Abstract
To address questions of whether endogenous BDNF acts differentially on inhibitory and excitatory neurons, and through what routes, we used chimera culture of cerebral cortical neurons derived from BDNF-/- mice and another type of transgenic mice that express green fluorescence protein and BDNF. Presynaptic BDNF transferred to both types of neurons, GABA-synthesizing enzyme-positive and -negative neurons. The latter neurons were confirmed to be glutamatergic with immunocytochemistry. Dendritic development of the former inhibitory neurons was promoted by endogenous BDNF transferred from presynaptic, excitatory neurons. In contrast, dendritic development of excitatory neurons was not related to the presence or absence of presynaptic BDNF, suggesting that BDNF acts on inhibitory neurons through an anterograde, transsynaptic route so as to promote dendritic development, whereas this is not the case in excitatory neurons.
Keywords: neurotrophin, brain-derived neurotrophic factor, dendritic growth, inhibitory neuron, visual cortex, chimera cell culture, green fluorescence protein
Introduction
Brain-derived neurotrophic factor (BDNF) is known to play a crucial role in development and plasticity of neuronal circuits in the CNS (for review, see Thoenen, 1995; McAllister et al., 1999; Bibel and Barde, 2000; Poo, 2001). In addition to actions on dendritic growth of pyramidal neurons in visual cortex (McAllister et al., 1995, 1996, 1997; Horch et al., 1999), BDNF has been reported to regulate development of inhibitory neurons containing GABA in the brain. For example, BDNF promotes the phenotype differentiation of GABAergic neurons in hippocampus and striatum (Ip et al., 1993; Nawa et al., 1993; Mizuno et al., 1994; Marty et al., 1996; Ivkovic and Ehrlich, 1999; Yamada et al., 2002), facilitates dendritic development of hippocampal GABAergic neurons in culture (Bartrup et al., 1997; Vicario-Abejon et al., 1998), increases the density of inhibitory synapses (Marty et al., 2000) or the size of inhibitory terminals (Bolton et al., 2000) of hippocampal neurons, and plays a role in activity-dependent regulation of inhibition at cortical and hippocampal synapses (Rutherford et al., 1997; Tanaka et al., 1997; Frerking et al., 1998). Furthermore, depletion and overexpression of BDNF in transgenic mice impairs dendritic growth of cerebellar Purkinje cells (Schwartz et al., 1997) and accelerates maturation of GABAergic neurons in visual cortex (Huang et al., 1999), respectively.
Because these results were obtained with exogenously applied BDNF or with the nonphysiological level of BDNF, however, an important question of whether endogenous BDNF in the physiological condition exerts such an action is not answered yet. Also, a route through which endogenous BDNF acts on inhibitory neurons is not clarified, although glutamatergic or catecholaminergic neurons were suggested to be a source of BDNF (Nawa et al., 1995; Altar et al., 1997; Marty et al., 1997; Fawcett et al., 2000). Furthermore, another question of whether endogenous BDNF acts on inhibitory neurons in a different way from its action on excitatory neurons has not explicitly been answered yet.
To address these questions, we took an advantage of neurons derived from BDNF knock-out mice, because in these neurons one can detect an uptake of endogenous BDNF from other neurons of wild-type mice if both types of neurons are cocultured. In this preparation, however, it is practically difficult to differentiate neurons of knock-out mice from those of wild-type mice, because BDNF can be transferred from cell to cell. To overcome this problem, we prepared a mixed cell culture with another type of transgenic mice in which all the cells express green fluorescence protein (GFP) (Okabe et al., 1997; Fujikawa et al., 2000). In this chimera culture of neurons prepared from the different types of transgenic mice, we could unambiguously identify neurons having the potential to express endogenous BDNF with GFP tag and those lacking the potential under a fluorescence microscope. Combined with immunocytochemistry with antibody to GABA-synthesizing enzyme, we found that GABAergic neurons require BDNF of presynaptic, excitatory neurons for development of their dendrites, but excitatory neurons do not require such presynaptic BDNF.
Materials and Methods
Chimera culture of neurons. Neonatal GFP mice (C57BL/6; provided by Dr. M. Okabe, Genome Information Research Center, Osaka University, Suita 565-0871, Japan) and mice (C57BL/6; provided by Regeneron Pharmaceuticals, Tarrytown, NY) that were confirmed to be BDNF-/- mice with genotyping were anesthetized with ketamine (>30 mg/kg, i.p.) and then killed by cervical dislocation at postnatal days 2–3. Genotyping of neonatal mice was performed in the same way as described previously (Itami et al., 2000). The experimental procedures met the regulation of the Animal Care Committee of Osaka University Graduate School of Medicine. Neurons derived from BDNF-/- and GFP mice were cultured on the same glial feeder layers that had been prepared previously from BDNF-/- mice. The density of GFP and BDNF-/- neurons were 25–50 cells/cm 2 and ∼1000 cells/cm 2, respectively. The detailed method of culturing neurons was described previously (Kohara et al., 2001). All experiments were performed 14–21 d after plating. In part of the experiments, anti-BDNF antibody (30 μg/ml; Promega, Madison, WI) was added to the culture medium at 2 d after plating to neutralize endogenous BDNF.
Injection of plasmid cDNA of DsRed or Neurobiotin. In part of the experiments, plasmid cDNAs of DsRed (DsRed-Express; Clontech, Palo Alto, CA) were injected into the nucleus of neurons through micropipettes at the concentration of 1 μg/μl to trace axons of the neurons under observation. Axon terminals of injected neurons were visualized, as reported previously (Kohara et al., 2001). In another series of the experiments, 10% N-(2-aminoethyl)biothinamide hydrochloride (Neurobiotin;Vector Laboratories, Burlingame, CA) in PBS was injected into neurons through micropipettes. Neurobiotin was visualized by NeutrAvidin conjugated Alexa 350 (1: 1000; Molecular Probes, Eugene, OR).
