Abstract
Ideally, learning-related changes should be investigated while they occur in vivo, but physical accessibility and stability limit intracellular studies. Experiments with insects and crabs demonstrate their remarkable capacity to learn and memorize visual features. However, the location and physiology of individual neurons underlying these processes is unknown. A recently developed crab preparation allows stable intracellular recordings from the optic ganglia to be performed in the intact animal during learning. In the crab Chasmagnathus, a visual danger stimulus (VDS) elicits animal escape, which declines after a few stimulus presentations. The long-lasting retention of this decrement is mediated by an association between contextual cues of the training site and the VDS, therefore, called context-signal memory (CSM). CSM is achieved only by spaced training. Massed training, on the contrary, produces a decline of the escape response that is short lasting and, because it is context independent, is called signal memory (SM). Here, we show that movement detector neurons (MDNs) from the lobula (third optic ganglion) of the crab modify their response to the VDS during visual learning. These modifications strikingly correlate with the rate of acquisition and with the duration of retention of both CSM and SM. Long-term CSM is detectable from the response of the neuron 1 d after training. In contrast to MDNs, identified neurons from the medulla (second optic ganglion) show no changes. Our results indicate that visual memory in the crab, and possibly other arthropods, including insects, is accounted for by functional changes occurring in neurons originating in the optic lobes.
Keywords: visual learning and memory, in vivo intracellular recordings, insects, crustacean, escape response, Chasmagnathus
Introduction
Adaptive behavior in a cluttered visual environment requires that arthropods, like vertebrates, extract information from the visual scene. Behavioral experiments suggest that principles similar to those occurring in mammals underlie the analysis of visual patterns in arthropods (Srinivasan et al., 1993; Mizunami et al., 1998), whereas electrophysiological recordings reveal the existence of neurons that are, in many respects, functionally similar to those of the mammalian cortex (O'Carroll, 1993; Glantz, 1998). Recent experiments with insects (Judd and Collet, 1998; Liu et al., 1999; Giurfa et al., 2001; Tang and Guo, 2001) and crabs (Tomsic et al., 1998; Pedreira et al., 2002; Pedreira and Maldonado, 2003) revealed their remarkable capacity to categorize, learn, and memorize visual features, but individual neurons subserving these abilities have never been identified. The visual neuropils of insects and crustaceans are homologous, comprising three ganglia, each with parallel retinotopic organization (Strausfeld and Naässel, 1981). Because of the accessibility to these ganglia and the mechanical stability provided by the hard carapace, semiterrestrial crabs offer special advantages for recording intracellularly in the intact animal (Berón de Astrada et al., 2001). Semiterrestrial crabs are highly visual and reactive creatures with behaviors that are easy to evoke and quantify in the laboratory. In the shore crab Chasmagnathus granulatus, the sudden movement of a visual danger stimulus (VDS) above the animal elicits a strong escape reaction. But a few presentations of this stimulus at spaced intervals (spaced training) result in an enduring suppression of the escape response that is specific for the training stimulus (Lozada et al., 1990). The adaptive value of this learning ability has been discussed previously (Tomsic et al., 1993). Although at the beginning of our studies with Chasmagnathus we termed this phenomenon “habituation” (Lozada et al., 1990; Tomsic et al., 1993), later investigations demonstrated that it was a rather more complex form of memory. In fact, the long-term suppression of the escape response is exhibited only if the animal is tested in the same environment where it had been trained (i.e., in the context where the stimulus proved to be innocuous) (Tomsic et al., 1998). This and other results (Tomsic et al., 1998; Pedreira et al., 2002; Pedreira and Maldonado, 2003) indicated that the memory produced by spaced training is determined by an association between the VDS and the context; therefore, we call this associative memory the context-signal memory (CSM). Conversely, few presentations of the VDS at short intervals (massed training) result in a rapid suppression of the escape response that is only short lasting. This type of memory, which is context independent, is called signal memory (SM) (for review, see Maldonado, 2002).
In the crayfish, movement detector fibers appear to be the only cell type emerging from the eyestalk with the appropriate response properties to trigger a defense reflex (Glantz, 1974a), and, in addition, they modify their sensitivity during repeated presentation of a moving object (Glantz, 1974b). Therefore, these cells are the most attractive candidates to investigate the neural processes underlying the escape response of Chasmagnathus. Itis noteworthy that, until recently, movement detector neurons (MDNs) of crustaceans have not been investigated using intracellular recordings, and, therefore, their location and anatomy were unknown. A recent morphological and physiological characterization of a group of MDNs from the lobula (third optic neuropil) of Chasmagnathus demonstrates their selective responses to stimuli moving above the animal (Berón de Astrada and Tomsic, 2002). Here, we investigate the relationship of these cells to the visually elicited escape response and to the two types of memory described above. Worthy of note, the study was performed by recording intracellularly the neuronal activity in a living animal, at the time it was actually learning.
Materials and Methods
Animals. Animals were adult male C. granulatus crabs (2.7–3.0 cm across the carapace; weight, ∼17.0 gm) collected in the rías (narrow coastal inlets) of San Clemente del Tuyú, Argentina, and transported to the laboratory, where they were lodged in plastic tanks (35 × 48 × 27 cm) filled to a 2 cm depth with diluted marine water to a density of 20 crabs per tank. Water used in tanks and other containers during the experiments was prepared using hw-Marinex (Winex, Hamburg, Germany; salinity, 10–14 ‰; pH of 7.4–7.6) and maintained within a range of 22–24°C. The holding and experimental rooms were maintained on a 12 hr light/dark cycle (lights on 7:00 A.M. to 7:00 P.M.). Animals were fed rabbit pellets (Nutrientes S.A., Buenos Aires, Argentina) every 3 d, and the water was changed after feeding. Experiments were performed within the first 2 weeks after the animal's arrival, throughout the entire year, and between 8:00 A.M. and 7:00 P.M. Each crab was used in only one experiment. Experimental procedures are in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
In most crustaceans, the eyestalks contain the retina and five neuropils. These have been traditionally called the lamina, external medulla, internal medulla, terminal medulla, and hemiellipsoid body. The first three neuropils laying behind the retina (lamina, external medulla, and internal medulla) are considered by Strausfeld and Nässel (1981) to be clearly homologous with the lamina, medulla, and lobula of the insects (Osorio and Bacon, 1994; Strausfeld, 1998), and they suggest that these terms be used as well for the crustacean neuropils. They use the term “lateral protocerebrum” to refer collectively to the terminal medulla and hemiellipsoid body complex. This allows the distinction between the first three retinotopic neuropils and the more proximal protocerebral regions and hemiellipsoid body complex. We have adopted this terminology.
