Abstract
Prostaglandin (PG)E2 promotes the wakeful state when administered into the posterior hypothalamus, in which the histaminergic tuberomammillary nucleus (TMN) is located. To explore the neurotransmitter mechanisms responsible for PGE2-induced wakefulness in rats, we examined the effect of PGE2 on the activity of the histaminergic system and the involvement of PGE2 receptor subtypes in the response. PGE2 perfusion in the TMN at doses of 100, 200, and 400 pmol/min for 2 hr significantly increased histamine release from the medial preoptic area and frontal cortex in a dose-dependent manner, as measured by in vivo microdialysis. Among the agonists of the four distinct subtypes of PGE2 receptors (EP1–4) tested, only the EP4 receptor agonist (ONO-AE1-329) mimicked the excitatory effect of PGE2 on histamine release from both the medial preoptic area and frontal cortex. Perfusion of either PGE2 or the EP4 agonist into the TMN at a dose of 200 pmol/min for 1 hr increased histidine decarboxylase activity, histidine decarboxylase mRNA level, and histamine content in the hypothalamus. In situ hybridization revealed that EP4 receptor mRNA was expressed in histidine decarboxylase-immunoreactive neurons of the TMN region. Furthermore, EP4 agonist perfusion into the TMN induced wakefulness. These findings indicate that PGE2 induces wakefulness through activation of the histaminergic system via EP4 receptors.
Keywords: prostaglandin E2, EP receptors, histamine, histidine decarboxylase, wakefulness, microdialysis, rat
Introduction
Prostaglandin (PG)E2 is produced in the brains of various mammals including humans (Hayaishi, 1991) and exerts an awaking effect in rats (Matsumura et al., 1989a,b) and monkeys (Onoe et al., 1992) after administration into the brain. As determined by microdialysis and simultaneous recordings of the electroencephalogram (EEG) in freely moving rats, endogenous PGE2 levels in the hypothalamus were reported to be significantly higher during wakefulness than during slow-wave sleep, suggesting that PGE2 is involved in physiological sleep–wake regulation (Gerozissis et al., 1995). However, the neurotransmitters responsible for the PGE2-induced wakefulness still remain to be elucidated.
The tuberomammillary nucleus (TMN) of the posterior hypothalamus is the sole source of histaminergic innervation of the mammalian CNS. Histaminergic output from the TMN is considered to play a crucial role in mediating arousal (for review, see Monti, 1993; Lin, 2000; Haas and Panula, 2003). For example, arousal is produced by the pharmacological augmentation of histaminergic transmission (Lin et al., 1988, 1989, 1990). Conversely, sleep is promoted by pharmacological blockade of central histaminergic receptors (Kiyono et al., 1985; Nicholson et al., 1985; Tasaka et al., 1989); by inhibition of histidine decarboxylase (HDC), a key enzyme for histamine biosynthesis (Kiyono et al., 1985; Itowi et al., 1991); and by hyperpolarization of the TMN with GABAergic agonists (Lin et al., 1989; Sallanon et al., 1989; Nelson et al., 2002). Lesions of the posterior hypothalamus were reported to produce hypersomnolence (Nauta, 1946; Sallanon et al., 1988); however, no long-lasting effect on wakefulness was observed after lesions of the histaminergic system in rats (Chou et al., 2001) or of the posterior hypothalamus in cats (Denoyer et al., 1991). Furthermore, histamine release from the anterior hypothalamus of rats shows a circadian variation associated with that of locomotor activity (Mochizuki et al., 1992). The action site of PGE2 to promote and maintain the wakeful state was demonstrated to be the posterior hypothalamus, as revealed by using an in vivo microdialysis method in monkeys (Onoe et al., 1992). This location is clearly distinct from the action site of the febrile effect of PGE2, which is in the anterior hypothalamus. In addition, we found previously that the expression of c-Fos in the TMN was positively correlated with the amount of wakefulness in rats (Scammell et al., 1998, 2001). On the basis of these observations, histamine may be a key neurotransmitter involved in the arousal effect of PGE2.
In the present study, we investigated the effects of PGE2 perfusion into the TMN on the release of histamine in the rat brain by in vivo microdialysis and measured the activity and mRNA level of HDC. We found that application of PGE2 to the TMN not only induced histamine release but also activated histamine biosynthesis. It is well known that the diversity and specificity of the effects of PGE2 are defined by functionally distinct subtypes of PGE2 receptors (EP) classified into four types (EP1, EP2, EP3, and EP4) (Narumiya et al., 1999). Here, we used highly selective agonists for each subtype of EP receptor and clarified that PGE2-induced histaminergic activation was mediated by EP4 receptors, and that activation of these receptors in the TMN induced wakefulness in rats.
Materials and Methods
Male Sprague Dawley rats (Shizuoka Laboratory Animal Center, Shizuoka, Japan), weighing 280–320 gm, were used for the experiments. One rat was used for one experiment only, and not used repeatedly. They were housed at a constant temperature (24 ± 0.5°C), with a relative humidity of 60 ± 2%, and on an automatically controlled 12 hr light/dark cycle (lights on at 8:00 A.M.), and they had ad libitum access to food and water. The experimental protocols were approved by the Animal Research Committee of Osaka Bioscience Institute. PGE2 was obtained from Cayman Chemical (Ann Arbor, MI). EP1, EP2, EP3, and EP4 agonists (ONO-DI-004, ONO-AE1-259, ONO-AE-248, and ONO-AE1-329, respectively) were generous gifts from Ono Pharmaceutical (Osaka, Japan). The chemical structures, specificities, and potencies of these four EP agonists were reported previously (Narumiya and FitzGerald, 2001; Shibuya et al., 2002). All of the other chemicals were of analytical grade.