Immunocytochemistry. For immunocytochemical staining, neurons were fixed usually with 4% paraformaldehyde (PFA; Sigma, St. Louis, MO) and 4% sucrose in PBS, pH 7.0, for 30 min at room temperature. For analysis of dendritic morphology, neurons were fixed with 4% PFA and 4% sucrose in PBS, pH 7.4, for 20 min at room temperature. The cells were incubated with PBS containing 0.2% Triton-X (Sigma) for 1 min and blocked by 10% goat serum in PBS for 1 hr at 37°C. Then, anti-MAP2 monoclonal antibody (isotype:IgG1, 1:250; Sigma), anti-synaptotagmin monoclonal antibody (isotype:IgG2b, 1:200; Calbiochem, San Diego, CA), anti-GAD65 monoclonal antibody (isotype:IgG2a, 1:1000; Chemicon, Temecula, CA), anti-BDNF rabbit polyclonal antibody (2 μg/ml; provided by Dr. R. Katoh-Semba, Institute for Developmental Research, Kasugai 480-0392, Japan) (Katoh-Semba et al., 1997, 2001), anti-GFP chicken polyclonal antibody (1:1000; Chemicon), or anti-GFP rabbit polyclonal antibody (1:1000; Molecular Probes) was applied for 2 hr at 37°C. MAP2, synaptotagmin, and GAD65 were visualized by isotype-specific secondary antibody conjugated with Alexa 350 (1:200; Molecular Probes), Alexa 546 (1:2000), and Alexa 647 (1:200). GFP and BDNF were visualized by anti-chicken secondary antibody conjugated with Alexa 488 (1:1000) and anti-rabbit secondary antibody conjugated with Cy5 (1: 200; Chemicon). GFP has its own fluorescence but it is gradually fading during observation. Once GFP is immunocytochemically stained with anti-GFP antibody, however, the fluorescence does not fade so quickly. Therefore, immunocytochemical staining of GFP was necessary for observation of neurites of neurons. Fluorescent signals were observed with a 40×/1.3 numerical aperture oil immersion objective (Plan Flour; Nikon, Tokyo, Japan) attached to an inverted epifluorescence microscope and captured by a cooled CCD camera (C4742–95; Hamamatsu Photonics, Hamamatsu, Japan). This system consisted of 1024 × 1024 pixels, each of which corresponded to 0.17 × 0.17 μm with the 40× objective. Filters (UV2EC, B2EC, and G2EC; Nikon; XF110; Omega Optical, Brattleboro, VT) were used for four-color immunofluorescence detection. Fluorescence data were analyzed further with an Aquacosmos system (Hamamatsu Photonics). In part of the experiments, a confocal fluorescence microscope (E600FN; Nikon; Radiance 2000; Bio-Rad, Hercules, CA) was used to obtain thin-sliced images of stained neurons.
Measurement of fluorescent signal. The fluorescence intensity of BDNF was measured on a square window (30 × 30 pixels; 5.1 × 5.1 μm) placed on the soma of a neuron under observation, and the mean fluorescence intensity of 900 pixels was calculated by subtracting the noise of the CCD camera system that was detected in the complete darkness. The window was placed randomly on the soma in the blind condition, i.e., the window was placed by an experimenter who had not seen any BDNF image of the neuron, and then the fluorescence intensity was measured automatically by the Aquacosmos system. As control, the soma of another neuron that was not contacted by GFP-positive terminals was randomly selected in the same culture dish. The intensity of fluorescence in such a control neuron was calculated in the same way as above. Because the soma of control neurons had some background fluorescence, its fluorescence intensity was expressed as 100%, and that of test neurons was normalized to this value.
Analysis of morphology. After immunocytochemical staining, neurons, the dendrites of which did not overlap with dendrites of other neurons, were randomly selected from the same dishes. After having recorded fluorescent images, neurons were incubated with anti-mouse IgG1-conjugated biotin for 1 hr at 37°C. Then, an ABC kit (Vector Laboratories) was used for visualization of MAP2. Neurolucida (MicroBrightField, Williston, VT) attached to an upright microscope (E600; Nikon) was used for drawing dendrites of neurons. The quantitative assessment of dendritic morphology was done with an analyzing software, Neuroexplore (MicroBrightField).
Results
Transfer of endogenous BDNF from presynaptic terminals to postsynaptic neurons
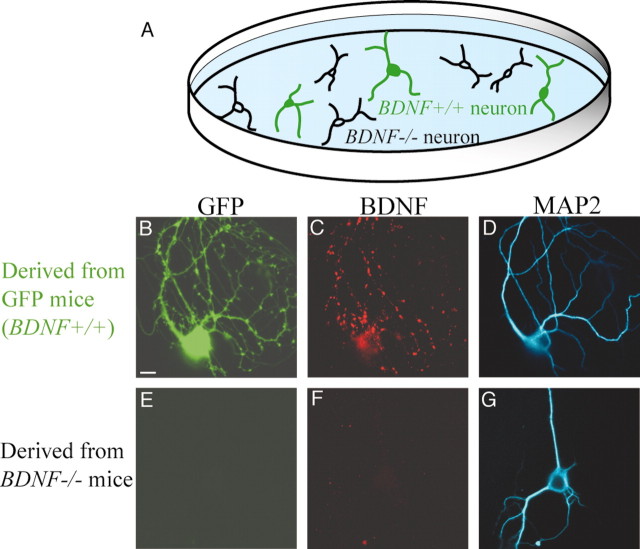
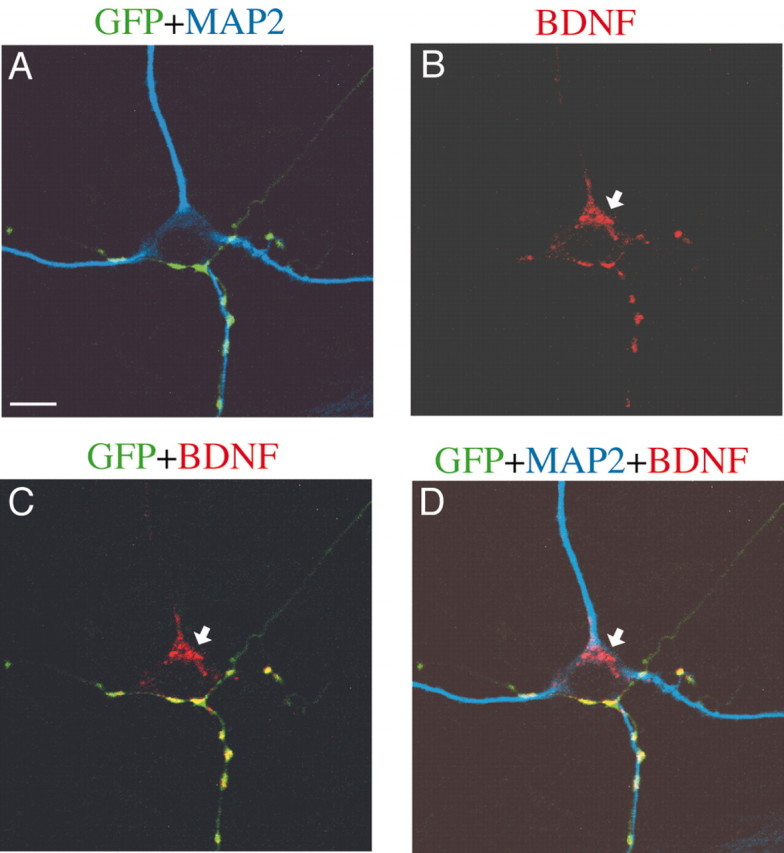
We prepared chimera culture of cortical neurons from homozygously knock-out mice of BDNF gene (BDNF-/- mice) and from GFP mice (Fig. 1A). In this culture, GFP-positive neurons are expected to have the potential to express endogenous BDNF, whereas GFP-negative neurons are not. In fact, we confirmed that the former neurons expressed endogenous BDNF in a punctuated manner in neurites (Fig. 1B,C), whereas the latter neurons did not have any sign of endogenous BDNF (Fig. 1E,F). Such a GFP-negative neuron became visible by immunocytochemistry with antibody to MAP2, which is a marker of somatodendritic region of neurons (Fig. 1G).