Visual stimulus and recording procedures. Behavioral and electrophysiological experiments were both performed inside a Faraday cage completely covered to prevent outside visual stimuli from reaching the animal. The ceiling and walls of the cage were painted uniform white. The surface of the vibration-damped table (Technical Manufacturing Corporation, Peabody, MA) was painted black. Illumination within the cage was provided by a lamp oriented toward the roof of the cage, providing light on the compound eye with an intensity of 200 mW/m 2. The VDS (Fig. 1 A, B) consisted of the horizontal displacement of a black rectangular screen (7 × 25 cm), driven by a motor at an angular velocity of 82°/sec. The screen was located 25 cm above the crab, and its movement was controlled from outside the cage by a computer. The motion cycle, which consisted of a 90° clockwise and counterclockwise excursion from the starting position and back, was completed in 2.2 sec. Each recording trial lasted 9 sec and was composed of two cycles separated by 2 sec (see Fig. 3). The trial was built in this way to make certain that the passing screen enters the crab's visual field twice from two opposite sides during each trial, thus ensuring animals are similarly stimulated regardless of their positions inside the recording container. This moving stimulus evokes a strong escape reaction and has been used for >10 years in our laboratory to study the escape response of Chasmagnathus as well as its modification by learning. In nature, Chasmagnathus is preyed on by different species of seabirds that approach the crab using different strategies. Consequently, the visual features of a predatory attack vary widely for the crab. Because crabs have to cope with predators approaching at different speeds and even with sudden changes of speeds, MDNs seem to be divided into several classes tuned to respond to different velocities (Wiersma et al., 1982). An object moving along an angular trajectory, as that performed by the VDS, encompasses multiple tangential velocities and, consequently, can stimulate several of these classes of neurons simultaneously. This may explain why the VDS proved to be so efficient for eliciting a reliable escape response in crabs. In addition, given the variability in the receptive field among MDNs (see Results), the use of a moving stimulus that encompasses a large portion of the dorsal-receptive field provided a useful way of optimizing the collection of electrophysiological data for the present study.
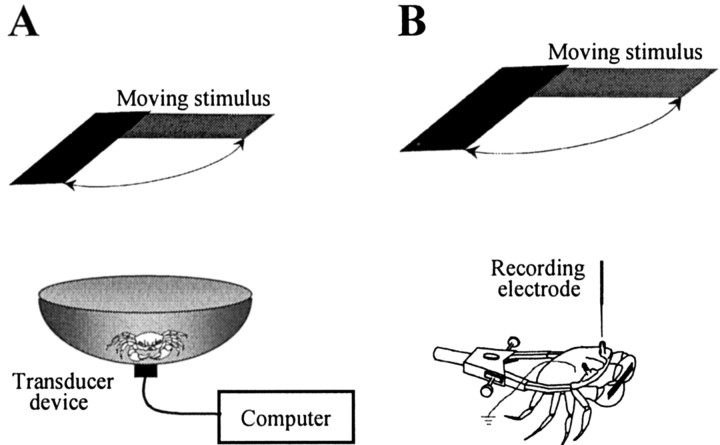
Figure 1.
Experimental setups and recording procedures. A, Behavioral experiments were conducted in an actometer consisting of a bowl connected to a transducer device, such that locomotion by the crab was translated into voltage changes. Voltage signals were digitized and recorded in a computer. B, For intracellular recording, the crab was held in an adjustable clamp that allowed free movements of the walking legs. The eyestalks were cemented to the carapace, and a small piece of cuticle was removed from the tip of one eye without damaging the ommatidial area. A glass microelectrode was advanced through this opening.
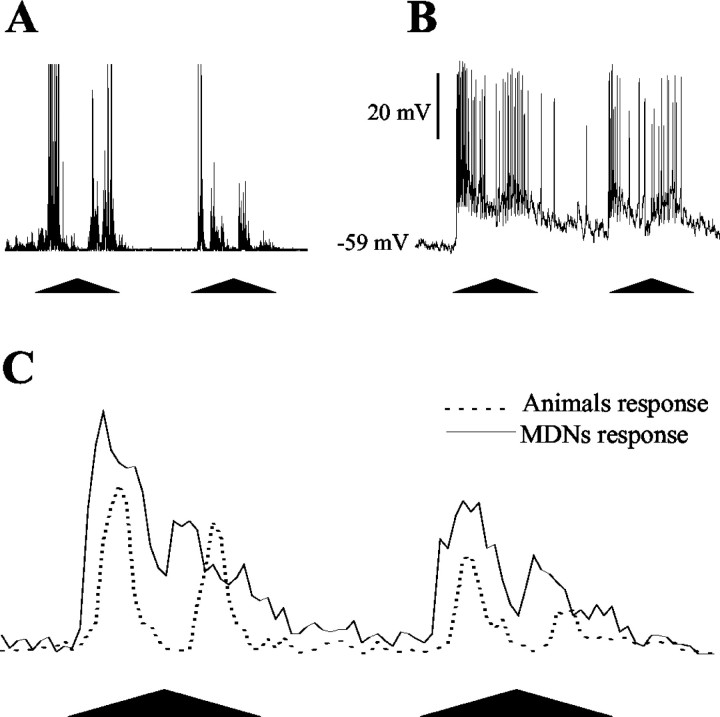
Figure 3.
The visually elicited behavioral and neuronal responses. The stimulus and the data recording system used in behavioral and electrophysiological experiments were the same, which allowed us to compare both types of responses in the time domain. A, Recording (in arbitrary units) of the escape response to the moving visual stimulus during one trial. B, Recording of the response of a MDN to the same stimulation. C, Peristimulus time histograms of the behavioral and neuronal responses derived from population data (escape responses: n = 40, one per crab; neuronal responses: n = 40, one per crab). The number of voltage peaks (behavior) or action potentials (neurons) elicited by the visual stimulus was counted. Bins are 100 msec. Each trial comprises two cycles of stimulus movement separated by 2 sec.
Behavioral experiments were conducted in an actometer (Fig. 1 A) consisting of a bowl-shaped container with a steep concave wall 12 cm high (top diameter, 23 cm; floor diameter, 9 cm), covered to a depth of 0.5 cm with marine water. The container was connected to a transducer device such that locomotion by the crab inside the container was translated into voltage changes. These signals were digitized and recorded in a computer (see below).
