Abstract
Stimulation of dopamine (DA) receptors in the striatum is essential for voluntary motor activity and for the generation of plasticity at corticostriatal synapses. In the present study, mice lacking DA D1 receptors have been used to investigate the involvement of the D1-like class (D1 and D5) of DA receptors in locomotion and corticostriatal long-term depression (LTD) and long-term potentiation (LTP). Our results suggest that D1 and D5 receptors exert distinct actions on both activity-dependent synaptic plasticity and spontaneous motor activity. Accordingly, the ablation of D1 receptors disrupted corticostriatal LTP, whereas pharmacological blockade of D5 receptors prevented LTD. On the other side, genetic ablation of D1 receptors increased locomotor activity, whereas the D1/D5 receptor antagonist SCH 23390 decreased motor activity in both control mice and mice lacking D1 receptors.
Endogenous DA stimulated D1 and D5 receptors in distinct subtypes of striatal neurons to induce, respectively, LTP and LTD. In control mice, in fact, LTP was blocked by inhibiting the D1–protein kinase A pathway in the recorded spiny neuron, whereas the striatal nitric oxide-producing interneuron was presumably the neuronal subtype stimulated by D5 receptors during the induction phase of LTD.
Understanding the role of DA receptors in striatal function is essential to gain insights into the neural bases of critical brain functions and of dramatic pathological conditions such as Parkinson's disease, schizophrenia, and drug addiction.
Keywords: basal ganglia, behavior, in vitro electrophysiology, interneurons, long-term depression, long-term potentiation, nitric oxide
Introduction
Dopamine (DA) signaling in the striatum plays a central role in a variety of motor and cognitive activities. Abnormal striatal DAergic transmission is involved in several neuropsychiatric diseases, such as parkinsonism, schizophrenia, and drug addiction (Berke and Hyman, 2000; Lewis and Lieberman, 2000; Obeso et al., 2000). Endogenous DA, released from midbrain DA neurons, modulates striatal function by interacting with DA receptors. Among the various subtypes of DA receptors, the D1-like family has been involved in the regulation of motor activity and in the expression of activity-dependent synaptic plasticity at corticostriatal synapses. Accordingly, pharmacological inhibition of D1like receptors reduces spontaneous motor activity (Meyer et al., 1993; Vallone et al., 2000) and prevents both long-term depression (LTD) and long-term potentiation (LTP) (Calabresi et al., 1992a, 2000; Centonze et al., 2001; Kerr and Wickens, 2001). To date, however, it is still unknown which member of the D1-like family of DA receptors (D1 or D5) mediates these actions of DA in the striatum. In this respect, both D1 and D5 receptors are expressed in the striatum (Bergson et al., 1995; Surmeier et al., 1996; Yan and Surmeier, 1997; Rivera et al., 2002a), are positive regulators of adenylyl cyclase activity (Stoof and Kebabian, 1981; Grandy et al., 1991; Sunahara et al., 1991; Tiberi et al., 1991; Vallone et al., 2000), and might be, in principle, equally important for both motor activity and ordered synaptic plasticity. However, the evidence that the quantitative ratios of these receptors differ significantly in the various neuronal populations of the striatum (Bergson et al., 1995; Surmeier et al., 1996; Yan and Surmeier, 1997; Rivera et al., 2002a) supports the concept that they serve distinct physiological roles.
In the present study, therefore, we used mice in which the expression of DA D1 receptors was selectively disrupted to analyze the involvement of D1 and D5 receptors in locomotor activity and corticostriatal LTD and LTP.
Materials and Methods
Male wild-type (WT) and D1 DA receptor knock-out (D1-/-) mice (Xu et al., 1994) (2–3 months of age) were used for all the experiments.
Locomotor activity. For locomotor activity studies, we used a multicage activity meter system (Digiscam Animal Activity Monitor; Columbus Instruments, Columbus, OH). This apparatus consisted of eight individual mice cages (21 × 21 × 30 cm), equipped with two sets (one above the other) of eight photocell beams per side spaced 2.5 cm to measure horizontal and vertical activity. WT and D1-/- mice were habituated to the cages for 3 consecutive days, and basal activity was recorded for 3 hr on the following day. The motor-suppressing effect of SCH 23390 (a D1/D5 receptor antagonist) at the doses of 30, 50, 100, and 300 μg/kg was tested in both mouse genotypes in 3 hr sessions. Each group was composed of eight animals, and each animal was used as its own control. All injections were administered intraperitoneally, in 1 ml/100 gm body weight/volume, and SCH 23390 (Tocris Cookson, Bristol, UK) was dissolved in saline.
Electrophysiology. Intracellular and whole-cell patch-clamp electrophysiological recordings were performed in vitro from brain slices. The preparation and maintenance of coronal corticostriatal slices have been described previously (Calabresi et al., 1997, 2000; Centonze et al., 2002b). Briefly, coronal slices (200–300 μm) were prepared from tissue blocks by use of a vibratome. The slices included the neostriatum and the neocortex. A single slice was transferred to a recording chamber and submerged in a continuously flowing Krebs' solution (32–33°C, 2–3 ml/min) gassed with a 95% O2 and 5% CO2 mixture. The composition of the solution was as follows (in mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, and 25 NaHCO3.