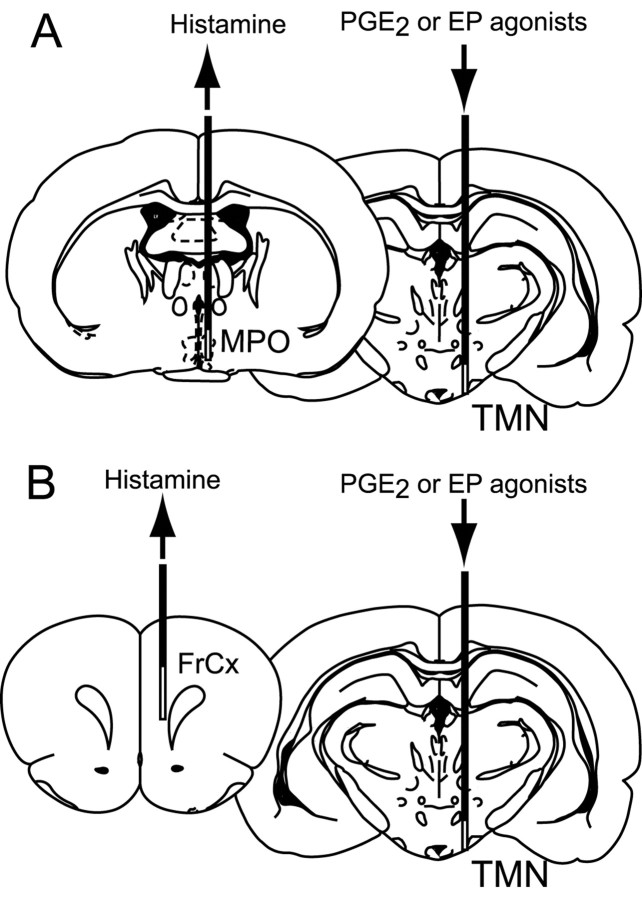
Histamine release from the medial preoptic area and frontal cortex after perfusion with PGE2or EP receptor agonist into the TMN. Rats were anesthetized with urethane (1.2 gm/kg, i.p.). Two microdialysis probes (PC-10, CMA/Microdialysis, Stockholm, Sweden) were inserted stereotaxically, as shown in Figure 1, one into the TMN [anteroposterior (AP), -4.5 mm; dorsoventral (DV), -9.2 mm; left–right (LR), -0.8 mm from the bregma according to the atlas of Paxinos and Watson (1997); membrane length, 2 mm] for administration of PGE2 or EP agonists, and the other into the medial preoptic area (MPO) (AP, -1.0 mm; DV, -8.6 mm; LR, -0.5 mm; membrane length, 2 mm) or the frontal cortex (FrCx) (AP, +3.2 mm; DV, -4.8 mm; LR, -1.0 mm; membrane length, 3 mm) for collecting the extracellular histamine. Both probes were perfused with artificial CSF (ACSF) [composition (in mm): 140 NaCl, 3 KCl, 1.0 MgCl2, 1.3 CaCl2, 2 Na2HPO4, and 0.2 NaH2PO4, pH 7.4] at a flow rate of 2 μl/min. PGE2 and EP agonists were dissolved in dimethyl sulfoxide (DMSO) to make the stock solution and then diluted in ACSF to the concentrations needed. The vehicle was the ACSF solution containing 0.5% DMSO. Two hours after insertion of the microdialysis probes, dialysates were continuously collected from the MPO or FrCx at 20 min intervals (40 μl each) for 1 hr before the perfusion, during the perfusion of the drugs for 2 hr, and until 2 hr after the end of perfusion. The dialysates were kept at -20°C until the histamine assay could be conducted. According to our in vitro calibration test, the relative recovery of PGE2 was 6.0 ± 0.6%.
Figure 1.
Schematic representation of the implantation sites for microdialysis probes. Coronal sections are from the stereotaxic atlas of Paxinos and Watson (1997). PGE2 or EP agonist was administered into the TMN through a microdialysis probe with a membrane (unshaded area) of 2 mm length, and histamine was monitored by another probe implanted in the MPO (A) (membrane length, 2 mm) or the FrCx (B) (membrane length, 3 mm).
Preparation of brain tissue for analyses of histamine content, and HDC activity and mRNA expression. Animals were decapitated after PGE2 or the EP4 agonist had been perfused into the TMN at doses of 200 and 400 pmol/min for 1 hr as described above. The brains were removed, and the unilateral hypothalamus of the perfusion side was dissected. The control rats were perfused with the vehicle for 1 hr. The tissues for analysis of histamine content and HDC activity were stored at -84°C until assayed, whereas samples used for Northern blotting were immediately homogenized in Isogen (Nippon Gene, Toyama, Japan) for extracting RNA, frozen, and kept at -84°C until blotting experiments could be conducted.
Determination of the histamine concentration by HPLC-fluorometry. The histamine concentrations in the perfusates and the hypothalamus homogenates were determined by HPLC-fluorometry (Yamatodani et al., 1985; Huang et al., 1999).
The dissected hypothalamus was weighed and homogenized in 4 vol of ice-cold 0.1 m potassium phosphate, pH 6.8, containing 0.01 mm pyridoxal 5′-phosphate, 0.2 mm dithiothreitol, 1% polyethylene glycol (average molecular weight, 300), and 100 μg/ml phenylmethylsulfonyl fluoride. The homogenate (100 μl) was mixed with 50 μl of 9% perchloric acid containing 5 mm Na2-EDTA and was centrifuged at 10,000 × g for 15 min at 4°C. The supernatant (35 μl) was injected into the HPLC system. The content of tissue histamine was expressed as nanomoles per gram of wet tissue weight in absolute values.
Determination of the HDC activity. The HDC activity was determined by the method described previously (Huang et al., 1998). The homogenate (800 μl) was centrifuged twice at 10,000 × g for 15 min at 4°C, and the resultant supernatant was dialyzed three times against 100 vol of 0.1 m potassium phosphate, pH 6.8, at 4°C. The enzyme solution was incubated at 37°C for 2 hr with 0.25 mm l-histidine. The amount of histamine was quantified by HPLC-fluorometry. The protein content was measured with a Protein Quantification Kit-Wide Range (Dojindo Molecular Technologies, Tokyo, Japan), with bovine serum albumin used as the standard. The HDC activity was expressed as picomoles per minute per milligram of protein.
Northern blotting for detection of HDC mRNA in the hypothalamus. RNA was extracted from the unilateral hypothalamus treated with PGE2, EP4 agonist, or vehicle. Total RNA (20 μg/lane) was separated on a 1% (w/v) agarose gel containing 2.2 m formaldehyde and blotted onto a Hybond N + membrane (Amersham Biosciences, Buckinghamshire, UK). The membrane was prehybridized for 2 hr in buffer, pH 7.4, containing 0.15 m NaCl, 10 mm NaH2PO4, 1 mm EDTA, 5× Denhardt's solution, 50% (v/v) formamide, 0.5% (w/v) SDS, and 100 μg/ml salmon sperm DNA, and was then incubated with [32P]cDNA probe for HDC (Joseph et al., 1990) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA overnight at 42°C. The membrane was washed twice for 20 min each time at 55°C, and then the signal was visualized and quantified with an FLA2000 fluorescence imaging analyzer (Fuji Photo Film, Tokyo, Japan).
In situ hybridization for EP4mRNA and HDC immunostaining in the TMN. After transcardiac perfusion with PBS followed by PBS containing 10% formalin solution (Sigma, St. Louis, MO), the brains were removed, placed in 30% sucrose solution in PBS, and kept there for 2 d at 4°C. The frozen brains were cut into 20 μm coronal sections in a cryotome and hybridized with digoxigenin (DIG)-labeled EP4 riboprobe, as reported previously by Hatanaka (1997). The signal was developed by using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate reagents (Roche Diagnostics, Mannheim, Germany). The EP4 riboprobe was generated from the full-length rat EP4 cDNA (Sando et al., 1994) (GenBank accession number D28860) subcloned into pBluescript II vector provided by Dr. Y. Sugimoto (Kyoto University, Kyoto, Japan), after digestion with XhoI and EcoRI for antisense and sense DIG-labeled riboprobes, respectively.
