, 1996). Although the use of osmotic pumps allows control over the timing and concentration of drugs applied to the inner ear, the main disadvantage is that the animal undergoes an invasive surgical procedure, which may alter inner ear function.
Systemic treatment with zVAD along with an ototoxic agent also provided partial protection of hair cells, as assessed by morphological evaluation of hair cells and behavioral responses. Many inner ear disorders can be treated by systemic administration of drugs (e.g., andrenocorticoid steroids) as a course of therapy. It is possible that other factors such as affinity for crossing the blood–brain barrier or the local titer of zVAD in the inner ear influenced our findings after systemic treatments. Future studies could evaluate the effects of increased zVAD dosage on vestibular function and protection from ototoxic insult. Although hair cell protection from systemic administration of zVAD was less than that observed after direct application to the inner ear, the drug did significantly reduce aminoglycoside-induced ototoxicity.
To quantify the densities of surviving hair cells, we used two different counting methods: stereocilia bundles versus calretinin-labeled hair cells. Because stereocilia bundles are necessary for hair cell function, quantification of calretinin-labeled cells (which also labels the cuticular plate or the cell body if there is a missing stereocilia bundle) might overestimate the number of functional surviving hair cells. Although the trends observed using both counting methods were similar (Fig. 5), there were almost twice as many stereocilia bundles in the scanning electron microscopy (SEM) densities as those observed in the calretinin-labeled tissue. The hair cell densities obtained from the calretinin labeling were consistent with data obtained using calretinin and other hair cell markers including hair cell antigen and lectins (Goodyear et al., 1999; Warchol, 2001; Matsui et al., 2002a). The density differences between the two counting methods could be attributed to the shrinkage that occurs during the dehydration process when the tissue is prepared for SEM. To estimate shrinkage, the surface areas from the utricles processed for SEMs were measured. The average area was 1.026 ± 0.086 mm2, which is ∼54% smaller than the surface area of an immunolabeled utricle from comparably aged animals (Goodyear et al., 1999). When we measured the surface areas of the utricles taken from the contralateral ear (tissue processed for calretinin immunohistochemistry), the SEM-prepared tissue was 58% smaller than the immunolabeled tissue. When this shrinkage is taken into consideration, the hair cell densities from the SEM counts are similar to those in the calretinin-labeled tissue.
Correlation of hair cell density and HVOR/OVAR measurements: are the hair cells functional?
The HVOR gains measured in chickens before drug treatments were similar to those observed in other lateral-eyed species. For example, rabbits and pigeons both have HVOR gains near 0.6 at 1 Hz (Baarsma and Collewijn, 1975; Barmack, 1981; Anastasio and Correia, 1988; Dickman et al., 2000), with phase leading head velocity. At the mid-range stimulus frequencies tested (0.5–2 Hz), the leads were generally on the order of 10°. OVAR steady-state responses measure otolith function and have been demonstrated in many animal species, including rats, rabbits, monkeys, and pigeons (Baarsma and Collewijn, 1975; Hess and Dieringer, 1990; Angelaki and Hess, 1996; Dickman and Angelaki, 1999; Maruta et al., 2001). The OVAR dynamic responses reported here are similar to those reported for pigeons (Dickman and Angelaki, 1999). The spatial phase of the OVAR response in our study was shifted by 90° relative to the pigeon data because of the difference in the coordinate reference frame used. For pigeons, phases were expressed relative to the bird's line of sight, whereas the phases presented here were expressed relative to the animal's head position.