Figure 1.
Chimera cell culture prepared from two types of transgenic mice. A, Schematic illustration of chimera culture of cortical neurons derived from BDNF-/- and GFP mice. The latter neurons were labeled with GFP and had the potential to express endogenous BDNF (BDNF+/+). B, GFP image of a cortical neuron derived from a GFP mouse. C, Immunocytochemical BDNF image of the neuron shown in B. D, MAP2 image of the neuron shown in B and C. E, No GFP fluorescence signal in a cortical neuron derived from a BDNF-/- mouse was observed. This neuron was located in the same dish as above. F, No BDNF immunoreactivity in the neuron shown in E was observed. G, MAP2 image of the neuron shown in E and F. Scale bar: (in B) B–G, 10 μm.
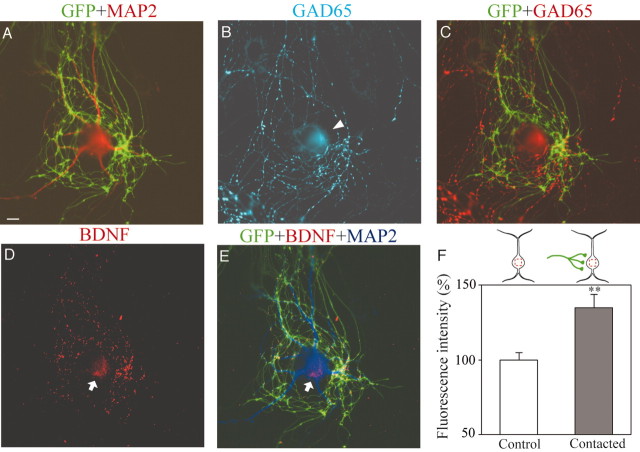
To identify GABAergic neurons, we stained neurons with antibody to GAD65, which is known to be a synthesizing enzyme of GABA in presynaptic terminals (Erlander and Tobin, 1991). With this immunocytochemistry, we could unambiguously detect GABAergic neurons. Then, we found that endogenous BDNF transferred from presynaptic terminals to postsynaptic GABAergic neurons. An example of this finding is shown in Figure 2. As shown in Figure 2A, axon branches of a GFP-positive neuron (BDNF+/+), the soma of which was outside the frame of this picture, made a net-like terminal arborization, which surrounded and contacted a MAP2-positive neuron (red). This postsynaptic neuron was confirmed to be a GABAergic neuron, because the immunoreactivity to antibody against GAD65 was clearly seen in its cell body (Fig. 2B, arrowhead). As shown in Figure 2A, this neuron was GFP negative and, thus, judged as derived from a BDNF knock-out mouse. In Figure 2B, neurites containing GAD65-positive puncta or varicosities were seen around the soma of the GABAergic neuron. These neurites were judged to be axon branches of this and other GABAergic neurons, because they were not stained with anti-MAP2 antibody (Fig. 2A). The superimposed picture in Figure 2C indicates that GFP-positive axon branches were GAD65 negative and, thus, not GABAergic. In Figure 2D,E, it is seen that BDNF-positive puncta were located in presynaptic axon branches. Also, we found that the soma of the GABAergic neuron contained BDNF (Fig. 2D,E, arrows). This suggests that endogenous BDNF was transferred from the presynaptic, GFP-positive axon branches to the postsynaptic GABAergic neuron, because this postsynaptic neuron was derived from a BDNF-/- mouse and, thus, should not produce endogenous BDNF by itself.
Figure 2.
Transfer of endogenous BDNF to postsynaptic GABAergic neurons. A, Superposition of GFP image (green) and MAP2 image (red) of cultured neurons. A GFP-negative postsynaptic neuron (red) was contacted by GFP-positive axon branches and their terminals (green). Scale bar, 10 μm. B, GAD65 image of the same frame as in A. The arrowhead indicates the soma. C, Superposed image of GFP signals (green) and GAD65 signals (red). D, Endogenous BDNF image of the same frame as the others. The arrow indicates the soma of the postsynaptic neuron. E, Superposed image of GFP signals (green), BDNF signals (red), and MAP2 signals (blue). F, Mean value of intensity of BDNF signal in two groups of neurons. The top schematically shows the method to measure the fluorescence intensity of BDNF signal in neurons. The mean values obtained from GABAergic neurons that did not contact GFP-positive terminals (□) and contacted such terminals (▪). Vertical bars indicate SEM. Double asterisks indicate statistical significance of the difference from the control at p < 0.01 (unpaired t test).
There is a possibility that BDNF signals that were detected in the soma region of neurons might be located in the pericellular space that was foreground or background of the soma. To minimize such a possibility, a very thin section (1.2 μm thick) of image was obtained with a confocal microscope in part of the experiments. As indicated by arrows in Figure 3B–D, BDNF signals were seen in the soma of a postsynaptic neuron. Also, it was seen that the BDNF signals in the soma were not overlapped with GFP signals, whereas those in neurites were mostly overlapped (Fig. 3C,D). These results confirmed that BDNF was present in the soma of the postsynaptic neuron.
Figure 3.
Intraneuronal BDNF visualized by confocal microscopy. All pictures were single focal sections of 1.2 μm thickness. A, Superposed image of GFP signals (green) and MAP2 signals (blue). B, BDNF image (red) of the same frame as A. The arrow shows BDNF signals in the cell body. C, Superposed image of GFP and BDNF. BDNF signal in the cell body (arrow) was not superposed with GFP-positive neurites. D, Superposed image of GFP, MAP2, and BDNF. Scale bar: (in A) 10 μm.
To quantify the transfer of BDNF to postsynaptic neurons, the intensity of fluorescence was measured in the soma of the neurons that were contacted by GFP-positive axon terminals (Fig. 2F, insets). As control, the soma of another neuron that was not contacted by GFP-positive terminals was randomly selected in the same culture dish. Because the soma of control neurons had some background fluorescence, its fluorescence intensity was expressed as 100%, and that of test neurons was normalized to this value. The mean fluorescence intensity of the somata of 10 GABAergic neurons that were contacted by GFP-positive axons was 135.0 ± 8.5% (mean ± SEM), which was significantly (p < 0.01; unpaired t test) larger than that of the seven control neurons (Fig. 2F). This indicates that the postsynaptic GABAergic neuron contains endogenous BDNF that has probably been transferred from GFP-positive, BDNF+/+ presynaptic axons.