For intracellular recordings, sharp electrodes were used. They were filled with 2 m KCl (30–60 MΩ). An Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) was used for recordings in either current-clamp or voltage-clamp mode. For synaptic stimulation, bipolar electrodes were used. The stimulating electrode was located in either the cortical areas close to the recording electrode or the white matter between the cortex and the striatum. As the conditioning high-frequency stimulation (HFS), we used three trains (3 sec duration, 100 Hz frequency, at 20 sec intervals).
An Axopatch 1D amplifier (Axon Instruments) was used for whole-cell patch-clamp recordings. Currents steps were generated using pClamp version 8.0 software (Axon Instruments). The striatum could be readily identified under low-power magnification, whereas individual neurons were visualized in situ using a differential interference contrast (Nomarski) optical system. This used an Olympus Optical (Tokyo, Japan) BX50WI non-inverted microscope with 40× water immersion objective combined with an infrared filter, a monochrome CCD camera (model 4912; Cohu, San Diego, CA), and a personal computer-compatible system for analysis of images and contrast enhancement (WinVision 2000; Delta Sistemi, Rome, Italy). Recordings were made with borosilicate glass pipettes (1.8 mm outer diameter; 3–5 MΩ) containing the following (mm): 125 K +-gluconate, 10 NaCl, 1.0 CaCl2, 2.0 MgCl2, 1 BAPTA, 19 HEPES, 0.3 guanosine triphosphate, and 2.0 Mgadenosine triphosphate, adjusted to pH 7.3 with KOH.
Quantitative data on post-tetanic or post-treatment modifications are expressed as percentage of the controls, the latter representing the mean of responses recorded during a stable period (15–30 min) before tetanic stimulation or drug application. Each data point in the graphs in the figures was obtained from at least five single neurons. Student's t test and χ 2 test (for paired and unpaired observations) were used to compare the means, and ANOVA was used when multiple comparisons were made against a single control group.
Drugs were applied by dissolving them to the desired final concentration in saline and by switching the perfusion from control saline to drugcontaining saline. The drugs used were as follows: APV, CNQX, SCH 23390, S-nitroso-N-acetylpenicillamine (SNAP), 7-nitroindazole monosodium salt (7-NINA) (Tocris Cookson), DA, nifedipine, quinpirole, SKF 38393 (Sigma-RBI, St. Louis, MO), H89 (Calbiochem, La Jolla, CA), and zaprinast (Rhône-Poulenc Rorer, Dagenham, UK).
Immunocytochemistry. For immunocytochemical studies, WT (n = 4) and D1-/- mice (n = 5) were deeply anesthetized with sodium pentobarbital and perfused transcardially with 4% paraformaldehyde made in 0.1 m phosphate buffer, pH 7.4. Brains were removed, postfixed in the same solution for 2 hr at 4°C, cryoprotected with 30% sucrose in 0.1 m PBS, frozen in dry ice, and sectioned (25 μm thick) in a freezing microtome. Coronal sections through the striatum were collected and stored in PBS with 0.02% sodium azide at 4°C for immunocytochemistry. All studies were approved by the appropriate animal care committee.
To localize D5 DA receptors, a polyclonal rabbit antibody against the D5 receptor subtype (1:2000 dilution) was developed and characterized at the Cajal Institute (Rivera et al., 2002a; Centonze et al., 2003). Somatostatin (SS) interneurons in the striatum correspond to neurons that express nitric oxide synthase (NOS) (Vincent et al., 1983). Therefore, to localize NOS-positive neurons, we used a monoclonal rat antiserum against somatostatin (1:100 dilution; provided by Drs. J. Rodrigo and A. C. Cuello, Cajal Institute, Consejo Superior de Investigaciones Científicas, Madrid, Spain) (Milstein and Cuello, 1983). Primary antibodies were diluted in 0.1 m PBS, with 0.2% Triton X-100 (PBS-TX), 1% bovine serum albumin (BSA), and 0.1% sodium azide.
Free-floating double-labeling immunocytochemistry was performed in sections from WT and D1-/- mice (Rivera et al., 2002a,b). Briefly, nonspecific binding sites in the sections were blocked with 5% BSA in PBS-TX for 30 min. Sections were washed and incubated with our rabbit polyclonal anti-D5 antiserum and with the monoclonal anti-SS antibody for 48 hr at 4°C. Sections were then incubated with two secondary antibodies, Alexa 598-conjugated goat anti-rabbit (red; Molecular Probes, Eugene, OR) and with goat anti-rat CY2 (green; Amersham Biosciences, Buckinghamshire, UK) diluted 1:500 in PBS-TX for 1 hr in the dark. In each experiment, the specificity of staining was monitored by incubating control sections with one primary antibody and then with both secondary antibodies to detect any cross-reaction between them. Sections were mounted in PBS/glycerol (1:1) and 2% 1,4-diazabicyclo-[2.2.2]octane (Sigma-Aldrich Química SA, Madrid, Spain), coverslipped, and observed by laser confocal microscopy (TCS-NT; Leica, Wetzlar, Germany).