Five days of streptomycin treatment resulted in substantial hair cell loss and a greatly reduced vestibular VOR function. The decrease in the VOR response immediately after streptomycin treatment was directly correlated with hair cell loss, as determined by hair cell counts from the crista and otolith organs. Our results demonstrating a reduction in the HVOR response after streptomycin treatment are similar to those reported previously (Carey et al., 1996). In addition, our data suggest that streptomycin treatment significantly reduces OVAR responses. Although the reduction in OVAR responses paralleled the HVOR responses, the reduction in OVAR responses after streptomycin treatment was not as strong. If hair cell survival were the chief determinant of HVOR/OVAR gain, then gain would be directly proportional to hair cell density. Carey and colleagues (1996) did not find a strict correlation between hair density and the gain of HVOR. In their study, the HVOR in the streptomycin-treated animals had gains of <0.1, although ∼40% of hair cells were still present. A similar trend was observed in our data. A measurable response was present after streptomycin treatment in both studies but was vastly undercompensatory for gaze stabilization.
Hair cell loss in the saccule was comparable with damage in the horizontal canal crista. In contrast, much less hair cell loss was evident in the utricle (Fig. 5). Because the tilt axis for OVAR stimulation was only 11.5° relative to earth vertical, the gravitoinnertia acceleration stimulus was acting primarily on utricular hair cells, because of the geometrical orientation of the maculas. In addition, correlations between hair cell loss and OVAR reduction suggest that the utricle and not the saccule was primarily responsible for the observed OVAR responses, despite the significant loss of saccular hair cells (J. D. Dickman, unpublished data). One surprising observation was that streptomycin-treated animals had normal gains at the highest OVAR stimulus-frequency tested (0.333 Hz). At the higher frequencies, perhaps the remaining functional hair cells in the utricle and saccule were sufficiently stimulated to elicit a normal gain.
Administration of zVAD along with streptomycin resulted in increased hair cell survival as well as improved HVOR and OVAR responses. These results strongly suggest that zVAD has a protective effect on both cell structure and vestibular function. Systemic injection of zVAD resulted in less hair cell protection than did direct interlabyrinthine application. Nevertheless, both cell survival and vestibular compensatory eye-movement gains were significantly improved when compared with treatment with streptomycin alone. Several factors may account for reduced vestibular function in zVAD/streptomycin-treated animals as compared with controls. Aminoglycosides are known to block the mechanotransduction channel in both auditory and vestibular hair cells (Ohmori, 1985; Kimitsuki et al., 1994), and the clearance of aminoglycosides from the inner ear fluids and hair cells requires weeks in mammals (Aran et al., 1995). Because the animals were tested immediately after 5 d of drug therapy, it is possible that some of the mechanotransduction channels were blocked by streptomycin in the endolymph, resulting in reduced functional capacity. Functional testing after the aminoglycosides had been cleared from the inner ear was not feasible in our study, because chickens readily regenerate their vestibular hair cells after streptomycin treatment (Weisleder and Rubel, 1992, 1993), and regenerated hair cells are known to restore vestibular function within 1–3 weeks after streptomycin treatment (Jones and Nelson, 1992; Carey et al., 1996). It is also possible that streptomycin may have altered the physiological state of some hair cells. Sympathetic neurons deprived of nerve growth factor (NGF) in vitro are rescued by application of the general caspase inhibitor BAF (Deshmukh et al., 1996). One study has examined the electrophysiological properties of NGF-deprived SCG neurons from rats and mice after rescue by BAF (Werth et al., 2000). Under those conditions, NGF deprivation reduced the resting membrane potentials by 9 mV and prolonged action potentials by >50%. To date, there has been no published study of rescue of sympathetic neurons via caspase inhibitors in vivo. Comparable reduction in hair cell membrane potentials would lead to reduced sensitivity to head movements. It is unlikely that the zVAD itself affected either the HVOR or OVAR because both the gain and phase values in zVAD-treated animals were identical to control measurements.
In summary, local or systemic administration of the caspase inhibitor zVAD promotes the survival of functional sensory hair cells in the presence of toxic insult and may provide a basis for future therapeutic interventions.