There is a possibility that BDNF detected in the soma of GABAergic neurons might be transferred retrogradely from a GFP-positive neuron. To test such a possibility, we visualized 6 GABAergic neurons with the method of direct intranuclear injection of plasmid cDNAs of DsRed fluorescent protein (Kohara et al., 2001) and another 10 GABAergic neurons with the method of intracellular injection of a tracer, Neurobiotin. With either method, we could trace axons of the neurons until their terminals. Because we did not find a notable difference in visualization of neurons between these two methods, the data were combined. Altogether, six neurons were contacted by GFP-positive afferents. Axon terminals of three neurons contacted GFP-positive, excitatory neurons, and the other three did not. Ten GABAergic neurons were not contacted by GFP-positive afferents. Axon terminals of five of these neurons contacted GFP-positive, excitatory neurons, and those of the other five did not. It is to be noted that the former five neurons that had the possibility to receive BDNF only through the retrograde route did not have detectable BDNF signal in their cell bodies. To quantify these results, the background fluorescence intensity of the soma of the former five neurons was compared with that of the latter five neurons that had no possibility to receive BDNF through either route. The mean fluorescence intensity of the former neurons was 95.9 ± 4.1% of that of the latter neurons. The difference was insignificant (unpaired t test; p > 0.05). Thus, these results suggest that the possibility of retrograde transport is negligible in our preparations, although we cannot completely exclude such a mechanism.
An intercellular transfer of endogenous BDNF in the anterograde direction was detected also in GAD65-negative neurons that were most likely excitatory neurons. In part of the experiments, neurons were immunocytochemically stained with antibody to phosphate-activated glutaminase, which is glutamate-synthesizing enzyme in the transmitter pool of cortical neurons (Kaneko and Mizuno, 1988). We observed that all of the GAD65-negative neurons were reactive to this antibody, indicating that GAD65-negative neurons were glutamatergic neurons. The mean fluorescence intensity of the somata of 16 GAD65-negative neurons that were contacted by GFP-positive axons was 154.8 ± 16.3% of that of another 14 GAD65-negative neurons that were not contacted by GFP-positive axons. The difference was statistically significant (p < 0.01; unpaired t test). These results indicate that presynaptic endogenous BDNF transferred to both inhibitory and excitatory neurons.
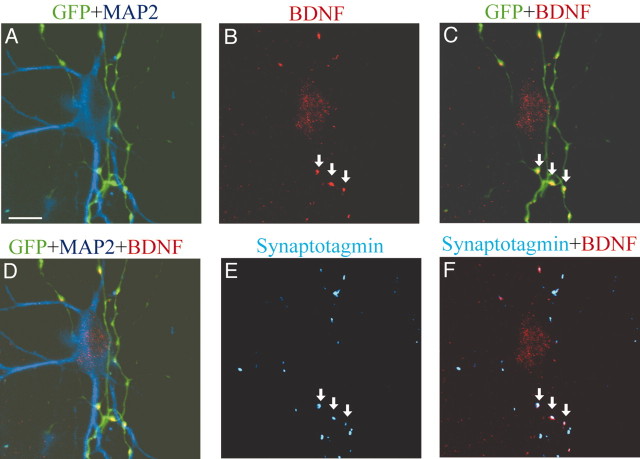
Then, we tested whether BDNF actually exists in presynaptic axon terminals. For this, we stained neurons immunocytochemically with antibody against a presynaptic marker protein, synaptotagmin (Fig. 4E). Some BDNF signals were localized in GFP-positive axons, so that they appeared to be yellow puncta (Fig. 4C, arrows). As shown in Figure 4F, such clusters of BDNF-positive puncta were mostly colocalized with synaptotagmin-positive presynaptic terminals.
Figure 4.
Existence of endogenous BDNF in presynaptic axon terminals. A, Superposed image of GFP signals (green) and MAP2 signals (blue). B, BDNF image (red) of the same frame as A. The arrows show clusters of BDNF signals. Faint BDNF signal was seen in the soma of the postsynaptic neuron, as shown in D. C, Superposed image of GFP and BDNF. BDNF puncta were colocalized with GFP-positive neurites (arrows). D, Superposed image of GFP, MAP2, and BDNF. E, Synaptotagmin image of the same frame as the others. The arrows indicate the same puncta as in B, C, and F. F, Superposed image of synaptotagmin (blue) and BDNF (red). BDNF puncta indicated by arrows were colocalized with synaptotagmin. Scale bar: (in A) 10 μm.
Promoted growth of dendrites of GABAergic neurons by presynaptic BDNF
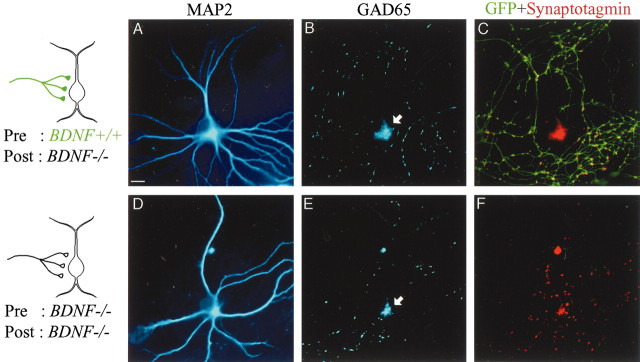
To assess actions of endogenous BDNF on postsynaptic GABAergic neurons, we quantitatively analyzed the dendritic morphology of neurons that were derived from BDNF-/- mice. As shown in Figure 5, the dendritic morphology of neurons that were immunoreactive to anti-GAD65 antibody (Fig. 5B,E) was visualized by immunocytochemistry with anti-MAP2 antibody (Fig. 5A,D). The neuron shown in Figure 5A was contacted by GFP-positive (BDNF+/+) axon terminals, as shown by yellow puncta in Figure 5C, in which the picture stained with anti-GFP antibody (green) was superimposed with that with anti-synaptotagmin antibody (red). The neuron shown in Figure 5D was contacted by GFP-negative axon terminals that were shown by synaptotagmin-postive puncta (F). In Figure 5A,D, it is obvious that the GABAergic neuron contacted by GFP-positive (BDNF+/+) terminals had much more abundant dendritic branches than the other neuron contacted by GFP-negative axons (BDNF-/-). The total picture of dendrites of another pair of GABAergic neurons is shown in Figure 6A. Again, it is obvious that the neuron contacted by GFP-positive (BDNF+/+) terminals had well developed dendrites (right), whereas that contacted by GFP-negative axons (BDNF-/-) had relatively poor dendrites (left).
Figure 5.
Promoted growth of dendrites of GABAergic neurons by presynaptic BDNF. A, MAP2 image of a neuron contacted by a GFP-positive axon (BDNF+/+), as schematically shown on the left. B, GAD65 image of the same frame as in A. The arrow shows the cell body. C, Superposition of GFP images (green) and synaptotagmin images (red) of the same frame as A and B. D, MAP2 image of a neuron contacted by a GFP-negative axon (BDNF-/-), as schematically shown on the left. E, GAD65 image of the same frame as in D. The arrow shows the cell body. F, Superposition of GFP images (green) and synaptotagmin images (red) of the same frame as D and E. Because both the presynaptic and postsynaptic neurons were GFP negative, there was no green signal. Scale bar: (in A), 10 μm.