The cryosections were incubated with guinea pig anti-HDC antibody (1:5000; Euro-Diagnostica, Malmő, Sweden) in PBS containing 0.01% (v/v) Triton X-100 and 10% normal goat serum at 4°C for 20 hr. The HDC immunoreactivity was detected with biotin-labeled anti-guinea pig IgG antibody (1:200; Vector Laboratories, Burlingame, CA) and horseradish peroxidase-conjugated avidin (Vectastain kit; Vector Laboratories), and stained with 3,3′-diaminobenzidine.
For double labeling for EP4 mRNA and HDC immunoreactivity, cryosections were hybridized with the DIG-labeled cRNA probe for EP4, and then incubated first with horseradish peroxidase-conjugated Fab fragment of anti-DIG antibody (Roche Diagnostics) at 4°C for 20 hr and then with biotinyl tyramide solution (TSA Biotin System; PerkinElmer Life Sciences, Boston, MA) at room temperature for 7 min. Visualization was achieved by incubation with streptavidin–Alexa Fluor 488 conjugate (1: 500; PerkinElmer Life Sciences). HDC was then immunostained by incubation of the same sections at 4°C for 20 hr with guinea pig anti-HDC antibody (1:5000; Euro-Diagnostica) followed by Texas Red-conjugated anti-guinea pig IgG (1:200; Vector Laboratories). These signals were observed under a DM IRE2 fluorescence microscope (Leica, Wetzler, Germany) and merged with MetaMorph System (Universal Imaging, Downingtown, PA).
EEG and electromyogram recordings during EP4agonist perfusion into the TMN in rats. Under pentobarbital anesthesia (50 mg/kg, i.p.), rats underwent surgery for implantation of electrodes for EEG and electromyogram (EMG) recordings and placement of a guide cannula for the microdialysis probe, as described previously (Huang et al., 2001). Briefly, a guide cannula (outer diameter, 0.6 mm) with an indwelling stylet was directed stereotaxically into the TMN. The coordinates of the guide tip were as follows: AP, -4.5 mm; LR, -0.8 mm; and DV, -7.2 mm from bregma, according to the atlas of Paxinos and Watson (1997). When perfusion was started, the stylet was replaced by the dialysis probe, which was protruded 2 mm beyond the guide tube. The cannula and electrodes were fixed on the skull with dental cement and anchored to the skull with four stainless-steel screws. Two stainless-steel wire electrodes for EMG recordings were placed into the neck muscles. Postoperatively, each rat was allowed 10 d of recovery, after which it was transferred to a soundproof recording chamber and connected to EEG–EMG recording cables for 3 d of habituation to the experimental conditions.
At least 20 hr before the recording session, the stylet of the microdialysis guide cannula was replaced by a microdialysis probe (PC-12; CMA/Microdialysis) consisting of a semipermeable membrane having a tip length of 2 mm, an outer diameter of 0.5 mm, and a molecular cutoff size of 20 kDa. The probe was continuously perfused with ACSF at a flow rate of 2 μl/min. The EEG–EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 16–128 Hz), digitized at a sampling rate of 128 Hz, and recorded by using the data acquisition program SleepSign (Kissei Comtec, Nagano, Japan) as described previously (Huang et al., 2001). Baseline and experimental recordings were taken in each rat for two consecutive 24 hr periods, starting at 8:00 A.M. From 9:00 to 11:00 A.M. on the experimental day, the TMN in the experimental groups was perfused with the EP4 agonist at a dose of 100, 200, or 400 pmol · 2 μl -1 · min -1, or with EP1, EP2, or EP3, one at a single dose of 400 pmol · 2 μl -1 · min -1; and ACSF containing 0.5% DMSO was applied to the control group.
Vigilance state analysis. Vigilance states were automatically classified off-line in 10 sec epochs into wake, rapid eye movement (REM) and non-REM (NREM) sleep by SleepSign, according to the standard criteria (Huang et al., 2001). As a final step, defined sleep–wake stages were examined visually and corrected, if necessary.
Histological verification. When an experiment was over, the rats were killed with an overdose of pentobarbital sodium and perfused through the microdialysis probes with a pontamine sky blue dye solution (0.5% w/v) to verify the site of PGE2 or EP agonist administration.
Statistical analysis. All data were expressed as the mean ± SEM (n = 5–6). The statistical significance of the effect of PGE2 or EP agonists on HDC activities, mRNA expression, histamine release, and histamine contents were assessed by one-way ANOVA followed by Fisher's PLSD test, except as otherwise stated. The time course of histamine release was assessed by two-way ANOVA. For vigilance studies, amounts of the different sleep–wake states were analyzed by the paired t test, with each animal serving as its own control. In all of the cases, p < 0.05 was taken as the level of significance.
Results
Effects of PGE2 perfusion into the TMN on histamine release from the MPO and FrCx
To examine activation of the histaminergic nervous system after perfusion of PGE2 into the TMN, we monitored histamine release from the MPO and FrCx, both of which have been implicated in the arousal effect of histamine (Lin, 2000; Lin et al., 1994, 1996).
Histamine output became stable 2 hr after implantation of the probe. Thus, the mean value of histamine output found during the next 1 hr was defined as the basal release, and the subsequent fractions were expressed as percentages of this value (Fig. 2A,B).
Figure 2.
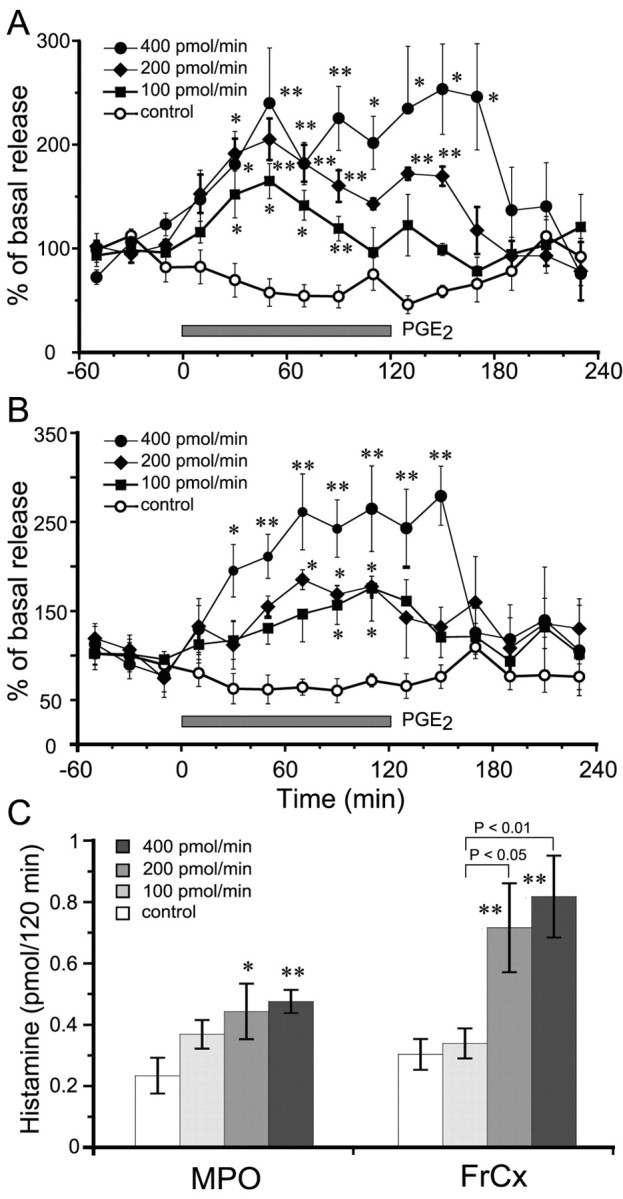
Histamine release from the MPO and FrCx after perfusion of PGE2 in the TMN for 2 hr. A, Time courses of histamine release from the MPO. B, Time courses of histamine release from the FrCx. The open circles, filled squares, filled diamonds, and filled circles stand for the groups treated with vehicle control and with PGE2 at doses of 100, 200, and 400 pmol/min, respectively. The horizontal filled bar indicates the duration of PGE2 perfusion. C, Dose dependency of histamine released from the MPO and FrCx during 2 hr PGE2 perfusion. The open, light gray, medium gray, and dark gray bars stand for the groups treated with vehicle control and with PGE2 at doses of 100, 200, and 400 pmol/min, respectively. Each value represents the mean ± SEM of five or six rats. *p<0.05; **p<0.01, significantly different from the control, as assessed by two-way (A, B) or one-way (C) ANOVA followed by the PLDS test.