Footnotes
This work was supported by The National Organization for Hearing Research Foundation, the Division of Biology and Biomedical Sciences (Washington University), and National Institutes of Health (NIH) Individual National Research Service Award Fellowship F31 DC05082 (J.I.M.). Other funding sources include Howard Hughes Medical Institute Medical Student Research Fellowship 57003555 (A.H.), the Deafness Research Foundation (D.A.C.), NIH Grant DC02386 (J.D.D.), NIH core facilities Grant P30 DC 04665, NIH Grant DC03576, and National Aeronautics and Space Administration Grant NAG2-1364 (M.E.W.). We thank Debbie Corliss and Dr. Petula Coutinho for their excellent surgical assistance. We thank Dr. Dora Angelaki and Dr. Eugene Johnson Jr for helpful comments and discussion.
Correspondence should be addressed to either of the following: Mark E. Warchol, Central Institute for the Deaf, 4560 Clayton Avenue, St. Louis, MO 63110-1549, E-mail: mwarchol@cid.wustl.edu; or J. David Dickman, Central Institute for the Deaf, 4560 Clayton Avenue, St. Louis, MO 63110-1549, E-mail: ddickman@cid.wustl.edu.
J. I. Matsui's present address: Harvard University, Department of Molecular and Cellular Biology, The Biological Laboratories, 16 Divinity Avenue, Cambridge, MA 02138.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236111-12$15.00/0
References
- Anastasio TJ, Correia MJ ( 1988) A frequency and time domain study of the horizontal and vertical vestibulo-ocular reflex in the pigeon. J Neurophysiol 59: 1143–1161. [DOI] [PubMed] [Google Scholar]
- Angelaki DE ( 1992) Two-dimensional coding of linear acceleration and the angular velocity sensitivity of the otolith system. Biol Cybern 67: 511–522. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Hess BJ ( 1996) Three-dimensional organization of otolithocular reflexes in rhesus monkeys. I. Linear acceleration responses during off-vertical axis rotation. J Neurophysiol 75: 2405–2424. [DOI] [PubMed] [Google Scholar]
- Aran JM, Chappert C, Dulon D, Erre JP, Aurousseau C ( 1995) Uptake of amikacin by hair cells of the guinea pig cochlea and vestibule and ototoxicity: comparison with gentamicin. Hear Res 82: 179–183. [DOI] [PubMed] [Google Scholar]
- Baarsma EA, Collewijn H ( 1975) Eye movements due to linear accelerations in the rabbit. J Physiol (Lond) 245: 227–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Fashena DS, Naeger RA ( 2000) Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci USA 97: 11722–11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH ( 1981) A comparison of the horizontal and vertical vestibulo-ocular reflexes of the rabbit. J Physiol (Lond) 314: 547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL ( 1993) Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res 70: 167–172. [DOI] [PubMed] [Google Scholar]
- Carey JP, Fuchs AF, Rubel EW ( 1996) Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J Neurophysiol 76: 3301–3312. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW ( 2003) Hair cell death in the avian basilar papilla: characterization of the in vitro model and caspase activation. J Assoc Res Otolaryngol 4: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW ( 2002) Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci 22: 8532–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson Jr EM ( 1996) Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol 135: 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE ( 1999) Three-dimensional organization of vestibular-related eye movements to off-vertical axis rotation and linear translation in pigeons. Exp Brain Res 129: 391–400. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Beyer M, Hess BJ ( 2000) Three-dimensional organization of vestibular related eye movements to rotational motion in pigeons. Vision Res 40: 2831–2844. [DOI] [PubMed] [Google Scholar]
- Dye BJ, Frank TC, Newlands SD, Dickman JD ( 1999) Distribution and time course of hair cell regeneration in the pigeon utricle. Hear Res 133: 17–26. [DOI] [PubMed] [Google Scholar]
- Forge A ( 1985) Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res 19: 171–182. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L ( 2000) Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res 139: 97–115. [DOI] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT ( 2002) Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog's saccule. J Neurobiol 50: 81–92. [DOI] [PubMed] [Google Scholar]