Intracellular recordings from the optic lobe were performed as described by Berón de Astrada et al. (2001). The crab was firmly held in an adjustable clamp that allowed free movements of the walking legs but reduced movements of the chelae (Fig. 1 B). The eyestalks were cemented to the carapace at an angle of ∼70° from the horizontal line, which corresponds to their normal seeing position. A tangential cut performed with a sharp scalpel was made to remove a small piece of thin cuticle (∼500 μm in diameter) from the tip of the eyestalk without causing damage to the ommatidial area. The crab was held inside the actometer with half its body submerged in water using magnetic holding devices. The glass microelectrode was then positioned and advanced through the opening in the cuticle. Microelectrodes (borosilicate glass; outer diameter, 1.2 mm; inner diameter, 0.68 mm) were pulled on a Brown–Flaming micropipette puller (P-77; Sutter Instrument, Novato, CA), yielding tip resistances of 40–60 MΩ when filled with 3 m KCl. A bridge balance amplifier was used for intracellular recordings (Axoclamp 2B; Axon Instruments, Foster City, CA). The output of the amplifier was monitored on an analog oscilloscope, digitized at 1.5 kHz (Digidata 1200; Axon Instruments) and recorded in a computer for subsequent analysis. Experiments were all performed at the membrane resting potential. In a series of experiments aimed at assessing changes in neuronal excitability during training, 500 msec pulses of depolarizing current were delivered through the recording microelectrode. For each cell, the current required to elicit 6–12 spikes during the pulse was established before starting the training and was kept constant throughout. A single pulse was delivered 10 sec before every training trial.
Experimental protocols. Both behavioral and electrophysiological experiments began after a black curtain was lowered in the front part of the cage and then after the animal remained visually undisturbed for 10 min. In electrophysiological experiments, the adaptation period began after the impaled cell was identified as a MDN from the lobula by its response to motion above the animal. Such a neuron was usually encountered within a few minutes of probing. Because our interest was to investigate MDNs that are possibly involved with the escape response of Chasmagnathus elicited by the VDS moving overhead, we limited our study to those neurons with receptive fields, at least in part, in the dorsal portion of the eye. MDNs that responded to movements in different positions around the equator of the eye, but not in the dorsal part, were not taken into account for the present study (Berón de Astrada and Tomsic, 2002).
Two different training protocols were used according to previous behavioral and pharmacological studies (Pedreira et al., 1998; Hermitte et al., 1999). Massed training consisted of repeated trials once every 2 sec. Spaced training consisted of the trials being presented once every 3 min. Because each trial lasted 9 sec (see above), a 15 trial session of massed training was concluded in ∼2 min, whereas an equivalent session of spaced training lasted 45 min. Long-term memory experiments included a training and a testing session separated by 24 hr. During the day of training, control animals received the same experimental manipulations as trained animals, except for the presentation of the moving stimulus. No recordings were performed during training. At the test day, responses to the stimulus were evaluated in both control and trained animals. In electrophysiological experiments, the response of a MDN to the first presentation of the stimulus was recorded after a resting period of 10 min. Only one neuron per animal was evaluated. Cells were recorded by one of us unaware of the treatment received by the animal on the day of training.
In the present study, the response of MDNs was assessed in restrained crabs. In this condition, although spontaneous leg movements occur, leg movements in an attempt to escape the VDS seldom happen (Berón de Astrada and Tomsic, 2002). Nevertheless, it has been shown previously that restrained crabs trained with repeated presentations of the VDS are able to learn and exhibit normal memory (Tomsic et al., 1991; Tomsic, 1993).
Data analysis. The stimulus control and the data recording system used in behavioral and electrophysiological experiments were the same, which allowed us to compare both types of responses in the time domain. The escape response was transduced and recorded as a train of voltage changes, with peaks corresponding to running steps of the escape reaction. Hence, the behavioral response in a trial was estimated by the number of voltage peaks recorded during the periods of visual motion stimulation (cycles 1 + 2). Likewise, the neuronal response in a trial was estimated by the number of spikes recorded during the periods of stimulation (cycles 1 + 2). The stimulus-evoked EPSP was measured as the area of the electrical response after action potentials had been digitally removed from the records. On repeated trial experiments, successive responses were normalized to the first response of the series. Data reported in the text and figures are mean ± SEMs.
Results
The MDNs of the crab
In Chasmagnathus, as in the crayfish (Glantz, 1994, 1998; Glantz and Bartels, 1994; Glantz et al., 1995), sustaining, dimming, and several classes of nonspiking interneurons show strong responses to changes in light intensity and, to a lesser extent, show some motion sensitivity. A distinctive class of spiking neurons, however, shows responses that reveal a clear-cut preference for moving stimuli compared with stimuli consisting in stationary changes in the intensity of light (Fig. 2).
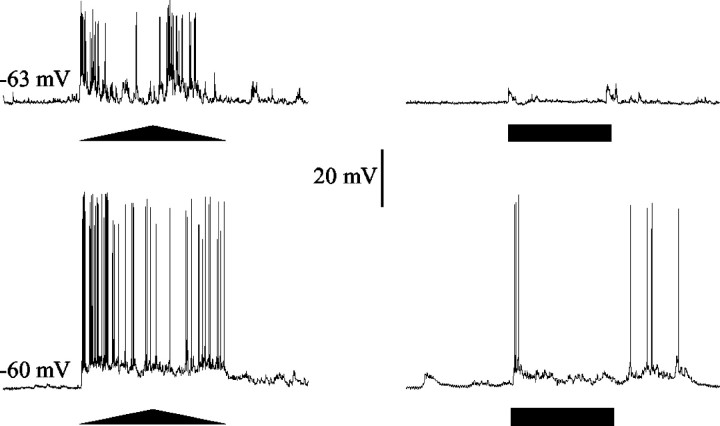
Figure 2.
Responses to stationary and to moving stimuli in MDNs of the crab. MDNs show a much greater sensitivity to a moving target (triangle) than to overall changes in luminosity (bar). Each row shows the response of a single cell to the VDS (left trace) and to a pulse of light (right trace). The horizontal black bars represent 1 sec duration of light stimulation. In this and subsequent illustrations, the two top sides of the triangles below the traces represent the clockwise and counterclockwise movements of the stimulus contained in a cycle, and the base of the triangle represents 2.2 sec of record.