Figure 6.
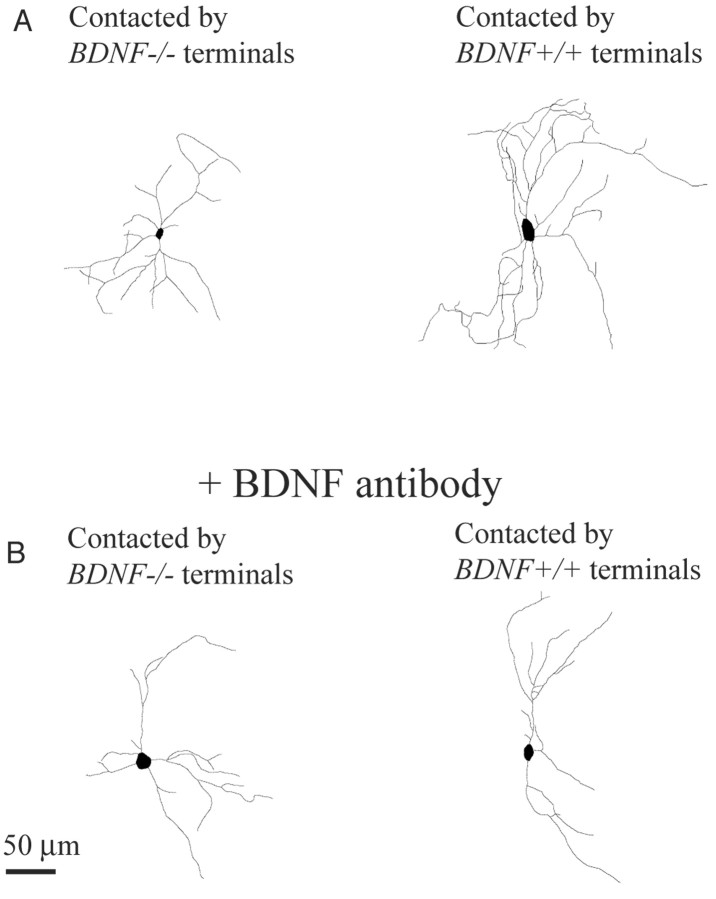
Morphology of dendrites of GABAergic neurons and effects of anti-BDNF antibody. A, Left, Dendritic morphology of a GABAergic neuron (BDNF-/-) that was contacted by GFP-free (BDNF-/-) axons. Right, Dendritic morphology of another GABAergic neuron (BDNF-/-) that was contacted by GFP-positive (BDNF+/+) axons. B, Dendritic morphology of GABAergic neurons (BDNF-/-) treated with anti-BDNF antibody. Left and right, Neurons were contacted by GFP-free axons (BDNF-/-) and GFP-positive axons (BDNF+/+), respectively.
To quantify this finding, we calculated three parameters of dendritic morphology of each neuron (Fig. 7). The mean total length of dendrites of 14 GABAergic neurons that were contacted by GFP-positive axons (BDNF+/+) and that of other 12 GABAergic neurons that were contacted by GFP-negative axons (BDNF-/-) were 2376.7 ± 225.5 μm and 1345.2 ± 152.9 μm, respectively (Fig. 7A, left two columns). The difference between these two values was statistically significant (p < 0.01; ANOVA). The mean numbers of dendritic branch points of the neurons contacted by GFP-positive and GFP-negative axons were 22.2 ± 2.0 and 13.3 ± 1.9, respectively (Fig. 7B, left two columns). The difference between these two values was, again, significant (p < 0.01; ANOVA). In contrast, the number of primary dendrites was not affected by presynapic BDNF (Fig. 7C, left two columns). The mean numbers of primary dendrites of the neurons contacted by GFP-positive and GFP-negative axons were 7.0 ± 0.5 and 6.2 ± 0.6, respectively. These results suggest that endogenous BDNF, which probably was supplied from presynaptic terminals, may promote branch formation of dendrites of postsynaptic GABAergic neurons.
Figure 7.
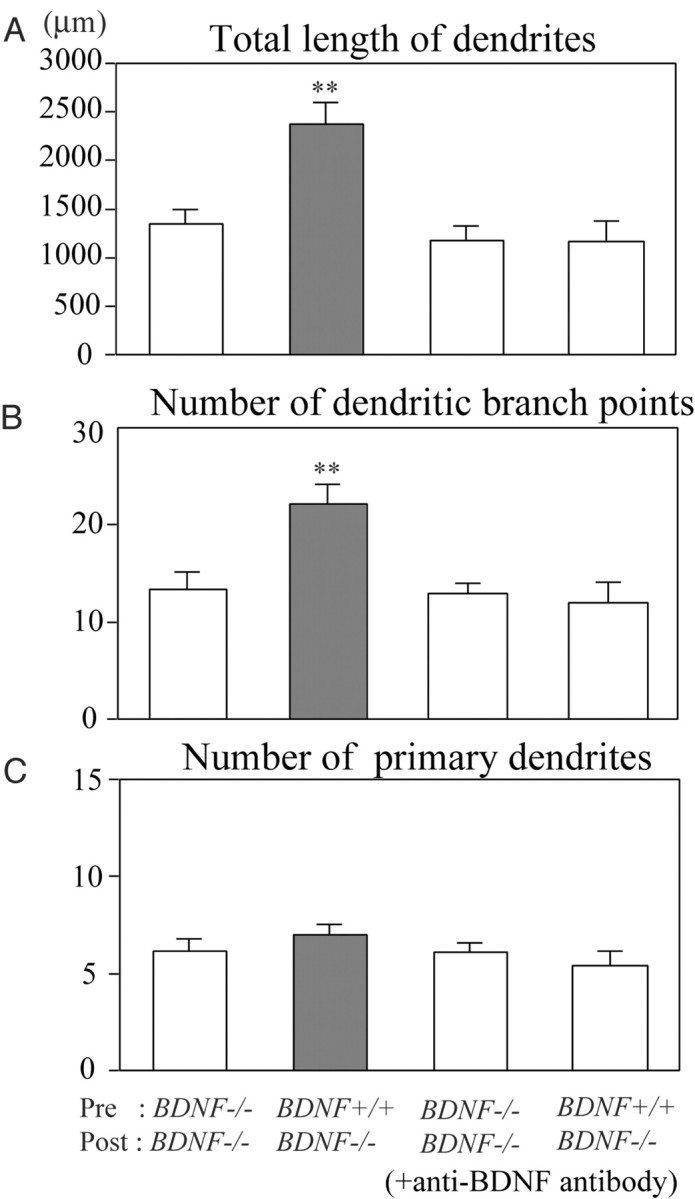
Quantitative assessment of actions of presynaptic BDNF on dendrites of GABAergic neurons. A, Mean values of the total length of dendrites of neurons contacted by GFP-negative axons (BDNF-/-) (leftmost column) and GFP-positive axons (BDNF+/+) (hatched column) in the control medium and corresponding values of neurons treated with anti-BDNF antibody (third and rightmost columns). Vertical bars indicate SEM. Double asterisks in A and B indicate statistical significance of the difference at p < 0.01 (ANOVA). B, Mean number of dendritic branch points per neuron. C, Mean number of primary dendrites per neuron. In B and C, neurons are grouped in the same way as in A.