Results
Effects of D1-like receptor blockade on locomotor activity of WT and D1-/- mice
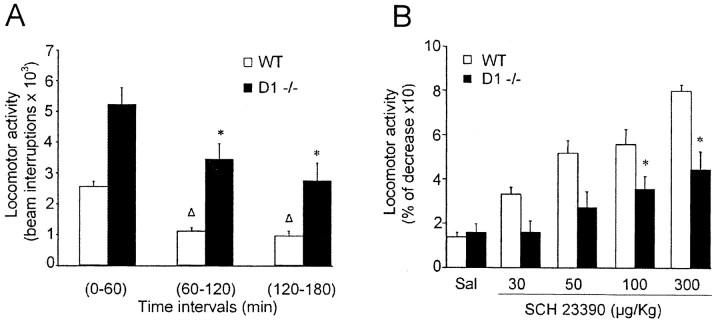
In the absence of any pharmacological challenge, D1-/- mice were significantly more active than their WT counterparts when tested for locomotor activity. According to a previous report (Xu et al., 1994), D1-/- mice interrupted photocell beams more frequently than control mice throughout the whole period of observation (Fig. 1A).
Figure 1.
Locomotor activity of WT and D1-/- mice. A, Histogram illustrating the basal locomotion activity of WT and D1-/- mice for a 3 hr period in intervals of 60 min. Note that, in all of the three intervals, D1-/- mice were significantly more active than their WT counterparts. Δ and * indicate a significant reduction of photocell beam interruptions compared with the first hour interval for WT and D1-/- mice, respectively. Δp < 0.01; *p < 0.05. B, Effects of SCH 23390 (30, 50, 100, or 300 mg/kg, i.p.) on locomotor activity recorded from WT and D1-/- mice for 60 min after treatment. Each bar graph represents mean ± SEM of cumulative photocell beam interruptions for the first 60 min after treatment; n = 8 mice. *p < 0.05, indicates a significant reduction of photocell beam interruptions induced by SCH 23390 when compared with basal activity (Student's t test).
To extend our investigation to the potential role of D5 receptors on motor activity, we also analyzed the motor effects of SCH 23390, a D1/D5 receptor antagonist, in both WT and D1-/- mice. In WT mice, administration of SCH 23390 strongly suppressed motor activity, as indicated by a dose-dependent reduction of photocell beam interruptions. The percentage of reduction after 60 min of treatment was ∼30% with the lowest dose used (30 μg/kg) and ∼80% with the highest dose (300 μg/kg). Although the pharmacological effect of SCH 23390 in D1-/- mice was less pronounced, also in this genotype SCH 23390 induced a reduction of locomotor activity (35 and 45% for 100 and 300 μg/kg, respectively), suggesting a facilitatory action of D5 receptors on motor activity (Fig. 1B).
LTD and LTP in WT and D1-/- mice
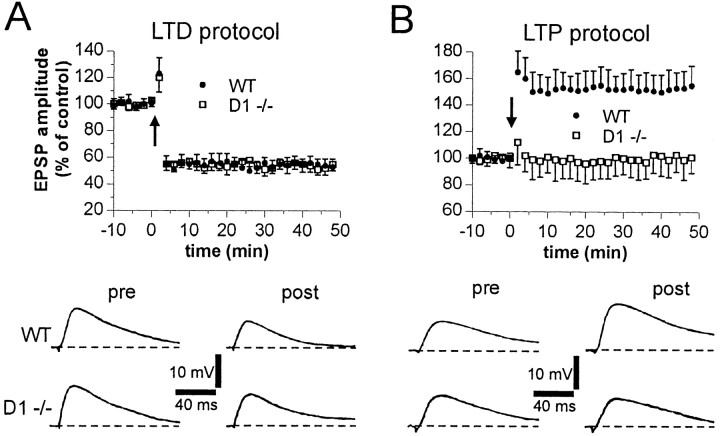
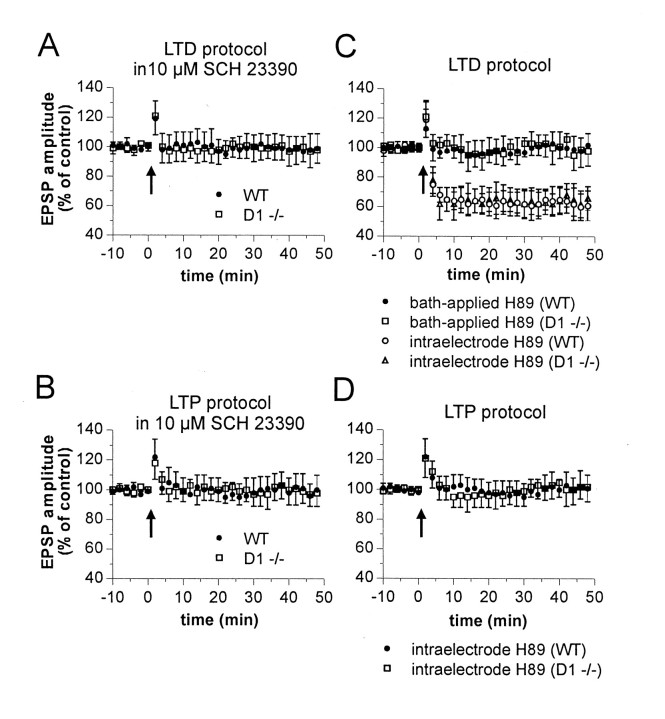
Intracellular recordings from electrophysiologically identified striatal spiny neurons (Calabresi et al., 1992a, 1997, 2000; Centonze et al., 1999; Kerr and Wickens, 2001) were performed from WT and D1-/- slices during the activation of corticostriatal terminals. HFS of these terminals was able to produce long-term changes (LTD or LTP) in EPSP amplitude of control mice. As reported previously (Calabresi et al., 1997, 2000), HFS protocol was delivered in the presence of 1.2 mm external magnesium to optimize the appearance of LTD, whereas LTP induction was favored by the removal of this ion from the bathing solution. Both forms of synaptic plasticity were present in WT mice (n = 9 for LTD; n = 10 for LTP). Conversely, whereas corticostriatal LTD was still present in slices prepared from D1-/- mice (n = 11; p < 0.001), LTP was