The characteristics of these MDNs of Chasmagnathus have been described previously (Berón de Astrada and Tomsic, 2002). However, before starting to analyze the role of these neurons in the escape response elicited by the VDS, it is convenient to summarize their main features. Intracellular dye injections revealed that MDNs of the crab arborize extensively in the lobula and in the lateral protocerebrum. In the lobula, the dendritic tree is organized as a series of tangential branches that run parallel to each other, suggesting that the cell collects the information arriving through the columnar organization over a wide field. The somata is located in the cell body cluster, laying beneath the lobula. The axon projects centripetally and leaves the optic ganglia through the protocerebral tract. The response to a moving stimulus consists of a strong discharge of action potentials frequently superimposed on noisy graded potentials (Fig. 2). The response of each neuron is highly consistent on repeated stimulation, but such consistency can be obtained only when the stimuli are separated by long intervals. Repeated stimulation at intervals of <10 min produces a reduction of the response (see below). The magnitude of the graded potentials and spikes varies widely among neurons, in part because of differences in the site of impalement. The extent and location of the receptive field varies among MDNs; hence, the profile of the response to the VDS reflects some variability among MDNs. As is characteristic in all movement-sensitive neurons, including those of vertebrates, the movement-elicited response of MDNs of Chasmagnathus is relatively independent of the background intensity and of the contrast between target and background. The responses of most MDNs do not appear to be directionally sensitive to stimuli moving overhead (cf. Berón de Astrada and Tomsic, 2002).
Temporal analysis of the behavioral and the neuronal response
To begin analyzing the role of MDNs on the visually elicited escape response and on its modification by learning, we first compared the neuronal activity evoked by the VDS with the behavioral activity elicited by the same stimulus (Fig. 3A,B). The peristimulus time histograms of Figure 3C show that the firing profile of the cells corresponds well with that of the behavioral reaction, whereas a comparison of the times taken to reach half of the maximum responses in the first cycle reveals that, on average, the neuronal reaction anticipates the behavioral response by ∼120 msec. This suggests that MDNs take part in the circuitry that controls the escape response elicited by stimuli moving overhead. We then explored whether MDNs are involved in visual learning induced by repeated stimulation.
The response of MDNs during learning and short-term memory
Memory formation is known to depend on the frequency of trial presentations (Hintzman, 1974). In different animals and using various learning tasks, it has been shown that short intertrial intervals (massed training) result in short or intermediate memories, whereas long intertrial intervals (spaced training) result in longer-lasting memories (Tully et al., 1994; Menzel et al., 2001). In the crab, massed and spaced training, respectively, generate the SM and the CSM described above. These two different types of memory have been distinguished in the crab at the behavioral (Pedreira et al., 1998), pharmacological (Hermitte et al., 1999), and molecular (Freudenthal and Romano, 2000) levels. Therefore, we investigated whether the two memories could be revealed at the level of response of the MDN.
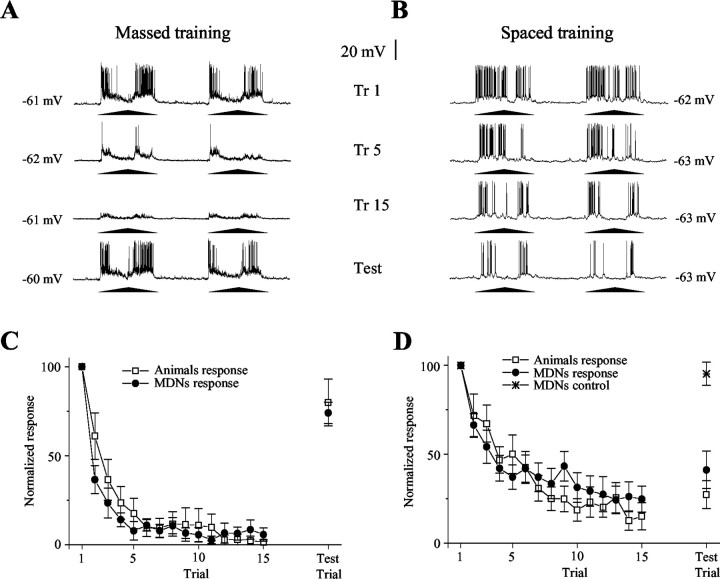
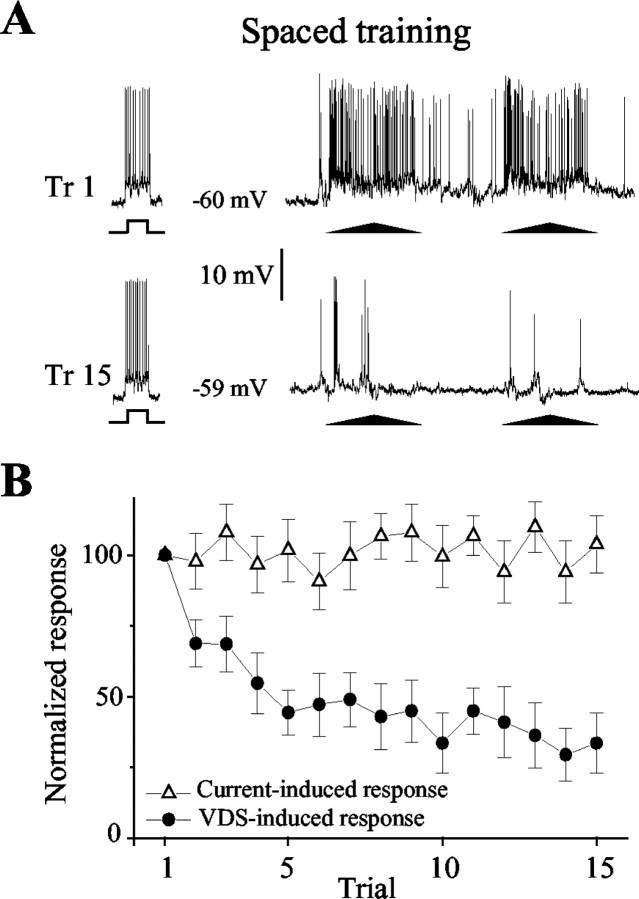
We found that the rates of reduction and recovery of the response of MDNs in both massed and spaced training coincide with the changes occurring at the behavioral level. Massed training yields a fast and profound reduction of the responses, which recover completely after 15 min at both the behavioral and neuronal levels. In contrast, spaced training results in a slower and shallower reduction of the responses, but the changes last longer (Fig. 4). The intracellular recordings showed no changes in the membrane resting potential either during massed (trial 1, 62.8 ± 2.8 mV; trial 15, 60.4 ± 2.3 mV) or spaced (trial 1, 63.4 ± 3 mV; trial 15, 63.8 ± 3.3 mV) training. In both training conditions, the analysis of the VDS-evoked EPSP showed a significant reduction. Interestingly, whereas in massed training the percentage of reduction of the EPSP parallels the percentage of reduction of the number of evoked spikes (percentage of initial response at trial 15: EPSP, 12 ± 4%; spikes, 10 ± 4%), in spaced training the EPSPs were reduced to a much lesser extent than the spikes (percentage of initial response at trial 15: EPSP, 62 ± 4%; spikes, 34 ± 6%). A reduction in the number of spikes without any modification of the EPSP could have been a convincing indication for a postsynaptic locus of plasticity. However, because there is not a simple relationship between the magnitude of the EPSP and the number of evoked spikes, the lesser reduction of the EPSP compared with that of spikes observed in spaced training does not allow us to make a conclusion on the site of plasticity.