As shown in Figure 2A, PGE2 perfusion into the TMN induced a significant increase in histamine release from the MPO in a dose-dependent manner. Perfusion with 100, 200, and 400 pmol/min produced significant elevation of histamine release beginning at 40 min after the start of the PGE2 perfusion; and, at 60 min, the release reached its maximal level, which was 140, 220, and 240% of the baseline level (0.049 ± 0.006 pmol/20 min), respectively. In the 100 and 200 pmol/min groups, the increased level gradually returned to the basal level within 1 hr after the end of PGE2 perfusion. However, when PGE2 was perfused at a dose of 400 pmol/min, the maximal level of histamine increase was sustained for 1 hr after the PGE2 perfusion had ended.
Histamine release from the FrCx was also increased in a dose-dependent manner, when PGE2 was perfused into the TMN (Fig. 2B). PGE2 perfusion at doses of 100 and 200 pmol/min induced significant histamine release from 100 and 80 min, respectively, after the start of the PGE2 perfusion, and this level of release was maintained for 40 and 60 min, respectively, with the maximal elevation of 150% of the baseline (0.061 ± 0.006 pmol/20 min). PGE2 perfusion at 400 pmol/min significantly increased the histamine release from the FrCx at 40 min after the start of the perfusion to a peak of 250% of the baseline value at 80 min. The highest levels were sustained for 40 min after the end of the perfusion, and then the histamine level quickly returned to the baseline level.
We calculated the histamine released from the MPO and FrCx during PGE2 perfusion for 2 hr and found that PGE2 significantly increased histamine release in a dose-dependent manner compared with the control. PGE2 at 100 pmol/min tended to increase histamine release, but there was no statistical difference from the controls (Fig. 2C).
We monitored the core body temperature during EP agonist or PGE2 perfusion into the TMN regions through a microdialysis probe and found that the core body temperature remained unchanged, suggesting that the drug is delivered into the TMN region distinct from the action site of the febrile effect of PGE2.
Effects of EP agonists on the histamine release from the MPO and FrCx
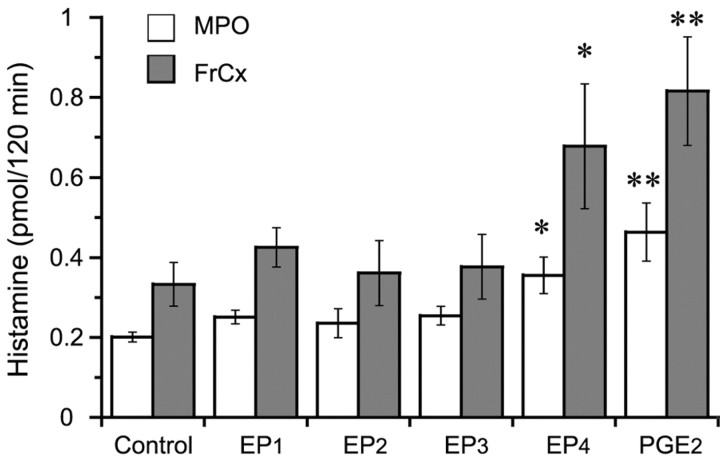
To define the EP receptor subtype involved in PGE2-induced histamine release, we used newly developed EP agonists that are highly specific for their respective EP receptor. When each agonist or PGE2 was perfused into the TMN at a dose of 200 pmol/min for 2 hr, histamine released from the MPO and FrCx during the perfusion was significantly increased in the EP4 agonist- or PGE2-treated groups, whereas no significant increase in the histamine release was observed in the other three groups treated with the agonists for EP1, EP2, and EP3, compared with the control (Fig. 3). There was no significant difference in the amount of histamine released from the MPO and FrCx between the EP4 agonist- and PGE2-treated groups (Fig. 3). These results strongly suggest that the PGE2-induced histamine release is mediated by the EP4 receptor.
Figure 3.
Amounts of histamine released from the MPO (open bars) and FrCx (filled bars) after perfusion of the TMN with four distinct EP receptor agonists or PGE2 at the same dose of 200 pmol/min for 2 hr. Each value is expressed as the absolute amount of released histamine during these perfusions for 2 hr and as the mean ± SEM of five or six rats. *p < 0.05; **p < 0.01, significantly different from their respective control, as assessed by one-way ANOVA followed by the PLDS test.
Localization of EP4 mRNA in the TMN
When we examined the distribution of EP4 mRNA in the rat posterior hypothalamus by in situ hybridization, a strong positive signal was observed in the TMN. The distribution profile of HDC-immunoreactive neurons (Fig. 4A,D) in adjacent serial coronal sections was almost identical to that of those containing EP4 mRNA (Fig. 4B,E). No positive signal was found in the control section hybridized with the sense probe (Fig. 4C). In sections double stained for HDC immunoreactivity and EP4 mRNA (Fig. 4F,G), almost all of the HDC-positive neurons of the TMN region were positive for EP4 mRNA (Fig. 4H), whereas many neurons in other regions were positive for only EP4 mRNA. These results indicate that the EP4 receptor subtype is expressed in HDC-immunoreactive neurons of the TMN region.
Figure 4.