absent in these mutants (n = 13; p > 0.05) (Fig. 2). These data indicate that D1 receptors are critically important for LTP induction but are unnecessary for LTD. LTD induction, in fact, rather required D5 receptor stimulation, because this form of synaptic plasticity was blocked in D1-/- mice (n = 6), as well as in their WT counterparts (n = 6), by preincubation (7–10 min) with the D1 and D5 receptor antagonist SCH 23390 (10 μm). As reported previously (Calabresi et al., 2000), SCH 23390 was also able to fully prevent corticostriatal LTP in WT mice (n = 5) (Fig. 3A,B).
Figure 2.
Role of DA D1 receptors in the expression of HFS-induced corticostriatal LTD and LTP. The graphs summarize the results from intracellular experiments performed in the presence (A) and absence (B) of external magnesium from WT (filled circles) and D1-/- (open squares) slices. The arrows indicate when HFS was delivered. The bottom parts of both A and B show EPSPs recorded in four neurons from WT and D1-/- slices immediately before (pre) and 20 min after (post) HFS. RMPs were as follows: -86 mV (A; WT and D1-/-) and -84 mV (B; WT and D1-/-).
Figure 3.
Involvement of the D1–PKA pathway in corticostriatal LTD and LTP of WT and D1-/- mice. The graphs summarize the results obtained from intracellular experiments performed in WT and D1-/- mice in the presence (A, C) and absence (B, D) of external magnesium. In A and B, 10 μm SCH 23390 was applied at least 10 min before HFS (arrows). C, D, Intracellular application of H89 (100 μm) prevented corticostriatal LTP but not LTD in both WT and D1 -/- mice. Corticostriatal LTD, conversely, was blocked in the two experimental groups only when this PKA inhibitor (10 μm, 10 min) was applied in the bathing solution.
The number of striatal neurons expressing the NMDAR1 subunit of NMDA receptors is decreased in D1-/- mice (Ariano et al., 1998), and the stimulation of D1 receptors has been found to potentiate NMDA receptor responsiveness in these cells (Cepeda et al., 1998). These findings might well account for the loss of NMDA receptor-dependent LTP seen in D1-/- mice. Thus, to address this issue, we measured the amplitude, half-decay time, and duration of the membrane depolarizations induced by HFS in WT and D1-/- mice during the induction protocol of corticostriatal LTP. These parameters did not significantly differ in the two classes of animals. The amplitude was 42 ± 6mVinWT(n = 10) and 40 ± 5 mV in D1-/- mice (n = 13; p > 0.05). The half-decay time was 3.0 ± 0.4 sec in WT (n = 10) and 3.0 ± 0.6 sec in mutants (n = 13; p > 0.05). The duration was 8.1 ± 2 sec in WT (n = 10) and 8.2 ± 3 sec in mutants (n = 13; p > 0.05) (data not shown). In an additional set of experiments, we investigated the effects of the L-type calcium channel blocker nifedipine (10 μm, 10 min; n = 5) on the expression of LTP in WT mice. Although this agent has been reported to inhibit the D1 receptormediated potentiation of NMDA currents (Cepeda et al., 1998), it failed to prevent corticostriatal LTP (p < 0.01) (data not shown).