Figure4.
Changes in MDNs reflect learning as well as short- and long-term memory. A, Fifteen trials of massed training cause a rapid and deep reduction of the response of the MDN that, however, recovers completely in <15 min (test). B, The same amount of spaced training results in a slow decrease of the response that, however, endures until the test. Trials 1, 5, and 15 and the test trial are shown. Each training trial comprises two cycles of stimulus movement separated by 2 sec. C, Averaged neuronal responses as those shown in A (n = 10 neurons from 10 crabs) compared with averaged behavioral responses (n = 20 crabs) evoked by massed training. D, Averaged neuronal responses as those shown in B (n = 12 neurons from 12 crabs) compared with averaged behavioral responses(n=20crabs)evoked by spaced training. Control(n=8)refers to MDNs from crabs that received only the first trial and the test trial. Although massed and spaced training induce different response changes, within each training condition the behavioral and neural performances appear identical. Responses were normalized to the first response of each recording. Graphs show means ± SEMs.
In spaced training, the neuronal responses were recorded for >1 hr. This length of intracellular recording in an intact living animal is certainly exceptional, and it is even more so considering that during the experiment the crab occasionally displays conspicuous movements of its legs. Thus, to ensure that the reduction of the neuronal response observed during training was a consequence of training and not a result of cell damage, we evaluated responses by the MDN to the visual stimulus after nearly 2 hr of impalement without training. No change in the response was detected (Fig. 4D, star). This control demonstrates that the reduction of the response observed in MDNs was specifically caused by repeated presentation of the visual stimulus and not because of other factors. Moreover, the change of response is specific for the moving stimulus, because the response of the neuron to a pulse of light measured before and after the repeated presentation of the training stimulus was not affected.
The response of MDNs during long-term memory
In the experiments described above, changes in the response of the MDN were assessed up to 15 min after training. Because spaced training induces a memory that lasts for several days (Hermitte et al., 1999), we investigated whether the neuronal changes observed 15 min after training persisted for at least 24 hr after training. Thus, was long-term memory in the crab reflected by long-term change in the response of MDNs? To determine this, we trained crabs with 30 spaced trials and 24 hr later compared their behavioral and neuronal responses with those of control animals (see Materials and Methods). The comparisons show that training similarly affected the behavioral and the neuronal responses. Trained crabs showed a level of escape response (498 ± 97; n = 40 crabs) significantly lower than control animals (840 ± 100; n = 40 crabs; p < 0.05; two-sample t test; two-sided p value), whereas their MDNs showed a number of evoked spikes (87.74 ± 9.4; n = 50 neurons from 50 crabs) significantly lower than that from control animals (118.82 ± 10.6; n = 46 neurons from 46 crabs; p < 0.03; two-sample t test; two-sided p value). The results demonstrate that long-term CSM, which is expressed as a reduction in the intensity of the escape reaction, is correlated with the long-term reduction in the response of MDNs.
Analysis of the reduction in the response of the MDN
In the mollusk Hermissenda, learning was shown to induce changes in the response of identified neurons that were related to modifications of the membrane resting potential and the membrane conductance (i.e., that were caused by an alteration of the general excitability of the cell) (Alkon, 1980; Alkon et al., 1982). To evaluate this possibility, we assessed the excitability of the MDN during spaced training by using pulses of depolarizing current delivered through the recording microelectrode (see Materials and Methods). The results of these experiments were conclusive: the clearcut reduction in the response to the natural stimulus (the visual stimulus) was not caused by a widespread reduction in the excitability of the neuron, because the response to the pulse of current was unmodified by the training (Fig. 5). In this and the previous experiment, no changes in the membrane resting potential were observed to occur during training. As in the previous experiment, measurements of the VDS-evoked EPSP revealed a significant reduction during training. Therefore, two possibilities might account for the changes observed in the response of MDNs. First, the biophysical changes might actually be occurring in the MDN, but in local domains away from the recording site, which would make them undetectable by the microelectrode (Borst and Haag, 2002). Second, the changes in the response of the MDN might be the consequence of changes occurring in presynaptic neurons. To investigate the latter possibility, we performed the following experiments.
Figure 5.
The change of response of the MDN is not accounted for by a general reduction of the neuronal excitability. A pulse of depolarizing current was delivered intracellularly to the MDN 10 sec before each trial during spaced training. A, The response of a MDN to the pulse of current (left record) and to the VDS (right record) at trials 1 and 15. Whereas the response to the VDS declines on repetitive stimulation, the response to the pulse of current remains unchanged. B, Averaged neuronal responses from experiments such as that shown in A (n = 8 neurons from 8 crabs). Graphs show means ± SEMs.