To confirm that such an effect of contact with GFP-positive (BDNF+/+), presynaptic terminals was induced by extracellularly released BDNF, we applied anti-BDNF antibody, which blocks function of BDNF, to chimera culture preparations. As shown in Figure 6B (right), a GABAergic neuron cultured with anti-BDNF antibody had relatively poor dendrites even when it was contacted by GFP-positive (BDNF+/+) terminals. The complexity of their dendritic arborization seemed to be about the same as that of another GABAergic neuron that was contacted by GFP-negative axons (BDNF-/-) (Fig. 6B, left). This is seen in the quantitative analysis of the three parameters of dendrites (Fig. 7A–C, right two columns). The mean total lengths of dendrites of the two groups of GABAergic neurons that were treated with anti-BDNF antibody were 1177.8 ± 149.5 μm and 1173.9 ± 209.8 μm, respectively (Fig. 7A, right two columns; n = 8 and 9, respectively). The mean numbers of dendritic branch points of the two groups of neurons were 13.0 ± 1.1 and 12.0 ± 2.2, respectively (Fig. 7B, right two columns). The mean numbers of primary dendrites of the two groups of neurons were 6.1 ± 0.5 and 5.4 ± 0.7, respectively (Fig. 7C, right two columns). In any of these three parameters, there was no significant difference between the two groups of GABAergic neurons, to which anti-BDNF antibody was applied.
In this quantitative analysis, it is also seen that the total length of dendrites and the number of dendritic branch points of the GABAergic neurons that were contacted by GFP-negative afferents were not reduced by the treatment with the anti-BDNF antibody (Fig. 7A,B, compare the third columns with the leftmost columns). There was no statistically significant difference (unpaired t test; p > 0.05) between these two columns in any of the three parameters (Fig. 7).
No effect of presynaptic BDNF on dendritic growth of excitatory neurons
Finally, we examined effects of presynaptic BDNF on the dendritic morphology of GAD65-negative neurons and found that the presence or absence of presynaptic BDNF is not related to dendritic growth of such excitatory neurons. Examples of GAD65-negative neurons are shown in Figure 8. The GAD65 negativity was obvious, in particular, in their somata (Fig. 8B,E, arrows), although the neuron in Figure 8B seemed to be surrounded by GAD65-positive puncta that were assumed to be GABAergic axon terminals. These neurons were judged as derived from BDNF-/- mice, because they were GFP negative (Fig. 8C,F). The seemingly yellow staining of some regions of the neuron shown in Figure 8F was because of GFP-positive presynaptic terminals, the soma of which was outside the frame of this picture. As seen in Figure 8A,D, the extent of dendritic arborization of the BDNF-negative excitatory neuron contacted by a GFP-positive axon (BDNF+/+) (Fig. 8D) was about the same as that of the neuron contacted by a GFP-negative axon (BDNF-/-) (Fig. 8A).
Figure 8.
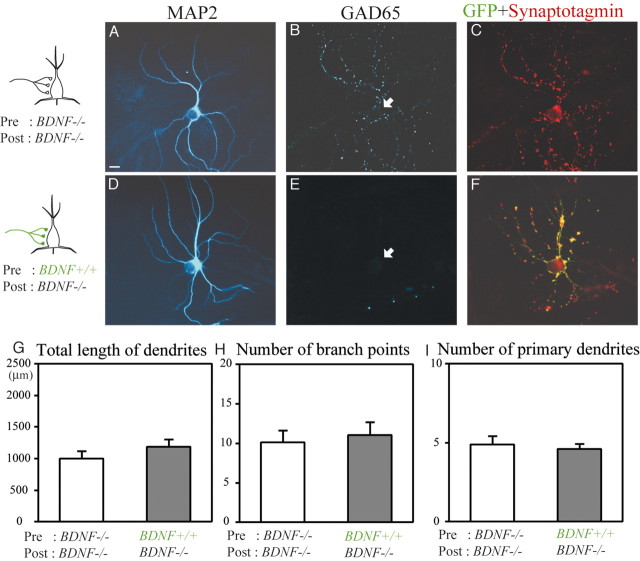
No effect of presynaptic BDNF on growth of dendrites of excitatory neurons. A, D, MAP2 images of a BDNF-/- neuron that was contacted by BDNF-/- axons and BDNF+/+ axons, respectively. B, E, GAD65 images of the neurons shown in A and D, respectively. The arrows indicate the location of the soma. Note the lack of label in the cell body of both neurons. C, F, Superposed image of GFP and synaptotagmin signals of the neurons shown in A and D, respectively. Scale bar: (in A) 10 μm. G, Mean value of the total length of dendrites of excitatory neurons contacted by GFP-negative axons (BDNF-/-) (open column) and by GFP-positive axons (BDNF+/+) (hatched column). Vertical bars indicate SEM. H, Mean number of branch points of dendrites per neuron. I, Mean number of primary dendrites per neuron.
The dendritic morphology of these two groups of excitatory neurons that were derived from (BDNF-/-) mice was analyzed in the same way as in Figure 7 (Fig. 8G–I). The total length of dendrites of 17 excitatory neurons contacted by GFP-positive axons (1181.5 ± 123.1 μm) was not significantly (unpaired t test; p > 0.05) different from that of another 16 excitatory neurons contacted by GFP-negative axons (998.1 ± 120.0 μm) (Fig. 8G). The mean numbers of dendritic branch points (11.1 ± 1.6) and primary dendrites (4.6 ± 0.3) of the excitatory neurons with GFP-positive axons were not significantly different from those of the neurons with GFP-negative axons (10.1 ± 1.5 and 4.9 ± 0.5, respectively) (Fig. 8H,I) (unpaired t test; p > 0.05). Thus, the presence or absence of presynaptic BDNF had no effects on dendritic development of postsynaptic, excitatory neurons.
Discussion
It is well established that GABAergic neurons do not have mRNA of BDNF and, thus, cannot produce BDNF by themselves (Ernfors et al., 1990; Cellerino et al., 1996; Rocamora et al., 1996; Gorba and Wahle, 1999). In the present study, we have found that such GABAergic neurons had a clear signal of the existence of BDNF in their cell bodies. This BDNF is judged to have been transferred from presynaptic GFP-positive axons that contain endogenous BDNF, because only the GABAergic neurons that were contacted by GFP-positive axon terminals had significant signals, but those that were not contacted by such terminals had not. A previous report also suggested that interneurons could incorporate BDNF that might be released from pyramidal cells in rat hippocampus (Schmidt-Kastner et al., 1996). However, they did not show that their interneurons were GABAergic and also did not provide direct evidence that BDNF was actually transferred to those neurons from pyramidal neurons.