Effects of protein kinase A inhibition in corticostriatal LTP and LTD of WT and D1-/- mice
Activation of both D1 and D5 receptors results in the stimulation of protein kinase A (PKA) activity. Therefore, we tested the effects of the selective PKA inhibitor H89 on both corticostriatal LTD and LTP. In WT mice, the intracellular injection of H89 (100 μm) fully blocked LTP (n = 6; p > 0.05) but failed to prevent HFSinduced LTD in both WT mice (n = 5; p < 0.01) and D1-/- animals (n = 5; p < 0.01). In contrast, when H89 (10 μm; n = 4 for each experimental condition) was added to the perfusing solution, LTP of WT mice and LTD of both WT and D1-/- mice were prevented (p > 0.05 for both conditions) (Fig. 3C,D). These findings confirm the requirement of PKA stimulation for corticostriatal LTP and LTD in both WT and D1-/- mice and indicate that LTP induction required the stimulation of the D1–PKA pathway in the projection neuron, whereas LTD depended on the activation of D5–PKA signaling in a neuronal subtype other than the recorded spiny neuron.
Expression of D5 receptors in NOS-positive striatal neurons
The SS- and NOS-positive interneuron is a major candidate for the action of DA during the induction phase of corticostriatal LTD. Physiological studies, in fact, have suggested that intrastriatal release of nitric oxide (NO) is a critical factor for LTD induction, by promoting through a feedforward mechanism the stimulation of the cGMP–PKG pathway in spiny projection neurons (Calabresi et al., 1999). In line with this idea, DA D1-like receptor stimulation depolarizes putative NO-producing interneurons (Centonze et al., 2002a) and increases striatal levels of cGMP (Altar et al., 1990; Morris et al., 1997). The inability of D1 receptor disruption in preventing LTD, therefore, might depend on a normal sensitivity to DA of D1-/- SS-positive interneurons.
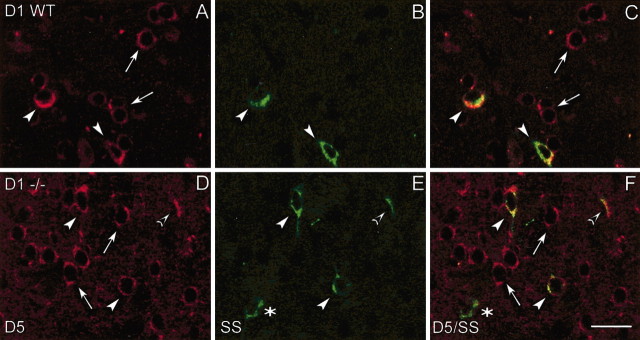
To address this issue, we performed double-labeling immunofluorescence experiments in both WT and D1-/- mice to investigate whether SS-positive interneurons (which correspond to NOS-positive neurons) (Vincent et al., 1983) of the striatum expressed D5 receptors. Although a small population of SS-positive neurons lacked D5 receptors (Fig. 4B,C, asterisks), the large majority of both WT and D1-/- SS-positive cells expressed D5 receptors (Fig. 4C,F, arrowheads). These receptors were also present in other medium- and large-sized neurons on the striatum, as shown previously (Rivera et al., 2002a).
Figure 4.
Confocal laser photomicrographs illustrating the colocalization of D5 receptors with SSin WT and D1-/- mice. A, D, Striatal neurons that express D5 receptors in WT and D1-/- mice. B, E, Striatal interneurons containing SS in WT and D1-/- mice. C, F, Paired images show double-labeled cells with D5 /SS in WT and D1-/- mice. White arrows indicate single-labeled cells with D5 receptor antiserum, asterisks indicate single-labeled cells with SS antibody, and arrowheads indicate double-labeled D5 /SS cells in the corresponding images. Note that D5 receptors are often expressed in SS-positive neurons, but not all SS-positive neurons contain D5 receptors; see asterisks in B and C. Same observation was made in wild-type mice. Scale bar, 25 μm.
Effects of DA receptor stimulation in low-threshold spike interneurons of D1-/- mice
SS- and NOS-positive interneurons correspond to the electrophysiologically identified low-threshold spike (LTS) cells (Kawaguchi, 1993; Kawaguchi et al., 1995). Thus, we recorded from these neurons in striatal slices of D1-/- mice. The electrophysiological features of LTS interneurons of D1-/- mice were unique among striatal cells and consisted essentially in low resting membrane potential (-58 ± 2 mV), exceptionally high input resistance (520 ± 166 MΩ, measured with patch-clamp electrodes), and in the ability to generate, in addition to fast spikes, large plateau depolarizations and low-threshold calcium spikes during depolarizing current pulses and immediately after hyperpolarization (Fig. 5A). These features did not significantly differ from those reported previously (Kawaguchi, 1993; Koos and Tepper, 1999; Centonze et al., 2002a).
Figure 5.