The response of different classes of visual interneurons during learning
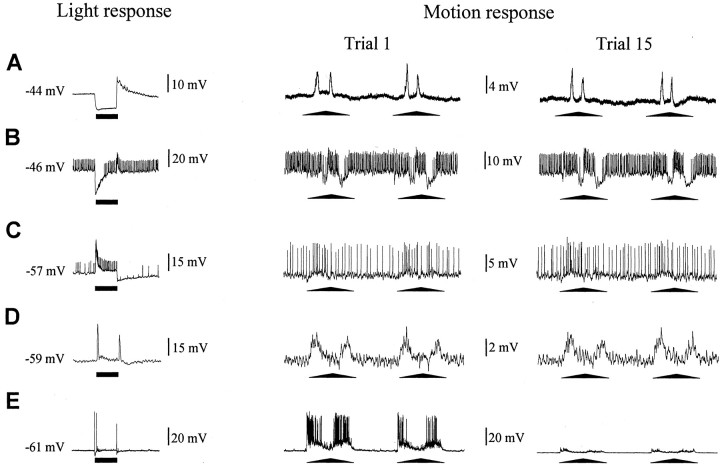
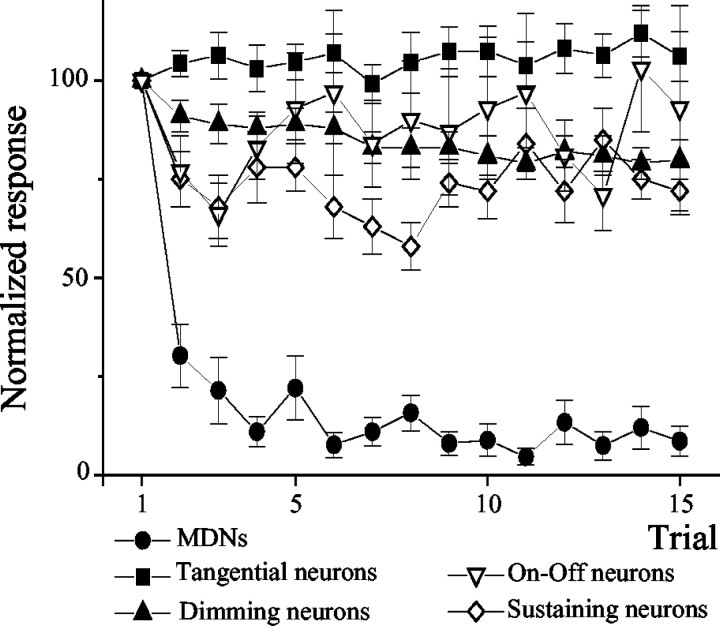
The receptive fields of neurons from the lobula are usually wider than those of neurons supplying it from the second optic neuropil (the medulla). MDNs presumably pool the retinotopic information, which reaches the lobula through the second optic chiasm (Kirk et al., 1982). Certainly, motion sensitivity is already present in neurons at the level of the medulla (Glantz, 1998), but these neurons are more sensitive to stationary changes of light intensity than to motion (Berón de Astrada and Tomsic, 2002) (Fig. 6A–E). Consequently, crucial steps in visual field integration and motion processing must occur between the medulla and lobula. Having detected changes in neurons in the lobula, we next investigated the responses to repeated presentations of the moving stimulus in neurons from the medulla. Because the presynaptic neurons to MDNs have not been determined, we decided to investigate several classes of interneurons that represent part of the morphological and physiological diversity that can be found in the medulla. The study was performed on four different classes of nonspiking and spiking medullary interneurons described previously, which are easy to impale and to distinguish by their response to a pulse of light (Berón de Astrada et al., 2001). These neurons were selected for the following reasons: (1) tangential neurons are local medullary interneurons; (2) sustaining and dimming neurons are, like MDNs, projecting interneurons, the axons of which leave the optic ganglia through the protocerebral tract; and (3) on–off neurons arborize in the medulla and send projections into the same region of the lobula where MDNs have their collator dendrites (our unpublished results). When stimulated with the VDS, these interneurons showed relatively weak but measurable graded or spiking responses. However, these responses did not change on repeated stimulation (Figs. 6A–D, 7). The steady-state responses of these neurons from the medulla, therefore, contrast markedly with the modification in the response of MDNs from the lobula (Figs. 6E, 7).
Figure 6.
Repeated visual stimulation does not affect the response of neurons from the second optic ganglion. Responses of different types of neurons to a pulse of light permitting their functional identification(left traces). Responses at the first(middle trace)and the last(right trace)trial showing the apparent lack of effect of successive stimulation with the moving stimulus(massed training) on the response of the different medulla interneurons. The three traces in each row correspond to the same neuron. A, Tangential neuron. B, Dimming neuron. C, Sustaining neuron. D, On–Off neuron. E, An MDN showing the characteristic response reduction. The bars below traces represent 1 sec pulse of light. Note that responses are displayed at different voltage scales. Graphs show means ± SEMs.
Figure 7.
Averaged results from recordings such as those shown in Figure 6. The figure shows the averaged results from recordings taken from tangential neurons (n = 10), dimming neurons (n = 11), sustaining neurons (n = 6), on–off neurons (n = 7), and MDNs (n = 8) during massed training. On repeated stimulation, the response of MDNs decreases dramatically by the second trial. In comparison, the responses of nonspiking and spiking interneurons from the second neuropil remain invariant. In nonspiking interneurons, the response was estimated as the area of the stimulus-elicited postsynaptic potentials. Graphs show means ± SEMs.
Discussion
Despite of the great deal of interest in studying visual memory in arthropods and the claim on the presumed simplicity of their nervous systems, identified neurons supporting visual learning have not been described in any arthropod. The present study on the crab shows that changes in the response of a group of identified MDNs closely reflect the behavioral changes that occur during learning, and that the persistence of these changes corresponds with memory retention. The neuronal changes induced by spaced training remain for at least 24 hr. The results suggest that MDNs are important elements of the neural substrate supporting short- and long-term visual memory in the crab.
Neuroplasticity within the arthropod optic ganglia
Most studies of visual motion processing by the arthropod optic lobes have focused on neurons that mediate optomotor behaviors, the responses of which remain essentially unmodified on repeated stimulation (Borst and Haag, 2002). Such studies have contributed to the notion that optic ganglia are sensory neuropils exclusively devoted to image processing. One consequence of this has been the tacit assumption that neural plasticity serving visual memory necessarily occurs at levels deeper than the optic lobes. However, the lobula is a center of high-level visual integration and, as in mammalian cortex, many of its neurons show binocular integration (Sztarker, 2000; Borst and Haag, 2002). There is also evidence that in the crab certain of these neurons even integrate tactile information (Berón de Astrada and Tomsic, 2002). Therefore, it should not be surprising that neurophysiological changes underlying learning and memory indeed take place in the lobula. In the locust, it has been shown that the response of the lobula giant MDN declines on repetitive visual stimuli (O'Shea and Rowell, 1975; Bacon et al., 1995). The modification of the response of the lobula giant neuron was assessed during a short time; therefore, it is not known whether the change persists for a long time, as has been shown here for the MDN of the crab.