- Goode CT, Carey JP, Fuchs AF, Rubel EW ( 1999) Recovery of the vestibulocolic reflex after aminoglycoside ototoxicity in domestic chickens. J Neurophysiol 81: 1025–1035. [DOI] [PubMed] [Google Scholar]
- Goode CT, Maney DL, Rubel EW, Fuchs AF ( 2001) Visual influences on the development and recovery of the vestibuloocular reflex in the chicken. J Neurophysiol 85: 1119–1128. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Gates R, Lukashkin AN, Richardson GP ( 1999) Hair-cell numbers continue to increase in the utricular macula of the early posthatch chick. J Neurocytol 28: 851–861. [DOI] [PubMed] [Google Scholar]
- Haustein W ( 1989) Considerations on Listing's law and the primary position by means of a matrix description of eye position control. Biol Cybern 60: 411–420. [DOI] [PubMed] [Google Scholar]
- Hepp K ( 1990) On Listing's law. Commun Math Phys 132: 285–292. [Google Scholar]
- Hess BJ ( 1990) Dual-search coil for measuring 3-dimensional eye movements in experimental animals. Vision Res 30: 597–602. [DOI] [PubMed] [Google Scholar]
- Hess BJ, Dieringer N ( 1990) Spatial organization of the maculo-ocular reflex of the rat: responses during off-vertical axis rotation. Eur J Neurosci 2: 909–919. [DOI] [PubMed] [Google Scholar]
- Hess BJ, Dieringer N ( 1991) Spatial organization of linear vestibuloocular reflexes of the rat: responses during horizontal and vertical linear acceleration. J Neurophysiol 66: 1805–1818. [DOI] [PubMed] [Google Scholar]
- Hinshaw HC, Feldman WH ( 1945) Streptomycin in treatment of clinical tuberculosis: a preliminary report. Proc Mayo Clin 20: 313–318. [Google Scholar]
- Jones TA, Nelson RC ( 1992) Recovery of vestibular function following hair cell destruction by streptomycin. Hear Res 62: 181–186. [DOI] [PubMed] [Google Scholar]
- Jørgensen JM ( 1981) On a possible hair cell turn-over in the inner ear of the caecilian Ichthyophis glutinosus. Acta Zool 62: 171–186. [Google Scholar]
- Jørgensen JM ( 1989) Number and distribution of hair cells in the vestibular macula of some avian species. J Morphol 201: 187–204. [DOI] [PubMed] [Google Scholar]
- Jørgensen JM ( 1991) Regeneration of lateral line and inner ear vestibular cells. Ciba Found Symp 160: 151–163. [DOI] [PubMed] [Google Scholar]
- Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Uemura T ( 1994) The effects of ototoxic drugs on mechano-electric transduction channels in chick cochlear hair cells. Eur Arch Otorhinolaryngol 251 [Suppl 1]: S53–56. [DOI] [PubMed] [Google Scholar]
- Kuntz AL, Oesterle EC ( 1998) Transforming growth factor alpha with insulin stimulates cell proliferation in vivo in adult rat vestibular sensory epithelium. J Comp Neurol 399: 413–423. [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A ( 1995) Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol 355: 405–417. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM ( 2000a) Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience 99: 333–342. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM ( 2000b) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288: 335–339. [DOI] [PubMed] [Google Scholar]
- Liu W, Staecker H, Stupak H, Malgrange B, Lefebvre P, Van De Water TR ( 1998) Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. NeuroReport 9: 2609–2614. [DOI] [PubMed] [Google Scholar]
- Loddick SA, MacKenzie A, Rothwell NJ ( 1996) An ICE inhibitor, z-VAD DCB attenuates ischaemic brain damage in the rat. NeuroReport 7: 1465–1468. [DOI] [PubMed] [Google Scholar]
- Maruta J, Simpson JI, Raphan T, Cohen B ( 2001) Orienting otolith-ocular reflexes in the rabbit during static and dynamic tilts and off-vertical axis rotation. Vision Res 41: 3255–3270. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Oesterle EC, Stone JS, Rubel EW ( 2000) Characterization of damage and regeneration in cultured avian utricles. J Assoc Res Otolaryngol 1: 46–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME ( 2002a) Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci 22: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Warchol ME ( 2002b) Hair cell survival following aminoglycoside treatment with caspase inhibitors in vivo. Paper presented at the 25th Annual Midwinter Research meeting of the Association for Research in Otolaryngology. St. Petersburg, FL, January 2002.