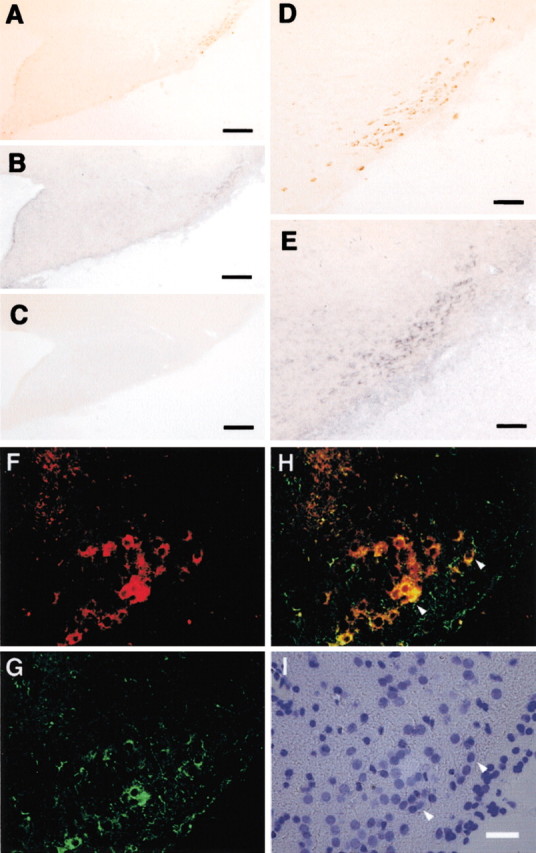
Photomicrographs showing the immunoreactivity for HDC (A, D) and the mRNA signal for EP4(B,E) in adjacent serial sections around the TMN. C, Control section hybridized with the sense probe. The cryosection was double labeled to indicate the localization of HDC (red) (F) and EP4 mRNA (green) (G). Both F and G are merged in H. Differential interference contrast, 4′,6′-diamidino-2-phenylindole-stained image is shown in I. Arrowheads point to HDC and EP4 double-positive neurons. Scale bars: A–C, 200 μm; D, E, 65 μm; (in I) F–I, 25 μm.
Effects of PGE2 and EP4 agonist perfusion into the TMN on histamine contents and on HDC activity and expression of HDC mRNA in the hypothalamus
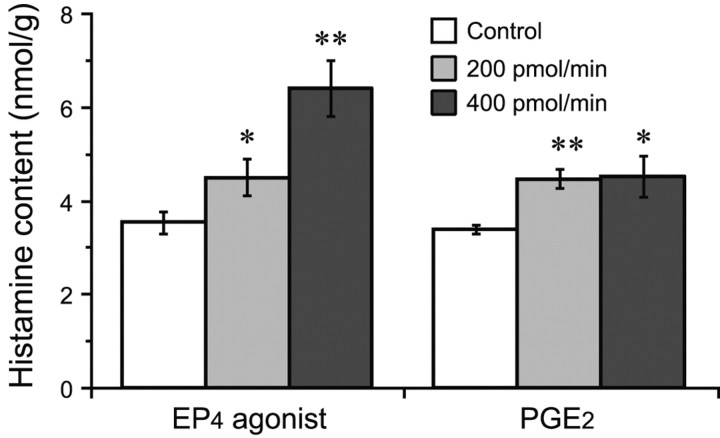
Figure 5 shows the changes in the histamine content of the hypothalamus 1 hr after PGE2 or EP4 agonist perfusion of the TMN. PGE2 perfusion at doses of 200 and 400 pmol/min significantly increased the histamine content by 31 and 33%, respectively, compared with that of the control group. Similarly, the EP4 agonist at doses of 200 and 400 pmol/min also increased the histamine content, by 27 and 67%, respectively. The other EP agonists did not induce any increase in the histamine content in the hypothalamus (data not shown).
Figure 5.
Histamine content in the hypothalamus after perfusion of the TMN with PGE2 or EP4 agonist at doses of 200 and 400 pmol/min for 1 hr. The control groups were perfused with ACSF containing 0.5% DMSO. Histamine content is expressed as nanomoles per gram of wet weight and as the mean ± SEM of five or six rats. The open, gray, and filled bars stand for the groups treated with vehicle control and with EP4 agonist or PGE2 at doses of 200 and 400 pmol/min, respectively. *p < 0.05; **p < 0.01, significantly different from the control, as assessed by one-way ANOVA followed by the PLDS test.
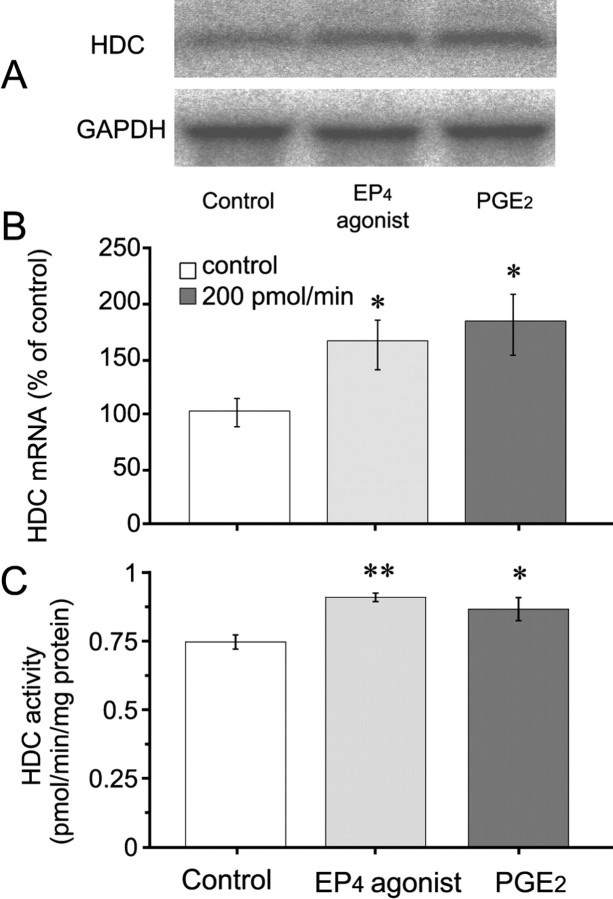
Northern blot analysis revealed that the HDC probe hybridized with a 2.6 kb transcript obtained from total RNA preparations from the hypothalamus (Fig. 6A). After 1 hr perfusion of the TMN with PGE2 or the EP4 agonist at a dose of 200 pmol/min, HDC mRNA expression in the hypothalamus was significantly increased, by 83 and 69%, respectively, compared with the control expression (Fig. 6B). HDC activity was also increased after PGE2 or the EP4 agonist perfusion of the TMN at a dose of 200 pmol/min, by 17% and 21%, respectively (Fig. 6C). No significant change in HDC activity or histamine content was observed after treatment with the three other kinds of EP agonists (data not shown).
Figure 6.
Increase in expression of HDC mRNA and HDC activity in the hypothalamus after perfusion of the TMN with PGE2 or EP4 agonist at a dose of 200 pmol/min for 1 hr. A, Northern blot analysis of HDC and GAPDH mRNAs in the hypothalamus treated with PGE2 or EP4 agonist. Each lane contained 20 μg of total RNA. B, The mRNA for HDC was quantified as a ratio to GAPDH mRNA by Northern blotting. The HDC mRNA content is indicated as a percentage of the control. C, HDC activity was measured in the homogenate of the hypothalamus. Control rats were perfused with ACSF containing 0.5% DMSO. The open, gray, and filled bars stand for the groups treated with vehicle control, EP4 agonist, and PGE2, respectively. Values are expressed as the mean ± SEM of five rats. *p < 0.05, significantly different from the control, as assessed by one-way ANOVA followed by the PLDS test.