The fact that we detected changes in the responses of lobula MDNs of the crab does not necessarily imply that neuroplasticity actually occurs in these cells. The changes might result from modifications in the MDN, from modifications in the activity of neurons directly or indirectly presynaptic to the MDN, or from modifications in both presynaptic and postsynaptic neurons. We did not detect modifications in parameters such as the membrane resting potential or the excitability of the MDN that could be related to the change of response to the VDS during training. However, this does not rule out the possibility that the physiological mechanism underlying this change may occur in the MDN. In particular, the postsynaptic modification could consist of biophysical events restricted to the synaptic input area. In such a case, electrophysiological recordings from sites remote to the synaptic region would not reveal that modification. The experiments performed on the four different classes of medullary neurons showed that none of the responses of the neurons was affected by training. Some of these neurons are thought to be presynaptic to the MDN, in particular the one here called on–off neuron, which sends projections into the same region of the lobula where MDNs have their collator dendrites. In the locust, experiments suggested that the changes underlying the response decline take place at the synapses (in the presynaptic and/or postsynaptic sites) linking unidentified incoming medulla neurons to the dendrites of the lobula giant MDN (O'Shea and Rowell, 1975). We cannot exclude the existence of medullary neurons presynaptic to the MDN, different from the ones that we recorded here. With this caveat in mind, our results are in agreement with those from the locust, suggesting that the modifications responsible for the changes observed in the response of wide-field MDNs occur at the level of the synapses between these lobula neurons and their presynaptic medullary neurons.
In contrast to the present account, the change of response occurring in the lobula giant neuron from the locust has not been studied in relation to the actual modification of a behavioral response (i.e., it has not been related to a learning process). The current study shows, for the first time, evidence that, in arthropods, short- and long-term plasticity underlying certain visual learning and memories already occurs within the optic lobes.
The role of the MDN in the CSM
In insects, mushroom bodies have been implicated as participating in contextual aspects of visual learning (Mizunami et al., 1998; Liu et al., 1999). For instance, in Drosophila, normal flies and mutant flies with miniature mushroom bodies both show similar abilities to learn a visual discrimination task. However, whereas normal animals can generalize the visual learned task to a different contextual situation, mutant flies cannot (Liu et al., 1999). This, among other results, suggests that different components of a single memory, such as that supporting generalization, may be stored in different parts of the arthropod brain. In the crab, long-term CSM is determined by an association between the VDS and the context. Consequently, substantial changes of the visual environment between training and test prevent the CSM from being evoked. In the present account, the study on the response of MDNs from trained crabs was performed, keeping constant the contextual environment between training and test. Under such conditions, the neuronal response reflected the behavioral expression of CSM. An important question that remains is whether the effect of changing the context on evoking the memory would be reflected by the performance of MDNs. Another important question to be addressed is to what extent MDNs reflect stimulus generalizations. In the crab, the learning acquired from the presentation of the VDS in a certain position can be generalized to the appearance of the VDS in a relatively different position above the animal. Preliminary results suggest that a similar degree of stimulus generalization would take place in MDNs
(J. Sztarker, personal communication). In summary, although MDNs appear as central elements regarding the acquisition and retention of visual memory in the crab, more research is certainly needed before their role in CSM can be completely defined.
Recording brain activity in vivo during learning is fundamental to understanding how memories are formed (Faber et al., 1999). However, intracellular recordings are rarely achieved in intact, behaving organisms (cf. Birt et al., 2003). It is notable that, in the present study, the neuronal changes were assessed by intracellular recordings in the living animal at the same time it was learning. Moreover, after the recording, the crab remains healthy and, several days after the experiment, no behavioral differences are observed with respect to nontreated animals. Thus, the visual memory abilities of crabs, their relatively simple and accessible nervous system, and the recording stability that can be achieved with their neurons provide an opportunity for uncovering neurophysiological and possibly molecular events that occur in identifiable neurons during learning.
Footnotes
This work was supported by the National Research Council of Argentina, by the University of Buenos Aires, and by Fundación Antorchas. We thank Drs. Nicholas Strausfeld, Raymond Glantz, and Héctor Maldonado for fruitful discussions and corrections to this manuscript.
Correspondence should be addressed to Dr. Daniel Tomsic, Laboratorio de Neurobiología de la Memoria, Departamento de Fisiología, Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Pabellón 2 Ciudad Universitaria (1428), Buenos Aires, Argentina. E-mail: tomsic@fbmc.fcen.uba.ar.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238539-08$15.00/0
References
- Alkon DL ( 1980) Membrane depolarization accumulates during acquisition of an associative behavioral change. Science 210: 1375-1376. [DOI] [PubMed] [Google Scholar]
- Alkon DL, Lederhendler I, Shoukimas JL ( 1982) Primary changes of membrane currents during retention of associative learning. Science 215: 693-695. [DOI] [PubMed] [Google Scholar]
- Bacon JP, Thompson KS, Stern M ( 1995) Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J Neurophysiol 74: 2739-2743. [DOI] [PubMed] [Google Scholar]
- Berón de Astrada M, Tomsic D ( 2002) Physiology and morphology of visual movement detector neurons in a crab (Decapoda: Brachyura). J Comp Physiol [A] 188: 539-551. [DOI] [PubMed] [Google Scholar]