The present study further indicates that BDNF that has been transferred in such a way promotes dendritic development of GABAergic neurons, because the neurons contacted by GFP-positive axon terminals had well developed dendrites, but those not contacted by such terminals had relatively poor dendrites. The better development of dendrites of the former neurons is thought to result from intercellularly transferred BDNF, because anti-BDNF antibody that was applied through the culture medium and, thus, expected to neutralize BDNF in the extracellular space blocked such a proliferous action.
There is a possibility that GABAergic neurons might receive BDNF through their axons from GFP-positive, postsynaptic neurons and such target-derived BDNF might exert the proliferous action, because BDNF is known to be transported also retrogradely from postsynaptic neurons to presynaptic axon terminals (Causing et al., 1997; Marty et al., 1997; Watson et al., 1999). This possibility seems unlikely, however, for two reasons. First, in the present study, none of the GABAergic neurons that did not receive GFP-positive afferents but sent their axons to other GFP-positive, excitatory neurons had detectable BDNF signal in their cell bodies. If endogenous BDNF is transferred in the retrograde direction, we should have detected BDNF signal in such GABAergic neurons. Second, if target-derived BDNF has some effects on GABAergic neurons, the functional block of released BDNF by the anti-BDNF antibody should have affected dendritic development of the GABAergic neurons that were contacted by GFP-negative terminals. The results shown in Figure 7 indicate, however, that the application of the antibody did not exert significant actions on the dendritic development of this group of GABAergic neurons (Fig. 7A–C, compare the third with leftmost columns).
In the present chimera culture preparations, the densities of GFP/BDNF-positive and -negative neurons were different. If the density of the former neurons was higher than that of the latter neurons, the number of GFP/BDNF-positive terminals might have exceeded that of GFP/BDNF-negative terminals. Consequently, GABAergic neurons that were contacted by the former terminals might have developed their dendrites better simply because of the greater number of contacts, irrespectively of BDNF transfer. To minimize this possibility, we set the ratio of the density of GFP/BDNF-positive and -negative cells at 1:20–40. In this condition, the number of GFP/BDNF-positive terminals was much less than that of negative terminals. Nevertheless, GFP/BDNF-positive terminals exerted the promoting action on dendritic growth of postsynaptic GABAergic neurons. Therefore, the possibility mentioned above seems unlikely in the present preparations.
Because GABAergic neurons do not have mRNA of BDNF as mentioned previously, the transfer of BDNF from excitatory neurons may be crucial for development of dendritic arborization of GABAergic neurons. In visual cortex in vivo, it is known that most, if not all, GABAergic neurons are contacted by axons of pyramidal neurons (Kisvarday, 1992; Johnson and Burkhalter, 1996). Also, it is well established that neocortical pyramidal neurons express BDNF (Yan et al., 1997; Friedman et al., 1998). Thus, it seems reasonable to assume that endogenous BDNF released from pyramidal cell axons acts on GABAergic neurons in visual cortex in vivo. However, previous studies in hippocampal neurons suggested that BDNF transferred retrogradely from excitatory neurons to GABAergic neurons may promote growth of the latter neurons (Marty et al., 1996, 1997). The present results have demonstrated, however, that the anterograde transfer of BDNF from excitatory neurons to GABAergic neurons plays such a role, although we cannot exclude any retrograde transfer. Anterogradely transferred BDNF, then, may activate the local protein synthesis that leads to growth of dendrites of postsynaptic neurons, as suggested (Aakalu et al., 2001; Takei et al., 2001).
In excitatory neurons, the transcellular transfer of BDNF seems to be not so important in the development of their dendrites, because the dendritic development was not correlated with the existence of presynaptic BDNF. This raises a possibility that the expression of functionally active or inactive BDNF receptors such as full-length and truncated TrkB in excitatory neurons might be different from that of inhibitory neurons. To our knowledge, there was no study in which the two types of receptors were differentially stained, except for a study in which full-length TrkB and pan TrkB including truncated type were differentially stained in the hippocampal formation of adult rats (Drake et al., 1999). In this study, no marked difference was reported in localization of immunoreactivity between full-length and pan TrkBs, although the intensity of reactivity was different at various subcellular sites. Thus, a reason why transferred BDNF was less important in dendritic development of excitatory neurons is not clear in the present study. Because excitatory neurons can produce BDNF by themselves, however, it is to be noted that endogenous BDNF may act on this type of neurons, in part at least, through an autocrine loop, as suggested previously (Kokaia et al., 1993; Miranda et al., 1993; Horch et al., 1999). From the present results, it is possible to suggest that the different actions of BDNF on excitatory and inhibitory synapses that were reported previously (Rutherford et al., 1998; Schinder et al., 2000) may be executed through the distinct pathways (i.e., anterograde, transsynaptic route to GABAergic neurons and autocrine route to glutamatergic neurons).
Because the release and transcellular transfer of BDNF are known to depend on neuronal activity (Goodman et al., 1996; Balkowiec and Katz, 2000; Hartmann et al., 2001; Kohara et al., 2001; Kojima et al., 2001; Lever et al., 2001; Gartner and Staiger, 2002), the transfer of BDNF to GABAergic neurons is assumed to be activity dependent. It is suggested that maturation of GABAergic neurons regulates the beginning of the critical period during which visual cortical neurons are highly sensitive to an alteration in inputs (Hensch et al., 1998). Furthermore, an overexpression of BDNF induces a precocious critical period in mouse visual cortex probably through its action on GABAergic neurons (Hanover et al., 1999; Huang et al., 1999). Thus, it is possible to suggest that the activity-dependent transfer of endogenous BDNF to GABAergic neurons may promote their maturation so as to play a role in the onset of the critical period of the developing visual cortex.
It was reported that another neurotrophin, NT-3, abolished the growth-promoting effect of BDNF on pyramidal neurons in slice culture preparations of visual cortex of young ferrets (McAllister et al., 1997). A question of whether NT-3 and BDNF have such an antagonist action also on GABAergic neurons should be addressed in a future study. Finally, it is to be noted that chimera culture preparations of neurons derived from different kinds of transgenic mice are a useful tool to elucidate the functional significance of a given bioactive molecule. Although conditional knock-out systems using Cre-loxP and specific promoter have been developed to reveal the local functions of target proteins (Tsien et al., 1996; Minichiello et al., 1999; Xu et al., 2000a,b; Iwasato et al., 2000), it is difficult to delete them from particular synapses of given neuronal circuits. In chimera cultures of neurons, in contrast, it is easy to make neuronal circuits in which particular molecules are lacking in visually identifiable circuits. In fact, we have successfully demonstrated that dendritic development of inhibitory cortical neurons is regulated by presynaptic BDNF.