EP4 agonist perfusion into the TMN increased wakefulness in rats
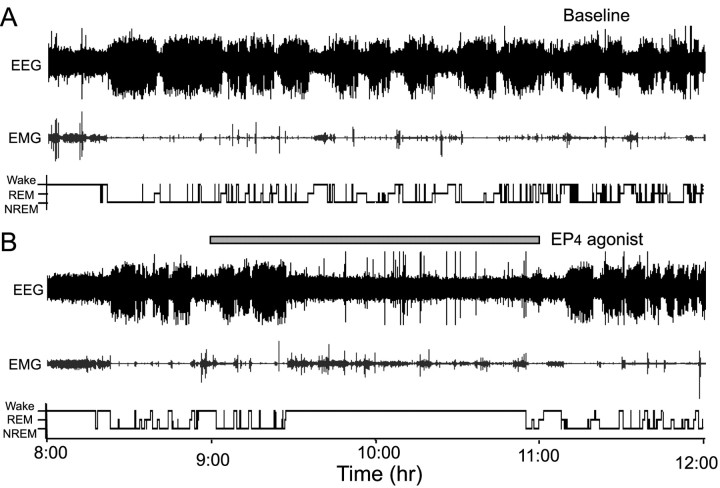
We perfused the TMN with the EP4 agonist through a microdialysis probe for 2 hr from 9:00 to 11:00 A.M. to investigate the changes in the sleep-stage distribution. Typical examples of polygraphic recordings and corresponding hypnograms from one of rats given the EP4 agonist at a dose of 400 pmol/min are shown in Figure 7. During the period of 9:00 to 11:00 A.M., this rat spent more time in sleep under the baseline condition (Fig. 7A). When the EP4 agonist was perfused on the experimental day, the animal still slept for the first 30 min of the perfusion and then awoke and remained awake until ∼10 min after the end of the drug administration (Fig. 7B). During the wakefulness induced by EP4 agonist, there was a short period of NREM sleep with 1–2 min episodes.
Figure 7.
Typical examples of polygraphic recordings and corresponding hyponograms in a rat before and after the administration of EP4 agonist at a dose of 400 pmol/min. A, Baseline day. B, Experimental day. The horizontal filled bar indicates the duration of EP4 agonist perfusion.
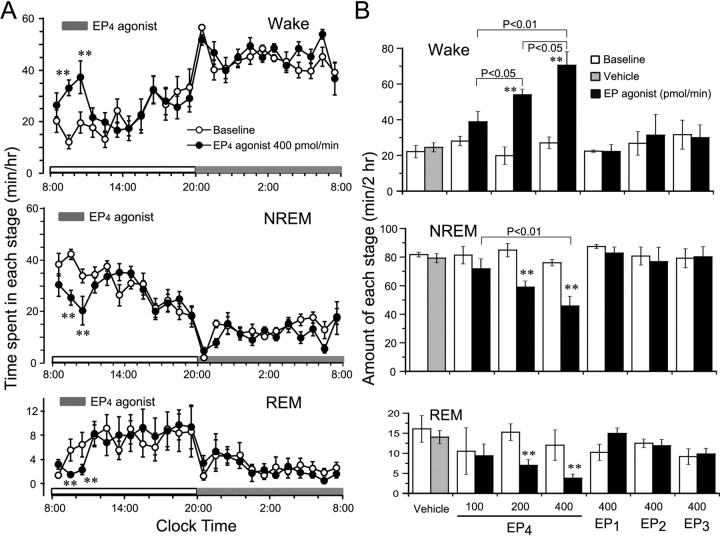
As shown in Figure 8A, EP4 agonist perfusion at 400 pmol/min significantly increased the wake time by 2.7- and 2.1-fold during the first and second hour of perfusion, when the amount of wakefulness was compared with that of the baseline day. This enhancement of wakefulness was concomitant with decreases in NREM and REM sleep. The EP4 agonist (400 pmol/min) decreased NREM sleep by 37 and 39%, and REM sleep by 73 and 63%, during the first and second hour of perfusion, respectively. There was no additional disruption of sleep architecture during the subsequent period. Similar time course profiles were observed with the middle concentration (200 pmol/min) of the EP4 agonist. Although the EP4 agonist at a dose of 100 pmol/min tended to increase the time spent in wakefulness, there was no significant difference between this value and the baseline one (data not shown).
Figure 8.
Sleep-stage distribution produced by EP agonist perfusion of the rat TMN. A, Time course changes in the 400 pmol/min EP4 agonist treatment group. Each circle represents the hourly mean ± SEM of wakefulness, NREM sleep, or REM sleep. Open and filled circles stand for the baseline and experimental day profiles, respectively. The short horizontal filled bars indicate the perfusion between 9:00 and 11:00 A.M. on the experimental day. The long horizontal open and filled bars on the x-axes indicate the 12 hr light and dark periods, respectively. B, Total time spent in wakefulness, NREM sleep, and REM sleep for 2 hr during the perfusion. Open, gray, and filled bars show the profiles of baseline days, and of experimental days treated with vehicle and EP agonists, respectively. Values are the means ± SEM (n = 5 or 6 in EP4 agonist treatment; n = 4 in each EP1, EP2, and EP3 agonist treatment group). *p < 0.05; **p < 0.01 by the paired t test. The statistical significance of amounts of each stage in EP4 agonist-treated groups was assessed by one-way ANOVA followed by the PLDS test.
We calculated the total time spent in wakefulness, NREM sleep, and REM sleep during the 2 hr perfusion (Fig. 8B). The EP4 agonist given at 200 and 400 pmol/min significantly increased the total amounts of wake time during those 2 hr by 2.3- and 2.6-fold, and reduced NREM sleep by 29 and 39%, and REM sleep by 54 and 68%, respectively. EP4 agonist (100 pmol/min) increased the total amounts of wake time during those 2 hr by 38%, but not with statistical significance. However, when the TMN was perfused with the vehicle solution or any of the three other EP agonists at a dose of 400 pmol/min, there was essentially no difference between the values obtained and their respective baseline one. These results clearly indicate that perfusion of the TMN with the EP4 agonist increased wakefulness and concomitantly reduced NREM and REM sleep.
Discussion
Application of PGE2 to the TMN activates the histaminergic system via EP4 receptors
When we perfused PGE2 into the TMN through microdialysis probes, PGE2 significantly increased histamine release from both the MPO and FrCx. These increases observed in the discrete regions of the brain were supposed to result from activation of the ascending histaminergic projection, suggesting that the application of PGE2 to the TMN induces the histamine release widely in the brain. Electrical stimulation of the TMN reportedly induced histamine release from neuronal terminals in the anterior hypothalamus (Mochizuki et al., 1991; Okakura-Mochizuki et al., 1996). Here, we found that chemical stimulation of the TMN with PGE2 also increased the histamine release in the brain, which is consistent with the observation that infusion of PGE2 into the third ventricle could increase the turnover rate of hypothalamic histamine in rats (Kang et al., 1999). However, we also revealed that PGE2 perfusion into the TMN increased the histamine content and mRNA expression and activity of HDC in the hypothalamus, suggesting that PGE2 increases histamine biosynthesis there.