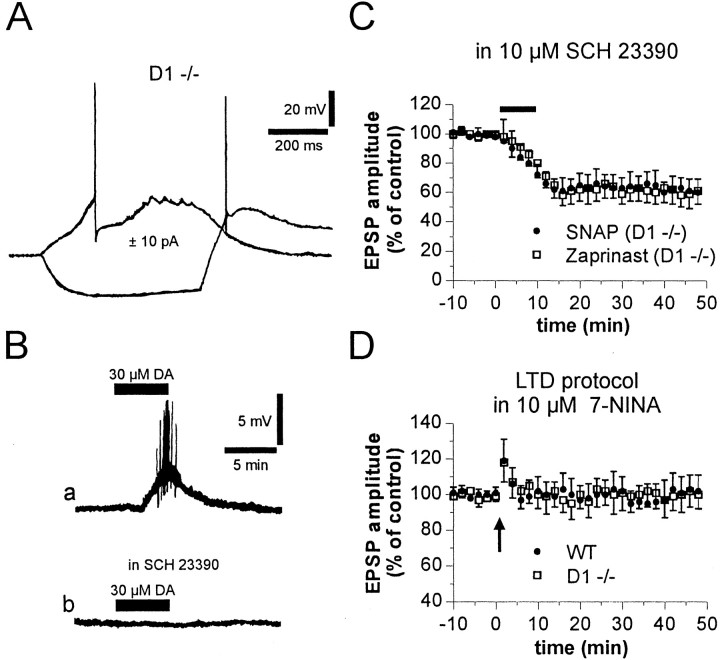
Involvement of NO-producing cells in striatal LTD recorded in D1-/- mice. A, Electrophysiological responses of a D1 -/- LTS neuron to the injection of depolarizing and hyperpolarizing current steps (RMP, -59 mV). B, Bath application of 30 μm DA induced in a D1-/- LTS cell a membrane depolarization, which led to firing discharge (a). In the same cell, the excitatory effect of DA, applied at the same concentration 15 min after the washout, was fullysuppressedby5minpreincubationwith10μmSCH23390(b). RMP,-61mV.C, Pharmacological blockade of D1-like receptors by 10μm SCH 23390 failed to prevent corticostriatal LTD induced in D1 -/- mice by bath application (7 min) of the NO donor SNAP (100μm) or the cGMP phosphodiesteraseinhibitorzaprinast(15μm).D, Theneuronalnitricoxidesynthaseinhibitor7-NINA(7–10min at 10μm) prevented corticostriatal LTD in both WT and D1-/- mice.
In all tested D1-/- LTS cells (three of three), application of 30 μm DA (4–8 min) elicited a slow and reversible membrane depolarization, which was sufficient to initiate a train of action potentials. This excitatory effect was likely mediated by D5 receptors, because it was mimicked by the D1-like receptor agonist SKF 38393 (10 μm; n = 3; p < 0.01) but not by the D2-like receptor agonist quinpirole (10 μm; n = 3; p > 0.01) and was blocked by 10 μm SCH 23390 (Fig. 5B).
Role of the NO–cGMP pathway in corticostriatal LTD recorded from D1-/- mice
The idea that the integrity of striatal LTD in D1-/- mice depends on an intact response to DA of NO-producing interneurons predicts that, also in D1-/- mice, striatal LTD requires NO–cGMP pathway stimulation downstream D5 receptors. In D1-/- mice, therefore, we studied the effect of the NO donor SNAP (100 μm) and the selective cGMP phosphodiesterase inhibitor zaprinast (15 μm) on corticostriatal EPSP amplitude recorded in the presence of 10 μm SCH 23390. As shown in Figure 5C, incubation (7 min) with SNAP (n = 4) or zaprinast (n = 6) induced corticostriatal LTD in D1-/- slices bathed with SCH 23390 (p < 0.01 for both experimental conditions). In addition, as reported in control animals (Calabresi et al., 1999), also in D1-/- mice HFS-induced LTD was fully prevented by the NOS inhibitor 7-NINA (10 μm; n = 5; p > 0.05) (Fig. 5D).
Discussion
In the present study, we provided evidence that the two members of D1-like family of DA receptors exert distinct roles in motor activity and corticostriatal synaptic plasticity. In particular, whereas genetic ablation of D1 receptors blocked LTP induction and increased locomotor activity, pharmacological blockade of D1/D5 receptors by SCH 23390 prevented LTD and inhibited motor activity in both WT and D1-/- mice.
Complex regulation of locomotor activity by D1 and D5 receptors
In D1-/- mice, locomotion was increased, whereas SCH 23390, aD1 and D5 receptor blocker, caused in the two groups of animals suppression of motor activity. SCH 23390, however, was less effective and less potent in D1-lacking animals than in control mice in inhibiting motor activity, suggesting that D1 receptors also exert a permissive action on D5 receptor function. To explain the dual role of D1 receptors in motor control, it can be speculated that D1 receptors synergize with D5 receptors when the two receptors are activated in the same cellular subtype, but they oppose to each other when activated in distinct neuronal populations. This hypothesis is supported by the following data. First, both D1 and D5 receptors are positive regulators of cAMP levels (Grandy et al., 1991; Sunahara et al., 1991; Tiberi et al., 1991; Vallone et al., 2000) and therefore cooperate in triggering common cellular events when coexpressed. In this respect, D5 receptors are also expressed in medium spiny neurons of the striatum (Rivera et al., 2002a; present study), a neuronal subtype particularly enriched in D1 receptors (Gerfen et al., 1990; Le Moine et al., 1991; Surmeier et al., 1992, 1996; Aizman et al., 2000). Second, D1 and D5 receptors are primarily expressed in different striatal cell populations (Bergson et al., 1995; Surmeier et al., 1996; Yan and Surmeier, 1997; Rivera et al., 2002a), suggesting that they can also exert distinct physiological actions. Noticeably, NOS-positive neurons, which express D5 receptors and are stimulated by D1-like receptor agonists, do not have D1 receptors (Le Moine et al., 1991; Kawaguchi et al., 1995; Rivera et al., 2002a) and cause longterm inhibition of the excitability of striatal spiny neurons, thereby contrasting the direct LTP-favoring effects of D1 receptors on these cells. In this line, DAergic activation of NOS-positive cells causes a long-term inhibition of medium spiny neurons detected by a reduction of c-Fos expression in striatal matrix neurons (Moratalla et al., 1996a,b).