Footnotes
This work was supported by a grant-in-aid for Scientific Research on Priority Areas–Advanced Brain Science Project from the Ministry of Education, Science, Sports and Culture of Japan (T.T.). We express many thanks to Drs.M. Okabe, R. Katoh-Semba, T. Kaneko, T. Torashima, Y. Hata, and K. Souya for providing GFP mice, anti-BDNF antibody, anti-glutaminase antibody, and technical advices of immunostaining, drawing neuronal dendrites, and confocal microscopy, respectively.
Correspondence should be addressed to Dr. Tadaharu Tsumoto, Division of Neurophysiology (D-14), Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita 565-0871, Japan. E-mail: ttsumoto@nphys.med.osakau.ac.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236123-09$15.00/0
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM ( 2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ ( 1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856–860. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM ( 2000) Activity-dependent release of endogenous brain-derived neurotrophic factor from sensory neurons detected by ELISA in situ J Neurosci 20: 7417–7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrup JT, Moorman JM, Newberry NR ( 1997) BDNF enhances neuronal growth and synaptic activity in hippocampal cell cultures. NeuroReport 8: 3791–3794. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA ( 2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14: 2920–2935. [DOI] [PubMed] [Google Scholar]
- Bolton MM, Pittman AJ, Lo DC ( 2000) Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci 20: 3221–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD ( 1997) Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 18: 257–267. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Maffei L, Domenici L ( 1996) The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci 8: 1190–1197. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL ( 1999) Ultrastructural localization of full-length trkB immunoreactivity in rat hipppcampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci 19: 8009–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ ( 1991) The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res 16: 215–226. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H ( 1990) Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron 5: 511–526. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA ( 2000) Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci 20: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA ( 1998) Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol 80: 3383–3386. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Black IB, Kaplan DR ( 1998) Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: an immnunocytochemical study. Neuroscience 84: 101–114. [DOI] [PubMed] [Google Scholar]
- Fujikawa N, Tominaga-Yoshino K, Okabe M, Ogura A ( 2000) Depolarization-dependent survival of cultured mouse cerebellar granule neurons is strain-restrained. Eur J Neurosci 12: 1838–1842. [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V ( 2002) Neurotrophin secretion from hippocampal neurons evoked by long-term potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA 99: 6386–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F ( 1996) Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci 7: 222–238. [DOI] [PubMed] [Google Scholar]
- Gorba T, Wahle P ( 1999) Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci 11: 1179–1190. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP ( 1999) Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci 19: RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V ( 2001) Postsynaptic release of brain-derived neurotrophic factor induced by high frequency synaptic stimulation. EMBO J 20: 5887–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF ( 1998) Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HE, Kruttgen A, Portbury SD, Katz LC ( 1999) Destablization of cortical dendrites and spines by BDNF. Neuron 23: 353–364. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S ( 1999) BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755. [DOI] [PubMed] [Google Scholar]
- Ip NY, Li Y, Yancopoulos GD, Lindsay R ( 1993) Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci 13: 3394–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami C, Mizuno K, Kohno T, Nakamura S ( 2000) Brain-derived neurotrophic factor requirement for activity-dependent maturation of glutamatergic synapse in developing mouse somatosensory cortex. Brain Res 857: 141–150. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich ME ( 1999) Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro J Neurosci 19: 5409–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S ( 2000) Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature 406: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RR, Burkhalter A ( 1996) Microcircuitry of forward and feedback connections within rat visual cortex. J Comp Neurol 368: 383–398. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Mizuno N ( 1988) Immunohistochemical study of glutaminase-containing neurons in the cerebral cortex and thalamus of the rat. J Comp Neurol 267: 590–602. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K ( 1997) Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem 69: 34–42. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Inaguma Y, Ichisaka S, Hata Y, Tsumoto T, Iwai M, Mikoshiba K, Kato K ( 2001) Induction of brain-derived neurotrophic factor by convulsive drugs in the rat brain: involvement of region-specific voltage-dependent calcium channels. J Neurochem 77: 71–83. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF ( 1992) GABAergic networks of basket cells in the visual cortex. Prog Brain Res 90: 385–405. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T ( 2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291: 2419–2423. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H ( 2001) Biological characterization and optical imaging of BDNF-GFP suggest an activity-dependent local release of BDNF in neurites of cultured hippocampal neurons. J Neurosci Res 64: 1–10. [DOI] [PubMed] [Google Scholar]
- Kokaia ZJ, Bengson M, Matsis M, Kokaia M, Persson H ( 1993) Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA 90: 6711–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M ( 2001) Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci 21: 4469–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Berninger B, Carroll P, Thoenen H ( 1996) GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron 16: 565–570. [DOI] [PubMed] [Google Scholar]
- Marty S, Berzaghi MD, Berninger B ( 1997) Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci 20: 198–202. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C ( 2000) Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci 20: 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC ( 1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15: 791–803. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC ( 1996) Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17: 1057–1064. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC ( 1997) Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18: 767–778. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC ( 1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22: 295–318. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfe D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R ( 1999) Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24: 401–414. [DOI] [PubMed] [Google Scholar]
- Miranda RJ, Sorabji F, Torrand-Allerand D ( 1993) Neuronal colocaliztion of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA 90: 6439–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Carnahan Y, Nawa H ( 1994) Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165: 243–256. [DOI] [PubMed] [Google Scholar]
- Nawa H, Bessho Y, Carnahan Y, Nakanishi S, Mizuno K ( 1993) Regulation of neuropeptide expression in cultured cerebral cortical neurons by brain-derived neurotrophic factor. J Neurochem 60: 772–775. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C ( 1995) BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci 7: 1527–1535. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y ( 1997) “Green mice” as a source of ubiquitous green cells. FEBS Lett 407: 313–319. [DOI] [PubMed] [Google Scholar]
- Poo MM ( 2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E ( 1996) Upregulation of BDNF mRNA in the barrel cortex of adult mice after sensory stimulation. J Neurosci 16: 4411–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, DeVan A, Lauer HM, Turrigiano GG ( 1997) Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci 17: 4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG ( 1998) BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21: 521–530. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Berninger M, Poo MM ( 2000) Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 25: 151–163. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Wetmore C, Olson L ( 1996) Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience 74: 161–183. [DOI] [PubMed] [Google Scholar]
- Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA ( 1997) Abnormal cerebellar development and foliation in BDNF-/- mice reveals a role for neurotrophins in CNS patterning. Neuron 19: 269–281. [DOI] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H ( 2001) Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes. J Biol Chem 276: 42818–42825. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N ( 1997) Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17: 2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H ( 1995) Neurotrophins and neuronal plasticity. Science 270: 593–598. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S ( 1996) Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87: 1317–1326. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RDG, Segal M ( 1998) Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci 18: 7256–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA ( 1999) Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci 19: 7889–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF ( 2000a) The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci 20: 6888–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang A, McConnell SK, Stryker MP, Reichardt LF ( 2000b) Cortical degeneration in the absence of neurotrophin signaling: Dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron 26: 233–245. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N ( 2002) Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 22: 7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA ( 1997) Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78: 431–448. [DOI] [PubMed] [Google Scholar]