To determine the EP receptor subtype involved in PGE2-induced activation of the histaminergic system, in this study, we used newly developed selective EP receptor agonists. We selected perfusion doses of PGE2 and the four EP agonists according to their values of binding affinity, agonist activity (EC50), and the doses inducing the maximal effects. PGE2 at doses of 100, 200, and 400 pmol/min induced histamine release in a dose-dependent manner, and perfusion with PGE2 at >400 pmol/min did not further elevate its effect. Among the EP agonists tested, the EP2, EP3, and EP4 agonists have binding affinities better than or similar to PGE2 binding to their respective receptors, but the EP1 agonist showed a lower affinity than PGE2 and a lower EC50 than the other three EP agonists (Maruyama and Ohuchida, 2000; Narumiya and FitzGerald, 2001). We tried to increase the dose of the EP1 agonist, but it was difficult for us to exceed 400 pmol/min because of its poor solubility. When the TMN was perfused with each EP agonist, only the EP4 agonist markedly increased histamine release from both the MPO and FrCx, whereas EP1, EP2, and EP3 agonists had little effect on histamine release, indicating that the PGE2-induced histamine release can be attributed to the action of the EP4 receptor subtype in the TMN. The presence of PGE2 receptors in the TMN is a key factor for activation of the histaminergic system. Our previous reports showed that the TMN contains a high density of binding sites of [3H]PGE2 (Watanabe and Hayaishi, 1988; Matsumura et al., 1990). Our present in situ hybridization study showed the expression of EP4 mRNA in the HDC-immunoreactive-positive neurons of the TMN region, further indicating that the EP4 receptor in the TMN region was involved in the PGE2-induced histamine release.
The EP4 receptor is the most recently identified EP subtype and is positively coupled to adenylate cyclase (Coleman et al., 1994; Narumiya et al., 1999). This receptor has been demonstrated to mediate the action of PGE2 within specific nuclei of the brain in response to circulating interleukin-1β (Zhang and Rivest, 2000) and to be induced in the paraventricular nucleus of the hypothalamus during fever responses to lipopolysaccharide and interleukin-1, both of which are presumably mediated by PGE2 (Oka et al., 2000). Our present study strongly suggests that the EP4 receptor is involved in PGE2-induced histamine release and wakefulness in the CNS.
PGE2-induced wakefulness is mediated by the EP4 receptor
Gerozissis et al. (1995) determined the PGE2 concentration in the perfusates recovered by microdialysis of the rat hypothalamus to be 500 pg/ml during NREM sleep and 600 pg/ml during wakefulness in freely moving rats. From their data, the extracellular level of PGE2 in the rat hypothalamus was calculated to be ∼0.3 μm, because the relative recovery of PGE2 through their microdialysis probes was ∼5%. In our studies, we perfused PGE2 solution of 100–400 pmol · 2 μl-1 · min-1 through the microdialysis probe, and the concentrations of these compounds outside the probe were calculated to be 3–12 μm according to our 6% relative recovery. Therefore, the PGE2 concentration used in our microdialysis study is considered to be approximately one order of magnitude higher than the extracellular level of PGE2 in the rat hypothalamus without stimulation. However, the EEG profiles after administration of EP4 agonist in the rat hypothalamus were indistinguishable from those during physiological wakefulness, suggesting that PGE2 and EP4 agonist induced natural physiological wakefulness.
Here, we found that activation of EP4 receptors in the TMN region induced wakefulness. In contrast, when infused into the subarachnoid space surrounding the ventral surface of the basal forebrain during the nocturnal hours, an EP4 agonist could inhibit wakefulness (Yoshida et al., 2000), suggesting that the effect of EP4 agonist on sleep–wake regulation is site dependent. Activation of histaminergic neurons induces EEG desynchronization or wakefulness (Kiyono et al., 1985; Lin et al., 1994, 1996; Huang et al., 2001). Recently, we demonstrated that orexin A activated the TMN to increase histamine release and induce wakefulness more quickly and strongly than an EP4 agonist (Huang et al., 2001). However, the relationship between orexin A and PGE2 remains to be clarified. EP4 knock-out mice might be useful for additional confirmation of the present study, but it is difficult for us to use these mice for sleep studies, because they suffer from patent ductus arteriosus (Narumiya and FitzGerald, 2001) and usually die very young. Conditional knock-out animals with posterior hypothalamus-selective depletion of EP4 will be useful for future study.
In conclusion, the application of PGE2 to the TMN increased both histamine release and synthesis in the brain. These effects were mimicked by engagement of the EP4 receptors. When an EP4 agonist was applied to the TMN, the arousal effect was induced with a reduction in NREM and REM sleep, indicating that PGE2 induces wakefulness through activation of the histaminergic system via EP4 receptors.
Footnotes
This study was supported in part by grants from the Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation (Y.U.), the Japan Space Forum (Y.U.), the Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.U.), Takeda Science Foundation (Y.U.), Sankyo Foundation (T.M.), the Grants-in-Aid for Japan Society for the Promotion of Science Fellows program (Z.-L.H. and T.O.), the Ministry of Health and Welfare of Japan (Grant 100107) (O.H.), and Osaka City. We thank Ono Pharmaceutical Company for providing EP agonists, Dr. Y. Sugimoto (Kyoto University) for providing rat EP4 cDNA, Drs. N. Eguchi and M. Ikeda for their valuable discussion, and Dr. M. Sakata, S. Matsumoto, and Y. Kuwahata for their excellent technical assistance.