Other interpretations of our results are also possible. Accordingly, the postulated inhibitory role of D1 receptors only emerges from the increased motor activity seen in D1-/- mice, although it could in principle result from a variety of developmental or compensatory changes. In addition, the evidence that the D1/D5 receptor antagonist SCH 23390 inhibited locomotor activity in both WT and D1-/- and that this action was more pronounced in WT might also suggest that D1 receptors exert only stimulatory actions on locomotor activity by synergizing with D5 receptors. The use of the recently generated mice lacking D5 receptors (Holmes et al., 2001; Hollon et al., 2002) could help to clarify this important issue.
Complex regulatory role of DA receptors in corticostriatal synaptic plasticity
Repetitive stimulation of corticostriatal pathway can induce either LTD or LTP both in vivo (Charpier and Deniau, 1997; Reynolds and Wickens, 2000) and in vitro (Calabresi et al., 1992a,b; Dos Santos Villar and Walsh, 1999; Partridge et al., 2000). Persistent changes in synaptic strength in the striatum are considered as neural correlates of specific motor abilities, although more recently have been involved in several other aspects of brain activity, such as reward-related learning (Berke and Hyman, 2000; Reynolds et al., 2001), maturation of neural circuitry during development (Choi and Lovinger, 1997), and drug addiction (Berke and Hyman, 2000; Hyman and Malenka, 2001; Nestler, 2001).
A solid achievement in the field of basal ganglia physiology is the requirement of DA for corticostriatal synaptic plasticity. The unavailability of pharmacological agents able to target selectively specific members of the two subfamilies of DA receptors, however, makes essential the use of mutant mice to identify the receptor subtypes involved in these physiological activities. On the basis of this consideration, mice lacking DA D2 receptors have been used previously for electrophysiological experiments in vitro. These experiments allowed to identify the D2 receptor as the specific member of the D2-like subfamily involved in the facilitatory effect on LTD and in the restraining action on LTP (Calabresi et al., 1997). Together, these results with the present data and with the observations that both D1- and D2-like antagonists block locomotor activity (Vallone et al., 2000) and striatal LTD (Calabresi et al., 1992a,c), it is possible to conclude that D5 and D2 receptors cooperate functionally to facilitate motor activity and striatal LTD, whereas D1 and D2 receptors are the receptor subtypes mainly involved in striatal LTP and, possibly, motor inhibition. It is also possible, however, that the role of D5 receptors in LTD only emerges as a compensatory mechanism after D1 receptor ablation and that it is negligible in control conditions. Again, the use of D5 receptor-lacking mice will prove useful to clarify this issue.
Concluding remarks
Current models of the basal ganglia organization propose that the striatum is an important component of motor, cognitive, and limbic circuits. This nucleus takes part in several brain activities by processing the flow of information arising from different neocortical areas and projecting to the thalamus (Bergman et al., 1998). DA plays a crucial role in these processes by affecting the activity of striatal cells through multiple mechanisms. Interestingly, a complex interplay between different subtypes of DA receptors is required for both striatal motor control and synaptic plasticity, possibly involving different subpopulations of striatal neurons.
Investigating the receptor and cellular mechanisms involved in striatal motor control and synaptic plasticity is an essential requirement to understand the neural bases of critical brain functions and of dramatic pathological conditions, such as Parkinson's disease, schizophrenia, and drug addiction.
Footnotes
Correspondence should be addressed to either of the following: Paolo Calabresi, Clinica Neurologica, Dipartimento di Neuroscienze, Università di Tor Vergata, Via Montpellier 1, 00133 Rome, Italy, E-mail: calabre@uniroma2.it; or Rosario Moratalla, Instituto Cajal, Consejo Superior de Investigaciones Científicas, Avda Dr. Arce 37, 28002 Madrid, Spain, E-mail: moratalla@cajal.csic.es
Copyright © 2003 Society for Neuroscience 0270-6474/03/238506-07$15.00/0
This work has been supported by Ministerio de Ciencia y Tecnologìa Grant SAF200-0122, Plan National Sobre Drogas, and Fundación La Caixa (Spain) (R.M.) and Consiglio Nazionale delle Ricerche and Ministero della Salute, Progetto Finalizzato Schizofrenia (Italy) (P.C.). We thank Sonia Villa for her help with the behavioral experiments.