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA ( 1997) Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci 15: 631–643. [DOI] [PubMed] [Google Scholar]
- Ohmori H ( 1985) Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol (Lond) 359: 189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS, Li XJ, Stieg PE, Yuan J, Penney JB, Young AB, Cha JH, Friedlander RM ( 1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399: 263–267. [DOI] [PubMed] [Google Scholar]
- Raff M ( 1998) Cell suicide for beginners. Nature 396: 119–122. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Messana EP, Cotanche DA ( 2000a) Effect of violation of the labyrinth on the sensory epithelium in the chick cochlea. Hear Res 141: 155–164. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Messana EP, Nedder AP, Cotanche DA ( 2000b) Endotracheal isoflurane anesthesia for chick auditory surgery. Hear Res 141: 165–168. [DOI] [PubMed] [Google Scholar]
- Robinson DA ( 1963) A method of measuring eye movements using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145. [DOI] [PubMed] [Google Scholar]
- Rogers JH ( 1989) Two calcium-binding proteins mark many chick sensory neurons. Neuroscience 31: 697–709. [DOI] [PubMed] [Google Scholar]
- Ruan RS, Leong SK, Mark I, Yeoh KH ( 1999) Effects of BDNF and NT-3 on hair cell survival in guinea pig cochlea damaged by kanamycin treatment. NeuroReport 10: 2067–2071. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM ( 1997) Caspases: intracellular signaling by proteolysis. Cell 91: 443–446. [DOI] [PubMed] [Google Scholar]
- Shoji F, Yamasoba T, Magal E, Dolan DF, Altschuler RA, Miller JM ( 2000) Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res 142: 41–55. [DOI] [PubMed] [Google Scholar]
- Si X, Zakir M, Dickman JD ( 2003) Afferent innervation of the utricular macula in pigeons. J Neurophysiol 89: 1660–1677. [DOI] [PubMed] [Google Scholar]
- Song BB, Schacht J ( 1996) Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res 94: 87–93. [DOI] [PubMed] [Google Scholar]
- Van Optstal J ( 1993) Representation of eye position in three dimensions. In: Multisensory control of movement (Berthoz A, ed), pp 27–41. Oxford: Oxford UP.
- Warchol ME ( 2001) Lectin from Griffonia simplicifolia identifies an immature-appearing subpopulation of sensory hair cells in the avian utricle. J Neurocytol 30: 253–264. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW ( 1992) Hair cell regeneration in the avian vestibular epithelium. Exp Neurol 115: 2–6. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW ( 1993) Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol 331: 97–110. [DOI] [PubMed] [Google Scholar]
- Wersäll J ( 1956) Studies on the structure and innervation of the sensory epithelium of the cristae ampullaris in the guinea pig. A light and electron microscopic investigation. Acta Otolaryngol [Suppl] 126: 1–85. [PubMed] [Google Scholar]
- Werth JL, Deshmukh M, Cocabo J, Johnson Jr EM, Rothman SM ( 2000) Reversible physiological alterations in sympathetic neurons deprived of NGF but protected from apoptosis by caspase inhibition or Bax deletion. Exp Neurol 161: 203–211. [DOI] [PubMed] [Google Scholar]
- Zakir M, Huss D, Dickman JD ( 2003) Afferent innervation patterns of the saccule in pigeons. J Neurophysiol 89: 534–550. [DOI] [PubMed] [Google Scholar]