Correspondence should be addressed to Dr. Yoshihiro Urade, Department of Molecular Behavioral Biology, Osaka Bioscience Institute, 6-2-4 Furuedai, Suita, Osaka 565-0874, Japan. E-mail: uradey@obi.or.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235975-09$15.00/0
References
- Chou TC, Gerashchenko D, Saper CB, Shiromani PJ ( 2001) Loss of histaminergic neurons does not produce hypersomnolence. Soc Neurosci Abstr 27: 1.1378. [Google Scholar]
- Coleman RA, Smith WL, Narumiya S ( 1994) International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229. [PubMed] [Google Scholar]
- Denoyer M, Sallanon M, Buda C, Kitahama K, Jouvet M ( 1991) Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res 539: 287–303. [DOI] [PubMed] [Google Scholar]
- Gerozissis K, De Saint Hilaire Z, Orosco M, Rouch C, Nicolaidis S ( 1995) Changes in hypothalamic prostaglandin E2 may predict the occurrence of sleep or wakefulness as assessed by parallel EEG and microdialysis in the rat. Brain Res 689: 239–244. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P ( 2003) The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 4: 121–130. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y ( 1997) Early molecular specification in the hippocampal rudiment: isolation of genes expressed in a region-specific manner in the embryonic telencephalon. Brain Res Dev Brain Res 98: 65–73. [DOI] [PubMed] [Google Scholar]
- Hayaishi O ( 1991) Molecular mechanisms of sleep-wake regulation: roles of prostaglandins D2 and E2 FASEB J 5: 2575–2581. [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Watanabe H, Kagoshima M, Maeyama K ( 1998) Biphasic elevation of plasma histamine induced by water immersion stress, and their sources in rats. Eur J Pharmacol 360: 139–146. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Watanabe H, Maeyama K ( 1999) Activation of sensory nerves participates in stress-induced histamine release from mast cells in rats. Neurosci Lett 270: 181–184. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O ( 2001) Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA 98: 9965–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itowi N, Yamatodani A, Kiyono S, Hiraiwa ML, Wada H ( 1991) Effect of histamine depletion on the circadian amplitude of the sleep-wakefulness cycle. Physiol Behav 49: 643–646. [DOI] [PubMed] [Google Scholar]
- Joseph DR, Sullivan PM, Wang YM, Kozak C, Fenstermacher DA, Behrendsen ME, Zahnow CA ( 1990) Characterization and expression of the complementary DNA encoding rat histidine decarboxylase. Proc Natl Acad Sci USA 87: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Yoshimatsu H, Kurokawa M, Ogawa R, Sakata T ( 1999) Prostaglandin E2 mediates activation of hypothalamic histamine by interleukin-1β in rats. Proc Soc Exp Biol Med 220: 88–93. [DOI] [PubMed] [Google Scholar]
- Kiyono S, Seo ML, Shibagaki M, Watanabe T, Maeyama K, Wada H ( 1985) Effects of α-fluoromethylhistidine on sleep-waking parameters in rats. Physiol Behav 34: 615–617. [DOI] [PubMed] [Google Scholar]
- Lin JS ( 2000) Brain structure and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med Rev 4: 471–503. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Jouvet M ( 1988) Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology 27: 111–122. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M ( 1989) A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res 479: 225–240. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Arrang JM, Garbarg M, Schwartz JC, Jouvet M ( 1990) Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res 523: 325–330. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Jouvet M ( 1994) Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci 6: 618–625. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Sakai K, Jouvet M ( 1996) Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J Neurosci 16: 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Ohuchida S ( 2000) Selective agonists and antagonists for prostaglandin E2 receptor subtypes. Tanpakushitsu Kakusan Koso 45: 1001–1007. [PubMed] [Google Scholar]
- Matsumura H, Honda K, Goh Y, Ueno R, Sakai T, Inoue S, Hayaishi O ( 1989a) Awaking effect of prostaglandin E2 in freely moving rats. Brain Res 481: 242–249. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Honda K, Choi WS, Inoue S, Sakai T, Hayaishi O ( 1989b) Evidence that brain prostaglandin E2 is involved in physiological sleep-wake regulation in rats. Proc Natl Acad Sci USA 86: 5666–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Watanabe Y, Onoe H, Hayaishi O ( 1990) High density of prostaglandin E2 binding sites in the anterior wall of the 3rd ventricle: a possible site of its hyperthermic action. Brain Res 533: 147–151. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Yamatodani A, Okakura K, Takemura M, Inagaki N, Wada H ( 1991) In vivo release of neuronal histamine in the hypothalamus of rats measured by microdialysis. Naunyn Schmiedebergs Arch Pharmacol 343: 190–195. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H ( 1992) Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav 51: 391–394. [DOI] [PubMed] [Google Scholar]
- Monti JM ( 1993) Involvement of histamine in the control of the waking state. Life Sci 53: 1331–1338. [DOI] [PubMed] [Google Scholar]
- Narumiya S, FitzGerald GA ( 2001) Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest 108: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F ( 1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- Nauta WJH ( 1946) Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol 9: 285–316. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M ( 2002) The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci 5: 979–984. [DOI] [PubMed] [Google Scholar]
- Nicholson AN, Pascoe PA, Stone BM ( 1985) Histaminergic systems and sleep. Studies in man with H1 and H2 antagonists. Neuropharmacology 24: 245–250. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB ( 2000) Relationship of EP1–4 prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol 428: 20–32. [DOI] [PubMed] [Google Scholar]
- Okakura-Mochizuki K, Mochizuki T, Yamamoto Y, Horii A, Yamatodani A ( 1996) Endogenous GABA modulates histamine release from the anterior hypothalamus of the rat. J Neurochem 67: 171–176. [DOI] [PubMed] [Google Scholar]
- Onoe H, Watanabe Y, Ono K, Koyama Y, Hayaishi O ( 1992) Prostaglandin E2 exerts an awaking effect in the posterior hypothalamus at a site distinct from that mediating its febrile action in the anterior hypothalamus. J Neurosci 12: 2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1997) The rat brain in stereotaxic coordinates. San Diego: Academic.
- Sallanon M, Sakai K, Buda C, Puymartin M, Jouvet M ( 1988) Increase of paradoxical sleep induced by microinjections of ibotenic acid into the ventrolateral part of the posterior hypothalamus in the cat. Arch Ital Biol 126: 87–97. [PubMed] [Google Scholar]
- Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M ( 1989) Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience 32: 669–683. [DOI] [PubMed] [Google Scholar]
- Sando T, Usui T, Tanaka I, Mori K, Sasaki Y, Fukuda Y, Namba T, Sugimoto Y, Ichikawa A, Narumiya S, Nakao K ( 1994) Molecular cloning and expression of rat prostaglandin E receptor EP2 subtype. Biochem Biophys Res Commun 200: 1329–1333. [DOI] [PubMed] [Google Scholar]
- Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O ( 1998) Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2 Proc Natl Acad Sci USA 95: 7754–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O ( 2001) An adenosine A2A agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107: 653–663. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Setiadji SV, Ibrahim N, Harayama N, Maruyama T, Ueta Y, Yamashita H ( 2002) Involvement of postsynaptic EP4 and presynaptic EP3 receptors in actions of prostaglandin E2 in rat supraoptic neurones. J Neuroendocrinol 14: 64–72. [DOI] [PubMed] [Google Scholar]
- Tasaka K, Chung YH, Sawada K, Mio M ( 1989) Excitatory effect of histamine on the arousal system and its inhibition by H1 blockers. Brain Res Bull 22: 271–275. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Hayaishi O ( 1988) Quantitative autoradiographic localization of prostaglandin E2 binding sites in monkey diencephalon. J Neurosci 8: 2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T ( 1985) High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr 344: 115–123. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Matsumura H, Nakajima T, Mandai M, Urakami T, Kuroda K, Yoneda H ( 2000) Prostaglandin E (EP) receptor subtypes and sleep: promotion by EP4 and inhibition by EP1/EP2 NeuroReport 11: 2127–2131. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rivest S ( 2000) A functional analysis of EP4 receptor-expressing neurons in mediating the action of prostaglandin E2 within specific nuclei of the brain in response to circulating interleukin-1β. J Neurochem 74: 2134–2145. [DOI] [PubMed] [Google Scholar]