R.M. and P.C. contributed equally to this work.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A ( 2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3: 226-230. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boyar WC, Kim HS ( 1990) Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol 181: 17-21. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Drago J, Sibley DR, Levine MS ( 1998) Striatal excitatory amino acid receptor subunit expression in the D1A-dopamine receptordeficient mouse. Dev Neurosci 20: 237-241. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E ( 1998) Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci 21: 32-38. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS ( 1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15: 7821-7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE ( 2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515-532. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G ( 1992a) Long-term depression in the striatum: physiological and pharmacological characterization. J Neurosci 12: 4224-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G ( 1992b) Long-term potentiation in the striatum is unmasked by removing the voltage-dependent blockade of NMDA receptor channel. Eur J Neurosci 4: 929-935. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Mercuri NB, Bernardi G ( 1992c) Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett 142: 95-99. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik J-H, Centonze D, Mercuri NB, Bernardi G, Borrelli E ( 1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci 17: 4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G ( 1999) A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci 19: 2489-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P ( 2000) Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci 20: 8443-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G ( 1999) Unilateral dopamine denervation blocks corticostriatal LTP. J Neurophysiol 82: 3575-3579. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P ( 2001) Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci 13: 1071-1077. [DOI] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P ( 2002a) Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci 15: 2049-2052. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Baunez C, Borrelli E, Pisani A, Bernardi G, Calabresi P ( 2002b) Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology 26: 164-175. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martín AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P ( 2003) Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci 23: 6245-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS ( 1998) Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol 79: 82-94. [DOI] [PubMed] [Google Scholar]
- Charpier S, Deniau JM ( 1997) In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA 94: 7036-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM ( 1997) Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA 94: 2665-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Villar F, Walsh JP ( 1999) Modulation of long-term synaptic plasticity at excitatory striatal synapses. Neuroscience 90: 1031-1041. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma Jr FJ, Sibley DR ( 1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429-1432. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang YA, Bourier C, Zhou QY, Johnson RA, Allen L, Buck K, Bunzow JR, Salon J, Civelli O ( 1991) Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci USA 88: 9175-9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young III WS, Westphal H, Jose PA, Sibley DR ( 2002) Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci 22: 10801-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN ( 2001) Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci 115: 1129-1144. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC ( 2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2: 695-703. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y ( 1993) Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC ( 1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18: 527-535. [DOI] [PubMed] [Google Scholar]
- Kerr JND, Wickens JR ( 2001) Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol 85: 117-124. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM ( 1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467-472. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Bloch B ( 1991) Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA 88: 4205-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA ( 2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325-334. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Cottrell GA, Van Hartesveldt C, Potter TJ ( 1993) Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats. Pharmacol Biochem Behav 44: 429-432. [DOI] [PubMed] [Google Scholar]
- Milstein C, Cuello AC ( 1983) Hybrid hybridomas and their use in immunohistochemistry. Nature 305: 537-540. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Xu M, Tonegawa S, Graybiel AM ( 1996a) Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA 93: 14928-14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM ( 1996b) Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17: 147-156. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM ( 1997) Dunamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacology 36: 1589-1599. [DOI] [PubMed] [Google Scholar]
- Nestler EJ ( 2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119-128. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW ( 2000) Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci 23: S8-S19. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang K-C, Lovinger DM ( 2000) Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol 84: 1422-1429. [DOI] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR ( 2000) Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience 99: 199-203. [DOI] [PubMed] [Google Scholar]
- Reynolds JNJ, Hyland BL, Wickens JR ( 2001) A cellular mechanism of reward-related learning. Nature 413: 67-70. [DOI] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvàez JA, de la Calle A, Moratalla R ( 2002a) Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci 16: 2049-2058. [DOI] [PubMed] [Google Scholar]
- Rivera A, Cuellar B, De la Calle A, Moratalla R ( 2002b) Differential distribution of dopamine D4 receptors in the striosome/matrix compartments of the striatum. J Neurochem 80: 219-229. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Guan HC, OìDowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HHM, Niznik HB ( 1991) Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350: 614-619. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Stefani A, Kitai ST ( 1992) Dopamine receptor subtypes co-localize in rat striatonigral neurons. Proc Natl Acad Sci USA 89: 10178-10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z ( 1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16: 6579-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW ( 1981) Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature 294: 366-368. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Gonidot N, Bertrand L, Yang-Feng Jr TL, Fremeau RT, Caron MG ( 1991) Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA 88: 7491-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Picetti P, Borrelli E ( 2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24: 125-132. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Johansson O, Hokfelt T, Skirboll L, Elde RP, Terenius L, Kimmel J, Goldstein M ( 1983) NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin-and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol 217: 252-263. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S ( 1994) Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79: 729-742. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ ( 1997) D5 dopamine receptors enhance Zn 2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19: 1115-1126. [DOI] [PubMed] [Google Scholar]