- Berón de Astrada M, Sztarker J, Tomsic D ( 2001) Visual interneurons of the crab Chasmagnathus studied by intracellular recordings in vivo. J Comp Physiol [A] 187: 37-44. [DOI] [PubMed] [Google Scholar]
- Birt D, Aou S, Woody CD ( 2003) Intracellularly recorded responses of neurons of the motor cortex of awake cats to presentations of pavlovian conditioned and unconditioned stimuli. Brain Res 969: 205-216. [DOI] [PubMed] [Google Scholar]
- Borst A, Haag J ( 2002) Neural networks in the cockpit of the fly. J Comp Physiol [A] 188: 419-437. [DOI] [PubMed] [Google Scholar]
- Faber T, Joerges J, Menzel R ( 1999) Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci 2: 74-78. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Romano A ( 2000) Participation of REL/NF-κB transcription factors in long-term memory in the crab Chasmagnathus Brain Res 855: 274-281. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Zhang S, Arnim J, Menzel R, Srinivasan MV ( 2001) The concepts of “sameness” and “difference” in an insect. Nature 410: 930-932. [DOI] [PubMed] [Google Scholar]
- Glantz RM ( 1974a) Defense reflex and motion detector responsiveness to approaching targets: the motion detector trigger to the defense reflex pathway. J Comp Physiol 95: 297-314. [Google Scholar]
- Glantz RM ( 1974b) Habituation of the motion detectors of the crayfish optic nerve. J Neurobiol 5: 489-510. [DOI] [PubMed] [Google Scholar]
- Glantz RM ( 1994) Directional selectivity in a nonspiking interneuron of the crayfish optic lobe: evaluation of a linear model. J Neurophysiol 72: 180-193. [DOI] [PubMed] [Google Scholar]
- Glantz RM ( 1998) Directionality and inhibition in crayfish tangential cells. J Neurophysiol 79: 1157-1166. [DOI] [PubMed] [Google Scholar]
- Glantz RM, Bartels A ( 1994) The spatiotemporal transfer function of crayfish lamina monopolar neurons. J Neurophysiol 71: 2168-2182. [DOI] [PubMed] [Google Scholar]
- Glantz RM, Wyatt C, Mahncke H ( 1995) Directionally selective motion detection in the sustaining fibers of the crayfish optic nerve: linear and nonlinear mechanisms. J Neurophysiol 74: 142-152. [DOI] [PubMed] [Google Scholar]
- Hermitte G, Pedreira ME, Tomsic D, Maldonado H ( 1999) Context shift and protein synthesis inhibition disrupt long-term habituation after spaced, but not massed, training in the crab Chasmagnathus Neurobiol Learn Mem 71: 34-49. [DOI] [PubMed] [Google Scholar]
- Hintzman DI ( 1974) Theoretical implications of the spacing effect. Theories in cognitive psychology (Solso RL, ed), pp 77-99. Hillsdale, NJ: Erlbaum.
- Judd SPD, Collet TS ( 1998) Multiple stored views and landmark guidance in ants. Nature 392: 710-714. [Google Scholar]
- Kirk MD, Waldrop B, Glantz RM ( 1982) The crayfish sustaining fibers. I. Morphological representation of visual receptive fields in the second optic neuropil. J Comp Physiol [A] 146: 175-179. [Google Scholar]
- Liu L, Wolf R, Ernst R, Heisenberg M ( 1999) Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400: 753-756. [DOI] [PubMed] [Google Scholar]
- Lozada M, Romano A, Maldonado H ( 1990) Long-term habituation to a danger stimulus in the crab Chasmagnathus granulatus Physiol Behav 47: 35-41. [DOI] [PubMed] [Google Scholar]
- Maldonado H ( 2002) Crustaceans as models to investigate memory illustrated by extensive behavioral and physiological studies in Chasmagnathus In: The crustacean nervous system (Wiese K, ed), pp 314-327. Berlin: Springer.
- Menzel R, Manz G, Menzel R, Greggers U ( 2001) Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn Mem 8: 198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunami M, Weibrecht JM, Strausfeld NJ ( 1998) Mushroom bodies of the cockroach: their participation in place memory. J Comp Neurol 402: 520-537. [PubMed] [Google Scholar]
- O'Carroll D ( 1993) Feature-detecting neurons in dragonflies. Nature 362: 541-543. [Google Scholar]
- O'Shea M, Rowell CH ( 1975) Protection from habituation by lateral inhibition. Nature 254: 53-55. [DOI] [PubMed] [Google Scholar]
- Osorio D, Bacon JP ( 1994) A good eye for arthropod evolution. BioEssays 16: 419-424. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H ( 2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38: 863-869. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Romano A, Tomsic D, Lozada M, Maldonado H ( 1998) Massed and spaced training build up different components of long-term habituation in the crab Chasmagnathus Anim Learn Behav 26: 32-43. [Google Scholar]
- Pedreira ME, Pérez-Cuesta L, Maldonado H ( 2002) Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J Neurosci 22: 8305-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan MV, Zhang SW, Rolfe B ( 1993) Is pattern vision in insects mediated by ‘cortical' processing? Nature 362: 539-540. [Google Scholar]
- Strausfeld NJ ( 1998) Crustacean—insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav Evol 52: 186-206. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Nässel DR ( 1981) Neuroarchitectures serving compound eyes of crustacea and insects. In: Vision in invertebrates, Vol VII 16B, Handbook of sensory physiology (Autrum H, ed), pp 1-132. Berlin, Heidelberg, New York: Springer.
- Sztarker J ( 2000) Interneuronas monoculares y binoculares: indicios funcionales de la organización circuital del sistema visual en el cangrejo Chasmagnathus. PhD thesis, University of Buenos Aires.
- Tang S, Guo A ( 2001) Choice behavior of Drosophila facing contradictory visual cues. Science 294: 1543-1546. [DOI] [PubMed] [Google Scholar]
- Tomsic D ( 1993) Percepción nociceptiva y habituación a un estímulo visual de peligro en el cangrejo Chasmagnathus. PhD thesis, University of Buenos Aires.
- Tomsic D, Rakitin A, Maldonado H ( 1991) Morphine and GABA: effect on perception, escape response and long term habituation to a danger stimulus in the crab Chasmagnathus Brain Res Bull 26: 699-706. [DOI] [PubMed] [Google Scholar]
- Tomsic D, Massoni V, Maldonado H ( 1993) Habituation to a danger stimulus in two semiterrestrial crabs. Ontogenic, ecological and opioid system correlates. J Comp Physiol [A] 173: 621-633. [Google Scholar]
- Tomsic D, Pedreira ME, Romano A, Hermite G, Maldonado H ( 1998) Context-US association as a determinant of long-term habituation in the crab Chasmagnathus Anim Learn Behav 26: 196-209. [Google Scholar]
- Tully T, Preat T, Boyton SC, Del Vecchio M ( 1994) Genetic dissection of consolidated memory in Drosophila Cell 79: 35-47. [DOI] [PubMed] [Google Scholar]
- Wiersma CAG, Roach JLM, Glantz RM ( 1982) Neural integration in the optic system. In: The biology of the crustacea, Vol 4, Neural integration and behavior (Sandeman DC, Atwood HL, eds), pp 1-31. New York: Academic.